Abstract
The cell is the basic structural unit of all living organisms. Most of the cells forming the human body share the basic components, but there are many categories that have specific light and electron microscopic characteristics. This review shed a light on these cell categories and their morphologies. Stem cell category is the cells responsible for the regeneration of damaged or lost cells, whereas protein-secreting cells are those responsible for the production and secretion of proteins. Protein-secreting cells have specific characters such as basophilic cytoplasm and vesicular nucleus by light microscope; these are confirmed by an electron microscope that shows rough endoplasmic reticulum, Golgi apparatus, secretory granules, and mitochondria. Steroid secreting, ion transporting, and contracting categories have specific morphology. Phagocytic cells such as macrophages and neutrophils are characterized by the presence of many lysosomes and phagosomes. Supporting cells are very important category as they not only support but also usually have another function such as myelin formation in Schwann, blood–brain barrier in astrocytes, or modification of response as in Pacinian corpuscle. Many cells showed interrelated characters between different categories, for example, phagocytic cells are able to contract to perform their function in fighting microorganism. Although we have trillions of cells, yet they only fall in some categories. Each cell category has specific morphological characters confirmed by ultrastructural characters. They all adapted to perform the desired functions.
Keywords: Categories, cells, human body, morphology
INTRODUCTION
The basic structural and functional unit of the living organism is the cell. The cell is a model of the whole organism represented in a very small unit. The human body is formed of 30 trillion of cells.[1] These cells are falling in about 215 types and 4 types of tissues. The tissues are simple as they are only connective tissue, epithelial tissue, nervous, and muscular tissue.[2] Human organization as any living organism is running upward from cells to the system.[3]
The cells are falling into certain categories stem cells, supporting cells, secretory cells exocrine, secretory cells endocrine, secretory cells matrix, phagocytic cells, killing cells, ion-transporting cells, antigen-presenting cells, contracting cells, endothelial cells, sensory cells, and nerve cells.[2,4,5]
The cell categories have different organelles to adapt their categories.
STEM CELL CATEGORY
It is the first category in which the cells are regenerative to other types of cells. These cells are characterized by having pale nucleus, basophilic cytoplasm due to the presence of free ribosomes, and some mitochondria seen by an electron microscope. Let's see stem cell in our systems:[2,5]
The central nervous system has stem cell discovered recently especially at the third and lateral ventricle, hippocampus formation. Stem cell in nervous tissue proliferates to neuroglia cells.[6,7,8]
The respiratory system has stem cells from the nose to alveoli. Starting from stem cells of the olfactory mucosa in the nose, stem cell of the respiratory epithelium stem, cells of bronchioles are called Clara cells. Finally, alveoli Type II are the stem cells of alveoli.[9,10]
As regard the integumentary system, the stratum basale in the skin is the stem cell of the epidermis, cells of hair pulp of hair follicle are the stem cells of hair, stem cell of sebaceous glands, and finally, stem cells of sweat glands and their ducts.[11]
As regard the digestive tract, we start by basal layer of oral epithelium, stem cell of taste bud, stem cell of the gastric gland, crypt base cell, and stem cell of the colon.[12]
The liver has an oval cell at the bile ductules of portal tracts responsible for the regeneration of the liver. The exocrine pancreas has stem cell called centroacinar cell at the beginning of ducts of serous acini.[13,14]
The urinary system has basal cells within its epithelium, but the damage of the renal tubules is regenerated by the adjacent cells’ division.[15,16]
The endocrine system has specific cells for the regeneration of endocrine cells. The pituitary gland has chromophobe cells as stem cells. C-cell of the pancreas regenerates other cells of islets of the pancreas. Thyroid, parathyroid, and adrenal glands are regenerated by the activity of the adjacent cell division.[17] Blood cells are regenerated by bone marrow stem cells which are called hemopoietic stem cells.[18]
Stem cells of connective tissue are called pericytes and located around the capillaries, whereas stem cells of skeletal muscle are called satellite cells and present around the skeletal muscle. Specialized connective tissue like bone and cartilage have osteogenic cells and chondrogenic cells as stem cells, respectively.[9,19,20]
Stem cells are able to divide symmetrically to compensate for themselves and asymmetrically to give newly differentiated cells that committed to a certain type and function.[21]
PROTEIN-SECRETING CELL CATEGORY
This category is relatively wide, so I prefer to subdivide them into four subcategories: luminal protein, endocrine protein, matrix protein, and special two cells.
Luminal protein
Those secrete to lumens of systems such as digestive, genital, and respiratory systems.
Here, we have serous acini, mucous acini, and mixed acini cells of salivary, oral lingual, palatine, pharyngeal, and esophageal glands.
Move to the gastric glands, you will found: mucous neck cells, surface mucous cells, and chief cells. Pancreatic serous acini, goblet cells of intestine and Paneth cells of small intestine are all considered examples of this subcategory. Columnar absorptive cells secrete enzymes, but they suspend them on the cell membrane to digest different food staffs. Goblet cells of ducts of gall bladder and pancreatic exocrine duct are another example.[2,22]
In the respiratory system, the goblet cells of respiratory epithelium secrete mucous which is formed mainly of water, glycoprotein, proteoglycan, lipid, and DNA.
Clara cell of bronchioles also secretes two components, surface active agent lipoprotein with surfactant like functions and Clara cell protein CC16. Alveolar cell Type II secretes surfactant lipoprotein that prevents alveolar and alveolar sacs collapse. The secretion of this cell is formed of phospholipid, protein, and lipids. The urethra has the bulbourethral glands, and intraepithelial glands also secrete mucous to the urethra. The fallopian tube contains peg cell that secretes mucous to the tube for the nourishment of fertilized ovum.[23,24]
Endocrine protein
It is the second category of protein secreting cells where the protein is released to blood and lymphatics. It is formed of endocrine glands and endocrine cells embedded in different systems. Endocrine cells embedded in systems and organs are called enteroendocrine cells. They are located in the stomach, small intestine, large intestine, and respiratory bronchi. In the GIT, there are many types of cells named by letter, whereas in the respiratory system, we have small granule cell type or Kulchitsky cells. Kulchitsky cells are respiratory representatives of the general class of enteroendocrine cells of the gut and gut derivatives. They are of two types; one produces catecholamine and the other produces polypeptide hormones such as serotonin, calcitonin, and gastrin-releasing peptide (bombesin).[5]
The main category of endocrine protein-secreting cells is all protein-secreting cells present in the endocrine glands all over the body. Anterior pituitary has five cells in its anterior lobe and two in its posterior lobe. Those of anterior lobe are somatotrophes, mammotrophes, thyrotrophes, gonadotrophes and corticotrophes.The posterior pituitary stores and secretes the hormones synthesized by nervous nuclei of hypothalamus paraventricular and supraoptic nuclei; they are called neurosecretory granules.[25]
The pineal gland has the pinealocytes that secrete melatonin at night and serotonin in the morning.[26]
The thyroid gland has two cells thyrocytes and parafollicular cells or also called C-cell. Thyrocytes secrete thyroid hormones, whereas C-cells secrete calcitonin.
Parathyroid has chief cell and oxyphil cell, but oxyphil cell is not a protein-secreting cell.[27] The pancreas has the cells of islets of Langerhans alpha, beta, and delta cells.[28]
The adrenal medulla has chromaffin cells which are responsible for adrenaline and noradrenaline secretion.[29] Moreover, the juxtaglomerular cells of the kidney are responsible for the secretion of renin which converts angiotensinogen to angiotensin I.[30]
Matrix protein
The third category is the cells of connective tissue and blood that secrete protein, glycoprotein, or proteoglycan to the matrix of the connective tissue where their secretion forms the extracellular matrix.
Fibroblasts and fibrocytes secrete collagen Type I, IV, V and elastic fibers in addition to fibronectin and proteoglycan.[31]
Chondroblasts and chondrocytes secrete collagen Type II, chondroitin sulfate, and chondronectin. Reticular cells secrete collagen Type III (Sun et al., 2011).[32]
Osteoblasts and osteocytes secrete collagen type I, osteonectin, sialoprotein, and matrix vesicles for calcification.[33]
Mast cells store and release heparin and histamine leukotrienes and chondroitin sulfate.[26]
Immune cells such as granular leukocytes, monocytes, T lymphocytes, and macrophages are responsible for the secretion of cytokines, lymphokines, and interferon. B lymphocytes change to plasma cells secrete gamma globulins of five types, immunoglobulins A, M, G, E, and D.[34]
Liver cells secrete albumin, beta globulins, and fibrinogen. The albumin protein is essential for the hydrostatic pressure of blood and fluid balance. Fibrinogen is essential for the clotting mechanism.[2,35]
Cellular protein with extracellular function
The fourth category includes the cellsthat synthesize protein performing its function while it is located within the cell. The first example is granulosa cells of ovarian follicles, the second example is the columnar absorptive cells of small intestine. Sertoli cells secrete androgen-binding protein that traps androgen to stimulate spermatogenesis. These three cells are best to be described as protein-synthesizing cells and not protein secreting as they secrete protein, and it performs its function where it is located inside the cell.[5]
Morphology of protein-secreting cells
These cells appear basophilic by routine stain due to the presence of ribosomes, rough endoplasmic reticulum, Golgi apparatus, mitochondria, and secretory granules [Figure 1]. There are some exceptions to the mentioned morphology as in liver and Sertoli cells due to high mitochondrial content.[2]
Figure 1.
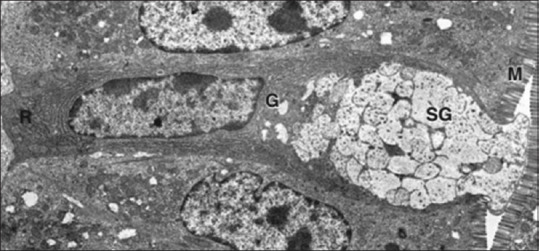
The EM of goblet cell with microvilli (M), rough endoplasmic reticulum (R), Golgi complex (G), and secretory granules (SG)[2]
Steroid-secreting cells
Adrenal cortex zona glomerulosa cells secrete mineralocorticoids, zona fasciculata secretes glucocorticoids, and zona reticularis cells secrete sex hormones. Theca cells of the ovarian follicles and interstitial cells of the ovary secrete androgen hormones. Interstitial cells of Leydig in the testis are located between seminiferous tubules, and they secrete androgen hormones.[36,37]
The morphology of these cells secreting steroid is correlated to the function. By light microscope, these cells have acidophilic cytoplasm and vesicular nuclei. By electron microscope, they contain numerous mitochondria with tubular cristae, smooth endoplasmic reticulum, glycogen, and lipid droplets. Some free ribosomes are found within these cells to synthesize the enzymes required for steroid formation [Figure 2].[2]
Figure 2.
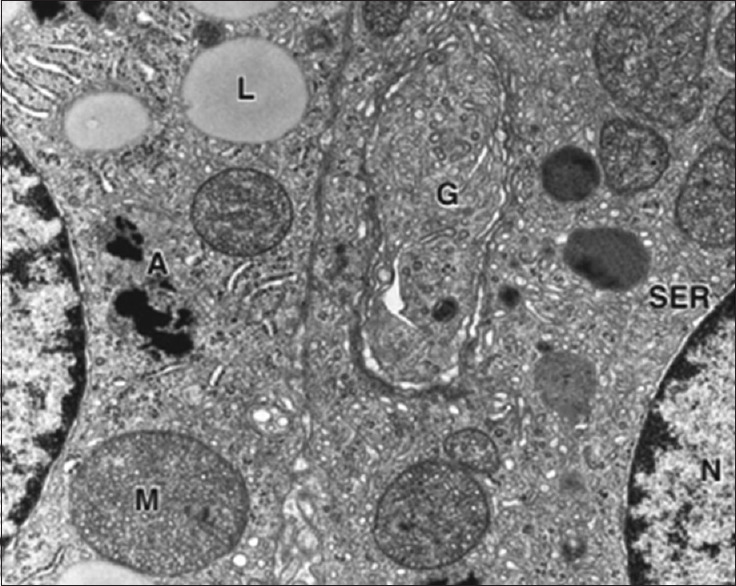
Steroid-secreting cells with the smooth endoplasmic reticulum, mitochondria (M), and lipid droplets (L)[2]
ION TRANSPORTING CELLS
These cells are responsible for maintaining certain electrolyte concentration within the lumen. These functions require great energy production, so they have greater mitochondria content. The cells falling in this category are as follows:
Ependymal cells lining the ventricles secrete the cerebrospinal fluid. Cells lining the endolymphatic canal of the inner ear secrete the endolymph with high potassium and low sodium content.[38,39] Stria vascularis of scala media of the inner ear maintains the endolymph of cochlea.[40] Outer pigmented and inner nonpigmented layers of the ciliary body of the eye are considered of multiple categories. The ciliary epithelium has three functions: secrete aqueous humor fluid with plasma like composition without protein, maintain the composition of aqueous by tight junction between apices of inner nonpigmented cells, and the last function is the formation of zonular proteins of suspensory ligaments of the lens.[41]
Parietal cells are considered ion transporting cells as it secretes hydrochloric acid to the gastric lumen to lower the PH to 2. It also secretes intrinsic factor that binds vitamin B12 to facilitate its absorption.[42,43]
Morphology of ion-transporting cells is the universe in containing numerous mitochondria and in addition to the usual amount. The lateral borders of ion-transporting cells are connected together by tight junction, adherent junction, and desmosomes. The cells need mitochondria to create ion concentration and junctional complex to maintain this ion change.[5]
CONTRACTILE CELLS
The contractile cells are complex and need a separate review, but to summarize, we can define the contracting cell as any cell that changes its shape and position. There are three types of contractile cells: the muscular cells, dividing cells, and moving cells. Contraction by itself requires actin filaments to be present within the cell; actin is microfilament 5 nm in diameter and formed of G actin protein arranged in double-twisted filamentous actin. Actin filament presents in all cells in our bodies, but it has different arrangement that differs from cell to other.[2]
The muscular tissue is formed of skeletal muscle, cardiac muscle, and smooth muscle. Striated muscle contains myofibrils with alternating dark and light bands. The basic contractile unit of striated muscles is the sarcomere in which the actin and myosin II are interacted to contract and relax. Sarcomere is the distance between two successive Z lines. Z line is formed of actinin protein that binds actin in cardiac and smooth muscle. Striated muscle has many actin-associated proteins to distribute the force of contraction evenly on the cell membrane like dystrophin the smooth muscle in reverse has no sarcomeres but it contains actin and myosin that are arranged randomly throughout the cytoplasm of the muscle fiber. Dense bodies regulate actin-intermediate filaments interaction. Dense bodies are similar to the Z line in striated muscle. Thick filaments are bipolar in striated muscle fibers but side polar in smooth muscle fibers.[44,45]
The dividing cells at the stage of cytokinesis contain actin-myosin interaction at the cleavage furrow. This actin-myosin interaction enables the dividing cells to give two newly separate cells.[46]
The moving cells also have a greater amount of actin and myosin, especially at the cell membrane to facilitate movement. Moving cells like macrophage and neutrophil move easily to bacteria and cell debris to get rid of them.
The myosin in muscle fibers and dividing cells is from Type II category, whereas myosin in moving cells is of Type I category. The difference between the two types is beyond the scope of this review.[47]
The point of importance in this review is the way of stimulation in skeletal muscle and smooth. The skeletal is stimulated by the somatic motor nerve to the motor endplate. The electrical stimulus reaches to triad system (T tubule and sarcoplasmic reticulum) to release calcium to sarcomere that initiates muscle contraction. In smooth muscle, the stimulus could be mechanical, electrical, or chemical.[35,48] Mechanical stretch of smooth or hormones come through autonomic nerve stimulation or hormones attached to the corresponding receptors on the cell membrane of the smooth muscle so the contraction is slow.[49]
PHAGOCYTIC CELLS
They are sharing the protein secreting type and phagocytic type as they phagocytose the bacteria and cellular debris; moreover, they secrete cytokines to combat different pathogens. There are many types and categories of phagocytic cells; monocyte in blood, macrophage of connective tissue, Langerhans's cell of the epidermis, Von Kupffer cells of the liver, microglia cells of the central nervous system, neutrophils, and eosinophils.[50,51]
The morphology of any phagocytic cell is nearly similar except the shape and size.
Any phagocytic cell contains numerous phagocytic vacuoles, numerous lysosomes in addition to the usual organelles when examined by an electron microscope.
By light microscope, these categories are highly variable due to affinity to stains. Macrophage stained grayish blue by H and E and specifically by toluidine blue, whereas neutrophils and eosinophils are stained by Leishman stain. Neutrophils are phagocytic cells but contain in addition to lysosomes, specific granules, and tertiary granules. Specific granules have collagenase, complement activator, and antimicrobial enzymes, whereas tertiary granules have phosphatase and metalloproteinases. Eosinophils also have specific granules beside the lysosomes. Here, the specific granules contain major basic, neurotoxin, peroxidase, and cationic proteins. As regard the nucleus of any phagocytic cell, it varies from cell to cell and varies according to the level of virulence and aggression. In granular leukocytes, the nucleus of neutrophil is multilobed or segmented while that of eosinophil is bilobed. The nucleus of monocytes or macrophages is kidney shaped. In macrophages and monocytes with high phagocytic activity, the shape of nuclei will change to horse-shoe shape. The cell membrane of macrophage also shows processes, while during aggressive phagocytosis the cell membrane will show lamellipodia or even microvilli.[2,5]
SUPPORTING AND PROTECTING CELLS
Supporting cell category includes those cells involved in support of other cells, surround other cells, or protect other cells. Most of those cells require an electron microscope and immunostaining for demonstration, but routine H and E stain could help in some. Most of supporting cells perform additional functions in addition to support.[52] Astrocytes of the central nervous system have nutritive and microenvironmental adjustment functions in addition to support. Astrocytes share in blood–brain barrier through their feet close relation to brain blood vessels. Oligodendroglia cells and Schwann cells are supporting cells for axons of nerve cells. Oligodendroglia supports and forms myelin sheath around axons of the central nervous system. Schwann cells do the same but for the axons of peripheral nerves. Myelin sheath protects the axons of neurons and speeds up the conduction of impulses.[47]
Reticular epithelial cells of the thymus are of six types, they support bone marrow-derived immature T lymphocytes during maturation and acquiring competencies. Reticular epithelial cells secrete thymic hormones that enhance the maturation of lymphocytes. The most important function of these cells is the formation of blood thymic barrier. Reticulocyte forms tight junction to protect the developing lymphocytes within the thymus from different antigens until full maturation.
Keratinocytes of the epidermis are covering cells that protect the whole surface of the body. They are arranged in 5 layers; stratum basale, spinosum, granulosum, lucidum, and corneum in thick skin sites and 4 layers in thin skin sites. Keratinocytes support the adjacent cells such as melanocytes, Merkel cells, and Langerhans cells [Figure 3].[5,11,53,54]
Figure 3.
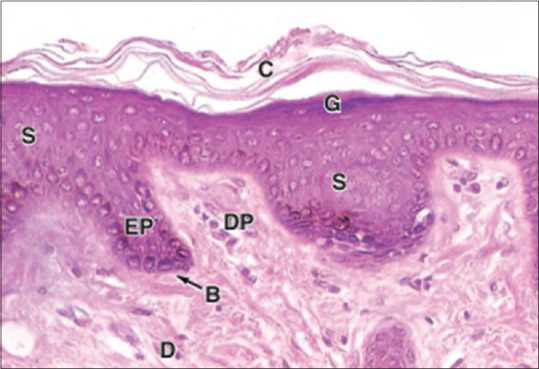
Keratinocytes in different layers stratum basale (B), stratum spinosum (S), granulosum (G), corneum (C), dermis (D)[2]
Supporting cells are present in all sensory organs as macula sacculi, macula auriculi, crista ampullaris, organ of Corti, taste bud, olfactory mucosa, and retina. They acquire different shapes, but mostly, they are tall columnar in shape.[55,56]
Modified Schwann cells are also present around the nerve endings in peripheral receptors to modify their functions. Many examples we have in our bodies such as corpuscles Meissner's, Pacinian, Ruffini, and end pulp of Krause. Here, the supporting cells modify the sensory impulse perceived. In the Meissner corpuscle, the nerve ending receives the fine touch, whereas in Pacinian, it receives pressure and vibration.[57,58]
Morphology of supporting cells is similar; they contain well-developed cytoskeleton and have an adaptable shape for their corresponding function [Figure 4]. They could be demonstrated by immunohistochemistry for specific intermediate filament. Astrocytes have glial fibrillar acidic protein, whereas keratinocytes have many types of keratin. Electron microscopic examination confirms their shape and numerous well-developed cytoskeletons as well as the junctions present between adjacent cells.[2,5]
Figure 4.
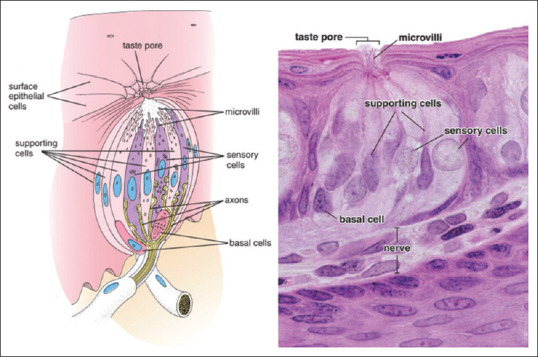
Supporting cells of taste bud[2]
MISCELLANEOUS AND INTERRELATED CELLS
Many cells could be allocated into more than one category of the above. However, we classify according to most eminent function for each. Here, we will describe some cells that have interrelated activity. Immune cells are protein secretory cells that release cytokines and lymphokines to affect each other or bone marrow to stimulate leukocytosis, but at the same time, the cytotoxic cell could kill the tumor cells or virally infected cells.[34,53]
Phagocytic cells are also moving cells so they are contracting cells; they release cytokines and digest the bacteria and parasites. Supporting cells have functions beyond the support as mentioned before. Nerve cells are secretory cells that affect adjacent other adjacent cells through synapses. Nerve cells also release hormones to bloodstream as neurosecretory cells in the hypothalamus and adrenal medulla. Osteoclasts of bone are phagocytic cells that erode the organic matrix and release the calcium content of bone to the bloodstream under hormonal control.[50,51]
The endothelial cells are a specialized cell that binds to each other to form semipermeable barrier between blood plasma and interstitial space. Endothelial cells control the bidirectional exchange of molecules by simple and active diffusion, receptor-mediated endocytosis, and transcytosis. These cells provide nonthrombogenic surface. They secrete regulators of vascular tone, interleukins of immune response, and growth factors.[59,60,61]
Cells of muscle spindle are highly specialized with double functions. The nuclear bag and nuclear chain are modified cells to contract and simultaneously send impulses to motor neuron cells to stimulate muscle contraction.[62,63]
SUMMARY AND CONCLUSION
Finally, the human body is formed of trillions of cells. Although all cells are formed of the plasma membrane, cytoplasm, and nucleus, yet they are classified into different categories according to their tasks inside the body. The cells of the body are highly variable to orchestrate different functions, finally, maintain homeostasis of the body.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bianconi E, Piovesan A, Facchin F, Beraudi A, Casadei R, Frabetti F, et al. An estimation of the number of cells in the human body. Ann Hum Biol. 2013;40:463–71. doi: 10.3109/03014460.2013.807878. [DOI] [PubMed] [Google Scholar]
- 2.Mescher AL Junqueira LC. Junqueira’s Basic Histology: Text and Atlas. 2018 [Google Scholar]
- 3.Alberts, Bruce, Alexander D, Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter. Molecular Biology of the Cell. Sixth edition. New York, NY: W. W. Norton & Company; 2014. [Google Scholar]
- 4.Gartner LP. 7th ed. Philadelphia: Wolters Kluwer; 2018. Color Atlas and Text of Histology. [Google Scholar]
- 5.Ross MH, Pawlina W. 7th ed. China: Wolters Kluwer Health; 2016. Histology: A Text and Atlas : With Correlated Cell and Molecular Biology. [Google Scholar]
- 6.Azevedo, Frederico A, Ludmila R.B. Carvalho, Lea T. Grinberg, José Marcelo Farfel, Renata E.L. Ferretti, Renata E.P. Leite, Wilson Jacob Filho, Roberto Lent, Suzana Herculano-Houzel. “Equal Numbers of Neuronal and Nonneuronal Cells Make the Human Brain an Isometrically Scaled-up Primate Brain.”. The Journal of Comparative Neurology. 2009;513:532–41. doi: 10.1002/cne.21974. https://doi.org/10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- 7.Mohamed, Ghada F, Mohamed Abd Elrahman Ahmed, Hany K K Mostafa. “Effect of Diet Restriction on the Survival of Myenteric Neurons in Jejunum of Aging Male Albino Rat. Histological and Immunohistochemical Study.”. Egypt. J. Histol. 2009;32:368–78. [Google Scholar]
- 8.Zhang J, Jiao J. Molecular biomarkers for embryonic and adult neural stem cell and neurogenesis. Biomed Res Int. 2015;2015 doi: 10.1155/2015/727542. ID: 727542 (14 pages) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu X, Liu G, Halim A, Ju Y, Luo Q, Song AG. Mesenchymal stem cell migration and tissue repair. Cells. 2019;8:784. doi: 10.3390/cells8080784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kyte SL, Gewirtz DA. The influence of nicotine on lung tumor growth, cancer chemotherapy, and chemotherapy-induced peripheral neuropathy. J Pharmacol Exp Ther. 2018;366:303–13. doi: 10.1124/jpet.118.249359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khalaf G, Mostafa HK. Histological and immunohistochemical study on the effect of passive smoking on the skin of adult male albino rats and the possible protective role of nigella sativa oil. Egypt J Histol. 2012;35:87–94. [Google Scholar]
- 12.Mostafa HK. Effect of the overcrowding on fundus of stomach in adult male albino rats with special reference to the protective role of Bran. J Histol Egypt. 2010;33:479–88. [Google Scholar]
- 13.Tanimizu N. Isolation of bipotential liver progenitor cells from neonatal mouse liver. Methods Mol Biol. 2019;1905:9–17. doi: 10.1007/978-1-4939-8961-4_2. [DOI] [PubMed] [Google Scholar]
- 14.Tanimizu N, Mitaka T. Re-evaluation of liver stem/progenitor cells. Organogenesis. 2014;10:208–15. doi: 10.4161/org.27591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chuah JK, Zink D. Stem cell-derived kidney cells and organoids: Recent breakthroughs and emerging applications, Biotechnol Adv. 2017;35:150–67. doi: 10.1016/j.biotechadv.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Kim YK, Nam SA, Yang CW. Applications of kidney organoids derived from human pluripotent stem cells. Korean J Intern Med. 2018;33:649–59. doi: 10.3904/kjim.2018.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nair, Gopika G, Jennifer S. Liu, Holger A. Russ, Stella Tran, Michael S. Saxton, Richard Chen, Charity Juang, et al. “Recapitulating Endocrine Cell Clustering in Culture Promotes Maturation of Human Stem-Cell- Derived ß Cells.”. Nature Cell Biology. 2019;21:263–74. doi: 10.1038/s41556-018-0271-4. no. 2. https:// doi.org/10.1038/s41556-018-0271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrison SJ, Scadden TD. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–34. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kruminis-Kaszkiel E, Osowski A, Bejer-Oleńska E, Dziekoński M, Wojtkiewicz J. Differentiation of human mesenchymal stem cells from wharton's Jelly towards neural stem cells using a feasible and repeatable protocol. Cells. 2020;9:739. doi: 10.3390/cells9030739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu L, Liu Y, Sun Y, Wang B, Xiong Y, Lin W, et al. Tissue source determines the differentiation potentials of mesenchymal stem cells: A comparative study of human mesenchymal stem cells from bone marrow and adipose tissue. Stem Cell Res Ther. 2017;8:275. doi: 10.1186/s13287-017-0716-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alberts B. 4th ed. New York, NY: Garland Science; 2013. Essential Cell Biology. [Google Scholar]
- 22.Korot’ko GF. Exo- and endosecretive digestive glands of enzymes as modulators of secretion. Eksp Klin Gastroenterol. 2010;12:81–6. [PubMed] [Google Scholar]
- 23.Aasi SZ, Leffell DJ, Lazova RZ. New York, NY: Springer New York; 2013. Atlas of Practical Mohs Histopathology. [Google Scholar]
- 24.Kimura S, Nio-Kobayashi J, Kishimoto A, Iwanaga T. The broad distribution of GP2 in mucous glands and secretory products. Biomed Res. 2016;37:351–8. doi: 10.2220/biomedres.37.351. [DOI] [PubMed] [Google Scholar]
- 25.Cao D, Ma X, Zhang WJ, Xie Z. Dissection and Coronal Slice Preparation of Developing Mouse Pituitary Gland. J Vis Exp. 2017;129:e56356 (5pages). doi: 10.3791/56356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fedorova EA, Sufieva DA, Grigorev IP, Korzhevskii DE. Mast cells of the human pineal gland. Adv Gerontol Uspekhi Gerontol. 2018;31:484–9. [PubMed] [Google Scholar]
- 27.Mohebati A, Shaha AR. Anatomy of thyroid and parathyroid glands and neurovascular relations. Clin Anat. 2012;25:19–31. doi: 10.1002/ca.21220. [DOI] [PubMed] [Google Scholar]
- 28.Dolenšek J, Rupnik MS, Stožer A. Structural similarities and differences between the human and the mouse pancreas. Islets. 2015;7:e1024405. doi: 10.1080/19382014.2015.1024405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Fayez MA, Atteya M, Mohamed RA, Ahmed AM, Alroalle AH, Salah Khalil M, et al. Adrenal medulla of AS/AGU rats: A histological and immunohistochemical study. Folia Morphol (Warsz) 2017;76:28–37. doi: 10.5603/FM.a2016.0036. [DOI] [PubMed] [Google Scholar]
- 30.Perlewitz A, Persson AE, Patzak A. The juxtaglomerular apparatus. Acta Physiol Oxf Engl. 2012;205:6–8. doi: 10.1111/j.1748-1716.2012.02429.x. [DOI] [PubMed] [Google Scholar]
- 31.Chapman MA, Meza R, Lieber RL. Skeletal muscle fibroblasts in health and disease. Differentiation. 2016;92:108–15. doi: 10.1016/j.diff.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun, Lin, Xiuli Wang, David L. “A 3D Cartilage - Inflammatory Cell Culture System for the Modeling of Human Osteoarthritis.”. Biomaterials. 2011;32:5581–89. doi: 10.1016/j.biomaterials.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X, Wang Z, Duan N, Zhu G, Schwarz EM, Xie C. Osteoblast-osteoclast interactions. Connect Tissue Res. 2018;59:99–107. doi: 10.1080/03008207.2017.1290085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sabado RL, Balan S, Bhardwaj N. Dendritic cell-based immunotherapy. Cell Res. 2017;27:74–95. doi: 10.1038/cr.2016.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gartner LP, Hiatt JL, Gartner LP. 1st ed. Philadelphia, Pa: Saunders/Elsevier; 2011. Concise Histology. [Google Scholar]
- 36.Shen WJ, Azhar S, Kraemer FB. Lipid droplets and steroidogenic cells. Exp Cell Res. 2016;340:209–14. doi: 10.1016/j.yexcr.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamaguchi T, Fujikawa N, Nimura S, Tokuoka Y, Tsuda S, Aiuchi T, et al. Characterization of lipid droplets in steroidogenic MLTC-1 Leydig cells: Protein profiles and the morphological change induced by hormone stimulation. Biochim Biophys Acta. 2015;1851:1285–95. doi: 10.1016/j.bbalip.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 38.Delgehyr N, Meunier A, Faucourt M, Bosch Grau M, Strehl L, Janke C, et al. Ependymal cell differentiation, from monociliated to multiciliated cells. Methods Cell Biol. 2015;127:19–35. doi: 10.1016/bs.mcb.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Kyrousi C, Lygerou Z, Taraviras S. How a radial glial cell decides to become a multiciliated ependymal cell. Glia. 2017;65:1032–42. doi: 10.1002/glia.23118. [DOI] [PubMed] [Google Scholar]
- 40.Liu H, Li Y, Chen L, Zhang Q, Pan N, Nichols DH, et al. Organ of Corti and Stria Vascularis: Is there an Interdependence for Survival? PLoS One. 2016;11:e0168953. doi: 10.1371/journal.pone.0168953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng AK, Civan MM, To CH, Do CW. cAMP Stimulates Transepithelial Short-Circuit Current and Fluid Transport Across Porcine Ciliary Epithelium. Invest Ophthalmol Vis Sci. 2016;57:6784–94. doi: 10.1167/iovs.16-20127. [DOI] [PubMed] [Google Scholar]
- 42.Kopic S, Murek M, Geibel JP. Revisiting the parietal cell. Am J Physiol Cell Physiol. 2010;298:C1–C10. doi: 10.1152/ajpcell.00478.2009. [DOI] [PubMed] [Google Scholar]
- 43.Rusak E, Chobot A, Krzywicka A, Wenzlau J. Anti-parietal cell antibodies Diagnostic significance. Adv Med Sci. 2016;61:175–9. doi: 10.1016/j.advms.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 44.Chellini F, Tani A, Zecchi-Orlandini S, Sassoli C. Influence of platelet-rich and platelet-poor plasma on endogenous mechanisms of skeletal muscle repair/Regeneration. Int J Mol Sci. 2019;20:683. doi: 10.3390/ijms20030683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hany K. Effect of diabetes mellitus on the structure of skeletal muscle in adult male Albino rats and the protective role of chromium: Histological and immunohistochemical study. Egypt J Histol. 2008;31:341–53. [Google Scholar]
- 46.Forcina L, Cosentino M, Musarò A. Mechanisms regulating muscle regeneration: Insights into the interrelated and time-dependent phases of tissue healing. Cells. 2020;9:2117–11. doi: 10.3390/cells9051297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gartner LP, Hiatt JL, Strum JM. 6th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2011. Cell Biology and Histology. [Google Scholar]
- 48.PhD, Dongmei Cui MD, John P, Naftel PhD, William P. Daley MD, James C. Lynch PhD, Duane E. Haines PhD, Gongchao Yang MD, Jonathan D, Fratkin MD. First edition. Philadelphia: LWW; 2010. Atlas of Histology with Functional and Clinical Correlations. [Google Scholar]
- 49.Lee FP, Chao PZ, Wang HW. Vardenafil inhibiting parasympathetic function of tracheal smooth muscle. J Chin Med Assoc. 2018;81:631–5. doi: 10.1016/j.jcma.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 50.Davies LC, Rice CM, McVicar DW, Weiss JM. Diversity and environmental adaptation of phagocytic cell metabolism. J Leukoc Biol. 2019;105:37–48. doi: 10.1002/JLB.4RI0518-195R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Foote JR, Patel AA, Yona S, Segal AW. Variations in the Phagosomal Environment of Human Neutrophils and Mononuclear Phagocyte Subsets. Front Immunol. 2019;10:188. doi: 10.3389/fimmu.2019.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taatjes DJ, Mossman BD. Totowa, N.J: Humana Press; 2006. Cell Imaging Techniques: Methods and Protocols. [Google Scholar]
- 53.Roberts NA, Adams BD, McCarthy NI, Tooze RM, Parnell SM, Anderson G, et al. Prdm1 regulates thymic epithelial function to prevent autoimmunity. J Immunol. 2017;199:1250–60. doi: 10.4049/jimmunol.1600941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roberts N, Horsley V. Developing stratified epithelia: Lessons from the epidermis and thymus. Wiley Interdiscip Rev Dev Biol. 2014;3:389–402. doi: 10.1002/wdev.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kinnamon SC, Finger TE. Recent advances in taste transduction and signaling. F1000Res. 2019;8:2117–11. doi: 10.12688/f1000research.21099.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pangrsic T, Singer JH, Koschak A. Voltage-gated calcium channels: Key players in sensory coding in the retina and the inner ear. Physiol Rev. 2018;98:2063–96. doi: 10.1152/physrev.00030.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abdo, Hind, Laura Calvo-Enrique, Jose Martinez Lopez, Jianren Song, Ming-Dong Zhang, Dmitry Usoskin, Abdeljabbar El Manira, Igor Adameyko, Jens Hjerling-Leffler, Patrik Ernfors. Science. Vol. 365. (New York, N.Y.): “Specialized Cutaneous Schwann Cells Initiate Pain Sensation.”; pp. 695–99. no. 6454. [DOI] [PubMed] [Google Scholar]
- 58.Ma KH, Svaren J. Epigenetic Control of Schwann Cells. Neuroscientist. 2018;24:627–38. doi: 10.1177/1073858417751112. [DOI] [PubMed] [Google Scholar]
- 59.Al-Soudi A, Kaaij MH, Tas SW. Endothelial cells: From innocent bystanders to active participants in immune responses. Autoimmun Rev. 2017;16:951–62. doi: 10.1016/j.autrev.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 60.Shao, Ying, Jason Saredy, William Y. Yang, Yu Sun, Yifan Lu, Fatma Saaoud, Charles Drummer, et al. “Vascular Endothelial Cells and Innate Immunity.”. Arteriosclerosis, Thrombosis, and Vascular Biology. 2020;40:e138–52. doi: 10.1161/ATVBAHA.120.314330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sturtzel C. Endothelial cells. Adv Exp Med Biol. 2017;1003:71–91. doi: 10.1007/978-3-319-57613-8_4. [DOI] [PubMed] [Google Scholar]
- 62.Ellaway PH, Taylor A, Durbaba R. Muscle spindle and fusimotor activity in locomotion. J Anat. 2015;227:157–66. doi: 10.1111/joa.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mayer WP, Murray AJ, Brenner-Morton S, Jessell TM, Tourtellotte WG, Akay T. Role of muscle spindle feedback in regulating muscle activity strength during walking at different speed in mice. J Neurophysiol. 2018;120:2484–97. doi: 10.1152/jn.00250.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]


