Abstract
Background:
Using DAs for preference-sensitive decisions is an evidence-based way to improve patient-centered decisions. Reimbursement mandates have increased the need for DAs in ICD care, although none have been formally evaluated. The objectives were to develop and pilot implantable cardioverter-defibrillator (ICD) decision aids (DAs) for patients considering primary prevention ICDs.
Methods:
Development Phase: An expert panel, including patients and physicians, iteratively developed four DAs: a one-page Option Grid™ conversation aid, a four-page in-depth paper tool, a 17-minute video, and an interactive website. Trial Phase: At three sites, patients with heart failure who were eligible for primary prevention ICDs were randomly assigned 2:1 to intervention (received DAs) or control (usual care). We conducted a mixed-methods evaluation exploring acceptability and feasibility.
Results:
Twenty-one eligible patients enrolled (15 intervention). Most intervention participants found the DAs to be unbiased (67%), helpful (89%), and would recommend them to others (100%). The pilot was feasible at all sites; however, using clinic staff to identify eligible patients was more efficient than chart review. Although the main goals were to measure acceptability and feasibility, intervention participants trended towards increased concordance between longevity values and ICD decisions (71% concordant vs. 29%, p = .06). Participants preferred the in-depth paper tool and video DAs. Access to a nurse during the decision-making window encouraged questions and improved participant-perceived confidence.
Conclusions:
Participants felt the DAs provided helpful, balanced information that they would recommend to other patients. Further exploration of this larger context of DA use and strategies to promote independent use related to electrophysiology (EP) visits are needed.
Keywords: defibrillation-implantable cardioverter-defibrillator, heart failure, patient decision aid, qualitative, shared decision making
1 |. BACKGROUND
Over 200,000 implantable cardioverter-defibrillators (ICD) are implanted annually in the US,1 including new devices and replacement procedures (i.e., generator changes for ICDs at end of battery life). In appropriately selected patients, ICDs reduce mortality from sudden cardiac death (SCD) resulting in roughly a 7% absolute increase in survival over 5 years.2–5 However, a number of potential clinical and quality of life (QOL) threats exist. Recent studies on programming ICDs have found ways to minimize the burdens of inappropriate shocks.6 Patients who do get shocked have described it as “getting kicked in the chest by a mule,”7 leading some to have their device removed for fear of repeated shocks.8 Some studies suggest that patients with ICDs have more heart failure admissions,9 a lower QOL–particularly if shocked by their devices10,11–and increased anxiety, depression, and post-traumatic stress disorder.12 Furthermore, if not properly deactivated, ICDs can cause unnecessary suffering at the end of life.13–16
Unfortunately, research also suggests problems with current ICD decision making. An integrative review of patient perspectives highlighted a paternalistic approach to decision making, with providers failing to provide information patients need to engage in a truly shared decision making process.7,17–19 Patients with ICDs frequently report never having had a conversation about periprocedural risks, expected benefits, potential QOL problems,7 or end of life preferences.20 With traditional approaches to patient-provider communication, patients tend to overestimate the benefits of ICDs, underestimate the risks, and are under informed about device deactivation.18 Recent policy changes highlight the importance of good shared decision making. For instance in October 2018 the Centers for Medicare and Medicaid Services mandated that “a formal shared decision making encounter must occur between the patient and an independent physician…using an evidence-based decision tool on ICDs prior to initial ICD implantation.”21 This mandate reflects the importance of this unmet need and a call to help address concerns regarding insufficient shared decision making between patients and clinicians.22
Decision aids (DAs) come in many forms including paper, video, interactive web sites, and telenovelas. While each has been shown to improve decision making in research settings,23 little work has been done to elucidate the optimal strategy for real world shared decision making between DA distribution alone and DA distribution alongside engaging patients in meaningful dialogue.24 Guided by the International Patient DA Standards25,26 and following the Ottawa Decision Support Framework,27 we developed a suite of four DAs consisting of: a one-page Option Grid conversation aid, a four-page in-depth pamphlet, a 17-minute video, and an interactive website. The purpose of this pilot study was to test the acceptability and feasibility of four DAs supporting decision making for primary prevention ICDs, through exploring patient perspectives and reporting on preliminary efficacy.
2 |. METHODS
2.1 |. Overview
The study consisted of two phases: Phase 1 was the development of four DAs in different formats (see Figure 1 for full development process) from February 2012 to September 2014; and Phase 2 was a mixed-methods randomized pilot test conducted over 6 months from September 2014 through March 2015 at three sites in the Denver Metro area. This research was approved by the Colorado Multiple Institutional Review Board (IRB) and the IRB at Kaiser Institute for Health Research.
FIGURE 1.
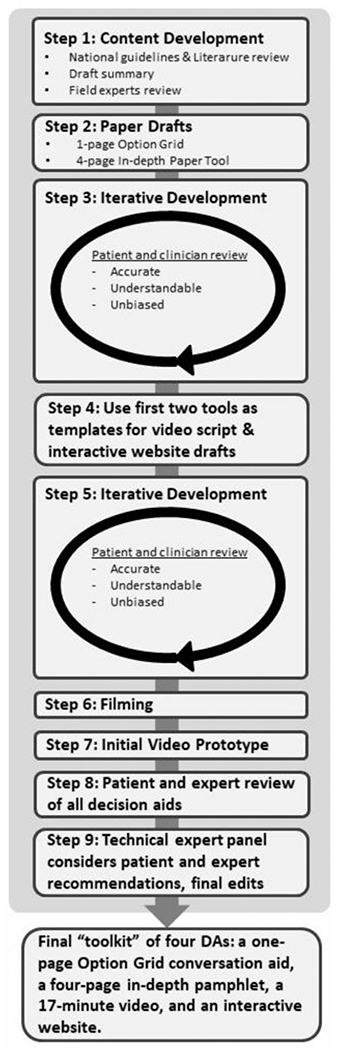
Development process for toolkit of four decision aids
2.2 |. Phase 1: Development of four DAs
Prior to DA development, we reviewed national guidelines and literature for published studies on the use of ICDs as a preventative measure against SCD. We created a summary of the evidence, which was reviewed by field experts.28 The consolidation of this evidence provided the foundation for the decision tools. The core development team consisted of a geriatric and palliative medicine physician, a cardiologist, an electrophysiologist specializing in the care of patients with ICDs, and an art director specializing in decision theory. With the assistance of a health literacy expert, we aimed to ensure all tools would be easy to read and understand at a 5th grade reading level. Development was based on the Ottawa Decision Support Framework27 and followed the principles as outlined in the International Patient DA Standards.29 Although updated IPDAS guidelines were published after this study was conducted, this study still follows high levels of user-centered design by including “(1) preprototype involvement of potential end users, (2) iterative responsiveness involving cycles of testing and refinement, and (3) involvement of other experts in development, such as health professionals. When reviewing the UCD-11, we found that our decision aid development process followed all measures of the UCD-11 and scored and 11 out of 11. The authors also believe the methods for these DAs design followed medium process with many completed elements of the maximal process.30 Ultimately, a “toolkit” of four decision tools was created: (1) a one-page Option Grid conversation aid, (2) a more in-depth paper pamphlet, (3) a video, and (4) an interactive website.
Prototypes of the 1-page option grid and 4-page in-depth pamphlet were developed over eight iterative cycles, with each rendition qualitatively reviewed by a mix of patients (both patients who accepted and declined ICDs, n = 28), patient focus groups (n = 3), patient stakeholders (n = 7), local research groups (n = 4), and a panel of field experts (n = 14). Early in the pilot, we discovered that due to ongoing concerns from the Office of the Inspector General about inappropriate ICD implantation, there was a very small window between when a patient became eligible and when they received an ICD (many patients were waiting until they were 90 days after a myocardial infarction). To account for this, the trial had to be paused and the DAs modified to stress to participants that they might be referred for an ICD rather than definitively stating that they will be referred an ICD.
The development team mirrored a similar method from a left ventricular assistance device development team, meeting bimonthly during the feedback process to digest reactions and recommendations and to decide how to address the feedback.31 Clinician and patient feedback were included in each iteration to guide the development team discussions towards end-user products. A detailed audit trail of decisions was maintained28 and made public on the website where the DAs are available (www.patientdecisionaid.org). Once feedback saturation was reached (i.e., no new ideas emerged from feedback), the paper versions were used as a template for an interactive website that included patient video clips and interactive questions. A video script was drafted and tested with patients and clinical experts before filming began. A male cardiologist served as the primary voice for the video, while a female narrator was used for graphic voiceover. Patient clips used on the website and in the video were selected carefully to include only process and experience narratives (e.g., “I thought about the risks and benefits of surgery while making my decision to get an ICD”); outcome narratives were not used as they can be overly influential (e.g., “Getting an ICD was the best thing I did for my health”).32
2.3 |. Phase 2: Pilot randomized trial
2.3.1 |. Settings
The trial of these DAs was conducted at three sites in order to capture the feasibility of the toolkit in settings with varied ICD care models and clinical populations: (1) University of Colorado Hospital (UC Health), a large tertiary care academic system; (2) Kaiser Permanente (KPCO), a not-for-profit vertically-integrated managed care system; and (3) the Eastern Colorado Veterans’ Health Care System (VA).
2.3.2 |. Participants and recruitment
Patients were invited to join the study if they met the New York Heart Association’s well-accepted indications for consideration of a primary prevention ICD (NYHA class II or III heart failure and left ventricle ejection fraction ≤35%2), were at least 18 years of age, and spoke English as a primary language. For both UC Health and KPCO, research coordinators identified eligible patients for recruitment by screening electrophysiology (EP) referrals made to the EP department and by screening individual EP schedules for future appointments. At the VA site, the nurse coordinator who scheduled primary prevention ICD referrals provided the VA study coordinator with eligible patients. Research staff contacted eligible patients via telephone to provide patients with more information about the study. Patients who agreed to participate were scheduled for their baseline visit either over the phone or in person at a location convenient for the participant. Participants provided informed consent and HIPAA authorization prior to participation and received a $25 gift card for each study visit (maximum of $75 total). Figure 2 shows the process for participants being enrolled and randomized in the study.
FIGURE 2.

Consort diagram of participants through pilot trial [Color figure can be viewed at wileyonlinelibrary.com]
2.3.3 |. Design and procedures
Prior to the appointment with EP, eligible patients were recruited via telephone, then provided informed consent and HIPAA authorization prior to being randomly assigned 2:1 to an intervention or control group with the goal of recruiting 60 patients. The control group received “usual care,” in which participants received any pamphlets or communication normally given by the treatment facility. The intervention group received the toolkit via overnight FedEx shipping. Patients in the intervention group were told they could use any or all of the DAs in the toolkit. Patients then completed quantitative survey measures and qualitative interviews over three visits: prior to meeting the EP (Visit 1), 1 month after meeting with the EP (Visit 2), and 3 months after Visit 1 (Visit 3). If a participant did not meet with an EP within 1 month of enrolling, Visit 2 was skipped.
2.3.4 |. Measures
Acceptability of the decision tools as a group was measured using a modified version of a DA acceptability questionnaire,33 which asked about the amount of information, the degree to which the information was balanced vs. biased, the clarity of information, the helpfulness of the DA, and whether the individual would recommend using the DA to others. Feasibility was determined both through the qualitative interviews with patients and our team’s experiences and field notes working with the staff. Patients’ knowledge of ICDs was measured using a 20-item questionnaire.34 Decision quality values items were asked two different ways. First, patients were asked to reflect on the importance of individual values on a scale of 1 (Not Important) through 10 (Very Important) for 5 topics: (1) avoiding sudden death, (2) dying quickly, (3) avoiding advancing heart failure, (4) avoiding surgery, and (5) avoiding being shocked. Second, participants chose between the following values trade-off scale: “If you were able to choose how to live the rest of your life, on a scale of 1–10 with 1 being “Die quickly from any cause, such as dying in your sleep, and not live as long,” and 10 being “live as long as possible with heart failure symptoms worsening over time,” what number would you pick?” 35,36 Participants were evaluated on their Decision Choice,37,38 Decision Conflict,39 Decision regret,40 the Control preferences scale41, decision participation, demographics, and health literacy.42
2.3.5 |. Qualitative interviews
We conducted semi-structured qualitative telephone interviews with patients 1 month after the EP visit and again 3 months after enrollment. The digitally recorded interviews lasted 11–53 min. We chose a qualitative descriptive design43 to evaluate patient perspectives regarding the acceptability and feasibility of the intervention (a toolkit of four DAs to support decision making for ICDs). The interview guide assessed which DAs patients preferred and why; if and how participants used certain DAs; reasons they did not use specific DAs; preferred delivery methods; and what advice they could offer to improve the DAs or the dissemination process. Following the Ottawa decision framework, we asked about decisional needs and, if a decision was made, how participants felt about their choices. Participants were interviewed by trained study coordinators. We used an iterative approach, adding further questions to probe on information gleaned from the earlier interviews in an effort to deepen later interviews.
2.3.6 |. Data analysis
Descriptive frequencies such as percentages, means, and standard deviations were calculated for all quantitative measures. T-tests and χ2 analyzes were conducted to detect differences between control and intervention participants. Value-treatment congruence was scored such that those who scored 1–4 were considered to want to die quickly; those who scored 6–10 were considered to want to live as long as possible, and those that scored a 5 were considered neutral. We identified patient congruence between values and actions if a patient wanted to die quickly (1–4) and chose not to get the ICD implanted, or if a patient wanted to live as long as possible (6–10) and chose to get the ICD implanted. If a person scored a neutral value, congruence was scored as neutral.35,36
For the qualitative data, we used a well-established team-based inductive and deductive approach43 to analyze the interview data, interviewer reflection summaries, and analytic notes from team meetings. The team coded the interviews using the interview guide to create a-priori (deductive) codes and patients’ responses to inform inductive code creation, and using Atlas.ti V7.5 as the qualitative analysis program to organize and code data. The data were then iteratively discussed with the larger team (all interviewers plus DM, a geriatrician, and JJ, an experienced qualitative researcher). As preliminary themes emerged through bimonthly meetings, the team considered site variations and alternative explanations through a process of data immersion and re-immersion. Differences were resolved through team discussion, resulting in consensus on key themes. Each site data set was then examined as a whole to determine workflow patterns and patient interactions that were specific to that site.
3 |. RESULTS
3.1 |. Phase 1: DA development
Ultimately, a “toolkit” of four DA was created including: (1) a one-page Option Grid conversation aid, (2) an in-depth paper tool, (3) a video, and (4) an interactive website (see Table 1). The prototypes were developed iteratively with each rendition reviewed by a mix of patients (both ICD accepters and decliners, n = 28), three patient focus groups, patient stakeholders (n = 7), four local research groups and a panel of field experts including electrophysiologists and clinicians (n = 14). A literacy expert (AGB) ran the final reading level of each DA. For each DA (including the video script), the text was evaluated for readability, with the goal of ensuring that materials would not exceed a 5th or 6th grade reading level to keep them accessible.44–46 Materials were also examined for other features that can influence understandability (e.g.,medical jargon; use of white space and headers; inclusion of simple, labeled graphics).
TABLE 1.
Brief description of toolkit DAs
| Tool | Features | IPDAS score | ||
|---|---|---|---|---|
| Qualifying | Certification | Quality | ||
| Option grid | A one-page list of common questions regarding ICD implantation and CHF comparing staying with current medical treatment to getting an ICD. It is designed to facilitate discussion between patient and provider on the pros and cons to getting the device. | 7/7 | 8/9 | 8/9 |
| In-depth paper pamphlet | Four-page, color brochure describing heart failure progression, the purpose of an ICD plus the risks and benefits of ICD implantation. This DA highlights the trade-off of living longer with dying quickly allowing readers to reflect upon how each option align with their treatment goals. | 7/7 | 9/9 | 9/9 |
| Video | This engaging 17-minute video allows patient to hear directly from a Cardiologist and Electrophysiologist regarding the device, implantation, risks and benefits and trade-offs. Viewers hear testimonials from both ICD decliners and recipients of the ICD, providing a more personal perspective on the decision-making process. | 7/7 | 8/9 | 8/9 |
| Website | An interactive version of the in-depth paper tool, the website also includes a “test your understanding” section to reinforce information uptake. Patients can print out their responses along with any questions that came to mind while viewing the website. It incorporates patient video clips discussing reimplantation and deactivation of the ICD. | 7/7 | 9/9 | 9/9 |
The information provided was consistent in content across all DAs. Narratives from patients provided the information and were carefully selected to be informative but not sensational or dramatic. Prior work led to some information being highlighted, such as shocks (i.e., what it feels like, how often does it happen)18, the misconception that ICDs improve heart failure symptoms7 (but certain ICDs with cardiac resynchronization therapy are available and should be discussed with the physician), and deactivation of an ICD18,47. All the DAs featured tools for values clarification and trade-offs with imagined futures and narratives.48 The developers emphasized that patients indeed have a choice, and that careful consideration of their values is important in helping them make this difficult decision.
3.2 |. Phase 2: Pilot randomized trial
Thirty-two patients were approached for the study across all three sites. Twenty-one patients were enrolled with 15 randomly assigned to the intervention group. Table 2 summarizes the sociodemographic and clinical characteristics of the sample. In general, more men (85.7%) enrolled in the study. Intervention and control patients were similar in age (M = 65.1, SD = 8.8, M = 70.0, SD = 5.5, respectively, t(19) = 1.3, ns) and ages for both groups ranged between 51 and 80. The majority of patients enrolled were VA patients (52.3%). Few patients were of Hispanic or Latino decent (14.3%) and the majority self-identified as White (71.4%).
TABLE 2.
Description of the sample
| Demographic characteristics | Intervention (N = 15) | Control (N = 6) | |
|---|---|---|---|
| Age (M, SD) | 65.1 (8.8) | 70.0 (5.5) | t(19) = 1.3, ns |
| Ejection fraction (M%, SD) | 27.2 (6.5) | 29.6 (7.8) | t(19) = 0.7, ns |
| Gender | |||
| Male (%) | 13 (86.7) | 5 (83.3) | |
| Female (%) | 2 (13.13) | 1 (16.7) | |
| Clinic | |||
| University of Colorado Hospital | 4 (26.7) | 1(16.7) | |
| Denver VA Hospital | 7 46.7) | 4 (66.7) | |
| Kaiser Permanente of Colorado | 4 (26.7) | 1 (16.7) | |
| Hispanic descent (Yes, %) | 3 (20.0) | 0 (0.0) | |
| Race | |||
| American Indian or Alaskan Native | 1 (6.7) | 0 (0.0) | |
| Black or African American | 2 (13.3) | 0 (0.0) | |
| White | 9 (60.0) | 6 (100) | |
| Multiracial or other | 3 (20.0) | 0 (0.0) | |
| Marital status | |||
| Married | 6 (40.0) | 5 (83.3) | |
| Divorced | 4 (26.7) | 0 (0.0) | |
| Separated | 1 (6.7) | 0 (0.0) | |
| Widowed | 3 (20.0) | 0 (0.0) | |
| Single, never married | 1 (6.7) | 1 (16.7) | |
| Highest education | |||
| Less than high school | 1 (6.7) | 0 (0.0) | |
| High school diploma or GED | 4 (26.7) | 0 (0.0) | |
| Some college | 7 (46.7) | 3 (50.0) | |
| College graduate | 1 (6.7) | 1 (16.7) | |
| Any post-grad work | 2 (13.3) | 2 (33.3) | |
| Employment | |||
| Full-time | 2 (13.3) | 0 (0.0) | |
| Part-time | 8 (53.3) | 2 (33.3) | |
| Retired | 3 (20.0) | 4 (66.7) | |
| On disability | 2 (13.3) | 0 (0.0) | |
| Household income | |||
| <$20,000 | 5 (33.3) | 0 (0.0) | |
| $20,000-$40,000 | 4 (40.0) | 2 (33.3) | |
| $40,000-$60,000 | 1 (6.7) | 2 (33.3) | |
| $60,000-$80,000 | 3 (20.0) | 0 (0.0) | |
| >$80,000 | 0 (0.0) | 2 (33.3) | |
| Ischemic heart failure | |||
| Yes | 10 (66.7) | 4 (66.7) | |
| Class of heart failure | |||
| I | 1 (6.7) | 0 | |
| II | 2 (13.3) | 2 (33.3) | |
| II-III | 2 (13.3) | 1 (16.7) | |
| III | 8 (53.3) | 3 (50.0) | |
| Comorbidities (Yes, %) | |||
| Anxiety | 0 (0.0) | 2 (33.3) | |
| Depression | 3 (20.0) | 1 (16.7) | |
| Cancer | 2 (13.3) | 1 (16.7) | |
| COPD | 3 (20.0) | 1 (16.7) | |
| Dementia | 0 (0.0) | 0 (0.0) | |
| Diabetes | 7 (46.7) | 3 (50.0) | |
| Coronary artery disease | 10 (66.7) | 4 (66.7) | |
| Hypertension | 9 (60.0) | 3 (50.0) | |
| Kidney disease | 3 (20.0) | 4 (66.7) | |
| Liver disease | 1 (6.7) | 0 (0.0) | |
| Stroke | 1 (6.7) | 0 (0.0) | |
| Admitted to hospital in last 12 Mo (Yes, %) | 12 (80.0) | 4 (66.7) | |
| Gone to emergency department in last 12 Mo | 11 (73.3) | 3 (50.0) | |
| Seen primary care physician in last 12 Mo (Yes, %) | 15 (100.0) | 6 (100.0) | |
| Years diagnosed with heart problem | |||
| Within past 2 Y | 5 (33.3) | 1 (16.7) | |
| 2-4 Y | 0 (0.0) | 0 (0.0) | |
| 4 or more Y | 8 (53.3) | 5 (83.3) | |
| Unsure | 2 (13.3) | 0 (0.0) | |
| Hospital anxiety and depression scale (M, SD) | |||
| Anxiety | 5.9 (3.8) | 6.2 (2.0) | t(18) = 0.2, ns |
| Depression | 5.1 (4.1) | 5.0 (4.5) | t(18) = 0.1, ns |
| ICD implanted | |||
| Yes | 6 (40.0) | 4 (66.7) | |
| No | 5 (33.3) | 0 (0.0) | |
| Undecided | 4 (26.7) | 2 (33.3) | |
3.2.1 |. Feasibility and acceptability results
Acceptability results can be seen in Table 3. Of the 15 intervention participants, nine provided acceptability feedback. The majority (67%) found the DAs to be unbiased, while 22% thought they were biased toward ICDs, and 11% thought they were biased toward not getting an ICD. Furthermore, 89% found the DAs helpful and 100% would recommend them to others. Five of the 21 patients were found to be no longer eligible for an ICD for primary prevention during the study due to improved ejection fractions.
TABLE 3.
Acceptability
| Intervention (N = 9) | |
|---|---|
| Amount of information | N (%) |
| Much less than I needed | 1 (11.1) |
| A little less than I needed | 1 (11.1) |
| About the right amount | 7 (77.8) |
| Balance of information | |
| Clearly slanted toward getting ICD | 1 (11.1) |
| A little slanted toward getting ICD | 1 (11.1) |
| Completely balanced | 6 (66.7) |
| A little slanted toward not getting ICD | 1 (11.1) |
| Clearly slanted toward not getting ICD | 0 (0.0) |
| Clarity of information | |
| Everything was clear | 3 (33.3) |
| Most things were clear | 5 (55.6) |
| Some things were clear | 1 (11.1) |
| Helpfulness of decision aid | |
| Very helpful | 4 (44.4) |
| Somewhat helpful | 4 (44.4) |
| A little helpful | 1 (11.1) |
| Would you recommend the decision aid | |
| I would definitely recommend | 6 (66.7) |
| I would probably recommend | 3 (33.3) |
3.2.2 |. Qualitative interviews
Through the qualitative interviews, most participants stated that they preferred the in-depth paper tool and video DAs over the Option Grid conversation aid and website. Those who preferred the written tools mentioned the ease of referring back to information provided earlier in the text, which was more difficult to do using video. At least half of the patients reviewed the tools prior to meeting with their EP. Although some patients reviewed the tools by themselves, others viewed one or more of the tools with a family member (or gave them the CD or link to video). Patients felt that sharing these tools helped empower them to share a discussion with a family member or caregiver, or sometimes to justify their decision about an ICD. Patients also found that the tools stimulated additional questions to ask at their next clinic visit. One said: [It] guided me in the right direction…I had enough information to ask intelligent questions, which I didn’t have prior to your stuff. Participants reported feeling more confident during their visit after reviewing the toolkit. Patients found it helpful to review the toolkit after their visit. One patient shared: I mean, to be honest with you, when the doctor talks to you, and then you can come home on your own time and read what you sent to me… it was easier to digest. Because you know, sometimes at the doctor’s you are scared to death. Participants stated that receiving all four tools at once was too much, as one participant said, “There was a lot of stuff and it was kind of repetitious. So, I think you kinda overdid it. I mean I understood the many different thoughts, but they were kinda all saying the same things in all three.” In contrast, at least one person emphasized that no tool can answer all possible questions, and several pointed out that the tools should never be used without a conversation with the doctor.
3.2.3 |. Quantitative results
Primary pilot outcomes are summarized in Table 4. Intervention participants did not have significantly greater knowledge about ICDs (M = 14.00, SD = 2.62) than controls (M = 11.60, SD = 3.13, ns) at 1-month follow-up. Knowledge scores did not differ at 3-month follow-up either (Intervention: M = 14.3, SD = 2.5, Control: M = 13.4, SD = 2.1, ns). Values-treatment concordance was significantly different between groups at the 1-month follow-up such that intervention participants were more likely to show concordance (83.3%) between their values and their ICD decision than did control participants (0.0%, Fisher’s Exact = 0.048). However, this difference was not evident at the 3-month follow-up (66.7%, 50.0%, respectively, Fisher’s Exact = 1.00).
TABLE 4.
Pilot outcomes
| Primary outcomes | Intervention (M, SD) | Control (M, SD) | t-test |
|---|---|---|---|
| Pre-test knowledge scores | 11.9 (2.6) | 12.2 (1.3) | t(19) = 0.21, ns |
| 1-mo test knowledge scores | 14.0 (2.6) | 11.6 (3.1) | t(13) = 1.57, ns |
| 3-mo test knowledge scores | 14.3 (2.5) | 13.4 (2.1) | t(12) = 0.72, ns |
| 1-mo values concordance | N = 6 (%) | N = 3 (%) | Fisher’s exact = 0.048 |
| Congruent | 5 (83.3) | 0 (0.0) | |
| Incongruent | 1 (16.7) | 3 (100) | |
| 3-mo values concordance | n (%) | n (%) | Fisher’s exact = 1.00 |
| Congruent | 4 (66.7) | 2 (50.0) | |
| Incongruent | 2 (33.3) | 2 (50.0) | |
| Secondary outcomes | Intervention | Control | |
| 1-mo decision participation (% active) | 71.4% | 30% | X2 = 1.54, ns |
| 1-mo decision conflict | 17.8 (15.0) | 24.4 (25.4) | t(13) = 0.64, ns |
| 1-mo decision regret | 21.9 (16.2) | 16.0 (19.2) | t(11) = 0.59, ns |
| 3-mo decision participation (% Active) | 50.0% | 60.0% | X2 = 0.12, ns |
| 3-mo decision conflict | 22.2 (19.7) | 26.3 (25.4) | t(12) = 0.33, ns |
| 3-mo decision regret | 15.6 (11.8) | 19.0 (19.2) | t(11) = 0.40, ns |
Decision conflict scores range: 0–100.
Decision regret scores range: 0–100.
Secondary outcomes are summarized in Table 4. Intervention and control participants did not significantly differ in the active approach to decision making at either follow-up time point. At the 1-month follow-up Intervention patients did not have significantly different levels of decision conflict (M = 17.81, SD = 14.97) than controls (M = 24.38, SD = 25.35, t(13) = 0.64, ns) or decision regret (M = 21.88, SD = 16.24, M = 16.00, SD = 19.17, respectively, t(11) = 0.59, ns). This was also true at 3-month follow-up (decision conflict: intervention M = 22.2, SD = 19.7, control M = 26.3, SD = 25.4, t(12) = 0.33, ns; decision regret: intervention M = 15.6, SD = 11.8, control M = 19.0, SD = 19.2, t(11) = 0. 40, ns).
4 |. DISCUSSION
4.1 |. Summary of results
Qualitatively, participants found the tools to be highly acceptable and all participants would recommend them to others. Patients reported that the DAs made them feel more confident at clinical visits with an electrophysiologist, stimulated questions during the visit, and reduced anxiety after the visit when they could review the tools. Participants felt that receiving all four tools at once was overwhelming, and preferred the in-depth paper and video DAs over the website and Option Grid DAs. The intervention group showed nonsignificant improvements across several measures including knowledge and concordance between their ICD decision and their values. While this was a pilot trial focused on acceptability and feasibility that was not powered to fully test these differences, it is reassuring that signal outcomes were in the hypothesized direction.
4.2 |. Acceptability discussion
An iterative development process including stakeholders with patient groups and physicians created a toolkit of four DA that were found to be largely unbiased, feasible, and 100% recommended by patients. With 22% of respondents finding the DA biased towards ICDs, 11% finding the DA biased against ICDs, and the majority of respondents (67%) feeling the DAs were unbiased, the development team felt this was a well-balanced toolkit of DAs. 100% of participants said that they would recommend the DAs to others. Traditional feasibility trials of DAs focus on patient acceptability, but this trial was unique to also include clinician perspectives through the development phase to ensure acceptable use in clinic settings. There were differences between values expressed by patients and physicians, so we learned that you have to develop DAs for both end user groups.49 Including all stakeholder perspectives is important for real-world use and implementation.50
4.3 |. Feasibility discussion
Previous research has shown that multi-level engagement is important for successful implementation of any intervention.51 Additionally, engaging the clinical teams in the implementation will likely lead to more sustainable implementation of the DAs. Similarly, previous research demonstrated that although coaching can be an effective strategy for implementation, it is difficult to sustain after funding ends.24 This pilot trial was designed to be pragmatic with the goal of understanding how patients would use DAs on their own.
4.4 |. Limitations
This study has several limitations. First, it is a small pilot study and is not powered to allow us to make conclusions about quantitative outcomes. However, much was learned from the development process including the rich qualitative content from patient interviews about the acceptability of the DAs. Being a small pilot study, our sample was also primarily male (85%). Second, sending four tools at once might have led to information overload although it did allow us to get participants real-world assessment of the usefulness of the various formats. Third, the majority of participants were white and these results may not be generalizable to populations in more diverse settings. Finally, our goal was to enroll 60 patients but we under-enrolled, this was partially due to decreasing numbers of initial ICD patients and the need to modify the DAs after the beginning of the trial.
5 |. CONCLUSIONS
This trial has important real-world implications for the ever-increasing need for DAs in clinical practice, highlighted by recent policy changes with Centers for Medicare and Medicaid Services.21 Patients in this trial found these DAs to be acceptable, unbiased, and would recommend to others. Importantly, the DAs were seen as most effective when delivered upstream of a clinic visit. This empowered the participants to have time to review, share with caregivers, stimulate questions for better conversations during the visit, and reduce anxiety after the visit. Participants preferred the in-depth paper tool and video DAs over an Option Grid conversation aid and interactive website. Future studies need to be conducted to determine the real-world effectiveness of these DAs. We are currently conducting an NIH trial (#R01HL136403) to determine both effectiveness and implementation of these DAs in real-world settings.
CONFLICT OF INTEREST
Financial support for this study was provided entirely by the Patient Centered Outcomes Research Institute (IP2 PI000116) and the National Institutes of Health (K23AG040696). Bryan Wallace is supported National Institutes of Health: National Heart, Lung, and Blood Institute (R01HL136403). Dr. Knoepke is supported National Institutes of Health: National Heart, Lung, and Blood Institute (1K23HL153892) and the American Heart Association (18CDA34110026). Dr. Allen receives grant funding from American Heart Association, National Institutes of Health, and the Patient Centered Outcomes Research group; and consulting fees from ACI Clinical, Amgen/Cytokinetics, Boston Scientific, and Novartis. Glyn Elwyn has edited and published books that provide royalties: Shared Decision Making (Oxford University Press) and Groups (Radcliffe Press). Glyn Elwyn’s academic interests are focused on shared decision making and coproduction. He owns copyright in measures of shared decision making (collaboRATE) and care integration (integRATE), a measure of experience of care in serious illness (consideRATE), a measure of goal setting coopeRATE, a measure of clinician willingness to do shared decision making (incorpoRATE), an observer measures of shared decision making (Observer OPTION-5 and Observer OPTION-12). He is the Founder and Director of &think LLC which owns the registered trademark for Option Grids™ patient decision aids; Founder, Director of SHARPNETWORK LLC, a provider of online training for shared decision making, consultant to EBSCO Health, Bind On-Demand Health Insurance, and Chief Clinical Research Scientist to abridge AI Inc. The authors declare no conflict of interest. The PCORI and NIH had no role in the design or development of the tools, the methods by which they were created, the analyses conducted, or the decision to publish findings.
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
DATA AVAILABILITY STATEMENT
The authors will share all data (in a de-identified format) with clinicians or researchers under an appropriate data use agreement.
REFERENCES
- 1.Mond HG, Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009–a World Society of Arrhythmia’s project. Pacing Clin Electrophysiol. 2011;34:1013–1027. [DOI] [PubMed] [Google Scholar]
- 2.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. [DOI] [PubMed] [Google Scholar]
- 3.Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure.[see comment]. N Engl J Med. 2004, 350(21): 2140–2150. [DOI] [PubMed] [Google Scholar]
- 4.Moss AJ,Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. [DOI] [PubMed] [Google Scholar]
- 5.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. [DOI] [PubMed] [Google Scholar]
- 6.Moss AJ, Schuger C, Beck CA, et al. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med. 2012; 367:2275–2283. [DOI] [PubMed] [Google Scholar]
- 7.Lewis KB, Stacey D, Matlock DD. Making decisions about implantable cardioverter-defibrillators from implantation to end of life: an integrative review of patients’ perspectives. Patient. 2014;7:243–260. [DOI] [PubMed] [Google Scholar]
- 8.Kovacs AH, Feigofsky S, Goff JS, et al. Implantable cardioverter defibrillator implant-explant-implant case study: addressing the psychological adjustment to multiple shocks. Clin Cardiol. 2006;29:274–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldenberg I, Moss AJ, Hall WJ, et al. Causes and consequences of heart failure after prophylactic implantation of a defibrillator in the multicenter automatic defibrillator implantation trial II. Circulation. 2006;113:2810–2817. [DOI] [PubMed] [Google Scholar]
- 10.Dunbar SB, Dougherty CM, Sears SF, et al. Educational and psychological interventions to improve outcomes for recipients of implantable cardioverter defibrillators and their families: a scientific statement from the American Heart Association. Circulation. 2012;126:2146–2172. [DOI] [PubMed] [Google Scholar]
- 11.Mark DB, Anstrom KJ, Sun JL, et al. Quality of life with defibrillator therapy or amiodarone in heart failure. N Engl J Med. 2008;359:999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sears SF Jr., Conti JB. Psychological aspects of cardiac devices and recalls in patients with implantable cardioverter defibrillators. Am J Cardiol. 2006;98:565–567. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein N, Carlson M, Livote E, Kutner JS. Brief communication: management of implantable cardioverter-defibrillators in hospice: a nationwide survey. Ann Intern Med. 2010;152:296–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein NE, Lampert R, Bradley E, Lynn J, Krumholz HM. Management of implantable cardioverter defibrillators in end-of-life care. Ann Intern Med. 2004;141:835–838. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein NE, Mehta D, Siddiqui S, et al. “That’s like an act of suicide” patients’ attitudes toward deactivation of implantable defibrillators. J Gen Intern Med. 2008;23 (Suppl 1):7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldstein NE, Mehta D, Teitelbaum E, Bradley EH, Morrison RS. “It’s like crossing a bridge” complexities preventing physicians from discussing deactivation of implantable defibrillators at the end of life. J Gen Intern Med. 2008;23 (Suppl 1):2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poole JE, Johnson GW, Hellkamp AS, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359:1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matlock DD, Nowels CT, Masoudi FA, et al. Patient and cardiologist perceptions on decision making for implantable cardioverter-defibrillators: a qualitative study. Pacing Clin Electrophysiol. 2011;34:1634–1644. [DOI] [PubMed] [Google Scholar]
- 19.Matlock DD, Keech TA, McKenzie MB, Bronsert MR, Nowels CT, Kutner JS. Feasibility and acceptability of a decision aid designed for people facing advanced or terminal illness: a pilot randomized trial. Health Expect. 2014;17:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson JH, Thylén I, Moser DK. Shared decision-making about end-of-life care scenarios compared among implantable cardioverter defibrillator patients: a national cohort study. Circ Heart Fail. 2019;12:e005619. 10.1161/CIRCHEARTFAILURE.118.005619 Epub 2019 Oct 11. [DOI] [PubMed] [Google Scholar]
- 21.Decision memo for implantable cardioverter defibrillators (CAG-00157R4). 2019. (Accessed 07/15/2019). https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&NCAId=288
- 22.Knoepke CE, Mandrola JM. Don’t be afraid: using an ICD means having difficult conversations. Am Heart Assoc. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4(4)CD001431. 10.1002/14651858.CD001431.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elwyn G, Scholl I, Tietbohl C, et al. “Many miles to go…”: a systematic review of the implementation of patient decision support interventions into routine clinical practice. BMC Med Inform Decis Mak. 2013;13:(Suppl 2)S14. 10.1186/1472-6947-13-S2-S14 Epub 2013 Nov 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elwyn G, O’Connor A, Stacey D, et al. International Patient Decision Aids Standards (IPDAS) collaboration. Developing a quality criteria framework for patient decision aid: online international Delphi consensus process. Br Med J. 2006;333:417–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joseph-Williams N, Newcombe R, Politi M, et al. Toward minimum standards for certifying patient decision aids: a modified Delphi consensus process. Med Decis Making. 2014;34:699–710. [DOI] [PubMed] [Google Scholar]
- 27.Ottawa decision support framework. Accessed June 21, 2015, 2015, at http://decisionaid.Ohri.Ca/docs/develop/odsf.Pdf.)
- 28.Supporting Evidence for the Development of ICD Decision Aids. 2019. Accessed 2/25/2021 https://patientdecisionaid.org/wp-content/uploads/2016/06/2019.04.16-Evidence-Document-DECIDE-ICD.pdf.)
- 29.Elwyn G, O’Connor A, Stacey D, et al. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. BMJ. 2006;333:417. 10.1136/bmj.38926.629329.AE Epub 2006 Aug 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Witteman HO, Maki KG, Vaisson G, et al. Systematic development of patient decision aids: an update from the IPDAS collaboration. Med Decis Making. 2021; 19:272989X211014163. 10.1177/0272989X211014163 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allen LA, McIlvennan CK, Thompson JS, et al. Effectiveness of an intervention supporting shared decision making for destination therapy left ventricular assist device: the DECIDE-LVAD randomized clinical trial. JAMA Intern Med. 2018;178(4):520–529. 10.1001/jamainternmed.2017.8713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaffer VA, Zikmund-Fisher BJ. All stories are not alike: a purpose-,content-, and valence-based taxonomy of patient narratives in decision aids. Med Decis Making 2013;33:4–13. [DOI] [PubMed] [Google Scholar]
- 33.Barry MJ, Fowler FJ Jr., Mulley AG Jr., Henderson JV Jr., Wennberg JE. Patient reactions to a program designed to facilitate patient participation in treatment decisions for benign prostatic hyperplasia. Med Care. 1995;33:771–82. [DOI] [PubMed] [Google Scholar]
- 34.Feldman-Stewart D, Brundage M. A conceptual framework for patientΓ Çôprovider communication: a tool in the PRO research tool box. Quality Life Res. 2009;18:109–114. [DOI] [PubMed] [Google Scholar]
- 35.Sepucha KR, Levin CA, Uzogara EE, Barry MJ, O’Connor AM, Mulley AG. Developing instruments to measure the quality of decisions: early results for a set of symptom-driven decisions. Patient Educ Couns. 2008;73:504–510. [DOI] [PubMed] [Google Scholar]
- 36.Sepucha K, Ozanne E, Silvia K, Partridge A, Mulley AG Jr., An approach to measuring the quality of breast cancer decisions. Patient Educ Couns. 2007;65:261–269. [DOI] [PubMed] [Google Scholar]
- 37.O’Connor AM, Tugwell P, Wells GA, et al. Randomized trial of a portable, self-administered decision aid for postmenopausal women considering long-term preventive hormone therapy. Med Decis Making. 1998;18:295–303. [DOI] [PubMed] [Google Scholar]
- 38.O’Connor AM, Tugwell P, Wells GA, et al. A decision aid for women considering hormone therapy after menopause: decision support framework and evaluation. Patient Educ Couns. 1998;33:267–279. [DOI] [PubMed] [Google Scholar]
- 39.O’Connor AM. Validation of a decisional conflict scale. Med Decis Making. 1995;15:25–30. [DOI] [PubMed] [Google Scholar]
- 40.Brehaut JC, O’Connor AM, Wood TJ, et al. Validation of a decision regret scale. Med Decis Making. 2003;23:281–292. [DOI] [PubMed] [Google Scholar]
- 41.Degner LF, Sloan JA, Venkatesh P. The control preferences scale. Can J Nurs Res. 1997;29:21–43. [PubMed] [Google Scholar]
- 42.Bass PF III, Wilson JF, Griffith CH. A shortened instrument for literacy screening. J Gen Intern Med. 2003;18:1036–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fereday J, Muir-Cochrane E. Demonstrating rigor using thematic analysis: A hybrid approach of inductive and deductive coding and theme development. Int J Qualitative Methods. 2010;5:80–92. [Google Scholar]
- 44.Ratzan S Introduction In: Selden CR, Zorn M, Ratzan SC, Parker RM, eds. National Library of Medicine Current Bibliographies in Medicine: Health Literacy. Vol. NML, Pub. No CBM 2000-1. 2000. Bethesda, MD. National Institutes of Health, US Department of Health and Human Services. 2016. [Google Scholar]
- 45.Kutner M, Greenburg E, Jin Y, Paulsen C. The Health Literacy of America’s Adults: Results from the 2003 National Assessment of Adult Literacy. NCES 2006-483. National Center for Education Statistics; 2006. [Google Scholar]
- 46.Berkman ND, Sheridan SL, Donahue KE, et al. Health literacy interventions and outcomes: an updated systematic review. Evidence Report/Technol Assessment. 2011;199:1–941. [PMC free article] [PubMed] [Google Scholar]
- 47.Green AR, Jenkins A, Masoudi FA, et al. Decision-making experiences of patients with implantable cardioverter defibrillators. Pacing Clin Electrophysiol. 2016;39:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Payne JW, Bettman JR, Schkade DA. Measuring constructed preferences: towards a building code. J Risk Uncertainty. 1999;19:243–270. [Google Scholar]
- 49.Brownson RC, Jacobs JA, Tabak RG, Hoehner CM, Stamatakis KA. Designing for dissemination among public health researchers: findings from a national survey in the United States. Am J Public Health. 2013;103:1693–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joseph-Williams N, Abhyankar P, Boland L, et al. What works in implementing patient decision aids in routine clinical settings? A rapid realist review and update from the international patient decision aid standards collaboration. Med Decis Making. 2020;272989X20978208. 10.1177/0272989X20978208 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chan WV, Pearson TA, Bennett GC, et al. ACC/AHA special report: clinical practice guideline implementation strategies: a summary of systematic reviews by the NHLBI implementation science work group: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;69:1076–1092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors will share all data (in a de-identified format) with clinicians or researchers under an appropriate data use agreement.


