Abstract
Objectives:
Despite being a cheap, easy, and commonly used technique for screening early development of cervical cancer, collective evidence on the effect of visual inspection with acetic acid (VIA) for reducing cervical cancer mortality and incidence are conflicting. We conducted a systematic review and meta-analysis to determine the effectiveness of VIA screening on cervical cancer mortality and incidence.
Methods:
We searched PubMed, Embase, Cochrane library (Cochrane Database of Systematic Reviews & Cochrane Central Register of Controlled Trials), World Health Organization’s (WHO) International Clinical Trials Registry Platform, and Google Scholar to identify studies conducted among women with no history of cervical cancer that assessed effectiveness of VIA on the cervical cancer mortality and incidence. Random effects model was used to estimate incident rate ratio and sensitivity analysis was conducted using Bayesian methods.
Results:
Of the included 4 studies, three were cluster randomized trials from India and one was quasi-experimental study done in Thailand. Duration of follow-up ranged from 7 to 12 years. Based on 3 trials, pooled rate-ratio for cervical cancer mortality and all-cause mortality was 0.68 (95% CI: 0.56–0.81, I2=0%) and 0.91 (0.85–0.97, I2=57%), respectively. Pooled rate-ratio of invasive cervical cancer was 0.94 (95% CI: 0.67 – 1.30, I2=84%). Likewise, there was non-significant reduction in incidence of stage IB, >=stage II, and unknown stage cervical cancer.
Conclusions:
VIA screening may lead to reduction in cervical cancer and all-cause mortality in long run. However, the effectiveness of VIA in preventing invasive cervical cancer is inconclusive.
Key Words: Cervical cancer, VIA, mortality, incidence, screening, early cancer diagnosis
Introduction
Cervical cancer is the fourth most common cancer in women (Cervical cancer statistics) with an age-standardised global estimated incidence of 13.1 per 100,000 women in 2018 (Arbyn et al., 2020). Cervical cancer is largely a preventable disease and affects more than 250,000 women in low and medium HDI (Human Development Index) countries every year (GLOBOCAN, 2020). Screening by various techniques has been investigated as a potential strategy to reduce this burden. Screening strategies are expected to detect early stages and reduce progression to invasive cervical cancer and associated mortality.
Countries that were successful in implementing a national screening program using cytology (Pap test) showed reduction in the cervical cancer mortality and incidence (Laara et al., 1987; Aklimunnessae et al., 2006; Maine et al., 2011; Landy et al., 2016). HPV-DNA testing also showed very high sensitivity and specificity for screening cervical cancer (Kuhn et al., 2000; Koliopoulos et al., 2017; Sangrajrang et al., 2017; Thomsen et al., 2020). WHO has advocated the use of HPV-DNA testing or cytology as the main screening tool for cervical cancer subject to the resources and infrastructure available (WHO 2013). Also, various modelling studies have also shown the utility of cervical screening in reducing the mortality due to cervical cancer (Su et al., 2013; Landy et al., 2016). Although cytology and HPV-DNA testing have shown promising results for cervical cancer screening (Kuhn et al., 2000; Sangrajrang et al., 2017; Thomsen et al., 2020; Laara et al., 1987; Aklimunnessae et al., 2006; Landy et al., 2016), they have faced operational challenges like high implementation and execution costs, requirement of specialist doctors, and laboratory support (WHO, 2002; Catarino et al.,2015).
VIA is an alternative to the above-mentioned methods which is carried out by applying 5% acetic acid over the cervix and then the acetowhite lesions can be seen with naked eye indicating precancerous lesion (ICMR, 2019). VIA is operationally more feasible on account of it being simple and easy to use and needing little training but its effectiveness in terms of reducing invasive cancer and morality is uncertain (WHO, 2012; Sullivan et al., 2015). Poli et al in 2015 from the rural areas of South India showed that VIA is safe and effective in resource limited settings (Poli et al., 2015). However, previous randomized controlled trials have reported conflicting results regarding the effect of VIA screening on cervical cancer mortality and incidence (Sankaranarayanan et al., 2009; Shatri et al., 2014; Thomsen et al., 2020). Some trials have shown that there is a significant reduction in mortality after VIA screening while others have failed to prove this. Similar is the case with the effect of VIA screening on cervical cancer incidence. A comprehensive examination of all evidence on relevant outcomes of screening by VIA is warranted. Therefore, we conducted this systematic review to assess the effectiveness of VIA based cervical cancer screening on mortality and cervical cancer incidence.
Materials and Methods
Literature search strategy
A comprehensive literature search was carried out for studies published since inception to 31st July 2020 in the following databases: Medline via PubMed, Embase, Cochrane library (Cochrane Database of Systematic Reviews & Cochrane Central Register of Controlled Trials), World Health Organization’s (WHO) International Clinical Trials Registry Platform, and Google Scholar. Search strategies for PubMed, EMBASE, Cochrane library, and Google Scholar are provided in the supplementary appendix.
Selection criteria
Inclusion criteria were: 1) Study population being women with no history of cervical cancer, 2) intervention or exposure group received screening for cervical cancer by VIA method, 3) any study design that has a comparator groups to assess the effect of cervical cancer screening 4) comparator group received standard care, 5) sufficient data was available in the article to calculate incident rate ratio for incidence and mortality, and 6) studies in English language or a summary in English. Exclusion criteria were: 1) abstracts, conference proceedings, and reviews, 2) modelling studies, and 3) studies not conducted on humans. (Supplementary appendix).
Study selection
All retrieved articles from the databases were uploaded to the Rayyan software (Ouzzani et al., 2016). Duplicates were removed after verifying the most recent and complete version. Two independent reviewers (AL and RAD) screened all the titles and abstracts of retrieved records. Studies were marked as ‘included’ if they satisfied the selection criteria, marked ‘excluded’ if they failed to satisfy the selection criteria and marked as ‘may be’ in case of doubt. Any disagreements about selection were resolved by the third author (SAR). Full-text studies were retrieved for the selected abstracts. Reference lists of the retrieved studies were also searched for additional sources. Final inclusion in the review was based on full-text reading.
Data extraction
A pretested spreadsheet-based data extraction form was used to collect information on authors, year of publication, study-setting, sample size, screening interval, adherence rate, follow-up duration, number of cases and number of deaths in intervention and control group. Extraction was done by two authors (AL and RAD) independently and checked for consistency by the third author.
Risk of bias assessment
Cochrane Collaboration’s tool for assessing risk of bias was used to assess the quality of the RCTs included in the systematic review and meta-analysis (RoB 2016). Risk of bias were assessed on the following domains: (1) random sequence generation, (2) allocation concealment, (3) blinding of participants and personnel, (4) blinding of outcome assessment, (5) incomplete outcome data, (6) selective reporting, and (7) other bias. Our judgements on these domains were categorized as ‘low’ risk of bias, ‘high’ risk of bias or ‘unclear’ risk of bias. ROBINS-I tool was used for assessing risk of bias of non-randomized study (Sterne et al.,2016). For non-randomized study, biases were assessed on following domains: (1) confounding, (2) selection of participants, (3) classification of interventions, (4) deviation from intended interventions, (5) missing data, (6) measurement of outcomes, and (7) selection of the reported result. Two independent reviewers (AL and RAD) evaluated the risk of bias of all the included studies in the review. Any disagreement was resolved by RSA.
Statistical analysis
We provided summary estimates of incident rate ratio for mortality and incidence of various stages of cervical cancer and used 95% confidence interval (CI) to gauge the precision of the summary estimates. Heterogeneity was assessed by Cochrane’s Q statistic test and I2 statistic (percentage of residual variation attributed to heterogeneity). I² value > 50% was considered to indicate presence of heterogeneity. Random-effects meta-analysis was performed in R v. 4.0.3. In addition, Bayesian meta-analysis was performed to assess the consistency of estimates and calculate prediction intervals. Publication bias was assessed by visual inspection of funnel plots. The review protocol was registered with PROSPERO - CRD42020201355 and the article written according to the PRISMA guideline (Table S3).
Results
Study selection
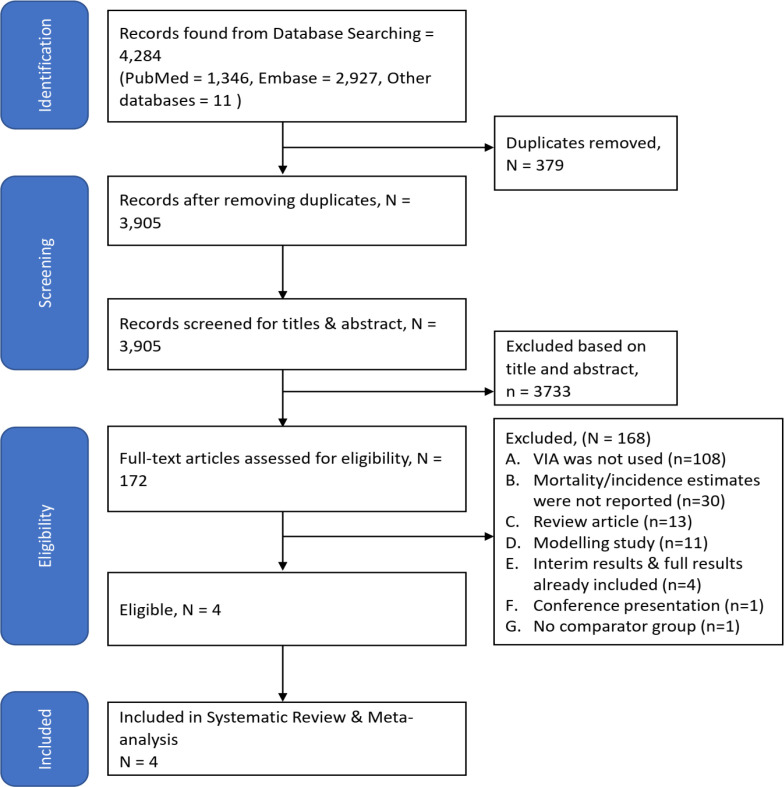
Overall, 4,284 studies were retrieved from the identified databases. After removing duplicates, 3,905 studies were screened using abstracts. In the next step, 172 full-text articles were screened. Finally, four studies satisfied the eligibility criteria and were included in this systematic review and out of which three trials were included in meta-analysis (Figure 1, Table S1).
Figure 1.
PRISMA Flow-Chart
Characteristics of studies included in the meta-analysis
Of the included studies, one was a quasi-experimental study and three were cluster-randomized controlled trial (RCT). The quasi-experimental study was done in three provinces of Thailand between 1997 and 2006 (Chumworathayi et al.,2010). In February 2000, the cervical cancer screening program using VIA was launched in Roi Et province. Incidence of cervical cancer in Roi Et province was compared with the same in two nearby provinces. After the introduction of screening program, the incidence was significantly higher than the nearby provinces. The incidence in Roi Et province in the year 2006 increased to almost three times its baseline value (1996) (Table 1). However, this study did the report the numbers needed for calculation of incidence and therefore could not be included in the quantitative synthesis.
Table 1.
Characteristics of Included Studies
| S No | Author | Study design | Start year | End year | City | State/ Province | Country | Rural or urban | Clusters of | Age group/ range included |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Sankaranarayanan 200726 | Cluster-randomized trial | 2000 | 2006 | Dindigul | Tamil Nadu | India | Rural | Panchayats | 30-59 years |
| 2 | Sankaranarayanan 200920 | Cluster-randomized trial | 2000 | 2007 | Osmanabad | Maharashtra | India | Rural | Group of villages having PHCs | 30-59 years |
| 3 | Shastri 201421 | Cluster-randomized trial | 1998 | 2011 | Mumbai | Maharashtra | India | Urban | Slums | 35-64 years |
| 4 | Chumworathayi 201025 | Quasi-experimental study | 1997 | 2006 | Roi Et | Roi Et | Thailand | Both | NA | NA |
| S No | Author | Subjects | Method of screening | No. of times screening was done | Screening interval (months) | Screening done by | Post screening intervention | Duration of follow-up (years) | No. participated in control group | No. participated in intervention group |
| 1 | Sankaranarayanan 200726 | Healthy, intact uterus, no H/O cervical cancer | VIA | 1 | NA | Female health workers | VIA positive had immediate colposcopy and biopsy | 7 | 30,958 | 49,311 |
| 2 | Sankaranarayanan 200920 | Healthy, intact uterus, no H/O cervical cancer | VIA | 1 | NA | ANMs | VIA positive had immediate colposcopy and biopsy | 8 | 31,488 | 34,074 |
| 3 | Shastri 201421 | Without previous H/o cervical/ breast/ any other malignancy | VIA | 4 | 24 months | Primary health workers | VIA positive were sent to clinic for colposcopy, and Pap followed by biopsy | 12 | 69,227 | 75,360 |
| 4 | Chumworathayi 201025 | NA | VIA | Not given | NA | Not given | VIA and cryotherapy and subsequent referral | No active follow-up. Cases were observed for 7 years in the intervention arm | NA | NA |
All three cluster-RCTs were conducted in India (two in western India and one in southern India). Two studies were done in rural areas and one in urban area. The screening test was same in all three trials i.e. visual inspection of cervix by 5% acetic acid (Sankaranarayanan et al., 2007; Sankaranarayanan et al., 2009; Shastri et al., 2014). However, in one trial screening was done four times at an interval of 24 months while in the other two, it was done only once. Screening was done by frontline healthcare workers in all three trials. The duration of follow-up ranged from 7 to 12 years. The number of participants in control and intervention groups ranged from 30,000 to 75,000, approximately (Table 1).
All three trials were assessed as having ‘unclear risk of bias’ in allocation concealment domain, whereas other domains like random sequence generation, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias showed ‘low risk of bias’. (Figure S1) The non-randomised study showed ‘serious risk of bias’ in the domains of confounding and selection of participants and ‘moderate risk of bias’ in the selection of reported result domain (Table S2).
VIA screening and mortality
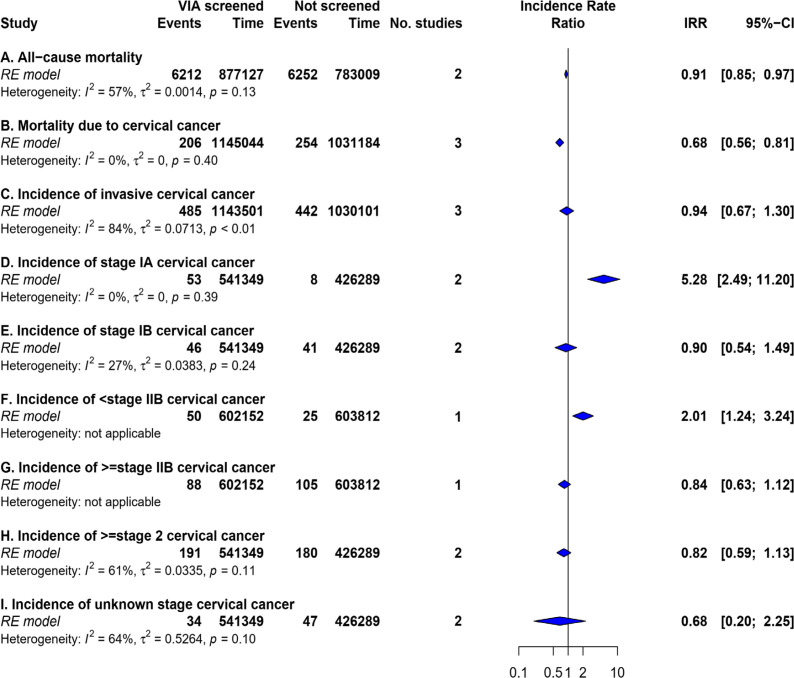
Pooled incident rate-ratio of cervical cancer mortality was 0.68 (95% CI: 0.56 – 0.81, p-value <0.01, n = 3). (Table 2, Figure 2) For the all-cause mortality, pooled incident rate-ratio was 0.91 (95% CI: 0.85 – 0.97, p-value<0.01, n = 2) (Table 2, Figure 2). Bayesian meta-analysis showed a 95% prediction interval for all-cause mortality and mortality due to cervical cancer as 0.520-1.700 and 0.398-1.653 respectively (Figure S4).
Table 2.
Incidence and Mortality Estimates (Random Effects Model)
| Name | Intervention group | Control group | Incidence rate ratio | CI Start | CI End | Weight | Chi-square | P value of chi2 | I square | Tau-Square | Z | P(Z) | df | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Events | Total (Person-years) | Events | Total (Person-years) | ||||||||||||
| Mortality | |||||||||||||||
| Mortality due to cervical cancer | 206 | 1145044 | 254 | 1031184 | 0.675 | 0.562 | 0.812 | 100 | 1.846 | 0.397 | 0 | 0 | 4.181 | 0 | 2 |
| Shastri 2014 | 67 | 602697 | 98 | 604228 | 0.685 | 0.502 | 0.935 | 35.12 | |||||||
| Sankaranarayanan 2009 | 56 | 267917 | 64 | 248175 | 0.811 | 0.566 | 1.16 | 26.361 | |||||||
| Sankaranarayanan 2007 | 83 | 274430 | 92 | 178781 | 0.588 | 0.437 | 0.791 | 38.519 | |||||||
| All-cause mortality | 6212 | 877127 | 6252 | 783009 | 0.909 | 0.85 | 0.972 | 100 | 2.336 | 0.126 | 57.191 | 0.001 | 2.78 | 0.005 | 1 |
| Sankaranarayanan 2007 | 1303 | 274430 | 977 | 178781 | 0.869 | 0.8 | 0.944 | 36.283 | |||||||
| Shastri 2014 | 4909 | 602697 | 5275 | 604228 | 0.933 | 0.898 | 0.97 | 63.717 | |||||||
| Incidence of cervical cancer | |||||||||||||||
| Incidence of invasive cervical cancer | 485 | 1143501 | 442 | 1030101 | 0.936 | 0.673 | 1.3 | 100 | 12.911 | 0.002 | 84.51 | 0.071 | 0.397 | 0.692 | 2 |
| Sankaranarayanan 2007 | 167 | 274023 | 158 | 178394 | 0.688 | 0.554 | 0.855 | 33.652 | |||||||
| Sankaranarayanan 2009 | 157 | 267326 | 118 | 247895 | 1.234 | 0.972 | 1.566 | 32.665 | |||||||
| Shastri 2014 | 161 | 602152 | 166 | 603812 | 0.973 | 0.783 | 1.208 | 33.683 | |||||||
| Incidence of stage IA cervical cancer | 53 | 541349 | 8 | 426289 | 5.278 | 2.486 | 11.203 | 100 | 0.732 | 0.392 | 0 | 0 | 4.332 | 0 | 1 |
| Sankaranarayanan 2007 | 18 | 274023 | 1 | 178394 | 11.718 | 1.564 | 87.779 | 13.971 | |||||||
| Sankaranarayanan 2009 | 35 | 267326 | 7 | 247895 | 4.637 | 2.06 | 10.438 | 86.029 | |||||||
| Incidence of stage IB cervical cancer | 46 | 541349 | 41 | 426289 | 0.9 | 0.543 | 1.493 | 100 | 1.375 | 0.241 | 27.274 | 0.038 | 0.408 | 0.683 | 1 |
| Sankaranarayanan 2009 | 31 | 267326 | 26 | 247895 | 1.106 | 0.657 | 1.862 | 61.159 | |||||||
| Sankaranarayanan 2007 | 15 | 274023 | 15 | 178394 | 0.651 | 0.318 | 1.332 | 38.841 | |||||||
| Incidence of >=stage 2 cervical cancer | 191 | 541349 | 180 | 426289 | 0.819 | 0.591 | 1.134 | 100 | 2.538 | 0.111 | 60.601 | 0.033 | 1.205 | 0.228 | 1 |
| Sankaranarayanan 2007 | 105 | 274023 | 98 | 178394 | 0.698 | 0.53 | 0.919 | 51.854 | |||||||
| Sankaranarayanan 2009 | 86 | 267326 | 82 | 247895 | 0.973 | 0.719 | 1.316 | 48.146 | |||||||
| Incidence of unknown stage cervical cancer | 34 | 541349 | 47 | 426289 | 0.676 | 0.203 | 2.251 | 100 | 2.783 | 0.095 | 64.065 | 0.526 | 0.637 | 0.524 | 1 |
| Sankaranarayanan 2007 | 29 | 274023 | 44 | 178394 | 0.429 | 0.269 | 0.686 | 64.487 | |||||||
| Sankaranarayanan 2009 | 5 | 267326 | 3 | 247895 | 1.546 | 0.369 | 6.467 | 35.513 | |||||||
| Incidence of <stage IIB cervical cancer | 50 | 602152 | 25 | 603812 | 2.006 | 1.241 | 3.241 | 100 | 0 | 1 | 0 | 0 | 2.841 | 0.004 | 0 |
| Shastri 2014 | 50 | 602152 | 25 | 603812 | 2.006 | 1.241 | 3.241 | 100 | |||||||
| Incidence of >=stage IIB cervical cancer |
88 | 602152 | 105 | 603812 | 0.84 | 0.633 | 1.116 | 100 | 0 | 1 | 0 | 0 | 1.203 | 0.229 | 0 |
| Shastri 2014 | 88 | 602152 | 105 | 603812 | 0.84 | 0.633 | 1.116 | 100 | |||||||
Figure 2.
Summary Estimates of Effectiveness of VIA Screening for Cancer Incidence and Mortality
VIA screening and incidence of cervical cancer
Pooled incident rate-ratio of invasive cervical cancer was 0.94 (95% CI: 0.67 – 1.30, p-value=0.69, n = 3) (Figure 2) with a 95% prediction interval of 0.392 – 2.392. (Figure S4) For stage IA, IB, and ≥II cervical cancer, pooled incident rate-ratio was 5.28 (95% CI: 2.49 – 11.20, p-value<0.01, n = 2), 0.90 (95% CI: 0.54 – 1.49, p-value=0.68, n=2), and 0.82 (95% CI: 0.59 – 1.13, p-value=0.23, n=2), respectively as shown in Figure S2.
Publication bias
The small number of studies precluded any reliable estimation of publication bias but the funnel plots appeared to be symmetrical on visual inspection (Figure S3) .
Discussion
Our review showed a statistically significant reduction in all-cause mortality and cervical cancer mortality after VIA screening. However, incidence of invasive cervical cancer was not affected by the VIA screening, at least within the follow-up period studied.
The 2009 study by Sankaranarayanan et al., (2009) showed that the cervical cancer mortality reduction after VIA screening was not significant in contrast to the other two included trials (Shastri et al., 2014; Sankaranarayanan et al., 2007). Similar was the case with the estimates reported by these trials for incidence of invasive cervical cancer. On pooling these conflicting evidences, we were able to show that VIA screening leads to 32% reduction in cervical cancer mortality and 9% reduction in all-cause mortality. However, the reduction in incidence of invasive cervical cancer was non-significant. This was contradictory to the quasi-experimental study conducted in Roi Et province of Thailand (Chumworathayi et al., 2010). The Thai study was a non-randomized study, and the duration of follow-up was also less. These factors along with lead time bias might have contributed to the difference in the results. Lead time bias is a well-known phenomenon responsible for increased incidence of cancer post-screening (Board et al., 2003). Overall, based on our pooled estimates it can be said that the current evidence does not support the expectation that VIA screening will lead to a reduction in incidence of invasive cervical cancer.
In our review, we also pooled estimates of stage-specific incidence of cervical cancer. There was a significant increase in the incidence of stage IA and <IIB cervical cancer. However, the incidence of stage IB, >=IIB and >II cervical cancer non-significantly reduced in the screening group. This pattern could possibly be attributed to the screening process itself and to the lead time bias because it is expected that a community screening program will lead to an increased detection in early stages of cancer, especially in the initial stages of implementation. This could also possibly explain the significant increase in the incidence of invasive cervical cancer in the quasi-experimental study done in Thailand (Chumworathayi et al., 2010). The success of a screening program is therefore, decided by long term effects on incidence and mortality outcomes rather than short term increases in case-finding.
We compared our results with the previously published systematic reviews on cervical cancer screening. Jansen et al conducted a systematic review in 2020 to understand the impact of organised cervical cancer screening on cervical cancer mortality in Europe. No randomized controlled trials from Europe were found. Therefore, only observational studies were included in the review. There was a 41% to 92% reduction in cervical cancer mortality among women attending organized screening vs non-attenders. This was close to our estimates. However, in these studies, the method of screening was not VIA. Moreover, all these studies were observational studies done in Europe only and the pooled estimates were not calculated (Jansen et al., 1990). Pierson et al also investigated the effect of cervical cancer screening on mortality and incidence in 2013 by conducting a systematic review and meta-analysis. They included studies from around the globe. The only RCT in this review concluded that the screening using cytology or HPV testing caused significant reduction in cervical cancer mortality. However, the reduction in incidence of invasive cervical cancer was not significant. This was similar to our study although the screening method in the study was cytology and HPT testing. A UK based cohort study had demonstrated that there is a 62% reduction in incidence of invasive cervical cancer among women who had one cytology test in the last 6-66 months compared to unscreened women. Likewise, pooled results of case-control studies had demonstrated that the women with cervical cancer had significantly lesser odds of having undergone cytology screening. The observed difference could be attributed to the cohort and case-control study designs which were rated as “low” and “very low” quality of evidence in the review. Also, the screening technique is these studies was cytology and the setting was developed countries (Peirson et al., 2013). These factors collectively are the possible reasons for the differences observed.
Credibility of findings
A number of factors vouch for the credibility of our findings. We searched multiple databases for retrieving relevant studies. Secondly, risk of bias was assessed for all studies using a standard tool and the results are presented for the clear comprehension of the results. Lastly, we performed Bayesian meta-analysis to assess the robustness of the estimates to account for the small number of studies and estimate the prediction interval of a future study.
Implications of the study
Our results indicate that the VIA screening may lead to a reduction in all-cause mortality and cervical cancer mortality in the long term. However, there was no effect on overall incidence of invasive cervical cancer. This is important finding for the policy makers as cervical cancer is the biggest contributor to cancer deaths in WHO Africa region, and second biggest contributor in WHO-SEARO region (GLOBOCAN, 2020). Majority of developed countries have already implemented cervical cancer screening programmes in their cancer control programme and have been able to reduce the incidence and mortality of cervical cancer using either cytology or HPV testing as a cancer screening modality (Hakama et al., 1985; Hamakam et al., 1986). However, majority of developing countries have not been able to implement these screening because of the operational challenges associated with these two modalities (Catarino et al., 2015). The implementation challenges associate with VIA includes human resource shortage, issues with the equipment, poor paper based record system, poor follow-up of patients, quality of VIA screening, continuous supervision, non-standardised training curricula, cost for training the man power, high inter-operator variability and low sensitivity among older women with endocervical lesions (Silkensen et al., 2018; Chary et al., 2014; Moon et al., 2012). A study conducted by Poli et al. in India concluded that VIA can be used to triage women who test positive for HPV, and can be preferred in low-resource areas as the results are available immediately, which would allow treatment in a single visit. They have also mentioned that the VIA triage significantly improved the positive predictive value. The WHO recommends VIA triage, in resource limited settings (WHO, 2013; Poli et al., 2017).
VIA has been tested as a modality for screening of cervical cancer in developing countries and has proved its mettle in terms of reducing mortality (Sullivan et al., 2015). The findings of our study further substantiate the fact that VIA screening leads to reduction of cervical cancer mortality. VIA is now being used as a screening tool for cervical cancer in many LMICs (Gupta et al., 2017). WHO has also recommended the use of VIA for screening of cervical cancer in developing countries (WHO, 2012). However, the prediction intervals suggested that the point estimate may lie on either side of the null value. This is a matter of concern because VIA is being considered for large-scale implementation for cervical cancer screening in resource constrained settings (WHO screening 2013).
Strengths and limitations
To the best of our knowledge, this is the first systematic review and meta-analysis that estimated the effect of VIA screening over cervical cancer mortality and incidence. Secondly, we used a standard search strategy using multiple databases to ensure that all relevant published studies were included. Thirdly, in our study, removal of duplicates, screening of studies, and its documentation was done in a reliable and reproducible manner using Rayyan. Fourthly, we included RCTs as well as quasi experimental studies so as to generate the comprehensive evidence related to the topic. Lastly, we also performed the Bayesian meta-analysis to estimate prediction intervals (Rover, 2020).
There were a few limitations in our study. We did not consider studies reported in languages other than English, but we believe this will not affect our findings since majority of the studies done globally, are published in English language. We could not perform sub-group and sensitivity analyses in our review due to the small number of available studies. Similarly, we could also not perform meta-regression and therefore, could not examine the sources heterogeneity in the pooled estimates.
Conclusions and policy recommendations
Reviewing the current evidence on the effectiveness of VIA screening has shown that benefits are more likely for mortality outcomes within a span of a decade but for outcomes like incidence of invasive cervical cancer, a longer follow-up duration might be required. Hence scaling up VIA testing in resource poor setting will lead to reduction in mortality due to cervical cancer.
Author Contribution Statement
Conceptualization: Ayush, Rizwan, Roy, Cherian, Durgesh, Rama, Baridalyne. Methodology: Ayush, Roy, Rizwan, Cherian, Durgesh, Rama, Baridalyne. Investigation: Ayush, Roy. Data Curation: Ayush, Roy. Writing - Original Draft: Ayush, Roy, Rizwan. Writing -Review and Editing: Rizwan, Ayush ,Roy, Cherian, Durgesh, Rama, Baridalyne. Visualization: Rizwan, Ayush, Cherian, Rama. Supervision: Rizwan, Cherian, Durgesh.
Acknowledgements
We are thankful to the authors of the included studies for generating valuable and useful data.
Ethical Approval
This study is exempt from ethical review and approval. It is a meta-analysis aggregating freely available data from published research. The informed consent and all due ethical proceedings were already satisfied by the authors of the individual eligible articles.
Data availability
The data underlying this article are available in the article and in its online Supplementary Material. Registered in ROSPERO - CRD42020201355
Conflict of interest
None.
References
- Aklimunnessa K, Mori M, Khan MMH, et al. Effectiveness of cervical cancer screening over cervical cancer mortality among Japanese women. Jpn J Clin Oncol. 2006;36:511–8. doi: 10.1093/jjco/hyl060. [DOI] [PubMed] [Google Scholar]
- Arbyn M, Weiderpass E, Bruni L, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8:e191–203. doi: 10.1016/S2214-109X(19)30482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Board Curry SJ, Byers T, et al. Potential of screening to reduce the burden of cancer [Internet]. Fulfilling the Potential of Cancer Prevention and Early Detection. National Academies Press (US) 2003. Available from: https://www.ncbi.nlm.nih.gov/books/NBK223933. [PubMed]
- Chumworathayi B, Blumenthal PD, Limpaphayom KK, et al. Effect of single-visit VIA and cryotherapy cervical cancer prevention program in Roi Et, Thailand: A preliminary report. J Obstet Gynaecol Res. 2010;36:79–85. doi: 10.1111/j.1447-0756.2009.01089.x. [DOI] [PubMed] [Google Scholar]
- Chary AN, Rohloff PJ. Major challenges to scale up of visual inspection-based cervical cancer prevention programs: the experience of Guatemalan NGOs. Glob Health Sci Pract. 2014;2:307–17. doi: 10.9745/GHSP-D-14-00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catarino R, Petignat P, Dongui G, Vassilakos P. Cervical cancer screening in developing countries at a crossroad: Emerging technologies and policy choices. World J Clin Oncol. 2015;6:281–90. doi: 10.5306/wjco.v6.i6.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervical cancer statistics [Internet] World Cancer Research Fund. 2018. [ [cited 2020 Dec 29]]. Available from: https://www.wcrf.org/dietandcancer/cancer-trends/cervical-cancer-statistics.
- Gupta R, Gupta S, Mehrotra R, Sodhani P. Cervical cancer screening in resource-constrained countries: Current Status and Future Directions. Asian Pac J Cancer Prev. 2017;18:1461–7. doi: 10.22034/APJCP.2017.18.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLOBOCAN 2020: New Cancer Data | UICC [Internet] [[cited 2020 Dec 29]]. Available from: https://www.uicc.org/news/globocan-2020-new-cancer-data.
- Hakama M, Chamberlain J, Day NE, Miller AB, Prorok PC. Evaluation of screening programmes for gynaecological cancer. Br J Cancer. 1985;52:669–73. doi: 10.1038/bjc.1985.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICMR-NICPR| Training manual on Visual Inspection of Acetic Acid, 2019. Available at: https://main.icmr.nic.in/sites/default/files/Books/viamanual.pdf.
- Jansen EEL, Zielonke N, Gini A, et al. Effect of organised cervical cancer screening on cervical cancer mortality in Europe: a systematic review. Eur J Cancer Oxf Engl. 1990;127:207–23. doi: 10.1016/j.ejca.2019.12.013. [DOI] [PubMed] [Google Scholar]
- Kuhn L, Denny L, Pollack A, et al. Human papillomavirus DNA testing for cervical cancer screening in low-resource settings. JNCI J Natl Cancer Inst. 2000;92:818–25. doi: 10.1093/jnci/92.10.818. [DOI] [PubMed] [Google Scholar]
- Koliopoulos G, Nyaga VN, Santesso N, et al. Cytology versus HPV testing for cervical cancer screening in the general population. Cochrane Database Syst Rev. 2017;10:8. doi: 10.1002/14651858.CD008587.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lăără E, Day NE, Hakama M. Trends in mortality from cervical cancer in the Nordic countries: association with organised screening programmes. Lancet Lond Engl. 1987;1:1247–9. doi: 10.1016/s0140-6736(87)92695-x. [DOI] [PubMed] [Google Scholar]
- Landy R, Pesola F, Castañón A, Sasieni P. Impact of cervical screening on cervical cancer mortality: estimation using stage-specific results from a nested case-control study. Br J Cancer. 2016;115:1140–6. doi: 10.1038/bjc.2016.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon TD, Silva-Matos C, Cordoso A, et al. Implementation of cervical cancer screening using visual inspection with acetic acid in rural Mozambique: successes and challenges using HIV care and treatment programme investments in Zambézia Province. J Int AIDS Soc. 2012;15:17406. doi: 10.7448/IAS.15.2.17406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maine D, Hurlburt S, Greeson D. Cervical cancer prevention in the 21st century: Cost Is Not the Only Issue. Am J Public Health. 2011;101:1549–55. doi: 10.2105/AJPH.2011.300204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan — a web and mobile app for systematic reviews. Sys Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirson L, Fitzpatrick-Lewis D, Ciliska D, Warren R. Screening for cervical cancer: a systematic review and meta-analysis. Syst Rev. 2013;24 doi: 10.1186/2046-4053-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli UR, Bidinger PD, Gowrishankar S. Visual inspection with Acetic Acid (VIA) screening program: 7 Years Experience in Early Detection of Cervical Cancer and Pre-Cancers in Rural South India. Indian J Community Med Off Publ Indian Assoc Prev Soc Med. 2015;40:203–7. doi: 10.4103/0970-0218.158873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RoB 2: A revised Cochrane risk-of-bias tool for randomized trials . 2016. [[cited 2020 Aug 28]]. Available from: https://www.unisa.edu.au/contentassets/72bf75606a2b4abcaf7f17404af374ad/rob2-0_indiv_main_guidance.pdf. [DOI] [PubMed]
- Röver C. Bayesian Random-Effects Meta-Analysis Using the bayesmeta R Package. J Stat Softw. 2020;93:1–51. [Google Scholar]
- Sankaranarayanan R, Esmy PO, Rajkumar R, et al. Effect of visual screening on cervical cancer incidence and mortality in Tamil Nadu, India: a cluster-randomised trial. Lancet Lond Engl. 2007;370:398–406. doi: 10.1016/S0140-6736(07)61195-7. [DOI] [PubMed] [Google Scholar]
- Su S-Y, Huang J-Y, Ho C-C, Liaw Y-P. Evidence for cervical cancer mortality with screening program in Taiwan, 1981–2010: age-period-cohort model. BMC Public Health. 2013;13:13. doi: 10.1186/1471-2458-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaranarayanan R, Jayant K, Muwonge R, et al. HPV screening for cervical cancer in rural India. N Engl J Med. 2009:10. doi: 10.1056/NEJMoa0808516. [DOI] [PubMed] [Google Scholar]
- Shastri SS, Mittra I, Mishra GA, et al. Effect of VIA screening by primary health workers: randomized controlled study in Mumbai, India. J Natl Cancer Inst. 2014;106:dju009. doi: 10.1093/jnci/dju009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;12:355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangrajrang S, Laowahutanont P, Wongsena M, et al. Comparative accuracy of Pap smear and HPV screening in Ubon Ratchathani in Thailand. Papillomavirus Res Amst Neth. 2017;3:30–5. doi: 10.1016/j.pvr.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silkensen SL, Schiffman M, Sahasrabuddhe V, Flanigan JS. Is it time to move beyond visual inspection with acetic acid for cervical cancer screening? Glob Health Sci Pract. 2018;6:242–6. doi: 10.9745/GHSP-D-18-00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen LT, Kjær SK, Munk C, et al. Clinical performance of human papillomavirus (HPV) testing versus cytology for cervical cancer screening: Results of a Large Danish Implementation Study. Clin Epidemiol. 2020;12:203–13. doi: 10.2147/CLEP.S243546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. WHO guidelines for screening and treatment of precancerous lesions for cervical cancer prevention. Geneva: World Health Organization; 2013. [PubMed] [Google Scholar]
- WHO. Prevention of cervical cancer through screening using visual inspection with acetic acid (VIA) and treatment with cryotherapy [Internet] [ [cited 2020 Dec 29]]. Available from: https://www.who.int/reproductivehealth/publications/cancers/9789241503860/en /
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available in the article and in its online Supplementary Material. Registered in ROSPERO - CRD42020201355