Abstract
Objective:
Chemotherapeutic combinational approaches would be more efficient in decreasing toxicity of drug, preventing tumor progression in relation to either drug alone. Hence, the aim of this study is to constract magnetic PLGA/PEG nanoparticles (NPs) co-loaded with Metformin (Met) and Silibinin (Sil) to investigate their cytotoxicity as well as their impact on mRNA expression levels of leptin and leptin receptor genes in A549 lung cancer cells.
Materials and Methods:
The synthesized NPs were characterized by FTIR, FE-SEM, and VSM and then, MTT assay was utilized to assess and compare the cytotoxicity of various concentrations of the chemotheruptic molecules in pure and nanoformulated forms as well as in alone and combination state after 48 h exposure time. Moreover, the mRNA levels of leptin and its receptor genes expression were studied by quantitative real-time PCR. By co-encapsulation of Met and Sil into PLGA/PEG/ Fe3O4, cytotoxic efficiency of the compounds considerably augmented for all concentrations.
Results:
Cytotoxicity assay displayed that combination of Met and Sil had a synergistic concentration-dependent effect on A549 lung cancer cells. Moreover, qPCR data revealed that the expression levels of the leptin and leptin receptor was considerably reduced with increasing concentrations of drug-encapsulated magnetic NPs, especially Met/Sil-encapsulated PLGA/PEG/ Fe3O4 NPs.
Conclusion:
Present preliminary study shows that co-incorporating Met, Sil, Fe3O4 into PLGA/PEG NPs might provide a more promising and safe treatment strategy for lung cancer.
Key Words: Silibinin, Metformin, Magnetic PLGA/PEG nanoparticles, Lung cancer, Leptin
Introduction
Cancer is an uncontrolled development of cells with the potential to attack, or spread to, other tissues and organs of the body (Jeddi et al., 2019; Nikmanesh et al., 2020; Abbasi et al., 2021) . Lung cancer is one of the most common and dangerous kinds of cancer throughout the world (Sheervalilou et al., 2016; Mellatyar et al., 2018) . Lung cancer as a malignant tumor characterized through unchecked cell growth in the lung tissue (Adlravan et al., 2021) . Different kinds of non-small cell lung cancer, are the mainly common types of lung cancer (Samadzadeh et al., 2021) . Three standard therapeutic approaches of lung treatment options including surgery, chemotherapy and, radiation therapy usually are used according to the type and stage of cancer (Farajzadeh et al., 2018). But the use of some these treatment strategies are limited due to the lack of considerable progress on survival rate and the metastatic tumors(Maasomi et al., 2017). Moreover, among the therapeutic strategies, particularly chemotherapy has some undesirable side effects such as nausea, fatigue, neutropenia, anemia and hair loss (Abotaleb et al., 2018). Hence developing novel treatment methods with minimal side effects is crucial. Nowadays, various natural compounds or phytochemicals have been isolated, and their potential anti-tumor efficiency have been assessed (Farajzadeh et al., 2018; Jafari-Gharabaghlou et al., 2018). Among them, some phytochemicals such as Silibinin (Sil) and Metformin (Met) showed promising chemopreventive potential against different malignancies in both in vitro and in vivo studies (Chatran et al., 2018; Amirsaadat et al., 2021).
Sil, a flavonoid derived from Silybum marianum, has been thoroughly assessed for preventing the growth of different malignancies via wide in vitro and in vivo examinations. Sil alone and in combination with other chemotherapeutic molecules exhibited effective anticancer activity via inhibition of cancer cell proliferation, angiogenesis and epigenetic-associated processes (Pourgholi et al., 2021). Met is a standard clinical drug to choose for treating polycystic ovary syndrome and type 2 diabetes mellitus (Mogheri et al., 2021) . In vivo and in vitro researches have exposed that Met has a direct anticancer effect through decreasing the proliferation and triggering the apoptosis of cancer cells (Zakikhani et al., 2006). Recent advances for efficient treatment of cancer are based on an combining of various chemotherapeutic molecules with different mechanism of actions (Dai et al., 2017). Besides, suppressing a single target does not definitely eradicate cancer cell, thus applying a combination of molecular-targeted drugs have been reported (Nagaraj and Datta, 2010). Combination therapy, mainly in the elimination of obstacles such as tumor resistance and low drug effectiveness can be effective to achieve better clinical outcomes to the single drug (Cukierman and Khan, 2010). Leptin, a product of Ob gene secreted by adipose tissue that regulates the balance between orexigenic and anorexigenic by binding to hypothalamic receptors and controls appetite, energy expenditure, and body mass composition (Ahima and Flier, 2000). The role of leptin in lung cancer is not clear (Dutta et al., 2012).
Primary findings proved the presence of ObRl in squamous cell carcinoma (SCC) of the lung and human lung tissue in which leptin lead to an increase in cell growth through the ERK1/2 pathway (Tsuchiya et al., 1999). Besides, it was found that ERK1/2 pathway is induced in A549 cells via leptin (Xu et al., 2018)
Numerous studies have showed that polymeric nanoparticles (NPs) significantly contribute for improving the co-delivery efficiency of chemotherapeutic agents due to their easy penetration into cell membranes, decreasing the off-target effects, and lysosomal escaping following endocytosis (Hashemi et al., 2021; Mousazadeh et al., 2021) . As a biomaterial approved by FDA, poly lactic-co-glycolic acid (PLGA) is known for biocompatibility and ability to biodegrade due to its long-term clinical trials (Firouzi-Amandi et al., 2018; Javan et al., 2019). The structure and the solubility of drug molecules-controlled drug release rate. Moreover, the hydrophilic block of polyethylene glycol (PEG) has showed great potential in decreasing the protein adsorption, opsonization and subsequent clearance as well as increasing blood circulation. there have been significant achievements in the application of NPs in disease diagnostic and highly efficient targeted therapy, it is vital that the systems are biocompatible and capable of being multifunctional for recognition of specific target tumor sites (Serati-Nouri et al., 2021) . In the current work, we aimed to evaluate the cytotoxicity efficiency of Met and Sil co-encapsulated into PLGA/PEG/Fe3O4 NPs against A549 human lung cancer cells. Also, the impact of the co-loaded magnetic PLGA/PEG NPs on the expression of leptin and leptin receptor genes as a possible molecular mechanism of their anticancer activity were investigated.
Materials and Methods
Materials
The A549 lung cell line (lung adenocarcinoma cells) obtained from National Center for Genetic and Biological Resources of Iran. FeCl2. 4H2O, FeCl3.6H2O) and Ammonia solution (25wt %) were obtained from Fluka (Buchs, Switzerland). Silibinin, Metformin, Roswell Park Memorial Institute (RPMI) 1640, Fetal Bovine Serum (FBS), MTT powder, Streptomycin/penicillin, dimethyl sulfoxide (DMSO) and dichloromethane (DCM) were gained from from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Preparation of PLGA/PEG/Fe 3 O 4 NPs
The ring-opening polymerization method was utilized to synthesize of PLGA–PEG copolymers. Pre-determined quantity of glycolide, D, L-lactide, and PEG4000 were melted at 120°C under nitrogen. Next, Sn(Oct)2 (0.01 mmoL) was added and the polymerization reaction was continued for 2 h (Yang et al., 2006). Next, the generated product was dissolved in dichloromethane, and finally, the precipitation process followed in cold diethyl ether solvent
Preparation of drug-loaded NPs
For encapsulation of Met/Sil in PLGA/PEG/Fe3O4 NPs, double emulsion method was utilized. Firstly, PLGA/PEG (250 mg) was dissolved in in dichloromethane (DCM, 5 ml). PVA (20 ml, 0.5% w/v) including Met and Sil (20 mg) was added to the organic phase and emulsified with sonicator probe with the power of 10 W for 45 seconds to produce w/o/w emulsion. Then, to evaporate the organic phase, the obtained emulsion was stirred in room temperature and the remaining solution was centrifuged for 40 minutes at 15,000 rpm and freeze-dried for the elimination of all of the residual solvents
NPs characterization
The field emission scanning electron microscopy (FE-SEM) (MIRA3 TESCAN, Czech) was applied to determine NPs morphology The Dynamic Light Scattering (DLS) was utilized to analyze the NPs average size. The structure confirmation of PLGA/PEG/Fe3O4 NPs was investigated via Fourier transform infrared (FTIR) spectroscopy (BRUKER series). The Vibrating Sample Magnetometer (VSM) is based on vibrating a sample magnetometer (VSM; Lakeshore Cryotronics, Westerville, OH) magnetization curves were measured at room temperature.
In vitro release study
The behavior discharge of agents from magnetic NPs was studied at pH 7.4. 3 mg of Met/Sil encapsulated magnetic NPs was scattered in 20 mL PBS and added in a dialysis tubing, and then incubated at 37oC with stirring at 70 rpm. Predetermined time points, 2mL of fresh PBS was added to keep the constant volume to each sample. The released of Met and Sil measured using Lambda 950 Visible-UV spectrophotometer at 237 and 288 nm and the Met and Sil released concentration was calculated according to the standard curve.
Drug encapsulation efficiency and drug loading
To determine the efficiency of drug encapsulation, after the synthesis of drug-loaded NPs, the supernatant of the tube was isolated and the quantity of non-entrapped drugs assessed via was defined via Visible-UV spectrophotometer at 237 and 288 nm wavelength for Sil and Met respectively. In the following, the percent of entrapped drug (Encapsulation Efficiency; EE) and drug loading (DL) were computed by employing the following formulas:
| (1) |
| (2) |
Cytotoxicity Assay
The A549 lung cell line were cultured in RPMI-1640 media plus 10% FBS and 1% streptomycin/penicillin and incubated at 5% humidity in sterile flasks. MTT assay was utilized to study the viability of cells. Briefly, A549 lung cancer cells (7,000 /well) were seeded in 96-well plates and incubated to help cell attachment. After 24 h, cells were exposed to Met, Sil, Met/Sil, Met-loaded PLGA/PEG/ Fe3O4 NPs, Sil-loaded PLGA/PEG/ Fe3O4 NPs, Met/Sil-loaded PLGA/PEG/ Fe3O4 NPs for 48 h. Next, MTT solution (0.5 mg/mL, 200 µL) was added to all wells and incubated for 4 hours. After incubation for 4 h at 37°C, the medium was evacuated and DMSO (100 μL) was included in each well. Finally, rate of absorbance was assessed through a microplate reader (Star fax 3200 (Awareness Technology Inc.) at 570 nm.The data on mean± SD (n = 3) were displayed in each test.
Real-time PCR assay
A549 cells were plated into 6-well plate and treated with free and nanofurmulated drugs for 48 h and and total RNA was extracted using RNeasy mini kit (Qiagen, USA) according to the manufacturer’s procedure. RNA quality and quantity determined by a NanoDrop (ThermoScientific, USA). For the cDNA synthesis, an appropriate proportion of RNA was taken from each sample and reverse transcribed through RevertAid First strand cDNA synthesis Kit (Fermentas, St Leon-Rot, Germany). Real-Time PCR system was followed using Syber Green Master Mix (BioMolecular Systems, Australia) for the target genes applying the selected primers. Relative expression of each gene was normalized via housekeeping gene (GAPDH) and quantified utilizing 2−ΔΔCt method. All the experiments were done in triplicate.
After treating A549 cells with different concentrations of the NPs for 48 h. Total RNA was extracted using TRIzol reagent (Invitrogen, USA). According to producer’s instructions, Nanodrop was verified the purity and quantity of total RNA. RevertAid™ First Strand cDNA synthesis kit (Thermo Fisher Scientific, MA, USA) was used for synthesizing cDNA from 1 μg RNA. Finally, the 2-ΔΔCt method was applied to determine the relative quantification and the gained data were normalized to the levels of the β-actin gene as a housekeeping control.
Statistical analysis
Statistical significance was done using GraphPad Prism® version 8.0 software, USA.The results were evaluated using one-way ANOVA Analysis of Variance. All the samples were analyzed in triplicates and the results provide as mean ± standard deviation (SD) for n = 3. The level of significance was considered by p-value. Statistically, P value <0.05 was considered significant.
Results
Characterization of nanoparticles
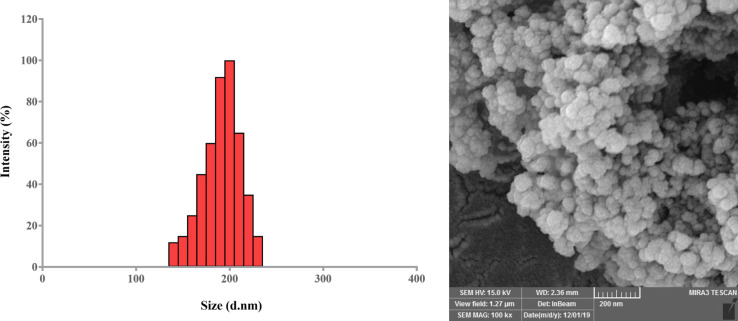
Spherical morphology homogeneously distributed without any significant aggregation in the synthesized NPs was confirmed via FE-SEM analysis. The micrographs of Met/Sil-encapsulated PLGA/PEG/ Fe3O4 NPs are shown in Figure 1. The sizes of Met/Sil-loaded PLGA/PEG/ Fe3O4 NPs were about 160-220 nm. The dispersion of the NPs was greatly improved. DLS data analysis showed a uniform distribution of particles with an average size of 140 nm, a polydispersity index of 0.157 and zeta potential of −23.4±3.6 mV for PLGA/PEG/ Fe3O4 NPs. Moreover, it was found that PLGA/PEG/ Fe3O4 NPs loaded with Met and Sil had an average size of 180 and 190 nm, respectively.
Figure 1.
A) DLS histogram showing the size distribution of Met/Sil-loaded PLGA/PEG/ Fe3O4 NPs. The average size ranged from 160 to 220 nm. B) Field emission scanning electron microscopy (FE-SEM) image of surface morphology of Met/Sil-loaded PLGA/PEG/ Fe3O4
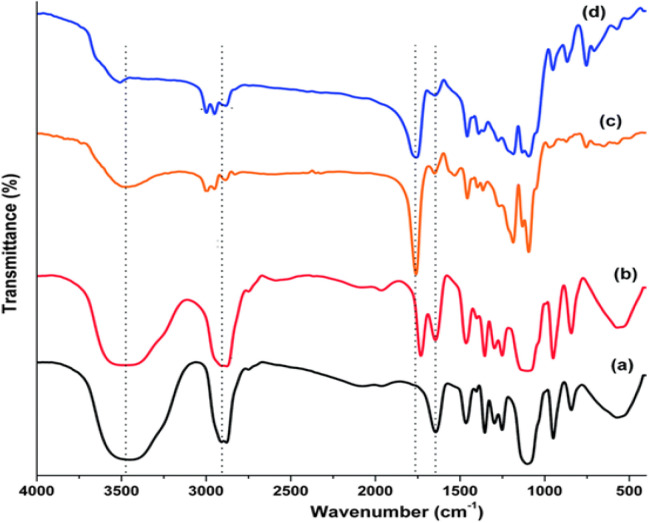
Chemical structures of the synthesized NPs were studied by FTIR spectroscopy (Figure 2). The stretching vibration mode of Fe–O bonds in Fe3O4 assigned to the absorption peaks at 720cm-1. The absorption band at 3509.9 cm-1 belonged to hydroxyl groups at the PEG end in the copolymer from which PEG homopolymer has been eliminated. The bands at 2885 cm-1 due to stretch vibration C–H, and a strong band at 1635 cm-1 is due to C=O stretch. Absorption at 1090.6 cm-1 belonged to C–O stretch. These absorption bands indicate that the encapsulation of drugs in magnetic nanoparticles was successful.
Figure 2.
FTIR Spectra of (A) PLGA/PEG/ Fe3O4 NPs, (B) Sil-loaded PLGA/PEG/ Fe3O4 NPs, (C) Met-loaded PLGA/PEG/Fe Fe3O4 NPs, and (D) Met/Sil-loaded PLGA/PEG/ Fe3O4 NPs
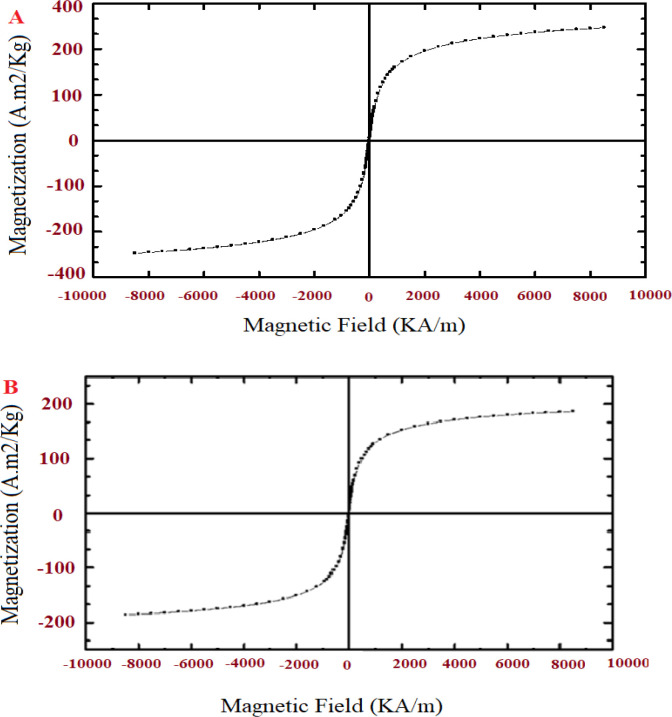
To measure the magnetic futures of the pure Fe3O4 NPs and Sil/Met encapsulated in PLGA/PEG/F3O4 NPs, a vibrating sample magnetometer (VSM) technique was used at room temperature. According to the hysteresis curve in Figure 3A, the saturation value of magnetization of the pure Fe3O4 NPs was found to be 56 emu/g. Also, in Figure 3B, the saturation value of magnetization of the Sil/Met-encapsulated PLGA/PEG/ Fe3O4 NPs is lower than the saturation value of magnetization of the pure Fe3O4 NPs presented (9 emu/g). This difference indicates that the co-encapsulation of Fe3O4, Sil and Met in PLGA/PEG copolymers was successful. After the elimination of the magnetic field, no magnetic activity was observed in the magnetic polymer NPs. This property of magnetic NPs is very important in its applications in biomedical and bioengineering fields.
Figure 3.
Magnetic Hysteresis Curve of Pure Fe3O4 NPs (A), and Met/Sil-loaded PLGA/PEG/F Fe3O4 NPs (B).
Drug release profile
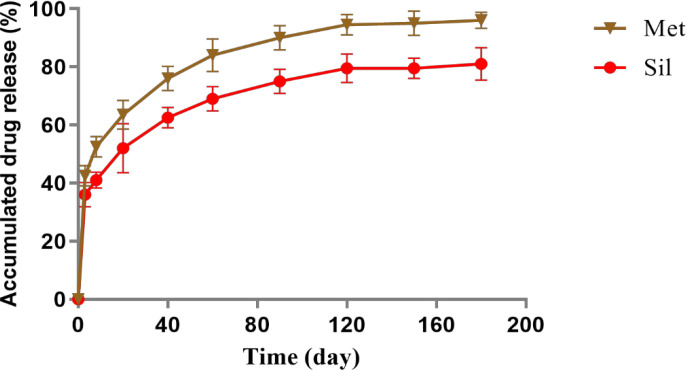
In this study, the release rate of Met and silibinin from Met/Sil-loaded PLGA/PEG/ Fe3O4 NPs were investigated at pH 7.4 (Figure 4) Up to 180 hours, the release rate of Sil and MET from the the nanoparticulate drug release systems were about 65% and 85% respectively. Both theraputic molecules have been shown to exhibit an intial burst release for the first 8 hours followed with slow rlease rate. Furthermore, the results showed that the release of hydrophic Sil molecules from the NPs were slower than hydrophilic Met molecules.
Figure 4.
Dischage Profile of Sil and Met from Met/Sil-loaded PLGA/PEG/ Fe3O4 NPs at pH 7.4. The data are presented as mean ± SD (n =3).
Drug loading (DL) and determination of the entrapment efficiency (EE)
Nanoencapsulation efficiency plays very essential role in delivery of drug by NPs. The nanoencapsulation efficiency of Met, Sil and Sil/Met were found to be 75.4 ± 2.6%, 83.28% and 85.39%, respectively. Moreover, the capacity of drug loading of Sil and Met found to be 13.6 ± 3.1% and 12.3 ± 4.2%, respectively. It seems that the high encapsulation efficacy of these natural antitumor drugs is due to their hydrophobic nature.
Cell viability
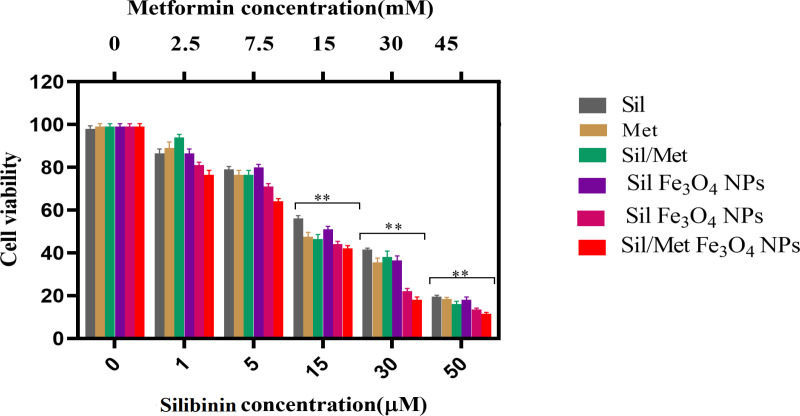
Based on the IC50 obtained from the cytotoxicity assay, data analysis showed that IC50s of pure Met, pure Sil, pure Met/Sil, Met-loaded PLGA/PEG/ Fe3O4 NPs, Sil-loaded PLGA/PEG/ Fe3O4 NPs, and Met/Sil-loaded PLGA/PEG/ Fe3O4NPs on A549 lung cancer cell line is 14.31 mM, 35.92 μM, 17.63, 26.37, 12.03, 10.51 μM for 48 h MTT assays (Figure 5), respectively. These finding for the treatment of A549 cells, different concentrations of pure Met, pure Sil, and nanocapsulated in PLGA/PEG/ Fe3O4 NPs have an inhibitory effect on cancer cells at 48 hours. With increasing concentration, the toxicity of nano-encapsulated Met and Sil was higher than the toxicity of pure Met and pure Sil. The effect of the combination agent at 48 hours showed that the synergistic combinations of two agents had a higher synergistic effect compared to the individual agent as well as were able to overome toxicity and other side effects of single agent (P<0.05) which express an interaction between the two agents. Therefore, these results revealed that the use of this agents in the form of pure and nanocapsulated, even at the lowest dilution, have synergistic effects.
Figure 5.
(A) in vitro cytotoxicity of pure Met, pure Sil, pure Met/Sil, Met-loaded PLGA/PEG/F Fe3O4 NPs, Sil-loaded PLGA/PEG/ Fe3O4 NPs, and Met/Sil-loaded PLGA/PEG/ Fe3O4 NPs against A549 cells incubated for 48 h. The data are presented as mean ± SD (n = 3).
Gene experssion analysis
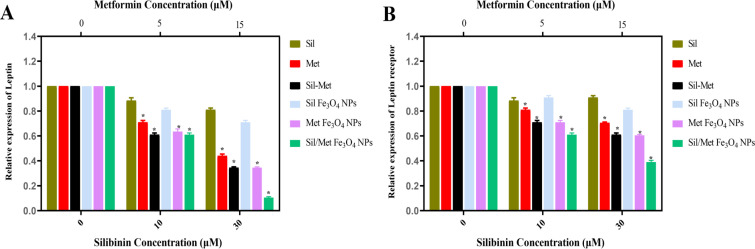
Real-time PCR was used to quantify leptin gene expression in A549 cell line treated with pure and magnetic nanoform of Met and Sil (Figure 6). GAPDH region normalized and calculated by the 2-∆∆ct method to assess the level of leptin and leptin receptor gene expression changes between the control and treated A549 cells. The expression level of leptin and its receptor genes were declined by all types of treatments. It was found that nano-encapsulated forms of Met and Sil in PLGA/PEG/ Fe3O4 NPs significantly decreased leptin gene expression as compared to alone and combination forms of chemotheruptic molecules. the highest decrease in leptin and its receptor gene expressions were detected on the cells treated with Met/Sil-loaded PLGA/PEG/ Fe3O4 NPs.
Figure 6.
Expression Levels of (A) leptin and (B) leptin receptor in A549 lung cancer cells by treatment with different concentrations of pure Met, pure Sil, pure Met/Sil, Met-loaded PLGA/PEG/ Fe3O4 NPs, Sil-loaded PLGA/PEG/ Fe3O4 NPs, and Met/Sil-loaded PLGA/PEG/ Fe3O4 NPs. *p < 0.05 vs. other groups was considered significant. The data are presented as mean ± SD (n = 3)
Discussion
To reduce deaths from lung cancer, expand new treatments or prevent them is very important. The importance of targeted drug delivery for antitumor drug and supressing side effects on normal cells is essential (Akbari et al., 2021). Combination therapy is of great interest due to the synergistic effect of the drugs (Moorthi and Kathiresan, 2013). The use of Sil as a potential compound in the treatment of various cancers have reported by many researches (Singh and Agarwal, 2006). These effects appear to be due to the ability of Sil to suppress cell proliferation, prevent cell division, increase expression of cell cycle inhibitors, increase apoptosis and suppress transcription factors (Singh and Agarwal, 2006). Some of the signaling pathways affected by Sil are MAPK and AKT (Singh and Agarwal, 2004). Met exerts its anticancer effect by activating the AMPK pathway . Met prevents cancer cell mitosis by inducing immune activation and thereby reducing growth factor signaling (Faramarzi et al., 2019). MicroRNA222 suppression induced by Met administration leads to increased levels of p27 and p57 molecules and consequently disrupts cell cycle in tumor cell . Leptin is made by adipose tissue in the human body and released into the blood(D’Marco et al., 2020) . Magnetic NPs can be used in the treatment of cancer cells through hyperthermia (Kobayashi, 2011). One of the approaches is used in the treatment of cancer is magnetic hyperthermia. This therapeutic method of hyperthermia, the fluid containing Magnetic NPs is injected into the cancerous tissu (Thiesen and Jordan, 2008). By creating an alternating magnetic field, these particles vibrate and generate heat, increasing the temperature of the cancerous tissue (Latorre and Rinaldi, 2009). This method will make chemotherapy work better (Salloum et al., 2008). As a result, it raises the temperature of tissue to 4 and causes the cancer cells to be completely destroyed (Johannsen et al., 2010). Research has shown that the use of magnetic NPs causes the entire cancer tissue to be affected by heat (Deatsch and Evans, 2014). Leptin reduces the production of the hypothalamic neuropeptide Y, which is a potent stimulus of appetite (thereby reduces appetite) (Shen et al., 2009). The leptin receptor is a membrane receptor belonging to the class 1 cytokine family Leptin exerts its function through it (Bain et al., 2014). Both leptin and its receptor are involved in mitogenic factors in breast cancer (Artac and Altundag, 2012). Therefore, they can be considered as targets for the treatment of lung cancer.
As the impact of NPs size on the biopharmaceutical aspects and discharge efficiency of drug, it was evaluated by DLS and SEM observations (Mollazade et al., 2011). Also, it has been suggested that most cells are capable of passing NPs with a diameter smaller than 400 nm through their membranes.. In terms of charge the surface chemistry of NPs play a crucial role in influencing the stability amount of NPs emulsions and the interaction between the cell membrane and NPs (Mohandesnezhad et al., 2020). The higher zeta potential value causes natural colloidal, long term stability of NPs due to negative repulsion. Therefore, it seems that the measurement of Met and Sil loaded PLGA/PEG/ Fe3O4 NPs will be acceptable for achieving longevity in blood and passive targeting mechanism of cancer cells. There was a important difference between the treated and control cells in the levels of leptin and leptin receptor gene expression, both of which were declined in the treated cells in relative to the control cells (Nejati-Koshki et al., 2014). Magnetic NPs can be used in therapeutic cancer cells by hyperthermia and agents can be loaded into magnetic NPs and used in targeted cancer treatment (Sivakumar et al., 2014; Nejati et al., 2021). Previous research has shown that the combination of Magnetic NPs with Met or Sil with other drugs has potent anti-cancer effects on cancer cells (Rasouli et al., 2020). The results of the present study were also in line with the results of the above research. The combination of Met and Sil with increased suppression of leptin gene expression in a cellular model of lung cancer. In this study, cytotoxicity assay showed that Met and Sil had a lethal effect on A549 cells in a concentration-dependent manner and nano-Met/Sil significantly reduced IC50 in 48 h. With increasing concentration, nano-encapsulated forms of Met and Sil have been shown to be more toxic than pure Met and Sil. The effect of the combination drug after 48 hours showed that the combination of the two drugs had a higher synergismic effect compared to the single drug. The results have shown that the use of these compounds were pure and nano encapsulated, even at the lowest dilution with a synergistic effect. Real-time PCR results revealed that Met and Sil, inhibited leptin and its receptor gene expression in a dose-dependent manner. In addition, Met and Sil co-loaded in PLGA/PEG/ Fe3O4 NPs reduced significantly the expression level of the leptin and its receptor gene.
In conclusion, in the current work, magntic PLGA/PEG loaded with Met and Sil were developed to examine the impact of Met and Sil on growth and proliferatiion of lung cancer cells. Based on our results magntic PLGA/PEG loaded with Met and Sil was able to kill lung cancer cells more rapidly than other groups, thus potentially this strategy can decrease side effects and cytotoxicity. Furthermore, according to our outcomes nano-formulation of Met and Sil with magntic PLGA/PEG can suppress leptin gene expression and its recepror of human lung cancerous A549 cells more that free form, therefore nano-coencapsulated state of Met and Sil has significant potential to use as complement drug for treatment of lung cancer. These results imply that loading of Met and Sil in magntic PLGA/PEG NPs as we have displayed here could result in hopeful candidates for treatment of lung cancer with a specific final goal of reducing drug resistance related to the current clinically used therapeutics.
Author Contribution Statement
SE and LF as the main colleague contributed to performing experiments, data analysis, and manuscript writing; JD and MH as main colleagues were involved in manuscript writing; DM and ZN as the executive of the project was involved in conception and design, manuscript writing, and supervised the manuscript preparation.
Acknowledgments
Also, authors would like to thank the Stem Cell Research Center for the use of their laboratory facilities.
Funding
This study was financially supported by grant No: 995537 of the National Institute for Medical Research Development
Conflict of Interest
The authors declare that they have no competing interests.
References
- Abbasi A, Dadashpour M, Alipourfard I. Calculation of radium-223 and actinium-225 α-emitter radiopharmaceuticals dose rates in treatment of metastatic castration-resistant prostate cancer. J Cancer Res Ther. 2021;17:348. doi: 10.4103/jcrt.JCRT_892_18. [DOI] [PubMed] [Google Scholar]
- Abotaleb M, Kubatka P, Caprnda M, et al. Chemotherapeutic agents for the treatment of metastatic breast cancer: An update. Biomed Pharm. 2018;101:458–77. doi: 10.1016/j.biopha.2018.02.108. [DOI] [PubMed] [Google Scholar]
- Adlravan E, Nejati K, Karimi MA, et al. Potential activity of free and PLGA/PEG nanoencapsulated nasturtium officinale extract in inducing cytotoxicity and apoptosis in human lung carcinoma A549 cells. J Drug Deliv Sci Technol. 2021;61:102256. [Google Scholar]
- Ahima RS, Flier JS. Leptin. Ann Rev Physiol. 2000;62:413–37. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- Akbari E, Mousazadeh H, Sabet Z, et al. Dual drug delivery of trapoxin A and methotrexate from biocompatible PLGA-PEG polymeric nanoparticles enhanced antitumor activity in breast cancer cell line. J Drug Deliv Sci Technol. 2021;61:102294. [Google Scholar]
- Amirsaadat S, Jafari-Gharabaghlou D, Alijani S, et al. Metformin and Silibinin co-loaded PLGA-PEG nanoparticles for effective combination therapy against human breast cancer cells. J Drug Deliv Sci Technol. 2021;61:102107. [Google Scholar]
- Artac M, Altundag K. Leptin and breast cancer: an overview. Med Oncol. 2012;29:1510–4. doi: 10.1007/s12032-011-0056-0. [DOI] [PubMed] [Google Scholar]
- Bain G, Collie-Duguid E, Murray GI, et al. Tumour expression of leptin is associated with chemotherapy resistance and therapy-independent prognosis in gastro-oesophageal adenocarcinomas. Br J Cancer. 2014;110:1525–34. doi: 10.1038/bjc.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatran M, Pilehvar-Soltanahmadi Y, Dadashpour M, et al. Synergistic anti-proliferative effects of metformin and silibinin combination on T47D breast cancer cells via hTERT and cyclin D1 inhibition. Drug Res. 2018;68:710–6. doi: 10.1055/a-0631-8046. [DOI] [PubMed] [Google Scholar]
- Cukierman E, Khan DR. The benefits and challenges associated with the use of drug delivery systems in cancer therapy. Biochem Pharmacol. 2010;80:762–70. doi: 10.1016/j.bcp.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Marco L, Puchades MJ, Gorriz JL, et al. Epicardial adipose tissue, adiponectin and leptin: a potential source of cardiovascular risk in chronic kidney disease. Int J Mol Sci. 2020;21:978. doi: 10.3390/ijms21030978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W, Wang X, Song G, et al. Combination antitumor therapy with targeted dual-nanomedicines. Adv Drug Deliv Rev. 2017;115:23–45. doi: 10.1016/j.addr.2017.03.001. [DOI] [PubMed] [Google Scholar]
- Deatsch AE, Evans BA. Heating efficiency in magnetic nanoparticle hyperthermia. J Magn Magn Mater. 2014;354:163–72. [Google Scholar]
- Dutta D, Ghosh S, Pandit K, et al. Leptin and cancer: Pathogenesis and modulation. Indian J Endocrinol Metab. 2012;16:S596. doi: 10.4103/2230-8210.105577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farajzadeh R, Pilehvar-Soltanahmadi Y, Dadashpour M, et al. Nano-encapsulated metformin-curcumin in PLGA/PEG inhibits synergistically growth and hTERT gene expression in human breast cancer cells. Artif Cells Nanomed Biotechnol. 2018;46:917–25. doi: 10.1080/21691401.2017.1347879. [DOI] [PubMed] [Google Scholar]
- Faramarzi L, Dadashpour M, Sadeghzadeh H, et al. Enhanced anti-proliferative and pro-apoptotic effects of metformin encapsulated PLGA-PEG nanoparticles on SKOV3 human ovarian carcinoma cells. Artif Cells Nanomed Biotechnol. 2019;47:737–46. doi: 10.1080/21691401.2019.1573737. [DOI] [PubMed] [Google Scholar]
- Firouzi-Amandi A, Dadashpour M, Nouri M, et al. Chrysin-nanoencapsulated PLGA-PEG for macrophage repolarization: Possible application in tissue regeneration. Biomed Pharmacother. 2018;105:773–80. doi: 10.1016/j.biopha.2018.06.037. [DOI] [PubMed] [Google Scholar]
- Hashemi B, Firouzi-amandi A, Amirazad H, et al. Emerging importance of nanotechnology-based approaches to control the COVID-19 pandemic; focus on nanomedicine iterance in diagnosis and treatment of COVID-19 patients. J Drug Deliv Sci Technol. 2021;2021:102967. doi: 10.1016/j.jddst.2021.102967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari-Gharabaghlou D, Pilehvar-Soltanahmadi Y, Dadashpour M, et al. Combination of metformin and phenformin synergistically inhibits proliferation and hTERT expression in human breast cancer cells. Iran J Basic Med Sci. 2018;21:1167. doi: 10.22038/IJBMS.2018.30460.7345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javan N, Khadem Ansari MH, Dadashpour M, et al. Synergistic antiproliferative effects of co-nanoencapsulated curcumin and chrysin on mda-mb-231 breast cancer cells through upregulating mir-132 and mir-502c. Nutr Cancer. 2019;71:1201–13. doi: 10.1080/01635581.2019.1599968. [DOI] [PubMed] [Google Scholar]
- Jeddi F, Alipour S, Najafzadeh N, et al. Reduced levels of miR–28 and miR–200a act as predictor biomarkers of aggressive clinicopathological characteristics in gastric cancer patients. Galen Med J. 2019;8:e1329. doi: 10.31661/gmj.v8i0.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannsen M, Thiesen B, Wust P, et al. Magnetic nanoparticle hyperthermia for prostate cancer. Int J Hyperth. 2010;26:790–5. doi: 10.3109/02656731003745740. [DOI] [PubMed] [Google Scholar]
- Kobayashi T. Cancer hyperthermia using magnetic nanoparticles. Biotechnol J. 2011;6:1342–7. doi: 10.1002/biot.201100045. [DOI] [PubMed] [Google Scholar]
- Latorre M, Rinaldi C. Applications of magnetic nanoparticles in medicine: magnetic fluid hyperthermia. Puerto Rico Health Sci J. 2009:28. [PubMed] [Google Scholar]
- Maasomi ZJ, Soltanahmadi YP, Dadashpour M, et al. Synergistic anticancer effects of silibinin and chrysin in T47D breast cancer cells. Asian Pac J Cancer Prev. 2017;18:1283. doi: 10.22034/APJCP.2017.18.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellatyar H, Talaei S, Pilehvar-Soltanahmadi Y, et al. 17-DMAG-loaded nanofibrous scaffold for effective growth inhibition of lung cancer cells through targeting HSP90 gene expression. Biomed Pharm. 2018;105:1026–32. doi: 10.1016/j.biopha.2018.06.083. [DOI] [PubMed] [Google Scholar]
- Mogheri F, Jokar E, Afshin R, et al. Co-delivery of metformin and silibinin in dual-drug loaded nanoparticles synergistically improves chemotherapy in human non-small cell lung cancer A549 cells. J Drug Deliv Sci Technol. 2021;66:102752. [Google Scholar]
- Mohandesnezhad S, Pilehvar-Soltanahmadi Y, Alizadeh E, et al. In vitro evaluation of Zeolite-nHA blended PCL/PLA nanofibers for dental tissue engineering. Mater Chem Phys. 2020;252:123152. [Google Scholar]
- Mollazade M, Zarghami N, Nasiri M, et al. Polyamidoamine (PAMAM) encapsulated curcumin inhibits telomerase activity in breast cancer cell line. Clin Biochem. 2011;13:S217. [Google Scholar]
- Moorthi C, Kathiresan K. Fabrication of highly stable sonication assisted curcumin nanocrystals by nanoprecipitation method. Drug Invent Today. 2013;5:66–9. [Google Scholar]
- Mousazadeh H, Pilehvar-Soltanahmadi Y, Dadashpour M, et al. Cyclodextrin based natural nanostructured carbohydrate polymers as effective non-viral siRNA delivery systems for cancer gene therapy. J Control Release. 2021;330:1046–70. doi: 10.1016/j.jconrel.2020.11.011. [DOI] [PubMed] [Google Scholar]
- Nagaraj NS, Datta PK. Targeting the transforming growth factor-β signaling pathway in human cancer. Expert Opin Investig Drugs. 2010;19:77–91. doi: 10.1517/13543780903382609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejati-Koshki K, Akbarzadeh A, Pourhassan-Moghaddam M. Curcumin inhibits leptin gene expression and secretion in breast cancer cells by estrogen receptors. Cancer Cell Int. 2014;14:66. doi: 10.1186/1475-2867-14-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejati K, Dadashpour M, Gharibi T, et al. Biomedical applications of functionalized gold nanoparticles: A Review. J Clust Sci. 2021;2021:1–16. [Google Scholar]
- Nikmanesh F, Sarhadi S, Dadashpour M, et al. Omics integration analysis unravel the landscape of driving mechanisms of colorectal cancer. Asian Pac J Cancer Prev. 2020;21:3539. doi: 10.31557/APJCP.2020.21.12.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourgholi A, Dadashpour M, Mousapour A, et al. Anticancer potential of silibinin loaded polymeric nanoparticles against breast cancer cells: Insight into the Apoptotic Genes Targets. Asian Pac J Cancer Prev. 2021;22:2587–96. doi: 10.31557/APJCP.2021.22.8.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasouli S, Montazeri M, Mashayekhi S, et al. Synergistic anticancer effects of electrospun nanofiber-mediated codelivery of Curcumin and Chrysin: Possible application in prevention of breast cancer local recurrence. J Drug Deliv Sci Technol. 2020;55:101402. [Google Scholar]
- Salloum M, Ma R, Weeks D, et al. Controlling nanoparticle delivery in magnetic nanoparticle hyperthermia for cancer treatment: experimental study in agarose gel. Int J Hyperther. 2008;24:337–45. doi: 10.1080/02656730801907937. [DOI] [PubMed] [Google Scholar]
- Samadzadeh S, Mousazadeh H, Ghareghomi S, et al. In vitro anticancer efficacy of Metformin-loaded PLGA nanofibers towards the post-surgical therapy of lung cancer. J Drug Deliv Sci Technol. 2021;61:102318. [Google Scholar]
- Serati-Nouri H, Rasoulpoor S, Pourpirali R, et al. In vitro expansion of human adipose-derived stem cells with delayed senescence through dual stage release of curcumin from mesoporous silica nanoparticles/electrospun nanofibers. Life Sci. 2021;285:119947. doi: 10.1016/j.lfs.2021.119947. [DOI] [PubMed] [Google Scholar]
- Sheervalilou R, Ansarin K, Fekri Aval S, et al. An update on sputum Micro RNA s in lung cancer diagnosis. Diagn Cytopathol. 2016;44:442–9. doi: 10.1002/dc.23444. [DOI] [PubMed] [Google Scholar]
- Shen Y, Wang Q, Zhao Q, et al. Leptin promotes the immune escape of lung cancer by inducing proinflammatory cytokines and resistance to apoptosis. Mol Med Rep. 2009;2:295–9. doi: 10.3892/mmr_00000099. [DOI] [PubMed] [Google Scholar]
- Singh RP, Agarwal R. A cancer chemopreventive agent silibinin, targets mitogenic and survival signaling in prostate cancer. Mutat Res Fundam Mol Mech Mutagen. 2004;555:21–32. doi: 10.1016/j.mrfmmm.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Singh RP, Agarwal R. Prostate cancer chemoprevention by silibinin: bench to bedside. Molecular Carcinogenesis: Published in cooperation with the University of Texas MD Anderson Cancer Center. 2006;45:436–42. doi: 10.1002/mc.20223. [DOI] [PubMed] [Google Scholar]
- Sivakumar B, Aswathy RG, Sreejith R, et al. Bacterial exopolysaccharide based magnetic nanoparticles: a versatile nanotool for cancer cell imaging, targeted drug delivery and synergistic effect of drug and hyperthermia mediated cancer therapy. J Biomed Nanotechnol. 2014;10:885–99. doi: 10.1166/jbn.2014.1820. [DOI] [PubMed] [Google Scholar]
- Thiesen B, Jordan A. Clinical applications of magnetic nanoparticles for hyperthermia. Int J Hyperther. 2008;24:467–74. doi: 10.1080/02656730802104757. [DOI] [PubMed] [Google Scholar]
- Tsuchiya T, Shimizu H, Horie T, et al. Expression of leptin receptor in lung: leptin as a growth factor. Eur J Pharmacol. 1999;365:273–9. doi: 10.1016/s0014-2999(98)00884-x. [DOI] [PubMed] [Google Scholar]
- Xu M, Cao F-L, Li N, et al. Leptin induces epithelial-to-mesenchymal transition via activation of the ERK signaling pathway in lung cancer cells. Oncol Lett. 2018;16:4782–8. doi: 10.3892/ol.2018.9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Park S-B, Yoon H-G, et al. Preparation of poly ɛ-caprolactone nanoparticles containing magnetite for magnetic drug carrier. Int J Pharm. 2006;324:185–90. doi: 10.1016/j.ijpharm.2006.06.029. [DOI] [PubMed] [Google Scholar]
- Zakikhani M, Dowling R, Fantus IG, et al. Metformin is an AMP kinase–dependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66:10269–73. doi: 10.1158/0008-5472.CAN-06-1500. [DOI] [PubMed] [Google Scholar]