Abstract
Background:
This study assessed the hepatic vein waveform (HVW) and mean maximum portal vein velocity (MM-PVV) on Doppler ultrasound in patients with liver cirrhosis (LC) and compared it with that of age and sex-matched controls. It correlated the degree of HVW abnormality and MM-PVV changes with liver function based on Child-Turcotte-Pugh (CTP) to determine which was more predictive of CTP.
Methods:
Sixty patients with LC and 60 healthy controls were consecutively recruited into this study. Each patient was classed based on the CTP system after relevant tests. Doppler evaluation of the hepatic vein (HV) and MM-PVV were performed. HVW obtained was classified either into triphasic, biphasic, or monophasic.
Results:
Sixty cirrhotic and 60 age-matched control subjects aged 19–69 and 18–69 years, respectively, completed this study. All control subjects had a normal HVW pattern while 46 (76.7%) cirrhotic subjects had abnormal HVW (P < 0.001). The MM-PVV was significantly lower in cirrhotic subjects than in controls; 22.8 cm/s versus 33.6 cm/s (P < 0.001). The degree of HVW abnormality among cirrhotics showed a significant positive correlation with CTP (r = 0.283, P = 0.029). MM-PVV on the other hand showed no correlation with CTP class (r = −0.124; P = 0.346). Linear regression showed that HVW was a significant predictor of hepatic dysfunction based on CTP.
Conclusion:
Changes in the waveform pattern of the HVs are a good predictor of the derangement of hepatic function in patients with LC than changes in PVV. HVW pattern could therefore serve as an adjunct to CTP class in hepatic function assessment.
Keywords: Hepatic vein waveform, liver cirrhosis, portal vein velocity, ultrasound
INTRODUCTION
There are several ways of assessing liver function in patients with liver cirrhosis (LC). Liver biopsy and histology though the gold standard are invasive, associated with risk of patient morbidity and mortality, and samples a small portion of the liver which may not represent an ongoing pathological process.[1,2] In our environment, noninvasive clinical and biochemical methods are mainly used. These are computed into a scoring system known as Child-Turcotte-Pugh (CTP) score.[3,4]
B-mode ultrasound is also used to follow-up patients with LC and to screen for malignant transformation. It, however, provides little information on hepatic function. Conversely, Doppler ultrasound demonstrates the hemodynamics of the portal venous system, hepatic artery, and veins, which reflect hepatic functional status.[2] Doppler indices commonly used for the evaluation of cirrhosis and portal hypertension include measurement of portal and splenic venous blood flow velocity and resistivity indices of splenic, hepatic, and superior mesenteric arteries. However, these indices are plagued by poor reproducibility and accuracy.[5]
Doppler ultrasound of the hepatic vein (HV) has been suggested as an additional tool that will help to increase the diagnostic accuracy of these parameters.[6,7] In healthy subjects, the hepatic vein waveform (HVW) is normally described as triphasic, although it has four components: a retrograde A-wave, an antegrade S-wave, a transitional V wave (could be antegrade, retrograde, or neutral), and an antegrade D-wave corresponding to atrial contraction, ventricular systole, atrial overfilling, and opening of the tricuspid valve, respectively.[8,9] Changes in the liver parenchyma impair the compliance of the wall of the HV resulting in the alteration of the normal phasic waveform. Initially, there is loss of the retrograde A-wave resulting in a biphasic waveform. With worsening fibrosis, complete loss of phasicity occurs with flattening of the waveform pattern.[10]
This study aimed to evaluate the HVW of patients with LC using ultrasound in comparison with control subjects. The mean maximum portal vein velocity (MM-PVV) was also measured. Both parameters were compared independently with the severity of liver disease based on the CTP class, and then, an attempt was made to determine which of them was more predictive of liver disease.
SUBJECTS AND METHODS
This is a case-controlled, comparative nonrandomized study in which 60 study subjects, above the age of 18 years, with LC along with 60 healthy age and sex-matched control subjects were consecutively recruited over 12 months – from July 2017 to June 2018. This study was approved by our Institutional Review Board of Obafemi Awolowo University Teaching Hospitals Complex, Ile-Ife.(approval number: ERC/2017/01/02) Informed consent was obtained from all study subjects.
Diagnosis of LC was made based on a combination of typical clinical features and biochemical findings as well as typical B-mode ultrasound features such as shrunken liver size with irregular outline, ascites, and portal hypertension. The control group was comprised of apparently healthy adult volunteers or subjects who had no clinical signs and symptoms of liver or renal disease. Subjects with heart failure, HV or inferior vena cava (IVC) obstruction, hepatocellular carcinoma, or other liver masses were excluded.
Relevant biodata, history, and physical examination findings were obtained. The subjects’ weight (kg) and height (M) were measured and were body mass index calculated. Hepatic encephalopathy was graded based on the West Haven Grading System from Grade 0 to Grade 4.[11]
Venous blood was obtained from cirrhotic subjects following an overnight fast. Laboratory parameters that were done include liver function test, serum electrolytes, urea and creatinine, prothrombin time/international normalized ratio (INR), and serum albumin.
Sonographic assessment/technique
Patients and controls were scanned following an overnight fast of at least 6–8 h to reduce excess bowel gas that may obscure the vascular structures. Real-time US examinations were performed with a 3.5-MHz transducer (Toshiba Real-time Ultrasound scanner Model TUS-F30 with Doppler facilities) by the lead researcher.
With the patient in the supine position, B-mode ultrasound of the hepatic parenchyma was done first to assess the liver span, echogenicity, echotexture, and surface nodularity. Pulsed Doppler evaluation of HV was then performed in the transverse plane and the probe manipulated (either through an intercostal approach or a subcostal approach over the right hypochondrium), until the star-like confluence of the HVs to the IVC was visualized. The HV evaluation was done after nonforced (quiet) expiration.[10,12] Doppler signals were, in general, obtained from the right[5,13] or middle[6,10] HVs at a distance of 3–6 cm from the junction of the HV and the IVC to increase the impact of hepatic parenchyma changes on the HVWs instead of the IVC flow.[7] The left HV was not evaluated because the pulsatility within it is greater than that in the middle and right HVs,[13,14] which can lead to flow artifacts of the Doppler sonographic signal.[15] The HVW assessments were made on the lowest frequency range possible without aliasing. In addition, the lowest possible wall filter was utilized, the sample volume set at 2–5 mm, and angles of insonation <60°.
The HVW was classified into three groups according to the Doppler signal characteristics:
Type 0, triphasic waveform; the presence of a short phase of reversed flow
Type I, biphasic waveform; decreased amplitude of the phasic oscillations without the short phase of reversed flow
Type II, monophasic waveform; complete flat waveform [Figure 1].
Figure 1.
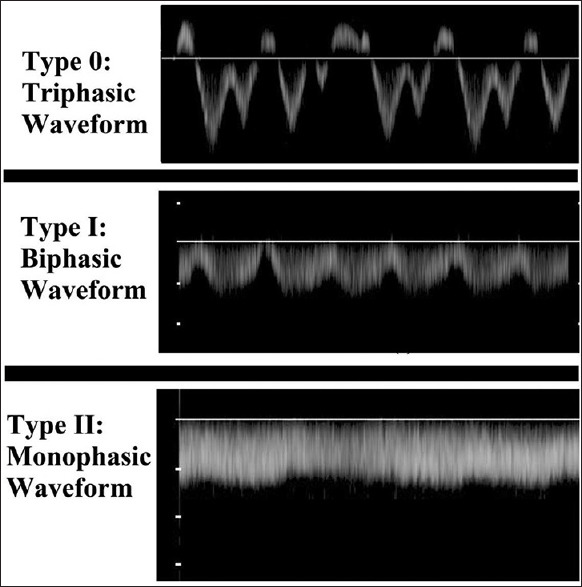
Spectral waveform of 3 cirrhotic study subjects showing the classification of hepatic vein waveform patterns
The transverse diameter of the main PV was measured at its midpoint. The direction of flow was noted. The maximum velocity was measured using the same Doppler settings as described above. Three values of the maximum PVV were obtained and the average was taken to get the MM-PVV. To decrease the effect of respiration on the portal blood flow, measurements were obtained during a short time of breath-holding.[16]
Other parameters taken were spleen span and the presence of ascites. Splenomegaly was defined as spleen span >12 cm.[8] Ascites were graded according to Haktanir et al.[17] criteria into 3 categories: Absent, mild, and moderate-to-severe.
For cirrhotic patients, disease severity was assessed using the CTP score, which took into account five conventional clinical (hepatic encephalopathy and ascites) and laboratory (albumin, prothrombin time, and bilirubin values) parameters.
Data and statistical analysis
The data obtained were analyzed using IBM Statistical Package for the Social Sciences (IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY, USA). Differences in the distribution of various HVWs between control subjects and cirrhotic patients and among the various groups of cirrhotic patients were analyzed with the Chi-square (χ2) or Fisher's exact test as appropriate. Differences in the CTP ordinal score were analyzed with the χ2 or Fisher's exact test as appropriate for trend. To compare means, student's t-test was performed. For multiple values, ANOVA was used. The Spearman correlation was used to analyze the relationship of CTP class with the changes in the HVW and MM-PVV. Linear regression was used to evaluate the degree of relationship between CTP class and the Doppler parameters, HVW and MM-PVV. The level of statistical significance was set at P < 0.05.
RESULTS
A total of 120 study subjects comprising 60 cirrhotic and 60 age- and-sex-matched healthy controls subjects completed this study. The distribution of cirrhotic subjects showed two age peaks; 40–49 and ≥60 years. The cirrhotic patients comprised 44 (73.3%) males and 16 (26.7%) females with a male-female ratio of 2.8:1.
Table 1 shows the Doppler characteristics of subjects and controls. There was a statistically significant difference between the HVW of cirrhotic subjects and controls. Abnormal HVW was present in 76.6% (46) of cirrhotic subjects [Figure 2]. All control subjects had triphasic HVW pattern.
Table 1.
Comparison of Doppler ultrasound characteristics of cirrhotic subjects and controls
| Variables | Cirrhotics (n=60) | Controls (n=60) | Statistics | df | P |
|---|---|---|---|---|---|
| HVW, n (%) | |||||
| Triphasic | 14 (23.3) | 60 (100) | 86.931 | 2 | <0.001* |
| Biphasic | 20 (33.3) | 0 | |||
| Monophasic | 26 (43.4) | 0 | |||
| PVD (cm) | |||||
| Mean±SD | 1.30±0.25 | 0.94±0.18 | 9.319 | 118 | <0.001# |
| Range | 0.79-1.79 | 0.57-1.36 | |||
| n (%) | |||||
| Diameter <1.30 | 27 (45.0) | 58 (96.7) | 38.763 | 1 | <0.001* |
| Diameter ≥1.30 | 33 (55.0) | 2 (3.3) | |||
| Portal vein velocity (cm/s) | |||||
| Mean±SD | 22.8±10.4 | 33.6±11.1 | -5.530 | 118 | <0.001# |
| Range | 0.0-44.0 | 15.3-70.2 | |||
| n (%) | |||||
| Velocity ≥15 | 46 (76.7) | 60 (100) | 15.894 | 1 | <0.001* |
| Velocity <15 | 14 (23.3) | 0 (0.00) |
*Chi-square/Fisher’s exact test statistic was used to compare proportions, #Independent samples t-test was used to compare means. PVD: Portal vein diameter, SD: Standard deviation, HVW: Hepatic vein waveform
Figure 2.
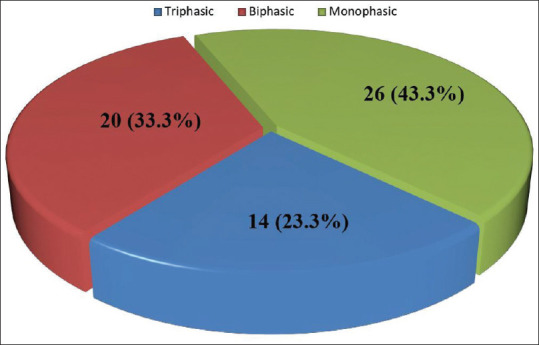
Pie chart showing the distribution of hepatic vein waveform in cirrhotic subjects
The control subjects had a significantly higher MM-PVV than cirrhotic subjects [Table 1]. All control subjects had MM-PVV ≥15 cm/s while 23.3% of cirrhotic subjects had significantly reduced MM-PVV <15 cm/s. The cirrhotic patients had hepatopetal portal blood flow except for 2 subjects who demonstrated no flow in the PVs.
No significant relationship was noted between HVW and other ultrasound parameters MM-PVV, PV diameter, liver span, splenic span, and presence of ascites [Table 2]. MM-PVV showed a significant negative correlation with PV diameter [Table 3] with r = −0.272 and P = 0.035. It showed no correlation with liver span or splenic span. No relationship was also noted between MM-PVV and the presence or absence of ascites.
Table 2.
Association between hepatic vein waveform, other ultrasound, and biochemical parameters in cirrhotic subjects
| Variables | Triphasic (n=14) | Biphasic (n=20) | Monophasic (n=26) | P |
|---|---|---|---|---|
| MM-PVV (cm/s) mean±SD | 25.4±8.3 | 23.9±11.6 | 20.5±10.3 | 0.319# |
| Velocity ≥15 | 13 (92.9) | 16 (80.0) | 17 (65.4) | 0.134* |
| Velocity <15 | 1 (7.1) | 4 (20.0) | 9 (34.6) | |
| PVD (cm), mean±SD | 1.3±0.2 | 1.2±0.3 | 1.4±0.3 | 0.211# |
| Diameter <1.30 | 5 (35.7) | 12 (60.0) | 10 (38.5) | 0.252* |
| Diameter ≥1.30 | 9 (64.3) | 8 (40.0) | 16 (61.5) | |
| Ascites, n (%) | ||||
| Absent | 6 (42.9) | 4 (20.0) | 3 (11.5) | 0.221* |
| Mild | 1 (7.1) | 4 (20.0) | 5 (19.2) | |
| Moderate to severe | 7 (14.3) | 12 (30.0) | 18 (19.2) | |
| Liver span (cm), mean±SD | 11.6±1.6 | 13.0±2.7 | 11.9±2.4 | 0.185# |
| Splenic span (cm), mean±SD | 15.2±3.6 | 14.9±3.4 | 15.2±3.6 | 0.827# |
| Span <12.0 | 3 (21.4) | 4 (20.0) | 4 (15.4) | 0.836* |
| Span >12.5 | 11 (78.6) | 16 (80.0) | 22 (84.6) | |
| Bilirubin (µmol/l), mean±SD | 42.5±26.9 | 36.5±22.2 | 54.5±33.0 | 0.101# |
| Albumin (g/l), mean±SD | 27.9±9.1 | 27.1±6.8 | 30.1±5.8 | 0.319# |
| PT (s), mean±SD | 17.5±3.2 | 17.4±3.4 | 18.9±3.6 | 0.266# |
| INR, mean±SD | 1.5±0.3 | 1.4±0.3 | 1.6±0.4 | 0.265# |
| Na (mmol/l), mean±SD | 129.9±6.1 | 125.0±22.8 | 130.9±5.4 | 0.346# |
| K (mmol/l), mean±SD | 3.6±0.5 | 3.8±0.5 | 3.9±0.6 | 0.405# |
| Creatinine (µmol/l), mean±SD | 84.6±15.3 | 86.2±20.0 | 82.6±19.6 | 0.814# |
| Urea (mmol/l), mean±SD | 5.5±2.3 | 6.1±2.5 | 7.3±4.0 | 0.212# |
*Chi-square/Fisher’s exact test statistic was used to compare proportions, #One-way ANOVA was used to compare means. PVD: Portal vein diameter, PT: Prothrombin time, INR: International normalized ratio, SD: Standard deviation, MM-PVV: Mean maximum portal vein velocity
Table 3.
Association between mean maximum portal vein velocity other ultrasound parameters and biochemical parameters
| Variables | Statistics | P |
|---|---|---|
| PVD | −0.272* | 0.035 |
| Liver span | 0.077* | 0.559 |
| Spleen span | −0.190* | 0.147 |
| Ascites, n (%) | 0.962# | 0.388 |
| Bilirubin (µmol/l) | 0.116* | 0.376 |
| Albumin (g/l) | −0.092* | 0.486 |
| PT (s) | −0.176* | 0.178 |
| INR | −0.175* | 0.182 |
| Na (mmol/l) | 0.157* | 0.231 |
| K (mmol/l) | −0.280* | 0.030 |
| Creatinine (µmol/l) | −0.087* | 0.511 |
| Urea (mmol/l) | −0.096* | 0.468 |
*Pearson correlation coefficient, #One-way ANOVA. PVD: Portal vein diameter, PT: Prothrombin time, INR: International normalized ratio
HVW pattern did not show any significant correlation with serum bilirubin, albumin, creatinine, urea, sodium, and potassium levels [Table 2]. It was also unrelated to prothrombin time and INR.
Similarly, (MM-PVV) showed no correlation with serum bilirubin, albumin, creatinine, urea, and sodium levels [Table 3]. It, however, showed a significant negative correlation with serum potassium levels; r = −0.280 and P = 0.030.
Table 4 shows the distribution of CTP classes based on HVW. The proportion of subjects with triphasic waveform pattern in Class A was more than those in Classes B and C. Similarly, Class C had significantly more subjects with monophasic waveform than Classes A and B. HVW also showed a significant positive correlation with CTP class with r = 0.283 and P = 0.029. In contrast, no significant relationship or correlation is noted between MM-PVV and CTP class [Table 5].
Table 4.
Relationship between hepatic vein waveform and Child-Pugh classes/scores
| Variables | Triphasic (n=14) | Biphasic (n=20) | Monophasic (n=26) | Statistics | df | P |
|---|---|---|---|---|---|---|
| Child-Pugh class | ||||||
| A | 5 (35.7) | 1 (5.0) | 1 (3.8) | 12.836* | 4 | 0.012 |
| B | 4 (28.6) | 12 (60.0) | 10 (38.5) | |||
| C | 5 (35.7) | 7 (35.0) | 15 (57.7) | |||
| - | r= 0.283** | 0.029 | ||||
| Bilirubin score (µmol/l), n (%) | ||||||
| <34 | 7 (50.0) | 11 (55.0) | 8 (30.8) | 9.610* | 4 | 0.048 |
| 34-50 | 3 (21.4) | 6 (30.0) | 3 (11.5) | |||
| >50 | 4 (28.6) | 3 (15.0) | 15 (57.7) | |||
| - | r=269** | 0.037 | ||||
| Albumin score (g/l), n (%) | ||||||
| >35 | 4 (28.6) | 2 (10.0) | 3 (11.5) | 8.839* | 4 | 0.065 |
| 30-35 | 4 (28.6) | 5 (25.0) | 15 (57.7) | |||
| <30 | 6 (42.9) | 13 (65.0) | 8 (30.8) | |||
| INR score, n (%) | ||||||
| <1.7 | 11 (78.6) | 16 (80.0) | 16 (61.5) | 3.060* | 4 | 0.548 |
| 1.7-2.3 | 3 (21.4) | 4 (20.0) | 4 (34.6) | |||
| >2.3 | 0 | 0 | 1 (3.8) | |||
| Ascites score, n (%) | ||||||
| None | 6 (42.9) | 4 (20.0) | 3 (11.5) | 5.715* | 4 | 0.221 |
| Mild | 1 (7.1) | 4 (20.0) | 5 (19.2) | |||
| Moderate-to-severe | 7 (50.0) | 12 (60.0) | 18 (69.2) | |||
| HE score, n (%) | ||||||
| None | 14 (100.0) | 20 (100.0) | 23 (88.5) | 4.130* | 2 | 0.127 |
| Grade I - II | 0 | 0 | 3 (11.5) | |||
| Grade III - IV | 0 | 0 | 0 |
*Chi-square test statistic was used to compare proportions, #One-way ANOVA was used to compare means, **Spearman’s rho correlation coefficient. HE: Hepatic encephalopathy, INR: International normalized ratio
Table 5.
Relationship between mean maximum portal vein velocity and Child-Pugh classes/scores
| Variables | Statistics | df | P |
|---|---|---|---|
| Child-Pugh class | |||
| Class A versus B versus C | 0.494# | 2 | 0.613 |
| Class A versus B | 1.020* | 31 | 0.315 |
| Class A versus C | 0.920* | 32 | 0.364 |
| Class B versus C | −0.043* | 51 | 0.966 |
| Total Child-Pugh score | −0.124** | 0.346 | |
| Bilirubin score | 0.198# | 2 | 0.821 |
| Albumin score | 0.202 | 2 | 0.817 |
| INR score | 1.298# | 2 | 0.281 |
| Ascites score | 0.962# | 2 | 0.388 |
| HE score | 1.323# | 1 | 0.255 |
*t-test was used to compare means, **Spearman rho correlation coefficient, #One-way ANOVA was used to compare means. HE: Hepatic encephalopathy, INR: International normalized ratio
On linear regression, HVW was a significant predictor of hepatic dysfunction based on CTP class. The regression equation was as follows: CTP class = 2.014 + 0.266 × HVW, R2= 0.097, F = 6.252, P = 0.015. PVV on the other hand was not a significant predictor of CTP class with P = 0.496 [Table 6].
Table 6.
Linear regression analysis of hepatic vein waveform and portal vein velocity versus Child–Pugh class
| Dependent variable: Child-Pugh class | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| R 2 | F | df | P | Constant | b1 | |
| Independent variable | ||||||
| HVW | 0.097 | 6.252 | 1 | 0.015 | 2.014 | 0.266 |
| PVV | 0.008 | 0.469 | 1 | 0.496 | 2.467 | −0.006 |
PVV: Portal vein velocity, HVW: Hepatic vein waveform
DISCUSSION
This study showed normal triphasic hepatic venous waveform in all the control subjects. This was similar to the results from other studies.[5,6,10,12,13,18,19] On the other hand, Shapiro et al.[20] observed triphasic waveform in 90.7% of healthy subjects, while 9.3% had abnormal tracing. This may be attributable to technique since theirs was done under breath-hold which could lead to inadvertent Valsalva maneuver with consequent increased intraabdominal pressure and dampening of the tracing.[8,9] Coulden et al.[14] corroborated this in their study in which normal subjects with triphasic waveform had an absent reverse component of the waveform and subsequent progressive loss of pulsatility following increased intra-abdominal pressure.
In this study, 76.7% of cirrhotic subjects had abnormal HVW (33.3% biphasic and 43.3% monophasic), while 23.3% had normal (triphasic) waveform. This is comparable to that obtained in other studies.[10,12,13,21] Sudhamshu et al.[22] and Bolondi et al.,[6] however, had lower values of 60%, 50%, and 50%, respectively, while Bhutto et al.[23] and Baik et al.[5] had higher values of 92.3% and 92.0%, respectively. The reason for these differences is unclear although the breath-hold method employed by Bhutto et al. and Baik et al. might explain their higher values. Furthermore, Baik et al. recruited patients with both cirrhosis and a history of variceal bleeding; those with advanced disease. The etiology of cirrhosis might also account for these differences. For example, in the study by von Herbay et al.[13] and Sudhamshu et al.,[22] the major etiologic agent for cirrhosis was alcohol while hepatitis C virus infection was the main agent in the study by Bhutto et al.[23] In contrast, hepatitis B virus infection is the main causative agent of cirrhosis in this environment.[24,25]
Similar to results obtained in previous studies,[10,12,21,23,26] this study revealed a significant difference in the HVW pattern between cirrhotic subjects and controls with a P < 0.05. The degree of HVW abnormality among cirrhotic subjects also showed a significant relationship with hepatic dysfunction based on CTP class as noted in other studies.[6,23,27,28] In the study by Bhutto et al., the proportion of monophasic waveform by class was 90%, 73.91%, and 50% for Classes C, B, and A, respectively, which differed from that in this study which was 57.7%, 38.5%, and 3.8%, respectively. On the other hand, in the study by Antil et al., no patient in Class A had monophasic HVW while in Classes B and C, 33.3% and 87.0%, respectively, had monophasic HVW. The reason for these variations is unclear although the inhomogeneous affectation of the hepatic parenchyma by the cirrhotic process may account for it.[4] Comparable to findings by von Herbay et al.,[13] this study showed a decreasing incidence of triphasic waveform pattern with increasing CTP class. Ohta et al.[29] likewise observed that monophasic waveform correlated with high CTP score and poor survival rate. In contrast, Sudhamshu et al. in two separate studies[22,30] and Joseph et al.[26] did not demonstrate any relationship between CTP class and HVW. Monophasic waveform was rare in both studies by Sudhamshu et al. with only approximately 3% of subjects having monophasic HVW. His second study excluded subjects with hepatofugal portal blood flow secondary to large portosystemic shunts. This might likely have contributed to the rarity of monophasic waveform pattern in his studies since other studies have shown that loss of the normal triphasic pattern has high sensitivity in detecting large esophageal varices,[26,27] which is a major portosystemic shunt. Although Joseph et al.[26] did not make similar exclusion as Sudhamshu et al., they excluded patients with acute variceal hemorrhage and patients who had undergone endoscopic variceal ligation or sclerotherapy. Second, HVW assessment in their study was done under breath-hold.
In the index study as in other studies, PVV was significantly lower in cirrhotic subjects than in controls.[20,31,32,33] O’Donohue et al.[21] on the other hand observed no significant difference in the PVV of cirrhotic subjects and controls. This difference might be accounted for by his smaller sample. Furthermore, the evaluation of the PVV in their study was done during normal respiration in contrast to this study in which it was obtained during a short time of breath-holding. Contrary to the findings in this study, Iwao et al.[31] and Zironi et al.[33] noted a significant difference in the mean PVV between each CTP class. Annet et al.[32] and Kutlu et al.[34] on the other hand did not observe any correlation between mean PVV and CTP score. The reason for this wide variability in results from studies on PV is not clearly understood. It has been attributed to various factors such as equipment and observer variability, postural changes, exercise, cardiac output, postprandial effects, and the presence of collateral pathways.[8,21,35]
CONCLUSION
Changes in the waveform pattern of the HVs are a significant predictor of the presence of cirrhotic changes in the liver parenchyma. It also has significant relationship with the degree of derangements in hepatic function based on CTP score. Although reduction of PVV is significantly more in cirrhotic patients than in controls, it correlates less with the degree of changes in hepatic function than changes in HVW.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Macarini L, Stoppino LP. Radiologic assessment of liver fibrosis – Present and future. In: Practical Management of Chronic Viral Hepatitis. In Tech. 2013. pp. 115–6. https://www.intechopen.com/books/3312. [Google Scholar]
- 2.Goyal N, Jain N, Rachapalli V, Cochlin DL, Robinson M. Non-invasive evaluation of liver cirrhosis using ultrasound. Clin Radiol. 2009;64:1056–66. doi: 10.1016/j.crad.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749–61. doi: 10.1016/S0140-6736(14)60121-5. [DOI] [PubMed] [Google Scholar]
- 4.Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838–51. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baik SK, Kim JW, Kim HS, Kwon SO, Kim YJ, Park JW, et al. Recent variceal bleeding: Doppler US hepatic vein waveform in assessment of severity of portal hypertension and vasoactive drug response. Radiology. 2006;240:574–80. doi: 10.1148/radiol.2402051142. [DOI] [PubMed] [Google Scholar]
- 6.Bolondi L, Li Bassi S, Gaiani S, Zironi G, Benzi G, Santi V, et al. Liver cirrhosis: Changes of Doppler waveform of hepatic veins. Radiology. 1991;178:513–6. doi: 10.1148/radiology.178.2.1987617. [DOI] [PubMed] [Google Scholar]
- 7.Aubé C, Winkfield B, Oberti F, Vuillemin E, Rousselet MC, Caron C, et al. New Doppler ultrasound signs improve the non-invasive diagnosis of cirrhosis or severe liver fibrosis. Eur J Gastroenterol Hepatol. 2004;16:743–51. doi: 10.1097/01.meg.0000108357.41221.e5. [DOI] [PubMed] [Google Scholar]
- 8.Davis M, Chong WK. Doppler ultrasound of the liver, portal hypertension, and transjugular intrahepatic portosystemic shunts. Ultrasound Clin. 2014;9:587–604. [Google Scholar]
- 9.Scheinfeld MH, Bilali A, Koenigsberg M. Understanding the spectral Doppler waveform of the hepatic veins in health and disease. Radiographics. 2009;29:2081–98. doi: 10.1148/rg.297095715. [DOI] [PubMed] [Google Scholar]
- 10.Colli A, Cocciolo M, Riva C, Martinez E, Prisco A, Pirola M, et al. Abnormalities of Doppler waveform of the hepatic veins in patients with chronic liver disease: Correlation with histologic findings. AJR Am J Roentgenol. 1994;162:833–7. doi: 10.2214/ajr.162.4.8141001. [DOI] [PubMed] [Google Scholar]
- 11.Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy – Definition, nomenclature, diagnosis, and quantification: Final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716–21. doi: 10.1053/jhep.2002.31250. [DOI] [PubMed] [Google Scholar]
- 12.Arda K, Ofelli M, Çalikoglu U, Olçer T, Cumhur T. Hepatic vein Doppler waveform changes in early stage (Child-Pugh A) chronic parenchymal liver disease. J Clin Ultrasound. 1997;25:15–9. doi: 10.1002/(sici)1097-0096(199701)25:1<15::aid-jcu3>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 13.von Herbay A, Frieling T, Häussinger D. Association between duplex Doppler sonographic flow pattern in right hepatic vein and various liver diseases. J Clin Ultrasound. 2001;29:25–30. doi: 10.1002/1097-0096(200101)29:1<25::aid-jcu4>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 14.Coulden RA, Lomas DJ, Farman P, Britton PD. Doppler ultrasound of the hepatic veins: Normal appearances. Clin Radiol. 1992;45:223–7. doi: 10.1016/s0009-9260(05)80001-7. [DOI] [PubMed] [Google Scholar]
- 15.Dietrich CF, Lee JH, Gottschalk R, Herrmann G, Sarrazin C, Caspary WF, et al. Hepatic and portal vein flow pattern in correlation with intrahepatic fat deposition and liver histology in patients with chronic hepatitis C. Am J Roentgenol. 1998;171:437–43. doi: 10.2214/ajr.171.2.9694471. [DOI] [PubMed] [Google Scholar]
- 16.Iliopoulos P, Vlychou M, Karatza C, Yarmenitis SD, Repanti M, Tsamis I, et al. Ultrasonography in differentiation between chronic viral hepatitis and compensated early stage cirrhosis. World J Gastroenterol. 2008;14:2072–9. doi: 10.3748/wjg.14.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haktanir A, Cihan BS, Celenk C, Cihan S. Value of Doppler sonography in assessing the progression of chronic viral hepatitis and in the diagnosis and grading of cirrhosis. J Ultrasound Med. 2005;24:311–21. doi: 10.7863/jum.2005.24.3.311. [DOI] [PubMed] [Google Scholar]
- 18.Lo RH, Afif AM, Wang Y, Lau D, Ooi CC. A sonographic Doppler study of hepatic vein, portal vein and hepatic artery in liver cirrhosis: Correlation of hepatic haemodynamics and liver dysfunction. Clin Radiol. 2012;67:S10. doi: 10.1177/1742271X17721265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorka W, Kagalwalla A, McParland BJ, Kagalwalla Y, al Zaben A. Diagnostic value of Doppler ultrasound in the assessment of liver cirrhosis in children: Histopathological correlation. J Clin Ultrasound. 1996;24:287–95. doi: 10.1002/(SICI)1097-0096(199607/08)24:6<287::AID-JCU2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 20.Shapiro RS, Winsberg F, Maldjian C, Stancato-Pasik A. Variability of hepatic vein Doppler tracings in normal subjects. J Ultrasound Med. 1993;12:701–3. doi: 10.7863/jum.1993.12.12.701. [DOI] [PubMed] [Google Scholar]
- 21.O’Donohue J, Ng C, Catnach S, Farrant P, Williams R. Diagnostic value of Doppler assessment of the hepatic and portal vessels and ultrasound of the spleen in liver disease. Eur J Gastroenterol Hepatol. 2004;16:147–55. doi: 10.1097/00042737-200402000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Sudhamshu KC, Sharma D, Chataut SP. Hepatic vein waveforms in liver cirrhosis re-evaluated. Hepatol Int. 2010;5:581–5. doi: 10.1007/s12072-010-9226-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhutto AR, Abbasi A, Butt N, Khan A, Munir SM. Hepatic vein waveform in liver cirrhosis: Correlation with child's class and size of varices. J Pak Med Assoc. 2012;62:794–7. [PubMed] [Google Scholar]
- 24.Omuemu CE, Ndububa DA, Nnabuchi CV. Prevalence of hepatitis B surface antigen and anti-hepatitis C antibodies in chronic liver disease patients in a Nigerian Teaching Hospital. J Med Res Pract. 2012;1:58–60. [Google Scholar]
- 25.Musa BM, Bussell S, Borodo MM, Samaila AA, Femi OL. Prevalence of hepatitis B virus infection in Nigeria, 2000-2013: A systematic review and meta-analysis. Niger J Clin Pract. 2015;18:163–72. doi: 10.4103/1119-3077.151035. [DOI] [PubMed] [Google Scholar]
- 26.Joseph T, Madhavan M, Devadas K, Ramakrishnannair VK. Doppler assessment of hepatic venous waves for predicting large varices in cirrhotic patients. Saudi J Gastroenterol. 2011;17:36–9. doi: 10.4103/1319-3767.74465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antil N, Sureka B, Mittal MK, Malik A, Gupta B, Thukral BB. Hepatic venous waveform, splenoportal and damping index in liver cirrhosis: Correlation with child pugh's score and oesophageal varices. J Clin Diagn Res. 2016;10:C01–5. doi: 10.7860/JCDR/2016/15706.7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdallah TM, El Shelfa W, El-Sayed SB, Dawoud H. Hemodynamic changes of hepatic veins as predictors of large oesophageal varices in liver cirrhotic patients. Afro Egypt J Infect Endem Dis. 2014;4:32–40. [Google Scholar]
- 29.Ohta M, Hashizume M, Kawanaka H, Akazawa K, Tomikawa M, Higashi H, et al. Prognostic significance of hepatic vein waveform by Doppler ultrasonography in cirrhotic patients with portal hypertension. Am J Gastroenterol. 1995;90:1853–7. [PubMed] [Google Scholar]
- 30.Sudhamshu KC, Matsutani S, Maruyama H, Akiike T, Saisho H. Doppler study of hepatic vein in cirrhotic patients: Correlation with liver dysfunction and hepatic hemodynamics. World J Gastroenterol. 2006;12:5853–8. doi: 10.3748/wjg.v12.i36.5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwao T, Toyonaga A, Oho K, Tayama C, Masumoto H, Sakai T, et al. Value of Doppler ultrasound parameters of portal vein and hepatic artery in the diagnosis of cirrhosis and portal hypertension. Am J Gastroenterol. 1997;92:1012–7. [PubMed] [Google Scholar]
- 32.Annet L, Materne R, Danse E, Jamart J, Horsmans Y, Van Beers BE. Hepatic flow parameters measured with MR imaging and Doppler US: Correlations with degree of cirrhosis and portal hypertension. Radiology. 2003;229:409–14. doi: 10.1148/radiol.2292021128. [DOI] [PubMed] [Google Scholar]
- 33.Zironi G, Gaiani S, Fenyves D, Rigamonti A, Bolondi L, Barbara L. Value of measurement of mean portal flow velocity by Doppler flowmetry in the diagnosis of portal hypertension. J Hepatol. 1992;16:298–303. doi: 10.1016/s0168-8278(05)80660-9. [DOI] [PubMed] [Google Scholar]
- 34.Kutlu R, Karaman I, Akbulut A, Baysal T, Sigirci A, Alkan A, et al. Quantitative Doppler evaluation of the splenoportal venous system in various stages of cirrhosis: Differences between right and left portal veins. J Clin Ultrasound. 2002;30:537–43. doi: 10.1002/jcu.10114. [DOI] [PubMed] [Google Scholar]
- 35.Kok T, van der Jagt EJ, Haagsma EB, Bijleveld CM, Jansen PL, Boeve WJ. The value of Doppler ultrasound in cirrhosis and portal hypertension. Scand J Gastroenterol Suppl. 1999;230:82–8. doi: 10.1080/003655299750025598. [DOI] [PubMed] [Google Scholar]


