Abstract
Background
Renal involvement can complicate the course of inflammatory bowel disease (IBD). In this study, we aimed to analyze the extent of renal manifestations in patients with IBD (Crohn disease or ulcerative colitis) during the biologic era.
Material/Methods
Patients diagnosed with and followed up for IBD for a period covering 16 years were retrospectively analyzed. Patients who received IBD diagnosis with clinical, endoscopic, and histopathological findings and were older than 18 years were enrolled in the study. Demographic, clinical, laboratory, and treatment data were retrieved from the patients’ medical records.
Results
Of the 1874 patients analyzed, the diagnosis was ulcerative colitis in 1055 patients and Crohn disease in the remaining 819. Renal manifestations were found in 105 patients (5.6%), 55 (6.7%) of whom were diagnosed with Crohn disease and 50 (4.7%) with ulcerative colitis. Renal calculi was the most common renal manifestation for both Crohn disease and ulcerative colitis. Renal manifestations were related to disease activity and surgical resection history in patients with Crohn disease, whereas no such relationship was found in patients with ulcerative colitis.
Conclusions
Renal manifestations may be seen in up to 6% of patients with IBD, and patients with Crohn disease seems to have more risk than do patients with ulcerative colitis. Nephrolithiasis is the most common form of renal involvement in IBD and is closely associated with disease activity. This relationship between IBD and renal manifestations should be considered, especially when there are subtle renal symptoms.
Keywords: Colitis, Ulcerative; Crohn Disease; Inflammatory Bowel Diseases; Nephrolithiasis
Background
Inflammatory bowel disease (IBD) is an umbrella term that includes Crohn disease (CD) and ulcerative colitis (UC). It describes a group of disorders characterized by chronic inflammation of the gastrointestinal tract [1]. The exact underlying mechanism of IBD is not fully elucidated. An uncontrolled immune response, mostly mediated by aberrant T-cell function as a result of genetic and environmental factors, is thought to be important in the etiopathogenesis of the disease [2,3]. The prevalence of IBD is higher in industrialized countries (northern Europe and North America) than in non-industrialized countries. Overall, the global incidence of IBD is increasing and is especially driven by the increasing number of patients in newly industrialized countries [4]. Despite the increasing patient load, the introduction of biologics in therapy has revolutionized the management and outcomes of IBDs, compared to that in the pre-biologic era.
Extraintestinal manifestations are seen in 6% to 47% of patients diagnosed with IBD [5,6]. The skin, eye, joints, liver, and biliary tree are among the most common involvement sites and have been the subjects of detailed studies. Renal and urinary involvement have been also reported in 4% to 23% of the patients diagnosed with IBD in pre-biologic-era cohorts [7]. These patients most commonly present with nephrolithiasis [8,9], tubulointerstitial nephritis [10,11], glomerulonephritis [12–14], and secondary (AA) amyloidosis [15–17]. IBD usually precedes extraintestinal manifestations, and intestinal disease activity can influence the development and severity of extraintestinal manifestations. In addition to the contribution of a susceptible genetic background and effects of uncontrolled inflammation, agents used in the treatment of IBD can be related to renal complications [18,19]. The increasing number of patients and novel therapies (biologics) introduced in clinical practice have the potential to influence the burden of IBD-related renal manifestations.
In this study, our aim was to analyze the extent of renal manifestations, together with epidemiological, demographic, clinical, laboratory, and histopathological features, in patients followed up with a diagnosis of IBD in the biologic era in a high-volume center.
Material and Methods
Ethical Approval
The study protocol was approved by the Clinical Research Ethics Committee of Istanbul University Cerrahpasa (approval no. 313700) and conducted in accordance with the 1975 Declaration of Helsinki, as revised in 2013. Patient consent to participate was not required owing to the retrospective nature of the research.
Setting
The study was conducted in a tertiary care university hospital, and the medical records of the patients followed by the specialized IBD outpatient clinic for a period of 16 years were reviewed retrospectively by the same researcher.
Study Design
Adult patients (18 years of age and older) who were diagnosed with IBD clinically, endoscopically, and histopathologically were included in the study. Patients considered as having probable IBD and/or those who had a history of renal dysfunction or renal disorders before IBD diagnosis were excluded. Demographic data, comorbidities, laboratory, endoscopy and biopsy results, the duration and activity of the disease, medications used for IBD treatment, occurrence of renal involvement, and related factors were recorded. A control group was formed out of patients without renal manifestations who had the next protocol number to the patients with renal manifestations.
Data Collection and Definitions
In terms of demographic and clinical data, age, sex, educational status, tobacco and alcohol use, presence of comorbidities, duration of IBD, medications used for IBD, and history of IBD-related surgical interventions were recorded, in addition to the laboratory data, including glucose, urea, creatinine, uric acid, estimated glomerular filtration rate (e-GFR), albumin, ALT, AST, erythrocyte sedimentation rate, C-reactive protein (CRP), hemoglobin, white blood cells, and urinalysis.
The e-GFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration formula [20]. Albuminuria and proteinuria were measured with 24-h urine samples. The results of histopathological examination in patients who underwent renal biopsy or rectal biopsy were recorded. Data on kidney size, parenchyma echogenicity, presence of nephrolithiasis, and dilatation of the collecting system were also recorded using renal ultrasonography and/or abdominal computerized tomography examinations.
Microscopic hematuria (defined as 5 or more erythrocytes per high-power field in urine sediment microscopy), proteinuria (defined as 24-h urinary protein excretion over 300 mg/day), acute kidney injury (AKI, according to KDIGO criteria) [21], chronic kidney disease (CKD, defined as e-GFR <60 mL/min/1.73 m2 over 3 months), and nephrolithiasis were investigated as manifestations of renal involvement in the patients after the diagnosis of IBD.
Gastrointestinal involvement sites were determined using endoscopic findings, and patients were grouped according to the Montreal classification [22]. Clinical symptoms, laboratory markers, and recent endoscopic findings were considered when determining disease activity. Active disease reported by the endoscopist by evaluating parameters such as ulcer, erosion, and inflamed stenosis was accepted as endoscopic active disease. Endoscopic remission was considered as the conditions reported by the endoscopist as endoscopic remission. Among disease activity indices, the Crohn’s disease activity index was used for CD, and patients with scores lower than 150 were considered to be in remission, and those with scores equal to or higher than 150 to have active disease [23]. The UC activity index, defined by Seo et al [24], was used for assessing disease severity in UC; patients with scores lower than 120 were considered to be in remission and those with scores equal to higher than 120 to have active disease [25].
Statistical Analysis
Continuous variables were expressed as mean and standard deviation (SD). For comparison of the categorical variables between the groups, Pearson’s chi-square test and Fisher’s exact test were used. The normality of the quantitative variables was calculated with the Shapiro-Wilk test. Normally distributed variables were compared using the independent samples t test, while the comparison of the non-normally distributed variables was made with the Mann-Whitney U test. Statistical tests were performed using SPSS for Windows version 22.0 software (IBM Corp, Armonk, NY, USA). A P value <0.05 was considered statistically significant.
Results
Baseline Characteristics of the Study Population
Of the 1874 patients analyzed, the diagnosis was UC in 1055 patients and CD in the remaining 819. Renal manifestations were found in 105 patients, 55 (52.4%) of whom were diagnosed with CD and 50 (47.6%) with UC. In the control group, 45 (42.9%) patients had a diagnosis of CD and 60 (57.1%) a diagnosis of UC. Demographic characteristics and comorbidities of the patients with renal involvement and the control group are shown in Table 1. There were no significant differences in both groups in terms of sex. The presence of diabetes mellitus, hypertension, and analgesic use was higher in patients with UC with renal involvement compared with that of the control group. However, in patients with CD, there was no significant difference between the renal involvement and control groups in the trends of covariates (Table 1).
Table 1.
Demographic characteristics and comorbidities of inflammatory bowel disease patients with renal involvement and controls.
| Crohn’s disease | Ulcerative colitis | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Patients with renal involvement (n=55) | Control group (n=45) | p | Patients with renal involvement (n=50) | Control group (n=60) | p | |
| Age in years (mean±SD) | 45.7±12.6 | 42.1±12.1 | 0.116 | 51.5±15.0 | 46.9±12.6 | 0.084 |
|
| ||||||
| Male gender, (%) | 58.2 | 60.0 | 0.854 | 60.0 | 46.7 | 0.163 |
|
| ||||||
| Education status, (%) | ||||||
| Primary school | 38.5 | 22.7 | 30.4 | 43.1 | ||
| Secondary school | 11.5 | 13.6 | 0.340 | 13.0 | 17.2 | 0.378 |
| High school | 30.8 | 45.5 | 32.6 | 20.7 | ||
| University or higher | 19.2 | 18.2 | 23.9 | 19.0 | ||
|
| ||||||
| Tobacco use, (%) | 58.2 | 73.3 | 0.114 | 46.0 | 40.0 | 0.526 |
|
| ||||||
| Alcohol use, (%) | 10.9 | 8.9 | 0.738 | 12.0 | 13.3 | 0.835 |
|
| ||||||
| Analgesics use*, (%) | 12.7 | 8.9 | 0.542 | 20.0 | 6.7 | 0.037 |
|
| ||||||
| Comorbid diseases, (%) | ||||||
| Diabetes mellitus | 1.8 | 2.2 | 0.700 | 14.0 | 3.3 | 0.042 |
| Hypertension | 7.3 | 4.4 | 0.554 | 28.0 | 6.7 | 0.003 |
| Hyperlipidemia | 16.3 | 22.2 | 0.457 | 36.0 | 31.7 | 0.631 |
| Hyperuricemia | 5.5 | 2.2 | 0.411 | 6.0 | 1.7 | 0.226 |
Calculated for NSAID use. Significant p values are written in bold.
The most common presenting symptoms in IBD patients with renal involvement were diarrhea, bloody stools, abdominal pain, tenesmus, and nocturnal diarrhea (Table 2). As treatment, local and systemic steroids and immunomodulating agents (azathioprine and methotrexate) were primarily used, in addition to 5-ASA compounds and anti-TNF agents (Table 2).
Table 2.
The most common presenting symptoms and treatment characteristics of the patients.
| Crohn’s disease | Ulcerative colitis | |||||
|---|---|---|---|---|---|---|
| Patients with renal involvement (n=55) | Control group (n=45) | p | Patients with renal involvement (n=50) | Control group (n=60) | p | |
| Symptoms | ||||||
| Diarrhea ,% | 80.0 | 91.1 | 0.101 | 78.0 | 76.7 | 0.526 |
| Nocturnal diarrhea, % | 16.4 | 26.7 | 0.156 | 32.0 | 30.0 | 0.492 |
| Rectal bleeding, % | 27.3 | 20.0 | 0.710 | 92.0 | 85.0 | 0.257 |
| Abdominal pain, % | 72.7 | 66.7 | 0.330 | 46.0 | 56.7 | 0.779 |
| Tenesmus, % | 20.0 | 31.1 | 0.148 | 40.0 | 26.7 | 0.100 |
| Fever, % | 12.7 | 2.2 | 0.056 | 10.0 | 5.0 | 0.262 |
| Treatments | ||||||
| 5-ASA derivatives, % | 89.1 | 75.6 | 0.073 | 98.0 | 100.0 | 0.455 |
| Systemic steroids, % | 67.3 | 53.3 | 0.155 | 52.0 | 45.0 | 0.464 |
| Budesonide, % | 27.3 | 24.4 | 0.748 | NA | NA | NA |
| Azathioprine/MTX, % | 92.7 | 82.2 | 0.108 | 36.0 | 28.3 | 0.390 |
| Anti-TNF, % | 49.1 | 20.0 | 0.002 | 12.0 | 5.0 | 0.182 |
| Infliximab, % | 41.8 | 8.9 | <0.001 | 8.0 | 5.0 | 0.521 |
| Adalimumab, % | 7.3 | 11.1 | 0.504 | 4.0 | 0.0 | 0.204 |
5-ASA – 5-aminosalicylic acid; MTX – methotrexate; NA – not applicable; TNF – tumor necrosis factor. Significant p values are written in bold.
Renal Manifestations in Patients with IBD
The renal manifestations in patients with CD and UC are shown in Table 3. There was a trend toward more renal manifestations in patients with CD than with UC in the entire cohort (P=0.06). Microscopic hematuria, which was nearly 70% in both groups, was the most common urinary finding, and renal calculi was the most frequent renal manifestation in both groups (81.8% and 90.0% for CD and UC patients, respectively). A diagnosis of AA amyloidosis was made in 5 patients with CD after rectal biopsy and in 1 patient with kidney biopsy. Two of them had active perianal disease and the rest had poorly controlled inflammation, with high CRP levels. Three patients with UC who had undergone renal biopsy due to proteinuria and hematuria were diagnosed with IgA nephropathy. One patient had undergone renal biopsy after developing progressive renal dysfunction, and the histopathological examination revealed sulfasalazine-associated acute tubulointerstitial nephritis (Figure 1). The frequency and type of renal manifestations were not different between the CD and UC groups; however, amyloidosis was more common in patients with CD than in UC patients with renal manifestations. There was no significant difference between the groups in terms of renal manifestations seen in IBD patients who received and did not receive biologic treatment (P=0.605, P=0.860, respectively).
Table 3.
Renal manifestations in patients with Crohn disease and ulcerative colitis.
| Crohn’s disease (n=55) | Ulcerative colitis (n=50) | p | |
|---|---|---|---|
| Urinary findings, n (%) | |||
| Microscopic hematuria | 38 (69.1) | 37 (74.0) | 0.667 |
| Macroscopic hematuria | 10 (18.2) | 10 (20.0) | 0.812 |
| Proteinuria | 11 (20.0) | 4 (8.0) | 0.098 |
| Pathological findings, n (%) | |||
| Renal calculi | 45 (81.8) | 45 (90.0) | 0.273 |
| Secondary (AA) amyloidosis | 6 (10.9) | 0 (0.0) | 0.027 |
| Glomerulonephritis | 0 (0.0) | 3 (6.0) | 0.100 |
| Acute interstitial nephritis | 0 (0.0) | 1 (2.0) | 0.470 |
| Clinical entities, n (%) | |||
| Acute kidney injury | 4 (7.3) | 3 (6.0) | 0.794 |
| Chronic kidney disease | 4 (7.3) | 1 (2.0) | 0.365 |
Significant p values are written in bold.
Figure 1.
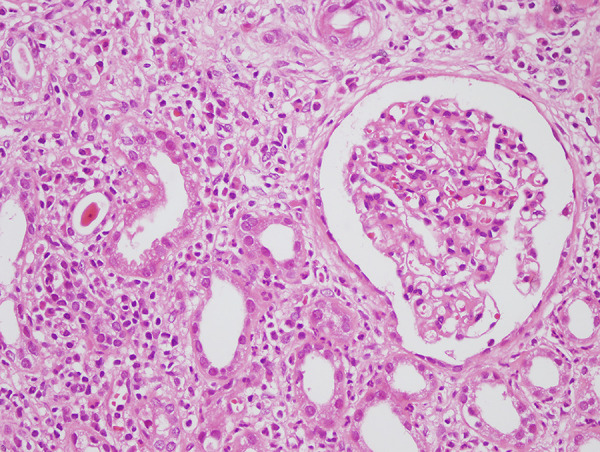
Acute interstitial nephritis (hematoxylin and eosin, magnification ×400).
Patients with CD and renal involvement had increased CRP levels and erythrocyte sedimentation rates and lower e-GFR and serum albumin levels compared to those without renal involvement (Table 4). There was no significant difference between the 2 CD groups regarding the site of involvement or disease behavior. In the CD group with renal involvement, the number of patients under anti-TNF treatment, patients with any history of IBD-related surgery, or patients with active disease were higher and the follow-up period was longer than in the control group.
Table 4.
Clinical, laboratory, and treatment data of Crohn disease patients with or without renal involvement.
| Crohn’s disease | Patients with renal involvement (n=55) | Control group (n=45) | p |
|---|---|---|---|
| Age in years at diagnosis (mean±SD) | 35.0±11.9 | 35.0±12.1 | 0.970 |
|
| |||
| Follow-up period in years (mean±SD) | 10.7±7.9 | 6.9±4.6 | 0.021 |
|
| |||
| Disease location, n (%) | |||
|
| |||
| Ileal | 10 (18.2) | 11 (24.4) | 0.253 |
|
| |||
| Colonic | 4 (7.3) | 5 (11.1) | |
|
| |||
| Ileocolonic | 41 (74.5) | 27 (60.0) | |
|
| |||
| Isolated upper gastrointestinal disease | 0 (0.0) | 2 (4.4) | |
|
| |||
| Perianal disease | 16 (29.1) | 9 (20.0) | |
|
| |||
| Disease behavior, n (%) | |||
|
| |||
| Non-stricturing/non-penetrating | 16 (29.1) | 22 (48.9) | 0.128 |
|
| |||
| Stricturing | 17 (30.9) | 10 (22.2) | |
|
| |||
| Penetrating | 22 (40.0) | 13 (28.9) | |
|
| |||
| Disease activity and treatment, n (%) | |||
|
| |||
| Active disease | |||
| Endoscopic active | 27 (49.1) | 12 (26.7) | 0.022 |
| Clinical active (CDAI ≥150) | 31 (56.4) | 18 (40.0) | 0.103 |
|
| |||
| Anti-TNF use | 27 (49.1) | 9 (20.0) | 0.003 |
|
| |||
| IBD related surgery | 32 (58.2) | 16 (35.6) | 0.024 |
|
| |||
| Laboratory parameters | |||
|
| |||
| Creatinine (mg/dL) | 0.9±0.4 | 0.8±0.2 | 0.028 |
|
| |||
| e-GFR (mL/min/1.73 m2) | 81.7±26.7 | 95.4±19.9 | 0.005 |
|
| |||
| Uric acid (mg/dL) | 4.6±1.4 | 4.2±1.4 | 0.310 |
|
| |||
| Albumin (g/dL) | 3.8±0.7 | 4.1±0.5 | 0.031 |
|
| |||
| Hemoglobin (g/dL) | 13.0±4.6 | 13.2±1.8 | 0.063 |
|
| |||
| ESR (mm/h) | 32.1±24.1 | 22.4±20.1 | 0.008 |
|
| |||
| CRP (mg/L) | 16.5±20.6 | 7.9±13.6 | <0.001 |
CDAI – Crohn’s Disease Activity Index; CRP – C-reactive protein; ESR – erythrocyte sedimentation rate; e-GFR – estimated glomerular filtration rate; TNF – tumor necrosis factor. Significant p values are written in bold.
Patients with UC and renal involvement were also found to have lower e-GFR and serum albumin levels compared to those without renal involvement. There was no significant difference between the 2 UC groups regarding CRP levels, erythrocyte sedimentation rates, site of involvement, disease activity, the use of anti-TNF treatment, and any history of IBD-related surgery (Table 5).
Table 5.
Clinical, laboratory, and treatment data of ulcerative colitis patients with or without renal involvement.
| Ulcerative colitis | Patients with renal involvement (n=50) | Control group (n=60) | p |
|---|---|---|---|
| Age in years at diagnosis (mean±SD) | 41.8±15.1 | 37.9±12.2 | 0.231 |
|
| |||
| Follow-up period in years (mean±SD) | 9.7±6.0 | 8.9±6.0 | 0.425 |
|
| |||
| Disease extent, n (%) | |||
|
| |||
| Proctitis | 11 (22.0) | 10 (16.7) | 0.706 |
|
| |||
| Left-sided colitis | 19 (38.0) | 22 (36.7) | |
|
| |||
| Extensive colitis | 20 (40.0) | 28 (46.6) | |
|
| |||
| Disease activity and treatment, n (%) | |||
|
| |||
| Active disease | |||
| Endoscopic active | 12 (24.0) | 13 (21.7) | 0.771 |
| Clinical active (UCAI ≥120) | 15 (30.0) | 12 (20.0) | 0.225 |
|
| |||
| Anti-TNF use | 6 (12.0) | 3 (5.0) | 0.182 |
|
| |||
| IBD related surgery | 3 (6.0) | 3 (5.0) | 0.579 |
|
| |||
| Laboratory parameters, (mean±SD) | |||
|
| |||
| Creatinine (mg/dL) | 0.9±0.4 | 0.8±0.2 | 0.016 |
|
| |||
| e-GFR (mL/min/1.73 m2) | 79.0±25.4 | 93.8±21.4 | 0.001 |
|
| |||
| Uric acid (mg/dL) | 4.6±1.5 | 3.9±1.5 | 0.068 |
|
| |||
| Albumin (g/dL) | 4.1±0.4 | 4.3±0.6 | 0.006 |
|
| |||
| Hemoglobin (g/dL) | 13.1±1.4 | 13.0±1.7 | 0.904 |
|
| |||
| ESR (mm/h) | 23.1±20.6 | 20.9±19.6 | 0.757 |
|
| |||
| CRP (mg/L) | 6.5±10.3 | 7.0±12.7 | 0.550 |
CRP – C-reactive protein; ESR – erythrocyte sedimentation rate; e-GFR – estimated glomerular filtration rate; TNF – tumor necrosis factor; UCAI – ulcerative colitis activity index. Significant p values are written in bold.
Discussion
In this study, we evaluated the extent of renal manifestations in patients with IBD. To the best of our knowledge, our study included the highest number of patients within a 16-year cohort during the biological era in IBD. According to previous studies, renal and urinary tract involvement occurs in 4% to 23% of patients with IBD, with renal calculi being the most common form of renal involvement [7,26]. In our study, renal involvement was found in 5.6% of the patients with IBD, a similar result to those obtained in previous studies. We observed that renal involvement was higher in patients with CD (6.7%) than in patients with UC (4.7%), although the difference did not reach significance. Surgical interventions, inflammatory burden, and perianal disease may have an effect in the tendency for more frequent renal manifestation in CD.
There are several studies showing an increased risk of nephrolithiasis among patients with IBD compared with the general population [27,28]. Diarrhea and malabsorption-related low urine volume and pH and increased intestinal oxalate absorption-related hyperoxaluria are the main mechanisms resulting in uric acid and calcium oxalate stones, which are 2 of the predominantly seen stone formations in patients with IBD. IBD-related surgery (total colectomy, intestinal bypass, and small bowel resection), active disease and extent of gastrointestinal tract involvement (especially ileocolonic involvement) are among the main risks for urinary stone formation in this patient group. The prevalence of nephrolithiasis is reported to be higher in adults than in children and in CD compared to UC probably due to disease duration and extent of involvement [7]. Kim et al examined 387 patients with CD, and urinary tract calculi were found in 18 (4.7%) patients, slightly lower than what we reported [29]. In another study, Cury et al showed that nephrolithiasis was present in 36 patients with CD and in 28 patients with UC (38% for both) [8]. In our study, 45 of 1055 (4.3%) patients with UC and 45 of 819 (5.5%) patients with CD were found to have nephrolithiasis. The variance in the prevalence among studies in the literature may be explained by the difference of studied patient groups (including surgical history, ileal involvement, and disease age) and regional diversities in genetic and environmental factors that influence lithogenesis. Additionally, renal colic can be hard to distinguish from abdominal pain, which is a common condition in CD, leading to delays in diagnosis [28].
Our findings demonstrated an association between disease activity in CD and renal manifestations. The CRP level, which is an established valuable laboratory test for determining disease activity in CD, was significantly elevated in CD patients with renal manifestations compared to in the control group. Anti-TNF use was significantly higher in the renal manifestations group, which might be explained by the higher disease activity and burden in these patients. This is consistent with previous studies pointing to a strong association between disease activity in CD and nephrolithiasis [8,30,29] as the most common renal manifestation in IBD. However, renal manifestations observed were not different when patients receiving and not receiving anti-TNF therapy were compared. The fact that anti-TNFs are generally used in IBD patients with uncontrolled inflammation who are at higher risk of renal manifestations, anti-TNFs have the ability to suppress inflammation and reduce renal manifestation risk in some of these patients, and anti-TNFS themselves have potential renal adverse effects may all be intertwined factors. Consequently, the retrospective design of the present study was not strong enough to separate these causes. Also, as expected, and parallel to the literature, ileocolonic involvement was the dominant involvement site for CD patients with renal involvement. We could not demonstrate any difference in levels of inflammatory markers in UC patients with and without renal manifestations. This may be explained by the relatively lower strength of these markers to show disease activity in UC because of the mainly mucosal (and rarely sub-mucosal) inflammation compared to transmural inflammation in CD [31,32]. Many studies have indicated that intestinal resection or extensive intestinal disease is associated with an increased risk of nephrolithiasis [33]. In our study, the incidence of surgical intervention was statistically significantly higher in CD patients with nephrolithiasis than in the control group, whereas no such difference was demonstrated in patients with UC. In our study, although 73.3% of CD patients with nephrolithiasis had ileocolonic involvement, no significant difference was found in comparison with the control group. This situation can be explained by the fact that the most common involvement in CD is ileocolonic.
AA amyloidosis is a rare complication of IBD, with renal amyloidosis known to be the most common lethal manifestation of IBD-associated amyloidosis [19]. Secondary amyloidosis tends to develop comparatively late in the natural history of IBD as a result of prolonged uncontrolled inflammation. While it may be present at the time of diagnosis in some patients with a long delay in diagnosis, it is often only apparent many years after the initial diagnosis of IBD. Previous studies showed that there was a significant correlation between the activity of IBD and the development of amyloidosis, and that the prevalence of amyloidosis in patients with CD was significantly higher than in those with UC [16,26]. Renal amyloidosis can manifest as nephrotic syndrome and/or renal insufficiency. In a study by Greenstein et al [15], amyloidosis was present in 15 out of 1709 (0.9%) patients with CD and in 1 out of 1341 (0.07%) patients with UC. Approximately two-thirds of the patients with amyloidosis had fistulae or abscesses. Tosca Cuquerella et al reported that amyloidosis was encountered in 4 out of 1201 (0.3%) patients with IBD, all of which had CD [16]. In a study by Sattianayagam et al [34], of the 26 IBD patients with amyloidosis, 22 had CD and 4 had UC. Of these patients diagnosed with CD, 15 had a history of intestinal resection and 10 had fistulas or abscesses. A higher incidence of IBD-associated amyloidosis in male patients was noted in these studies.
In our study, 6 (10.9%) patients with CD were diagnosed with AA amyloidosis. On the other hand, amyloidosis was not diagnosed in any patients with UC. Most patients with CD with renal amyloidosis were women, unlike the patients in the studies previously discussed. Other features of these patients with amyloidosis in the present study, such as the prevalence of ileocolonic involvement, fistulizing behavior, and high disease activity, were compatible with the literature. The high number of patients diagnosed with amyloidosis in our cohort may be related to the fact that our unit is a tertiary referral center where more resistant cases are followed and to the long follow-up period (10 years) in these patients with uncontrolled inflammation.
Glomerulonephritis is a rare complication of IBD. Different types of glomerulonephritis in IBD, including IgA nephropathy, crescentic glomerulonephritis, focal segmental glomerulosclerosis, membranous glomerulonephritis, minimal change disease, membranoproliferative glomerulonephritis, and mesangiocapillary glomerulonephritis, have been described [12,13,35]. In a histopathological study by Ambruzs et al, kidney biopsy results of 83 patients with IBD (45 CD, 38 UC) were reviewed, and IgA nephropathy was found to be the most common diagnosis (24%) [13]. This coexistence of IgA nephropathy and IBD may be due to changes in antigenic tolerance and dysregulation in antibody production in the background of chronic mucosal inflammation. In addition, some of the genes that play a role in IgA nephropathy are directly related to IBD susceptibility, while others are either related to the integrity of the epithelial barrier or regulation of its response to mucosal pathogens [36]. Previous studies showed that there is a correlation of glomerulonephritis with intestinal disease activity and recovery of kidney function has been reported after remission of IBD. It is thought that the decrease in intestinal inflammation leads to a decrease in chronic antigenic stimulation and an improvement in the course of glomerulonephritis [19]. In our study, 3 patients (6%) with UC had a diagnosis of glomerulonephritis, which were all histopathologically diagnosed with IgA nephropathy. These 3 patients underwent kidney biopsy due to persistent microscopic hematuria and accompanying non-nephrotic proteinuria. They all had active disease at the time of kidney biopsy.
Recent studies have shown that IBD activity is associated with tubulo-interstitial damage and that there is a positive correlation between tubular proteinuria and active intestinal disease [11,37]. Thus, tubulointerstitial nephritis was accepted as an extraintestinal manifestation of IBD. Other causes of tubulointerstitial nephritis are also common in patients with IBD, such as hyperoxaluria, renal amyloidosis, and hypokalemia due to chronic diarrhea [38]. In addition, renal dysfunction can develop due to the medical treatment of IBD. There are several reports of tubulointerstitial nephritis associated with 5-ASA treatment in IBD patients. In the study by Arend and Springate [18], in which 5-ASA-related cases of tubulointerstitial nephritis were reviewed, the male sex was predominant and there was an absence of specific findings on urinalysis. Despite positive responses to steroid therapy, the authors concluded that the risk of chronic and progressive renal injury was high [18]. In our study, a male patient with UC was diagnosed with 5-ASA-related tubulointerstitial nephritis. A kidney biopsy was performed because of progressive renal failure. After the diagnosis, 5-ASA was discontinued and oral prednisolone was started. After treatment, partial improvement in renal functions was observed.
Anti-TNF drugs are actively used in the treatment of many inflammatory diseases, such as in IBD. Although there are studies in the literature showing that anti-TNF agents do not have negative effects on kidney functions, few reports have reported that anti-TNF agents cause autoantibody formation and cause de novo glomerulonephritis [39]. As we discussed earlier, patients who generally require biologic therapy are resistant to conventional therapies, with poor control of inflammation. For this reason, as in our study, renal manifestations are not rarer with these treatments, contrary to what might be thought in the first place. On the other hand, although deep remission cannot be achieved in some patients, it may be possible to partially control the inflammation and reduce renal complications such as amyloidosis caused by uncontrolled inflammation to a certain extent with biological treatments.
While the form of renal involvement in IBD varies, any of these conditions can present as AKI or CKD. There are few studies that examine the association between IBD and renal failure. In a study by Lewis et al conducted on 251 patients with IBD (166 CD, 85 UC), 40 patients (15.9%) had renal failure (14 AKI, 26 CKD) [40]. It must be noted that the study was conducted in an inpatient setting in a tertiary healthcare center and may constitute patients with a more severe disease course. In a retrospective cohort study by Vajravelu et al, with a series of 17 807 patients with IBD, an increased risk of CKD, especially among younger patients was detected [41]. In our study, CKD developed in 5 patients with IBD (4 CD, 1 UC).
Some of the renal manifestations associated with IBD, such as kidney stones, can also be seen frequently in the general population. Again, these renal manifestations are frequently associated with diseases, such as hypertension and diabetes, which both have a higher prevalence than IBD. Therefore, these diseases can also contribute to the emergence of renal manifestations when co-occurring with IBD. The patient population and methodological characteristics of the study did not allow us to distinguish the effect of these confounding diseases.
A major strength of our study was that it was performed in a high-volume tertiary referral center that evaluated over 1800 patients in a 16-year period. We think that our study contributes to the literature by reporting the renal manifestations seen in IBD during the biological era. The limitations of this study were its retrospective design and that it was performed in a referral university hospital. It is possible that we might have recruited patients with more severe IBD. We might have overlooked some of the patients with AKI, as they were seen in the outpatient clinic at longer time intervals than the times used in the AKI definition. Additionally, there were some patients with mild proteinuria in whom renal biopsies were not indicated. This might have underestimated the rates of glomerular diseases and tubulo-interstitial nephritis. The shorter follow-up duration of the CD control group may have masked and underestimated the development of renal manifestations; this should also be stressed as a study limitation.
Conclusions
Renal manifestations may be seen in up to 6% of patients with IBD. Patients with CD seem to have more risk of renal manifestations than do patients with UC. Nephrolithiasis is the most common form of renal involvement in IBD and is closely associated with disease activity. This relationship between IBD and renal manifestations should be considered, especially when there are subtle renal symptoms. Such patients must be treated by a multidisciplinary team to minimize the risk of potential complications.
Acknowledgments
The study was presented as a poster at the 54th European Renal Association–European Dialysis and Transplant Association (ERA-EDTA) congress, organized in Madrid, Spain, from 3 to 6 June 2017 (available at https://academic.oup.com/ndt/issue/32/suppl_3).
Footnotes
Conflict of interest: None declared
Institution and Department Where Work Was Conducted
The work was done at Istanbul University-Cerrahpasa, Cerrahpasa Medical Faculty, Department of Gastroenterology.
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors, who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: None declared
References
- 1.Carter MJ, Lobo AJ, Travis SP, et al. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004;53(Suppl 5):V1–16. doi: 10.1136/gut.2004.043372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loddo I, Romano C. Inflammatory bowel disease: Genetics, epigenetics, and pathogenesis. Front Immunol. 2015;6:551. doi: 10.3389/fimmu.2015.00551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babickova J, Gardlik R. Pathological and therapeutic interactions between bacteriophages, microbes and the host in inflammatory bowel disease. World J Gastroenterol. 2015;21(40):11321–30. doi: 10.3748/wjg.v21.i40.11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet. 2017;390(10114):2769–78. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein CN, Blanchard JF, Rawsthorne P, et al. The prevalence of extraintestinal diseases in inflammatory bowel disease: A population-based study. Am J Gastroenterol. 2001;96(4):1116–22. doi: 10.1111/j.1572-0241.2001.03756.x. [DOI] [PubMed] [Google Scholar]
- 6.Mendoza JL, Lana R, Taxonera C, et al. [Extraintestinal manifestations in inflammatory bowel disease: Differences between Crohn’s disease and ulcerative colitis]. Med Clin (Barc) 2005;125(8):297–300. doi: 10.1157/13078423. [DOI] [PubMed] [Google Scholar]
- 7.Pardi DS, Tremaine WJ, Sandborn WJ, et al. Renal and urologic complications of inflammatory bowel disease. Am J Gastroenterol. 1998;93(4):504–14. doi: 10.1111/j.1572-0241.1998.156_b.x. [DOI] [PubMed] [Google Scholar]
- 8.Cury DB, Moss AC, Schor N. Nephrolithiasis in patients with inflammatory bowel disease in the community. Int J Nephrol Renovasc Dis. 2013;6:139–42. doi: 10.2147/IJNRD.S45466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ganji-Arjenaki M, Nasri H, Rafieian-Kopaei M. Nephrolithiasis as a common urinary system manifestation of inflammatory bowel diseases; A clinical review and meta-analysis. J Nephropathol. 2017;6(3):264–69. doi: 10.15171/jnp.2017.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Izzedine H, Simon J, Piette AM, et al. Primary chronic interstitial nephritis in Crohn’s disease. Gastroenterology. 2002;123(5):1436–40. doi: 10.1053/gast.2002.36613. [DOI] [PubMed] [Google Scholar]
- 11.Tokuyama H, Wakino S, Konishi K, et al. Acute interstitial nephritis associated with ulcerative colitis. Clin Exp Nephrol. 2010;14(5):483–86. doi: 10.1007/s10157-010-0294-z. [DOI] [PubMed] [Google Scholar]
- 12.Onime A, Agaba EI, Sun Y, et al. Immunoglobulin A nephropathy complicating ulcerative colitis. Int Urol Nephrol. 2006;38(2):349–53. doi: 10.1007/s11255-006-0061-y. [DOI] [PubMed] [Google Scholar]
- 13.Vegh Z, Macsai E, Lakatos L, et al. The incidence of glomerulonephritis in a population-based inception cohort of patients with inflammatory bowel disease. Dig Liver Dis. 2017;49(6):718–19. doi: 10.1016/j.dld.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 14.Ambruzs JM, Walker PD, Larsen CP. The histopathologic spectrum of kidney biopsies in patients with inflammatory bowel disease. Clin J Am Soc Nephrol. 2014;9(2):265–70. doi: 10.2215/CJN.04660513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenstein AJ, Sachar DB, Panday AK, et al. Amyloidosis and inflammatory bowel disease. A 50-year experience with 25 patients Medicine (Baltimore) 1992;71(5):261–70. doi: 10.1097/00005792-199209000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Tosca Cuquerella J, Bosca-Watts MM, Anton Ausejo R, et al. Amyloidosis in inflammatory bowel disease: A systematic review of epidemiology, clinical features, and treatment. J Crohns Colitis. 2016;10(10):1245–53. doi: 10.1093/ecco-jcc/jjw080. [DOI] [PubMed] [Google Scholar]
- 17.Elaziz MMA, Fayed A. Patterns of renal involvement in a cohort of patients with inflammatory bowel disease in Egypt. Acta Gastroenterol Belg. 2018;81(3):381–85. [PubMed] [Google Scholar]
- 18.Arend LJ, Springate JE. Interstitial nephritis from mesalazine: Case report and literature review. Pediatr Nephrol. 2004;19(5):550–53. doi: 10.1007/s00467-004-1411-6. [DOI] [PubMed] [Google Scholar]
- 19.Oikonomou K, Kapsoritakis A, Eleftheriadis T, et al. Renal manifestations and complications of inflammatory bowel disease. Inflamm Bowel Dis. 2011;17(4):1034–45. doi: 10.1002/ibd.21468. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–84. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 22.Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: Controversies, consensus, and implications. Gut. 2006;55(6):749–53. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freeman HJ. Use of the Crohn’s disease activity index in clinical trials of biological agents. World J Gastroenterol. 2008;14(26):4127–30. doi: 10.3748/wjg.14.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seo M, Okada M, Yao T, et al. An index of disease activity in patients with ulcerative colitis. The Am J Gastroenterol. 1992;87(8):971–76. [PubMed] [Google Scholar]
- 25.Higgins PD, Schwartz M, Mapili J, et al. Patient defined dichotomous end points for remission and clinical improvement in ulcerative colitis. Gut. 2005;54(6):782–88. doi: 10.1136/gut.2004.056358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ambruzs JM, Larsen CP. Renal manifestations of inflammatory bowel disease. Rheum Dis Clin North Am. 2018;44(4):699–714. doi: 10.1016/j.rdc.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Parks JH, Worcester EM, O’Connor RC, et al. Urine stone risk factors in nephrolithiasis patients with and without bowel disease. Kidney Int. 2003;63(1):255–65. doi: 10.1046/j.1523-1755.2003.00725.x. [DOI] [PubMed] [Google Scholar]
- 28.Gaspar SR, Mendonca T, Oliveira P, et al. Urolithiasis and Crohn’s disease. Urol Ann. 2016;8(3):297–304. doi: 10.4103/0974-7796.184879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim MJ, Woo SY, Kim ER, et al. Incidence and risk factors for urolithiasis in patients with Crohn’s disease. Urol Int. 2015;95(3):314–19. doi: 10.1159/000375536. [DOI] [PubMed] [Google Scholar]
- 30.Mukewar S, Hall P, Lashner BA, et al. Risk factors for nephrolithiasis in patients with ileal pouches. J Crohns Colitis. 2013;7(1):70–78. doi: 10.1016/j.crohns.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Chang S, Malter L, Hudesman D. Disease monitoring in inflammatory bowel disease. World J Gastroenterol. 2015;21(40):11246–59. doi: 10.3748/wjg.v21.i40.11246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bakkaloglu OK, Eskazan T, Celik S, et al. Can we predict mucosal remission in ulcerative colitis more precisely with a redefined cutoff level of C-reactive protein? Colorectal Dis. 2022;24(1):77–84. doi: 10.1111/codi.15940. [DOI] [PubMed] [Google Scholar]
- 33.Gkentzis A, Kimuli M, Cartledge J, et al. Urolithiasis in inflammatory bowel disease and bariatric surgery. World J Nephrol. 2016;5(6):538–46. doi: 10.5527/wjn.v5.i6.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sattianayagam PT, Gillmore JD, Pinney JH, et al. Inflammatory bowel disease and systemic AA amyloidosis. Dig Dis Sci. 2013;58(6):1689–97. doi: 10.1007/s10620-012-2549-x. [DOI] [PubMed] [Google Scholar]
- 35.Shaer AJ, Stewart LR, Cheek DE, et al. IgA antiglomerular basement membrane nephritis associated with Crohn’s disease: A case report and review of glomerulonephritis in inflammatory bowel disease. Am J Kidney Dis. 2003;41(5):1097–109. doi: 10.1016/s0272-6386(03)00208-7. [DOI] [PubMed] [Google Scholar]
- 36.Kiryluk K, Li Y, Scolari F, et al. Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat Genet. 2014;46(11):1187–96. doi: 10.1038/ng.3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poulou AC, Goumas KE, Dandakis DC, et al. Microproteinuria in patients with inflammatory bowel disease: is it associated with the disease activity or the treatment with 5-aminosalicylic acid? World J Gastroenterol. 2006;12(5):739–46. doi: 10.3748/wjg.v12.i5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mutalib M. Renal involvement in paediatric inflammatory bowel disease. Pediatr Nephrol. 2021;36(2):279–85. doi: 10.1007/s00467-019-04413-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corica D, Romano C. Renal involvement in inflammatory bowel diseases. J Crohns Colitis. 2016;10(2):226–35. doi: 10.1093/ecco-jcc/jjv138. [DOI] [PubMed] [Google Scholar]
- 40.Lewis B, Mukewar S, Lopez R, et al. Frequency and risk factors of renal insufficiency in inflammatory bowel disease inpatients. Inflamm Bowel Dis. 2013;19(9):1846–51. doi: 10.1097/MIB.0b013e31828a661e. [DOI] [PubMed] [Google Scholar]
- 41.Vajravelu RK, Copelovitch L, Osterman MT, et al. Inflammatory bowel diseases are associated with an increased risk for chronic kidney disease, which decreases with age. Clin Gastroenterol Hepatol. 2020;18(10):2262–68. doi: 10.1016/j.cgh.2019.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]


