Abstract
Background:
Natural killer T (NKT) cells are unconventional T cells that bridge innate and adaptive immunity. NKT cells have been implicated in the development of atopic dermatitis (AD).
Objective:
We aimed to investigate the role of NKT cells in AD development, especially in skin.
Methods:
Global proteomic and transcriptomic analyses were performed using human healthy controls (HC) and AD patients’ skin and blood. CXCR4 and CXCL12 expressions in skin NKT cells were analyzed in human AD and mouse AD models. By using parabiosis and intravital imaging, the role of skin CXCR4+ NKT cells was further evaluated in AD mouse models using CXCR conditionally deficient or CXCL12 transgenic mice.
Results:
CXCR4 and its cognate ligand CXCL12 were significantly upregulated in human AD skin by global transcriptomic and proteomic analyses. CXCR4+ NKT cells were enriched in AD skin and were consistently elevated in our AD mouse models. Allergen-induced NKT cells participate in cutaneous allergic inflammation. Predominant skin NKT cells were CXCR4+ and CD69+, similar to tissue-resident memory T (TRM) cells. Skin-resident NKT cells uniquely expressed CXCR4, unlike NKT cells in liver, spleen and lymph nodes. Skin fibroblasts were the main source of CXCL12. CXCR4+ NKT cells preferentially trafficked to CXCL12-rich areas, forming an enriched CXCR4+ NKTRM/CXCL12+ cell cluster, which developed in acute and chronic allergic inflammation in our AD mouse models.
Conclusions:
CXCR4+ NKTRM cells may form a niche that contributes to atopic dermatitis, where CXCL12 is highly expressed.
Keywords: Atopic dermatitis, Natural killer T cells, Tissue-resident memory T (TRM) cells, CXCR4, CXCL12, Thymic stromal lymphopoietin
Capsule Summary
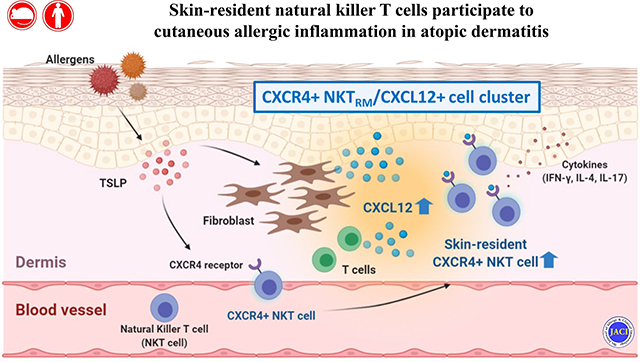
Skin-resident CXCR4+ NKT cells have a role in atopic dermatitis.
Graphical Abstract
INTRODUCTION
Atopic dermatitis (AD), a chronic inflammatory recurrent skin disease, is the most common skin disease among children, and its incidence has recently been increasing in adults.1,2 AD is associated with a defective skin barrier that facilitates allergic sensitization to food and environmental allergens, leading to pruritic, eczematous, and dry skin.3,4 Histologically, AD is characterized by infiltration of adaptive immune cells (T cells) and innate immune cells (eosinophils, mast cells, and natural killer T (NKT) cells) into skin, indicating that a combination of adaptive and innate immunity are involved in AD development.5 Innate allergic immune cells, including NKT cells or mast cells, are considered a principal innate immune axis and collaborate with several chemokines that are increased in AD.6–8 NKT cells share many cell-surface proteins with conventional T cells and NK cells, and serve as unconventional T cells bridging innate and adaptive immunity.9 More than 80% of NKT cells express invariant TCRα chain Vα24-Jα18 paired with Vβ11 in humans (or Vα14-Jα18 paired with Vβ8, Vβ7, or Vβ2 in mice) and are referred to as invariant NKT cells.10,11 Upon activation, NKT cells secrete a broad range of cytokines, including interleukin(IL)-4, interferon(IFN)-γ, and IL-17.5,12,13
A broad range of chemokines, responsible for leukocyte trafficking and homing, are produced when inflammation occurs.14 While NKT cells express several chemokine receptors,15–17 we found that skin-resident NKT cells express C-X-C chemokine receptor type 4 (CXCR4), specifically in AD skin of human and mice. We further studied CXCR4 and its cognate ligand, CXCL12 (also known as stromal cell-derived factor-1α, SDF-1α) axis in AD. CXCR4/CXCL12 interactions are involved in a variety of physiological processes, such as hematopoiesis,18 organogenesis,19 leukocyte trafficking,20 and skin inflammation.21 Recently, the CXCR4/CXCL12 axis was found to be involved in chronic psoriasis-like skin inflammation.21 In this stdy, we characterized NKT cells in human AD skin and in the skin of experimental AD mouse models. These NKT cells showed properties of skin residency. To further characterize these novel cells, we comprehensively explored their trafficking/migration, residency, and pathogenic role in the development of AD by intravital microscopy, KAEDE mice, parabiosis surgery, and CXCR4 deficient NKT cells.
METHODS
Intravital multiphoton microscopy
Mice were anaesthetized with ketamine and xylazine (10 μg/g) with repeated half doses as required, and the ear was depilated. The mice were mounted on a heating plate (CU301, Live Cell Instrument, Seoul, Korea) for ear imaging based on a customer designed chamber referenced in a previous protocol.22 Images were acquired with an upright LSM 7 MP (Carl Zeiss Microimaging). Images were acquired with a 20X/1.0PA water immersion objective. Fluorescence excitation fluorescence excitation was provided by a Mai Tai (690–1040 nm) HD DeepSee tunable laser (Spectra Physics, Santa Clara, CA, USA). GFP, HSG, and DsRed were excited at 880 nm using the Mai Tai laser. Raw imaging data were processed with Image J software (NIH, Bethesda, MD, USA). Cell migration was analyzed by automatic cell tracking aided by manual corrections. Data were used to plot graphs in Prism 5 (Graphpad, La Jolla, CA, USA). To generate videos, sequences exported from Zen (Carl Zeiss) were analyzed with Image J software.
Parabiosis
Parabiotic surgery was performed as described previously.23 Briefly, size- and age-matched female CD45.1 and CD45.2 mice were intraperitoneally anaesthetized with ketamine and xylazine (10 μg/g). Mice were shaved along corresponding lateral flanks and excess hair was wiped off with an alcohol prep pad. After skin disinfection by wiping with 70% ethanol followed by betadine solution, mirrored skin incisions were made from the olecranon to the knee joint of each mouse, and the subcutaneous fascia was bluntly dissected to create approximately 0.5 cm of free skin. A non-absorbable 5–0 VICRYL thread was placed through the olecranon and knee joints to secure the legs, and the dorsal and ventral skins were approximated by staples or continuous suture. Additional betadine solution was added to cover the full length of the incision. The mice were then kept on heating pads until recovery. Flunixin (2.5 μg/g) was subcutaneously injected as analgesic treatment by every 24 h for 48 h after surgery. Mice were then monitored everyday with gel supplied in the cages. After 4 weeks, parabiotic mice were surgically separated by a reversal of the above procedure before the next steps.
RESULTS
Increased CXCR4+ NKT cells/CXCL12 in AD skin
The epidermis of AD skin strongly expresses thymic stromal lymphopoietin(TSLP),24 and NKT cells are increased in the lesional skin of AD patients.6 However, the mechanism of how NKT cells are recruited into the lesional skin of AD and how TSLP interacts with NKT cells to migrate into the AD skin are unknown. To identify the factors involved in TSLP-activated NKT cells, we performed novel quantitative proteome analysis using a Tandem Mass Tag (TMT)-labeling method.25,26 Proteins extracted from NKT cells of un-treated, TSLP 6 h-treated, and TSLP 24 h-treated groups were labeled with the TMT isobaric tag individually, and then analyzed by 2D-liquid chromatography(LC)-tandem mass spectrometry (MS/MS) (Fig E1, A). Initially, we identified 1,404 proteins in TSLP-treated and un-treated NKT cells and performed Gene Ontology (GO) analysis (Fig E1, B). Among the 24 proteins considered as differentially expressed (Gaussian fit ratio ≤−0.37 or ≥0.37), only CXCR4 was found to be gradually upregulated in a time-dependent manner (Fig 1, A).
Fig 1. CXCR4+ NKT cells were increased in the lesional skin of AD patients.
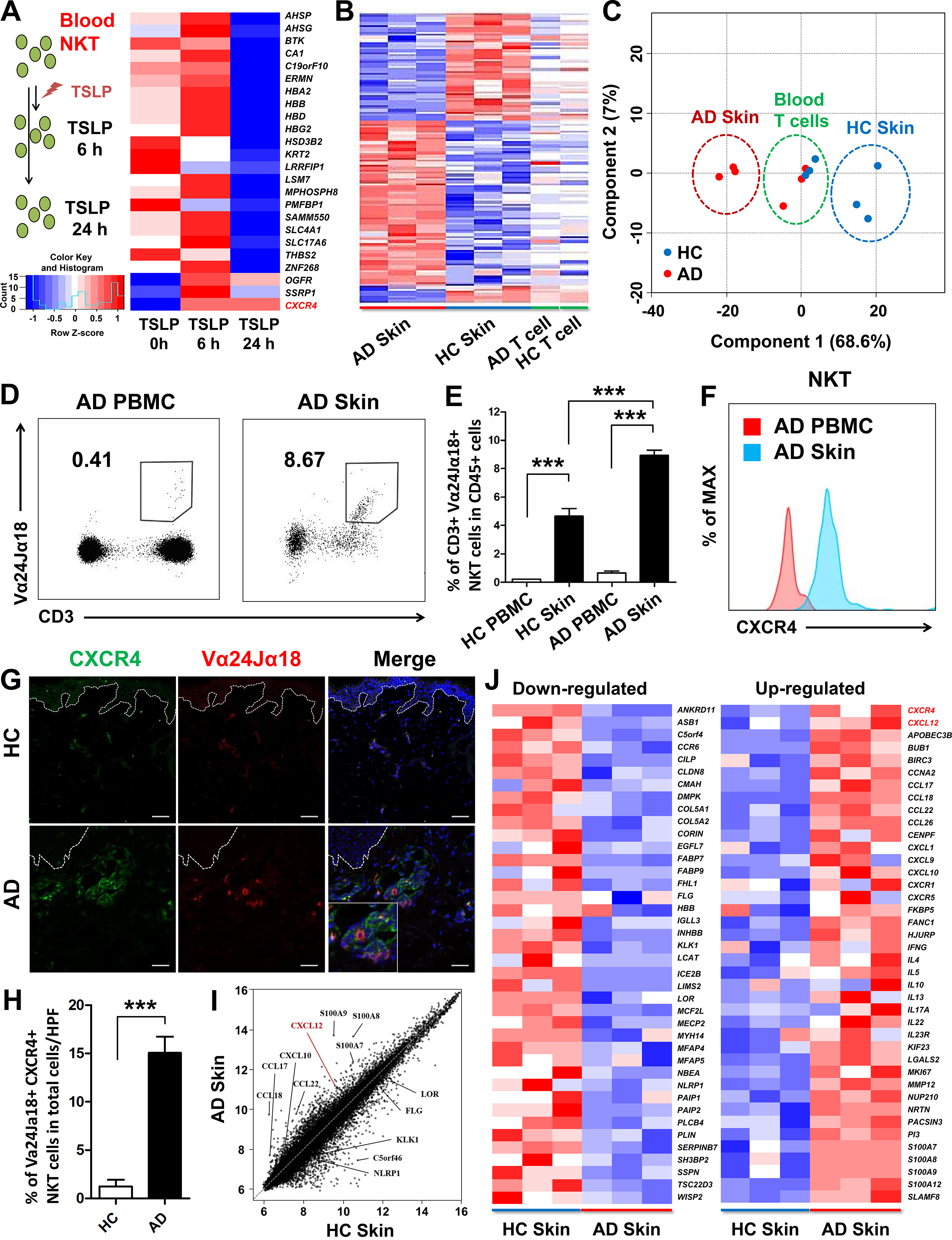
(A) Experimental scheme. NKT cells were sorted from HC blood by using this gating strategy: Live cells/CD45+/CD3+/Va24Ja18+. The purified NKT cells (>90%) were treated with TSLP for indicated times and proteins were extracted and analyzed by proteomics. Heat map showing 21 down-regulated and 3 up-regulated proteins identified using TMT labeling method. Microarray (B) and dot plot (C) analysis of AD patients and healthy controls (HC). Representative population (D) and CXCR4 expression (F) of NKT cells in AD PBMCs and AD skin. (E) Quantitative distribution of NKT cells in PBMCs and skin of HC and AD patients. Immunofluorescence staining (G) and quantification (H) of CXCR4+ NKT cells in AD and HC skin. Scatter plot (I) and heat map (J) showing selected genes up- and down-regulated in AD and HC skin (***p < 0.001). Scale bar = 20 μm.
TSLP contributes to a unique microenvironment in AD skin compared to blood; therefore, we further analyzed the global transcriptomes in skin and blood T cells from AD patients and healthy controls (HCs). Hierarchical clustering showed that large offsets of differentiated genes were transcriptionally unique between AD skin and HC skin (Fig 1, B). Principal component analysis also showed that AD skin(red) and HC skin(blue) formed two distinct clusters, while peripheral blood T cells from AD patients and HCs were poorly differentiated and formed a single cluster(green) (Fig 1, C). Vα24Jα18+ NKT cell populations were increased in lesional skin compared to in peripheral blood of AD patients (Fig 1, D). We also detected higher cell frequency of Vα24Jα18+ NKT cells in AD skin compared to HC skin (Fig 1, E). Interestingly, skin-resident NKT cells expressed higher CXCR4 levels than NKT cells in the blood of AD patients (Fig 1, F). CXCR4+ NKT cells infiltrated into the AD skin in much greater numbers compared to HC skin (Fig 1, G and H).
Further transcriptomic analyses of AD and HC skin showed that CXCL12, the cognate ligand for CXCR4, was significantly elevated in AD skin, alongside other chemokine genes, CCL17, CCL18, and other AD-related genes including S100A7, S100A8, and S100A9 (Fig 1, I and Fig E1, C).27,28 Our transcriptomic data were consistent with those of previous studies of down-regulated and up-regulated genes typically observed in AD skin (Fig 1, J).29,30 Taken together, these data demonstrate that CXCR4+Vα24Jα18+ NKT cells and CXCL12 are greatly increased in AD skin, suggesting that the CXCR4/CXCL12 axis is involved in recruiting NKT cells into AD skin.
CXCL12 expression in TSLP-activated fibroblasts and T cells
Because skin-resident NKT cells express high levels of CXCR4, we examined how Vα24Jα18+ NKT cells might interact with CXCL12, which was significantly elevated in AD skin. We found that Vα24Jα18+ NKT cells did not express CXCL12; however, skin NKT cells were in juxtaposition to other CXCL12-producing cells, suggesting that CXCR4+Vα24Jα18+ NKT cells might directly interact with surrounding CXCL12-producing cells (Fig E2, A). We next observed that CXCL12-producing cells in AD skin exhibited both elongated and round shapes. Thus, we stained these cells with anti-vimentin and anti-CD3 antibodies to distinguish between dermal fibroblasts and T cells. Significant CXCL12 production was observed in both fibroblasts (Fig E2, B) and T cells (Fig E2, D) of AD skin compared to HC skin. A higher percentage of dermal fibroblasts expressed CXCL12 (Fig E2, C) than T cells, though both produced CXCL12 in AD skin (Fig E2, E).
We further examined whether TSLP could induce T cells and fibroblasts to produce CXCL12, as both T cells and fibroblasts have TSLP receptors.31,32 TSLP-activated T cells produced more CXCL12 than non-activated T cells (Fig E3, A and B), and extracellular (secreted) CXCL12 was produced at high levels in the TSLP 24 h-treated group (Fig E3, C). In addition, fibroblasts produced more CXCL12 following TSLP treatment for 24 h, both intracellularly and extracellularly, compared to the non-treated group (Fig E3, D). To determine if CXCL12 affects the trafficking/migration properties of CXCR4+ NKT cells, we performed Transwell migration assays. NKT cells and T cells were sorted from human peripheral blood mononuclear cells (PBMCs) and pretreated with TSLP for 24 h. Following exposure to CXCL12, NKT cell migration was significantly increased compared to that of CXCL12 non-treated NKT cells, but not T cells (Fig E3, E), suggesting that CXCR4 expressing NKT cells may migrate into a CXCL12-rich area in AD skin.
CXCR4+ NKT and CXCR12+ cells in acute allergic inflammation mice
Next, we further questioned how CXCR4 expressing NKT cells interact with CXCL12+ cells in vivo in an acute allergic inflammation mouse model. CXCL12-DsRed transgenic mice were sensitized at the abdomen with dinitrofluorobenzene(DNFB) 5 days before ear challenge (Fig 2, A). We observed that only mice receiving DNFB-sensitization and challenged with DNFB showed significant ear swelling, whereas DNFB-sensitized mice challenged with fluorescein isothiocyanate(FITC) failed to develop ear swelling (Fig 2, B). Moreover, to confirm the allergen-induced NKT cells develop skin allergic inflammation specifically, we used the different purification strategies of NKT cells and transferred DNFB or oxazolone (OXA) sensitized TCRβ+/α-Galcer CD1d+ NKT cells into Rag1−/− (T and B cells deficient) recipients and cross challenged with different allergens. We observed that only recipients received DNFB or OXA sensitized NKTs and challenged with the corresponding allergen confer ear inflammation, otherwise failed to respond to un-experienced allergens (Fig E4). Taken together, these results indicate that those NKT cells, which have the memory of experienced antigen, may trigger skin allergic inflammation. Furthermore, Jα18−/− NKT deficient mice showed less ear swelling after challenging with DNFB compared with wild-type mice (Fig E5, A and B), highlighting the role of NKT cells in acute allergic inflammation.
Fig 2. CXCR4+ NKT and CXCR12+ cells in acute allergic inflammation mice.
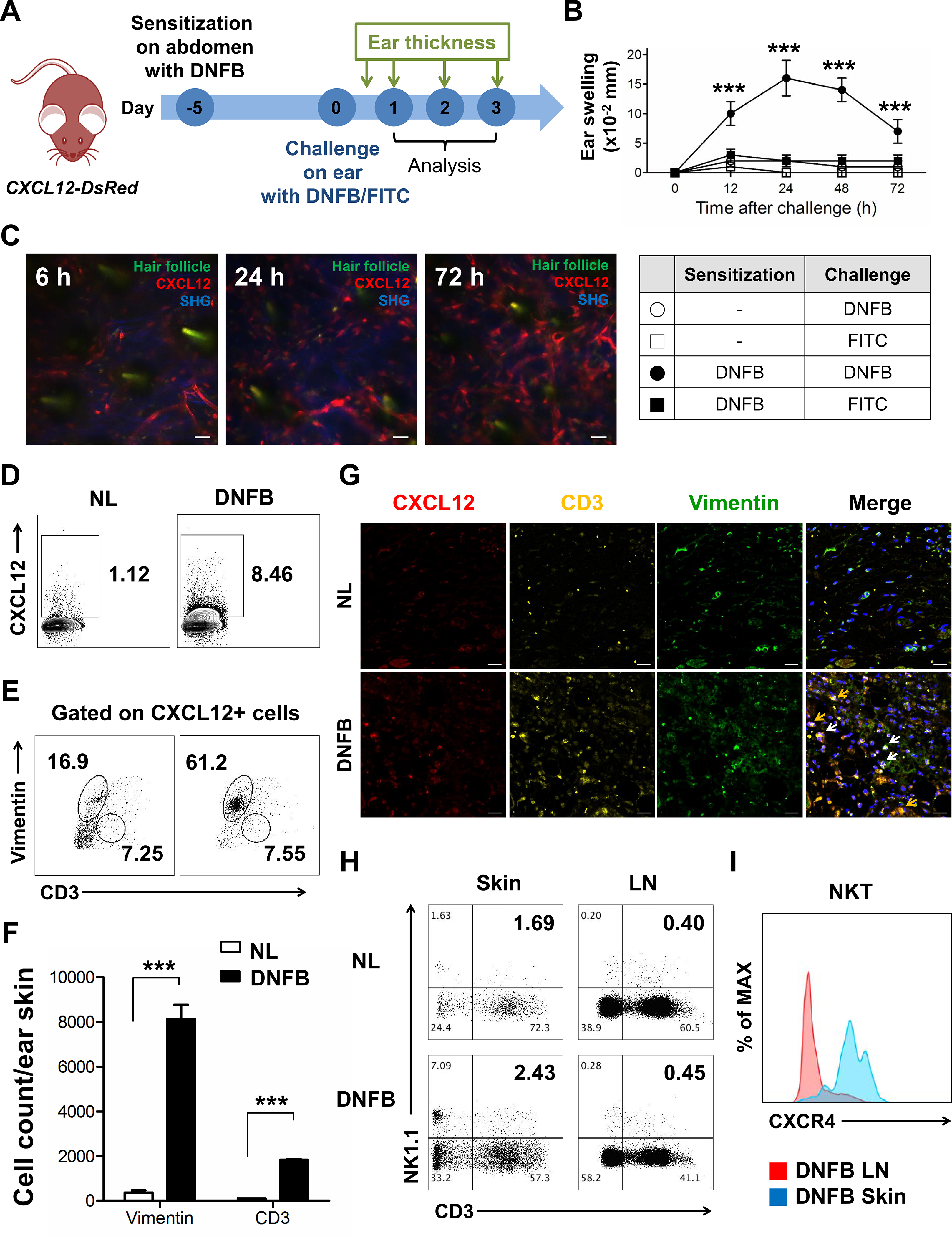
(A) Experimental scheme. CXCL12-DsRed mice were sensitized with DNFB 5 days before challenge at the ear. Ear thickness was measured for 3 consecutive days. (B) DNFB-induced cutaneous allergic inflammation in CXCL12-DsRed mice (***p < 0.001). (C) Representative intravital images of CXCL12-DsRed mice ear at 6, 24, and 72 h after DNFB challenge. (D) The CXCL12 expression in ear skin cells with or without DNFB challenge for 72 h. The frequency (E) and number (F) of fibroblasts and CD3 T cells in CXCL12-expressing ear skin cells with or without DNFB challenge for 72 h (***p < 0.001). (G) IF staining of DNFB-untreated normal (NL) and DNFB-challenged ear skin for 72 h. Yellow arrows represent merging of CD3 (yellow) and CXCL12 (red); white arrows represent merging of fibroblasts (green) and CXCL12 (red). (H) Proportion of CD3+ and NK1.1+ NKT cells in skin and LN of NL and DNFB-challenged mice. (I) CXCR4 expression of CD3+ and NK1.1+ NKT cells in LN and skin of DNFB-challenged mice. Data are representative of three experiments with n = 5 mice per group. Scale bar = 20 μm.
CXCL12-DsRed cells were induced from 6 to 72 h after DNFB challenge (Fig 2, C). We found that CXCL12+ cells were significantly induced at 72 h after DNFB challenge (Fig 2, D), and the major CXCL12-expressing cells were dermal fibroblasts (Fig 2, E) along with CXCL12+ CD3 T cells, which were also significantly increased (Fig 2, F). This is consistent with our results from human AD skin. We also observed that CXCL12+ fibroblasts and T cells were clustered and enriched at the same site in DNFB-challenged skin, indicating that both CXCL12+ cells concurrently interacted with CXCR4 expressing NKT cells (Fig 2, G). We next examined whether CXCR4+ NKT cells could be detected in DNFB-challenged skin. On day 3 after DNFB challenge, there was a large increase in the number of NKT cells in DNFB-treated skin, but we observed only a marginal increase in the number of NKT cells in DNFB-treated lymph nodes (LN) compared to in DNFB-untreated mice (Fig 2, H). NKT cells in DNFB-challenged skin expressed significantly higher levels of CXCR4 than those in the LN (Fig 2, I), consistent with our results in human. These CXCR4 expressing NKT cells produced significantly increased amounts of IL-4, IFN-γ, and IL-17 in DNFB-challenged skin (Fig E5, C), suggesting a pathogenic role for skin-resident NKT cells in our model.
To verify the pathogenic role of NKT cells in acute allergic inflammation, we adoptively transferred DNFB-sensitized splenic NKT cells (5 × 105) (Fig E6, A) into naïve recipient mice (CXCL12-DsRed) (Fig E6, B). Twenty-four hours after adoptive transfer, the right ears of recipient mice were challenged with DNFB, while vehicle was applied to the left ears, and then the amount of ear swelling was measured at different times (12, 24, 48, and 72 h) (Fig E6, C). We observed that recipient mice receiving DNFB-sensitized NKT cells and challenged with DNFB showed significant ear swelling comparable to positive control mice (sensitized, then challenged with DNFB). DNFB-sensitized mice without challenge, naïve mice challenged with DNFB, or recipient mice receiving DNFB-sensitized NKT cells without challenge were unresponsive. These results suggest that allergen-induced NKT cells can develop cutaneous inflammation in our acute allergic mouse model.
To further evaluate whether allergen-induced NKT cells proliferate, we stained the donor NKT cells with CSFE before adoptive transfer (Fig E6, B). We found that adoptively transferred DNFB-sensitized NKT cells quickly migrated into skin at day 1 after DNFB challenge and proliferated fully at day 3 compared to those without DNFB challenge (Fig E6, D). Interestingly, DNFB-sensitized NKT cells in skin-draining LN (dLN) began proliferating at day 1 even without DNFB challenge and proliferated fully at day 3 (with or without DNFB challenge), whereas DNFB-sensitized NKT cells in non-draining LN (non-dLN) showed minimal proliferation. These results indicate that these NKT cells induced by DNFB have skin homing property and maintain their proliferative potential for at least 10 days even without secondary challenge. We next observed that CXCR4 expressing NKT cells were significantly induced in DNFB-challenged skin time-dependently compared to non-challenged skin (Fig E6, E), suggesting that CXCR4+ NKT cell infiltration into acute allergic skin can be accelerated in a time-dependent manner by secondary DNFB challenge.
To address how allergen-induced NKT cells traffic to the skin in vivo in acute allergic inflammation, we adoptively transferred GFP+ DNFB-sensitized NKT cells into CXCL12-DsRed recipients, and then challenged with DNFB (Fig E7, A). By intravital imaging at 6 h after DNFB challenge, NKT cells infiltrated into the skin (Fig E7, B) and randomly migrated with a high velocity (Fig E6, E and F, and Video E1), at which time CXCR4 expression was not induced in NKT cells. At 24 h after DNFB challenge, we observed that GFP+ NKT cells showed reduced motility and began to localize to CXCL12-DsRed+ cells (Fig E7, E and F, and Video E2). At 72 h, GFP+ NKT cells exhibited a greater reduction in motility and more restricted migration, residing in a CXCL12-rich area, followed by formation of a green-red cell cluster (Fig E7, B and E and F, and Video E3), showing a unique pattern of CXCR4+ NKT cells clustering with CXCL12-expressing fibroblasts and T cells (Fig E7, C). We also found that GFP+ NKT cells infiltrated into the skin in a time-dependent fashion (Fig E7, D); at 72 h, GFP+ NKT cells expressed CXCR4, while NKT cells in LNs did not express CXCR4 (Fig E7, G). Taken together, our results suggest that CXCR4 expressing NKT cells traffic to a CXCL12-rich area and form a CXCR4+ NKT/CXCL12+ cell cluster, which is associated with acute allergic inflammation.
Allergen-induced NKT cells mediated cutaneous inflammation in AD mouse model
Next, to investigate the pathogenic role of allergen-induced NKT cells in a chronic AD mouse model, we adoptively transferred ovalbumin (OVA)-sensitized GFP+ NKT cells (5 × 105) into naïve B6 wild-type (WT) recipients 24 h before OVA plus cholera toxin (CT) challenge (Fig 3, A). The recipients showed slow but substantive ear swelling at day 7 comparable to the positive control group with OVA sensitization and challenge, while recipients administered OVA-sensitized NKT cells without challenge or mice with OVA sensitization alone did not respond (Fig 3, B), suggesting that NKT cells induced by chronic allergen exposure also mediate skin inflammation in the AD mouse model. To exclude the effect of conventional T cells, we conducted the same process but transferred OVA-sensitized NKT cells into Rag1−/− recipients. Our data showed that NKT cells mediated skin inflammation induced by OVA in absence of adaptive immunity, but the intensity of the skin inflammation was lower (Fig E8) when compared to the results shown in Fig 3, B, indicating that endogenous conventional T cells also participate in skin inflammation induced by OVA (Fig 3, B; Fig E8).
Fig 3. NKT cells developed cutaneous inflammation in chronic AD mouse model.
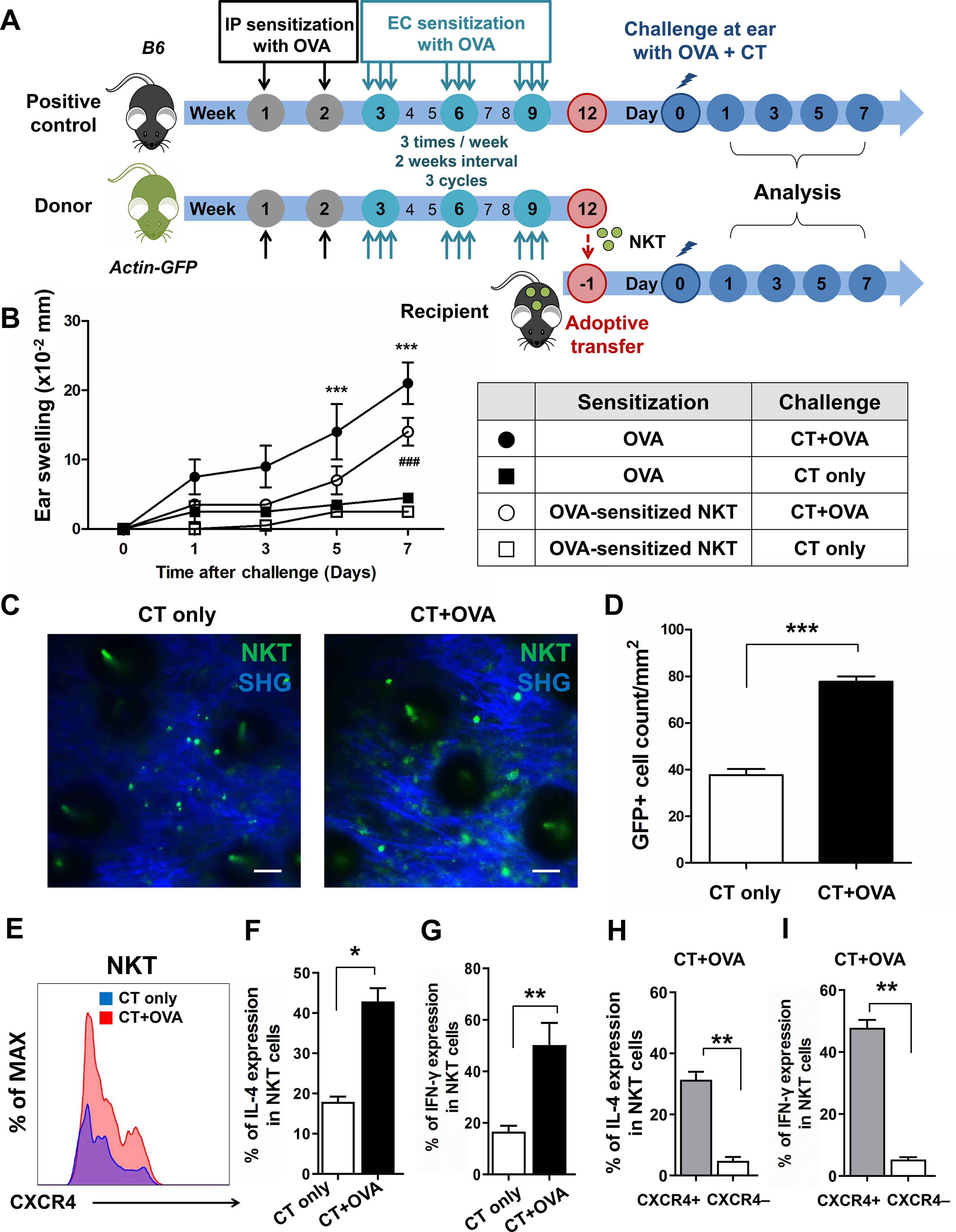
(A) Experimental scheme. B6 or GFP mice were intraperitoneally administered 50 μg of OVA or saline with alum once per week for 2 weeks. Mouse dorsal skin was tape-stripped and EC-sensitized with OVA three times per week at 2-week intervals 3 times. Three weeks later, the actin-GFP mice splenic NKT cells were sorted by Live cells/CD45+/CD3+/NK1.1+, and then intravenously injected into naïve B6 mice. Positive control B6 mice and recipient B6 mice were challenged 1 day later with 100 μg of OVA + CT. Ear thickness was analyzed on days 1, 3, 5, and 7. (B) Ear swelling of recipients (***p < 0.001, ###p < 0.001). (C) Representative intravital images of GFP+ NKT cells located in the ear with or without OVA challenge on day 7. Cell count (D), CXCR4 (E), IL-4 (F), and IFN-γ (G) expressions of GFP+ NKT cells in the ears with or without OVA challenge on day 7 (*p < 0.05, **p < 0.01, ***p < 0.001). Populations of IL-4 (H) and IFN-γ (I) in skin CXCR4+ and CXCR4− GFP+ NKT cells with OVA challenge on day 7 (**p < 0.01). Data are representative of two experiments with n = 8 mice per group. Scale bar = 20 μm.
By intravital microscopy, we found slowly migratory NKT cells in OVA-challenged ears at day 1 (Video E4). At day 7, we observed that a significant number of GFP+ NKT cells migrated into OVA-challenged ears compared to OVA-unchallenged ears (CT only) (Fig 3, C and D); clusters of GFP+ NKT cells were sessile and showed restricted motility, indicating that OVA-induced NKT cells can also form clusters (Video E5). This is consistent with our results in acute allergic inflammation. We also found that OVA-induced NKT cells in OVA-challenged skin expressed more CXCR4 than in unchallenged skin (Fig 3, E). These CXCR4 expressing NKT cells produced significantly higher levels of IL-4 and IFN-γ than CXCR4- NKT cells (Fig 3, F–I), supporting that chronically induced skin-resident NKT cells contribute to chronic AD development.
Skin-resident NKT cells expressed CXCR4 and CD69 resemble TRM cells
Our results for the skin and blood transcriptomes revealed the difference between skin and blood T cells (Fig 1, C), and our mouse model showed differences in CXCR4 expression in NKT cells in the skin compared to LN. To further evaluate these results, we compared the phenotype of NKT cells among the skin, liver, LN, and spleen from normal WT B6 mice. We found that the numbers of CD3+NK1.1+ NKT cells or CD1d restricted NKT cells were highest in the liver, followed by in the skin, spleen, and LN (Fig 4, A and Fig E9, A). When we measured CXCR4 expression in NKT cells, only skin NKT cells expressed CXCR4 (Fig 4, A, and Fig E9, B and C). In contrast, another marker for NKT cells, CXCR6, was highly expressed in all tissues including the liver and skin but was not skin-specific (Fig E9, D), indicating that CXCR4 may be a specific marker of skin-resident NKT cells. We also confirmed two purification strategies of NKT cells and there is no significant difference between the CD3+NK1.1+ NKT cells and TCRβ+α-Galcer CD1d+ NKT cells (Fig.E10 A and B). Recently, many different types of tissue-resident memory (TRM) T cells have been identified including skin-resident T cells23 and γδT cells.33 Thus, we evaluated the expression of CD69, a widely appreciated marker for TRM,34 in NKT cells from different tissues. CD69+ NKT cells were most abundant in the liver and skin, followed by the spleen and LN (Fig 4, A). In normal mouse skin, the predominant NKT cells were CD69+, and all CXCR4 expressing NKT cells were also CD69+(Fig 4, A), suggesting that CXCR4 expressing NKT cells represent a type of TRM in skin. Consistent with our mouse results, we observed that CXCR4+CD69+Vα24Jα18+ NKT cells could be identified in human AD skin compared to HC skin (Fig. 4, B and C), and skin-resident NKT cells expressed higher levels of CD69 than circulating NKT cells from AD patients (Fig 4, D). This indicates that CXCR4+CD69+ NKT cells are a type of TRM that contribute to AD development by forming clusters enriched for NKT cells in human AD skin (Fig 4, B).
Fig 4. Skin-resident NKT cells expressed CXCR4 and CD69.
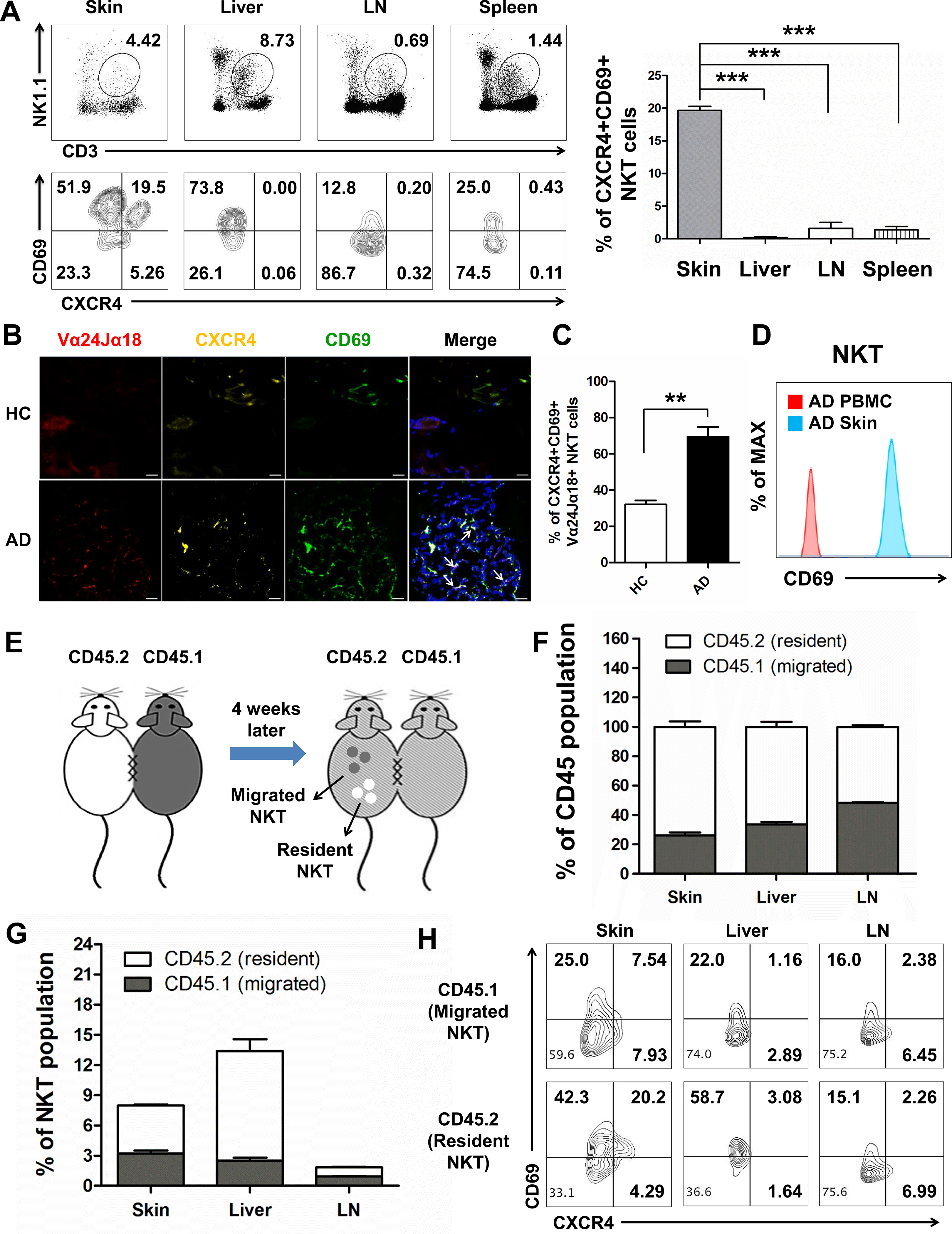
(A) Frequency of CD3 and NK1.1 double-positive NKT cells (UP), and their CXCR4 and CD69 expression (down) in skin, liver, LN, and spleen (n = 5 mice/group, three repeats). (***p < 0.001). (B) Expression of CXCR4 and CD69 in the Vα24Jα18+ NKT cells of human HC and AD skin. Merged staining of Vα24Jα18+ (red), CXCR4 (yellow), and CD69 (green) were observed in the lesional skin of AD patients marked with white arrow. Nuclei were stained with DAPI (blue). Original magnification X200. Scale bar = 20 μm. (C) Percentage of CXCR4+CD69+ NKT cells in HC and AD patient skin. (**p < 0.01). (D) Expression of CD69 in the skin and PBMC NKT cells of AD patient. (E) Parabiosis experimental scheme. CD45.1 and CD45.2 congenic mice were joined by parabiosis surgery. In CD45.2 host mice, CD45.1+ and CD45.2+ cells were considered as migrating cells and resident cells, respectively. (n = 5 pairs/ group, three repeats). (F) The CD45.1+/CD45.2+ cells from each parabiont distributed in skin, liver, and LN of CD45.2 host mice at week 4. (G) The frequency of CD45.1+/CD45.2+ CD3+NK1.1+ NKT cells in the skin, liver, and LN of CD45.2 parabiont. (H) CXCR4 and CD69 expression of CD45.1+/CD45.2+ CD3+NK1.1+ NKT cells pooled from the skin, liver and LN of CD45.2 parabiont.
To further verify the tissue-residency of skin NKT cells, CD45.1 and CD45.2 congenic mice were joined by parabiosis surgery. In this setting, re-circulating cells equilibrate between each parabiont 35, whereas tissue resident cells do not (Fig 4, E). At 4 weeks after parabiosis surgery, substantial chimerism of LN leukocytes was observed, while CD45+ cells from the skin and liver were dis-equilibrated between each parabiont (Fig 4, F). For NKT cells, drastic disequilibrium of NKT cells was maintained in the liver, and more CD45.2+ tissue-resident NKT cells were observed in the skin, whereas NKT cells of either CD45.1+ or CD45.2+ origin in the LN were present in a similar ratio (Fig 4, G). Host tissue-resident NKT cells in the liver expressed higher CD69 levels than NKT cells that had migrated from the partner (Fig 4, H). Skin-resident NKT cells also expressed higher levels of CD69 compared to migratory NKT cells, while LN NKT cells from both the host and partner showed low levels of CD69. Furthermore, skin-resident NKT cells expressed higher levels of CXCR4 than migratory NKT cells from the parabiotic partner (Fig 4, H), supporting the tissue-residency of CXCR4+CD69+ NKT cells in skin.
To further examine the different expression of CXCR4 between skin-resident and migrating NKT cells, we used KAEDE mice, which contain a photoconvertible green-fluorescent protein that irreversibly changes appears as red upon violet light exposure36. Immediately after exposure (After), all KAEDE+ skin cells red (Fig 5, A and B, and Video E6 and E7). At 24 h after exposure, we identified a population of rapidly moving KAEDE-green cells in the photoconverted ear, indicating their recent entry into the skin (Fig 5, A and B, and Video E8). Consistent with these results, we observed that KAEDE-red NKT cells expressed higher levels of CD69 and CXCR4 than KAEDE-green NKT cells (Fig. 5, C and D), confirming non-migratory CXCR4+CD69+ KAEDE-red NKT cells as a type of TRM cells in the skin.
Fig 5. Skin-resident NKT cells after photoconversion of KAEDE mice.
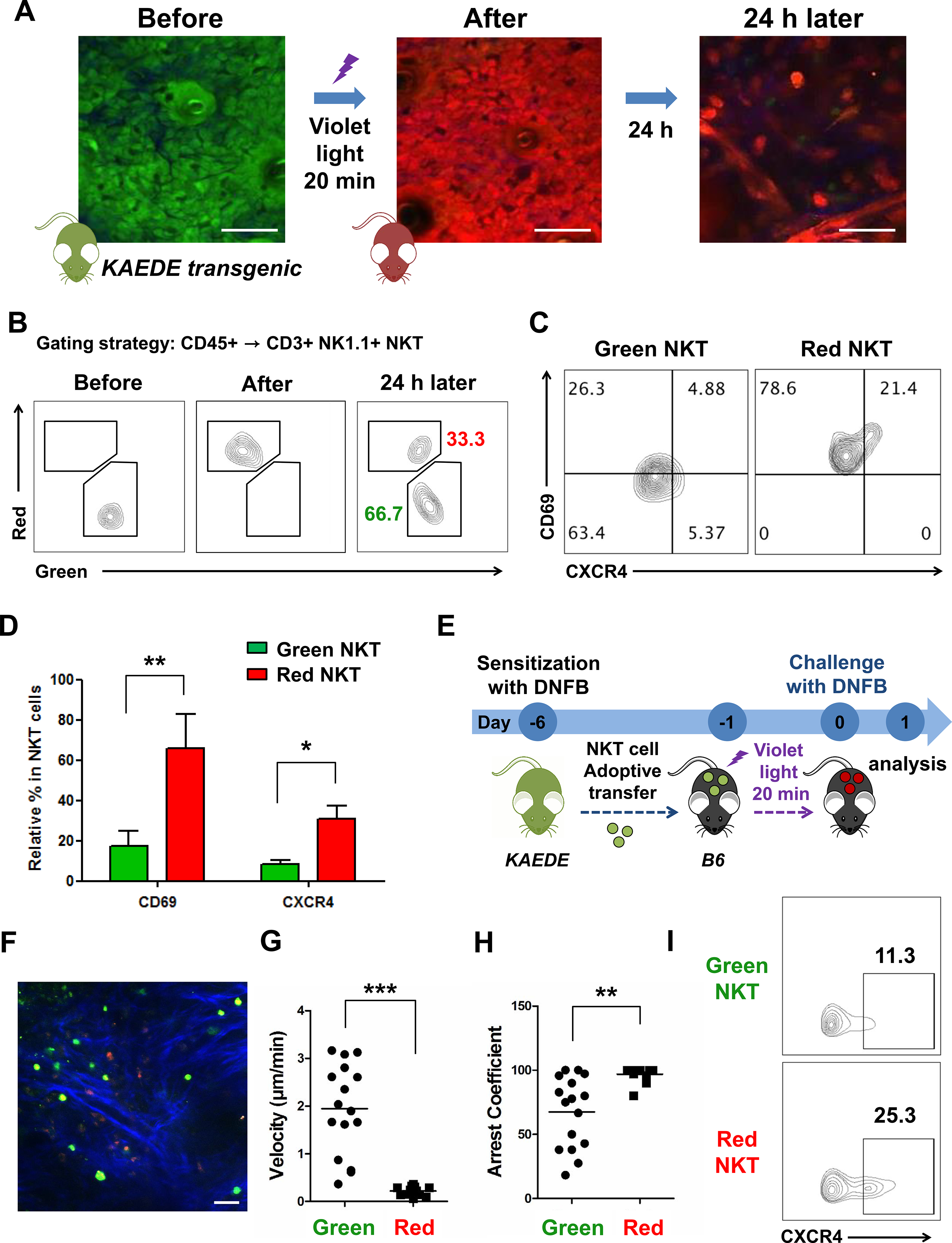
(A) Representative intravital images of KAEDE mice ear at indicated times after violet light exposure. (B) Proportion of KAEDE-red and green CD3+NK1.1+ NKT cells at indicated times. (C) CXCR4 and CD69 expression in KAEDE-red and green NKT cells. (D) Relative percentage of CD69+ and CXCR4+ NKT cells in KAEDE-red and green NKT cells (*p < 0.05, **p < 0.01). (E) Experimental scheme. DNFB-sensitized KAEDE mice were used as donors for adoptive transfer of NKT cells into naïve B6 recipients, half by intravenous injection and half by intradermal injection into the right ears. Next, the right ears were exposed to violet light for 20 min. After photoconversion for 6 h, the right ears were challenged with DNFB, while vehicle was applied to the left ones, and then live images were acquired at 24 h after photoconversion. (F) Representative intravital images of ear at 24 h after violet light exposure. Mean velocity (G) and arrest coefficients (H) of KAEDE-red and green NKT cells in DNFB-challenged ear at 24 h after violet light exposure. Points represent individual cells, and lines indicate means (**p < 0.01, ***p < 0.001). (I) CXCR4 expression in KAEDE-red and green NKT cells. Data are representative of three experiments with n = 5 mice per group. Scale bar = 20 μm.
To characterize the migratory patterns of skin NKT cells, we adoptively transferred DNFB-sensitized KAEDE NKT cells via intradermal injection (half) and intravenous injection (half) into B6 mice (Fig 5, E). The ears were exposed to violet light immediately, and then challenged with DNFB at 6 h after photo-conversion (Fig 5, E). We found both KAEDE-red and green NKT cells in DNFB-challenged ears at 24 h after violet light exposure (Fig 6, F), supporting the recent recruitment of KAEDE-green NKT cells from the circulation. Moreover, most KAEDE-red NKT cells were sessile, while KAEDE-green NKT cells were mobile (Fig 5, G and H, and Video E9). Consistent with our data above, most KAEDE-red NKT cells retained high CXCR4, a phenotype associated with skin-resident NKT cells, and distinct from KAEDE-green NKT cells that expressed lower levels of CXCR4, a phenotype of skin-migrated NKT cells (Fig 5, I).
Fig 6. CXCR4+ NKT cells developed cutaneous allergic inflammation.
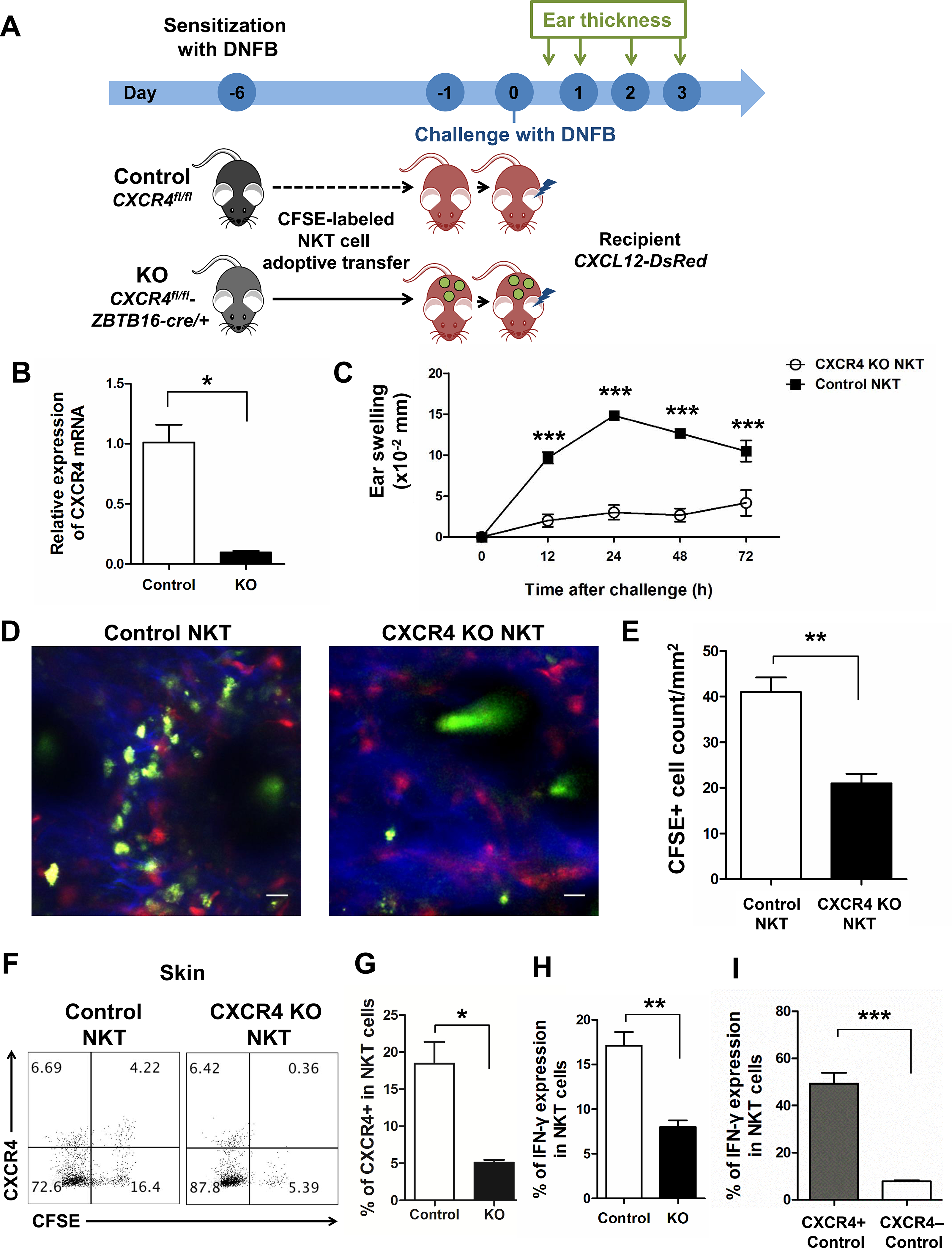
(A) Experimental scheme. CXCR4-flox and ZBTB16-GFP/Cre mice were crossed to generate pups (CXCR4fl/fl-ZBTB16cre/+) that were CXCR4 deficient in NKT cells. DNFB-sensitized splenic CD3+NK1.1+ NKT cells from CXCR4fl/fl (Control) and CXCR4fl/fl-ZBTB16cre/+ mice spleen were adoptively transferred into CXCR12-DsRed recipients after CFSE staining. After 24 h, the right ears of recipients were challenged with DNFB, while vehicle was applied to the left ears, and then ear swelling was measured at 12, 24, 48, and 72 h. (B) RT-PCR results of splenic NKT cells sorted from control and CXCR4 KO mice. (*p < 0.05). (C) Ear swelling of CXCL12-DsRed recipients at indicated times (***p < 0.001). Representative intravital images (D) and cell count (E) of control NKT cells and CXCR4 KO NKT cells in the ears challenged by DNFB for 72 h. (**p < 0.01). Expression (F) and percentage (G) of CXCR4+ NKT cells and IFN-γ expression (H) in adoptively transferred control and CXCR4 KO NKT cells in the skin (*p < 0.05, **p < 0.01). (I) IFN-γ expression of CXCR4+ and CXCR4− NKT cells in adoptively transferred control NKT cells pooled from ears (***p < 0.001). Data are representative of three experiments with n = 5 mice per group. Scale bar = 20 μm.
CXCR4 deficient NKT cells failed to mediate cutaneous allergic inflammation
To evaluate the function of CXCR4 in NKT cells, we crossed CXCR4-flox and ZBTB16-GFP/Cre mice to generate offspring (CXCR4fl/fl-ZBTB16cre/+) in which the CXCR4 gene is selectively deleted (CXCR4 KO) in NKT cells (Fig 6, A and B). Next, we sorted DNFB-sensitized splenic NKT cells from CXCR4fl/fl (Control) and CXCR4fl/fl-ZBTB16cre/+(CXCR4 KO) mice, and adoptively transferred these NKT cells into CXCR12-DsRed recipients after CFSE staining Fig 6, A). We observed that recipients administered DNFB-sensitized CXCR4 KO NKT cells did not demonstrate ear swelling between 12 h and 72 h after DNFB challenge compared to the WT control group (Fig 6, C). We also found that CXCR4 deficient NKT cells showed a significantly compromised ability to infiltrate the DNFB-challenged ear (Fig 6, D), while a much larger number of WT NKT cells (green) resided in the CXCL12-rich area (red), forming an enriched green-red cluster in DNFB-challenged ears at day 3 (Fig 6, D and E). We confirmed that CXCR4+ NKT cells migrated into DNFB-challenged skin of recipients that received WT NKT cells (Fig 6, F), but as expected most skin NKT cells did not express CXCR4 in the CXCR4 KO group administered DNFB-sensitized CXCR4 deficient NKT cells (Fig 6, F and G). WT NKT cells in DNFB-challenged skin of control group produced significantly higher levels of IFN-γ compared to CXCR4 deficient NKT cells (Fig 6, H). Furthermore, in the WT control group, CXCR4+ NKT cells were the major producers of IFN-γ, while CXCR4− NKT cells produced very low levels of IFN-γ in DNFB-challenged skin (Fig 6, I), strongly supporting a pathogenic role for CXCR4 expressing skin-resident NKT cells in the induction of cutaneous inflammation in AD.
DISCUSSION
CXCR4 is a 7-transmembrane domain and G-protein-coupled cell surface receptor,37 and was initially identified as a co-receptor for entry of T-tropic(X4) HIV viruses into CD4 T cells.38 CXCR4 was reported to be essential for developmental organogenesis,18,19 cancer metastasis,39 and immune cell trafficking.20 While CXCR4 mediated chemotaxis of NK cells has already been reported,40 our proteomics data suggested TSLP induced CXCR4 expression in NKT cells, suggesting a plausible mechanism for CXCR4 mediated NKT cell trafficking to AD skin enriched with TSLP. We demonstrated that CXCR4+ NKT cells infiltrated AD skin in greater numbers than in normal human skin. Interestingly, circulating NKT cells from AD patients expressed lower levels of CXCR4 compared to AD skin NKT cells (Fig 1, F), suggesting that CXCR4+ NKT cells have a skin-specific role in allergic inflammation. Furthermore, in parabiosis studies and in KAEDE mice, CXCR4+ NKT cells also expressed CD69+, an accepted marker of TRM in the skin, confirming the specific role of CXCR4+ NKTRM cells in the skin.
The migration of pathogenic T cells to the affected skin in inflammatory skin diseases is a pivotal event41 in the development of cutaneous manifestations in AD. T cell trafficking to inflamed skin has been widely studied.42 Cutaneous lymphocyte antigen and CCR4 are essential for skin homing T cells.43,44 In contrast to conventional T cells, however, the migration properties of NKT cells into the skin have not been thoroughly evaluated. Human NKT cells have different migration properties depending on their anatomic location and disease state. Liver NKT cells highly express CXCR6 and respond to CXCL16, accumulating in the liver and providing immune surveillance, anti-tumor activity, or promotion of inflammation and liver fibrosis.45,46 Kidney NKT cells expressing CXCR6 also attenuated severe nephritis in a murine model.47 CXCR6 was also expressed in skin NKT cells, but NKT cells in all tissues including the liver, LN, and spleen expressed high levels of CXCR6; thus, CXCR6 expression is a general property for NKT and is not skin-specific. In contrast, we found that CXCR4+ NKT cells were found predominantly in skin and were rarely present in the liver, LN, and spleen. Notably, all NKT cells in the liver, LN, and spleen were CXCR4−. Additionally, after adoptive transfer of allergen-induced NKT cells, CXCR4+ NKT cells migrated preferentially to the skin rather than the liver, LN, or spleen. Furthermore, KAEDE photo-conversion studies demonstrated that the KAEDE-red skin-resident NKT cells expressed higher levels of CXCR4 than migratory KAEDE-green NKT cells. This suggests that CXCR4 is a skin-specific chemokine receptor for NKTRM and that it contributes to pathogenic events by inducing infiltration of NKT cells into the inflamed CXCL12-enriched skin of AD.
CXCL12, a cognate ligand for CXCR4, plays a crucial role in the migration of various cell types, including immune cells, to sites of inflammation.14,48 CXCL12 is produced by bone marrow stromal cells,49 endothelial cells,50 and dermal fibroblasts.51 We demonstrated that vimentin+ dermal fibroblasts in AD skin expressed significantly higher levels of CXCL12 compared to normal human skin. Interestingly, T cells also contributed to the production of CXCL12 in AD skin. The tissue microenvironment of AD, which contains high levels of TSLP, may affect the production of CXCL12 in skin. TSLP strongly induced CXCL12 in dermal fibroblasts and T cells 24 h after TSLP treatment and was consistently elevated in our AD mouse model. Furthermore, in vivo imaging studies demonstrated that under the influence of CXCL12, CXCR4+ NKT cells patrolled and attached to CXCL12-expressing cells, forming a CXCR4+ NKT/CXCL12+ cell clusters in AD skin. This is consistent with previous in vivo imaging studies.50 Additionally, conditional deletion of CXCR4 in NKT cells significantly reduced the infiltration of NKT cells into the inflamed skin of our mouse model, further supporting an essential role for the CXCR4/CXCL12 axis in skin-resident NKT cells in AD.
Recently, various phenotypes of tissue-resident memory T cells have been identified in non-barrier and barrier tissues including the skin.34,52,53 TRM in the skin play a role in protective immunity against infection23,36,54,55 and a critical role in the development of chronic inflammatory skin diseases including psoriasis56,57 and AD.58 This is the first study to identify skin-resident NKT cells expressing CXCR4 and CD69 as a phenotype of TRM in AD. We further confirmed that CXCR4 expressing skin-resident NKT cells are pathogenic TRMs that contribute to the development of cutaneous allergic inflammation in AD. We confirmed this by several experiments using adoptive transfer of allergen-induced CXCR4+ and CXCR− CD3+NK1.1+ NKT cells and TCRβ+α-Galcer CD1d+ NKT cells59 in acute and chronic allergic inflammation using our AD model. CXCR4+CD69+ NKTRM cells and CXCL12+ cells in AD skin were increased and formed clusters, and we suggest that, CXCR4/CXCL12 inhibition can represent a therapeutic alternative for AD patients.
Supplementary Material
Key Messages.
CXCR4 and CXCL12 levels are consistently elevated in human and mouse AD skin.
Skin-resident CXCR4+ NKT cells form a cluster with CXCL12+ cells and are associated with allergic inflammation in AD.
Acknowledgements
We thank Dr. Pilhan Kim for (KAIST, Daejeon, Korea) Actin-GFP mice, Dr. Min-Geol Lee (Yonsei University College of Medicine, Seoul, Korea) for CD45.1+ mice, Dr. Tae-Gyun Kim (Yonsei University College of Medicine, Seoul, Korea) for Rag1−/− mice. Dr. Hye Young Kim (Seoul National University, Seoul, Korea) for Jα18−/− mice, Dr. Yoshihiro Miwa (College of Medicine, Tsukuba University) for KAEDE mice. This work was supported by NIH grants R01AI041707 (T.S.K.), R01AI127654 (T.S.K.), TR01AI097128 (T.S.K. and R.A.C.), R01AR063962 (R.A.C.), and the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science and ICT) (No. NRF-2017R1A2B4009568 (C.O.P)), grants of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI14C1324 (K.H.L and C.O.P), HI14C1799 (C.O.P)), the “Dongwha” Faculty Research Assistance Program of Yonsei University College of Medicine (6–2016-0129, C.O.P), and Amorepacific Grant (2018, C.O.P).
Abbreviations used
- AD
Atopic dermatitis
- CT
Cholera toxin
- CXCR4
C-X-C chemokine receptor type 4
- DNFB
Dinitrofluorobenzene
- FITC
Fluorescein isothiocyanate
- GFP
Green fluorescence protein
- GO
Gene Ontology
- HC
Healthy controls
- HPF
High power field
- IFN
Interferon
- IL
Interleukin
- LC
Liquid chromatography
- LN
Lymph nodes
- MS
Mass spectrometry
- NKT
Natural killer T
- OVA
Ovalbumin
- PBMC
Peripheral blood mononuclear cells
- TMT
Tandem Mass Tag
- TRM
Tissue-resident memory T
- TSLP
Thymic stromal lymphopoietin
- WT
Wild-type
Footnotes
Conflict of interest
All authors declare no conflicts of interest.
Competing interests
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bieber T Atopic dermatitis. N Engl J Med 2008;358:1483–94. [DOI] [PubMed] [Google Scholar]
- 2.Chu H, Shin JU, Park CO, Lee H, Lee J, Lee KH. Clinical Diversity of Atopic Dermatitis: A Review of 5,000 Patients at a Single Institute. Allergy Asthma Immunol Res 2017;9:158–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leung DY, Boguniewicz M, Howell MD, Nomura I, Hamid QA. New insights into atopic dermatitis. J Clin Invest 2004;113:651–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weidinger S, Novak N. Atopic dermatitis. Lancet 2016;387:1109–22. [DOI] [PubMed] [Google Scholar]
- 5.Park CO, Noh S, Jin S, Lee NR, Lee YS, Lee H, et al. Insight into newly discovered innate immune modulation in atopic dermatitis. Exp Dermatol 2013;22:6–9. [DOI] [PubMed] [Google Scholar]
- 6.Wu WH, Park CO, Oh SH, Kim HJ, Kwon YS, Bae BG, et al. Thymic stromal lymphopoietin-activated invariant natural killer T cells trigger an innate allergic immune response in atopic dermatitis. J Allergy Clin Immunol 2010;126:290–9, 9.e1–4. [DOI] [PubMed] [Google Scholar]
- 7.Allakhverdi Z, Comeau MR, Jessup HK, Yoon BR, Brewer A, Chartier S, et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med 2007;204:253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gros E, Bussmann C, Bieber T, Forster I, Novak N. Expression of chemokines and chemokine receptors in lesional and nonlesional upper skin of patients with atopic dermatitis. J Allergy Clin Immunol 2009;124:753–60 e1. [DOI] [PubMed] [Google Scholar]
- 9.Shi FD, Ljunggren HG, La Cava A, Van Kaer L. Organ-specific features of natural killer cells. Nat Rev Immunol 2011;11:658–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat Immunol 2010;11:197–206. [DOI] [PubMed] [Google Scholar]
- 11.Albacker LA, Chaudhary V, Chang YJ, Kim HY, Chuang YT, Pichavant M, et al. Invariant natural killer T cells recognize a fungal glycosphingolipid that can induce airway hyperreactivity. Nat Med 2013;19:1297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol 2013;13:101–17. [DOI] [PubMed] [Google Scholar]
- 13.Shimizuhira C, Otsuka A, Honda T, Kitoh A, Egawa G, Nakajima S, et al. Natural killer T cells are essential for the development of contact hypersensitivity in BALB/c mice. J Invest Dermatol 2014;134:2709–18. [DOI] [PubMed] [Google Scholar]
- 14.Schiraldi M, Raucci A, Munoz LM, Livoti E, Celona B, Venereau E, et al. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med 2012;209:551–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim CH, Johnston B, Butcher EC. Trafficking machinery of NKT cells: shared and differential chemokine receptor expression among V alpha 24(+)V beta 11(+) NKT cell subsets with distinct cytokine-producing capacity. Blood 2002;100:11–6. [DOI] [PubMed] [Google Scholar]
- 16.Thomas SY, Hou R, Boyson JE, Means TK, Hess C, Olson DP, et al. CD1d-restricted NKT cells express a chemokine receptor profile indicative of Th1-type inflammatory homing cells. J Immunol 2003;171:2571–80. [DOI] [PubMed] [Google Scholar]
- 17.Johnston B, Kim CH, Soler D, Emoto M, Butcher EC. Differential chemokine responses and homing patterns of murine TCR alpha beta NKT cell subsets. J Immunol 2003;171:2960–9. [DOI] [PubMed] [Google Scholar]
- 18.Chang CH, Hale SJ, Cox CV, Blair A, Kronsteiner B, Grabowska R, et al. Junctional Adhesion Molecule-A Is Highly Expressed on Human Hematopoietic Repopulating Cells and Associates with the Key Hematopoietic Chemokine Receptor CXCR4. Stem Cells 2016;34:1664–78. [DOI] [PubMed] [Google Scholar]
- 19.Ivins S, Chappell J, Vernay B, Suntharalingham J, Martineau A, Mohun TJ, et al. The CXCL12/CXCR4 Axis Plays a Critical Role in Coronary Artery Development. Dev Cell 2015;33:455–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arieta Kuksin C, Gonzalez-Perez G, Minter LM. CXCR4 expression on pathogenic T cells facilitates their bone marrow infiltration in a mouse model of aplastic anemia. Blood 2015;125:2087–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zgraggen S, Huggenberger R, Kerl K, Detmar M. An important role of the SDF-1/CXCR4 axis in chronic skin inflammation. PLoS One 2014;9:e93665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li JL, Goh CC, Keeble JL, Qin JS, Roediger B, Jain R, et al. Intravital multiphoton imaging of immune responses in the mouse ear skin. Nat Protoc 2012;7:221–34. [DOI] [PubMed] [Google Scholar]
- 23.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature 2012;483:227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol 2002;3:673–80. [DOI] [PubMed] [Google Scholar]
- 25.Thompson A, Schafer J, Kuhn K, Kienle S, Schwarz J, Schmidt G, et al. Tandem mass tags: a novel quantification strategy for comparative analysis of complex protein mixtures by MS/MS. Anal Chem 2003;75:1895–904. [DOI] [PubMed] [Google Scholar]
- 26.Li Z, Adams RM, Chourey K, Hurst GB, Hettich RL, Pan C. Systematic comparison of label-free, metabolic labeling, and isobaric chemical labeling for quantitative proteomics on LTQ Orbitrap Velos. J Proteome Res 2012;11:1582–90. [DOI] [PubMed] [Google Scholar]
- 27.Jin S, Park CO, Shin JU, Noh JY, Lee YS, Lee NR, et al. DAMP molecules S100A9 and S100A8 activated by IL-17A and house-dust mites are increased in atopic dermatitis. Exp Dermatol 2014;23:938–41. [DOI] [PubMed] [Google Scholar]
- 28.Park CO, Lee HJ, Lee JH, Wu WH, Chang NS, Hua L, et al. Increased expression of CC chemokine ligand 18 in extrinsic atopic dermatitis patients. Exp Dermatol 2008;17:24–9. [DOI] [PubMed] [Google Scholar]
- 29.Guttman-Yassky E, Suarez-Farinas M, Chiricozzi A, Nograles KE, Shemer A, Fuentes-Duculan J, et al. Broad defects in epidermal cornification in atopic dermatitis identified through genomic analysis. J Allergy Clin Immunol 2009;124:1235–44.e58. [DOI] [PubMed] [Google Scholar]
- 30.Suarez-Farinas M, Ungar B, Correa da Rosa J, Ewald DA, Rozenblit M, Gonzalez J, et al. RNA sequencing atopic dermatitis transcriptome profiling provides insights into novel disease mechanisms with potential therapeutic implications. J Allergy Clin Immunol 2015;135:1218–27. [DOI] [PubMed] [Google Scholar]
- 31.Rochman Y, Dienger-Stambaugh K, Richgels PK, Lewkowich IP, Kartashov AV, Barski A, et al. TSLP signaling in CD4(+) T cells programs a pathogenic T helper 2 cell state. Sci Signal 2018;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shin JU, Kim SH, Kim H, Noh JY, Jin S, Park CO, et al. TSLP Is a Potential Initiator of Collagen Synthesis and an Activator of CXCR4/SDF-1 Axis in Keloid Pathogenesis. J Invest Dermatol 2016;136:507–15. [DOI] [PubMed] [Google Scholar]
- 33.Jiang X, Park CO, Geddes Sweeney J, Yoo MJ, Gaide O, Kupper TS. Dermal gammadelta T Cells Do Not Freely Re-Circulate Out of Skin and Produce IL-17 to Promote Neutrophil Infiltration during Primary Contact Hypersensitivity. PLoS One 2017;12:e0169397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park CO, Kupper TS. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat Med 2015;21:688–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collins N, Jiang X, Zaid A, Macleod BL, Li J, Park CO, et al. Skin CD4(+) memory T cells exhibit combined cluster-mediated retention and equilibration with the circulation. Nat Commun 2016;7:11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park CO, Fu X, Jiang X, Pan Y, Teague JE, Collins N, et al. Staged development of long-lived T-cell receptor alphabeta TH17 resident memory T-cell population to Candida albicans after skin infection. J Allergy Clin Immunol 2018;142:647–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loetscher M, Geiser T, O’Reilly T, Zwahlen R, Baggiolini M, Moser B. Cloning of a human seven-transmembrane domain receptor, LESTR, that is highly expressed in leukocytes. J Biol Chem 1994;269:232–7. [PubMed] [Google Scholar]
- 38.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science 1996;272:872–7. [DOI] [PubMed] [Google Scholar]
- 39.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001;410:50–6. [DOI] [PubMed] [Google Scholar]
- 40.Noda M, Omatsu Y, Sugiyama T, Oishi S, Fujii N, Nagasawa T. CXCL12-CXCR4 chemokine signaling is essential for NK-cell development in adult mice. Blood 2011;117:451–8. [DOI] [PubMed] [Google Scholar]
- 41.Kupper TS, Fuhlbrigge RC. Immune surveillance in the skin: mechanisms and clinical consequences. Nat Rev Immunol 2004;4:211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu L, Fuhlbrigge RC, Karibian K, Tian T, Kupper TS. Dynamic programming of CD8+ T cell trafficking after live viral immunization. Immunity 2006;25:511–20. [DOI] [PubMed] [Google Scholar]
- 43.Fuhlbrigge RC, Kieffer JD, Armerding D, Kupper TS. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells. Nature 1997;389:978–81. [DOI] [PubMed] [Google Scholar]
- 44.Campbell JJ, Haraldsen G, Pan J, Rottman J, Qin S, Ponath P, et al. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature 1999;400:776–80. [DOI] [PubMed] [Google Scholar]
- 45.Wehr A, Baeck C, Heymann F, Niemietz PM, Hammerich L, Martin C, et al. Chemokine receptor CXCR6-dependent hepatic NK T Cell accumulation promotes inflammation and liver fibrosis. J Immunol 2013;190:5226–36. [DOI] [PubMed] [Google Scholar]
- 46.Mackay LK, Minnich M, Kragten NA, Liao Y, Nota B, Seillet C, et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science 2016;352:459–63. [DOI] [PubMed] [Google Scholar]
- 47.Riedel JH, Paust HJ, Turner JE, Tittel AP, Krebs C, Disteldorf E, et al. Immature renal dendritic cells recruit regulatory CXCR6(+) invariant natural killer T cells to attenuate crescentic GN. J Am Soc Nephrol 2012;23:1987–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lim K, Hyun YM, Lambert-Emo K, Capece T, Bae S, Miller R, et al. Neutrophil trails guide influenza-specific CD8(+) T cells in the airways. Science 2015;349:aaa4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kohara H, Omatsu Y, Sugiyama T, Noda M, Fujii N, Nagasawa T. Development of plasmacytoid dendritic cells in bone marrow stromal cell niches requires CXCL12-CXCR4 chemokine signaling. Blood 2007;110:4153–60. [DOI] [PubMed] [Google Scholar]
- 50.Pitt LA, Tikhonova AN, Hu H, Trimarchi T, King B, Gong Y, et al. CXCL12-Producing Vascular Endothelial Niches Control Acute T Cell Leukemia Maintenance. Cancer Cell 2015;27:755–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fedyk ER, Jones D, Critchley HO, Phipps RP, Blieden TM, Springer TA. Expression of stromal-derived factor-1 is decreased by IL-1 and TNF and in dermal wound healing. J Immunol 2001;166:5749–54. [DOI] [PubMed] [Google Scholar]
- 52.Watanabe R, Gehad A, Yang C, Scott LL, Teague JE, Schlapbach C, et al. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci Transl Med 2015;7:279ra39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gaide O, Emerson RO, Jiang X, Gulati N, Nizza S, Desmarais C, et al. Common clonal origin of central and resident memory T cells following skin immunization. Nat Med 2015;21:647–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu L, Zhong Q, Tian T, Dubin K, Athale SK, Kupper TS. Epidermal injury and infection during poxvirus immunization is crucial for the generation of highly protective T cell-mediated immunity. Nat Med 2010;16:224–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pan Y, Tian T, Park CO, Lofftus SY, Mei S, Liu X, et al. Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature 2017;543:252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schlapbach C, Gehad A, Yang C, Watanabe R, Guenova E, Teague JE, et al. Human TH9 cells are skin-tropic and have autocrine and paracrine proinflammatory capacity. Sci Transl Med 2014;6:219ra8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matos TR, O’Malley JT, Lowry EL, Hamm D, Kirsch IR, Robins HS, et al. Clinically resolved psoriatic lesions contain psoriasis-specific IL-17-producing alphabeta T cell clones. J Clin Invest 2017;127:4031–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hijnen D, Knol EF, Gent YY, Giovannone B, Beijn SJ, Kupper TS, et al. CD8(+) T cells in the lesional skin of atopic dermatitis and psoriasis patients are an important source of IFN-gamma, IL-13, IL-17, and IL-22. J Invest Dermatol 2013;133:973–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matsuda JL, Naidenko OV, Gapin L, Nakayama T, Taniguchi M, Wang CR, et al. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J Exp Med 2000;192:741–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


