Abstract
Purpose:
HPV-positive oropharyngeal squamous cell carcinoma (OPSCC) carries a favorable prognosis for patients, yet nearly 30% of patients will experience disease relapse. We sought to detail patterns of failure, associated salvage therapy, and outcomes for patients with recurrent HPV-positive OPSCC.
Methods and materials:
This is a single institution retrospective study of patients with recurrent HPV-positive OPSCC irradiated from 2002 to 2014. The primary study outcome was overall survival (OS, calculated using the Kaplan-Meier method). Secondary aims included patterns of first failure with descriptive details of salvage therapy. Solitary recurrences were defined as initial presentation of recurrence in a single site (primary, neck or oligometastatic), and multi-site was defined as local and regional and/or multiple sites of distant recurrence. Survival outcomes were compared using the log-rank test.
Results:
The cohort consisted of 132 patients. The median follow-up was 59 months for surviving patients. Estimated 2-year and 5-year OS rates were 47% and 32%, respectively. Comparative 2-year and 5-year OS rates were 65% and 46% versus 19% and 9% for the solitary group and multi-site group, respectively (p < .001).
Conclusions:
Patients with recurrent HPV-positive OPSCC experience 5-year survival of approximately 32%. However, patients with a “solitary” recurrence including disease at the primary site, neck or oligometastatic site have more favorable long-term outcomes.
Keywords: Head and neck cancer, Oropharyngeal cancers, HPV-positive, Oligometastatic disease, Squamous cell carcinoma, Patterns of failure, Salvage therapy, Patient outcomes
Introduction
Historically, cure rates for patients with oropharyngeal squamous cell cancer (OPSCC) were modest [1,2]. Patients not cured often succumbed to a primary site or nodal recurrence [1,2]. Additional therapy for persistent or recurrent disease led to infrequent cure. A minority of patients also would develop distant disease, which almost always would lead to a terminal event.
In recent decades there has been an increase in OPSCC associated with human papillomavirus (HPV) [3]. It is well known that there is a greatly improved prognosis for patients with HPV-positive tumors, and recurrent disease following treatment for HPV-positive OPSCC develops less frequently than for HPV-negative disease [4]. In the recent report of NRG Oncology RTOG 1016, approximately 24% of patients developed disease recurrence, with the pattern of recurrence roughly evenly divided between local-regional recurrences and distant metastases [5].
Comparing the progression-free survival from modern studies, which hovers between 64 and 78%, to the reported overall survival of 71–85%, it is clear that some patients can be salvaged after recurrence, but details regarding salvage treatments are lacking [5–7]. A secondary analysis of patients enrolled in RTOG 0129 and 0522 evaluated comparative survival in patients with p16-positive and p16-negative OPSCC after recurrence. This study demonstrated that while the patterns of recurrence with regards to location and time frame were similar, patients with p16-positive disease had a significantly improved 2-year overall survival with more than half the patients alive at 2 years [6].
Herein we sought to examine a large cohort of patients who were treated with primary (chemo)radiation for OPSCC, and suffered recurrent disease. We explore patterns of failure, but more importantly, we seek to define which presentations of recurrence are amenable to meaningful salvage therapy, and discuss modalities used in salvage treatment. Additionally, we evaluated outcomes beyond 2 years to determine if improved survival was related to a longer natural history of HPV-positive squamous cell cancer, or truly ridding a subset of patients of recurrent disease.
Methods
Patients
We reviewed all cases of patients undergoing definitive (chemo)radiation for HPV-positive oropharyngeal squamous cell carcinoma treated at our institution from 2002–2014. The University of Texas MD Anderson Cancer Center (MDACC) Institutional Review Board approved this study. Initial inclusion criteria included all patients ≥ 18 years old, diagnosed with non-metastatic p16 or HPV-positive OPSCC, who received their initial course of curative-intent radiation therapy at MDAAC. All patients included completed a full course of radiation treatment. Initial staging was based on the AJCC 7th edition staging manual. Based on this cohort, we identified patients with relapsed disease, which comprise the final study cohort.
None of the patients identified as having recurrent disease were excluded.
Initial treatment
Patients were initially treated with definitive radiotherapy (with or without chemotherapy) in the upfront setting. No patients underwent primary oncologic surgery for their initial disease (such as transoral robotic surgery (TORS)). For the initial course of radiation, patients were treated with standard techniques used at our center during the study interval, and radiation treatment plans were subjected to peer review as per departmental policy. Most commonly, patients were treated with intensity modulated radiotherapy (IMRT) or volumetric arc therapy (VMAT). The majority of patients were treated with concurrent chemotherapy and/or induction chemotherapy. Ipsilateral neck radiation for patients with well lateralized tonsil primaries was allowed per institutional practice patterns. All patients completed their initial prescribed course of radiotherapy. Patients who terminated treatment prematurely (due to adverse events or death) were excluded from analysis.
Patients were evaluated for planned neck dissection during early follow up after chemoradiation. Planned neck dissections were defined as a neck dissection within 6 months of primary treatment due to known unresolved, non-progressive, disease in the neck after primary radiation. Patients with positive findings of disease from these planned neck dissections were not considered to have recurrent disease, unless they had a subsequent cancer event.
Outcomes
The primary outcome of interest was overall survival. Patients were initially characterized by site of first recurrence into five cohorts including: local, regional, local-regional, distant (oligometastatic), and distant (multi-metastatic). Oligometastatic recurrence was defined as ≤ 3 metastatic deposits within a single organ system/lymph node region, not including any diffuse malignant processes (such as malignant pleural effusion). Patients with synchronous local and/or regional recurrence plus distant metastatic disease were included in the multi-metastatic cohort. Based on a posteriori finding, we subsequently analyzed our cohort by dichotomizing them into 2 groups: Solitary recurrences defined as initial presentation of recurrence in a single site (primary, neck or oligometastatic), and multi-site defined as local and regional and/or multiple site of distant recurrence.
Statistical analysis
Survival outcomes and disease recurrence (or control) were estimated using the Kaplan-Meier method. For all survival estimates presented, date of diagnosis of the initial relapse was used as the initial time point for analysis. Date of death or last follow-up alive was used to calculate overall survival, while date of subsequent relapse or last follow-up without relapse was used to estimate disease recurrence rates. Survival outcomes across cohorts were compared using the log-rank test. Chi-squared tests were used to test for differences regarding initial patient and treatment characteristics amongst cohorts. For patients with oligometastatic lung disease, we provide qualitative details regarding salvage therapy.
Statistical analyses were carried out using JMP Version 14.3, SAS Institute Inc (Cary, NC). P-values less than 0.05 were considered statistically significant.
Results
Patients and initial treatment
The cohort consisted of 132 patients. Median age at time of initial treatment was 57.5 years [Range: 41–84]. Median initial RT dose to the primary site was 70 Gy [Range: 66–70]. The majority of patients were treated with IMRT (n = 127, 96%), 2 patients were treated with 3D techniques (2%), and 3 patients were treated with IMPT (2%). Eight patients (6%) were treated with ipsilateral neck radiation. One hundred sixteen patients (88%), received some form of systemic therapy (cytotoxic chemotherapy or targeted therapy) either as concurrent therapy and/or induction therapy. Planned neck dissections were performed in 22 patients (17%). Further details about initial patient, tumor, and treatment characteristics are shown in Table 1.
Table 1.
Initial patient and treatment characteristics.
| All (n,%) | Local (28) | Regional (31) | Local Regional (n = 10) | Oligometastatic (n = 23) | Multi-metastatic (40) | P value | |
|---|---|---|---|---|---|---|---|
| T Category | |||||||
| T1 | 26 (20) | 4 (14) | 7 (23) | 0 | 7 (30) | 8 (20) | 0.09 |
| T2 | 51 (39) | 9 (32) | 15 (48) | 1 (10) | 8 (35) | 18 (45) | |
| T3 | 32 (24) | 8 (29) | 6 (19) | 5 (50) | 2 (9) | 11 (27.5) | |
| T4 | 23 (17) | 7 (25) | 3 (10) | 4 (40) | 6 (26) | 3 (7.5) | |
| N Category | |||||||
| N0 | 7 (5) | 4 (14) | 0 | 0 | 2 (9) | 1 (2.5) | 0.01 |
| N1 | 11 (8) | 5 (18) | 1 (3) | 1 (10) | 2 (9) | 2 (5) | |
| N2a | 5 (4) | 0 | 0 | 0 | 3 (13) | 2 (5) | |
| N2b | 61 (46) | 9 (32) | 16 (52) | 2 (20) | 10 (43) | 24 (60) | |
| N2c | 36 (27) | 9 (32) | 11 (35) | 4 (40) | 4 (17) | 8 (20) | |
| N3 | 12 (9) | 1 (4) | 3 (10) | 3 (30) | 2 (9) | 3 (7.5) | |
| Sex | |||||||
| Male | 120 (91) | 24 (86) | 27 (87) | 10 (100) | 22 (96) | 37 (92.5) | 0.37 |
| Female | 12 (9) | 4 (14) | 4 (13) | 0 | 1 (4) | 3 (7.5) | |
|
Smoking Status
|
|||||||
| Never | 58 (44) | 9 (32) | 16 (52) | 2 (20) | 11 (48) | 20 (50) | 0.33 |
| Former | 41 (31) | 8 (29) | 7 (22) | 5 (50) | 8 (35) | 13 (32.5) | |
| Current | 33 (25) | 11 (39) | 8 (26) | 3 (30) | 4 (17) | 7 (17.5) | |
| Concurrent Chemotherapys | |||||||
| Concurrent (Cisplatin) | 47 (36) | 13 (46) | 5 (16) | 6 (60) | 8 (35) | 15 (37.5) | 0.03 |
| Concurrent (Carboplatin) | 20 (15) | 1 (4) | 8 (26) | 3 (30) | 2 (9) | 6 (15) | |
| Concurrent (Cetuximab) | 34 (26) | 10 (36) | 10 (32) | 1 (10) | 3 (12) | 10 (25) | |
| None | 31 (23) | 4 (14) | 8 (26) | 0 | 10 (43) | 9 (22.5) | |
| Induction Chemotherapy | |||||||
| Yes | 67 (51) | 9 (32) | 21 (68) | 7 (70) | 10 (43) | 20 (50) | 0.05 |
| No | 65 (49) | 19 (68) | 10 (32) | 3 (30) | 13 (57) | 20 (50) | |
| Planned Neck Dissection | |||||||
| Yes, Negative | 12 (9) | 4 (14) | 1 (3) | 0 | 2 (9) | 5 (12.5) | 0.51 |
| Yes, Positive (no ECE) | 1 (1) | 0 | 0 | 0 | 0 | 1 (2.5) | |
| Yes, Positive (with ECE) | 9 (7) | 1 (4) | 3 (10) | 2 (20) | 1 (4) | 2 (5) | |
| No | 110 (83) | 23 (82) | 27 (87) | 8 (80) | 20 (87) | 32 (80) |
Recurrence
The median time interval between the initial treatment and date of recurrence was 14 months [Range: 2.7–132.6]; 54 patients (41%) had recurrence within the first year following treatment and 117 (89%) recurrences developed within 3 years of initial treatment. Twenty-eight patients (21%) experienced local recurrence alone, 31 (23%) had regional recurrence alone, 10 (8%) had synchronous local regional recurrence, 23 (17%) had distant oligometastatic disease and 40 (30%) had distant multi-metastatic disease (Table 2). Median time to recurrence did not vary significantly between patients when analyzed by site of recurrence.
Table 2.
Summary of patterns of recurrent disease, salvage therapy and outcome.
| Recurrence Site | Number of patients | Surgical salvage | DefinitiveRadiation | Disease control at treated Site | NED at last followup |
|---|---|---|---|---|---|
| Local | 28 | 15 | 5 | 14 | 10*** |
| Regional | 31 | 23** | 3 | 22 | 21 |
| Local Regional | 10 | 2 | 1 | 1 | 1 |
| Distant - oligometastatic | 23 | 9 | 9 | 10 | 9 |
| Distant - multimetastatic | 40* | 0 | 0 | 1 | 1 |
| Total | 132 | 49 | 18 | 48 | 42 |
Includes 7 patients with synchronous local and/or regional recurrence;
17 patients received adjuvant radiation and/or chemotherapy;
2 patients had subsequent regional relapse and treated with surgical salvage.
Outcomes
The median follow-up was 20 months for all patients [Range: 0.1–154], and 59 months for surviving patients [Range: 1–154].
Median overall survival time, measured from time of presentation with recurrent disease to death for the entire cohort was 21 months [95% CI: 15.1–28.2]. Estimated 2-year and 5-year overall survival rates were 47% and 32%, respectively (Fig. 1).
Fig. 1.
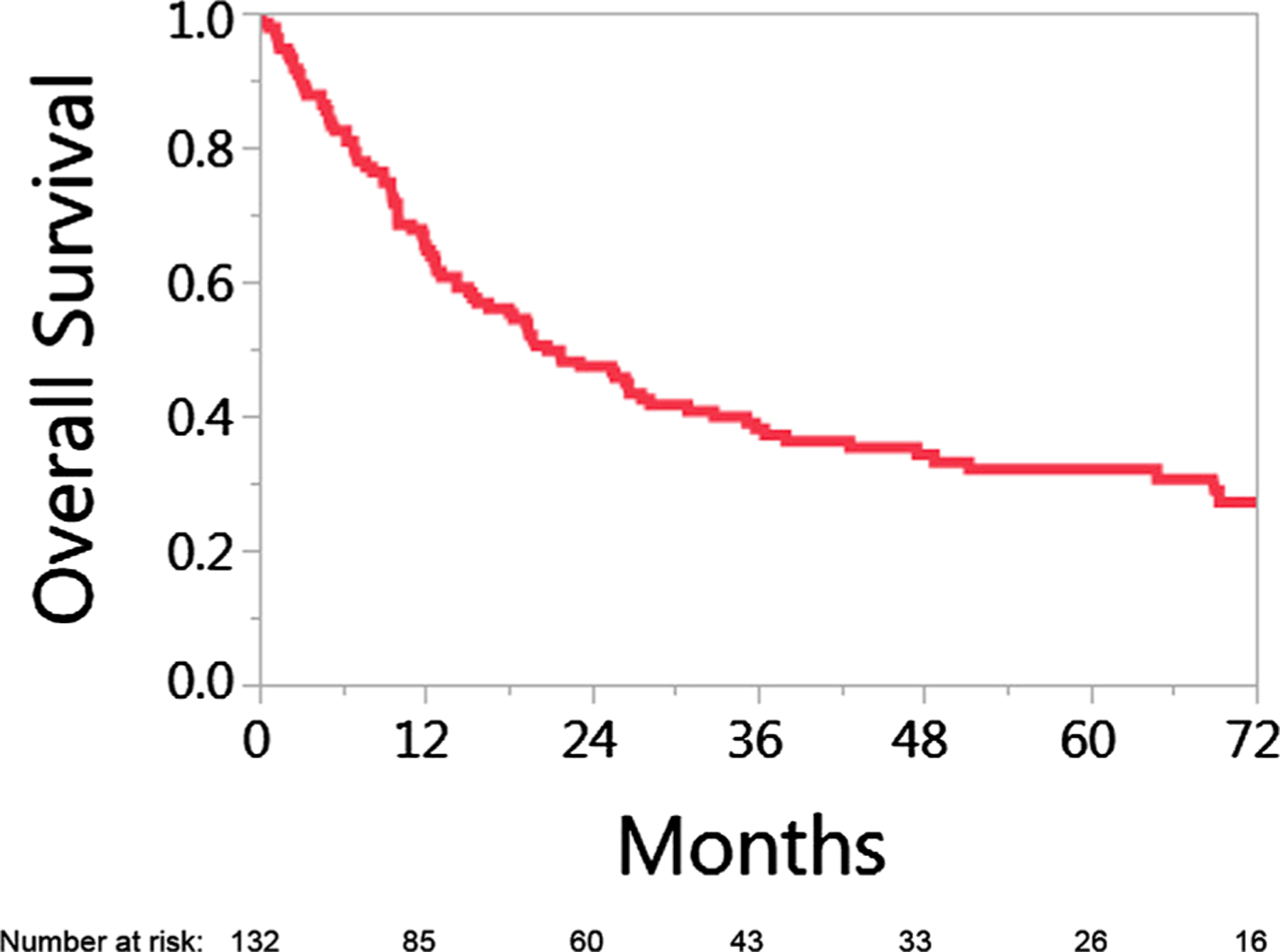
Overall survival for 132 patients with HPV associated oropharyngeal cancer who developed recurrent disease. Time 0 is time of diagnosis of relapse.
OS varied considerably for patients dependent on site of first failure. Median overall survival times for patients with local, regional, local-regional, oligometastatic, and multi-metastatic distant disease recurrence were: 35 months, 78 months, 7 months, 36 months, and 12 months respectively (p < 0.001). Kaplan-Meier survival curves for overall survival for patients in these 5 groups are shown in Fig. 2. Comparative 2-year and 5-year overall survival rates were 65% and 46% versus 19% and 9% for the solitary group and multi-site group, respectively (p < .001, Fig. 3).
Fig. 2.
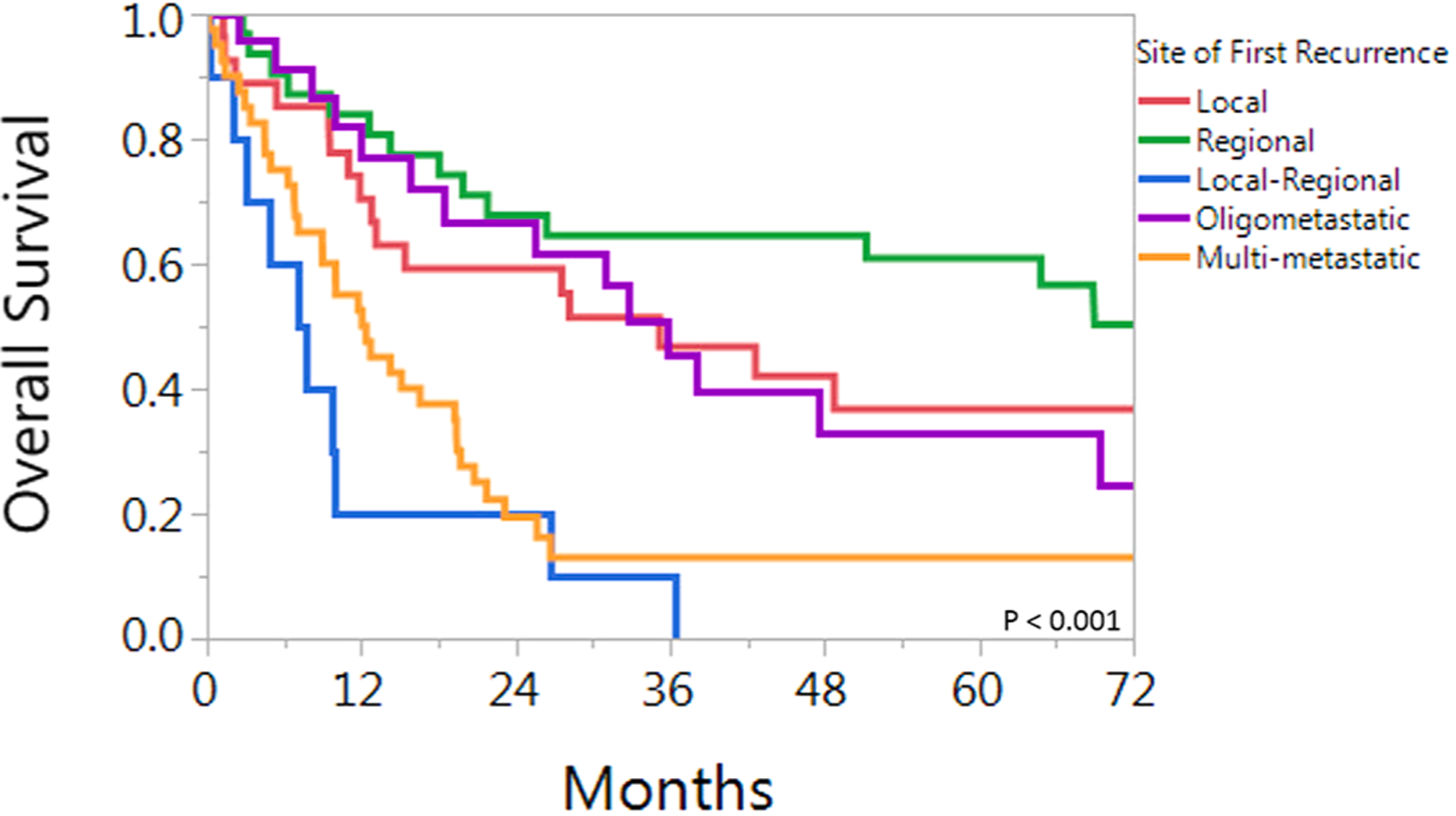
Overall survival for 132 patients with HPV associated oropharyngeal cancer who developed recurrent disease. Patients are grouped by site of first recurrence(s).
Fig. 3.
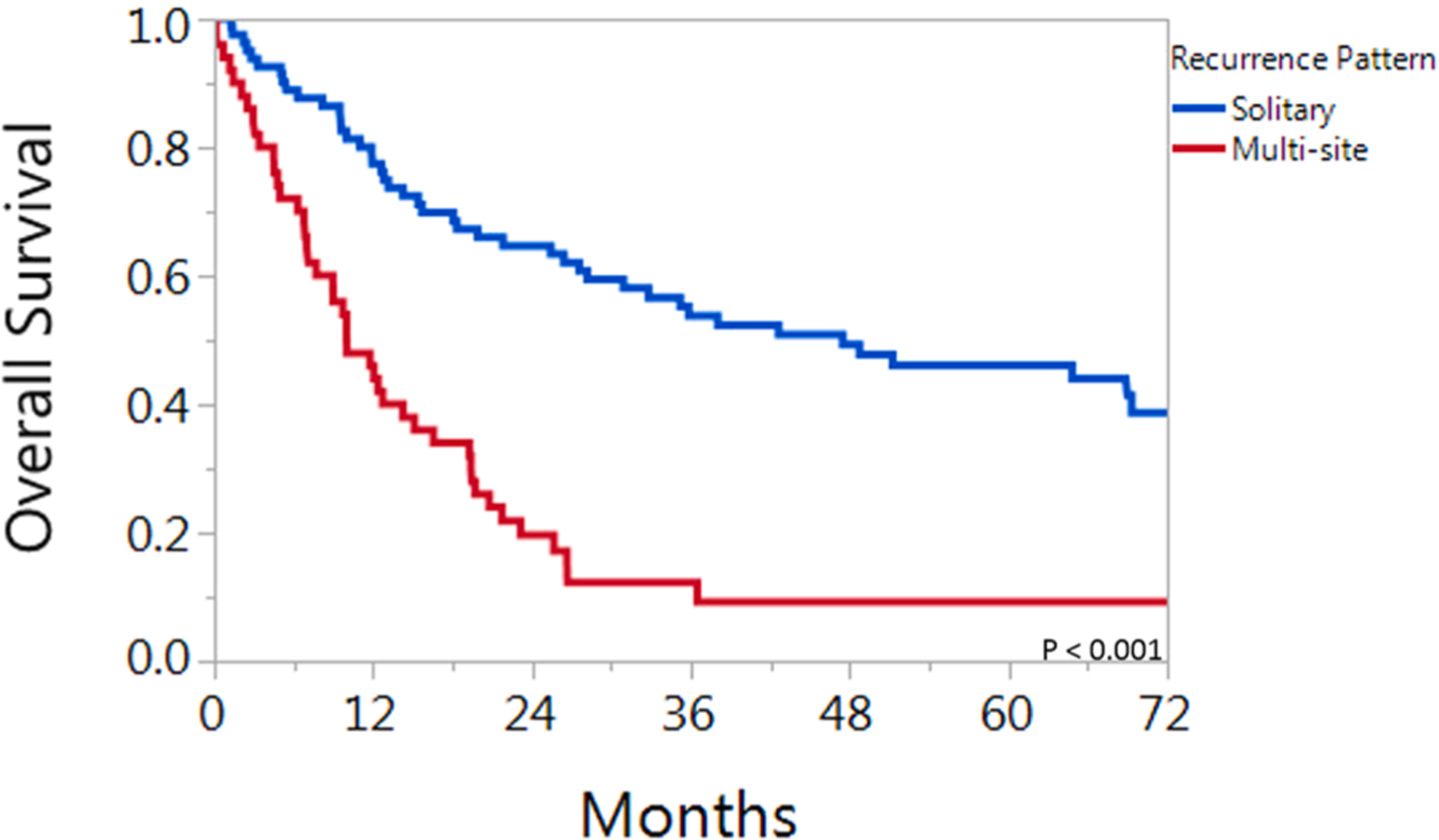
Overall survival for 132 patients with HPV associated oropharyngeal cancer who developed recurrent disease. Patients are grouped by solitary (including oligo-metastasis) versus multiple sites of recurrence.
Patients who developed recurrent disease in ≤ 12 months had median OS of 12 months [95% CI: 4–26.4] compared to 27 months [95% CI: 19.3–51.3] (p = 0.02) for those with a more prolonged time to recurrence. These differences were observed when further subgrouping by solitary vs. multisite was analyzed. In the solitary group, those with recurrence within 12 months had a 2-year overall survival rate of 56% compared to 71% for those whose recurrence manifested after 12 months (p = .045); for the multisite group the 2-year survival rates were 10% vs. 26% based on recurrence within or >12 months from initial treatment, respectively (p = .07).
Salvage therapy by site of recurrence
Details of salvage therapy are described below and summarized in Table 2.
Local
Twenty-eight patients had primary site recurrent disease. Fourteen patients (50%) had tonsil primaries and 14 (50%) had base of tongue primaries. The recurrent t-categories were: rT1, 10 patients, rT2, 6, rT3, 1 and rT4, 11. Fifteen patients had surgical resection (7 of whom also had free flap reconstruction), 5 had re-irradiation (3 with conventionally fractionated treatment, and 2 with stereotactic radiotherapy), and 8 had systemic treatment and/or supportive care. There was no difference in attempted surgical salvage based on primary disease site. Nine patients (90%) with rT1 were operated, while only 3 patients (30%) with rT4 were operated. Of the 5 patients re-irradiated, 1 had rT1, and the remainder rT4.
Fourteen patients (10 treated with surgery and 4 with re-irradiation) remain without evidence of local disease at last follow up (range 11–118 months from diagnosis of recurrence). Two of these patients had subsequent regional events requiring further therapy (1 surgery, 1 re-irradiation), and both remained without further disease. Four patients later developed distant disease and none were successfully salvaged.
Regional
Thirty-one patients had regional recurrence as site of first failure. The recurrent nodal categories were: rN1, 16 patients, rN2a, 3, rN2b, 9, and rN2c, 2.
Four patients had recurrence in a previously dissected neck of whom 3 had extranodal soft tissue and/or muscle extension noted at time of initial surgery. All 4 patients had disease recur less than 12 months after RT. None of these patients underwent further local therapy and all died with disease.
In the remaining 27 patients, recurrence occurred in a site of prior involved lymph nodes in 21 patients (81%). Three patients re-presented with disease in uninvolved (but electively treated) nodal sites, and 3 patients had disease recur in an untreated contralateral neck.
Twenty-three of these 27 patients (85%) with regional recurrence and an undissected neck underwent surgical salvage (with or without chemotherapy and/or post-operative radiotherapy (PORT)), 2 patients (7.5%) were treated with (chemo)radiation-based salvage, and 2 patients (7.5%) were treated with palliative/supportive care.
Amongst the 23 patients operated, 17 had adjuvant treatment. Seven had postoperative external beam and concurrent chemotherapy (including the 3 whose neck involved with recurrence was previously unirradiated), 5 had brachytherapy (with iridium192 catheters), 4 had combined high dose brachytherapy and external beam radiation, and one patient had preoperative chemotherapy. Twenty-one of these 23 patients had regional control of their disease at last follow-up, and the 5-year OS was 82%.
Three patients were treated with systemic therapy plus radiotherapy. Stereotactic radiation was used for an isolated RP recurrence and isolated level 2 recurrence. One patient treated with SBRT later had recurrence in the contralateral neck, and the other patient was without disease at last follow up. The third patient was treated with standard fractionation EBRT and developed a subsequent recurrence of disease in the treatment field.
Local regional
Recurrent stages for the 10 patients with combined local-regional failures were varied with T-categories ranging from T1-T4 and N-categories from N1-N2c. Salvage was only attempted in 3 patients; Two patients were treated with surgical salvage (one also treated with PORT) and 1 patient was treated with chemotherapy followed by re-irradiation. Seven patients were treated with palliative or supportive care. Only 1 patient treated with surgical salvage and PORT with low volume disease (rT1N1) achieved ultimate local-regional control and remains alive and without disease.
Oligometastatic disease
Sites of metastatic disease for the 23 patients with an oligometastatic metastatic presentation included: lung (n = 18), lung/hilum (n = 1), mediastinum (n = 1), brain (n = 2) and axilla (n = 1).
Among the 18 patients with oligometastatic disease of the lung, 13 had a solitary lesion, and 5 had either 2 (2) or 3 (3) lesions. Of the 13 with solitary lesions, 6 were treated with surgery (wedge resection or lobectomy), 6 with stereotactic radiation, and 1 person declined therapy. Six of the 12 treated patients were alive at last follow-up. All 5 patients with multiple lesions were treated with varying systemic regimens, and only 1 was treated with a local therapy too (stereotactic radiation). Only 1 of these 5 patients is alive at last follow-up.
Treatment was individualized for the 2 patients with thoracic nodal disease. One was treated with surgery and is alive at last follow-up. The second was treated with chemotherapy and radiation and subsequently died. Among the 2 patients with isolated brain disease one was treated with surgery followed by post-op stereotactic RT and one with stereotactic RT alone. Both remained controlled in the brain at last follow up, though one patient developed regional and distant metastatic disease 3 years after their brain recurrence. The patient with axillary recurrence was treated with axillary dissection and developed an additional recurrence in the axilla and other distant metastatic sites.
Multi-site metastatic disease
Forty patients had multi-metastatic disease (including 7 patients with synchronous local and/or regional recurrence); common sites of distant spread included lung, liver, distant nodal disease (mediastinum, abdomen), and bone. Thirty patients (75%) were treated with systemic therapy, 3 (7.5%) were treated with palliative local therapies, and 7 (17.5%) had unknown therapy. Only one patient (receiving chemotherapy alone for multiple bilateral lung metastases) remains alive without evidence of disease.
Discussion
Even with improved survival, upwards of 30% of patients with HPV-positive OPSCC may experience disease recurrence with modern treatment [7,8]. Our 2-year OS for all patients with recurrent disease approximates 50%, consistent with other reports in the literature [6,9–11]. With longer follow-up we demonstrated a 5-year OS rate of 32%, but perhaps more importantly, we have shown that patients presenting with a single site of recurrence had significantly better survival with a 5-year OS rate of 46%. In particular, patients who had isolated cervical nodal recurrences did very well.
Fakhry et al. grouped patients solely on local-regional recurrences vs. distant metastases and reported worse survival for patients with distant metastases [6]. However, this subgroup analysis included patients with either p16-positive or negative tumors. We could not reproduce this finding. Our study (restricted to patients with HPV-associated disease) analyzed recurrences with greater granularity and found that patients have longer survival if they have recurrence in a single site (i.e. local, regional, or oligometastatic), versus those who develop recurrences in multiple sites (i.e. local-regional or multi-metastatic).
Numerous studies have looked at the utility of salvage surgery for recurrent HNSCC, and patients who undergo surgical salvage have reported better outcomes [6,12,13]. In a small series of patients with relapsed HPV-positive OPSCC treated with upfront surgery, patients treated with aggressive salvage for LRR or DM had a 3-year cancer specific survival of 63% after loco-regional relapse and 100% after distant metastases [13]. The authors concluded that aggressive salvage treatment for loco-regional relapse and distant metastases should be recommended for appropriate candidates. This finding holds true in our study, as patients treated with surgical salvage of local or regional recurrences or oligometastases experienced improved survival.
There is an increasing body of literature supporting the use of local therapies in the oligometastatic disease setting to prolong survival in HNSCC patients [10,14–18]. Our study showed prolonged survival for patients treated with local therapies for oligometastatic lung disease, even in the setting of prior regional recurrence. Prospective trials evaluating multi-modality therapy for oligometastatic disease (specifically including local therapies such as surgery and radiotherapy) in HPV-positive OPSCC are warranted.
Interestingly, in our study, patients whose disease recurred distantly with an oligometastatic presentation had similar survival to patients with local recurrences. We suspect the reason for this is two-fold. Oligometastatic disease occurs in a previously untreated region, making surgery or stereotactic radiation likely safe and feasible with minimal morbidity from these types of procedures. Secondly, some local recurrences can be hard to salvage. This may be due to the complexity of surgery in a previously irradiated mucosal site and patients may require extensive surgeries with a higher risk of morbidity [10,19]. While the numbers were small, there were obvious differences in using surgery for patients with rT1 vs. rT4. It is certainly a shift in oncologic paradigms to view a metastatic relapse similar to a local relapse. In either scenario, our study suggests approximately 50% of patients will have durable disease control with aggressive local therapy.
The subgroup that appeared to have the best outcomes was the cohort with isolated regional recurrences. Further parsing of this subgroup was too small to make statistical inferences, but we did observe several features. First the small group that had recurrences in a surgically violated neck did very poorly. Whether this is a function of the biology of these cancers which tended to present with rapid recurrences, or a function of the neck already maximally treated with 2 local modalities is speculative. On the other extreme, the patients who had recurrences in the treatment naïve neck did well, and further adds to the question of who needs treatment to a clinically N0 neck in HPV associated disease. The group with the most patients were those who had surgery for their recurrence in an irradiated neck. Many received re-irradiation adjuvantly, but the techniques varied, so a specific recommendation regarding either the need for adjuvant treatment or the specific approach cannot be made.
Outcomes for patients who are treated with systemic therapy alone for recurrence or metastatic HNSCC are poor, with response rates around 20–25% [20,21]. Outcomes with systemic treatment alone in modern trials mirror the outcomes seen in our patients, with a median OS ≤ 1 year [9,12,22]. Given the growing body of literature, including the present study, which supports aggressive salvage therapy for appropriate patients coupled with the low response rate to systemic therapies, multi-disciplinary discussion should be held for patients presenting with recurrent HPV-positive OPSCC in an effort to identify patients who may benefit from more intensive therapy, particularly if they have small volume disease or solitary sites of recurrence.
Limitations of this study are those inherent to any retrospective review, as there is a risk of selection and treatment biases. For example, our patients with synchronous local and regional recurrences were generally approached with systemic therapy rather than surgery, and we can only speculate why these management choices were made. Patients presenting with lung disease that was biopsy positive for SCCa, but not tested for HPV or p16, were included in this study; thereby there may be some patients included who did not in fact have metastatic disease, but rather a metachronous primary lung cancer. Despite these limitations, the retrospective nature and individualized chart review allowed for this very granular series of HPV-positive OPSCC recurrences after primary (chemo)radiation with details regarding useful salvage therapies.
Conclusions
Patients with recurrent HPV-positive OPSCC experience 2- and 5-year overall survival rates of approximately 50% and 33%, respectively. Patients with a “solitary” recurrence including disease at the primary site, neck or oligometastatic site have relatively favorable outcomes, as their 5-year survival rate approximates 50%.
Acknowledgement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Funding sources and financial disclosures:
Dr. Fuller receives funding via the multidisciplinary Stiefel Oropharyngeal Research Fund of The University of Texas MD Anderson Cancer Center Charles and Daneen Stiefel Center for Head and Neck Cancer, as well as a multi-site National Science Foundation (NSF), Division of Mathematical Sciences, Joint NIH/NSF Initiative on Quantitative Approaches to Biomedical Big Data (QuBBD) Grant (NSF 1557679) and the NIH Big Data to Knowledge (BD2K) Program of the National Cancer Institute (NCI) Early Stage Development of Technologies in Biomedical Computing, Informatics, and Big Data Science Award (1R01CA214825). Dr. Gunn received a philanthropic gift from the Family of Paul W. Beach. Drs. Mohamed and Fuller received funding support from the National Institutes of Health (NIH)/National Institute for Dental and Craniofacial Research (1R01DE025248/R56DE025248). NIH/NCI (R21CA226200), and the NIH/NCI Early Phase Clinical Trials in Imaging and Image-Guided Interventions Program (1R01CA218148). Dr. Fuller received grant and/or salary support from: the Andrew Sabin Family Foundation; The Mike Hogg Foundation; National Institute of Biomedical Imaging and Bioengineering (NIBIB) Research Education Programs for Residents and Clinical Fellows Grant (R25EB025787-01); an NIH/NCI Cancer Center Support Grant (CCSG) Pilot Research Program Award from the UT MD Anderson CCSG Radiation Oncology and Cancer Imaging Program (P30CA016672); the NIH/National Cancer Institute Head and Neck Specialized Programs of Research Excellence Developmental Research Program Award (P50CA097007); a Paul Calabresi Clinical Oncology Program Award (K12 CA088084); a General Electric Healthcare/MD Anderson Center for Advanced Biomedical Imaging In-Kind Award; an Elekta AB/MD Anderson Department of Radiation Oncology Seed Grant; the Center for Radiation Oncology Research at MD Anderson Cancer Center Seed Grant; and the MD Anderson Institutional Research Grant (IRG) Program. Dr. Fuller has received speaker honoraria, grant support, and travel funding from Elekta AB.
Dr. Frank reports personal fees from Varian, grants and personal fees from C4 Imaging, grants from Eli Lilly, grants from Elekta, grants and personal fees from Hitachi, other from Breakthrough Chronic Care, personal fees from Augmenix, personal fees from National Comprehensive Cancer Center (NCCN), outside the submitted work.
Footnotes
Potential Conflicts of interest are listed here
XXX
Declaration of Competing Interest
There are no actual conflicts of interest to declare.
References
- [1].Calais G, et al. Randomized trial of radiation therapy versus concomitant chemotherapy and radiation therapy for advanced-stage oropharynx carcinoma. J Natl Cancer Inst 1999;91(24):2081–6. 10.1093/jnci/91.24.2081. [DOI] [PubMed] [Google Scholar]
- [2].Olmi P, et al. Locoregionally advanced carcinoma of the oropharynx: conventional radiotherapy vs. accelerated hyperfractionated radiotherapy vs. concomitant radiotherapy and chemotherapy–a multicenter randomized trial. Int J Radiat Oncol Biol Phys 2003;55(1):78–92. 10.1016/s0360-3016(02)03792-6. [DOI] [PubMed] [Google Scholar]
- [3].Mallen-St Clair J, Alani M, Wang MB, Srivatsan ES. Human papillomavirus in oropharyngeal cancer: the changing face of a disease. Biochim Biophys Acta 2016; 1866(2):141–50. 10.1016/j.bbcan.2016.07.005. [DOI] [PubMed] [Google Scholar]
- [4].Ang KK, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010;363(1):24–35. 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gillison ML, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet 2019;393(10166):40–50. 10.1016/S0140-6736(18)32779-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fakhry C, et al. Human papillomavirus and overall survival after progression of oropharyngeal squamous cell carcinoma. J Clin Oncol 2014;32(30):3365–73. 10.1200/JCO.2014.55.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ang KK, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol 2014;32(27):2940–50. 10.1200/JCO.2013.53.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Nguyen-Tan PF, et al. Randomized phase III trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas in the Radiation Therapy Oncology Group 0129 trial: long-term report of efficacy and toxicity (in eng). J Clin Oncol Dec 2014;32(34):3858–66. 10.1200/JCO.2014.55.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Argiris A, et al. Prognostic significance of human papillomavirus in recurrent or metastatic head and neck cancer: an analysis of Eastern Cooperative Oncology Group trials. Ann Oncol Jul 2014;25(7):1410–6. 10.1093/annonc/mdu167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Guo T, et al. Surgical salvage improves overall survival for patients with HPV-positive and HPV-negative recurrent locoregional and distant metastatic oropharyngeal cancer. Cancer 2015;121(12):1977–84. 10.1002/cncr.29323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Deeken JF, et al. Effect of multimodality treatment on overall survival for patients with metastatic or recurrent HPV-positive head and neck squamous cell carcinoma. Head Neck May 2015;37(5):630–5. 10.1002/hed.23644. [DOI] [PubMed] [Google Scholar]
- [12].de Ridder M, et al. Disease course after the first recurrence of head and neck squamous cell carcinoma following (chemo)radiation. Eur Arch Otorhinolaryngol, Oct 10 2019, doi: 10.1007/s00405-019-05676-2. [DOI] [PubMed]
- [13].Sims JR, et al. Management of recurrent and metastatic HPV-positive oropharyngeal squamous cell carcinoma after transoral robotic surgery. Otolaryngol Head Neck Surg Jul 2017;157(1):69–76. 10.1177/0194599817696304. [DOI] [PubMed] [Google Scholar]
- [14].Beckham TH, et al. Long-term survival in patients with metastatic head and neck squamous cell carcinoma treated with metastasis-directed therapy. Br J Cancer Nov 2019;121(11):897–903. 10.1038/s41416-019-0601-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Leeman JE, et al. Patterns of treatment failure and postrecurrence outcomes among patients with locally advanced head and neck squamous cell carcinoma after chemoradiotherapy using modern radiation techniques. JAMA Oncol 2017;3(11): 1487–94. 10.1001/jamaoncol.2017.0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Liu D, et al. Pulmonary metastasectomy for head and neck cancers. Ann Surg Oncol Sep 1999;6(6):572–8. 10.1007/s10434-999-0572-8. [DOI] [PubMed] [Google Scholar]
- [17].Shiono S, et al. Pulmonary metastasectomy for pulmonary metastases of head and neck squamous cell carcinomas. Ann Thorac Surg Sep 2009;88(3):856–60. 10.1016/j.athoracsur.2009.04.040. [DOI] [PubMed] [Google Scholar]
- [18].Kang HS, et al. Predictive factors for long-term survival in head and neck squamous cell carcinoma patients with distant metastasis after initial definitive treatment. J Cancer Res Clin Oncol Jan 2016;142(1):295–304. 10.1007/s00432-015-2043-x. [DOI] [PubMed] [Google Scholar]
- [19].Pang L, Jeannon JP, Simo R. Minimizing complications in salvage head and neck oncological surgery following radiotherapy and chemo-radiotherapy. Curr Opin Otolaryngol Head Neck Surg Apr 2011;19(2):125–31. 10.1097/MOO.0b013e3283440ee3. [DOI] [PubMed] [Google Scholar]
- [20].Morton RP, et al. Cisplatinum and bleomycin for advanced or recurrent squamous cell carcinoma of the head and neck: a randomised factorial phase III controlled trial. Cancer Chemother Pharmacol 1985;15(3):283–9. 10.1007/bf00263902. [DOI] [PubMed] [Google Scholar]
- [21].Mehra R, et al. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: pooled analyses after long-term follow-up in KEYNOTE-012. Br J Cancer Jul 2018;119(2):153–9. 10.1038/s41416-018-0131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cohen EEW, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet 2019;393(10167):156–67. 10.1016/S0140-6736(18)31999-8. [DOI] [PubMed] [Google Scholar]


