Abstract
Background
Nearly 6,000 multisystem inflammatory syndrome in children (MIS-C) have been reported in the United States by November 2021. Left ventricular global myocardial strain has been proved to be one of the best evidence of the diagnostic and prognostic implications for cardiac dysfunction. The global myocardial strain change of MIS-C in the acute phase was still unclear.
Methods
PubMed and other sources were searched. A network meta-analysis was conducted. MIS-C was divided into two groups according to left ventricular ejection fraction (LVEF): MIS-C with depressed ejection fraction (MIS-C dEF) and MIS-C with preserved ejection fraction (MIS-C pEF). Global longitudinal strain (GLS) and global circumferential strain (GCS) were compared among MIS-C, Kawasaki disease (KD), and healthy children.
Results
In total, nine case-control studies were included, published between 2014 and 2021. These studies involved 107 patients with MIS-C, 188 patients with KD, and 356 healthy children. After Bayesian analysis, MIS-C dEF group was found to have a lower LVEF, higher GLS and GCS than the KD groups. Both MIS-C pEF and KD had similar GLS and GCS, which were higher than healthy controls. There was no difference of LVEF among MIS-C pEF, KD, and healthy controls.
Conclusion
MIS-C dEF was more severe than KD, both in LVEF and global myocardial strain. MIS-C pEF and KD were similar with mild impaired left ventricular myocardial strain compared with the healthy children. Global myocardial strain may be a monitoring index for MIS-C.
Systematic Review Registration
[https://www.crd.york.ac.uk/prospero/], identifier [CRD42021264760].
Keywords: systemic inflammatory response syndrome, pediatric multisystem inflammatory disease, COVID-19 related, mucocutaneous lymph node syndrome, myocardial strain, meta-analysis
Introduction
As of December 21, 2021, 273 million confirmed Coronavirus Disease-19 (COVID-19) cases, and 5.3 million deaths were reported globally (1). Centers for Disease Control and Prevention (CDC) issued an advisory in May 2020 about the symptoms of the multisystem inflammatory syndrome in children (MIS-C) which is characterized by a hyperinflammatory syndrome with multiorgan dysfunction (2). This syndrome is also known as pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2, or pediatric multisystem inflammatory syndrome.
The current estimated incidence of MIS-C was estimated to be approximately 316 per 1,000,000 individuals < 21 years of age infected with COVID-19 (3). By the end of November 2021, CDC has received reports of nearly 6,000 MIS-C cases in the United States (4). More than 80% of patients with MIS-C present with cardiac involvement, including cardiac dysfunction, myocarditis, coronary artery abnormalities, valve regurgitation, pleural effusions, and cardiac arrhythmias (5).
Recently published articles summarize various similarities between MIS-C and Kawasaki disease (KD) (6, 7). Among children in developed countries, KD is the most common form of acquired cardiac disease (8). The estimated incidence of 250 cases per 1,000,000 children < 5 years in the North America. KD can lead to infiltration of inflammatory cells into medium-sized arteries, particularly the coronary arteries, with a risk of myocardial infarction and sudden death. In the acute phase, both MIS-C and KD have elevated cardiac markers, such as N-terminal pro-brain natriuretic peptide and troponin (9). Both MIS-C and KD could affect myocardial function in the acute phase (10, 11).
To assess the myocardial injury of MIS-C, speckle-tracking echocardiography (STE) with myocardial strain would be an ideal tool. Myocardial strain is a useful index of left ventricular deformation. The left ventricular global strain provides one of the best evidence on the diagnostic and prognostic implications for cardiac dysfunction (12). Some studies compared the myocardial strain using STE between MIS-C and KD (13, 14). Others compared the myocardial strain using STE between MIS-C and healthy children (15, 16). However, there was some inconsistency between the results, and sample sizes can affect the quality of evidence. In the scarcity of directly comparing studies, a network meta-analysis allows head-to-head comparisons indirectly through a common comparison group. Therefore, we conducted this network analysis to explore the myocardial strain of MIS-C.
Methods
The pre-registered protocol was implemented in the PROSPERO database (CRD42021264760). This manuscript was reported in accordance with PRISMA guideline (Supplementary Material).
Search Strategy
PubMed, MEDLINE, Web of Science, Embase, China national knowledge infrastructure (CNKI), and medRxiv.org from database were searched on 1 December 2021, to identify the relevant studies by two investigators. Keywords in the search strategy were (“Multisystem Inflammatory Syndrome” or “Kawasaki disease”), “pediatrics,” and “global myocardial strain” (Supplementary Table 1). In addition, we read the references of articles in search for literature that meets the criteria.
Data Extraction and Extraction
Original studies were eligible if the following criteria were met: (i) observational study in English or Chinese; and (ii) compared the global left ventricular myocardial strain [including global longitudinal strain (GLS), global circumferential strain (GCS)] among patients with MIS-C and KD and healthy children.
Original studies were ineligible if the following criteria were met: (i) global myocardial strain obtain using other methods, not speckle-tracking echocardiography; (ii) data acquired from KD patients were not during the acute phase before treatment; or (iii) single-arm study of MIS-C or KD.
The first author, the published year, number of participants in each group, median, global left ventricular myocardial strain, and left ventricular ejection fraction (LVEF) were extracted in the involved eligible studies.
Statistical Analysis
Before analysis, the quality of involved studies was evaluated using the Newcastle–Ottawa Scale. A score with a range of 0–9 was allocated to each study. Mean differences (MDs) and 95% CI were used to report the global myocardial strain and LVEF. We evaluated the myocardial strain using network meta-analysis. According to whether LVEF was normal or not, previous studies divided MIS-C into two groups: MIS-C with depressed ejection fraction (MIS-C dEF) and MIS-C with preserved ejection fraction (MIS-C pEF) (17–19). In this Bayesian network meta-analysis, a random-effects model and consistency model were applied (4 chains, 50,000 iterations, and 20,000 per chain). Inconsistency was assessed by the node-splitting method with Bayesian P-value. We analyzed the symmetry of a comparison-adjusted funnel plot to evaluate possible small sample effects, and used the Begg’s and Egger’s tests to evaluate publication bias in the included studies. A p-value < 0.05 was considered statistically significant for asymmetry. All the analyses were performed with the “gemtc” package in R software (R Foundation, Vienna, Austria, 4.0.2) and Stata (version 16.0; StataCorp, College Station, TX, United States).
Results
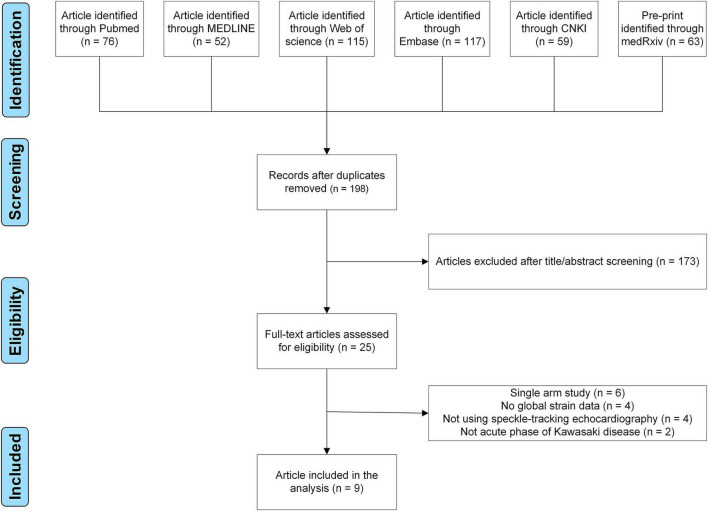
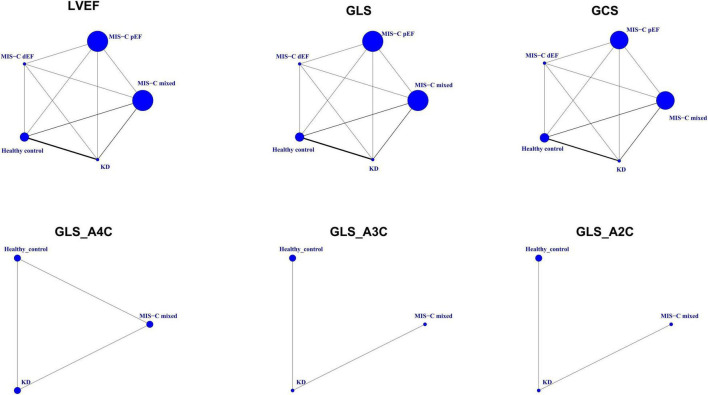
Finally, nine case-control studies were included (Figure 1; 13–16, 20–24). Evaluation of the quality of involved studies is presented in Supplementary Table 2. These nine studies conducted between 2014 and 2021 involved 651 participants: 107 patients with MIS-C, 188 patients with KD, and 356 healthy children (Table 1). Geometry of the network is shown in Figure 2.
FIGURE 1.
Flow chart of study selection.
TABLE 1.
Characteristics of involved studies and comparisons of global myocardial strain among multisystem inflammatory syndrome in children, Kawasaki disease, and healthy children.
| References | Group | Number of participants | Age (year) | Gender (male/female) |
| Xu et al. (20) | KD | 61 | 2.6 ± 2.1 | 45/16 |
| Healthy control | 50 | 2.7 ± 2.1 | 35/15 | |
| Wang et al. (21) | KD | 19 | 3.2 ± 2.1 | 13/6 |
| Healthy control | 56 | 3.2 ± 1.7 | 38/18 | |
| Azak et al. (22) | KD | 15 | 4.5 ± 3.1 | 11/4 |
| Healthy control | 15 | 5.7 ± 2.8 | 9/6 | |
| Wang et al. (23) | KD | 50 | 2.1 ± 1.7 | 28/22 |
| Healthy control | 30 | 2.2 ± 1.8 | 19/11 | |
| Zhang et al. (24) | KD | 11 | 2.9 ± 1.4 | 7/4 |
| Healthy control | 55 | 3.0 ± 1.2 | 35/20 | |
| Gaitonde et al. (13) | MIS-C mixed | 12 | 8.0 ± 4.4 | 9/3 |
| KD | 12 | 6.0 ± 2.2 | 8/4 | |
| Matsubara et al. (14) | MIS-C pEF | 16 | 11.4 ± 4.2 | 14/14 |
| MIS-C dEF | 12 | |||
| KD | 20 | 3.1 ± 1.3 | 15/5 | |
| Healthy control | 20 | 9.0 ± 2.3 | 11/9 | |
| Chang et al. (15) | MIS-C mixed | 43 | 10.1 ± 4.2 | 26/17 |
| Healthy control | 40 | 11.4 ± 3.7 | 23/17 | |
| Kobayashi et al. (16) | MIS-C mixed | 24 | 11.4 ± 6.3 | NR |
| Healthy control | 90 | Age-matched | NR |
KD, Kawasaki disease; MIS-C, multisystem inflammatory syndrome in children; MIS-C dEF, Multisystem Inflammatory Syndrome in Children with depressed ejection fraction; MIS-C pEF, Multisystem Inflammatory Syndrome in Children with preserved ejection fraction; NR, nor reported.
FIGURE 2.
Geometry of the network. Circles represent the intervention as a node in the network, size of circles corresponds to the number of participants included in each comparison, lines represent direct comparisons using studies, and the thickness of lines corresponds to the number of studies included in each comparison. GCS, global circumferential strain; GLS, global longitudinal strain; GLS_A2C, global longitudinal strain_apical two-chamber; GLS_A3C, global longitudinal strain_apical three-chamber; GLS_A4C, global longitudinal strain_apical four-chamber; KD, Kawasaki disease; LVEF, left ventricular ejection fraction; MIS-C, multisystem inflammatory syndrome in children; MIS-C dEF, Multisystem Inflammatory Syndrome in Children with depressed ejection fraction; MIS-C pEF, Multisystem Inflammatory Syndrome in Children with preserved ejection fraction.
Left Ventricular Ejection Fraction
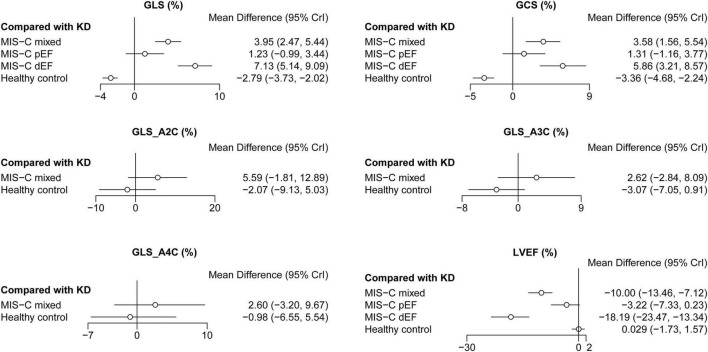
In total, eight studies reported the difference of LVEF among MIS-C, KD, and healthy control groups (13–15, 20–24). MIS-C mixed group and MIS-C dEF group had a lower LVEF than KD groups (Table 2). There was no difference of LVEF among MIS-C pEF, KD, and healthy control groups.
TABLE 2.
Head-to-head comparisons of left ventricular ejection fraction and global myocardial strain.
| Left ventricular ejection fraction | ||||||
| MIS-C mixed | ||||||
| −6.76 (−10.8, −2.83) | MIS-C pEF | |||||
| 8.17 (3.04, 13.37) | 14.94 (9.69, 20.25) | MIS-C dEF | ||||
| −10.02 (−13.2, −7.29) | −3.23 (−7.14, 0.16) | −18.2 (−23.31, −13.45) | Healthy control | |||
| −10.00 (−13.46, −7.12) | −3.21 (−7.33, 0.23) | −18.19 (−23.47, −13.34) | 0.03 (−1.73, 1.57) | KD | ||
|
| ||||||
|
Global longitudinal strain
| ||||||
| MIS-C mixed | ||||||
| 2.73 (0.34, 5.11) | MIS-C pEF | |||||
| −3.17 (−5.3, −1.03) | −5.9 (−8.41, −3.37) | MIS-C dEF | ||||
| 6.74 (5.38, 8.23) | 4.02 (1.9, 6.27) | 9.92 (8.04, 11.92) | Healthy control | |||
| 3.95 (2.47, 5.43) | 1.23 (−0.99, 3.44) | 7.13 (5.14, 9.09) | −2.79 (−3.73, −2.02) | KD | ||
|
| ||||||
|
Global circumferential strain
| ||||||
| MIS-C mixed | ||||||
| 2.28 (−0.31, 4.79) | MIS-C pEF | |||||
| −2.28 (−5.1, 0.4) | −4.57 (−7.48, −1.71) | MIS-C dEF | ||||
| 6.95 (5.16, 8.83) | 4.66 (2.38, 7.15) | 9.24 (6.73, 11.94) | Healthy control | |||
| 3.58 (1.56, 5.54) | 1.31 (−1.16, 3.77) | 5.86 (3.21, 8.57) | −3.36 (−4.68, −2.24) | KD | ||
|
| ||||||
|
Global longitudinal strain_apical four-chamber
| ||||||
| MIS-C mixed | ||||||
| 3.46 (−2.33, 9.92) | Healthy control | |||||
| 2.6 (−3.2, 9.67) | −0.98 (−6.55, 5.54) | KD | ||||
|
| ||||||
|
Global longitudinal strain_apical three-chamber
| ||||||
| MIS-C mixed | ||||||
| 5.69 (−1.02, 12.37) | Healthy control | |||||
| 2.62 (−2.84, 8.09) | −3.07 (−7.05, 0.91) | KD | ||||
|
| ||||||
|
Global longitudinal strain_apical two-chamber
| ||||||
| MIS-C mixed | ||||||
| 7.67 (−2.56, 17.78) | Healthy control | |||||
| 5.59 (−1.81, 12.89) | −2.07 (−9.13, 5.03) | KD | ||||
KD, Kawasaki disease; MIS-C, Multisystem Inflammatory Syndrome in Children; MIS-C dEF, Multisystem Inflammatory Syndrome in Children with depressed ejection fraction; MIS-C pEF, Multisystem Inflammatory Syndrome in Children with preserved ejection fraction. Data are mean difference (95% CrI) in the column-defining group compared with the row-defining group (e.g., the mean difference of left ventricular ejection fraction for MIS-mixed group compared with healthy control group is −10.02). Mean difference < 0 indicates the column-defining group had lower left ventricular ejection fraction or myocardial strain.
The Global Myocardial Strain
In total, eight studies reported the difference of GLS among MIS-C, KD, and healthy control groups (13–15, 20–24). Meanwhile, seven studies reported the difference of GCS among MIS-C, KD, and healthy control groups (13–15, 21–24). MIS-C mixed group and MIS-C dEF group had a higher GLS and GCS than KD groups (Figure 3). Both MIS-C pEF and KD had similar GLS and GCS, which were higher than healthy control (Table 2). There was no difference of GLS_A4C, GLS_A3C, and GLS_A2C among MIS-C mixed, KD, and healthy control groups (Figure 3).
FIGURE 3.
Forest plots of network meta-analysis of all studies. GCS, global circumferential strain; GLS, global longitudinal strain; GLS_A2C, global longitudinal strain_apical two-chamber; GLS_A3C, global longitudinal strain_apical three-chamber; GLS_A4C, global longitudinal strain_apical four-chamber; KD, Kawasaki disease; LVEF, left ventricular ejection fraction; MIS-C, multisystem inflammatory syndrome in children; MIS-C dEF, Multisystem Inflammatory Syndrome in Children with depressed ejection fraction; MIS-C pEF, Multisystem Inflammatory Syndrome in Children with preserved ejection fraction.
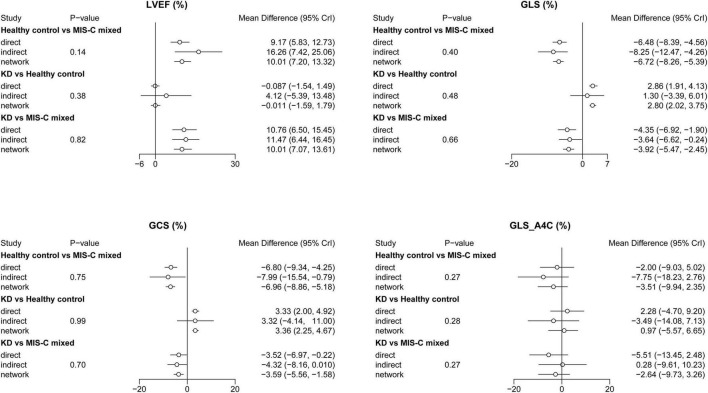
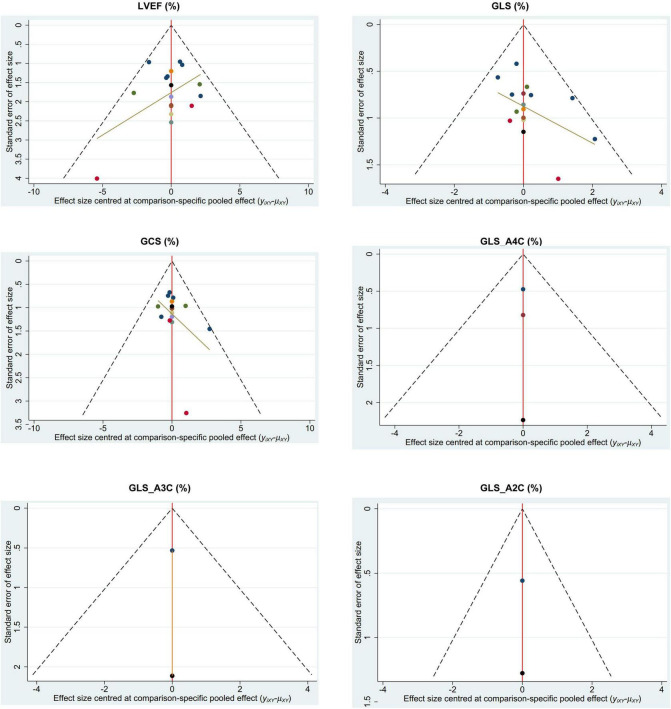
Inconsistency and Publication Bias
No inconsistency result had a level of P < 0.05. Therefore, the results of this study were not influenced by the inconsistencies (Figure 4). The assessment of publication bias was revealed in Table 3. No obviously publication bias was detected (Figure 5).
FIGURE 4.
Inconsistency test of network meta-analysis. GCS, global circumferential strain; GLS, global longitudinal strain; GLS_A4C, global longitudinal strain_apical four-chamber; KD, Kawasaki disease; LVEF, left ventricular ejection fraction; MIS-C, multisystem inflammatory syndrome in children.
TABLE 3.
Assessment of publication bias.
| Outcome | P-value of Begg’s test | P-value of Egger’s test |
| GLS | 0.837 | 0.140 |
| GCS | 0.620 | 0.414 |
| GLS_A4C | >0.999 | 0.312 |
| GLS_A3C | >0.999 | – |
| GLS_A2C | >0.999 | – |
| LVEF | 0.266 | 0.440 |
GCS, global circumferential strain; GLS, global longitudinal strain; GLS_A2C, global longitudinal strain_apical two-chamber; GLS_A3C, global longitudinal strain_apical three-chamber; GLS_A4C, global longitudinal strain_apical four-chamber; LVEF, left ventricular ejection fraction.
FIGURE 5.
Funnel plot of network meta-analysis. GCS, global circumferential strain; GLS, global longitudinal strain; GLS_A2C, global longitudinal strain_apical two-chamber; GLS_A3C, global longitudinal strain_apical three-chamber; GLS_A4C, global longitudinal strain_apical four-chamber; LVEF, left ventricular ejection fraction.
Discussion
This is the first meta-analysis regarding the changes of myocardial strain in patients with MIS-C, compared with KD and healthy children. The results of this network meta-analysis revealed that MIS-C dEF group had lower LVEF, higher GLS and GCS than KD. MIS-C pEF group had similar LVEF, GLS, and GCS with KD. MIS-C pEF and KD had preserved LVEF, impaired GLS, and GCS in the acute phase before treatment compared with the healthy controls. In this study, GLS and GCS served as explicit indexes for MIS-C, which seems more sensitive than LVEF.
Kawasaki disease is a well-known, self-limited, systemic inflammatory vasculitis that mainly affects small- and medium-sized arteries. Several previous studies confirmed impaired left ventricular myocardial strain of KD in the acute phase using STE (20, 22) or velocity vector imaging (25, 26). Our results found that KD had impaired GLS and GCS in the acute phase compared with the healthy children, consistent with those published articles (20, 22, 25, 26). The progressive structural alterations of the myocardium and coronary arteries were confirmed during the convalescent phase of KD in the previous studies (27–29). Therefore, some studies suggested that patients with KD who had clinical recovered, need long-term follow-up to detect long-lasting impaired left ventricular myocardial strain using STE (30–33) or cardiac magnetic resonance (34).
The summarized course of myocardial strain change in patients with KD may help to better understand the recovery of MIS-C due to MIS-C having some overlapping features with KD. Before LVEF decreased or symptoms appeared, myocardial strain has emerged as a promising parameter of subclinical myocardial dysfunction (35). Myocardial strain can be measured via echocardiography/STE or cardiac magnetic resonance (36). However, it may be challenging to perform cardiac magnetic resonance in MIS-C because young children do not cooperate and their clinical instability (10). In order to assess the functional state of the left ventricle, STE is the most suitable technique for measuring myocardial strain (12).
Patient with MIS-C usually have fever, laboratory evidence of inflammation, and multisystem organ involvement (10). Although shares similarities with KD, MIS-C also has part features of myocarditis and toxic-shock syndrome (36). MIS-C and KD are distinct syndromes. Current evidence indicates that MIS-C is the result of an exaggerated innate and adaptive immune response, characterized by a cytokine storm, and that it is triggered by prior SARS-CoV-2 exposure (36).
The clinical course for MIS-C appears to be more severe during the acute phase. The death rate of MIS-C is 0.87% (52/5,973), compared with 0.17% of KD (4, 37). All the children who died from MIS-C had severe cardiovascular involvement (5). Recently, a study with 539 patients with MIS-C found that 65.8% of patients have preserved LVEF and 34.2% of patients have depressed LVEF (19). LVEF may become a source of heterogeneity of MIS-C studies. Therefore, as suggested by the previous research (17–19), we divided MIS-C into two subgroups. Meanwhile, we strengthened the evidence of left ventricular deformation of MIS-C by using network meta-analysis and found the difference between these two groups: left ventricular dysfunction of MIS-C dEF group was more severe than KD, but MIS-C pEF group was similar with KD in the acute phase.
Several studies focused on the recovery of MIS-C with follow-up for short-term (38, 39), mid-term (19, 40, 41), and 1-year using conventional echocardiography (42). In total, 91.0% of patients with MIS-C had a normal LVEF by 30 days (19). LVEF of all patients with MIS-C needed 74–90 days to return to normal (19, 42). However, cardiac magnetic resonance studies did not find any abnormality in patients with MIS-C after 27 ± 14 days (43), or a 2-month follow-up (44). In our involved study, three studies performed the short-term follow-up (13, 14, 16). After treatment, LVEF and GCS normalized by 7–9 days of illness, impaired GLS persisted in most of the patients with MIS-C (14, 16), similar findings were reported by a recent MIS-C cohort study (45). GLS may be a good follow-up index for MIS-C. Thus, we recommend long-term, regular intervals, follow-up with myocardial strain echocardiography for MIS-C, supplemented with cardiac magnetic resonance if clinical conditions permit.
Global longitudinal strain is usually associated with adverse outcomes, especially in patients with myocarditis (46), heart failure (47), or cardiomyopathy (48). GLS may be a clinical predictor for MIS-C. In patients with higher GLS, they have higher risk of intensive care, mechanical ventilation, or inotropic support (16, 18, 45). Meanwhile, they may stay longer in intensive care unit or hospital (18, 45). Meanwhile, correlations of GLS with laboratory parameters in patients with MIS-C were reported, such as C-reactive protein, troponin, and brain natriuretic peptide, indicating the presence of myocarditis and cardiac dysfunction (13, 16, 45, 49). Therefore, the impaired myocardial strain on the initial echocardiography of patients presenting with KD-like illness may be a clue to differential diagnosis. Such patients require closer clinical observation for the development of cardiac dysfunction (13).
Limitation
First, this analysis was performed based on observational studies. The small sample size cannot be ignored. Not all involved studies mentioned this index of myocardial strain were normal distribution. All the aforementioned will affect the reliability of the results. Second, some underlying confounders, such as age, gender, sex, and body mass index, may become that were not adjustable. Third, no mid-term or long-term follow-up in all involved studies. Fourth, none of the involved studies focused on the potential impact of the coronary aneurysm on MIS-C. Fifth, the myocardial strain values may be measured by different echocardiography machines from the different manufacturers.
Conclusion
MIS-C dEF was more severe than KD, both in LVEF and global myocardial strain. MIS-C pEF and KD were similar with mild-impaired left ventricular myocardial strain, compared with the healthy children. Global myocardial strain may be a monitoring index for MIS-C.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
KL contributed to the data collection, data analysis, and manuscript writing. JY contributed to the data collection and data analysis. GS contributed to the project development and manuscript writing. All authors contributed intellectually to the work and read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.848306/full#supplementary-material
References
- 1.World Health Organization. Weekly Operational Update on COVID-19 [Internet]. (2021). Available online at: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19—21-december-2021 (accessed December 21, 2021). [Google Scholar]
- 2.Centers for Disease Control and Prevention. Information for Healthcare Providers About Multisystem Inflammatory Syndrome in Children (MIS-C). (2020). Available online at: https://www.cdc.gov/mis-c/hcp/ (accessed July 31, 2020). [Google Scholar]
- 3.Payne AB, Gilani Z, Godfred-Cato S, Belay ED, Feldstein LR, Patel MM, et al. Incidence of multisystem inflammatory syndrome in children among US persons infected with SARS-CoV-2. JAMA Netw Open. (2021) 4:e2116420. 10.1001/jamanetworkopen.2021.16420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Health Department-Reported Cases of Multisystem Inflammatory Syndrome in Children (MIS-C) in the United States. (2021). Available online at: https://covid.cdc.gov/covid-data-tracker/#mis-national-surveillance (accessed November 30, 2021). [Google Scholar]
- 5.Bowen A, Miller AD, Zambrano LD, Wu MJ, Oster ME, Godfred-Cato S, et al. Demographic and clinical factors associated with death among persons <21 years old with multisystem inflammatory syndrome in children-United States, February 2020-March 2021. Open Forum Infect Dis. (2021) 8:ofab388. 10.1093/ofid/ofab388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang QY, Xu BW, Du JB. Similarities and differences between multiple inflammatory syndrome in children associated with COVID-19 and Kawasaki disease: clinical presentations, diagnosis, and treatment. World J Pediatr. (2021) 17:335–40. 10.1007/s12519-021-00435-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee MS, Liu YC, Tsai CC, Hsu JH, Wu JR. Similarities and differences between COVID-19-related multisystem inflammatory syndrome in children and Kawasaki disease. Front Pediatr. (2021) 9:640118. 10.3389/fped.2021.640118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Council on, and prevention, diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American heart association. Circulation. (2017) 135:e927–99. 10.1161/CIR.0000000000000484 [DOI] [PubMed] [Google Scholar]
- 9.Zhou C, Zhao Y, Wang X, Huang Y, Tang X, Tang L. Laboratory parameters between multisystem inflammatory syndrome in children and Kawasaki disease. Pediatr Pulmonol. (2021) 56:3688–98. 10.1002/ppul.25687 [DOI] [PubMed] [Google Scholar]
- 10.Wu EY, Campbell MJ. Cardiac manifestations of multisystem inflammatory syndrome in children (MIS-C) following COVID-19. Curr Cardiol Rep. (2021) 23:168. 10.1007/s11886-021-01602-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hejazi OI, Loke YH, Harahsheh AS. Short-term cardiovascular complications of multi-system inflammatory syndrome in children (MIS-C) in adolescents and children. Curr Pediatr Rep. (2021) 9:93–103. 10.1007/s40124-021-00258-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song G, Zhang J, Wang X, Zhang X, Sun F, Yu X. Usefulness of speckle-tracking echocardiography for early detection in children with Duchenne muscular dystrophy: a meta-analysis and trial sequential analysis. Cardiovasc Ultrasound. (2020) 18:26. 10.1186/s12947-020-00209-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaitonde M, Ziebell D, Kelleman MS, Cox DE, Lipinski J, Border WL, et al. COVID-19-related multisystem inflammatory syndrome in children affects left ventricular function and global strain compared with Kawasaki disease. J Am Soc Echocardiogr. (2020) 33:1285–7. 10.1016/j.echo.2020.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsubara D, Kauffman HL, Wang Y, Calderon-Anyosa R, Nadaraj S, Elias MD, et al. Echocardiographic findings in pediatric multisystem inflammatory syndrome associated with COVID-19 in the United States. J Am Coll Cardiol. (2020) 76:1947–61. 10.1016/j.jacc.2020.08.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang JC, Matsubara D, Morgan RW, Diorio C, Nadaraj S, Teachey DT, et al. Skewed cytokine responses rather than the magnitude of the cytokine storm may drive cardiac dysfunction in multisystem inflammatory syndrome in children. J Am Heart Assoc. (2021) 10:e021428. 10.1161/JAHA.121.021428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi R, Dionne A, Ferraro A, Harrild D, Newburger J, VanderPluym C, et al. Detailed assessment of left ventricular function in multisystem inflammatory syndrome in children, using strain analysis. CJC Open. (2021) 3:880–7. 10.1016/j.cjco.2021.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alsaied T, Tremoulet AH, Burns JC, Saidi A, Dionne A, Lang SM, et al. Review of cardiac involvement in multisystem inflammatory syndrome in children. Circulation. (2021) 143:78–88. 10.1161/CIRCULATIONAHA.120.049836 [DOI] [PubMed] [Google Scholar]
- 18.Basu S, Kim EJ, Sharron MP, Austin A, Pollack MM, Harahsheh AS, et al. Strain echocardiography and myocardial dysfunction in critically ill children with multisystem inflammatory syndrome unrecognized by conventional echocardiography: a retrospective cohort analysis. Pediatr Crit Care Med. (2021). 23:e145–e152. 10.1097/PCC.0000000000002850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feldstein LR, Tenforde MW, Friedman KG, Newhams M, Rose EB, Dapul H, et al. Overcoming, characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA. (2021) 325:1074–87. 10.1001/jama.2021.2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu QQ, Ding YY, Lv HT, Zhou WP, Sun L, Huang J, et al. Evaluation of left ventricular systolic strain in children with Kawasaki disease. Pediatr Cardiol. (2014) 35:1191–7. 10.1007/s00246-014-0915-5 [DOI] [PubMed] [Google Scholar]
- 21.Wang RJ, Huang M, Xie LJ, Zhang YW, Chen C, Zhu DY, et al. [Evaluation of myocardial deformation in children with Kawasaki disease by two dimensional speckle tracking echocardiography]. Int J Pediatr. (2016) 43:638–42. 24859168 [Google Scholar]
- 22.Azak E, Cetin II, Gursu HA, Kibar AE, Surucu M, Orgun A, et al. Recovery of myocardial mechanics in Kawasaki disease demonstrated by speckle tracking and tissue Doppler methods. Echocardiography. (2018) 35:380–7. 10.1111/echo.13773 [DOI] [PubMed] [Google Scholar]
- 23.Wang YH, Tong MH, Wu TT, Lei HY, Wang JB, Ding XH. [The study of left ventricular systolic function in Kawasaki disease evaluated by two dimensional speckle tracking imaging]. Chin J Med Ultrasound. (2018) 15:692–9. [Google Scholar]
- 24.Zhang XL, Deng J, Chen J, Wang X, Du YL. [Value of two-dimensional speckle tracking echocardiography in evaluating left ventricular myocardial motor function of Kawasaki disease children at different phases]. Med J West China. (2019) 1:1269–72. [Google Scholar]
- 25.McCandless RT, Minich LL, Wilkinson SE, McFadden ML, Tani LY, Menon SC. Myocardial strain and strain rate in Kawasaki disease. Eur Heart J Cardiovasc Imaging. (2013) 14:1061–8. [DOI] [PubMed] [Google Scholar]
- 26.Hematian MN, Torabi S, MalaKan-Rad E, Sayadpour-Zanjani K, Ziaee V, Lotfi-Tolkaldany M. Noninvasive evaluation of myocardial systolic dysfunction in the early stage of Kawasaki disease: a speckle-tracking echocardiography study. Iran J Pediatr. (2015) 25:7. 10.5812/ijp.25(3)2015.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yutani C, Go S, Kamiya T, Hirose O, Misawa H, Maeda H, et al. Cardiac biopsy of Kawasaki disease. Arch Pathol Lab Med. (1981) 105:470–3. [PubMed] [Google Scholar]
- 28.Liu AM, Ghazizadeh M, Onouchi Z, Asano G. Ultrastructural characteristics of myocardial and coronary microvascular lesions in Kawasaki disease. Microvasc Res. (1999) 58:10–27. 10.1006/mvre.1999.2155 [DOI] [PubMed] [Google Scholar]
- 29.Mitani Y, Okuda Y, Shimpo H, Uchida F, Hamanaka K, Aoki K, et al. Impaired endothelial function in epicardial coronary arteries after Kawasaki disease. Circulation. (1997) 96:454–61. [PubMed] [Google Scholar]
- 30.Yu W, Wong SJ, Cheung YF. Left ventricular mechanics in adolescents and young adults with a history of Kawasaki disease: analysis by three-dimensional speckle tracking echocardiography. Echocardiography. (2014) 31:483–91. 10.1111/echo.12394 [DOI] [PubMed] [Google Scholar]
- 31.Martínez-García A, Ruiz-Esparza E, ázquez-Antona CV, ánchez-Cornelio CS, Ávila-Vanzzini N, Arias-Godínez JA, et al. Left ventricular longitudinal systolic strain in children with history of Kawasaki disease. Archivos de Cardiologia de Mexico. (2016) 86:196–202. 10.1016/j.acmx.2015.08.001 [DOI] [PubMed] [Google Scholar]
- 32.Dedeoglu R, Barut K, Oztunc F, Atik S, Adrovic A, Sahin S, et al. Evaluation of myocardial deformation in patients with Kawasaki disease using speckle-tracking echocardiography during mid-term follow-up. Cardiol Young. (2017) 27:1377–85. [DOI] [PubMed] [Google Scholar]
- 33.Lin Z, Zheng JJ, Chen EL, Ding TT, Yu W, Xia B. Assessing left ventricular systolic function in children with a history of Kawasaki disease. BMC Cardiovasc Disord. (2020) 20:9. 10.1186/s12872-020-01409-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bratis K, Hachmann P, Child N, Krasemann T, Hussain T, Mavrogeni S, et al. Cardiac magnetic resonance feature tracking in Kawasaki disease convalescence. Ann Pediatr Cardiol. (2017) 10:18–25. 10.4103/0974-2069.197046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tops LF, Delgado V, Marsan NA, Bax JJ. Myocardial strain to detect subtle left ventricular systolic dysfunction. Eur J Heart Fail. (2017) 19:307–13. 10.1002/ejhf.694 [DOI] [PubMed] [Google Scholar]
- 36.Sharma C, Ganigara M, Galeotti C, Burns J, Berganza FM, Hayes DA, et al. Multisystem inflammatory syndrome in children and Kawasaki disease: a critical comparison. Nat Rev Rheumatol. (2021) 17:731–48. 10.1038/s41584-021-00709-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vogel TP, Top KA, Karatzios C, Hilmers DC, Tapia LI, Moceri P, et al. Multisystem inflammatory syndrome in children and adults (MIS-C/A): case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. (2021) 39:3037–49. 10.1016/j.vaccine.2021.01.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jhaveri S, Ahluwalia N, Kaushik S, Trachtman R, Kowalsky S, Aydin S, et al. Longitudinal echocardiographic assessment of coronary arteries and left ventricular function following multisystem inflammatory syndrome in children. J Pediatr. (2021) 228:290–3.e1. 10.1016/j.jpeds.2020.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fabi M, Filice E, Biagi C, Andreozzi L, Palleri D, Mattesini BE, et al. Multisystem inflammatory syndrome following SARS-CoV-2 infection in children: one year after the onset of the pandemic in a high-incidence area. Viruses. (2021) 13:2022. 10.3390/v13102022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Capone CA, Misra N, Ganigara M, Epstein S, Rajan S, Acharya SS, et al. Six month follow-up of patients with multi-system inflammatory syndrome in children. Pediatrics. (2021) 148:e2021050973. 10.1542/peds.2021-050973 [DOI] [PubMed] [Google Scholar]
- 41.Barris DM, Keelan J, Ahluwalia N, Jhaveri S, Cohen J, Stern K, et al. Midterm outcomes and cardiac magnetic resonance imaging following multisystem inflammatory syndrome in children. J Pediatr. (2021). 241:237–41. 10.1016/j.jpeds.2021.10.009 [DOI] [PubMed] [Google Scholar]
- 42.Davies P, du Pre P, Lillie J, Kanthimathinathan HK. One-year outcomes of critical care patients post-COVID-19 multisystem inflammatory syndrome in children. JAMA Pediatr. (2021) 175:1281–3. 10.1001/jamapediatrics.2021.2993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bermejo IA, Bautista-Rodriguez C, Fraisse A, Voges I, Gatehouse P, Kang H, et al. Short-term sequelae of multisystem inflammatory syndrome in children assessed by CMR. JACC Cardiovasc Imaging. (2021) 14:1666–7. 10.1016/j.jcmg.2021.01.035 [DOI] [PubMed] [Google Scholar]
- 44.Webster G, Patel AB, Carr MR, Rigsby CK, Rychlik K, Rowley AH, et al. Cardiovascular magnetic resonance imaging in children after recovery from symptomatic COVID-19 or MIS-C: a prospective study. J Cardiovasc Magn Reason. (2021) 23:86. 10.1186/s12968-021-00786-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanil Y, Misra A, Safa R, Blake JM, Eddine AC, Balakrishnan P, et al. Echocardiographic indicators associated with adverse clinical course and cardiac sequelae in multisystem inflammatory syndrome in children with coronavirus disease 2019. J Am Soc Echocardiogr. (2021) 34:862–76. 10.1016/j.echo.2021.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sperlongano S, D’Amato A, Tagliamonte E, Russo V, Desiderio A, Ilardi F, et al. Acute myocarditis: prognostic role of speckle tracking echocardiography and comparison with cardiac magnetic resonance features. Heart Vessels. (2021). 37:121–31. 10.1007/s00380-021-01893-0 [DOI] [PubMed] [Google Scholar]
- 47.Grove GL, Pedersen S, Olsen FJ, Skaarup KG, Jorgensen PG, Shah AM, et al. Layer-specific global longitudinal strain obtained by speckle tracking echocardiography for predicting heart failure and cardiovascular death following STEMI treated with primary PCI. Int J Cardiovasc Imaging. (2021) 37:2207–15. 10.1007/s10554-021-02202-6 [DOI] [PubMed] [Google Scholar]
- 48.Rowin EJ, Maron BJ, Wells S, Burrows A, Firely C, Koethe B, et al. Usefulness of global longitudinal strain to predict heart failure progression in patients with nonobstructive hypertrophic cardiomyopathy. Am J Cardiol. (2021) 151:86–92. 10.1016/j.amjcard.2021.04.021 [DOI] [PubMed] [Google Scholar]
- 49.Sirico D, Basso A, Reffo E, Cavaliere A, Castaldi B, Sabatino J, et al. Early echocardiographic and cardiac MRI findings in multisystem inflammatory syndrome in children. J Clin Med. (2021) 10:13. 10.3390/jcm10153360 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.