Abstract
Atherogenesis is a multifactorial pathologic process influenced by genetics and environmental factors such as diet, exercise, stress, and other exposures. Estrogen receptors (ER) are expressed in cells of the arterial wall, suggesting that estrogen receptor ligands (estradiol, natural and pharmacologic ligands) may directly affect arterial biology and atherogenesis. Ligand bound estrogen receptor alpha and beta (ERα, ERβ) can influence physiology through direct binding to estrogen response elements in the DNA, through interactions with other transcription factors such as NF-κB, or through rapid effects not dependent on gene expression changes but instead through non-nuclear membrane sites involving ERα, ERβ, or G-coupled protein ER (GPER1).
Elucidation of potential direct effects of estrogens on the artery wall requires careful evaluation of arterial biologic responses to estrogens. We have developed a comprehensive approach to understand the mechanisms of estrogen action which employs histologic measures of the size and other characteristics of atherosclerotic lesions, immunohistochemical assessments of cellular composition, evaluation of chemical, molecular, and genomic changes in the arterial environment, and determination of the relationships between arterial estrogen receptor expression and atherogenesis. This approach can provide important insights into the mechanisms of action of estrogen and other mediators of atherogenesis.
Keywords: Atherosclerosis, Inflammation, Vascular biology, Gene expression, Macrophage, T cell, Estrogen receptor alpha and beta
1. Introduction
The presence of immune cells, particularly macrophages and T cells, is a common characteristic of developing atherosclerotic lesions [1]. Activated macrophages produce inflammatory cytokines that mediate pro-inflammatory effects through both innate and adaptive immunity. CD4+ T cells account for the majority of activated T cells in atheroma [2]. CD8+ T cells are less common and their role in atherogenesis is less well understood. T cells influence adaptive immunity by producing pro- and/or anti-inflammatory cytokines. T helper-1 (Th-1) cells, major activated T cells in lesions, produce pro-atherogenic cytokines such as interferon-γ (IFN-γ), TNF-α, and IL-2, whereas T helper-2 (Th-2) cells secrete anti-inflammatory cytokines IL-4 and IL-10 [1]. During early T cell development, and under certain circumstances, Th-1 and Th-2 cells can switch their cytokine expression patterns [3].
Estrogen appears to influence atherogenesis and cardiovascular disease through multiple mechanisms, since it alters inflammation, vasoactive molecules, lipid concentrations, antioxidant, coagulation, and fibrinolytic systems [4]. Estrogen therapy may produce beneficial effects on plasma lipoprotein concentrations, but these alterations explain less than half of the atheroprotective effects of estrogen [5, 6], suggesting beneficial effects which are independent of plasma lipids and involving direct effects on arterial biology. Anti-inflammatory effects of estrogens include reducing circulating levels of monocyte chemoattractant protein-1 (MCP-1), and vascular cell adhesion molecule-1 (VCAM-1) [7, 8], likely in part through transcription factor cross-talk involving estrogen receptor (ER) antagonism of NF-kB activity [9, 10]. ERα and ERβ are both present within the arterial wall, and there is evidence for direct effects of estrogens on arteries and arterial cells [11–13]. ERs are also expressed by immune cell populations [14].
Observational studies in women and experimental studies in animals provide support that estrogen protects against coronary heart disease (CHD) and atherosclerosis [5, 13, 15–17], and there is evidence from ER knockout models that the presence of ERα is important for this effect [18]. Evidence that atheroprotective effects of estrogens might be attenuated when initiated in late menopause arose from our own work in the ovariectomized nonhuman primate model [19, 20], later supported by data from the Women’s Health Initiative (WHI) which demonstrated lack of a beneficial effect of estrogen plus progestogen therapy (EPT) overall and potential adverse effects on clinical coronary heart disease outcomes in the first year of treatment of older postmenopausal women [21, 22]. The interval between menopause and initiation of estrogen therapy is important; women assigned to hormone therapy (HT) within 10 years of menopause had a hazard ratio of 0.76 (95 % CI 0.50, 1.16) compared to 1.28 (95 % CI 1.03, 1.58) for women assigned to HT 20 years or later after menopause [23]. The “timing hypothesis” regarding cardioprotective benefits of ET emerged from these and other data. Our recent work suggests that this may relate to a reduced ability of estrogen to influence macrophage accumulation and behavior in more advanced atherosclerotic plaques [24]; however, the exact mechanism(s) underlying this remain unclear. Thus, there are important questions to be answered regarding the relationships between risks and benefits of estrogen therapy and the timing of the initiation of therapy.
This chapter was developed to provide guidance to investigators in the evaluation of atherosclerosis and the effects of hormones and other agents in randomized preclinical studies in animal models. This chapter contains four methodological sections: (1) tissue collection and preservation, (2) morphologic assessments of fixed tissues, (3) immunohistochemical assessment of fixed and frozen tissues, and (4) biochemical assessments of frozen tissues. These general approaches and newer molecular approaches have evolved over decades of research studies at Wake Forest School of Medicine (formerly named Bowman Gray School of Medicine) and is broadly useful in studies of the effects of diet and other interventions on atherogenesis and other processes influenced by diet and hormones [13, 24–32]. Observations in the nonhuman primate within our group [13, 24, 31, 32] and in human subjects by others [33–35] suggest that estrogen receptor alpha is important in the atheroprotective effects of estrogens. Ongoing research should help to reveal the exact mechanisms by which this occurs.
2. Materials
2.1. Plasma or Serum Lipids
Blood draw supplies, EDTA Vacutainer (Becton, Dickinson & Co., BD) for plasma preparation, or red top serum collection Vacutainer (BD).
Clinical centrifuge.
Clinical Chemistry Autoanalyzer with reagents and validated calibrators for appropriate species (ACE ALERA autoanalyzer, Alfa Wasserman Diagnostic Technologies).
Total Cholesterol Reagent (ACE cholesterol, Alfa Wasserman Diagnostic).
HDL Cholesterol Reagent (ACE HDL-C, Alfa Wasserman Diagnostic Technologies).
Triglyceride Reagent (ACE Triglycerides, Alfa Wasserman Diagnostic Technologies).
2.2. Tissue Preparation
Lactated ringers or phosphate buffered saline (0.1 M phosphate, pH 7.4, PBS) for perfusion of the cardiovascular system and isolated arterial segments.
Ice cold PBS (4 °C) for tissue hydration and protection.
Scalpels, fine instruments including scissors, tweezers, weigh boats, thin cardboard strips.
OCT (Optimized Cutting Temperature Compound, Tissue-Tek®).
10 % neutral buffered formalin (NBF).
4 % paraformaldehyde buffered with 0.1 M NaPO4, pH 7.4.
Cryoprotectant buffer: 15 % sucrose-PBS.
70 % alcohol for receiving tissue transfers from fixative.
Dry ice and liquid nitrogen.
Tissue cassettes and cryomolds (Tissue-Tek).
Parafilm and aluminum foil for wrapping cryopreserved tissue sections.
Ultralow freezer for preservation of OCT and frozen tissues.
2.3. Histology/Immunohistochemistry
Chemical safety hood.
Microtome and blades.
Slides, slide covers, Permount.
Water bath, pressure cooker, and/or steamer.
Graded alcohols.
Histomorphometric software such as ImageJ (NIH) or Image-Pro Plus software22 (Media Cybernetics, Inc).
2.4. Immunohistochemistry (Frozen Sections)
Cryostat (−20 °C) for cutting 7–10 μm frozen sections from OCT embedded tissues.
Slides prepared for frozen sections: VECTABOND (Vector Laboratories)-coated slides or Fisher-Plus (Fisher Scientific) slides.
Solvents, acetone.
Appropriate primary and secondary antibodies and detection reagents.
Automation buffer (Tris buffered saline with non-ionic detergent, pH 7.4) commercially available (Genetex and other vendors).
PBS buffer (0.1 M phosphate, pH 7.4).
Monoclonal antibodies (MAb) to specific targets. MAbs that have been successfully used to detect cell type specific antigens in frozen sections of macaque tissue include: mouse anti-human CD4 (1:100 L200 clone, BD Pharmingen), mouse anti-human CD8 (1:10 RPA-T8 clone, BD Pharmingen), mouse anti-human Ham56 (1:1 Ham56, DAKO), and mouse anti-human CD68 (1:100 Y1/82A clone, BD Pharmingen).
Biotinylated secondary antibodies (Vector Laboratories, BioGenex, Inc., Southern Biotechnology), streptavidin-alkaline phosphatase and Vector Red substrate (VECTASTAIN-AP, Vector Laboratories). If sensitivity is an issue, horseradish peroxidase (HRP) conjugated secondary Ab and reagents can be used (VECTASTAIN Elite ABC, Vector Laboratories).
10 mM citrate, pH 6.0 for antigen retrieval.
2.5. Biochemical Assessments
Chemical safety hood for drying procedures.
Oven capable of steady temperatures of up to 110 °C.
Vacuum desiccator.
3:1 chloroform–methanol for lipid extraction.
Glass test tubes with Teflon coated caps for extractions and acid hydrolysis.
Kits or chemicals for assessment of cholesterol, cholesterol ester, hydroxyproline.
3. Methods
3.1. Plasma or Serum Lipids
Obtain blood from fasted animals in EDTA vacutainer tubes (for plasma) or red top tubes (for serum).
Centrifuge the blood sample at 1100–1300 × g for 10 min to separate plasma from circulating cells. Samples may be analyzed within 24 h or frozen at −70 °C for later analysis. Avoid freeze thawing of samples for most evaluations of plasma/ serum biomarkers.
Use an automated clinical chemistry autoanalyzer to measure total plasma cholesterol, HDL cholesterol, and triglyceride (see Notes 1 and 2). Alternatively, kits are available for the measurement of plasma lipids from many commercial vendors (e.g., Sigma-Aldrich, Thermo-Fisher).
Calculate the concentration of non-HDLc, which approximates the sum of low-density lipoprotein (LDL) cholesterol and very-low-density lipoprotein cholesterol, by subtracting HDLc from TPC.
3.2. Tissue Preparation
Approaches to tissue preparation vary with the primary intent for the use of the tissues and the species being studied. Historical approaches to evaluation of the extent, distribution, and pathologic characteristics of atherosclerotic plaques relied on whole body perfusion of an appropriate fixative at a pressure similar to the mean blood pressure of the species being studied; for monkey this is approximately 100 mmHg. This approach provides quality tissue preservation for histologic evaluation of the whole cardiovascular tree, but is not ideal for studies of the biochemistry or molecular biology and genomics of the tissues. We have largely adapted this approach so that some arterial sites are fixed for histology while others are preserved for molecular approaches by slam freezing, by preserving in RNAlater, by preserving in OCT (see below), or other methods. The arteries of the greatest clinical and translational interest are the epicardial coronary arteries (important for coronary heart disease risk), and the carotid bifurcation and internal carotid arteries because of their association with ischemic changes in brain associated with declining cognitive function and stroke. We have also focused on iliac arteries as a key tissue due to the similarities in atherosclerosis and responses to treatment between the iliac arteries and the coronary arteries. The abdominal aorta can also be evaluated as a key arterial site with propensity for aneurysm development.
Only pigs and Old World monkeys share with human beings the anatomical feature of primary epicardial arteries (left main, left circumflex, left anterior descending and right epicardial arteries). The coronary arteries of lower animals (New World monkeys, rabbits, and mice for example) begin branching at the aortic coronary ostia into multiple intramyocardial arteries. For that reason, mouse researchers often use the coronary ostia and subclavian arteries as surrogates of epicardial arteries. For species with epicardial arteries, the heart with its epicardial arteries should be evaluated. For other species, the aortic root, subclavian, innominate arteries, carotids, and aorta may be the focus.
Perfusion fixation of the heart and coronary arteries with 10 % neutral buffered formalin (or less commonly 4 % paraformaldehyde) for 1 h at 100 mmHg pressure (the approximate mean arterial pressure for nonhuman primates such as the cynomolgus macaque) helps to maintain the approximate in vivo geometry of the arteries for evaluation (see Note 3).
For the major epicardial coronary arteries, obtain five serial blocks, each 3 mm in length perpendicular to the long axis of the artery. These blocks of tissue will contain the segment of artery along with adjacent epicardial fat and myocardium from each of the left circumflex, the left anterior descending, and the right coronary arteries (Fig. 1).
Remove and process other arterial sites such as the aorta, iliac and mesenteric arteries, common carotid arteries, carotid bifurcations, and internal carotid arteries in accordance with desired approaches to analysis. Non-coronary arteries may be cleaned of adherent connective tissues in situ or after removal from the animal.
To best evaluate the atherosclerosis in carotid bifurcation and internal carotid arteries, the specimens should be unopened and processed like blocks of coronary artery. We generally take one block of carotid bifurcation and 2–3 blocks of internal carotid artery. For other arterial sites from longer less geometrically complex blood vessels such as aorta, common carotid, or iliac-femoral arteries, we generally open the excised arteries longitudinally, section into desired segments, and fix segments of artery flat on cardboard in 10 % neutral buffered formalin or 4 % paraformaldehyde, freezing other adjacent sections for angiochemical and molecular studies, and other sections in OCT for specialized IHC studies.
Subdivide arterial sites into segments in order to preserve arteries for maximal utilization by a broad array of methods. Convention can vary; one approach is to designate one segment for standard fixation for histology, another segment for embedding in OCT for future immunohistochemical or other analyses such as laser capture microdissection (LCM), and then one or more segments for freezing and/or preservation in RNAlater® for angiochemical or molecular/genomic analyses (see Figs. 1 and 2).
For freezing in OCT, fill a prelabeled plastic cryomold partly with OCT. Remove excess moisture from tissue section by touching on filter paper or a paper towel. Select best orientation for the tissue and place in tissue mold with OCT. Let tissue settle to the bottom of the mold (where sectioning will be initiated). Add OCT to cover tissue sections completely and fill the mold. Hold the mold with a hemostat and dip only the most bottom part into liquid nitrogen. It is important to not submerge. The OCT will turn white as it freezes. Once all of the OCT is frozen, wrap the mold in parafilm and aluminum foil, label, and store at −80 °C until use.
Fig. 1.
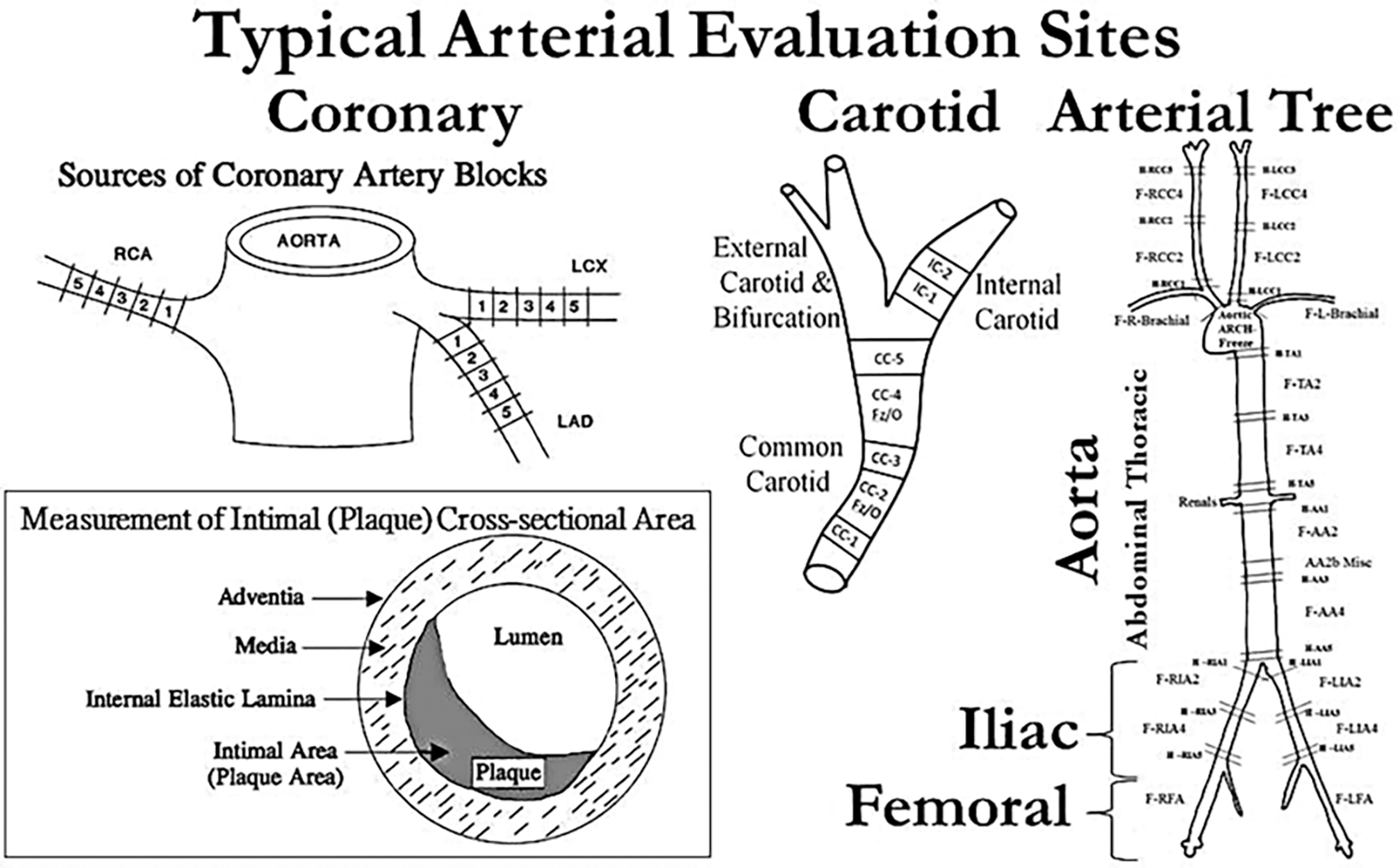
Diagram of typical arterial regions of interest in the heart (coronary arteries), neck (carotid arteries), and trunk (aorta and iliac arteries) and a representative sampling regimen from an experiment investigating the effects of hormones on atherosclerosis in coronary, central (aorta), and peripheral arteries. In addition, the inset contains a description of basic measurements of perfusion fixed coronary artery and atherosclerotic plaques
Fig. 2.
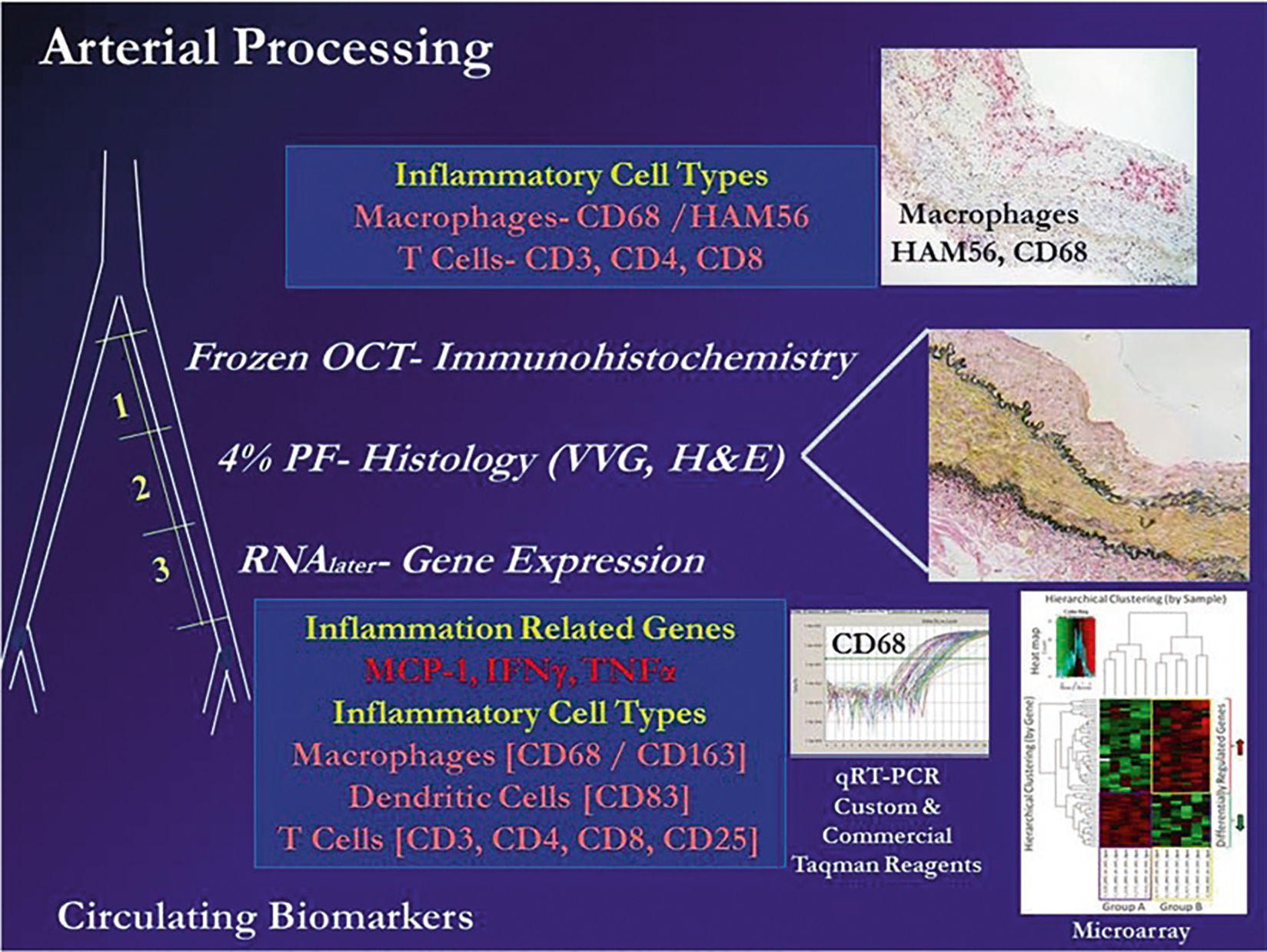
Diagram of approaches to evaluation of cellular, morphologic, and molecular phenotypes in an iliac artery biopsy or another arterial specimen obtained ante or post mortem in response to diet and interventions. A biopsy of the iliac artery can be obtained surgically and prepared for immunohistochemistry (image 1, cryopreserved in OCT embedding medium), histology (image 2, fixed with 4 % paraformaldehyde, moved to 70 % alcohol, and then embedded in paraffin), and for assessment of gene expression by qRT-PCR, microarray, or RNAseq (images 3 and 4, prepared in RNAlater® or slam frozen). Abbreviations: OCT Optimal Cutting Temperature freezing embedding medium, VVG Verhoeff-van Gieson stain, H and E hematoxylin and eosin. Macrophage markers CD68 and/or HAM56 (monoclonal antibody to human alveolar macrophages), CD163 (M2 marker hemoglobin-haptoglobin receptor), T cell markers CD3 (T cell lineage), CD4 (T helper cells), CD8 (NK cells), CD25 (Tregs). Dendritic cells CD83 [24, 31, 32]. In general, estrogen treatment initiated soon after the loss of estrogen (through ovariectomy) causes a 50–70 % inhibition of diet induced atherosclerotic plaque size in the coronary arteries on monkeys relative to untreated controls. Molecular and cellular responses to estrogen (reduced levels of arterial expression of inflammation and inflammatory cell associated markers) can be detected well before changes in lesion size can be observed; therefore, molecular approaches can significantly reduce sample sizes required for appropriate statistical power to see an effect
3.3. Histology
Transition formalin or paraformaldehyde fixed vascular tissues through graded alcohols. Tissues fixed in 4 % paraformaldehyde should be transitioned to 70 % alcohol within 24 h.
Embed the tissues in paraffin blocks in the appropriate orientation for later sectioning.
Cut 5 μm sections using a microtome. Float sections in a ~45 °C flotation bath to allow appropriate placement on glass slides.
Stain sections with Verhoeff-van Gieson (VVG) stain for accurate measurement of the cross-sectional area of the atherosclerotic lesion and other histologic parameters in the artery. VVG allows the identification of the internal and external elastic lamella (stained black), cell nuclei (stained black), and collagen (stained red). Sections can also be stained with hematoxylin and eosin (H and E), which stains cell nuclei blue and cytoplasm and extracellular matrix (ECM) various shades of pink. Mineral appears as blue on H and E, but if mineralization is a target it can be better detected with Von Kossa or Alizarin Red stains.
Determine plaque area/extent of atherosclerosis (mm2) of the artery section as the cross-sectional area of intimal lesions in each of the VVG stained sections of the artery segments (Fig. 1 [6]) by computer assisted histomorphometric methods using software such as ImageJ or Image-Pro Plus software22. For coronary arteries, digitally trace around the areas occupied by lesion, lumen, and media and guide the software to calculate the cross-sectional area. For peripheral arteries that are opened longitudinally, trace the areas of the lesion, lumen, and media as for coronary arteries. Also measure the length of the internal elastic lamella (IEL) of the sections (see Note 4).
Evaluate the length of the external elastic lamella (EEL) in arterial sections as a measure of artery size (although this does not appear to provide significant information above that of the IELA). Additional measures include the maximum intimal thickness (MxIT), the minimal intimal thickness (MinIT), and maximum and minimum medial thickness (MxMT, MinMT).
Differentiate fatty streaks from plaques by the following rule of thumb which relates to the proportions of lesion size to sublesion media thickness; plaques are defined as sites where the intimal thickness is at least 2× the medial thickness at that site.
For the determination of coronary artery atherosclerosis, make measurements using 15 blocks (each 3 mm in length) cut perpendicular to the long axis of the arteries, five serial blocks each from the left circumflex, the left anterior descending, and the right coronary arteries. From those sections each arterial site can be evaluated independently, or the plaque size can be averaged across some or all coronary artery beds to obtain mean coronary artery atherosclerosis. For the iliac arteries, a biopsy of one common iliac artery may be obtained to establish baseline measures before randomization to treatment and the contralateral iliac artery taken at necropsy for terminal measures [32, 36, 37]. Five micrometer sections from one to five blocks have generally been used to assess atherosclerosis in the iliac arteries (see Note 5). Carotid artery atherosclerosis can be assessed in both the left and right common carotid arteries (three blocks each), carotid bifurcations (one block each), and left and right internal carotid arteries (two blocks each) (Fig. 1).
To evaluate plaque complications histologically, take individual sections from blocks representative of the length of a specific artery site. For example, prepare four sections from each of five blocks of the three coronary arteries to be stained by VVG or H and E staining (described above) to provide a reasonable representation of the lesion composition in that artery.
Stain the sections with H and E and adjacent sections with VVG stains [7].
Count the density of leukocytes that have accumulated both within the adventitia and within the intimal plaque as an estimation of the degree of inflammation associated with the atherosclerotic plaque. Establish a subjective scoring system of 0–4 for the laboratory or for an experimental dataset, in which is assigned to the most extensively involved of those cases within the data set.
Estimate atheronecrosis as a percent of the cross-sectional area of the plaque.
Measure the fibro-muscular caps overlying the plaques histomorphometrically at points of maximum and minimum thickness.
Use trichrome reagent to stain for collagen, which can be estimated using color based segmentation of slides with ImageJ or ImageProPlus.
3.4. Immunohistochemistry
Immunohistochemical staining can be performed on fixed tissue embedded in paraffin or OCT-embedded tissues.
3.4.1. Fixed Tissues
For immunostaining of fixed tissues, cut 5 μm sections from the arterial block of interest.
Mount the sections on Superfrost Plus slides.
Deparaffinize the sections in xylenes.
Rehydrate the sections in graded alcohols.
To perform antigen retrieval, place the slides in 10 mM citrate (pH 6.0) in a 95–100 °C steamer, water bath, or pressure cooker for 20–30 min to expose epitopes of interest (see Note 6).
3.4.2. Frozen Tissues
Cut sections at 5–10 μm thickness on a Cryostat at the appropriate temperature. Generally the temperature should be close to −15 to −20 °C ±5 °C; colder temperatures may crack the OCT block.
Dry the slides overnight at room temperature in a vacuum desiccator.
Store the slides at −20 °C in a desiccated slide box inside a sealed zip-lock bag.
To prepare for immunohistochemical staining, bring the sections to room temperature while still in the sealed and desiccated bag to prevent condensation on tissue sections.
Fix the sections in cold acetone for 10 min, transfer to deionized water.
3.4.3. Staining of Fixed or Frozen Sections
Preincubate tissue sections with a blocking agent (normal serum or a protein solution) for 30 min at room temperature to prevent nonspecific Ab binding and reduce background noise.
Detect the epitopes of interest using commercially available primary monoclonal antibodies and associated reagents. Incubate the slides in primary antibody for 1.5 h at room temperature or overnight at 4 °C then wash with cold PBS or TBS buffer for 5 min.
To localize the primary antibodies, incubate tissue sections with appropriate biotinylated secondary antibody for 20 min at room temperature then wash with cold PBS or TBS buffer for 5 min.
Incubate sections with streptavidin-alkaline phosphatase for 20 min at room temperature, then wash with cold PBS or TBS buffer for 5 min.
Incubate sections with Vector Red substrate chromogen for 3 min (or until desired staining intensity is obtained) at room temperature then wash with cold PBS or TBS buffer (see Note 7).
Counterstain the sections with Mayer’s Hematoxylin or an alternative counterstain and examine by light microscopy.
Capture images with a motorized stage and tiling software (Image-Pro Plus) or obtain digital images at a core facility equipped with appropriate digital image capture equipment.
Quantify immunohistochemical cell staining using computer assisted morphometry (Image-Pro Plus Software, Media Cybernetics, Inc. Silver Springs, MD). A grid filter (in this case 8.5 μm2) can be applied to digitize images and each crosshatch within the intima evaluated for positive or negative staining. Contrast and color settings should be kept constant for all slides. Each point of intersection can then be evaluated according to staining character and location. Location should be defined as intima, media, or adventitia, and staining character classified as positive if immunostained or negative if not immunostained. Express staining as the percentage of intima occupied by positive cell staining, or as a percentage of total cells (nuclei), depending on the staining pattern observed.
3.5. Biochemical Assessments
Arteries can be assessed biochemically for content of lipids, calcium, collagen, and elastin [19]. Likewise, content of specific proteins can be assessed by Western blotting or proteomic approaches and RNA content can be examined by real time RT-PCR, microarray analysis, or RNAseq. These protein and RNA technologies are beyond the scope of this manuscript, but each hold the promise of providing additional insights into broad effects of estradiol and other endocrine modulators on cardiovascular and other diseases.
3.5.1. Arterial Lipid Composition
Pin tissue segments that will be used for chemical composition studies flat on a dissection board and photograph them for subsequent determination of lumen surface area.
Blot the surface of the tissue to remove surface liquid and obtain the wet weight of the tissue. Sections may then be extracted directly or frozen for future analysis.
Extract lipids from tissues with 20 volumes (vol/wt) of chloroform-methanol (3:1, vol/vol).
Rinse the tissues with additional chloroform: methanol and pool the extracts.
Determine the lipid free dry weight (LFDW) of the tissue by drying in vacuo until consistent weights are achieved.
Tissue cholesterol content (free and esterified) can be determined in the lipid extracts by the method of Rudel and Morris [38]. Kits employing this methodology are available commercially from Sigma-Aldrich (MAK043–1KT) and other vendors.
3.5.2. Arterial Calcium Content
Take the lipid-free dry artery from step 4 above and incubate it in 0.1 M HCl at 4 °C for 7 days to rehydrate and decalcify the tissue.
Determine the calcium content of the acid extract using Arsenazo III reagent and protocols supplied with Roche Reagents’ “Reagent for calcium” (Roche Diagnostic Systems) using a microtiter plate reader at 630 nm with background correction at 450 nm.
Prepare calcium standards and assay them under the same conditions as the samples (in 0.1 M HCl).
Dry the decalcified, delipidated tissue to a constant (reproducible) weight (decalcified LFDW).
3.5.3. Aortic Collagen and Elastin Content
Take the decalcified tissues from the above steps and rehydrate them in deionized water at 4 °C for several days.
Solubilize the collagen from the tissue by hot alkali extraction at 98 °C in 0.1 N NaOH for 50 min in a shaking water bath [39].
Separate insoluble material (elastin) from the soluble collagen by centrifugation at 1100 × g for 30 min.
Wash with 0.1 N NaOH and pool with original extract, and then with deionized water to remove salts, centrifuging between each wash as described above.
Dry insoluble material under vacuum to constant mass. This will give you arterial elastin content.
Acid hydrolyze the extracted collagen fraction in 6 M HCl at 110 °C for 17 h to produce free amino acids.
Determine the hydroxyproline content using an appropriate method such as that of Bergman and Loxley [40] to estimate collagen content. Kits employing this methodology are available commercially from Sigma-Aldrich (MAK008–1KT) and other vendors.
3.5.4. Expression of Data
Express angiochemical measurements on a concentration basis (milligrams per gram of wet or lipid-free dry weight) and on an area basis (mg/cm2 of lumen) (see Note 8).
3.6. Statistical Analysis
All data should be assessed for normality and transformed if necessary. Generally atherosclerosis data is not normally distributed and requires transformation prior to analysis. Since there are frequently animals which have no detectable lesion (0), we add a constant, usually 1, to the intimal area and take the log of that [log(Intimal Area + 1)].
4. Notes
HDL cholesterol concentrations can also be determined using the heparin-manganese precipitation procedure reported [41] as described in detail in the Manual of Laboratory Operations of the Lipid Research Clinics Program (1974). For cynomolgus macaques, the protocol should be modified to include the use of 2 M MnCl2 rather than the 1 M MnCl2 originally suggested for the Lipid Research Clinics. This modification facilitates the complete precipitation of low density lipoproteins, portions of which are resistant to precipitation in certain hyperlipoproteinemic monkeys when 1 M MnCl2 is used. The use of 2 M MnCl2 does not result in loss of HDL.
Cholesterol, HDLc, and triglycerides for the NHP work are standardized to calibrated controls from the Centers for Disease Control and Prevention-National Institutes of Health Lipid Standardization Program using reagents from Soloman Park Research Laboratory (Kirkland, WA) in conjunction with Northwest Lipid Metabolism and Diabetes Research Laboratories (NWLMDRL) at the University of Washington (Seattle) [42]. Intra- and inter-assay coefficients of variation should be less than 5 %.
For pressure perfusion fixation, we have developed special perfusion chambers which can be pressure regulated with applied air pressure. Alternatives include elevation of the perfusion buffers to a level above the tissue perfused which produces the desired perfusion pressure (for 10 % formalin at 100 mmHg, this is approximately 132 mm above the heart).
Artery size in longitudinally opened segments of peripheral arteries can be estimated by using the IEL length (IELL) as a circumference for mathematical derivation of the area encompassed by the IEL, the IEL area: IELA = IELL2/(4×π). This is an estimate, as distortion of the actual size of the arterial segment may occur when arteries are fixed without the perfusion fixation required to preserve arterial in situ shape and size. Lumen area can then be estimated by subtracting the intimal area from the IELA.
In the nonhuman primate, the common iliac arterial site has been shown to have similar plaque sizes in the left and right arteries (r = 0.97) and to be highly associated with coronary artery plaque extent (r = 0.86) [6].
Antigen retrieval methods are required for many targets and may need to be specialized depending on the antigen of interest. For citrate buffer antigen retrieval, see http://www.ihcworld.com/_protocols/epitope_retrieval/citrate_buffer.htm.
Alternatively, for difficult to detect antigens one can use streptavidin-horseradish peroxidase and DAB substrate for immunohistochemical detection.
In general, the use of wet weight of the tissue tends to underestimate amounts of a component per unit tissue, as increases in lipid and cell contents of the atherosclerotic aorta increase the wet weight of the tissue. The weight of the tissue after lipid extraction (LFDW, in grams) provides a more accurate estimate of the unit of tissue. However, tissue weight is dependent on the amount of connective tissue in the artery which is altered in response to atherogenesis as well as regression of atherosclerosis. Both of these lead to variable accumulation and loss of specific ECM components. The surface area of a tissue section (mm2) is less likely to be altered by chemical changes but is affected by vascular remodeling (shrinkage or enlargement) and is less accurately measured, especially in tissue that has been previously frozen.
Source of Funding
This project was supported by NIH grants AG18170 (TCR), AG28641 (TCR), and HL45666 (TBC).
References
- 1.Hansson GK, Robertson AK, Soderberg-Naucler C (2006) Inflammation and atherosclerosis. Annu Rev Pathol 1:297–329 [DOI] [PubMed] [Google Scholar]
- 2.Andersson J, Libby P, Hansson GK (2010) Adaptive immunity and atherosclerosis. Clin Immunol 134(1):33–46 [DOI] [PubMed] [Google Scholar]
- 3.Zhu J, Paul WE (2010) Heterogeneity and plasticity of T helper cells. Cell Res 20(1): 4–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendelsohn ME (2002) Protective effects of estrogen on the cardiovascular system. Am J Cardiol 89(12A):12E–17E [DOI] [PubMed] [Google Scholar]
- 5.Adams MR, Kaplan JR, Manuck SB et al. (1990) Inhibition of coronary artery atherosclerosis by 17-beta estradiol in ovariectomized monkeys. Lack of an effect of added progesterone. Arteriosclerosis 10(6):1051–1057 [DOI] [PubMed] [Google Scholar]
- 6.Clarkson TB, Anthony MS, Morgan TM (2001) Inhibition of postmenopausal atherosclerosis progression: a comparison of the effects of conjugated equine estrogens and soy phytoestrogens. J Clin Endocrinol Metab 86(1):41–47 [DOI] [PubMed] [Google Scholar]
- 7.Register TC, Cann JA, Kaplan JR et al. (2005) Effects of soy isoflavones and conjugated equine estrogens on inflammatory markers in atherosclerotic, ovariectomized monkeys. J Clin Endocrinol Metab 90(3):1734–1740 [DOI] [PubMed] [Google Scholar]
- 8.Register TC, Wagner JD, Zhang L, Hall J, Clarkson TB (2002) Effects of tibolone and conventional hormone replacement therapies on arterial and hepatic cholesterol accumulation and on circulating endothelin-1, vascular cell adhesion molecule-1, and E-selectin in surgically menopausal monkeys. Menopause 9(6):411–421 [DOI] [PubMed] [Google Scholar]
- 9.Speir E, Yu ZX, Takeda K, Ferrans VJ, Cannon RO 3rd (2000) Competition for p300 regulates transcription by estrogen receptors and nuclear factor-kappaB in human coronary smooth muscle cells. Circ Res 87(11): 1006–1011 [DOI] [PubMed] [Google Scholar]
- 10.Evans MJ, Eckert A, Lai K, Adelman SJ, Harnish DC (2001) Reciprocal antagonism between estrogen receptor and NF-kappaB activity in vivo. Circ Res 89(9):823–830 [DOI] [PubMed] [Google Scholar]
- 11.Gavin KM, Seals DR, Silver AE, Moreau KL (2009) Vascular endothelial estrogen receptor alpha is modulated by estrogen status and related to endothelial function and endothelial nitric oxide synthase in healthy women. J Clin Endocrinol Metab 94(9):3513–3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Register TC, Adams MR (1998) Coronary artery and cultured aortic smooth muscle cells express mRNA for both the classical estrogen receptor and the newly described estrogen receptor beta. J Steroid Biochem Mol Biol 64(3–4):187–191 [DOI] [PubMed] [Google Scholar]
- 13.Register TC (2009) Primate models in women’s health: inflammation and atherogenesis in female cynomolgus macaques (Macaca fascicularis). Am J Primatol 71(9):766–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shim GJ, Gherman D, Kim HJ et al. (2006) Differential expression of oestrogen receptors in human secondary lymphoid tissues. J Pathol 208(3):408–414 [DOI] [PubMed] [Google Scholar]
- 15.Grodstein F, Stampfer MJ, Manson JE et al. (1996) Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. N Engl J Med 335(7):453–461 [DOI] [PubMed] [Google Scholar]
- 16.Adams MR, Register TC, Golden DL, Wagner JD, Williams JK (1997) Medroxyprogesterone acetate antagonizes inhibitory effects of conjugated equine estrogens on coronary artery atherosclerosis. Arterioscler Thromb Vasc Biol 17(1):217–221 [DOI] [PubMed] [Google Scholar]
- 17.Zhao J, Imbrie GA, Baur WE et al. (2013) Estrogen receptor-mediated regulation of microRNA inhibits proliferation of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 33(2):257–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodgin JB, Krege JH, Reddick RL, Korach KS, Smithies O, Maeda N (2001) Estrogen receptor alpha is a major mediator of 17beta-estradiol’s atheroprotective effects on lesion size in Apoe−/− mice. J Clin Invest 107(3): 333–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Register TC, Adams MR, Golden DL, Clarkson TB (1998) Conjugated equine estrogens alone, but not in combination with medroxyprogesterone acetate, inhibit aortic connective tissue remodeling after plasma lipid lowering in female monkeys. Arterioscler Thromb Vasc Biol 18(7):1164–1171 [DOI] [PubMed] [Google Scholar]
- 20.Williams JK, Anthony MS, Honore EK et al. (1995) Regression of atherosclerosis in female monkeys. Arterioscler Thromb Vasc Biol 15(7):827–836 [DOI] [PubMed] [Google Scholar]
- 21.Anderson GL, Limacher M, Assaf AR et al. (2004) Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA 291(14):1701–1712 [DOI] [PubMed] [Google Scholar]
- 22.Rossouw JE, Anderson GL, Prentice RL et al. (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 288(3): 321–333 [DOI] [PubMed] [Google Scholar]
- 23.Rossouw JE, Prentice RL, Manson JE et al. (2007) Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 297(13):1465–1477 [DOI] [PubMed] [Google Scholar]
- 24.Sophonsritsuk A, Appt SE, Clarkson TB, Shively CA, Espeland MA, Register TC (2013) Differential effects of estradiol on carotid artery inflammation when administered early versus late after surgical menopause. Menopause 20(5):540–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bullock BC, Lehner ND, Clarkson TB, Feldner MA, Wagner WD, Lofland HB (1975) Comparative primate atherosclerosis. I. Tissue cholesterol concentration and pathologic anatomy. Exp Mol Pathol 22(2):151–175 [DOI] [PubMed] [Google Scholar]
- 26.Clarkson TB, Bond MG, Bullock BC, Marzetta CA (1981) A study of atherosclerosis regression in Macaca mulatta. IV. Changes in coronary arteries from animals with atherosclerosis induced for 19 months and then regressed for 24 or 48 months at plasma cholesterol concentrations of 300 or 200 mg/dl. Exp Mol Pathol 34(3):345–368 [DOI] [PubMed] [Google Scholar]
- 27.Clarkson TB, Lehner ND, Wagner WD, St Clair RW, Bond MG, Bullock BC (1979) A study of atherosclerosis regression in Macaca mulatta. I. Design of experiment and lesion induction. Exp Mol Pathol 30(3):360–385 [DOI] [PubMed] [Google Scholar]
- 28.Wagner WD, Clarkson TB (1975) Comparative primate atherosclerosis. II. A biochemical study of lipids, calcium, and collagen in atherosclerotic arteries. Exp Mol Pathol 23(1): 96–121 [DOI] [PubMed] [Google Scholar]
- 29.Wagner WD, St Clair RW, Clarkson TB (1980) A study of atherosclerosis regression in Macaca mulatta. II. Chemical changes in arteries from animals with atherosclerosis induced for 19 months then regressed for 24 months at plasma cholesterol concentrations of 300 or 200 mg/ dl. Exp Mol Pathol 32(2):162–174 [DOI] [PubMed] [Google Scholar]
- 30.Wagner WD, St Clair RW, Clarkson TB, Connor JR (1980) A study of atherosclerosis regression in Macaca mulatta: III. Chemical changes in arteries from animals with atherosclerosis induced for 19 months and regressed for 48 months at plasma cholesterol concentrations of 300 or 200 mg/dl. Am J Pathol 100(3):633–650 [PMC free article] [PubMed] [Google Scholar]
- 31.Walker SE, Adams MR, Franke AA, Register TC (2008) Effects of dietary soy protein on iliac and carotid artery atherosclerosis and gene expression in male monkeys. Atherosclerosis 196(1):106–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walker SE, Register TC, Appt SE et al. (2008) Plasma lipid-dependent and -independent effects of dietary soy protein and social status on atherogenesis in premenopausal monkeys: implications for postmenopausal atherosclerosis burden. Menopause 15(5):950–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Losordo DW, Kearney M, Kim EA, Jekanowski J, Isner JM (1994) Variable expression of the estrogen receptor in normal and atherosclerotic coronary arteries of premenopausal women. Circulation 89(4):1501–1510 [DOI] [PubMed] [Google Scholar]
- 34.Grumbach MM, Auchus RJ (1999) Estrogen: consequences and implications of human mutations in synthesis and action. J Clin Endocrinol Metab 84(12):4677–4694 [DOI] [PubMed] [Google Scholar]
- 35.Smith EP, Boyd J, Frank GR et al. (1994) Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med 331(16):1056–1061 [DOI] [PubMed] [Google Scholar]
- 36.Kaplan JR, Adams MR, Anthony MS, Morgan TM, Manuck SB, Clarkson TB (1995) Dominant social status and contraceptive hormone treatment inhibit atherogenesis in premenopausal monkeys. Arterioscler Thromb Vasc Biol 15(12):2094–2100 [DOI] [PubMed] [Google Scholar]
- 37.Melendez GC, Register TC, Appt SE, Clarkson TB, Franke AA, Kaplan JR (2015) Beneficial effects of soy supplementation on postmenopausal atherosclerosis are dependent on pretreatment stage of plaque progression. Menopause 22(3):289–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rudel LL, Morris MD (1973) Determination of cholesterol using o-phthalaldehyde. J Lipid Res 14(3):364–366 [PubMed] [Google Scholar]
- 39.Jackson DS, Cleary EG (1967) The determination of collagen and elastin. Methods Biochem Anal 15:25–76 [DOI] [PubMed] [Google Scholar]
- 40.Bergman I, Loxley R (1963) Two improved and simplified methods for the spectrophotometric determination of hydroxyproline. Anal Chem 35(12):1961–1965 [Google Scholar]
- 41.Burstein M, Samaille J (1960) On a rapid determination of the cholesterol bound to the serum alpha- and beta-lipoproteins. Clin Chim Acta 5:609. [DOI] [PubMed] [Google Scholar]
- 42.Myers GL, Kimberly MM, Waymack PP, Smith SJ, Cooper GR, Sampson EJ (2000) A reference method laboratory network for cholesterol: a model for standardization and improvement of clinical laboratory measurements. Clin Chem 46(11):1762–1772 [PubMed] [Google Scholar]


