Abstract
From a laboratory strain of the pea aphid, Acyrthosiphon pisum, we discovered a previously unknown facultative endosymbiotic bacterium. Molecular phylogenetic analysis based on 16S ribosomal DNA revealed that the bacterium is a member of the genus Spiroplasma. The Spiroplasma organism showed stable vertical transmission through successive generations of the host. Injection of hemolymph from infected insects into uninfected insects established a stable infection in the recipients. The Spiroplasma symbiont exhibited negative effects on growth, reproduction, and longevity of the host, particularly in older adults. Of 58 clonal strains of A. pisum established from natural populations in central Japan, 4 strains possessed the Spiroplasma organism.
Almost all aphids (Homoptera: Aphididae) possess an intracellular symbiotic bacterium, Buchnera sp., in the cytoplasm of mycetocytes (or bacteriocytes), hypertrophied cells in the abdomen specialized for endosymbiosis (1, 3). The aphids and their Buchnera symbionts are intimately mutualistic; the symbionts cannot survive when removed from the host cells, and the aphids suffer sterility or death when deprived of their symbionts (20, 23, 29). Aphids are thought to provide their Buchnera symbionts with a stable niche and nutrients, while Buchnera symbionts synthesize essential amino acids and other nutrients for the host (1, 7, 8). The evolutionary origin of the endosymbiotic association is believed to be quite ancient. Buchnera symbionts of various and distantly related aphid species are descended from a bacterium that was acquired by the common ancestor of extant aphids and have cospeciated with their hosts (27, 28). Phylogenetically, the Buchnera symbionts belong to the γ subdivision of the Proteobacteria (34). Because of their prevalence and importance in aphids, Buchnera spp. and the mycetocytes harboring them are often referred to as primary symbionts (P symbionts) and primary mycetocytes, respectively.
In addition to the Buchnera P symbionts, a number of aphids possess additional types of vertically transmitted endosymbiotic bacteria, which have been collectively referred to as secondary symbionts (S symbionts) or accessory symbionts (3, 10, 11, 15, 31). These additional bacteria are found in many, but not all, lineages of aphids, show remarkable differences in morphology, localization, and quantity between lineages, and are thought to be of polyphyletic evolutionary origins (3, 10, 11, 15, 31). They are often, but not always, harbored in different types of specialized cells, such as secondary mycetocytes and sheath cells, which constitute a mycetome (or bacteriome) with the primary mycetocytes in the abdomen of the insects, whereas other tissues and hemolymph may also be populated by them (4, 16). The microbiological nature of the S symbionts from various aphids is mostly unknown. To date, molecular phylogenetic studies on S symbionts of aphids have been conducted only on those of Acyrthosiphon pisum, Macrosiphum rosae, and Uroleucon complex (4, 5, 31, 34). Biological effects of the S symbionts on the host insects are largely unknown, although two facultative S symbionts of A. pisum were recently shown to have mostly negative effects on the growth and reproduction of the host (6). Some S symbionts exhibit partial infection in host populations, in which cases they are likely to be facultative endosymbiotic associates of a neutral or parasitic nature (4, 5, 16, 31). Other S symbionts exhibit complete infection in host populations and occupy a considerable biomass in the host body, in which cases they may be essential endosymbiotic companions in addition to the Buchnera P symbiont (10, 15, 31).
From the pea aphid, A. pisum, several different types of facultative S symbionts have been characterized. A rod- or tube-shaped bacterium closely related to Serratia spp. was identified in the secondary mycetocytes, sheath cells, and hemocoel of a number of American and Japanese strains and referred to as a pea aphid secondary symbiont (PASS) (4, 6), S symbiont (16, 34), or R-type symbiont (31). A rod-shaped bacterium which belongs to the genus Rickettsia was identified in the hemolymph of American strains of A. pisum and referred to as pea aphid Rickettsia (PAR) (5). Recently, two other endosymbiotic bacteria were reported from American strains of A. pisum and designated a T-type symbiont and a U-type symbiont (31). Therefore, the endosymbiotic organization of A. pisum can be formulated as the obligate P symbiont Buchnera together with one or a few optional S symbionts. A number of interesting subjects concerning the S symbionts have been little investigated, such as biological effects on the host, cooperative or antagonistic interactions with the P symbiont Buchnera, localization and population dynamics through the host's developmental course, etc. In order to understand the complex endosymbiotic relationships, a thorough grasp of the endosymbiotic microbiota in populations of A. pisum is essential.
In the present study, we report the discovery of a novel type of secondary endosymbiotic bacterium, which belongs to the genus Spiroplasma, from a Japanese strain of A. pisum. The microbiological nature, inheritance and transmission, effects on the host insect, and prevalence in natural populations of the Spiroplasma symbiont were investigated.
MATERIALS AND METHODS
Materials.
The Japanese strains of A. pisum used in this study are listed in Table 1. Seven laboratory strains are the same as those previously investigated (16). The other 58 strains are newly established isofemale lines collected by one of us (T.T.) from April to June 2000 at 43 localities covering central Japan. All these strains were reared on seedlings of the broad bean, Vicia faba, at 20°C in a long-day regimen, 16 h of light and 8 h of dark.
TABLE 1.
Japanese strains of the pea aphid A. pisum (Harris) used in this study
| Straina | Locality | Collection date | P symbiont | S symbiont | Spiroplasma |
|---|---|---|---|---|---|
| AIST | Tsukuba, Ibaraki | 7 April 1999 | + | − | − |
| EF99 | Suginami-ku, Tokyo | 22 April 1999 | + | − | − |
| HG99 | Bunkyo-ku, Tokyo | 22 April 1999 | + | + | − |
| ISb | No data | No data | + | + | − |
| MR88c | Morioka, Iwate | 15 August 1988 | + | + | − |
| SMb | No data | No data | + | − | + |
| TKC93d | Tokachi, Hokkaido | 23 June 1993 | + | + | − |
| ASRe | Ashiro-machi, Iwate | 29 June 2000 | + | + | − |
| AZ | Azuma-machi, Ibaraki | 10 May 2000 | + | − | − |
| BD | Bandai-machi, Fukushima | 6 June 2000 | + | − | − |
| BJ-1 | Kousei-cho, Shiga | 25 April 2000 | + | − | − |
| BJ-2 | Kousei-cho, Shiga | 25 April 2000 | + | − | − |
| GSe | Gosen, Niigata | 6 June 2000 | + | + | − |
| GY | Yoshioka-machi, Gunma | 27 May 2000 | + | − | − |
| HM-1 | Hashima, Gifu | 25 April 2000 | + | − | − |
| HM-2 | Hashima, Gifu | 25 April 2000 | + | − | − |
| HS | Higashisumiyoshi-ku, Osaka | 12 May 2000 | + | + | − |
| INT | Ina-machi, Ibaraki | 9 May 2000 | + | + | − |
| IT-1 | Toyoda-machi, Shizuoka | 25 April 2000 | + | + | − |
| IT-2 | Toyoda-machi, Shizuoka | 25 April 2000 | + | − | − |
| KH | Uenohara-machi, Yamanashi | 26 May 2000 | + | − | − |
| KNf | Kannari-machi, Miyagi | 30 June 2000 | + | − | − |
| KNMe | Kunimi-machi, Fukushima | 29 June 2000 | + | − | − |
| KRe | Koriyama, Fukushima | 6 June 2000 | + | − | − |
| KSe | Kurosaki-machi, Niigata | 6 June 2000 | + | + | − |
| KSM | Kasumigaura-machi, Ibaraki | 11 May 2000 | + | + | − |
| KT-1 | Kyoto, Kyoto | 25 April 2000 | + | − | − |
| KT-2 | Kyoto, Kyoto | 25 April 2000 | + | + | − |
| KW | Kishiwada, Osaka | 25 April 2000 | + | + | − |
| MB | Murasakibara, Kagoshima | 22 April 2000 | + | + | − |
| MD-1 | Matsudo, Chiba | 7 May 2000 | + | − | − |
| MD-2 | Matsudo, Chiba | 7 May 2000 | + | − | + |
| ME | Owatari-machi, Gunma | 25 May 2000 | + | + | − |
| MIH-1 | Miho-mura, Ibaraki | 10 May 2000 | + | − | − |
| MIH-2 | Miho-mura, Ibaraki | 10 May 2000 | + | + | − |
| ND-1 | Numadu, Shizuoka | 25 April 2000 | + | − | − |
| ND-2 | Numadu, Shizuoka | 25 April 2000 | + | + | − |
| NGg | Nagasaka-machi, Yamanashi | 26 May 2000 | + | + | − |
| NI | Nissin, Aichi | 25 April 2000 | + | − | + |
| NM | Nou-machi, Niigata | 26 May 2000 | + | + | − |
| OB | Obuse-machi, Nagano | 26 May 2000 | + | + | − |
| OT | Otsu, Shiga | 25 April 2000 | + | + | − |
| OY | Oyama, Tochigi | 26 May 2000 | + | − | − |
| SIT | Suita, Osaka | 25 April 2000 | + | − | − |
| SZK-1 | Suzuka, Mie | 26 April 2000 | + | + | − |
| SZK-2 | Suzuka, Mie | 26 April 2000 | + | + | − |
| TAG-1 | Taga-cho, Shiga | 25 April 2000 | + | + | − |
| TAG-2 | Taga-cho, Shiga | 25 April 2000 | + | + | − |
| TAN | Tanuma-machi, Tochigi | 25 May 2000 | + | + | − |
| TD-1 | Tamadukuri-machi, Ibaraki | 11 May 2000 | + | − | − |
| TD-2 | Tamadukuri-machi, Ibaraki | 11 May 2000 | + | + | − |
| TS | Toyoshina-machi, Nagano | 26 May 2000 | + | + | − |
| TT-1 | Toyota, Aichi | 25 April 2000 | + | − | − |
| TT-2 | Toyota, Aichi | 25 April 2000 | + | + | − |
| TU | Tsuchiura, Ibaraki | 10 May 2000 | + | − | − |
| URN-1 | Ureshino-machi, Mie | 26 April 2000 | + | − | − |
| URN-2 | Ureshino-machi, Mie | 26 April 2000 | + | − | − |
| WY-1 | Wakayama, Wakayama | 25 April 2000 | + | + | − |
| WY-2 | Wakayama, Wakayama | 25 April 2000 | + | − | − |
| YD | Yuda-machi, Iwate | 29 June 2000 | + | − | − |
| YI | Yokkaichi, Mie | 26 April 2000 | + | + | − |
| YR-1 | Yoro-machi, Gifu | 25 April 2000 | + | − | + |
| YR-2 | Yoro-machi, Gifu | 25 April 2000 | + | − | + |
| YY-1 | Yachiyo, Chiba | 30 April 2000 | + | + | − |
| YY-2 | Yachiyo, Chiba | 30 April 2000 | + | + | − |
The first seven strains were investigated previously (16); the rest are newly established lines (see Materials and Methods). Unless otherwise indicated, the original host plant was Vicia sativa. Numbers following strain names (e.g., BJ-1 and BJ-2) indicate that the strains were established from different insects collected at the same locality.
Original host plant unknown.
Original host plant, Pisum sativum.
Original host plant, Glycine max.
Original host plant, Tolifolium retens.
Original host plant, Tolifolium pratense.
Original host plant, Vicia amoena.
Molecular biological procedures.
The DNA of A. pisum and its endosymbionts was individually extracted from an insect using a QIAamp tissue kit (Qiagen). PCR amplification of eubacterial 16S ribosomal DNA (rDNA) using primers 16SA1 and 16SB1, cloning of the products, typing of the clones by restriction fragment length polymorphism (RFLP), and sequencing of the clones were conducted as previously described (16).
Molecular phylogenetic analysis.
A multiple alignment of 16S rDNA sequences was conducted using the program package Clustal W (33). The final alignment was inspected and corrected manually. Ambiguously aligned regions were excluded from the phylogenetic analysis. Nucleotide sites that included an alignment gap(s) were also omitted from the aligned data set. Neighbor-joining trees (30) were constructed with Kimura's two-parameter distance (25) using Clustal W. Maximum-parsimony trees were constructed using the program package PAUP 4.0b2 (32). A bootstrap test (9) was conducted with 1,000 resamplings.
Diagnostic PCR of laboratory strains.
Diagnostic PCR detection of 16S rDNA of the endosymbiotic bacteria was performed using specific reverse primers ApisP1 for the P symbiont Buchnera, PASScmp for the PASS, Rick16SR for Rickettsia spp., and TKSSsp for Spiroplasma spp., in combination with the universal forward primer 16SA1, under a temperature profile of 94°C for 2 min followed by 30 cycles of 94°C for 1 min, 55°C for 1 min, and 70°C for 1 min (Table 2).
TABLE 2.
PCR primers used in this study
| Target gene | Primer | Sequence (5′→3′) | Directiona | Specificity | Reference |
|---|---|---|---|---|---|
| 16S rDNA | 16SA1 | AGAGTTTGATCMTGGCTCAG | F | Universal | 12 |
| 16SB1 | TACGGYTACCTTGTTACGACTT | R | Universal | 12 | |
| ApisP1 | TTCCAGTGTGGCTGGTTA | R | Buchnera | This study | |
| Buch16S1F | GAGCTTGCTCTCTTTGTCGGCAA | F | Buchnera | This study | |
| Buch16S1R | CTTCTGCGGGTAACGTCACGAA | R | Buchnera | This study | |
| PASScmp | GCAATGTCTTATTAACACAT | R | PASS | 16 | |
| Rick16SR | CATCCATCAGCGATAAATCTTTC | R | Rickettsia | This study | |
| TKSSsp | TAGCCGTGGCTTTCTGGTAA | R | Spiroplasma | 14 | |
| dnaA | ApDnaAF1 | ATTCTTCAGTAAAAATGCTTGGA | F | Spiroplasma | Tsuchida et al., unpublished data |
| ApDnaAR1 | ACACATTTACTTCATGCTATTGA | R | Spiroplasma | Tsuchida et al., unpublished data | |
| groEL | GroEAF1 | CCTCAAGGCTGTGGCCG | F | PASS | Koga et al., unpublished data |
| GroEAR1 | TAGGCACCATCTCTGCAAACTC | R | PASS | Koga et al., unpublished data |
F, forward; R, reverse.
Examination of hemolymph.
The abdominal dorsa of adult aphids, the surfaces of which had been sterilized by washing with 70% ethanol and sterile water, were fixed onto glass slides with Scotch tape. The legs of the insects were removed by forceps, and hemolymph was collected from the injury with a glass capillary. About 1 μl of hemolymph was subjected to either DNA extraction with a QIAamp tissue kit or direct microscopic observation. For the latter procedure, the hemolymph was applied to microscope immersion oil on a glass slide, covered with a coverslip, and observed with a phase-contrast microscope. For reference, a male-killing Spiroplasma strain harbored by a Drosophila melanogaster strain was observed at the same time.
Injection of hemolymph.
Injection of hemolymph was performed under a dissecting microscope using ultrathin glass capillary tubes made by a microelectrode maker PN-3 (Narishige Scientific Instrument) connected to an air pump. Hemolymph of the donor insects, adults of the strain SM, was prepared as described above and sucked into a capillary tube. The capillary was inserted into the base of the second or third leg of recipient insects, third-instar nymphs of strain AIST, which were anesthetized in a stream of carbon dioxide. About 0.1 μl of hemolymph was injected into the recipients by air pressure. To confirm that injection itself does not damage or affect the recipient insects, we also transferred hemolymph between AIST insects and between SM insects as control treatments.
Evaluation of effects on the host insect.
To evaluate the effects of the Spiroplasma infection on the fitness parameters of the host insect, two insect lines of identical AIST genetic backgrounds with and without the Spiroplasma symbiont were established. One line was injected with hemolymph of strain SM and the other line was injected with hemolymph of strain AIST. We used these lines for experiments 6 months after the injection, when the former line, AIST-Sp+, had been stably infected with the Spiroplasma and the latter line, AIST-Sp−, was free of it. The insects were individually reared on seedlings of the broad bean at 20°C in a long-day regime, 16 h of light and 8 h of dark, and their fresh body weight, production of offspring, and life span were monitored.
Diagnostic PCR of newly established, field-collected strains.
The newly established strains of A. pisum collected from natural populations in Japan were examined for infection with Spiroplasma, PASS, and Buchnera by specific PCR detection. To prepare template DNA, a simple boiling-preparation method was adopted (17): an insect was squashed in a 0.5-ml plastic tube with 100 μl of SB (10 mM Tris-HCl [pH 8.0], 1 mM EDTA, 25 mM NaCl, 2 mg of proteinase K/ml), incubated at 55°C for 30 min, heated at 99°C for 10 min, and centrifuged, and the resultant supernatant was used for PCR detection. To confirm reproducibility of the results, two second- or third-instar nymphs from each strain were independently subjected to the analysis. Spiroplasma was detected using primers 16SA1 and TKSSsp, targeting 16S rDNA, and primers ApDnaAF1 and ApDnaAR1, targeting dnaA. PASS was detected using primers 16SA1 and PASScmp, targeting 16S rDNA, and primers GroEAF1 and GroEAR1, targeting groEL. Buchnera was detected using primers Buch16S1F and Buch16S1R, targeting 16S rDNA (Table 2). PCR conditions were 95°C for 4 min, followed by 35 cycles of 95°C for 30 s, 55°C for 30 s and 72°C for 1 min.
Nucleotide sequence accession numbers.
The 16S rDNA sequences of the Spiroplasma symbiont from A. pisum strains SM, MD-2, NI, YR-1, and YR-2 have been deposited in the DDBJ/EMBL/GenBank nucleotide sequence databases under accession numbers AB048263 and AB050443 to AB050446, respectively.
RESULTS
Analysis of 16S rDNA amplified from strain SM.
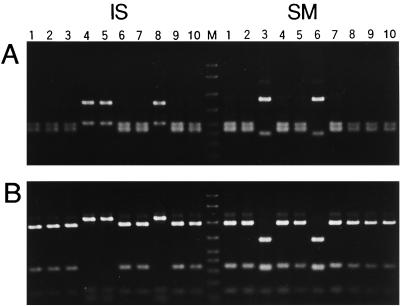
Almost the entire length of eubacterial 16S rDNA in strain SM was amplified by PCR, and the products were subjected to cloning. RFLP analysis of the clones revealed the presence of two major types of sequences (Fig. 1). Judging from the RFLP profiles, the first type was the Buchnera P symbiont. The RFLP profiles of the second type did not agree with those of PASS harbored by the strain IS.
FIG. 1.
RFLP analysis of bacterial 16S rDNA amplified and cloned from the total DNA of A. pisum strains IS and SM. (A) RsaI digestion; (B) HinfI digestion. For strain IS, lanes 1, 2, 3, 6, 7, 9, and 10 are the clones containing the P-symbiont sequence, while lanes 4, 5, and 8 are those containing the S-symbiont sequence. For strain SM, lanes 1, 2, 4, 5, 7, 8, 9, and 10 are the clones containing the P-symbiont sequence, while lanes 3 and 6 are those containing the Spiroplasma sequence. Lane M, DNA size markers (2,000, 1,500, 1,000, 700, 500, 400, 300, and 200 bp from top to bottom).
Molecular phylogenetic analysis.
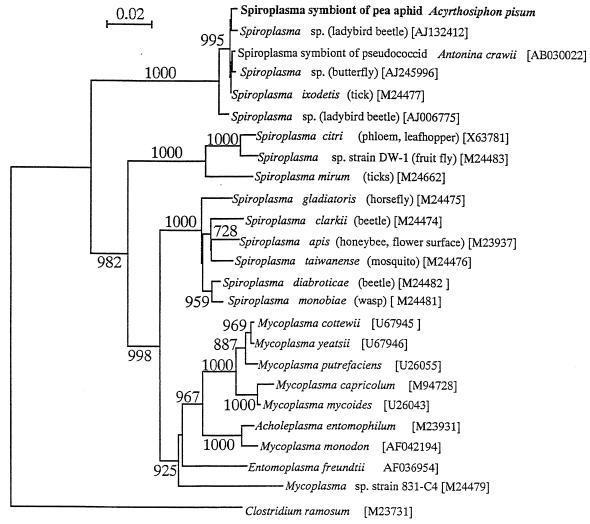
Three clones of the second type were sequenced. These sequences, 1,444 nucleotides in size, were identical to each other. The sequence exhibited very high levels of similarity to the sequences of Spiroplasma spp. in DNA databases. Molecular phylogenetic analysis demonstrated that the 16S rDNA sequence belongs to the clade of Spiroplasma and Mycoplasma in the Mollicutes (Fig. 2). The sequence was closely related to a Spiroplasma symbiont from a pseudococcid, Antonina crawii, a Spiroplasma strain from a tick, a male-killing Spiroplasma strain from a butterfly (Danaus chrysippus), and a male-killing Spiroplasma strain from the ladybird beetles Harmonia axyridis and Adalia punctata. From these results, it was demonstrated that the A. pisum strain SM harbors a bacterium of the genus Spiroplasma in addition to the P symbiont Buchnera.
FIG. 2.
Molecular phylogenetic analysis of the Spiroplasma symbiont from A. pisum strain SM based on 16S rDNA sequence. A total of 1,229 unambiguously aligned nucleotide sites were subjected to the analysis. A neighbor-joining phylogeny is shown; maximum-parsimony analysis gave essentially the same result. The bootstrap values obtained with 1,000 resamplings are shown at the nodes, although values less than 70% were omitted. The numbers in brackets are accession numbers.
Diagnostic PCR detection in laboratory strains of A. pisum
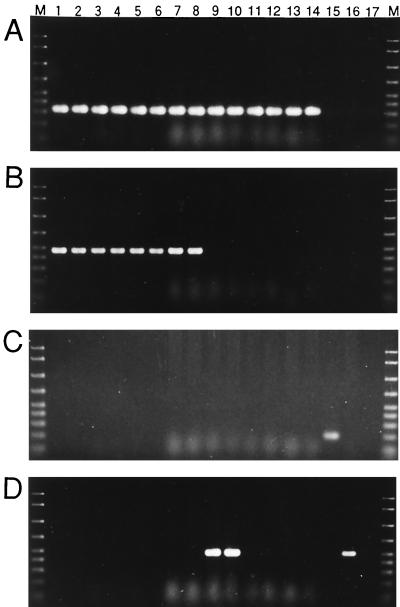
To examine the presence of the Spiroplasma in laboratory strains of A. pisum, we conducted diagnostic PCR experiments with specific reverse primers in combination with the universal forward primer 16SA1 (Fig. 3). When the specific primer ApisP1 was used, the P symbiont Buchnera was detected in all seven strains examined (Fig. 3A). When the specific primer PASScmp was used, PASS was detected in four strains: IS, MR88, TKC93, and HG99 (Fig. 3B). PCR performed with the specific primer Rick16SR demonstrated that none of the seven strains possessed PAR (Fig. 3C). Using the specific primer TKSSsp, the Spiroplasma was detected only in strain SM (Fig. 3D).
FIG. 3.
Diagnostic PCR detection of endosymbionts in laboratory strains of A. pisum. (A) Specific detection of the P symbiont Buchnera with primers 16SA1 and ApisP1. (B) Specific detection of PASS with primers 16SA1 and PASScmp. (C) Specific detection of PAR with primers 16SA1 and Rick16SR. (D) Specific detection of Spiroplasma spp. with primers 16SA1 and TKSSsp. Lanes 1 and 2, IS; lanes 3 and 4, TKC93; lanes 5 and 6, MR88; lanes 7 and 8, HG99; lanes 9 and 10, SM; lanes 11 and 12, EF99; lanes 13 and 14, AIST; lane 15, bruchid beetle (Kytorhinus sharpianus) containing a Rickettsia sp. (13); lane 16, pseudococcid (Antonina crawii) containing a Spiroplasma sp. (14); lane 17, no template control; lane M, DNA size markers (2,000, 1,500, 1,000, 700, 500, 400, 300, 200, and 100 bp from top to bottom). Although the results for only two individuals of each strain are shown, the reproducibility of the results was confirmed by examining more than 20 individuals of each strain.
Examination of hemolymph.
Diagnostic PCR of the hemolymph demonstrated that the Spiroplasma is present in the hemolymph of strain SM but absent in that of strain AIST (data not shown). Many known Spiroplasma members exhibit a characteristic spiral shape, up to 5 μm long, and active motility, with typical twitching movement, in the body fluid of host organisms (36, 37). We observed the hemolymph from unwinged adults of strain SM with a phase-contrast microscope. Numerous small motile particles were found in the hemolymph. However, these particles were not so long as the Spiroplasma symbiont of D. melanogaster and did not show the twitching movement typical of Spiroplasma. In addition, particles with a similar appearance were, though fewer, also found in the hemolymph of strain AIST (data not shown). At this stage, therefore, we are uncertain whether the particles are the Spiroplasma cells.
Artificial transmission by injection of hemolymph.
It has been reported that injection of hemolymph from infected to uninfected A. pisum can establish a stable infection of PASS and/or PAR in the latter (4, 5, 6). To examine whether the Spiroplasma is transferable in the same way, hemolymph from insects of the strain SM was injected into AIST individuals that are free of the Spiroplasma. Three AIST third-instar nymphs were injected with hemolymph of SM adults to generate three independent injected lines. The insects became adult around 5 days after the injection and began to deposit nymphs parthenogenetically, and the newborn nymphs were monitored for Spiroplasma infection by specific PCR (Table 3). Infected nymphs appeared 9 to 11 days after the injection. At later stages, all nymphs inherited the Spiroplasma. Ten first-instar nymphs per generation were examined for the presence of the Spiroplasma by specific PCR over two successive generations (Table 3). All the offspring tested inherited the Spiroplasma. To date, one of the lines has been maintained for 9 months through 32 generations and still exhibits 100% infection with the Spiroplasma (Table 3).
TABLE 3.
Artificial transmission of the Spiroplasma symbiont from strain SM to strain AIST by injection of hemolymph
| Strain | No. of Spiroplasma-positive nymphs/no. examined in:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Injected generation at no. of days after injection (days after birth)
|
Successive generations
|
|||||||||
| 5–6 (8–9) | 7–8 (10–11) | 9–11 (12–14) | 12–13 (15–16) | 14–15 (17–18) | 16–18 (19–21) | 19– (24–) | 1a | 2b | 32c | |
| AIST-Sp+ | 0/5 | 0/5 | 3/5 | 5/5 | 5/5 | 5/5 | 5/5 | 10/10 | 10/10 | 30/30 |
| AIST-Sp+2 | 0/5 | 0/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 10/10 | 10/10 | |
| AIST-Sp+3 | 0/5 | 0/5 | 4/5 | 5/5 | 5/5 | 5/5 | 5/5 | 10/10 | 10/10 | |
Established from a nymph deposited 12 to 13 days after injection in injected generation.
Established from a newly born nymph in generation 1.
Around 9 months after injection.
Effects on the host aphid.
To evaluate the effects of the Spiroplasma infection on the fitness parameters of the host insect, we compared fresh body weight, production of offspring, and life span of strain AIST-Sp+ with those of strain AIST-Sp−. These strains are infected with the Spiroplasma and uninfected, respectively, but have the same genetic background (Fig. 4; Table 4).
FIG. 4.
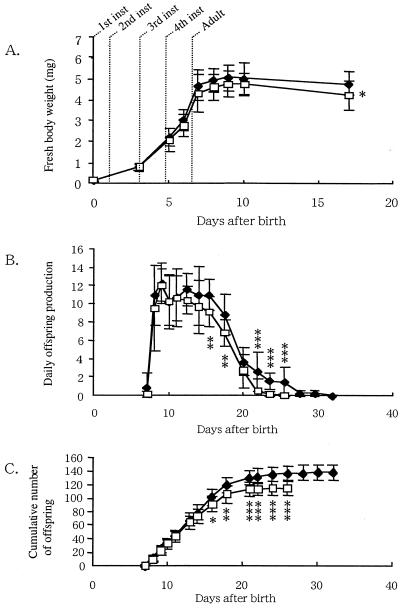
Effects of the Spiroplasma infection on the fitness parameters of the host insect through the developmental course. (A) Fresh body weight; (B) number of offspring per day; (C) cumulative number of offspring. Open rectangles show strain AIST-Sp+ infected with the Spiroplasma symbiont (n = 16), while filled diamonds show the uninfected strain AIST-Sp− (n = 19). Asterisks indicate statistically significant differences between AIST-Sp+ and AIST-Sp− (Mann-Whitney U test): ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001. Inst, instar.
TABLE 4.
Effects of Spiroplasma infection on the fitness parameters of the host insecta
| Strainb | n | Age at first reproduction (days after birth) | Total no. of offspring | Longevity (days) |
|---|---|---|---|---|
| AIST-Sp+ | 16 | 7.9 ± 0.3 | 113.3 ± 10.6 | 22.3 ± 2.0 |
| AIST-Sp− | 19 | 7.7 ± 0.5 | 138.1 ± 11.6c | 33.0 ± 3.4c |
Measurements were conducted 7 months after injection.
Sp+, with Spiroplasma; Sp−, without Spiroplasma.
Statistically significantly different from the AIST-Sp+ result (Mann-Whitney U test, P < 0.001).
(i) Fresh body weight.
Figure 4A shows the fresh body weight of strains AIST-Sp+ and AIST-Sp− over 17 days of development. The body weight of AIST-Sp− was slightly higher than that of AIST-Sp+, although the difference was significant only at 17 days.
(ii) Age of first reproduction.
The age of first reproduction of the AIST-Sp− was slightly earlier than that of the AIST-Sp+, although the difference was not significant (Table 4).
(iii) Longevity.
The longevity of strain AIST-Sp− was significantly greater than that of strain AIST-Sp+ (Table 4). The difference was mainly attributable to the higher mortality of older AIST-Sp+ adults.
(iv) Daily offspring production.
Figure 4B shows the number of offspring produced per day per insect of the strains AIST-Sp+ and AIST-Sp−. The number of offspring produced by AIST-Sp− was slightly higher than that by AIST-Sp+, and the difference was statistically significant as the age of the insects increased over 17 days.
(v) Cumulative number of offspring.
Figure 4C shows the cumulative number of offspring of the strains AIST-Sp+ and AIST-Sp−. The number of offspring in AIST-Sp− was significantly larger than that in AIST-Sp+ at the adult stage, because of the accumulation of the slight differences in daily reproduction and the difference in mortality as the adults aged. As a result, the total number of offspring in strain AIST-Sp− was significantly higher than that in strain AIST-Sp+ (Table 3).
Occurrence in strains of A. pisum from natural populations.
To examine the prevalence of the Spiroplasma symbiont, 58 isofemale clonal strains of A. pisum were established from 43 localities in central Japan and were examined by diagnostic PCR for infection with the Spiroplasma symbiont, PASS, and Buchnera (Table 1). As expected, Buchnera was detected in all the strains. Using two pairs of specific primers, PASS was consistently detected in 29 of 58 strains. In contrast, the Spiroplasma was found in only 4 of 58 strains using two pairs of specific primers. The 16S rDNA sequences of the Spiroplasma sp., amplified using the primers 16SA1 and TKSSsp, were determined for strains MD-2, NI, YR-1, and YR-2. The sequences were almost identical to the sequence from strain SM (data not shown).
DISCUSSION
To date, a number of endosymbiotic bacterial associates from A. pisum have been identified: Buchnera, PASS, PAR, T-type symbiont, U-type symbiont, and various types of gut bacteria (4, 5, 18, 19, 31, 34). In this study, we discovered a previously unknown type of endosymbiont, a Spiroplasma sp., from A. pisum. Almost all members of the genus Spiroplasma described so far are associated in some way with various types of insects and other arthropods (37, 38). As far as we know, this is the first characterization of Spiroplasma from a member of the Aphididae using molecular phylogenetic techniques.
In the phylogeny of the Mollicutes based on 16S rDNA (Fig. 2), members of the genus Spiroplasma did not constitute a single monophyletic group but formed three monophyletic clusters in a paraphyletic manner. The Spiroplasma symbiont of A. pisum belonged to the most basal cluster of the three groups. Members of the cluster were very closely related to each other based on 16S rDNA similarity. In addition to the symbiont of A. pisum, the cluster embraced the symbiont of a pseudococcid, male-killing agents from a butterfly and ladybird beetles, and the symbiont of a tick. In an ecological and evolutionary context, it may be significant that the coccids are phylogenetically closely related to aphids (35) and that ladybird beetles are among the most important predators of aphids (26). It also appears to be meaningful that these insects exhibit a female-biased sex ratio, either through cytoplasmic male killing, as in the butterfly (24) and the ladybird beetles (22), or through parthenogenesis, as in the aphid and the pseudococcid (2).
The Spiroplasma symbiont was detected in A. pisum strain SM, which had been maintained in a laboratory at Shimane Agricultural Experiment Station, Izumo, Japan, for at least 5 years, though its origin is obscure (Y. Narai, personal communication). During maintenance in our laboratory, all individuals of the strain examined were Spiroplasma positive. Injection of hemolymph from infected insects into uninfected insects easily established a stable Spiroplasma infection in the recipients (Table 3). The Spiroplasma was also detected from strains of A. pisum from natural populations in Japan (Table 1). These results strongly suggest that the Spiroplasma sp. is highly adapted to endosymbiosis in A. pisum and is capable of stable maintenance and vertical transmission in the host. It was reported that a facultative S symbiont of A. pisum, PASS, also occurs in M. rosae and is transferable to Acyrthosiphon kondoi by hemolymph injection (5, 6). It was also reported that three types of facultative S symbionts, the R (= PASS), T , and U types, were commonly detected in A. pisum and Uroleucon spp. (31). At present, the host range of the Spiroplasma symbiont is not known.
In our injection experiments, injected Spiroplasma was vertically transmitted to the offspring of the recipient. It should be noted, however, that there was a considerable time lag between the injection and the establishment of vertical transmission (Table 3). Newly molted adults did not transmit the Spiroplasma to their nymphs 5 to 6 days after injection, and partial vertical transmission was first detected 9 to 11 days after injection, followed by complete transmission. Two hypotheses can explain this pattern. One hypothesis is that there is a critical developmental stage at which the embryos can be infected with the Spiroplasma symbiont. Another hypothesis is that there is a threshold density of the Spiroplasma organisms which must be attained before the Spiroplasma sp. can infect the embryos in the ovarioles. To estimate which of these hypotheses is more appropriate, quantitative and histological studies on the course of Spiroplasma infection during the development of A. pisum are required.
The injection technique made it possible to evaluate the effects of Spiroplasma infection on host insects with the same genetic background. Since the comparison was conducted 7 months after the injection, the observed effects are not due to the injection itself but can be attributed to the presence or absence of Spiroplasma. Negative effects on the fitness parameters of the host insect were generally observed: decrease in body weight, reduced offspring production, and shortened life span (Fig. 4; Table 4). Most members of the genus Spiroplasma are known or suspected to be parasitic, although the degree of effects may be extremely diverse between different species and under different conditions, ranging from evidently detrimental through almost neutral to sometimes slightly beneficial (36, 37). It should be noted, however, that the negative effects on A. pisum were principally expressed in older adults (Fig. 4). Under natural conditions, the mortality of aphids is generally very high due to severe attacks by natural enemies, such as predators, parasitoids, and pathogens (26), which implies that old adult insects have a relatively low probability of contributing offspring. It is conceivable that the mitigated negative effects on young insects might have evolved as a strategy of Spiroplasma to ensure its own vertical transmission.
In this study, the Spiroplasma symbiont was detected in 7.7% (5 of 65) of Japanese clones tested; 1 of 7 laboratory strains and 4 of 58 newly established strains from natural populations. How the Spiroplasma symbiont is maintained at such a low frequency is an intriguing problem. Although the Spiroplasma symbiont is vertically transmitted with a high fidelity (Table 3), as long as the transmission rate is not strictly 100%, the rate of Spiroplasma infection must decline to zero as host generations proceed. In addition, this study has shown that the Spiroplasma symbiont has negative effects on the fitness of the host (Fig. 4; Table 4), which would rapidly drive out the Spiroplasma symbiont from host populations. Nevertheless, the Spiroplasma symbiont is certainly present in natural populations, suggesting that some processes must counter these effects. One possibility is that the Spiroplasma symbiont has positive effects on host fitness under particular environmental conditions, which confers a net selective advantage on the host. Another possibility is horizontal transmission of the Spiroplasma symbiont.
Recently, it was reported that another facultative S symbiont, PASS, has negative effects on the growth and reproduction of A. pisum (6), which is superficially reminiscent of the effects caused by the Spiroplasma. The intensity of the effects of PASS was dependent on environmental factors, such as temperature and host plant species. Interestingly, it was suggested that the presence of PASS may mitigate the detrimental effect of elevated temperature on the fecundity of the host (6), which may, at least partly, explain the prevailing occurrence of PASS in populations of A. pisum reported in previous studies (4, 31) and this study (Table 1). If the Spiroplasma symbiont exerts a similar positive effect on the fitness of the host, the effect might compensate for the disadvantages to ensure the persistence of the Spiroplasma in populations.
To date, there has been no evidence of horizontal transmission of the Spiroplasma sp. between A. pisum individuals under laboratory and natural conditions. Notably, some members of the genus Spiroplasma are maintained in cycles in the phloem of plants and the bodies of plant-sucking insects that act as vectors (36, 37). It may be possible that the Spiroplasma is sometimes transmitted from infected to uninfected A. pisum via plant phloem. Alternatively, horizontal transmission via parasitoids is also conceivable (21).
In the evolutionary history of aphids, the P symbiont Buchnera has been highly conserved and has played essential roles for the hosts. In addition to the P symbiont, various types of facultative endosymbiotic companions have been acquired repeatedly and independently in many lineages of aphids. Where did they come from? How were they acquired by the aphids? How did they find their place in an already established mutualistic system? What are their biological functions for the host? How are they maintained in host populations? What interactions exist between the host, Buchnera, and S symbionts? Are they cooperative, competitive or antagonistic? A number of interesting problems remain to be investigated.
ACKNOWLEDGMENTS
We thank K. Honda, H. Ishikawa, and Y. Narai for aphid samples, A. Sugimura, S. Kumagai, and K. Sato for technical and secretarial assistance, and T. Wilkinson for reading the manuscript.
This research was supported by the Industrial Science and Technology Frontier Program “Technological Development of Biological Resources in Bioconsortia” of the Ministry of International Trade and Industry of Japan and by the Program for Promotion of Basic Research Activities for Innovation Biosciences (ProBRAIN) of the Bio-Oriented Technology Research Advancement Institution.
REFERENCES
- 1.Baumann P, Baumann L, Lai C-Y, Rouhbakhsh D, Moran N A, Clark M A. Genetics, physiology and evolutionary relationships of the genus Buchnera: intracellular symbionts of aphids. Annu Rev Microbiol. 1995;49:55–94. doi: 10.1146/annurev.mi.49.100195.000415. [DOI] [PubMed] [Google Scholar]
- 2.Blackman R L. Reproduction, cytogenetics and development. In: Minks A K, Harrewijn P, editors. Aphids: their biology, natural enemies and control. 2A. Amsterdam, The Netherlands: Elsevier; 1987. pp. 163–195. [Google Scholar]
- 3.Buchner P. Endosymbiosis of animals with plant microorganisms. New York, N.Y: Interscience; 1965. [Google Scholar]
- 4.Chen D Q, Purcell A H. Occurrence and transmission of facultative endosymbionts in aphids. Curr Microbiol. 1997;34:220–225. doi: 10.1007/s002849900172. [DOI] [PubMed] [Google Scholar]
- 5.Chen D Q, Campbell B C, Purcell A H. A new Rickettsia from a herbivorous insect, the pea aphid Acyrthosiphon pisum (Harris) Curr Microbiol. 1996;33:123–128. doi: 10.1007/s002849900086. [DOI] [PubMed] [Google Scholar]
- 6.Chen D Q, Montllor C B, Purcell A H. Fitness effects of two facultative endosymbiotic bacteria on the pea aphid, Acyrthosiphon pisum, and the blue alfalfa aphid, A. kondoi. Entomol Exp Appl. 2000;95:315–323. [Google Scholar]
- 7.Dixon A F G. Aphid ecology. London, United Kingdom: Chapman & Hall; 1998. [Google Scholar]
- 8.Douglas A E. Nutritional interactions in insect-microbial symbioses: aphids and their symbiotic bacteria Buchnera. Annu Rev Entomol. 1998;43:17–37. doi: 10.1146/annurev.ento.43.1.17. [DOI] [PubMed] [Google Scholar]
- 9.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 10.Fukatsu T, Ishikawa H. Occurrence of chaperonin 60 and chaperonin 10 in primary and secondary bacterial symbionts of aphids: implications for the evolution of an endosymbiotic system in aphids. J Mol Evol. 1993;36:568–577. doi: 10.1007/BF00556361. [DOI] [PubMed] [Google Scholar]
- 11.Fukatsu T, Ishikawa H. Differential immunohistochemical visualization of the primary and secondary intracellular symbiotic bacteria of aphids. Appl Entomol Zool. 1998;33:321–326. [Google Scholar]
- 12.Fukatsu T, Nikoh N. Two intracellular symbiotic bacteria from the mulberry psyllid Anomoneura mori (Insecta, Homoptera) Appl Environ Microbiol. 1998;64:3599–3606. doi: 10.1128/aem.64.10.3599-3606.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukatsu T, Shimada M. Molecular characterization of Rickettsia sp. in a bruchid beetle, Kytorhinus sharpianus (Coleoptera: Bruchidae) Appl Entomol Zool. 1999;34:391–397. [Google Scholar]
- 14.Fukatsu T, Nikoh N. Endosymbiotic microbiota of the bamboo pseudococcid Antonina crawii (Insecta, Homoptera) Appl Environ Microbiol. 2000;66:643–650. doi: 10.1128/aem.66.2.643-650.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukatsu T, Watanabe K, Sekiguchi Y. Specific detection of intracellular symbiotic bacteria of aphids by oligonucleotide-probed in situ hybridization. Appl Entomol Zool. 1998;33:461–472. [Google Scholar]
- 16.Fukatsu T, Nikoh N, Kawai R, Koga R. The secondary endosymbiotic bacterium of the pea aphid Acyrthosiphon pisum (Insecta: Homoptera) Appl Environ Microbiol. 2000;66:2748–2758. doi: 10.1128/aem.66.7.2748-2758.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gloor G B, Preston C R, Johnson-Schlitz D M, Nassif N A, Phillis R W, Benz W K, Robertson H M, Engels W R. Type I repressors of P element mobility. Genetics. 1993;135:81–95. doi: 10.1093/genetics/135.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harada H, Oyaizu H, Ishikawa H. A consideration about the origin of aphid intracellular symbiont in connection with gut bacterial flora. J Gen Appl Microbiol. 1996;42:17–26. [Google Scholar]
- 19.Harada H, Oyaizu H, Kosako Y, Ishikawa H. Erwinia aphidicola, a new species isolated from pea aphid, Acyrthosiphon pisum. J Gen Appl Microbiol. 1997;43:349–354. doi: 10.2323/jgam.43.349. [DOI] [PubMed] [Google Scholar]
- 20.Houk E J, Griffiths G W. Intracellular symbiotes of Homoptera. Annu Rev Entomol. 1980;25:161–187. [Google Scholar]
- 21.Huigens E M, Luck R F, Klaassen R H, Maas M F, Timmermans M J, Stouthamer R. Infectious parthenogenesis. Nature. 2000;405:178–179. doi: 10.1038/35012066. [DOI] [PubMed] [Google Scholar]
- 22.Hurst G D D, von der Scheulenburg H G, Majerus T M O, Bertrand D, Zakharov I A, Baungaard J, Voelkl W, Stouthamer R, Majerus M E N. Invasion of one insect species, Adalia bipunctata, by two different male-killing bacteria. Insect Mol Biol. 1999;8:133–139. doi: 10.1046/j.1365-2583.1999.810133.x. [DOI] [PubMed] [Google Scholar]
- 23.Ishikawa H, Yamaji M. Symbionin, an aphid endosymbiont-specific protein. I. Production of insects deficient in symbiont. Insect Biochem. 1985;15:155–163. [Google Scholar]
- 24.Jiggins F M, Hurst G D, Jiggins C D, von der Schulenburg J H, Majerus M E. The butterfly Danaus chrysippus is infected by a male-killing Spiroplasma bacterium. Parasitology. 2000;120:439–446. doi: 10.1017/s0031182099005867. [DOI] [PubMed] [Google Scholar]
- 25.Kimura M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 26.Minks A K, Harrewijn P, editors. Aphids: their biology, natural enemies and control. 2A. Amsterdam, The Netherlands: Elsevier; 1988. [Google Scholar]
- 27.Moran N, Baumann P. Phylogenetics of cytoplasmically inherited microorganisms of arthropods. Trends Ecol Evol. 1994;9:15–20. doi: 10.1016/0169-5347(94)90226-7. [DOI] [PubMed] [Google Scholar]
- 28.Moran N A, Munson M A, Baumann P, Ishikawa H. A molecular clock in endosymbiotic bacteria is calibrated using the insect hosts. Proc R Soc Lond B. 1993;253:167–171. [Google Scholar]
- 29.Ohtaka C, Ishikawa H. Effects of heat treatment on the symbiotic system of an aphid mycetocyte. Symbiosis. 1991;11:19–30. [Google Scholar]
- 30.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 31.Sandström, J. P., J. A. Russell, J. P. White, and N. A. Moran. Independent origins and horizontal transfer of bacterial symbionts of aphids. Mol. Ecol., in press. [DOI] [PubMed]
- 32.Swofford D L. PAUP*: phylogenetic analysis using parsimony version 4.0b2. Sunderland, Mass: Sinauer Associates; 1999. [Google Scholar]
- 33.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Unterman B M, Baumann P, McLean D L. Pea aphid symbiont relationships established by analysis of 16S rRNAs. J Bacteriol. 1989;171:2970–2974. doi: 10.1128/jb.171.6.2970-2974.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Von Dohlen C D, Moran N A. Molecular phylogeny of the Homoptera: a paraphyletic taxon. J Mol Evol. 1995;41:211–223. doi: 10.1007/BF00170675. [DOI] [PubMed] [Google Scholar]
- 36.Whitcomb R F. The genus Spiroplasma. Annu Rev Microbiol. 1980;34:677–709. doi: 10.1146/annurev.mi.34.100180.003333. [DOI] [PubMed] [Google Scholar]
- 37.Whitcomb R F. The biology of spiroplasmas. Annu Rev Entomol. 1981;26:397–425. [Google Scholar]
- 38.Williamson D L, Whitcomb R F, Tully J G, Gasparich G E, Rose D L, Carle P, Bové J M, Heckett K J, Adams J R, Henegar R B, Konai M, Chastel C, French F E. Revised group classification of the genus Spiroplasma. Int J Bacteriol. 1998;48:1–12. doi: 10.1099/00207713-48-1-1. [DOI] [PubMed] [Google Scholar]