ABSTRACT
Some studies have shown that the betel nut Areca catechu L and “binahong” leaves Anredera cordifolia (Ten) Steenis have anti-parasite and wound healing properties. This study evaluated the effect of A. catechu nut and A. cordifolia leaves powder supplementation on faecal parasite number and type, histopathology of the intestine, caecum, associated organs, some serum biochemistry, and egg production of laying hens. Twenty-four 54-week-old ISA-brown laying hens from local layer farmers were assigned randomly into 4-treatment groups: 1) without supplementation (T0), 2) supplemented with 0.25% (T0.25%), 3) 0.5% (T0.5%), 4) 1.0% (T1.0%). We carried out the supplementation for 18 days by administering A. catechu nut powder for 3-days, and subsequently, A. cordifolia leaves powder for another 3-days for 3-rounds to control the parasite larvae. Faecal parasite count and type were enumerated at the beginning and end of treatment. Egg production was recorded daily during the 18 days experiment. Blood was sampled at the end of the experiment to determine serum albumin, globulin, and transaminases. The intestinal tract, liver, and spleen samples were collected at the end of the study for histopathological examination. Faecal Ascaridia galli in control hens increased by 87.5% after 18 days of the experiment, while A. catechu nut and A. cordifolia leaves powder supplementation prevented such an increase. Supplemented hens have a better reduction of Railentina cesticillus compared to control birds. Supplementation improved intestinal and other tissue histopathology, especially in the caecum (free of erosion), improving serum albumin and transaminases without affecting egg production.
KEYWORDS: Areca catechu, Anredera cordifolia, hens, endoparasites, buah pinang, binahong
1. Introduction
The challenges of global warming manifested by sudden changes between rainy and hot-humid weather in tropical climates combined with antibiotic resistance are amplifying environmental stress and hardship for local producers. Globally, the nematode infestation in laying hens is widespread [1]. Ascaridia galli infection is the most persistent infestation in layers [2–9]. Small-scale-independent farmers in a tropical country such as Indonesia raise commercial layers to produce commercial eggs as the primary income source. Layer farmers bred the most common hybrid chicken the ISA Brown to lay eggs for up to two years or as long as egg production is economically feasible. Ascaridia galli is not the only harboured endoparasites in commercial hens but also endoparasites such as coccidian [5–9]. To prevent microbial challenge, farmers use readily available feed.
Herbs and plant metabolites/extracts are gaining wide attention owing to their numerous health benefits, therapeutic characteristic, and nutraceutical properties [10–20]. Areca catechu is a species of palm widely found in the tropical Pacific, Asia, and parts of Africa [21,22]. In India and Indonesia, it is a traditional masticatory/chewing herbal medicine. The fruit or nut is locally known as “buah pinang” or betel nut. It comprises polyphenols – mainly flavonoids and tannins (11.1–29.8%), polysaccharides (17.3–25.7%), proteins (6.2–9.4%), fats (8.1–15.1%), fibres (8.2–15.4%), alkaloids (0.11–0.24%) and minerals (1.1–2.5%) [22,23]. The alkaloids include arecoline (7.5 mg/g weight), arecaidine (1.5 mg/g weight), guvacoline (2.0 mg/g weight) and guvacine (2.9 mg/g weight) [22,24] and recently acatechu A and acatechu B [25]. The arecoline is the primary active substance and the major toxic compound [22,24,26]. It has higher lipophilicity; therefore, it can cross the cell membrane quickly [27]. It induces apoptosis in normal human cells and causes oral mucosal fibrosis and cytotoxicity [28].
The anti-helminthic properties of the Areca nut have been demonstrated in numerous in vitro and in vivo studies. Ethanol extract of Areca nut powder added at 40% showed an effective reduction in the motility of the liver fluke (Fasciola spp.) in the petri dish, resulting in shrinkage and deformation of the body shape and shrunken edges of the tegument [29]. At 1 g/Kg body weight (approximately 1.5%), Areca nut powder effectively expels round and tapeworm and eggs from indigenous local chicken. However, at a higher dosage of more than 1.5%, the chicken was not in good condition [30]. Areca nut extract posseses anticoccidial activity by reducing faecal oocyte counts, mucosal damage, and caecal lesion in broiler chicken experimentally infected with Eimeria tenella [31]. The mechanism appeared to be mediated by nitric oxide production, which was up-regulated in the inflammatory stage (3-days post-infection) and down-regulated 6-days post-infection. Ethanol extract and ethyl acetate fraction of betel nut can efficiently increase the number of goblet cells in the colon and caecum of mice orally infected with parasitic worm T. muris infective eggs [32]. Numerous studies showed a protective effect of Areca nut ethanol extract on a wound (excision and burn) healing and were likely to be due to its phenolic antioxidant activity [33–37].
Anredera cordifolia (Ten) Steenis (“Binahong”) leaves comprise flavonoids (vitexin, isovitexin, morin, and myricetin) and the sapogenins ursolic acid [38]. It has antibacterial and antioxidant activities [38–41]. Some studies demonstrated that A. cordifolia leaves have healing qualities on some wounds (cut, burn, post-partum perineum) [42–50]. A topical application of ethanol extract of A. cordifolia leaves twice a day on a 2 cm long excision wound in Guinea pig enhanced the length of excision closure, compared to control with povidone-iodine 10% [45]. A granulation network and re-epithelialization of the open physical wound appeared to be involved in healing [50]. Thus, the wound healing properties could be beneficial to healing the intestine and associated organ lesions due to endoparasite infestation. A study conducted by our group recorded an anti-microbial/endoparasite activity of A. cordifolia leaves powder in Saanen goat with mastitis. It exhibited that it could reduce total faecal oocytes [51]. However, there was no detail on oocyte types and their possible mechanism.
As the in-feed drug has been banned or increasingly reduced in animal husbandry practice and layer hens are an important source of quality protein (egg) with long production time, the potential of A. catechu and A. cordifolia to prevent natural endo-parasitic infestation is warranted to be studied. In a recent work, we found that administration of very low dosage, i.e. 0.025% – 0.1% A. catechu nut powder and A. cordifolia leaves powder alternately in 42 weeks layer hens reduced liver transaminases that could indicate cell regeneration properties of both additives [44]. However, the low dosage was insufficient to reduce faecal A. galli and other endo-parasite numbers than control without supplementation [5]. Therefore, we further study the supplementation of A. catechu nut and A. cordifolia leaves powder alternately every 3-days in 54-weeks-old layer hens with a higher dosage, i.e. ten times our first study. We investigated the faecal endo-parasite number, some serum biochemistry, and egg production, but importantly the histopathology of the intestinal tract, liver, and spleen. The histopathological examination of the affected tissues may give a better understanding about the anti-endoparasite properties of both phytogenic additives.
2. Material and methods
2.1. Ethical statement
Our study was conducted in ISA Brown laying-hens obtained from the existing small-scale layer farmer, and the hens for supplementation were raised similarly in a wire-battery cage but separately. During the experiment, we applied animal ethics provided the hens with free access to drinking water and feed according to standard hens’ age. Hens were sacrificed at the end of supplementation by cutting the jugular vein according to standard animal welfare. Ethical Committee approved the protocols of this in vivo animal study with approval number: 133/EA/KEPK-FKM/2022.
2.2. Collection of plant material and preparation of powder
Fresh dry A. catechu nuts were obtained from a local market, and the pericarp was discarded. Fresh A. cordifolia leaves were collected from local farmers and air-dried. The dried plant materials were ground to a powder and filtered through a 25–50 mesh screen to get a homogenous powder. The dried plant powder was kept in a refrigerated container for further use. A detailed description of the preparation process has been provided elsewhere [5,44].
2.3. Supplementation of A. catechu nut and A. cordifolia leaves powder
We obtained the experimental hens from a small-scale (2500 hens) independent-layer farmer in Semarang-Central Java, Indonesia. The farmer raised Isa Brown hens in a typical V-type three-tiers battery bamboo housing. Each battery cage was 40 cm x 40 cm x 30 cm in size, which housed one hen per cage. The three-tiers battery housing was located in an open space (outdoor) with a roof to protect it from sun and rain. The experimental hens (24) were randomly selected based on age (54-week-old) and body weight (with an average body weight of 1.88 ± 0.02 Kg). We raised the selected hens in standard wire-battery housing separately. The 24-hens were adapted for two weeks, after which they were randomly assigned into four treatment groups: control with no supplementation (T0); supplemented with 0.25% A. catechu nut and 0.25% A. cordifolia leaves powder (T2.5%); supplemented with 0.5% A. catechu nut and 1.0% A. cordifolia leaves powder (T0.5%); supplemented with 1.0% A. catechu nut and 1.0% A. cordifolia leaves powder (T1.0%). Each group contained 6 hens; hence, there were 24 hens. Each hen was given a 130 g commercial layer diet (mesh) from the farmers (2900 Kkal/Kg ME, 17–19% crude protein, 3–11% crude lipid, 5–6% crude fibre, 3.5% calcium, and 0.45% phosphor, with no antibiotic nor coccidiostat) per day and free access to drinking water. Supplementation was carried out by mixing the powder in the diet so that each supplemented group received the respective dosage. We designed the supplementation of A. catechu nut powder for 3-days, subsequently A. cordifolia leaves powder for 3-days, and the alternate administration was carried out for a total of 18 days. Figure 1 depicts a summary of the supplementation design.
Figure 1.
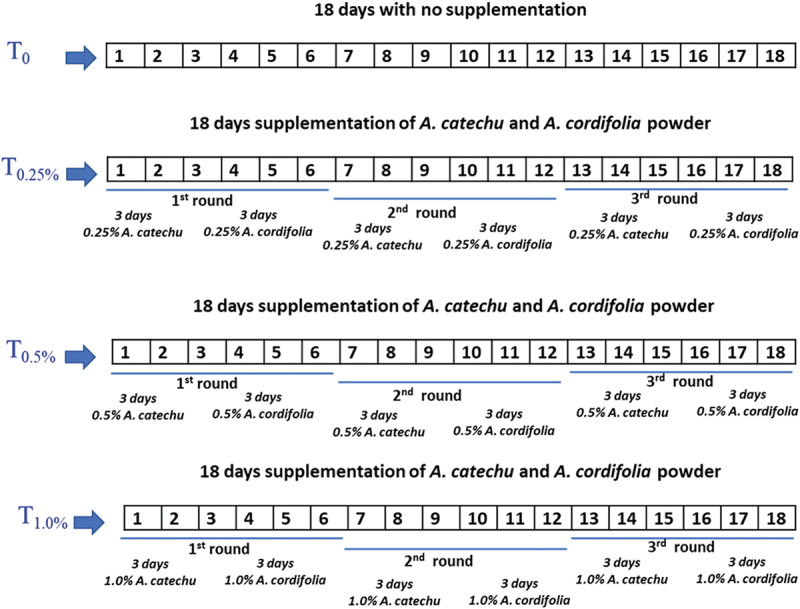
The supplementation designs. (T0): control without supplementation; (T0.25%): supplemented with 0.25% A. catechu nut and 0.25% A. cordifolia leaves powder; (T0.5%): supplemented with 0.5% A. catechu nut and 1.0% A. cordifolia leaves powder; (T1.0%): supplemented with 1.0% A. catechu nut and 1.0% A. cordifolia leaves powder. A total of 18 days supplementation consisted of first round administration of A.catechu for 3-days, followed by A. cordifolia for 3-days. Subsequently, followed by the second and third round which is the same as the first round i.e. administration of A.catechu for 3- days, followed by A. cordifolia for 3-days.
We designed a 3-days alternate supplementation based on the life cycle of endo-parasites and to control parasite larvae. We assumed that the first round of 3-days of betel nut (with anti-helminth potential) would kill the mature larvae. The next 3-days of A. cordifolia could recover the infected tissue with its wound healing properties, while allowing the larval stage of endoparasites to mature. The second round of 3-days betel nut supplementation would kill the mature larvae (from the larva of the first round), and then 3-days A. cordifolia would recover the tissue again. The third or final round of supplementation would kill the residual mature larvae from the previous round (round 2), and the following 3-days A. cordifolia supplementation provides the subsequent tissue recovery. To the best of our knowledge, no other publications has utilized a similar approach to ours in supplementing layer hens A. catechu and A. cordifolia except our previous study [44].
2.4. Collection of faecal samples, parasites identification, and enumeration
One day before phytogenic powder administration, we collected fresh faeces from each bird from all groups (total of 24 birds). Fresh faeces from each bird were immediately preserved in 10% formalin for parasite diagnosis [52]. The microscope was equipped with a camera, connected to a computer screen, and moved horizontally and vertically to scan the whole sample to identify and enumerate parasites. Parasites were grouped into types. The enumeration was carried out by experienced staff at the Laboratory of Animal Health Semarang city, Regional Office of Animal Husbandry and Health. Fresh faeces were collected at the end of the experiment from each bird from all groups (23 birds) and preserved in 10% formalin for endoparasite determination.
An increase or decrease in faecal parasite number for each type of parasite was calculated as follows:
Increase faecal parasite number (%) = 100 – [(faecal parasite number before treatment/faecal parasite number after treatment) x 100]
Reduction of faecal parasite number (%) = 100% – [(faecal parasite number after treatment/faecal parasite number before treatment) x 100]
2.5. Determination of serum albumin, globulin, AST, and ALT
At the end of the experiment, we collected blood samples from each bird of all groups (23 birds) through the brachial vein. Serum was immediately separated by centrifugation and stored frozen until analyses. The activity of alanine transaminase (ALT) and aspartate transaminase (AST) (previously known as serum glutamic pyruvic transaminase (GPT) and serum glutamic oxaloacetic transaminase (GOT)) were measured by the kinetic method according to the International Federation of Clinical Chemistry [10,11,13].
2.6. Histopathological examination
After blood sampling, birds were sacrificed by cutting the jugular vein according to Standard Animal Welfare. Duodenum, ileum, caecum, liver, kidney, and spleen were removed and preserved in buffered formalin. Preserved samples were embedded in paraffin, cut, and stained with Haematoxylin Eosin (HE) [12]. The samples were analysed by a histopathologist.
2.7. Egg production
We recorded each bird’s egg production and weight during 18 days of supplementation daily.
2.8. Statistical analysis
Excel and SPSS were used to conduct statistical analyses. Parasite numbers for each treatment group were compared to the control group (T0) before and after supplementation using a percentage of reduction, increase, or no change. A descriptive histopathological investigation was performed. Analyses of Variance (Anova) were performed on serum albumin, globulin, AST, and ALT data. Duncan’s multiple tests were carried out when the means among groups were significantly different. Paired T-tests were performed before and after supplementation for serum albumin, globulin, and transaminases. Significance was set at p ≤ 0.05.
3. Results
Table 1 shows the frequency of faecal parasite types and number before and after 18-day supplementation of A. catechu nut and A. cordifolia leaves powder. Before supplementation, helminth A. galli and R. cesticillus were observed in all hens with varying numbers in each group. It determines that the hens obtained from local farmers and raised in an outdoor battery housing have a varying endo-parasitic infestation.
Table 1.
The types and a total number of faecal parasites after 18 days supplementation of A. catechu seed and A. cordifolia leave powder alternately every 3-days in 54-week-old laying hens.
| T0 | T0.25% | T0.5% | T1.0% | |
|---|---|---|---|---|
| Before supplementation: | ||||
| Ascaridia galli | 1 | 8 | 4 | 0 |
| Railentina cesticillus | 7 | 13 | 10 | 11 |
| Tetrameres americana sp. | 0 | 0 | 0 | 0 |
| 2 types | 2 types | 2 types | 2 types | |
| After 18 days supplementation: | ||||
| Ascaridia galli | 8 | 7 | 4 | 0 |
| Railentina cesticillus | 1 | 0 | 0 | 0 |
| Tetrameres americana sp. | 0 | 0 | 1 | 0 |
| 2 types | 1 type | 2 types | 0 types | |
| Reduction or increase in endo-parasites number: | ||||
| A. galli | 87,5% increase | 12,5% reduction |
constant (4 parasites) |
constant (no parasites) |
| R.cesticillus | 85,7% reduction | 100% reduction | 100% reduction |
100% reduction |
| T. americana sp. | - | - | 1 (appear) | - |
Each group consisted of 6 hens, with 5 hens in control as one hen had accident and must be excluded.
(T0): control without supplementation; (T0.25%): supplemented with 0.25% A. catechu nut and 0.25% A. cordifolia leaves powder; (T0.5%): supplemented with 0.5% A. catechu nut and 1.0% A. cordifolia leaves powder; (T1.0%): supplemented with 1.0% A. catechu nut and 1.0% A. cordifolia leaves powder.
After 18-day supplementation, the control birds without supplementation have increased number of A. galli (87.5% increase). Interestingly, with 0.25% supplementation, the A. galli number was reduced by 12.5%, whereas with higher supplementation, there was no change in the number of A. galli. The reduction in the T0.25% group was slight, leaving 7-parasites (from 8), which was still higher compared to T0.5% and T1.0%. At 1.0% supplementation, the absence of A.galli was constant. In contrast, in the control group without supplementation showed an increase of 87.5% in A. galli number.
Table 2 represents a histopathological investigation of the duodenum, ileum, caecum, liver, kidney, and spleen after 18 days of supplementation of A. catechu nut and A. cordifolia leaves powder alternately in 54 weeks laying hens were presented in. In the control group, histopathology of the small intestine (duodenum, jejunum, ileum) and caecum displayed the presence of erosion (Er). In 0.5% supplemented groups, erosion was still present in the ileum and caecum to variable degrees. Strikingly, after 1% supplementation, no erosion can be detected in the caecum, and Figure 2 displays the samples of caecum histopathology of control and T1.0%).
Table 2.
Histopathological examination of duodenum, ileum, caecum, liver, spleen, after 18 days supplementation of A. catechu seed and A. cordifolia leave powder alternately in 54-week-old laying hens.
| Groups | T0 | T0.25% | T0.5% | T1.0% | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Replicate Birds | 1 | 2 | 3 | 4 | 5 | 6 | Total positive | 1 | 2 | 3 | 4 | 5 | 6 | Total positive | 1 | 2 | 3 | 4 | 5 | 6 | Total positive | 1 | 2 | 3 | 4 | 5 | 6 | Total positive | ||
| Duodenum | Er | - | + | + | + | + | x | 4/5 | + | + | - | + | + | + | 5/6 | + | + | - | + | + | + | 5/6 | + | + | + | + | + | + | 6 /6 | |
| I | + | - | - | - | - | x | 0/5 | + | - | - | - | - | - | 2/6 | - | - | - | - | - | - | 0/6 | - | - | - | - | - | - | 0/6 | ||
| Hlm | - | - | - | - | - | x | 0/5 | - | - | - | - | - | - | 0/6 | - | + | - | - | - | - | 1/6 | - | - | - | - | - | - | 0/6 | ||
| Jejunum | Er | + | + | + | + | + | x | 5/5 | + | + | + | + | + | + | 6/6 | + | + | + | + | + | + | 6/6 | + | + | + | + | + | + | 6 /6 | |
| Hr | - | - | - | - | - | x | 0/5 | - | + | - | - | - | - | 1/6 | - | - | - | - | - | - | 0/6 | - | - | - | - | - | - | 0/6 | ||
| I | + | - | - | - | - | x | 1/5 | + | - | - | - | - | - | 1/6 | - | - | - | - | - | - | 0/6 | - | - | - | - | - | - | 0/6 | ||
| Gc | - | - | - | - | - | x | 0/5 | - | - | - | - | - | - | 0/6 | - | + | - | + | - | - | 2/6 | - | - | - | - | + | - | 1/6 | ||
| C | - | - | - | - | - | x | 0/5 | - | - | - | - | - | - | 0/6 | - | - | - | - | - | - | 0/6 | - | - | - | + | - | - | 1/6 | ||
| Ileum | Er | + | + | + | + | + | x | 5/5 | + | + | + | + | + | + | 5/6 | - | - | - | + | + | + | 3/6 | + | + | - | - | - | + | 3/6 | |
| I | - | + | - | - | - | x | 1/5 | + | - | - | - | - | - | 1/6 | - | - | - | - | - | - | 0/6 | - | - | - | - | - | - | 0/6 | ||
| Gc | - | - | - | - | - | x | 0/5 | - | + | - | - | - | - | 1/6 | - | - | + | + | + | + | 4/6 | - | - | + | + | + | - | 3/6 | ||
| Hlm | - | - | - | - | - | x | 0/5 | - | - | - | - | - | - | 0/6 | + | - | - | - | - | - | 1/6 | - | - | - | - | - | - | 0/6 | ||
| NL | - | - | - | + | - | x | 1/5 | - | - | - | - | - | - | 0/6 | - | - | - | - | - | - | 0/6 | - | - | - | + | + | - | 2/6 | ||
| Caecum | Er | + | + | + | + | + | x | 5/5 | + | + | - | - | - | + | 3/6 | - | + | - | + | + | + | 4/6 | - | - | - | - | - | - | 0/6 | |
| I | + | - | + | - | - | x | 2/5 | + | - | - | - | - | + | 2/6 | - | - | - | - | - | - | 0/6 | - | - | - | - | - | - | 0/6 | ||
| Gc | - | - | - | + | + | x | 2/5 | - | + | - | - | - | - | 1/6 | - | + | - | - | + | - | 2/6 | - | - | - | - | - | - | 0/6 | ||
| C | - | - | - | - | - | x | 0/5 | - | - | - | - | - | - | 0/6 | + | - | - | - | - | - | 1/6 | - | - | - | - | - | - | 0/6 | ||
| Liver | N | + | + | + | + | - | x | 4/5 | - | - | - | + | - | - | 1/6 | - | - | - | - | + | - | 1/6 | - | + | - | + | + | 3/6 | ||
| Ic | + | + | + | + | - | x | 4/5 | - | + | - | + | + | + | 3/6 | + | + | - | + | + | + | 5/6 | + | - | - | + | - | - | 2/6 | ||
| Hr | - | - | - | - | + | x | 1/5 | - | - | - | - | - | - | 0/6 | - | - | - | - | - | - | 0/6 | - | - | - | - | - | - | 0/6 | ||
| C | - | - | - | - | - | x | 0/5 | - | - | - | - | - | - | 0/6 | - | - | - | - | - | - | 0/6 | - | - | - | - | + | - | 1/6 | ||
| Kidney | I | - | + | + | - | - | x | 2/5 | + | - | - | - | + | - | 2/6 | + | - | + | + | + | - | 4/6 | + | + | - | - | - | - | 2/6 | |
| N | - | - | - | - | - | x | 0/5 | - | - | - | - | - | - | 0/6 | - | - | - | + | - | - | 1/6 | - | - | - | - | - | + | 1/6 | ||
| C | - | - | - | - | - | x | 0/5 | - | - | - | - | - | - | 0/6 | - | - | - | - | - | - | 0/6 | + | - | - | - | - | - | 1/6 | ||
| Spleen | FL | - | - | + | + | - | x | 2/5 | - | - | - | - | - | - | 0/6 | - | - | - | - | - | - | 0/6 | - | - | - | - | - | - | 0/6 | |
| WP | - | - | - | - | - | x | 0/5 | + | - | + | - | - | - | 2/6 | + | - | - | + | + | - | 3/6 | - | - | - | + | - | + | 2/6 | ||
| I | - | - | - | - | - | x | 0/6 | - | - | - | - | - | - | 0/6 | - | - | + | - | - | - | 1/6 | - | - | - | - | - | - | 0/6 | ||
Notes: Each group consisted of 6 hens, with a control group of 5 hens (one was excluded due to accident)
Er = erosion, Hr = haemorrhage, I = inflammation; Ic = inflammatory cells around blood vessel, Hlm = helminth, GC = goblet cell, C = congestion; N = necrosis, FL = follicle lymphocytes, NL = nodular lymphoid, WP = white pulp
Each group consisted of 6 hens, with 5 hens in control as one hen had accident and must be excluded.
(T0): control without supplementation; (T0.25%): supplemented with 0.25% A. catechu nut and 0.25% A. cordifolia leaves powder; (T0.5%): supplemented with 0.5% A. catechu nut and 1.0% A. cordifolia leaves powder; (T1.0%): supplemented with 1.0% A. catechu nut and 1.0% A. cordifolia leaves powder.
Figure 2.
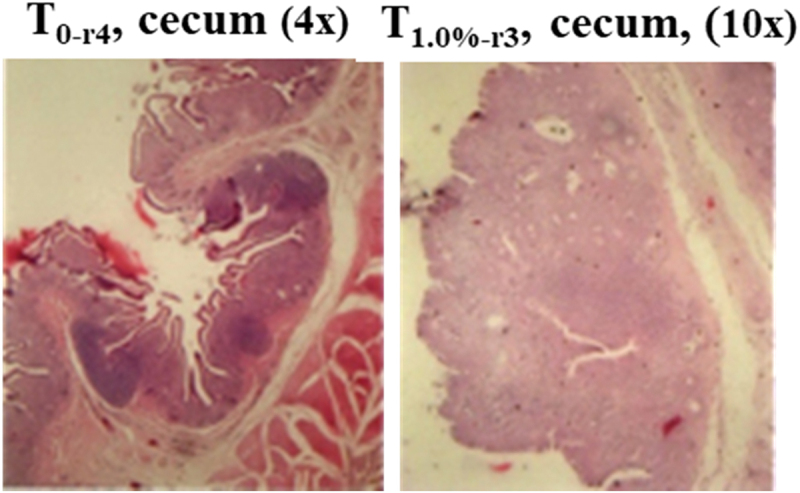
Samples of caecum histopathology showing heavy erosion in the control group without supplementation (T0 replicate-4, 4x magnification), and free of erosion in 1.0% A. catechu nut and 1.0% A. cordifolia leaves powder supplemented group (T1.0% replicate-3, 10x magnification). Histopathology of all groups were presented and summarized in Table 2
There was inflammation (I) in control duodenum, ileum, and caecum, but none after supplementation at 0.5% and 1%. In the ileum, goblet cells were not found in control birds but found in one of 0.25% supplemented birds, four of 0.5%, and 3- of 1% supplemented birds. There was helminth found in one bird with 0.5% supplementation. One control bird and two 1% supplemented birds had nodular lymphoid (NL).
Liver histopathology exhibited the presence of necrosis (N), infiltration of lymphocytes around blood vessels (Ic), congestion (C), and haemorrhage (H). Necrosis was found in the control liver (4/5 birds) and reduced after supplementation. Infiltration of lymphocytes (Ic) around blood vessels was found in four of 5 control birds. There were only two birds with Ic. after 1% supplementation. Congestion was found in one of 1% supplemented birds. Haemorrhage (Hr) was only found in one control bird and none in the supplemented group.
Several birds from all groups had inflammation in the kidney. There was necrosis in one of 0.5% and 1% of supplemented birds, while congestion (C) was found in one 1% supplemented bird. Spleen’s observation presented that follicle lymphocytes (FL) were found in two of five birds in the control group. No FL was observed after supplementation at all dosages. White pulp (WP) was observed in all supplemented birds but none in the control group. Inflammation was found only in 1 of 0.5% supplemented birds.
Table 3 presents the serum albumin, globulin, and transaminase levels of all groups before and after supplementation. There was a significantly higher level of globulin in groups supplemented with phytogenic additives before supplementation (p < 0.05). However, supplementation did not affect serum albumin, globulin, ALT, and AST (p > 0.05) in all groups.
Table 3.
Serum albumin, globulin, and transaminase in 54-week-old laying hens before and after 18 days alternate supplementation of A. catechu seed and A. cordifolia leaves powder.
| Supplementation |
p | ||||
|---|---|---|---|---|---|
| T0 | T0.25% | T0.5% | T1.0% | ||
| Albumin (g/dL) | |||||
| Before | 1.64 ± 0.34 | 1.86 ± 0.17 | 1.75 ± 0.20 | 1.73 ± 0.30 | 0.21 |
| After | 2.03 ± 0.08 | 2.22 ± 0.40 | 2.20 ± 0.21 | 2.21 ± 0.31 | 0.22 |
| P | 0.08 | 0.04* | 0.00* | 0.03* | |
| Globulin (g/dL) | |||||
| Before | 3.24 ± 1.74a | 5.76 ± 0.57b | 5.37 ± 1.06b | 5.50 ± 1.61b | 0.01* |
| After | 3.43 ± 1.69 | 4.10 ± 0.99 | 3.81 ± 1.13 | 4.60 ± 0.83 | 0.41 |
| P | 0.40 | 0.00* | 0.00* | 0.05* | |
| ALT (U/L) | |||||
| Before | 4.75 ± 3.45 | 6.26 ± 3.13 | 4.28 ± 1.04 | 5.55 ± 2.33 | 0.59 |
| After | 4.52 ± 3.07 | 2.93 ± 0.84 | 2.16 ± 0.50 | 3.46 ± 1.53 | 0.17 |
| P | 0.65 | 0.07 | 0.00* | 0.05* | |
| AST (U/L) | |||||
| Before | 299.2 ± 84.92 | 274.90 ± 54.58 | 229.53 ± 18.52 | 230.70 ± 38.68 | 0.31 |
| After | 202.06 ± 41.40 | 246.88 ± 45.62 | 205.13 ± 11.09 | 193. 23 ± 32.73 | 0.11 |
| P | 0.18 | 0.01* | 0.04* | 0.05* | |
AST: aspartate transaminase. ALT: alanine transaminase.
Each group consisted of 6 hens. with a control group of 5 hens (one was excluded due to accident)
Values represent the average of 6 hens (except control group with 5 hens) ± standard deviation
p with no superscript * means there is no effect of supplementation (p > 0.05) (Anova). Different superscript within the same row is significant at (p < 0.05) (post-hoc test).
P with superscript * means significantly different between before and after supplementation (T-test).
(T0): control without supplementation; (T0.25%): supplemented with 0.25% A. catechu nut and 0.25% A. cordifolia leaves powder; (T0.5%): supplemented with 0.5% A. catechu nut and 1.0% A. cordifolia leaves powder; (T1.0%): supplemented with 1.0% A. catechu nut and 1.0% A. cordifolia leaves powder.
Paired T-test revealed that serum albumin improved but serum globulin reduced significantly after supplementation (p < 0.05). Serum transaminase were significantly reduced after supplementation (p < 0.05).
Table 4 showed that all groups’ average total egg production and egg weight at the end of phytogenic powder supplementation was not significantly different (p > 0.05). However, the values are higher in all supplemented groups.
Table 4.
Egg production during 18 days supplementation of A. catechu seed and A. cordifolia Leaves powder in 54-week-old laying hens.
| Egg production | Supplementation |
p |
|||
|---|---|---|---|---|---|
| T0 | T0.25% | T0.5% | T1.0% | ||
| Average Egg Weight | 44.7 ± 27.8 | 58.83 ± 4.3 | 59.1 ± 5.6 | 47.9 ± 18.2 | 0.96 |
| Average Total Egg per Bird | 7.0 ± 5.1 | 9.2 ± 1.9 | 8.2 ± 6.1 | 8.6 ± 4.1 | 0.20 |
Each group consisted of 6 hens. with a control group of 5 hens (one was excluded due to accident).
Values represent the average of 6 hens (except control group with 5 hens) ± standard deviation.
Anova was done at p < 0.05. No superscript letter means there is no effect of supplementation on egg production.
(T0): control without supplementation; (T0.25%): supplemented with 0.25% A. catechu nut and 0.25% A. cordifolia leaves powder; (T0.5%): supplemented with 0.5% A. catechu nut and 1.0% A. cordifolia leaves powder; (T1.0%): supplemented with 1.0% A. catechu nut and 1.0% A. cordifolia leaves powde.
4. Discussion
Ascaridia galli is a parasitic roundworm that belongs to the phylum Nematoda. In our case, the birds obtained from local farmers were already infected with the parasite during their rearing. In the tropics, Ascaridia galli is the most prevalent parasite that affects layer husbandry [2–9, 53,54]. Infestation of A. galli in control birds without supplementation increased by 87.5% after 18 days (Table 1), indicating constant infection. On the other hand, a slight reduction occurred (12.5% reduction) at the lowest dosage of supplementation (0.25%) and a higher dosage of supplementation showed no change in faecal A. galli number. Hence, the supplemented groups that did not experience an increase in the number of faecal A. galli suggested that alternate supplementation of A. catechu and A. cordifolia every 3-days can prevent endo-parasite development thereby, preventing an increased parasitic growth that occurred in the control group without supplementation. Based on our methodological hypotheses, alternate supplementation allowed A. catechu nut powder (with anti-helminth potential) to kill the A. galli larvae, and followed by supplementation of A. cordifolia (with anti-wound healing activity) that healed some lesions resulting from the infection without interference from the A. catechu. After healing, another round of 3-days A. catechu nut re-supplementation again killed some more parasites, after which A.cordifolia was re-administered to continue the healing process (Figure 1). Thus, prevention of an increase of faecal A. galli in the supplemented group was likely attributed by the active alkaloid of Areca nut (arecoline, arecaidine, guvacoline, and guvacine), as shown by some studies [29–31]. When both supplements were administered as a mixture, the healing process became disturbed as a higher dosage of A. catechu (1%) could harm the organ’s cell lining and interfere with the healing process. Such harmful effect of A. catechu has been demonstrated and could be due to its alkaloid content [22,26]. Therefore, an alternate supplementation for 3-days would give time for each supplement to exert its biological function without interfering with one other.
Another possibility is that when the supplements were administered together, an antagonistic effect was seen, i.e. A. catechu, with its cytotoxic activity was capable of killing the larvae but was also cytotoxic to intestinal cells lining [28], that interfered with the healing action of A. cordifolia. A study [55] conducted by purposely infecting indigenous chicken with A. galli and supplementing a mixture of A. catechu nut and A. cordifolia leaf powder for only ten days showed that the supplemented group had more A. galli eggs per gram in the faeces and the duodenum than the control group. It was unclear why the mixture could not reduce A. galli in their study, but we speculated that the administration schedule and combined dosage play an important role. Therefore, our study added significant evidence that 1% supplementation of A. catechu and A. cordifolia powder alternately every 3-days resulted in a better reduction of faecal A. galli.
For R. cesticillus, it was naturally reduced by 85.7% in the control group (Table 1). However, all supplemented groups had reduced R. cesticillus by 100%, suggesting the ability of alternate supplementation to improve the reduction. Raillietina species are found in the jejunum and ileum of the chickens and can reduce growth, causing weakness and digestive tract obstruction, whereas their larval stage (cysticercoid) resides in various invertebrate intermediate hosts, such as ants, beetles, small mini-wasps, or termites [56–58]. Railentina cesticillus is known to spread via flies and cockroaches due to dirty housing [59–62]. With several thousand or more hens, some small-scale farmers have no assistance to do daily sanitation. Furthermore, the outdoor cage-housing with its daily excretes quickly attracted flies and cockroaches, facilitating the spread of the parasites. In control hens, a natural reduction is experienced due to our management, namely daily cleaning of all excretes, feed, and drinking containers. Therefore, the intermediate host was eliminated, and with clean feed and drinking water daily, the hens’ natural immune defence can work better, reducing faecal R. cesticillus in the control group. The supplemented group improved the natural reduction, indicating A. catechu nut and A cordifolia leave powder contribution.
In 0.5% of supplemented birds, one type of helminth T. americana sp. appeared after 18 days. Tetrameres americana sp. are small parasitic roundworms that infect chickens by ingesting an intermediate host, such as grasshoppers, cockroaches, earthworms, and water fleas. This type of helminth was already found in previous layers from the same farm [5]. Many studies show that helminth infestation can never be eradicated. It is due to its life cycle in which a fertile egg can be re-ingested via faeces and grow in the intestinal tract. Later, the eggs are shed along with the faeces, and the cycle is repeated. The appearance of T. americana in the 0.5% supplemented group could be from the expelled parasite from the intestine by the action of the active alkaloid of Areca nut (arecoline, arecaidine, guvacoline, and guvacine) powder [30]. The ability of Areca nut alkaloid to penetrate mucosal lining could reach the buried parasite, and the gastrointestinal peristaltic activity can expel it into the faeces [22,63].
Histopathological examination of all groups indicated that the intestinal and caecal samples of all the birds from the control group with no supplementation showed erosion (E) (Table 2). Erosion in these tissues is a sign of infection from helminth and cocci infestation, common in layers worldwide [5,44]. It also indicates that the laying hens from the farmers have already experienced chronic infections whose severity could not be determined. As the hen ingests the infective egg of A. galli, the first larva stage hatch in the proventriculus or duodenum. It moults into second and third-stage larvae in the lumen, with some attaching the mucosal lining and feeding. Lesions and haemorrhaging of the mucosa occur during the third stage of larval feeding, causing enteritis, anaemia, and diarrhoea [54,64]. In supplemented groups, erosion was still present in some hens in the duodenum, jejunum, and ileum, suggesting that infection to the organ from endo-parasite can only be healed partly by supplementation. We speculate that the healing is attributed to both phytogenic as both contain polyphenolic compounds with antioxidant properties and re-epithelialization activity [33,34,36,37].
Goblets cells appear at 0.5% and 1% supplementation in the jejunum, ileum, and caecum but not in the duodenum. At 1% supplementation, three hens did not show erosion, and the presence of goblet cells indicated the presence of regeneration as these cells function to produce and maintain mucus layers along the intestinal lining. Two birds in this group had lymph nodules, that indicated an ongoing immune response against endoparasites antigen. The parasites could be hiding under epithelial layers and cause reinfection to occur. Strikingly, for caecum, supplementation of the powder decreased erosion remarkably as compared to control with no supplementation. At 0.25% supplementation, half of the samples were free from erosion, and strikingly at 1%, all samples were free from erosion. It could be due to the inability of sporozoite and merozoite to develop due to A. catechu nut powder (Table 1, after 1% supplementation), while A. cordifolia assists in healing the damaged tissue [33–35,65,66]. As we have previously described, the mechanism could be due to the action of the active alkaloid of Areca nut (arecoline, arecaidine, guvacoline, and guvacine) powder [30]. The lipophilic nature of the alkaloid to penetrate the lipid bilayer of mucosal lining could reach and kill the buried parasite [22,63]. During 3-days of Areca nut administration, the cytotoxicity against parasites was more noticeable than the healing activity. Therefore, the next round of A. cordifolia leaves administration would further support the healing activity mediated by re-epithelialization. Furthermore, the ursolic acid of A. cordifolia can induce epidermal keratinocyte differentiation (via peroxisome proliferator-activated receptor-alpha) that also assists in healing the erosion in the caecum [67]. The caecum is the blind sac of hens before the excretion of undigested feed. The A. cordifolia leaves powder and Areca nut with its fibre could partly reach the caecum and stay longer and hence could act longer to heal all the erosion. It is warranted to study such a possibility further.
Necrosis in the liver showed that after 0.25 and 0.5% supplementation, there is only one tissue sample that showed necrosis. At 1% supplementation, three samples showed necrosis, which could be due to several factors. One is that increasing the dosage of A. catechu nut powder could be toxic to the liver. Arecoline of the beetle nut is cytotoxic and can cause fibrosis [68]. Necrosis appears due to an inflammatory response, after which regeneration occurs and continues with tissue remodelling. However, uncontrolled tissue remodelling can lead to fibrosis, which could occur at 1% supplementation. Another possibility is that at a lower dosage, Areca nut powder can function as an anthelmintic, antioxidant, antibacterial, and anti-inflammation [24,28,36,69]. Therefore, in 0.25% and 0.5% of supplemented birds, the number of liver samples with necrosis and lymphocytes infiltration around blood vessels was reduced.
The histopathology of the kidney showed that supplementation did not affect it, as the samples’ initial and final conditions were similar on average. The kidney functions to filter the blood, excreting the end product of metabolism and regulating the concentration of hydrogen ions and minerals in extracellular fluid. To the best of our knowledge, there have been no studies that relate kidneys to endoparasite infestation, and the presence of inflammation in some samples in each group indicated a normal condition of 56-weeks-old layers.
The spleen is a secondary lymphoid organ in chickens. The presence of follicle lymphocytes (FL) in the control group’s spleen indicated an immune response against an antigen. The antigen likely comes from the Marek virus, which usually infects layers worldwide, except in flocks purposely raised under pathogen-free conditions. Significant swelling in the visceral organ during organ sampling supports the possible Marek viral infection. Marek infection occurs in layer chicken from 1 to 3 weeks old with or without clinical signs, and this infection can reduce growth and egg production [12]. No follicle lymphocytes were found in all supplemented birds, but white pulp appeared. White pulp (WP) indicates the presence of coccidial infection, mainly from Emeria tennela. Reinfection stimulates lymphocytes to respond more quickly to proliferate, followed by an increase in diameter and weight of the white pulp [70]. Although no E. tennela was found in bird faecal samples, the remnants of inflammatory response in the spleen were still observable. White pulp appearance indicates that supplementation improves the immune response against endoparasite infection.
Our present results showed that serum albumin concentration after supplementation improved significantly (p < 0.05) (Table 3). As albumin synthesis occurs in hepatocytes, it indicates an improvement in liver function. The possible improvement is supported by the results that showed supplementation reduced transaminase significantly (p < 0.05). The result is also consistent with our previous study that showed reduced transaminase activity after alternate supplementation using both powders at one-tenth of the present dosage in 42-week-old layers [5,44]. All phytogenic taken via gastro-intestine (GIT) are carried to the liver. A higher dosage used in the present study could put a higher liver workload and cause cell damage, especially from the active alkaloid of Areca nut. However, the polyphenol as the main content of Areca nut (11.1–29.8%) is a well-known antioxidant that could counter-act cell damage by the active alkaloid (0.11–0.24%) [23] and radicals’ generation. Therefore, its use depends on the dosage, administration schedule, and combined use with another herb. Subsequent administration of A. cordifolia leaves with epithelialization activity 3-days after Areca nut administration [35] added further support in preventing and healing cells’ damage. Reduction in serum globulin after supplementation indicates a reduction in inflammation due to immune response to endoparasites. Reduction of inflammation is supported by the results of faecal parasites number and histopathology after supplementation, as described previously.
Our results support the anti-endoparasites and wound healing function of Areca nut and A. cordifolia leaves powder in vivo layer hens, especially in the caecum, improving serum albumin and transaminase without affecting egg production.
5. Conclusion
Our results demonstrated that supplementation of phytogenic Areca catechu nut and Anredera cordifolia leave powder alternately every 3-days for 18 days reduced faecal-endoparasites and improved histopathology endoparasites-affected tissues in layer hens, especially in the caecum. The dosage and alternative schedule of supplementation of A. catechu and A. cordifolia to reduce endoparasites and heal cells’ damage are still open for further studies.
Acknowledgments
We thank Ms. Eka Maya Kurniasih for formatting the article. This study is part of a collaborative initiative between researchers of Universitas Diponegoro, Semarang, Indonesia, and Tuft University, Boston, USA.
Funding Statement
This work is partially supported by the Faculty of Animal and Agricultural Sciences, Universitas Diponegoro, Semarang, Indonesia.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].Jansson DS, Nyman A, Vågsholm I, et al. Ascarid infections in laying hens kept in different housing systems. Avian Pathol. 2010;39(6):525–532. [DOI] [PubMed] [Google Scholar]
- [2].Kumar S, Garg R, Ram H, et al. Gastrointestinal parasitic infections in chickens of upper gangetic plains of India with special reference to poultry coccidiosis. J Parasitic Dis. 2015;39(1):22–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ogbaje CI, Agbo EO, Ajanusi OJ.. Prevalence of Ascaridia galli, Heterakis gallinarum and tapeworm infections in birds slaughtered in Makurdi township. Int J Poult Sci. 2012;11(2):103–107. [Google Scholar]
- [4].Grafl B, Polster S, Sulejmanovic T, et al. Assessment of health and welfare of Austrian laying hens at slaughter demonstrates influence of husbandry system and season. Br Poult Sci. 2017;58(3):209–215. [DOI] [PubMed] [Google Scholar]
- [5].Kusumanti E, Murwani R.. Reduction of fecal parasites by Arecha catechu L. seed and Anredera cordifolia (Ten) Steenis leaves powder in laying hens. Third International Conference on Tropical and Coastal Region Eco-Development (ICTCRED). IOP Conf. Series: Earth and Environmental Science, Yogyakarta, Indonesia. 2017; 116(18) March 2018: Article 012102. [Google Scholar]
- [6].Abd El-Maksoud HA, Abdel-Magid AD, El-Badry MA. Biochemical effect of coccidian infestation in laying hen. BENHA VET MED J. 2014;26(1):127‐133. [Google Scholar]
- [7].Hafiz AB, Muhammad AR, Muhammad AA, et al. Prevalence of Ascaridia galli in white leghorn layers and Fayoumi-Rhode Island red crossbred flock at government poultry farm Dina, Punjab, Pakistan. Trop Biomed. 2015;32(1):11–16. [PubMed] [Google Scholar]
- [8].Muhammad AA, Imran AK, Abdul A, et al. Prevalence of Ascaridia galli in white leghorn layers and Fayoumi-Rhode Island red crossbred flock at government poultry farm Dina, Punjab, Pakistan. Trop Biomed. 2015;32(1):11–16. [PubMed] [Google Scholar]
- [9].Sharma N, Hunt PW, Hine BC, et al. The impacts of Ascaridia galli on performance, health, and immune responses of laying hens: new insights into an old problem. Poult Sci. 2019;98(12):6517–6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Murwani R, Bayuardhi B. Broilers serum cholesterol and glutamic oxaloacetic transaminase and their relation to antibiotic in feed and medication programs in four broiler producers in Semarang region-Central Java, Indonesia. Int J Poult Sci. 2007;6(4):266–270. [Google Scholar]
- [11].Murwani R, Murtini S. Effect of chlortetracycline additive in broilers fed local diets on antibody titers to NDV vaccine. Int J Poult Sci. 2009;8(8):755–759. [Google Scholar]
- [12].Murtini S, Murwani R, Satrija F, et al. Anti Marek’s disease virus activity of Scurrulaoortiana (Tea Mistletoe) stem extract in Embryonic chicken eggs. Int J Poult Sci. 2010;9(9):879–885. [Google Scholar]
- [13].Murwani R, Indriani A, Wihardani K, et al. Blood biochemical indices and productivity of broilers on diet supplemented with mannan oligosaccharide, baker yeast, or combined baker yeast and noni leaves extracts. Int J Poult Sci. 2011;10(12):990–997. [Google Scholar]
- [14].Tanod WNL, Murwani R, Susanti S, et al. Addition of cashew (Annacardium occidentale) apple powder into diet can increase body weight and intestinal relative weight in broiler. Pak J Nutr. 2015;14(9):629–631. [Google Scholar]
- [15].Murwani R. Effect of corn or sorghum in combination with soybean meal or mungbean as feed ingredients on the serum antibody titres to NDV vaccine in broiler chickens. Int J Poult Sci. 2008a;7(5):497–501. [Google Scholar]
- [16].Murwani R. Aditif Pakan Aditif Alami Pengganti Antibiotika (feed additives: natural alternative to in-feed antibiotics). Semarang, Indonesia: Unnes Press; 2008b. [Google Scholar]
- [17].Sibero MT, Siswanto AP, Murwani R, et al. Antibacterial, cytotoxicity and metabolite profiling of crude methanolic extract from andaliman (Zanthoxylum acanthopodium) fruit. Biodiversitas. 2020;21(9):4147–4154. [Google Scholar]
- [18].Ebrahim AA, Elnesr SS, Abdel-Mageed MAA, et al. Nutritional significance of aloe vera (Aloe barbadensis Miller) and its beneficial impact on poultry. World’s Poult Sci J. 2020;76(4):803–814. [Google Scholar]
- [19].Dhama K, Sharun K, Gugjoo MB, et al. A comprehensive review on chemical profile and pharmacological activities of Ocimum basilicum. Food Rev Int. 2021;1–29. DOI: 10.1080/87559129.2021.1900230 [DOI] [Google Scholar]
- [20].El-Saadony MT, Zabermawi NM, Zabermawi NM, et al. Nutritional aspects and health benefits of bioactive plant compounds against infectious diseases: a review. Food Rev Int. 2021;1–23. DOI: 10.1080/87559129.2021.1944183 [DOI] [Google Scholar]
- [21].Cheriyan H, Monoj KK. Arecanut production scenario in India. Indian J Arecanut Spices Med Plants. 2014;16(4):3–11. [Google Scholar]
- [22].Xiaoxiao Chen, Yongzhi He, Yanru Deng . Chemical Composition, Pharmacological, and Toxicological Effects of Betel Nut. Evidence-Based Complementary and Alternative Medicine. 2021; Article ID. 1808081:7. 10.1155/2021/1808081 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [23].Shivashankar S, Dhanaraj S, Mathew AG, et al. Physical and chemical characteristics of processed Areca nuts. J Food Sci Technol. 1969;6:113–116. [Google Scholar]
- [24].Amudhan MS, Begum VH, Hebbar KB. A review on phytochemical and pharmacological potential of Areca catechu L. Seed. Int J Pharm Sci Res. 2012;3(11):4151–4157. [Google Scholar]
- [25].Cao M, Yuan H, Daniyal M, et al. Two new alkaloids isolated from traditional Chinese medicine Binglang the fruit of Areca catechu. Fitoterapia. 2019;138:104276. [DOI] [PubMed] [Google Scholar]
- [26].Deng JF, Ger J, Tsai WJ, et al. Acute toxicities of betel nut: rare but probably overlooked events. J Toxicol Clin Toxicol. 2001;39(4):355–360. [DOI] [PubMed] [Google Scholar]
- [27].Cai ZY, Li YC, Li LH, et al. Analysis of arecoline in Semen Arecae decoction pieces by microchip capillary electrophoresis with contactless conductivity detection. J Pharm Anal. 2012;2(5):356–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Peng W, Liu YJ, Wu N, et al. Areca catechu L. (Arecaceae): a review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J Ethnopharmacol. 2015;164:340–356. [DOI] [PubMed] [Google Scholar]
- [29].Yamson EC, Tubalinal GASP, Viloria VV, et al. Anthelmintic effect of betel nut (Areca catechu) and neem (Azadirachta indica) extract against liver fluke (Fasciola spp.). J Adva Vet Anim Res. 2019;6(1):44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tangalin MGG. Anthelmintic effects of processed mature betel nut as dewormer to native chicken and small ruminants (sheep and goats). Asian J Health Basic Res Sect. 2011;1:230–243. [Google Scholar]
- [31].Wang D, Zhou L, Li W, et al. Anticoccidial effects of areca nut (Areca catechu L.) extract on broiler chicks experimentally infected with Eimeria tenella. Exp Parasitol. 2018;184:16–21. [DOI] [PubMed] [Google Scholar]
- [32].Anto EJ, Lelo A, Ilyas S, et al. Effect of ethanol extract and ethyl acetate fraction of betel nut (Areca catechuL.) in Colonic goblet cells of mice (Mus musculus) given orally infective egg of Trichuris muris. Open Access Maced J Med Sci. 2020;8(B):637–642. [Google Scholar]
- [33].Azeez S, Amudhan S, Sachidananda M, et al. Wound healing profile of Areca catechu extracts on different wound models in Wistar rats. Kuwait Med J. 2007;39:48–52. [Google Scholar]
- [34].Verma DK, Bharat M, Nayak D, et al. Areca catechu: effect of topical ethanolic extract on burn wound healing in albino rats. Int J Pharmacol Clin Sci. 2012;1(3):74–78. [Google Scholar]
- [35].Miladiyah I, Prabowo BR. Ethanolic extract of Anrederacordifolia (Ten.) Steenis leaves improved wound healing in Guinea pigs. Universa Med. 2012;31(1):4–11. [Google Scholar]
- [36].Sazwi NN, Nalina T, Abdul Rahim ZH. Antioxidant and cytoprotective activities of piper betle, Areca catechu, Uncaria gambir and betel quid with and without calcium hydroxide. BMC Complement Altern Med. 2013;13(1):351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Amudhan MS, Begum VH. Protective effect of Areca catechu extract on ethanol induced gastric mucosal lesions in rats. Pharmacology. 2008;1:97–106. [Google Scholar]
- [38].Alba TM, Garlet de Pelegrin CM, Sobottka AM. Pharmacognosy, ethnobotany, ecology, pharmacology, and chemistry of Anredera cordifolia (Basellaceae): a review. Rodriguésia. 2020;71:e01042019. [Google Scholar]
- [39].Selawa W, Runtuwene MRJ, Citraningtyas G. Flavonoid content and total antioxidant capacity of binahong leaf ethanol extract. Pharmacon J Ilmiah Farmasi UNSRAT. 2013;2(1):2302–2493. [Google Scholar]
- [40].Khunaifi M Test of antibacterial activity of binahong leaf extract (Anredera cordifolia (Ten) Steenis) against Staphylococcus aureus and Pseudomonas aeruginosa bacteria. Doctoral dissertation, Universitas Islam Negeri Maulana Malik Ibrahim. 2015 [Google Scholar]
- [41].Wardani LK, Sulistyani N . Antibacterial activity test of ethyl acetate extract of binahong leaf (Anredera scandens (L.) Moq.) against Shigella flexneri with the profile of thin layer chromatography. J Ilmiah Kefarmasian. 2012;2(1): 1–16. http://journal.uad.ac.id/index.php/PHARMACIANA/article/view/636 [Google Scholar]
- [42].Prasetyo AT, Herihadi E. The application of moist exposed burn ointment (MEBO) and binahong leaves in treating partial thickness burn: a case report. J Plastik Rekonstruksi. 2013;3:142–146. [Google Scholar]
- [43].Baby AA, Raphael KR. Potential antimicrobial, anthelmintic, and antioxidant properties of Areca catechu L. Root. Int J Pharm Pharm Sci. 2014;6(6):486–489. [Google Scholar]
- [44].Marlani HP, Kusumanti E, Murwani R. Arechaatechu L. seed and Anredera cordifolia (Ten) Steenis leaf powder supplementation reduced serum transaminase in laying hens. Livestock Research for Rural Development. 2017;29. Article #94. Retrieved June 24, 2022, from http://www.lrrd.org/lrrd29/5/rmur29094.html [Google Scholar]
- [45].Miladiyah I, Prabowo BR. Ethanolic extract of Anredera cordifolia (Ten.) Steenis leaves improved wound healing in Guinea pigs. Universa Med. 2015;31(1):4–11. [Google Scholar]
- [46].Kaur G, Utami NV, Usman HA. Effect of topical application of binahong [Anredera cordifolia (Ten.) Steenis] leaf paste in wound healing process in mice. Althea Med J. 2014;1(1):6–11. [Google Scholar]
- [47].Wijayanti K, Esti RHS. Effectiveness of binahong decoction water (Anredera cordifolia (ten) steenis) for perineal wound healing at home delivery aesya grabag magelang. Int J Res Med Sci. 2017;5(5):1970–1975. [Google Scholar]
- [48].Airlangga KSG, Gorda IW, Dada IKA, et al. Binahong leaves accelerates the healing of burns on white rats. Bull Veteriner Udayana. 2019;11(1):8–78. [Google Scholar]
- [49].Ariani S, Loho L, Durry MF. Benefits of leaves binahong (Anredera Cordifolia (Ten) against establishment of network granulation and reepitalization rabbit open wound healing skin. J e-Biomedik (Ebm). 2013;1(2):914–919. [Google Scholar]
- [50].Kurniawan F. Effect of ointment binahong leaf extract (Anredera Cordifolia) ethanol fraction against healing physical wounds on rabbit (Canis Familiaris). Universitas Gadjah Mada. 2013. [Google Scholar]
- [51].Kusumanti E, Sugiharto S. Effect of dietary supplementation of binahong leaf meal, betel nut meal or their combination on serum albumin and globulin, fecal endoparasites and bacterial counts in milk of Saanen goats suffering from subclinical mastitis. Agric Nat Resour. 2017;51(5):415–419. [Google Scholar]
- [52].Parfitt JW, Bank AW. A method for counting Fasciola eggs in cattle faeces in the field. Vet Rec. 1997;87:180–182. [DOI] [PubMed] [Google Scholar]
- [53].Ananda RR. Studi infeksi nematoda pada ayam petelur (Gallus Gallus) Strain Isa Brown di Peternakan Mandiri Kelurahan Tegal Sari, Kecamatan Gading Rejo, Kab. Pringsewu, Lampung (A study of nematode infection of Isa Brown laying Hens in small-scale husbandry farmer in Tegal Sari village, Gading Rejo district, Pringsewu regency, Lampung). Bachelor thesis. Faculty of Mathematic and Natural Science: Lampung Indonesia. 2018 [Google Scholar]
- [54].Tanuwijaya PA, Febraldo D. Parasite Infections in Poultry Environments (case report on Gallus domesticus endoparasite). J Environ Sci Sustainable Dev. 2021;4(1):97–136. [Google Scholar]
- [55].Prastowo J, Herawati O, Ariyadi B, et al. Effects of Areca catechu seed and Anredera cordifolia leaf on Ascaridia galli infection in the domestic chicken (Gallus Gallus domesticus). Int J Poult Sci. 2017;16(12):494–499. [Google Scholar]
- [56].Permin A, Hansen JW. The epidemiology, diagnosis and control of poultry parasites. A FAO handbook. Rome Italy: Food and Agriculture Organization of the United Nations; 2003. p. 36–39. [Google Scholar]
- [57].Alenyorege B, Alexander A, Kosono A, et al. Termites as intermediate hosts for poultry worms. J Vet Adv. 2011;1:16–23. [Google Scholar]
- [58].Butboonchoo P, Wongsawad C, Rojanapaibul A, et al. Morphology and molecular phylogeny of Raillietina spp. (Cestoda: cyclophyllidea: davaineidae) from domestic chickens in Thailand. Korean J Parasitol. 2016;54(6):777–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hall HTB. Diseases and parasites of livestock in the tropics. 2nd ed. Longman Publishing Group, London; 1985. [Google Scholar]
- [60].Fraser CM, Bergeron JA, Mays A, et al. The merck veterinary manual a handbook of diagnosis, therapy, and disease prevention and control For the veterinarian. 7th ed. Amstutz HE, et al., Edited by, Rahway NJ: Merck & CO., Inc; 1991. [Google Scholar]
- [61].Yusof AM. Identification of cockroaches as mechanical vector for parasitic infections and infestations in Kuantan, Malaysia. J Entomol. 2018;15(3):143–148. [Google Scholar]
- [62].Gałęcki R, Sokół R, Oliveira PL. A parasitological evaluation of edible insects and their role in the transmission of parasitic diseases to humans and animals. PLoS ONE. 2019;14(7):e0219303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kaushal M, Mishra AK, Raju BS, et al. Betel quid chewing as an environmental risk factor for breast cancer. Mutat Res. 2010;703(2):143–148. [DOI] [PubMed] [Google Scholar]
- [64].Mullens BA, Murillo AC . Parasites in laying hen housing systems. In: Hester PY, editor. Egg Innovations and Strategies for Improvements. London, United Kingdom: Elsevier; 2017. pp. 597–606. 10.1016/B978-0-12-800879-9.00055-X [DOI] [Google Scholar]
- [65].Moura-Letts G, Villegas LF, Marçalo A, et al. In vivo wound-healing activity of oleanolic acid derived from the acid hydrolysis of Anredera diffusa. J Nat Prod. 2006;69(6):978–979. [DOI] [PubMed] [Google Scholar]
- [66].Morikawa T, Ninomiya K, Takamori Y, et al. Oleanane-type triterpene saponins with collagen synthesis-promoting activity from the flowers of Bellis perennis. Phytochemistry. 2015;116:203–212. [DOI] [PubMed] [Google Scholar]
- [67].Lim SK, Hong SP, Jeong SW, et al. Simultaneous effect of ursolic acid and oleanolic acid on epidermal permeability barrier function and epidermal keratinocyte differentiation via peroxisome proliferator-activated receptor-α. J Dermatol. 2007;34:625–634. [DOI] [PubMed] [Google Scholar]
- [68].Venkatesh D, Puranik RS, Vanaki SS, et al. Study of salivary arecoline in areca nut chewers. J Oral Maxillofacial Pathol. 2018;22(3):446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Jaiswal P, Kumar P, Singh VK, et al. Areca catechu L.: valuable herbal medicine against different health problems. Res J Med Plant. 2011;5:145e152. [Google Scholar]
- [70].Yunus M, Suwanti LT, Mustofa I, et al. The effects of Eimeria tenella infection on the spleen weight, size and white pulp diameters in the chicken’s primary infection and secondary infection. Vet Medika. 2008;1:13–16. [Google Scholar]


