ABSTRACT
Immunogenic cell death (ICD) involves the release of ATP, which can be destroyed by ectonucleotidases, converting it into immunosuppressive adenosine. Hence, inhibition of such ectonucleotidases is a strategy for enhancing ICD-elicited anticancer immunity. In a recent paper in Science Translational Medicine, Mao et al. report the construction of reactive oxygen-labile nanoparticles that bear two functionalities, namely (i) the capacity to sensitize cancer cells to near-infrared light (NIL) irradiation, hence inducing ICD in the context of photodynamic therapy, and (ii) the peculiarity to respond to NIL by releasing a pharmacological inhibitor of ectonucleotidases, hence enhancing intratumoral concentrations of ATP. In preclinical models, these nanoparticles are highly efficient in inducing anticancer immune responses.
The therapeutic outcome of oncological regimen depends on the onset of anticancer immune responses as they can be stimulated by radiotherapy, certain targeted drugs, a selected panel of chemotherapeutics, as well as local oncolytic agents, all of which are potent activators of immunogenic cell stress and cell death routines.1 Immunogenic cell death (ICD) is associated with the emission of a defined set of danger associated molecular patterns (DAMPs) by malignant cells that in their correct spatial and temporal appearance trigger the functional engagement of dendritic cells (DCs) and the consequent onset of T cell-mediated adaptive immunity. DAMPs emitted in the course of ICD include adenosine triphosphate (ATP) and annexin A1 (ANXA1), which serve as chemoattractant and homing signal for DCs, respectively. Calreticulin that translocates from the endoplasmic reticulum lumen (ER) on to the cytoplasmic membrane guides DC-mediated phagocytosis, and the exodus of high mobility group box 1 (HMGB1) triggers final antigen presenting cell (APC) maturation. Altogether ICD stimulates DC-mediated tumor antigen cross presentation to cytotoxic T lymphocytes (CTL), resulting in anticancer immunogenicity manifesting with interferon γ (IFN γ)-mediated tumor lysis and the generation of memory T cells facilitating disease control beyond treatment discontinuation.2
The net effect of the chemoattractant ATP on tumor immune infiltration in response to ICD inducers depends on multiple factors, including the presence of extracellular ATP-degrading enzymes such as the sequentially active ectonucleotidases, ectonucleoside triphosphate diphosphohydrolase 1 (ENTPD1, better known as CD39) and 5’-nucleotidase ecto (NT5E, better known as CD73), which convert ATP into AMP and AMP into immunosuppressive extracellular adenosine (ADO), respectively.3,4 Extracellular ADO favors neovascularization and metastatic dissemination by binding adenosine A2b receptor (ADORA2B) in the stroma and in tumor cells. Moreover, the ligation ADORA2B and adenosine A2a receptor (ADORA2A) by ADO modulates the functions of multiple immune cells including the inhibition of DC maturation and CTL function. Immunosuppression by extracellular ADO thus strongly subverts ICD-induced anticancer immunity.5
An accumulating body of preclinical evidence supports the notion that local administration of oncolytic regimen, such as intratumorally applied cytotoxicants, radiofrequency or photothermal ablation, high-intensity focused ultrasonography, photodynamic therapy, laser therapy, microwave and cryotherapy, facilitates the emission of ICD associated DAMPs, thus promoting anticancer immunity in particular when combined with systemic immunotherapies.6,7 Recently, the lab of Xin Ming at the Wake Forest University School of Medicine engineered reactive oxygen species (ROS)-responsive NP700 nanoparticles composed of the boronic acid-containing cationic polymer poly[(2-acryloyl)ethyl(p-boronic acid benzyl)diethylammonium bromide] (PDEAEA-PBA) and the photosensitizer-containing lipid polymer 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)-2000]-IRDye 700 (DSPE-PEG2000-IR700) that carry the ectonucleotidase inhibitor ARL67156 as payload. Local photodynamic therapy with near-infrared light (NIL) triggered the production of ROS, the consequent induction of ICD and the associated release of DAMPs. In parallel, ROS led to the liberation of ARL67156 from NP700 particles that further enhanced the immunostimulatory effect of extracellular ATP in vitro. This boosted T cell mediated immune responses translating into long-term survival in preclinical murine models. Altogether, NP700 led to a reprogramming of the tumor microenvironment (TME), rendering it responsive to programmed cell death protein 1 (PD-1)-targeted systemic immunotherapy8 (Figure 1).
Figure 1.
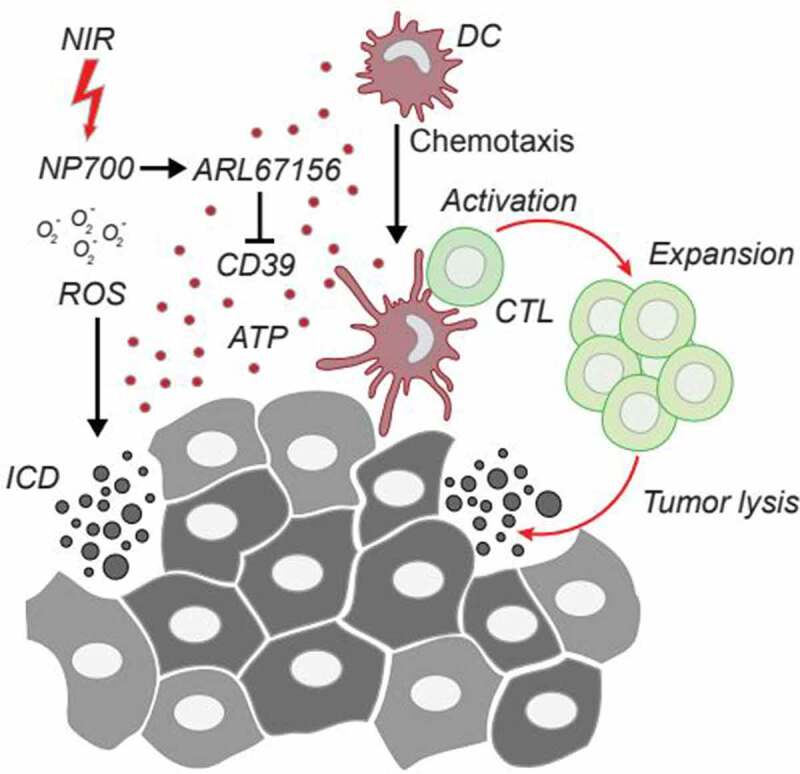
Local immunogenic cell death renders tumors responsive to immune checkpoint blockade. Locally applied near-infrared light (NIL) irradiation of NP700 nanoparticles triggers the production of reactive oxygen species (ROS) and the consequent induction of ICD. The concomitant release of the ectonucleotidase inhibitor ARL67156 payload further increases the ATP-mediated attraction of dendritic cells (DCs) thus eliciting full blown adaptive anticancer immunity with downstream tumor lysis by cytotoxic T lymphocytes (CTLs) In summary NP700 facilitates the reprogramming of the tumor microenvironment (TME) rendering it immunogenic and thus responsive to immune checkpoint blockade.
The sequential combination of ICD inducers with systemic immunotherapy has proven benefit over concomitant combination or monotherapies in preclinical models as well as in patients.9,10 It is thus tempting to postulate that locally increasing the concentration of ICD-associated DAMPs in the TME will help to further increase the number of patients that benefit from immune checkpoint blockade.
Acknowledgments
OK receives funding by the DIM ELICIT initiative of the Ile de France and Institut National du Cancer (INCa); GK is supported by the Ligue contre le Cancer (équipes labellisées, Program “Equipe labelisée LIGUE”; no. EL2016.LNCC (VT/PLP)); Agence National de la Recherche (ANR) – Projets blancs; ANR under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; AMMICa US23/CNRS UMS3655; Association pour la recherche sur le cancer (ARC); Association “Le Cancer du Sein, Parlons-en!”; Cancéropôle Ile-de-France; Chancellerie des universités de Paris (Legs Poix), Fondation pour la Recherche Médicale (FRM); a donation by Elior; European Research Area Network on Cardiovascular Diseases (ERA-CVD, MINOTAUR); Gustave Roussy Odyssea, the European Union Horizon 2020 Project Oncobiome; Fondation Carrefour; INCa; Inserm (HTE); Institut Universitaire de France; LeDucq Foundation; the LabEx Immuno-Oncology (ANR-18-IDEX-0001); the RHU Torino Lumière; the Seerave Foundation; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); and the SIRIC Cancer Research and Personalized Medicine (CARPEM).
Competing interests
OK is a cofounder of Samsara Therapeutics. GK has been holding research contracts with Daiichi Sankyo, Eleor, Kaleido, Lytix Pharma, PharmaMar, Osasuna Therapeutics, Samsara Therapeutics, Sanofi, Sotio, Tollys, Vascage and Vasculox/Tioma. GK has been consulting for Reithera. GK is on the Board of Directors of the Bristol Myers Squibb Foundation France. GK is a scientific co-founder of everImmune, Osasuna Therapeutics, Samsara Therapeutics and Therafast Bio. GK is the inventor of patents covering therapeutic targeting of aging, cancer, cystic fibrosis and metabolic disorders.
Data availability statement
All data that led to the conclusions in this manuscript have been referenced and all sources have been described.
References
- 1.Kroemer G, Galassi C, Zitvogel L, Galluzzi L.. Immunogenic cell stress and death. Nat Immunol. 2022;23:487–3. doi: 10.1038/s41590-022-01132-2. [DOI] [PubMed] [Google Scholar]
- 2.Yatim N, Cullen S, Albert ML. Dying cells actively regulate adaptive immune responses. Nat Rev Immunol. 2017;17:262–275. doi: 10.1038/nri.2017.9. [DOI] [PubMed] [Google Scholar]
- 3.Allard B, Allard D, Buisseret L, Stagg J. The adenosine pathway in immuno-oncology. Nat Rev Clin Oncol. 2020;17:611–629. doi: 10.1038/s41571-020-0382-2. [DOI] [PubMed] [Google Scholar]
- 4.Moesta AK, Li XY, Smyth MJ. Targeting CD39 in cancer. Nat Rev Immunol. 2020;20:739–755. doi: 10.1038/s41577-020-0376-4. [DOI] [PubMed] [Google Scholar]
- 5.Kepp O, Bezu L, Yamazaki T, Di Virgilio F, Smyth MJ, Kroemer G, Galluzzi L. ATP and cancer immunosurveillance. EMBO J. 2021;40:e108130. doi: 10.15252/embj.2021108130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kepp O, Marabelle A, Zitvogel L, Kroemer G. Oncolysis without viruses - inducing systemic anticancer immune responses with local therapies. Nat Rev Clin Oncol. 2020;17:49–64. doi: 10.1038/s41571-019-0272-7. [DOI] [PubMed] [Google Scholar]
- 7.Kepp O, Kroemer G. Pseudovirus for immunotherapy. Nat Cancer. 2020;1:860–861. doi: 10.1038/s43018-020-00107-2. [DOI] [PubMed] [Google Scholar]
- 8.Mao C, Yeh S, Fu J, Porosnicu M, Thomas A, Kucera GL, Votanopoulos KI, Tian S, Ming X. Delivery of an ectonucleotidase inhibitor with ROS-responsive nanoparticles overcomes adenosine-mediated cancer immunosuppression. Sci Transl Med. 2022;14:eabh1261. doi: 10.1126/scitranslmed.abh1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kroemer G, Kepp O. Small cell lung cancer responds to immunogenic chemotherapy followed by PD-1 blockade. Oncoimmunology. 2021;10:1996686. doi: 10.1080/2162402X.2021.1996686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamazaki T, Buque A, Ames TD, Galluzzi L. PT-112 induces immunogenic cell death and synergizes with immune checkpoint blockers in mouse tumor models. Oncoimmunology. 2020;9:1721810. doi: 10.1080/2162402X.2020.1721810. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data that led to the conclusions in this manuscript have been referenced and all sources have been described.


