Abstract
Background:
Naming impairment is commonly noted in individuals with aphasia. However, object naming receives more attention than action naming. Furthermore, most studies include participants with aphasia due to only one aetiology, commonly stroke. We developed a new assessment, the Hopkins Action Naming Assessment (HANA), to evaluate action naming impairments.
Aims:
Our aims were to show that the HANA is a useful tool that can (1) identify action naming impairments and (2) be used to investigate the neural substrates underlying naming. We paired the HANA with the Boston Naming Test (BNT) to compare action and object naming. We considered participants with aphasia due to primary progressive aphasia (PPA) or acute left hemisphere stroke to provide a more comprehensive picture of brain-behaviour relationships critical for naming. Behaviourally, we hypothesised that there would be a double dissociation between object and action naming performance. Neuroanatomically, we hypothesised that different neural substrates would be implicated in object vs. action naming and that different lesion-deficit associations would be identified in participants with PPA vs. acute stroke.
Methods & Procedures:
Participants (N=138 with PPA, N=37 with acute stroke) completed the BNT and HANA. Behavioural performance was compared. A subset of participants (N=31 with PPA, N=37 with acute stroke) provided neuroimaging data. The whole brain was automatically segmented into regions of interest (ROIs). For participants with PPA, the image variables were the ROI volumes, normalised by the brain volume. For participants with acute stroke, the image variables were the percentage of each ROI affected by the lesion. The relationship between ROIs likely to be involved in naming performance was modelled with LASSO regression.
Outcomes & Results:
Behavioural results showed a double dissociation in performance: in each group, some participants displayed intact performance relative to healthy controls on actions but not objects and/or significantly better performance on actions than objects, while others showed the opposite pattern. These results support the need to assess both objects and actions when evaluating naming deficits. Neuroimaging results identified different regions associated with object vs. action naming, implicating overlapping but distinct networks of regions. Furthermore, results differed for participants with PPA vs. acute stroke, indicating that critical information may be missed when only one aetiology is considered.
Conclusions:
Overall, the study provides a more comprehensive picture of the neural bases of naming, underscoring the importance of assessing both objects and actions and considering different aetiologies of damage. It demonstrates the utility of the HANA.
Keywords: aphasia, stroke, primary progressive aphasia, object naming, action naming
Introduction
While aphasia is most commonly caused by stroke, other aetiologies including brain tumor, traumatic brain injury, infection, and neurodegenerative diseases such as primary progressive aphasia (PPA) can also result in aphasia. Studying individuals with each of these causes of aphasia can provide complementary information about the neural bases of language. For example, many people with post-stroke aphasia have been observed who have difficulty linking word sounds and word meanings after focal damage to left posterior superior temporal cortex, supporting Wernicke’s classic observations that the area is critical for word comprehension (e.g., Hillis, Rorden, & Fridriksson, 2017). Similarly, individuals with semantic variant PPA exhibit impaired semantic memory along with disproportionate atrophy of the anterior temporal lobes, supporting the theory that those regions form a semantic hub (e.g., Lambon Ralph, Jefferies, Patterson, & Rogers, 2016), another component of the network underlying word comprehension. Converging evidence across diseases has also been reported (Faria et al., 2013). For instance, double dissociations between spoken and written naming have been observed in both stroke and PPA (e.g., Caramazza & Hillis, 1990; Ellis & Young, 1988; Gorno-Tempini et al., 2011; Hillis, Oh, & Ken, 2004; Miceli & Capasso, 1997; Rapp, Benzing, & Caramazza, 1997; Tainturier, Moreaud, David, Leek, & Pellat, 2001). However, results from different aetiologies are seldom compared directly. Comparing individuals with different aetiologies of damage can provide a more comprehensive picture of the brain-behavior relationships critical for language. It could be that similar patterns are observed regardless of aetiology if the same critical regions are damaged in both. Alternatively, it could be that complementary information is observed from different aetiologies since different regions are more likely to be damaged by stroke versus neurodegenerative disease (or other lesion types). Both complementary and converging evidence from different aetiologies can be informative. Here, we compare the neural substrates of object and action naming in PPA and acute stroke.
Naming impairments and their neural bases are commonly investigated in individuals with aphasia, both post-stroke (e.g., Arévalo, Lu, Huang, Bates, & Dronkers, 2011; Faroqi-Shah et al., 2014; Forseth et al., 2018; Fridriksson et al., 2018; Fridriksson, Fillmore, Guo, & Rorden, 2015; Gleichgerrcht et al., 2016; Halai, Woollams, & Lambon Ralph, 2017; Hillis et al., 2018; Hillis, Tuffiash, Wityk, & Barker, 2002; Hope, Leff, & Price, 2018; Lacey, Skipper-Kallal, Xing, Fama, & Turkeltaub, 2017; McKinnon et al., 2018; Mirman et al., 2015; Newhart, Ken, Kleinman, Heidler-Gary, & Hillis, 2007; Parkinson, Raymer, Chang, FitzGerald, & Crosson, 2009; Piras & Marangolo, 2007; Python, Glize, & Laganaro, 2018; Schwartz, Faseyitan, Kim, & Coslett, 2012; Schwartz et al., 2009; Thye & Mirman, 2018; Tochadse, Halai, Lambon Ralph, & Abel, 2018; Tsapkini, Frangakis, & Hillis, 2011; Walker et al., 2011; Weiss et al., 2016) and in those with PPA (e.g., Beber et al., 2019; Benetello et al., 2016; Budd et al., 2010; Cotelli et al., 2016; Gesierich et al., 2012; Gleichgerrcht, Fridriksson, & Bonilha, 2015; Henry & Grasso, 2018; Kemmerer, Rudrauf, Manzel, & Tranel, 2012; Leyton, Hodges, Piguet, & Ballard, 2017; Marcotte et al., 2014; Mesulam et al., 2013; Meyer, Faria, Tippett, Hillis, & Friedman, 2017; Migliaccio et al., 2016; Mion et al., 2010; Race et al., 2013; Riello et al., 2018; Schwartz et al., 2012; Sebastian et al., 2018; Snowden et al., 2018; Thompson, Lukic, King, Mesulam, & Weintraub, 2012). Furthermore, individuals with stroke and primary progressive aphasia have been observed whose naming impairment is more severe for objects than actions, while others display the opposite pattern of performance (e.g., Bak & Hodges, 2003; Beber et al., 2019; Berndt, Mitchum, Haendiges, & Sandson, 1997; Damasio & Tranel, 1993; Hillis et al., 2006, 2004; Hillis, Tuffiash, & Caramazza, 2002; Thompson et al., 2012; Zingeser & Berndt, 1990). This finding suggests that the difference between object and action naming is not just degree of difficulty, with actions being harder to name than objects, as has sometimes been assumed. Rather, at least some neural substrates of object and action naming seem to be in separate locations that can be damaged independently. Converging evidence from individuals with stroke and primary progressive aphasia as well as functional neuroimaging supports this claim. Many studies indicate that left posterior frontal regions support action naming, while left temporal cortex supports object naming (e.g., Benetello et al., 2016; Cotelli et al., 2016; DeLeon et al., 2007; Foundas, Daniels, & Vasterling, 1998; Gesierich et al., 2012; Halai et al., 2017; Hart & Gordon, 1990; Hillis et al., 2018; Hillis, Tuffiash, Wityk, et al., 2002; Hurley, Paller, Rogalski, & Mesulam, 2012; Kemmerer et al., 2012; Leff et al., 2009; Leyton et al., 2017; Martin, Haxby, Lalonde, Wiggs, & Ungerleider, 1995; Mesulam et al., 2013; Meyer et al., 2017; Migliaccio et al., 2016; Newhart et al., 2007; Piras & Marangolo, 2007; Race et al., 2013; Schwartz et al., 2009; Snowden et al., 2018; Tranel, Adolphs, Damasio, & Damasio, 2001; Tranel, Kemmerer, Adolphs, Damasio, & Damasio, 2003; Tranel, Manzel, Asp, & Kemmerer, 2008; Tsapkini et al., 2011; Tyler, Russell, Fadili, & Moss, 2001; Walker et al., 2011; Weiss et al., 2016)
Assessment of action naming is important not only to detect all cases of impaired naming, but also to understand other aspects of production. For instance, grammatical sentence production requires verb retrieval. However, aphasia assessments often focus on naming of objects alone, making them insensitive for detecting certain language deficits and providing an incomplete view of the neural regions involved in naming. Here, we address this gap by presenting a new assessment to evaluate action naming, the Hopkins Action Naming Assessment (HANA). While this assessment has been used in conjunction with a battery of other assessments in some published reports (de Aguiar et al., 2020; Keator et al., 2019, Keator et al., 2020; Long, Sebastian, Faria, & Hillis, 2018; Odolil et al., 2020; Purcell et al., 2017; Race et al., 2013; Riello et al., 2018; Rofes et al., 2019; Sebastian et al., 2016; Sebastian et al., 2018; Tippett et al., 2020; Tsapkini et al., 2018; Unal et al., 2020), the current study presents the first focused description of the assessment and its norming. The HANA consists of 30 black and white images of actions. The items are matched in frequency and length to the 30-item version of the Boston Naming Test (BNT; Mack, Freed, Williams, & Henderson, 1992) (see Table 1). The HANA can be given in conjunction with the BNT, which is already in frequent clinical use, providing diagnostically useful information about the relative strengths of action and object naming while requiring minimal time to be added to the diagnostic session.
Table 1.
Length and frequency of the Boston Naming Test and Hopkins Action Naming Assessment items.
| Frequency |
Length |
|||||
|---|---|---|---|---|---|---|
| item | CELEX English linguistic database | Kučera & Francis | Sydney Morning Herald | Thorndike & Lorge | letters | phonemes |
|
| ||||||
| BNT items | ||||||
| bed | 244.47 | 135 | 59.43 | 1236 | 3 | 3 |
| pencil | 15.75 | 36 | 5.38 | 186 | 6 | 5 |
| whistle | 9.66 | 4 | 8.87 | 211 | 7 | 4 |
| comb | 5.70 | 6 | 1.37 | 96 | 4 | 3 |
| saw | 387.88 | 353 | 159.85 | 2443 | 3 | 2 |
| helicopter | 10.56 | 2 | 15.44 | 0 | 10 | 10 |
| octopus | 1.45 | 1 | 2.43 | 9 | 7 | 7 |
| hanger | 0.78 | 0 | 1.15 | 16 | 6 | 5 |
| camel | 8.16 | 1 | 4.27 | 24 | 5 | 4 |
| pretzel | 0.34 | 0 | 0 | 0 | 7 | 6 |
| racquet | 0.78 | 1 | 3.11 | 0 | 7 | 5 |
| volcano | 3.46 | 2 | 3.33 | 22 | 7 | 7 |
| dart | 4.30 | 0 | 2.43 | 68 | 4 | 3 |
| globe | 10.28 | 15 | 12.16 | 43 | 5 | 4 |
| beaver | 2.07 | 3 | 1.32 | 28 | 6 | 5 |
| rhinoceros | 1.56 | 3 | 0.94 | 4 | 10 | 9 |
| igloo | 0.45 | 0 | 0.47 | 0 | 5 | 4 |
| dominoes | 0.56 | 0 | 0.21 | 0 | 8 | 7 |
| escalator | 1.34 | 0 | 1.49 | 0 | 9 | 9 |
| hammock | 0.84 | 5 | 0.51 | 39 | 7 | 5 |
| pelican | 0.78 | 0 | 3.41 | 5 | 7 | 7 |
| pyramid | 3.97 | 2 | 3.92 | 17 | 7 | 7 |
| unicorn | 0.67 | 0 | 0.77 | 0 | 7 | 7 |
| accordion | 0.84 | 1 | 1.37 | 10 | 9 | 7 |
| asparagus | 2.07 | 1 | 3.58 | 53 | 9 | 9 |
| latch | 2.68 | 5 | 0.38 | 15 | 5 | 3 |
| scroll | 3.13 | 0 | 1.45 | 14 | 6 | 5 |
| sphinx | 0.84 | 1 | 0.64 | 4 | 6 | 6 |
| trellis | 0.95 | 0 | 0.73 | 19 | 7 | 6 |
| protractor | 0 | 0 | 0 | 0 | 10 | 10 |
|
| ||||||
| mean | 24.211 | 19.2 | 10.014 | 152.1 | 6.6 | 5.8 |
| SD | 81.6581 | 67.82 | 30.3284 | 487.95 | 1.92 | 2.19 |
| range | 0–387.88 | 0–353 | 0–159.85 | 0–2443 | 3–10 | 2–10 |
|
| ||||||
| HANA items | ||||||
| run | 229.89 | 246 | 385.83 | 1643 | 3 | 3 |
| vacuum | 15.14 | 22 | 9.09 | 48 | 6 | 6 |
| suck | 10.00 | 5 | 3.03 | 49 | 4 | 3 |
| shave | 5.87 | 6 | 3.11 | 62 | 5 | 3 |
| point | 403.35 | 402 | 400.67 | 1377 | 5 | 4 |
| curtsy | 0.78 | 0 | 0.30 | 0 | 6 | 5 |
| crawl | 7.82 | 11 | 3.28 | 168 | 5 | 4 |
| hibernate | 0.39 | 2 | 0.13 | 5 | 9 | 7 |
| shear | 0.73 | 40 | 0.68 | 38 | 5 | 3 |
| underline | 2.85 | 2 | 2.22 | 4 | 9 | 7 |
| spill | 4.19 | 1 | 11.39 | 74 | 5 | 4 |
| peel | 10.56 | 3 | 6.78 | 91 | 4 | 3 |
| erase | 2.12 | 1 | 1.41 | 24 | 5 | 4 |
| sharpen | 1.96 | 1 | 1.75 | 32 | 7 | 5 |
| subtract | 2.46 | 2 | 0.60 | 6 | 8 | 8 |
| quack | 0.56 | 9 | 0.73 | 10 | 5 | 4 |
| juggle | 0.84 | 0 | 2.60 | 10 | 6 | 4 |
| extinguish | 0.78 | 1 | 1.19 | 16 | 10 | 10 |
| navigate | 0.67 | 1 | 1.54 | 13 | 8 | 7 |
| sled | 0.78 | 0 | 1.07 | 26 | 4 | 4 |
| carve | 2.18 | 3 | 4.05 | 82 | 5 | 3 |
| yawn | 2.79 | 2 | 2.22 | 67 | 4 | 3 |
| knit | 2.51 | 11 | 4.31 | 117 | 4 | 3 |
| dribble | 1.34 | 0 | 0.81 | 8 | 7 | 5 |
| knead | 1.17 | 1 | 0.21 | 10 | 5 | 3 |
| hypnotize | 0 | 0 | 0 | 17 | 9 | 8 |
| shred | 1.96 | 3 | 1.28 | 79 | 5 | 4 |
| prescribe | 3.58 | 5 | 2.13 | 42 | 9 | 8 |
| whisper | 14.92 | 12 | 4.56 | 673 | 7 | 6 |
| play | 276.37 | 205 | 413.04 | 2606 | 4 | 3 |
|
| ||||||
| mean | 33.619 | 33.2 | 42.334 | 246.6 | 5.9 | 4.8 |
| SD | 94.4779 | 89.77 | 121.2887 | 590.27 | 1.91 | 1.97 |
| range | 0–403.35 | 0–402 | 0–413.04 | 0–2606 | 3–10 | 3–10 |
|
| ||||||
| Welch two sample t-test results | ||||||
| t | −0.41 | −0.68 | −1.42 | −0.68 | 1.42 | 1.86 |
| df | 56.81 | 53.97 | 32.61 | 56.02 | 58.00 | 57.39 |
| p-value | .681 | .498 | .166 | .502 | .162 | .068 |
Notes: BNT= Boston Naming Test; HANA= Hopkins Action Naming Assessment; SD = standard deviation; df= degrees of freedom. Refer to the text for the normative sources of the frequency measures.
In the current study, we administered both the BNT and the HANA to individuals with acute left hemisphere stroke (N=37) and PPA (N=138). Our first aim was to show that the HANA is clinically useful for identifying action naming impairments, which are separate from the object naming impairments that can be diagnosed via the BNT. We hypothesised that a double dissociation between object and action naming would be observed, with some individuals showing deficits for objects but not actions and vice versa. Our other aim was to show that these assessments can be a useful tool for comparing the neural substrates associated with performance on object and action naming. To investigate this aim, we used neuroimaging data from a subset of the same participants (N=31 with PPA, N=37 with acute stroke). We hypothesised that different neural substrates would be implicated in object vs. action naming and that different lesion-deficit associations would be identified in participants with PPA vs. acute stroke. The last hypothesis is based on the premise that different regions of the brain are affected in PPA vs. stroke, allowing sufficient power to detect associations in different regions. For example, if few people with stroke have damage to the temporal pole, damage to that area is unlikely to be statistically associated with naming or other deficits, even if the left temporal pole is a critical node of the naming network. To assess as many areas as possible that might be important for naming, we included all variants of PPA, as different brain regions tend to be affected across variants (e.g., Gorno-Tempini et al., 2004, 2011), as well as across aetiologies (PPA vs stroke). The overall goal of the work presented here is to demonstrate the utility of the HANA and provide a more comprehensive picture of brain-behavior relationships critical for naming.
Materials and Methods
Participants
PPA
Participants (N=138, 73 females, mean age 68.7 ± 8.24 years, mean symptom duration 42.0 ± 26.64 months) who had been diagnosed with primary progressive aphasia were recruited from the senior author’s outpatient neurology clinic in accordance with the policies of the Johns Hopkins Medical Institutions IRB. Diagnosis of PPA was based on presentation with progressive language impairment that remains the most prominent symptom in the absence of other cognitive, behavior, or personality changes (Gorno-Tempini et al., 2011; Mesulam, 1982). Information about the participants with PPA, including demographic information and scores on the tasks of interest, is presented in Table 2.
Table 2.
Demographic and naming assessment data for participants with PPA
| participant | sex | age | education (years) | symptom duration (months) | scan-assessment interval (months) | BNT | HANA | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Participants with lvPPA | ||||||||
| L01 | female | 79 | 16 | 30 | −5 | 2 | 0 | |
| L02 | female | 73 | 18 | 108 | 0 | 3 | 9 | |
| L03 | male | 72 | 18 | 42 | 5 | 9 | 3 | |
| L04 | female | 76 | 18 | 12 | 1 | 10 | 13 | |
| L05 | male | 68 | 13 | 69 | −2 | 16 | 11 | |
| L06 | female | 68 | 18 | 36 | 4 | 16 | 15 | |
| L07 | male | 76 | 12 | 31 | 0 | 17 | 8 | * |
| L08 | female | 66 | 18 | 66 | 1 | 21 | 20 | |
| L09 | male | 51 | 12 | 39 | 3 | 23 | 25 | |
| L10 | male | 55 | 18 | 12 | 1 | 25 | 20 | |
| L11 | male | 72 | 20 | 24 | 0 | 25 | 22 | |
| L12 | female | 73 | 18 | 91 | 1 | 1 | ||
| L13 | female | 72 | 16 | 48 | 1 | 2 | ||
| L14 | female | 65 | 12 | 78 | 1 | 2 | ||
| L15 | female | 73 | 12 | 68 | 1 | 4 | ||
| L16 | female | 79 | 18 | 24 | 2 | 1 | ||
| L17 | male | 85 | 18 | 78 | 3 | 3 | ||
| L18 | male | 78 | 16 | 96 | 4 | 2 | ||
| L19 | female | 72 | 14 | 60 | 5 | 9 | ||
| L20 | female | 68 | 18 | 42 | 7 | 4 | ||
| L21 | female | 72 | 18 | 36 | 7 | 5 | ||
| L22 | female | 77 | 12 | 24 | 9 | 8 | ||
| L23 | male | 75 | 18 | 10 | 9 | 11 | ||
| L24 | female | 69 | 18 | 48 | 12 | 6 | ||
| L25 | male | 71 | 20 | 42 | 12 | 10 | ||
| L26 | female | 65 | 14 | 84 | 12 | 19 | ||
| L27 | male | 69 | 19 | 60 | 13 | 9 | ||
| L28 | female | 70 | 16 | 24 | 13 | 10 | ||
| L29 | male | 75 | 16 | 30 | 13 | 10 | ||
| L30 | female | 78 | 18 | 36 | 13 | 17 | ||
| L31 | female | 54 | 15 | 36 | 14 | 11 | ||
| L32 | female | 71 | 16 | 12 | 14 | 14 | ||
| L33 | female | 68 | 12 | 24 | 14 | 18 | ||
| L34 | female | 74 | 18 | 57 | 15 | 9 | ||
| L35 | female | 69 | 12 | 33 | 16 | 23 | ||
| L36 | male | 70 | 18 | 57 | 17 | 13 | ||
| L37 | male | 52 | 12 | 12 | 17 | 13 | ||
| L38 | male | 64 | 18 | 24 | 18 | 19 | ||
| L39 | male | 73 | 20 | 12 | 18 | 23 | ||
| L40 | female | 68 | 18 | 36 | 18 | 24 | ||
| L41 | female | 70 | 12 | 15 | 19 | 16 | ||
| L42 | female | 90 | 16 | 24 | 19 | 19 | ||
| L43 | male | 75 | 18 | 20 | 19 | |||
| L44 | male | 80 | 12 | 6 | 21 | 22 | ||
| L45 | female | 70 | 14 | 48 | 22 | 19 | ||
| L46 | female | 69 | 16 | 36 | 24 | 18 | ||
| L47 | female | 65 | 18 | 35 | 24 | 22 | ||
| L48 | male | 60 | 13 | 35 | 25 | 12 | * | |
| L49 | male | 73 | 20 | 77 | 25 | 13 | * | |
| L50 | female | 56 | 16 | 72 | 26 | 18 | * | |
|
| ||||||||
| mean | 70.3 | 16.1 | 42.3 | 13.8 | 12.5 | |||
| SD | 7.70 | 2.66 | 24.97 | 7.61 | 7.18 | |||
| range | 51–90 | 12–20 | 6–108 | 1–26 | 0–25 | |||
|
| ||||||||
| Participants with nfvPPA | ||||||||
| N01 | male | 77 | 20 | 18 | 4 | 2 | 3 | |
| N02 | female | 79 | 14 | 35 | 0 | 7 | 1 | |
| N03 | male | 72 | 14 | 52 | −4 | 8 | 3 | |
| N04 | male | 77 | 8 | 6 | 4 | 13 | 17 | |
| N05 | female | 65 | 16 | 48 | 4 | 15 | 13 | |
| N06 | female | 52 | 45 | 0 | 16 | 8 | ||
| N07 | male | 85 | 12 | 24 | 3 | 17 | 13 | |
| N08 | male | 77 | 18 | 58 | 1 | 27 | 25 | |
| N09 | male | 65 | 18 | 24 | 2 | 29 | 29 | |
| N10 | male | 62 | 20 | 12 | 2 | 30 | 23 | * |
| N11 | male | 73 | 16 | 22 | 0 | 30 | 29 | |
| N12 | male | 58 | 18 | 56 | −2 | 30 | 30 | |
| N13 | female | 85 | 12 | 22 | 5 | 6 | ||
| N14 | female | 78 | 16 | 60 | 5 | 12 | ||
| N15 | female | 65 | 16 | 91 | 6 | 3 | ||
| N16 | male | 69 | 18 | 96 | 12 | 9 | ||
| N17 | female | 65 | 12 | 30 | 12 | 13 | ||
| N18 | female | 62 | 16 | 6 | 12 | 15 | ||
| N19 | female | 78 | 3 | 24 | 17 | 14 | ||
| N20 | male | 48 | 12 | 81 | 18 | 13 | ||
| N21 | male | 52 | 18 | 60 | 18 | 14 | ||
| N22 | male | 66 | 20 | 18 | 20 | 22 | ||
| N23 | male | 64 | 18 | 18 | 21 | 16 | ||
| N24 | female | 67 | 16 | 31 | 22 | 20 | ||
| N25 | male | 76 | 18 | 42 | 22 | 21 | ||
| N26 | female | 74 | 12 | 30 | 23 | 16 | ||
| N27 | male | 81 | 16 | 36 | 23 | 20 | ||
| N28 | female | 71 | 18 | 59 | 23 | 22 | ||
| N29 | male | 73 | 18 | 48 | 25 | 20 | ||
| N30 | male | 61 | 16 | 54 | 25 | 21 | ||
| N31 | male | 74 | 12 | 24 | 25 | 21 | ||
| N32 | male | 65 | 18 | 36 | 25 | 21 | ||
| N33 | female | 82 | 12 | 54 | 27 | 21 | ||
| N34 | female | 77 | 18 | 97 | 28 | 22 | ||
| N35 | female | 67 | 16 | 84 | 29 | 26 | ||
| N36 | male | 63 | 16 | 29 | 27 | |||
| N37 | male | 67 | 19 | 46 | 29 | 28 | ||
| N38 | male | 70 | 19 | 10 | 29 | 28 | ||
| N39 | male | 52 | 18 | 12 | 30 | 21 | * | |
| N40 | male | 69 | 15 | 28 | 30 | 28 | ||
| N41 | male | 59 | 14 | 12 | 28 | |||
|
| ||||||||
| mean | 68.8 | 15.7 | 40.2 | 20.4 | 18.1 | |||
| SD | 9.14 | 3.46 | 24.93 | 8.46 | 8.05 | |||
| range | 48–85 | 3–20 | 6–97 | 2–30 | 1–30 | |||
|
| ||||||||
| Participants with svPPA | ||||||||
| S01 | female | 60 | 18 | 29 | 1 | 4 | 0 | |
| S02 | female | 68 | 16 | 68 | 0 | 6 | 0 | * |
| S03 | male | 71 | 20 | 42 | 2 | 9 | 1 | * |
| S04 | female | 77 | 12 | 36 | 0 | 9 | 9 | |
| S05 | male | 59 | 13 | 45 | 2 | 16 | 8 | |
| S06 | female | 62 | 18 | 48 | 4 | 27 | 29 | |
| S07 | female | 59 | 18 | 143 | 0 | 1 | ||
| S08 | female | 59 | 12 | 60 | 0 | 0 | ||
| S09 | female | 74 | 16 | 129 | 0 | 1 | ||
| S10 | male | 58 | 16 | 97 | 0 | 2 | ||
| S11 | male | 64 | 16 | 45 | 1 | 5 | ||
| S12 | male | 64 | 16 | 120 | 2 | 2 | ||
| S13 | female | 67 | 16 | 36 | 2 | 3 | ||
| S14 | male | 65 | 13 | 24 | 4 | 2 | ||
| S15 | female | 72 | 16 | 72 | 4 | 4 | ||
| S16 | female | 52 | 12 | 5 | 4 | |||
| S17 | female | 76 | 18 | 12 | 5 | 8 | ||
| S18 | female | 68 | 16 | 68 | 6 | 3 | ||
| S19 | female | 66 | 16 | 48 | 6 | 8 | ||
| S20 | male | 74 | 18 | 30 | 6 | 10 | ||
| S21 | male | 61 | 16 | 40 | 6 | 16 | * | |
| S22 | female | 61 | 18 | 31 | 8 | 1 | * | |
| S23 | male | 79 | 18 | 48 | 8 | 6 | ||
| S24 | female | 68 | 16 | 45 | 8 | 21 | * | |
| S25 | female | 74 | 16 | 24 | 9 | 9 | ||
| S26 | male | 55 | 18 | 36 | 10 | 11 | ||
| S27 | female | 61 | 18 | 60 | 15 | 10 | ||
| S28 | male | 79 | 12 | 24 | 18 | 9 | * | |
| S29 | female | 61 | 14 | 36 | 18 | 17 | ||
| S30 | female | 73 | 20 | 12 | 19 | 17 | ||
| S31 | male | 61 | 18 | 18 | 20 | 9 | * | |
| S32 | female | 62 | 12 | 48 | 21 | 10 | * | |
| S33 | male | 50 | 12 | 22 | 22 | 16 | ||
|
| ||||||||
| mean | 65.5 | 16.0 | 48.7 | 9.2 | 7.6 | |||
| SD | 7.60 | 2.37 | 32.45 | 7.33 | 6.87 | |||
| range | 50–79 | 12–20 | 12–143 | 0–27 | 0–29 | |||
|
| ||||||||
| Participants with unclassifiable PPA | ||||||||
| U01 | female | 62 | 16 | 41 | 0 | 27 | 30 | |
| U02 | female | 63 | 16 | 24 | 6 | 12 | ||
| U03 | female | 69 | 18 | 30 | 7 | 11 | ||
| U04 | male | 68 | 19 | 20 | 10 | 5 | ||
| U05 | female | 55 | 16 | 48 | 18 | 14 | ||
| U06 | male | 74 | 16 | 12 | 18 | 19 | ||
| U07 | female | 68 | 12 | 72 | 19 | 21 | ||
| U08 | male | 74 | 20 | 22 | 20 | 24 | ||
| U09 | male | 80 | 16 | 12 | 21 | 26 | ||
| U10 | female | 74 | 48 | 24 | 20 | |||
| U11 | male | 68 | 18 | 36 | 24 | 24 | ||
| U12 | male | 72 | 18 | 6 | 26 | 19 | ||
| U13 | female | 78 | 12 | 12 | 27 | 26 | ||
| U14 | female | 81 | 12 | 36 | 19 | |||
|
| ||||||||
| mean | 70.4 | 16.1 | 29.9 | 19.0 | 19.3 | |||
| SD | 7.26 | 2.66 | 18.30 | 7.23 | 6.83 | |||
| range | 55–81 | 12–20 | 6–72 | 6–27 | 5–30 | |||
|
| ||||||||
| All participants with PPA | ||||||||
| mean | 68.7 | 15.9 | 42.0 | 15.2 | 13.7 | |||
| SD | 8.24 | 2.84 | 26.64 | 8.82 | 8.44 | |||
| range | 48–90 | 3–20 | 6–143 | 0–30 | 0–30 | |||
Notes: Bolded lines represent participants who contributed data to the neuroimaging analysis. SD = standard deviation; scan-assessment interval (months)= number of months between scan and assessment, with negative values indicating scan before assessment and positive values indicating assessment before scan; BNT= number of correct responses on the Boston Naming Test, maximum score of 30; HANA= number of correct responses on the Hopkins Action Naming Assessment, maximum score of 30.
indicates a significant difference between performance on the BNT and HANA, Fisher’s Exact Test p<.05.
Summary values are reported for participants with each variant of PPA as well as the whole group that contributed behavioral data; summary values are not reported for the scan-test interval as they apply only to the subset of participants with neuroimaging data.
If possible, participants were classified as having logopenic (N=50), nonfluent (N=41), or semantic (N=33) variants using recent guidelines (Gorno-Tempini et al., 2011) on the basis of detailed language and cognitive assessments, history, comprehensive neurological examination, and available neuroimaging. However, fitting one of the classification types was not required for inclusion: 14 participants were unclassifiable (either because they did not have all of the core distinguishing features of any variant, as in cases of pure anomia, or because they had core features of more than one variant). As a group, the unclassifiable participants were significantly earlier in the course of PPA (mean symptom duration = 29.9 ± 18.30 months) than those with lvPPA (mean symptom duration = 42.3 ± 24.97 months; t(28)=−2.06, p<.05) or svPPA (mean symptom duration = 48.7 ± 32.45 months; t(41)=−2.52, p<.05); they may have progressed after these assessments to fit one of the classification types. Across classification types, participants were comparable on education and age, with the exception that participants with svPPA were significantly younger (mean age 65.5 ± 7.60 years) than participants with lvPPA (mean age 70.3 ± 7.70 years; t(69)=−2.80, p<.05) or unclassifiable PPA (mean age 70.4 ± 7.26 years; t(26)=−2.12, p<.05).
All 138 PPA participants completed the HANA; 135 also completed the BNT. A subset of 31 PPA participants (7 svPPA, 12 nfvPPA, 11 lvPPA, 1 unclassified; mean age 68.3 ± 8.68 years; 14 female) who had high resolution T1 weighted image (T1-WI) anatomical MRI scans within five months of the naming assessment were included in the neuroimaging analysis. The other PPA participants were excluded from this analysis because they did not have the required imaging.
Stroke
Participants (N=37, 14 females, mean age 56.5 ± 13.41 years) with acute left hemisphere ischemic stroke were enrolled at Johns Hopkins Hospital or Johns Hopkins Bayview Medical Center in accordance with the policies of the Johns Hopkins Medical Institutions IRB. All had MRI-confirmed stokes, with diffusion-weighted imaging (DWI) revealing the presence of unilateral left hemisphere infarct without hemorrhage on the initial scan. Participants were premorbidly fluent English speakers with normal or corrected-to-normal vision and hearing. They did not have history of dementia, previous symptomatic stroke, or other neurological disease affecting the brain, nor did they have reduced level of consciousness or ongoing sedation. All 37 acute stroke participants completed the HANA within five days of hospital admission; 34 also completed the BNT. All stroke participants were included in the neuroimaging analysis. Information about the participants with acute stroke, including demographic information and scores on the tasks of interest, is presented in Table 3.
Table 3.
Demographic and naming assessment data for participants with acute stroke
| participant | sex | age | education (years) | scan-stroke interval (days) | scan-admission interval (days) | scan-assessment interval (days) | BNT | HANA | |
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| A01 | male | 56 | 14 | 1 | 0 | −1 | 1 | 0 | |
| A02 | male | 43 | 12 | 2 | 1 | −3 | 1 | 2 | |
| A03 | male | 61 | 16 | 8 | 0 | −5 | 3 | 1 | |
| A04 | female | 54 | 18 | 0 | 0 | −2 | 3 | 3 | |
| A05 | female | 28 | 12 | 7 | 5 | 1 | 5 | 2 | |
| A06 | male | 53 | 12 | 1 | 0 | −4 | 8 | 9 | |
| A07 | male | 65 | 12 | 1 | 0 | −1 | 12 | 3 | * |
| A08 | female | 61 | 9 | 3 | 3 | 1 | 15 | 20 | |
| A09 | male | 37 | 16 | 0 | 0 | −4 | 16 | 16 | |
| A10 | female | 39 | 12 | 12 | 2 | 1 | 16 | 27 | * |
| A11 | male | 87 | 20 | 0 | 0 | −3 | 20 | 19 | |
| A12 | female | 65 | 14 | 8 | 1 | −1 | 21 | 26 | |
| A13 | male | 48 | 11 | 1 | 0 | −2 | 22 | 23 | |
| A14 | female | 49 | 12 | 2 | 0 | −4 | 23 | 18 | |
| A15 | male | 52 | 12 | 0 | 0 | −1 | 23 | 21 | |
| A16 | female | 53 | 14 | 7 | 6 | 4 | 23 | 22 | |
| A17 | female | 60 | 13 | 0 | 0 | −2 | 24 | 24 | |
| A18 | male | 65 | 18 | 2 | 2 | 0 | 24 | 26 | |
| A19 | male | 49 | 15 | 4 | 0 | −1 | 25 | 27 | |
| A20 | male | 46 | 12 | 4 | 3 | 0 | 26 | 23 | |
| A21 | female | 77 | 0 | 0 | 0 | 27 | 22 | ||
| A22 | female | 32 | 14 | 5 | 4 | 1 | 27 | 27 | |
| A23 | female | 56 | 12 | 0 | 0 | −2 | 27 | 28 | |
| A24 | male | 64 | 16 | 0 | 0 | −4 | 27 | 30 | |
| A25 | male | 43 | 13 | 6 | 0 | −3 | 28 | 22 | |
| A26 | male | 61 | 16 | 0 | 0 | −2 | 28 | 27 | |
| A27 | male | 60 | 14 | 1 | 1 | −2 | 28 | 29 | |
| A28 | male | 60 | 13 | 1 | 0 | 0 | 29 | 28 | |
| A29 | male | 57 | 14 | 1 | 0 | −1 | 29 | 28 | |
| A30 | male | 35 | 14 | 1 | 0 | −2 | 29 | 29 | |
| A31 | male | 70 | 13 | 0 | 0 | −4 | 29 | 29 | |
| A32 | male | 55 | 14 | 0 | 0 | −2 | 30 | 29 | |
| A33 | female | 60 | 14 | 0 | 0 | −2 | 30 | 30 | |
| A34 | male | 60 | 14 | 2 | 2 | 1 | 30 | 30 | |
| A35 | female | 71 | 2 | 1 | −2 | 0 | |||
| A36 | female | 81 | 14 | 0 | 0 | −5 | 3 | ||
| A37 | male | 78 | 30 | 0 | 0 | −1 | 29 | ||
|
| |||||||||
| mean | 56.5 | 14.3 | 2.2 | 0.8 | −1.5 | 20.9 | 19.8 | ||
| SD | 13.41 | 3.48 | 2.97 | 1.54 | 1.95 | 9.38 | 10.58 | ||
| range | 28–87 | 9–30 | 0–12 | 0–6 | −5–4 | 1–30 | 0–30 | ||
Notes: SD = standard deviation; scan-stroke interval (days) = number of days after onset of stroke symptoms that scan was obtained; scan-admission interval (days) = number of days after admission to hospital that scan was obtained. (This differs for some participants as compared to scan-stroke interval as participants sought treatment at different points relative to the onset of symptoms); scan-assessment interval (days)= number of days between scan and assessment, with negative values indicating scan before assessment and positive values indicating assessment before scan; BNT= number of correct responses on the Boston Naming Test, maximum score of 30; HANA= number of correct responses on the Hopkins Action Naming Assessment, maximum score of 30.
indicates a significant difference between performance on the BNT and HANA, Fisher’s Exact Test p<.05.
Controls
An additional twenty-six neurologically intact participants (20 female, mean age 64.0 ± 12.05 years) were recruited to serve as controls for behavioral testing.
Behavioural assessments
Object naming: Boston Naming Test (short form) [BNT]
Every other item from the original 60-item Boston Naming Test (Kaplan et al., 1983) was administered, following Mack et al. (1992). Participants named black and white line drawings of 30 objects. Uncued first responses were scored. The twenty-six controls tested here provided correct first responses to 22–29 items (mean=26.3 ± 2.48).
Action naming: Hopkins Action Naming Assessment [HANA]
Participants named black and white line drawings or photographs of 30 actions. These items were matched in frequency and length to the names of the objects in the BNT (Table 1) using N-Watch (Davis, 2005) and the MRC Psycholinguistic Database (Coltheart, 1981). Frequency measures were drawn from those reported by Kučera and Francis (1967), the CELEX English linguistic database (Baayen et al., 1995), the Syndey Morning Herald corpus (Dennis, 1995), and Thorndike and Lorge (1942). Welch two sample two-tailed t-tests showed that the two assessments did not differ on any of the length or frequency measures (all p-values > .05). Pilot testing of the assessment showed that each picture had at least 75% name agreement among a group of twenty young controls. The older controls tested in the present study provided correct first responses to 17–30 items (mean=23.9 ± 3.46). Actions generally have poorer name agreement compared to objects, as several action names can correctly describe most actions (e.g. yell, shout, scream). For both objects and actions, if the participant gave a name that was correct (synonymic) but not the target name, they were asked to give another name; but only the target, if named without a cue, was considered correct.
Behavioural Analysis
We dichotomised naming performance as normal or impaired relative to controls. The mean and standard deviation of controls was used to calculate standard scores. A standard score of z<−1.645 was considered significantly worse performance than controls (p<.05, one tailed) and classified as impaired. The pattern of intact vs. impaired performance on the object and action naming tasks was compared for all individual participants.
For each individual, we also compared accuracy of object vs. action naming using two-tailed Fisher’s Exact Tests.
Neuroimaging Analysis
PPA
Anatomical information was acquired using a MPRAGE T1 sequence on a 3T scanner. Scans occurred within five months of the cognitive testing reported here (mean scan-assessment interval: scans 1.0±2.32 months subsequent to assessment). Atlas-based analysis was used to segment each T1 MRI into 289 regions of interest (ROIs) using the MRI Cloud platform (www.mricloud.org). This type of analysis allows the anatomical parcellation defined in an atlas template image to be applied to the brain of each individual participant so that multiple regions of interest (ROIs) can be automatically segmented. Such an analysis ameliorates drawbacks of voxel-based analysis, which suffers from the noise resulting from the assumption that normalization is accurate enough to identify the same voxel in a standardised brain and the individual participants’, and of manual delineation of ROIs, which suffers from limited reproducibility and cannot be practically applied to whole brain analyses because of the amount of time necessary to apply such analyses (Faria et al., 2010). For each participant, the volume of brain tissue in each region was calculated in native space. Based on previous lesion and neuroimaging studies of naming, we selected 20 of the 289 regions resulting from the automatic parcellation as regions of interest (e.g., Benetello et al., 2016; Cotelli et al., 2016; DeLeon et al., 2007; Foundas, Daniels, & Vasterling, 1998; Gesierich et al., 2012; Gorno-Tempini et al., 2011; Halai et al., 2017; Hart & Gordon, 1990; Hillis et al., 2018; Hillis, Tuffiash, Wityk, et al., 2002; Hurley, Paller, Rogalski, & Mesulam, 2012; Kemmerer et al., 2012; Leff et al., 2009; Leyton et al., 2017; Martin, Haxby, Lalonde, Wiggs, & Ungerleider, 1995; Mesulam et al., 2013; Meyer et al., 2017; Migliaccio et al., 2016; Newhart et al., 2007; Piras & Marangolo, 2007; Race et al., 2013; Schwartz et al., 2009; Snowden et al., 2018; Tranel, Adolphs, Damasio, & Damasio, 2001; Tranel, Kemmerer, Adolphs, Damasio, & Damasio, 2003; Tranel, Manzel, Asp, & Kemmerer, 2008; Tsapkini et al., 2011; Tyler, Russell, Fadili, & Moss, 2001; Walker et al., 2011; Weiss et al., 2016). These regions consisted of the pars opercularis, pars orbitalis, and pars triangularis of the left inferior frontal gyrus; left precentral gyrus; left postcentral gyrus; left supramarginal gyrus; left angular gyrus; left superior temporal gyrus; right superior temporal gyrus; left superior temporal pole; right superior temporal pole; left middle temporal gyrus; right middle temporal gyrus; left middle temporal pole; right middle temporal pole; left inferior temporal gyrus; right inferior temporal gyrus; left fusiform gyrus; right fusiform gyrus; and left insula. The temporal lobe was subdivided such that the temporal pole consists of the regions of the superior and middle temporal gyri (labeled superior and middle temporal poles, respectively) lying anterior to a vertical plane through the anterior commissure, based on the anterior ending of the superior temporal sulcus. The superior and middle temporal poles were separated in the atlas template because they have different connectivity with other regions (Insausti et al., 1998; Kondo et al., 2003, 2005; Stefanacci et al., 1996). The volume for each ROI was normalised by cerebral volume, defined as total brain volume removing myelencephalon and cerebrospinal fluid (CSF), to control for relative regional atrophy. The ratio of cerebral to intracranial volume, defined as total brain volume removing myelencephalon but including CSF, was also calculated for use as a predictor in models to control for inter-individual brain size differences.
Acute stroke
Participants underwent MR imaging that included MPRAGE T1 anatomical scans as well as diffusion weighted imaging (DWI). Technicians blinded to the behavioral results used MRIcron (https://www.nitrc.org/projects/mricron) to trace areas of tissue disfunction, defined as areas of dense ischemia/infarct that were bright on DWI maps but dark on apparent diffusion coefficient (ADC) maps. Traced lesions were coregistered to T1 scans using SPM12 (https://www.fil.ion.ucl.ac.uk/spm/software/spm12). Lesioned tissue was replaced with tissue from the undamaged right hemisphere. The resulting artificial T1 image was then segmented using the automated MRICloud pipeline (https://braingps.mricloud.org/) that relies on a highly accurate large deformation diffeomorphic mapping algorithm to transform images between native space and atlas space and a multi-atlas fusion label algorithm that allows the use of multiple atlases to reduce segmentation and mapping inaccuracies. Via this pipeline, each individual’s scan was segmented into 289 regions of interest. The original lesion map was overlaid onto the resulting segmentation to calculate the percentage of tissue damaged in each of the 289 regions. As in the PPA analysis, we selected a smaller subset of regions for further analysis. From the list of ROIs used in the PPA analysis, seven were removed due to lack of variability: no participants had lesions in the six right hemisphere regions since unilateral left hemisphere stroke was an inclusion criterion, and none happened to have lesions in the left middle temporal pole. A minimum of five participants had lesions in the thirteen remaining ROIs used in the analyses of acute stroke data, consisting of the left inferior frontal gyrus pars opercularis, pars orbitalis, and pars triangularis; left precentral gyrus; left postcentral gyrus; left supramarginal gyrus; left angular gyrus; left superior temporal gyrus; left superior temporal pole; left middle temporal gyrus; left inferior temporal gyrus; left fusiform gyrus; and left insula. Total lesion volume was also calculated for use as a predictor in models to control for severity of stroke.
Relating neuroimaging and behavioural data
Least Absolute Shrinkage and Selection Operator (LASSO) regression (Tibshirani, 1996) was used to evaluate which ROIs were related to behavior on object and action naming. This method, which results in selection of a subset of covariates, was used instead of simpler multiple regression analyses as the neuroimaging data had high multicollinearity. That is, participants with damage in one region were highly likely to have damage in neighboring regions. LASSO regression is appropriate in this situation as it reduces over-fitting and provides for automated feature selection: when multiple highly correlated variables are entered into the model, only one is retained while the others are set to zero. This is helpful here where we have a large number of predictors relative to the sample size and where some of those predictors are closely related (Meinshausen & Yu, 2009). LASSO regression with standardised features was instantiated with the glmnet package in R (https://cran.r-project.org/web/packages/glmnet/index.html), using leave-one-out cross-validation to select the λ value that resulted in the minimum mean cross validated error. Inference testing and calculation of p-values for the features selected by the LASSO was conducted with the selectiveInference package in R (https://cran.r-project.org/web/packages/selectiveInference/selectiveInference.pdf). Separate analyses were conducted for each of the four combinations of object vs. action naming and PPA vs. acute stroke. Object naming models used number correct on the BNT as the dependent variable; action naming models used number correct on the HANA as the dependent variable. PPA models included cerebral to intracranial volume ratio as well as regional volume normalised by cerebral volume for each of the 20 ROIs as predictors. These models used one-tailed LASSO regression, considering only positive predictors where smaller regional volume (i.e. less healthy tissue) was associated with worse naming performance. Acute stroke models included total lesion volume as well as percent damage in each of the 13 ROIs as predictors. These models used one-tailed LASSO regression, considering only negative predictors where larger lesion volume (i.e. less healthy tissue) was associated with worse naming performance.
Results
Behavioural Results
We hypothesised that there would be a double dissociation in performance for object vs. action naming. To investigate this, we first established that the assessments identified participants with naming impairments. We separately considered participants whose aphasia was due to PPA vs. stroke. Performance is shown in Figures 1 and 2.
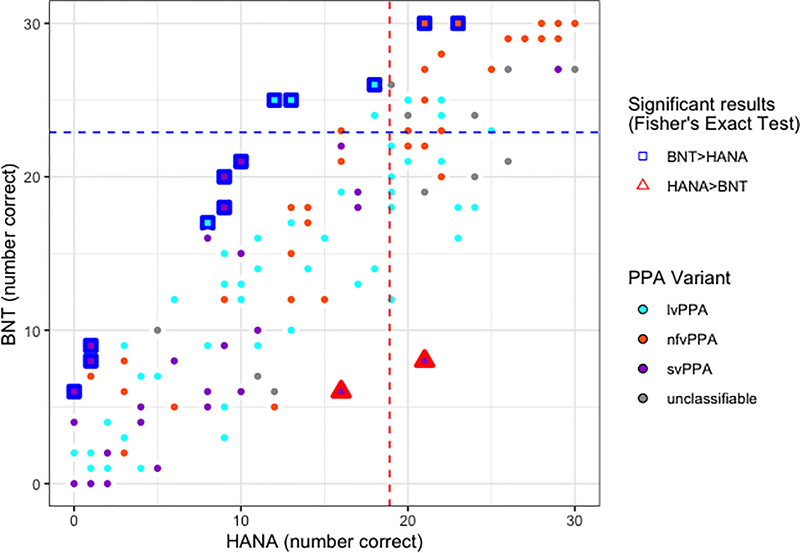
Figure 1.
Behavioural results for participants with PPA (N=135) who completed both the HANA and BNT assessments. Performance on the BNT is shown on the y-axis (maximum score=30) and performance on the HANA is shown on the x-axis (maximum score=30). Dot fill colours represent different PPA variants (cyan=lvPPA; orange=nfvPPA; purple=svPPA; gray=unclassifiable). Dashed lines represent cut-offs for normal performance at z>−1.645 relative to controls, blue for the BNT and red for the HANA. Participants who showed intact performance on the BNT but impaired performance on the HANA relative to controls appear in the upper left; those who showed the opposite pattern are shown in the lower right. Participants who performed significantly better on the BNT than the HANA according to Fisher’s Exact Test are highlighted with blue squares. Participants who performed significantly better on the HANA than the BNT according to Fisher’s Exact Test are highlighted with red triangles.
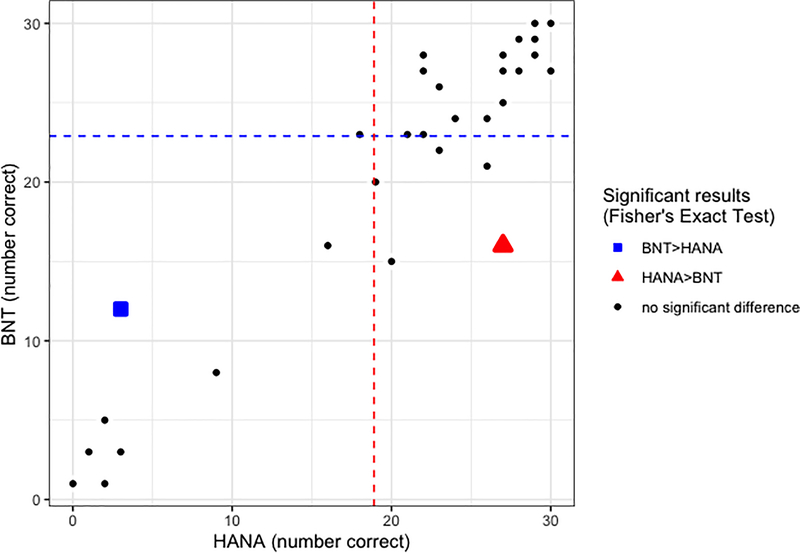
Figure 2.
Behavioural results for participants with stroke (N=34) who completed both the HANA and BNT assessments. Performance on the BNT is shown on the y-axis (maximum score=30) and performance on the HANA is shown on the x-axis (maximum score=30). Dashed lines represent cut-offs for normal performance at z>−1.645 relative to controls, blue for the BNT and red for the HANA. Participants who showed intact performance on the BNT but impaired performance on the HANA relative to controls appear in the upper left; those who showed the opposite pattern are shown in the lower right. The participant who performed significantly better on the BNT than the HANA according to Fisher’s Exact Test is highlighted with a blue square. The participant who performed significantly better on the HANA than the BNT according to Fisher’s Exact Test is highlighted with a red triangle.
On the BNT, the cut-off for normal performance was 23 based on the definition of normal performance as a standard score of z>−1.645. Correct naming of 22 or fewer items was considered impaired. Of the 135 participants with PPA who completed the BNT, 101 (75%) showed impaired performance on object naming. Likewise, 13 of the 34 (38%) with acute stroke were impaired on the BNT. On the HANA, the cut-off value derived from control performance was 19. Impaired performance on the HANA (i.e., correctly naming 18 or fewer items) was demonstrated by 89 of the 138 (64%) participants with PPA and 11 of the 37 (30%) with acute stroke who completed this assessment.
For a double dissociation to exist between two cognitive processes, there must be some individuals who demonstrate impaired performance on tasks tapping one function in the face of intact performance on tasks tapping the other function as well as other individuals who show the opposite pattern. Here, we would expect to find some individuals who show impaired performance on the BNT but not the HANA and vice versa to observe a double dissociation between object and action naming. We investigated this in two ways, the combination of which is stronger than either alone.
First, we looked at individuals’ patterns of performance on the two tasks defining impaired vs. intact performance with the cut-offs described above. We identified eighteen participants with PPA (ten lvPPA, two nfvPPA, one svPPA, and four unclassifiable) and five with acute stroke who showed impaired performance on the BNT but intact performance on the HANA (mean difference 2.9±4.12 more items correct on the HANA than the BNT; range 13 more items correct on the HANA than the BNT to 3 more items correct on the BNT than the HANA). We also identified the opposite dissociation of impaired action but not object naming, finding five participants with PPA (four lvPPA, one nfvPPA) and one with acute stroke who showed impaired performance on the HANA but not the BNT (mean difference 8.5±3.27 more items correct on the BNT than the HANA; range 5–13 more items correct on the BNT than the HANA). Together, these patterns of performance constitute a double dissociation.
Second, we directly compared performance on the two tasks, regardless of status relative to cut-offs, using Fisher’s Exact Tests to identify individuals who performed significantly better on one task than the other (p<.05). Such individuals would further support the finding of a double dissociation, ruling out the possibility that the findings reported above are an artifact of the specific cut-offs used to define intact vs. impaired performance. As expected, we observed both participants who performed significantly better on the HANA than on the BNT (two PPA [two svPPA], one acute stroke; mean difference 11.3±1.53 more items correct on the HANA than the BNT; range 10–13 more items correct on the HANA than the BNT) and participants who performed significantly better on the BNT than on the HANA (twelve PPA [four lvPPA, two nfvPPA, six svPPA], one acute stroke; mean difference 9.2±2.08 more items correct on the BNT than the HANA; range 6–13 more items correct on the BNT than the HANA).
To provide even stronger evidence of a double dissociation, we note that some individuals fit both criteria that we considered: they demonstrated intact performance on one task but impaired performance on the other task relative to controls AND their performance was significantly better on one task than the other. Under these combined criteria, we identified two individuals with deficits on object naming but not action naming (one PPA, one acute stroke; 8 items correct on the BNT vs. 21 correct on the HANA and 16 items correct on the BNT vs. 27 correct on the HANA) and three individuals with deficits on action naming but not object naming (three PPA; 26 items correct on the BNT vs. 18 correct on the HANA, 25 items correct on the BNT vs. 13 correct on the HANA, and 25 items correct on the BNT vs. 12 correct on the HANA). These behavioral results support the hypothesis that there is a double dissociation between object and action naming.
Neuroimaging Results
We hypothesised that different neural substrates would be implicated in object vs. action naming and for those with acute stroke vs. PPA. To investigate these hypotheses, we used LASSO regression models, one for each of the four combinations of object vs. action naming and PPA vs. acute stroke. Results are summarised in Tables 4 and 5.
Table 4.
LASSO results—PPA.
| BNT | HANA | |||||
|---|---|---|---|---|---|---|
| LASSO value | Adjusted Coefficient | p value | LASSO value | Adjusted Coefficient | p value | |
| model intercept | −6.142 × 10−16 | −7.101 × 10−16 | ||||
| cerebral to intracranial volume ratio | 0.303 | 0.327 | 0.096 | 0.416 | 0.515 | 0.091 |
| left inferior frontal gyrus-pars opercularis | ||||||
| left inferior frontal gyrus-pars orbitalis | ||||||
| left inferior frontal gyrus-pars triangularis | ||||||
| left postcentral gyrus | ||||||
| left precentral gyrus | ||||||
| left supramarginal gyrus | 0.186 | 0.276 | 0.252 | |||
| left angular gyrus | ||||||
| left superior temporal gyrus | ||||||
| right superior temporal gyrus | ||||||
| left superior temporal pole | 0.212 | 0.292 | 0.1 | 0.092 | 0.155 | 0.575 |
| right superior temporal pole | 0.119 | 0.101 | 0.462 | |||
| left middle temporal gyrus | ||||||
| right middle temporal gyrus | 0.072 | 0.161 | 0.494 | 0.030 | 0.161 | 0.567 |
| left middle temporal pole | ||||||
| right middle temporal pole | ||||||
| left inferior temporal gyrus | 0.020 | −0.042 | 0.893 | |||
| right inferior temporal gyrus | 0.054 | 0.064 | 0.462 | |||
| left fusiform gyrus | 0.271 | 0.285 | 0.081 | 0.198 | 0.28 | 0.243 |
| right fusiform gyrus | 0.030 | −0.022 | 0.716 | |||
| left insula | ||||||
Table 5.
Lasso results—acute stroke.
| BNT | HANA | |||||
|---|---|---|---|---|---|---|
| LASSO value | Adjusted Coefficient | p value | LASSO value | Adjusted Coefficient | p value | |
| model intercept | 1.367 × 10−16 | 1.455 × 10−16 | ||||
| total lesion volume | −0.225 | −0.167 | 0.072 | |||
| left inferior frontal gyrus-pars opercularis | ||||||
| left inferior frontal gyrus-pars orbitalis | ||||||
| left inferior frontal gyrus-pars triangularis | ||||||
| left postcentral gyrus | ||||||
| left precentral gyrus | ||||||
| left supramarginal gyrus | ||||||
| left angular gyrus | −0.524 | −0.555 | <.001 * | −0.405 | −0.487 | 0.728 |
| left superior temporal gyrus | ||||||
| left superior temporal pole | ||||||
| left middle temporal gyrus | −0.121 | −0.151 | 0.397 | |||
| left inferior temporal gyrus | ||||||
| left fusiform gyrus | ||||||
| left insula | −0.015 | −0.134 | 0.92 | |||
Note:
denotes significance at p<.05
Models for participants with PPA included all 20 regions of interest as predictors, as well as the ratio of cerebral to intracranial volume to control for brain size differences. These were one-tailed analyses in which only positive predictors were selected, reflecting relationships in which greater volume was indicative of better naming. For the model of object naming, we found that the volumes of left supramarginal gyrus, left superior temporal pole, right middle temporal gyrus, left inferior temporal gyrus, right inferior temporal gyrus, and left fusiform gyrus were associated with performance on the BNT, as was the ratio of cerebral to intracranial volume. None of the covariates were independent, statistically significant predictors of object naming in PPA, although there was a trend for cerebral to intracranial volume ratio (p=.096) and left fusiform gyrus volume (p=.081) to be independently predictive. For the model of action naming, we found that the volumes of left superior temporal pole, right superior temporal pole, right middle temporal gyrus, left fusiform gyrus, and right fusiform gyrus were associated with performance on the HANA, as was the ratio of cerebral to intracranial volume. None of the covariates were independent, statistically significant predictors of action naming in PPA, although there was a trend for larger ratio of cerebral to intracranial volume (p=.091) to be independently predictive. Results are summarised in Table 4 and visualised in Figure 3. Models for participants with acute stroke removed the regions of interest in which no participants had lesions, including the six right hemisphere ROIs and left middle temporal pole. Therefore, 13 regions of interest were included, as was total lesion volume to control for severity of stroke. One-tailed analyses were conducted in which only negative predictors were selected, reflecting relationships in which less damage was indicative of better naming. For the model of object naming, we found that larger percentage of tissue lesioned in the left angular gyrus and left middle temporal gyrus were associated with worse performance on the BNT. Greater damage to the left angular gyrus independently predicted object naming performance (p<.001). For the model of action naming, we found that larger percentage of tissue lesioned in the left angular gyrus and left insula were associated with worse performance on the HANA, as was larger total lesion volume. None of the covariates were independent, statistically significant predictors of action naming in acute stroke. Results are summarised in Table 5 and visualised in Figure 4.
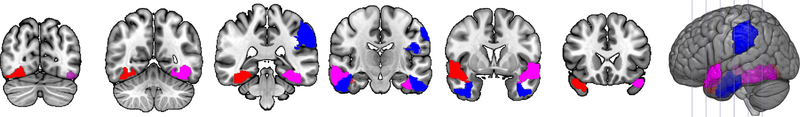
Figure 3.
Neuroimaging results for PPA. Regions in red were associated with action naming performance. Regions in blue were associated with object naming performance. Regions in magenta were associated with naming of both objects and actions. Slices are y=−73, −55. −36, −17, 2, and 21.
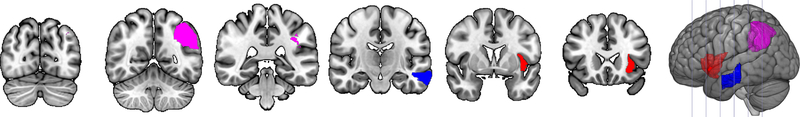
Figure 4.
Neuroimaging results for acute stroke. Regions in red were associated with action naming performance. Regions in blue were associated with object naming performance. Regions in magenta were associated with naming of both objects and actions. Slices are y=−73, −55. −36, −17, 2, and 21.
Comparing the results of the object and action naming models provides a test of the hypothesis that different neural substrates are involved in object vs. action naming. For participants with PPA, we did find regions that were involved with both, namely left superior temporal pole, right middle temporal gyrus, and left fusiform gyrus, as well as the ratio of cerebral to intracranial volume. However, in line with our hypothesis, we also found regions that were associated with object naming only: left supramarginal gyrus, left inferior temporal gyrus, and right inferior temporal gyrus. There were also regions associated with action naming only: right superior temporal pole and right fusiform gyrus. For participants with acute stroke, we again found a region commonly associated with both object and action naming: left angular gyrus. There were differences in the neural substrates for the two types of naming, as left middle temporal gyrus was associated with objects but not actions, while left insula and total lesion volume were associated with actions but not objects.
Comparing the results of the models of PPA and acute stroke participants provides a test of the hypothesis that distinct regions of damage are statistically associated with deficits in naming when considering individuals from these different populations. Of the eight regions associated with object naming in either aetiology, none were identified in both stroke and PPA. Likewise, of the seven regions associated with action naming in either aetiology, none were identified for both stroke and PPA. The regions that were associated with both object and action naming in PPA were distinct from those that were associated with both types of naming in stroke. Thus, in line with our hypotheses, different neural substrates were identified for PPA and acute stroke.
Discussion
In the present study, we paired a novel assessment of action naming, the HANA, with an established assessment of object naming in order to demonstrate their combined utility and explore the neural bases of naming. Clinical evaluations of naming are often (although not always) limited by considering only object naming; we expanded the scope of our project to compare matched picture naming assessments of actions and objects. Studies of the brain regions underlying language in neurological populations are often limited by including only participants with damage due to one aetiology; we expanded the scope of our project to compare participants with language deficits due to PPA as well as acute left hemisphere stroke. Looking at both object and action naming in multiple aetiologies in the same study affords us an unusual perspective from which we were able to investigate three hypotheses: (1) there is a behavioral double dissociation between object and action naming; (2) different neural substrates are involved in object vs. action naming; and (3) different lesion-deficit associations are identified in those with PPA vs. acute stroke. Our results confirmed these hypotheses.
First, we were able to demonstrate the utility of the HANA in identifying participants with action naming impairments. Of the 175 participants who completed the HANA, 100 were impaired relative to controls. Furthermore, there was a double dissociation such that some participants were impaired on object naming but not on action naming, while others were impaired on action naming but not object naming. This was the case regardless of whether we defined impairment dichotomously based on the performance of healthy controls or whether we looked at relative impairment defined as significantly better performance on one task than the other. Each of these methods has limitations. Using cut-offs based on control performance may lead to classifying individuals whose performance is similar across tasks as showing a dissociation because their scores on one task happen to fall just above the cut-off while their scores on the other happen to fall just below the cut-off. Using significance testing may lead to classifying individuals who perform well on both tasks as having a dissociation when their performance on one task is numerically better than the other even though both are in the range of healthy controls. Some participants may be identified as significantly better on the BNT than the HANA due to chance since the relatively small group of control participants found naming of objects easier than naming of actions (the cut-off for normal performance was 4 items higher for the BNT than the HANA), However, combining these methods mitigates these limitations, increasing confidence that true double dissociations were observed: some participants showed intact performance on one task relative to controls that was significantly better than their performance on the other task, which was classified as impaired relative to controls, while others showed the opposite pattern. This finding underscores the clinical importance of assessing and treating naming not only of objects but also of actions. Evaluating only one type may lead to missing functionally important deficits; treating object naming exclusively, which is typical of aphasia therapy, may have implications for sentence formulation. It was not the case that actions were always harder to name than objects as has sometimes been asserted. This result was not surprising in light of double dissociations previously reported in the literature. However, it does demonstrate that the HANA is a useful tool for evaluating action naming. As shown here, it is sensitive to action naming impairments in participants with aphasia and acute stroke. It is relatively quick to administer and score clinically. It is matched to a widely used standardised assessment of object naming, the Boston Naming Test, and performance on the two can be compared to evaluate naming of objects and actions.
Second, we showed that, beyond their clinical utility in diagnosing impairments, the HANA and BNT can be used to investigate the neural bases of naming. Different regions were identified as related to object naming performance vs. action naming performance in the different groups. Based on previous studies, we expected left temporal cortex to be critical for object naming and left posterior frontal regions to be critical for action naming. This was not the pattern that we uncovered: instead, damage to left temporal regions was implicated in poor performance on both object and action naming, and damage to left frontal regions was implicated in poor performance on neither object nor action naming. This divergence from expectations may be due to inadequate power in specific areas or to the statistical methods applied. LASSO regression reduces multicollinearity by selecting only the most predictive variable among those that are highly correlated. Regions that are in fact critical may have been eliminated if they were strongly correlated with those selected by the model. Although the pattern of regions we identified did not match our hypotheses, we did find distinct networks of areas involved in each type of naming. In participants with PPA, left supramarginal gyrus, left inferior temporal gyrus, and right inferior temporal gyrus were associated with object naming but not action naming, while right superior temporal pole and right fusiform gyrus were associated with action naming but not object naming. The left hemisphere regions related to object naming have previously been identified as involved in naming (e.g., Damasio & Tranel, 1993; Daniele et al., 1994; DeLeon et al., 2007; Hart & Gordon, 1990; Migliaccio et al., 2016; Mirman et al., 2015; Race et al., 2013; Riello et al., 2018; Schwartz et al., 2012; Thye & Mirman, 2018). The identification of right hemisphere homologues of left hemisphere language regions (right inferior temporal gyrus, right superior temporal pole, and right fusiform gyrus) may indicate that participants initially can compensate by recruiting right hemisphere regions; it is only when atrophy increases bilaterally that naming deficits become more severe (e.g., Rohrer et al., 2013).
In participants with acute stroke, left middle temporal gyrus was associated with object naming but not action naming, while left insula and total lesion volume were associated with action naming but not object naming. The left middle temporal gyrus has previously been implicated in object naming (Faroqi-Shah et al., 2014; Fridriksson et al., 2018; Hillis, Tuffiash, Wityk, et al., 2002; Piras & Marangolo, 2007; Python et al., 2018; Riello et al., 2018; Schwartz et al., 2009; Thye & Mirman, 2018; Tochadse et al., 2018). Larger total lesion volume was associated with worse performance on actions but not objects, which may indicate that action naming was a more difficult, cognitively demanding task for many – although, as described above, not all – of the participants. Similarly, previous work has shown that ischemic strokes that involve the insula are larger than strokes that exclude the insula and therefore are associated with more common and persistent deficits (Kodumuri et al., 2016). Furthermore, the left insula has been associated with action naming in the past (e.g., Benetello et al., 2016; Hillis et al., 2006; Kemmerer et al., 2012; Race et al., 2013; Tranel et al., 2001).
Note that we did also identify common regions that were critical for both object and action naming, as damage was predictive of poor performance on both. For participants with PPA, these regions consisted of left superior temporal pole, right middle temporal gyrus, and left fusiform gyrus, as well as ratio of cerebral to intracranial volume. For acute stroke, greater damage to the left angular gyrus was related to poor performance on both. It makes sense that common regions would exist as many of the cognitive processes underlying naming of the two word classes are shared, and these regions have previously been identified in studies of naming (e.g., Benetello et al., 2016; Damasio & Tranel, 1993; Daniele et al., 1994; DeLeon et al., 2007; Faria et al., 2014; Faroqi-Shah et al., 2014; Foundas et al., 1998; Fridriksson et al., 2018; Gleichgerrcht et al., 2015, 2016, Gorno-Tempini et al., 2004, 2011; Halai et al., 2017; Hillis, Tuffiash, Wityk, et al., 2002; Hurley et al., 2012; Kemmerer et al., 2012; Lambon Ralph et al., 2016; Mesulam et al., 2013; Meyer et al., 2017; Migliaccio et al., 2016; Mion et al., 2010; Newhart et al., 2007; Piras & Marangolo, 2007; Race et al., 2013; Raymer et al., 1997; Riello et al., 2018; Schwartz et al., 2009; Snowden et al., 2018; Thye & Mirman, 2018; Tranel et al., 2001, 2003, 2008; Tsapkini et al., 2011; Tyler et al., 2001; Walker et al., 2011).
Third, different regions were identified for participants with different aetiologies of brain damage. No single region was associated with both object and action naming in both PPA and acute stroke. Rather, all regions identified in the analyses were associated with naming only for participants with PPA or only for participants with acute stroke. This finding underscores the importance of considering converging evidence. For instance, many of the regions that we identified in the PPA participants were in the right hemisphere. It would not be possible to identify such regions in the typical participant with aphasia due to left hemisphere stoke since these regions are not infarcted. Similarly, some regions are less likely to be damaged in stroke than in PPA based on the blood supply of the brain. For example, the temporal poles are rarely damaged in stroke unless the infarct is quite large, while they are the predominant site of atrophy in semantic variant PPA. Examining the neural bases of cognition in only one of these populations may lead to missing important regions; comparing the two provides a more complete picture. One reason that there may be distinct regions associated with naming in the two aetiologies is that there are different distributions of underlying cognitive deficits in the two groups. For example, more patients with PPA are likely to have impaired naming due to impairments of conceptual semantics.
This study has several limitations. First, using a picture naming task as an assessment of action naming is complicated by the fact that depicting most actions necessarily involves depicting objects (e.g., the picture of “vacuum” includes a vacuum cleaner and a person using it). If a participant is impaired on object processing, they may also appear to be impaired on action processing because of this confound. Additionally, actions take place over time, which is difficult to depict in a static two-dimensional picture. However, we were able to find a double dissociation between the object and action naming tasks, indicating that, while this limitation may have weakened effects, it did not completely mask them. Future work may benefit from development of assessments where video clips of actions (and objects) are used as stimuli for naming instead of static pictures.
Next, it was not possible to directly compare the participants with PPA and acute stroke using exactly the same neuroimaging measures and thus we could not directly compare the two groups within the same statistical model. This is because it is impossible to determine the precise percentage of a region that is atrophied in PPA without having an earlier, premorbid scan of the same individual to compare with the later damaged scan. Since we did not have access to such data for all participants, we used a neuroimaging measure that it was possible to observe: volume of the region as a percentage of cerebral volume. It is more straightforward to measure the amount of damage in acute stroke as it is possible to visualise and trace the lesioned tissue on diffusion-weighted scans. Unlike the case of chronic stroke, there is no distortion of the brain shape due to encephalomalacia in acute stroke. In the future, studies could improve the assessment of the relationship between damage and behavior in PPA by relating longitudinal changes in brain volume over time from multiple scans to longitudinal changes in behavior over the same period (as in Faria, Sebastian, Newhart, Mori, & Hillis, 2014). Longitudinal tracking of brain and behavior may be of particular interest in PPA because it is a chronic condition that may include changes reflecting some plasticity and reorganization as individuals attempt to adapt to neurodegeneration.
Another important limitation is the sample size that was included in the neuroimaging analysis. Although behavioral data were available for a relatively sizable sample of participants with PPA, contemporaneous neuroimaging data were not available for many of the participants. Similarly, the sample size was relatively small for the acute stroke participants. It is possible that the results were overfitted by the LASSO model. However, we preferred this statistical approach to simpler regression models because it addressed the multicollinearity inherently present in the data (e.g., participants with damage in one anatomical region are likely to have damage in nearby regions as well due to biological processes). While the LASSO model reduced multicollinearity by eliminating highly correlated variables, we do note that some information may have been lost in the process if more than one of the highly correlated regions makes an important contribution to naming. Therefore, we cannot make any claims about the regions we did not identify in the analyses: it is possible that they were not identified due to the type of model used or because there was inadequate power in terms of number of patients with atrophy or lesions in those areas. For example, typically only individuals with nfvPPA have frontal atrophy, whereas individuals with both svPPA and lvPPA have atrophy in temporal areas (e.g., Gorno-Tempini et al., 2004). However, the finding that distinct lesion-deficit associations were identified for objects vs. actions within each aetiology cannot be due to this power issue, because data from the same patients were used in detecting object and action naming deficits. It is likely that naming of objects and actions depends on partially overlapping neural networks; here we identified a subset of the critical regions for each and for both. We may have also missed key brain areas because we did not attempt to identify the white matter tracts critical for naming objects or actions. We plan to continue collecting data on these tasks, and data from larger samples in the future may be able to address these concerns.
Furthermore, the results show the importance of comparing individuals with aphasia due to different underlying causes of brain damage as complementary information can be obtained. However, this was limited to only two aetiologies: PPA and acute stroke. Expanding this work to other aetiologies such as tumor, focal traumatic brain injury, and infection (e.g., herpes simplex virus encephalitis), or to temporary lesions created with repetitive transcranial magnetic stimulation in future work is likely to provide further information to enrich understanding of the brain-behavior relationships critical for naming. Lesion-deficit association in each of these populations has challenges and limitations.
Conclusions
The present study introduced a new assessment, the Hopkins Action Naming Assessment, that can be used in conjunction with the Boston Naming Test not only to better diagnose naming impairments but also to investigate the neural bases of naming. Comparing the association between naming performance and damage to neural regions of interest in both PPA and stroke provides a more comprehensive picture of the brain-behavior relationships critical for naming than could be obtained by studying either in isolation. Complementary information can be obtained by including analyses of both PPA and acute stroke, likely because different regions tend to be damaged in these populations. Likewise, as double dissociations between object and action naming performance were observed in both populations, identifying lesion-deficit associations for both word classes yields a more comprehensive picture of regions critical for naming.
Acknowledgments
Funding
This work was supported by the National Institutes of Health [grant numbers R01DC05375, R01DC011317, and R00DC015554].
Footnotes
Declaration of Interest
Declarations of interest: none
References
- Arévalo AL, Lu CC, Huang LBY, Bates EA, & Dronkers NF (2011). Action and Object Processing in Brain-Injured Speakers of Chinese. Neuropsychology, 25(6), 792–805. 10.1037/a0024272 [DOI] [PubMed] [Google Scholar]
- Baayen RH, Piepenbrock R, & van Rijn H (1995). The CELEX Lexical Database. Release 2 (CD-ROM), Linguistic Data Consortium, University of Pennsylvania, Philadelphia, PA. [Google Scholar]
- Bak TH, & Hodges JR (2003). Kissing and dancing — a test to distinguish the lexical and conceptual contributions to noun / verb and action / object dissociation. Preliminary results in patients with frontotemporal dementia. Journal of Neurolinguistics, 16, 169–181. [Google Scholar]
- Beber BC, Mandelli ML, Santos MAS, Binney RJ, Miller B, Chaves MLF, Gorno-Tempini ML, & Shapiro KA (2019). A behavioral study of the nature of verb–noun dissociation in the nonfluent variant of primary progressive aphasia. Aphasiology, 33(2), 200–215. 10.1080/02687038.2018.1461799 [DOI] [Google Scholar]
- Benetello A, Finocchiaro C, Capasso R, Capitani E, Laiacona M, Magon S, & Miceli G (2016). The dissociability of lexical retrieval and morphosyntactic processes for nouns and verbs: A functional and anatomoclinical study. Brain and Language, 159, 11–22. 10.1016/j.bandl.2016.05.005 [DOI] [PubMed] [Google Scholar]
- Berndt RS, Mitchum CC, Haendiges AN, & Sandson J (1997). Verb Retrieval in Aphasia. Brain and Language, 106(56), 68–106. [DOI] [PubMed] [Google Scholar]
- Budd MA, Kortte K, Cloutman L, Newhart M, Gottesman RF, Davis C, Heidler-Gary J, Seay MW, & Hillis AE (2010). The nature of naming errors in primary progressive aphasia versus acute post-stroke aphasia. Neuropsychology, 24(5), 581–589. 10.1037/a0020287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramazza A, & Hillis AE (1990). Where do semantic errors come from? Cortex, 26(1), 95–122. [DOI] [PubMed] [Google Scholar]
- Coltheart M (1981). The MRC Psycholinguistic Database, Quarterly Journal of Experimental Psychology, 33A, 497–505. [Google Scholar]
- Cotelli M, Manenti R, Paternicò D, Cosseddu M, Brambilla M, Petesi M, Premi E, Gasparotti R, Zanetti O, Padovani A, & Borroni B (2016). Grey Matter Density Predicts the Improvement of Naming Abilities After tDCS Intervention in Agrammatic Variant of Primary Progressive Aphasia. Brain Topography, 29(5), 738–751. 10.1007/s10548-016-0494-2 [DOI] [PubMed] [Google Scholar]
- Damasio AR, & Tranel D (1993). Nouns and verbs are retrieved with differently distributed neural systems. Proceedings of the National Academy of Sciences, 90(June), 4957–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniele A, Giustolisi L, Silveri MC, Colosimo C, & Gainotti G (1994). Evidence for a possible neuroanatomical basis for lexical processing of nouns and verbs. Neuropsychologia, 32(11), 1325–1341. 10.1016/0028-3932(94)00066-2 [DOI] [PubMed] [Google Scholar]
- Davis CJ (2005). N-Watch: A program for deriving neighborhood size and other psycholinguistic statistics. Behavior Research Methods, 37, 65–70. [DOI] [PubMed] [Google Scholar]
- de Aguiar V, Zhao Y, Ficek BN, Webster K, Rofes A, Wendt H, Frangakis C, Caffo B, Hillis AE, Rapp B, & Tsapkini K (2020). Cognitive and language performance predicts effects of spelling intervention and tDCS in Primary Progressive Aphasia. Cortex, 124, 66–84. 10.1016/j.cortex.2019.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeon J, Gottesman RF, Kleinman JT, Newhart M, Davis C, Heidler-Gary J, Lee A, & Hillis AE (2007). Neural regions essential for distinct cognitive processes underlying picture naming. Brain, 130(5), 1408–1422. 10.1093/brain/awm011 [DOI] [PubMed] [Google Scholar]
- Dennis S (1995). The Sydney Morning Herald Word Database. Noetica: Open Forum, 1(4), http://psy.uq.edu.au/CogPsych/Noetica/. [Google Scholar]
- Ellis AW, & Young AW (1988). Human cognitive neuropsychology. In Ellis AW & Young AW (Eds.), Human Cognitive Neuropsychology. Erlbaum. [Google Scholar]
- Faria AV, Crinion JT, Tsapkini K, Newhart M, Davis C, Cooley S, Mori S, & Hillis AE (2013). Patterns of dysgraphia in primary progressive aphasia compared to post-stroke aphasia. Behavioural Neurology, 26(1), 21–34. 10.3233/BEN-2012-110237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria AV, Sebastian R, Newhart M, Mori S, & Hillis AE (2014). Longitudinal imaging and deterioration in word comprehension in primary progressive aphasia: Potential clinical significance. Aphasiology, 28(8–9), 948–963. 10.1080/02687038.2014.911241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria AV, Zhang J, Oishi K, Li X, Jiang H, Akhter K, Hermoye L, Lee S-K, Hoon A, Stashinko E, Miller MI, van Zijl PCM, & Mori S (2010). Atlas-based analysis of neurodevelopment from infancy to adulthood using diffusion tensor imaging and applications for automated abnormality detection. NeuroImage, 52(2), 415–428. 10.1016/j.neuroimage.2010.04.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faroqi-Shah Y, Kling T, Solomon J, Liu S, Park G, & Braun A (2014). Lesion analysis of language production deficits in aphasia. Aphasiology, 28(3), 258–277. 10.1080/02687038.2013.853023 [DOI] [Google Scholar]
- Forseth KJ, Kadipasaoglu CM, Conner CR, Hickok G, Knight RT, & Tandon N (2018). A lexical semantic hub for heteromodal naming in middle fusiform gyrus. Brain, 141(7), 2112–2126. 10.1093/brain/awy120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foundas AL, Daniels SK, & Vasterling JJ (1998). Anomia: Case Studies with Lesion Localization. Neurocase, 4(1), 35–43. [Google Scholar]
- Fridriksson J, Den Ouden DB, Hillis AE, Hickok G, Rorden C, Basilakos A, Yourganov G, & Bonilha L (2018). Anatomy of aphasia revisited. Brain, 141(3), 848–862. 10.1093/brain/awx363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J, Fillmore P, Guo D, & Rorden C (2015). Chronic Broca’s aphasia is caused by damage to Broca’s and wernicke’s areas. Cerebral Cortex, 25(12), 4689–4696. 10.1093/cercor/bhu152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesierich B, Jovicich J, Riello M, Adriani M, Monti A, Brentari V, Robinson SD, Wilson SM, Fairhall SL, & Gorno-Tempini ML (2012). Distinct neural substrates for semantic knowledge and naming in the temporoparietal network. Cerebral Cortex, 22(10), 2217–2226. 10.1093/cercor/bhr286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleichgerrcht E, Fridriksson J, & Bonilha L (2015). Neuroanatomical foundations of naming impairments across different neurologic conditions. Neurology, 85(3), 284–292. 10.1212/WNL.0000000000001765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleichgerrcht E, Fridriksson J, Rorden C, Nesland T, Desai R, & Bonilha L (2016). Separate neural systems support representations for actions and objects during narrative speech in post-stroke aphasia. NeuroImage: Clinical, 10, 140–145. 10.1016/j.nicl.2015.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, Johnson JK, Weiner MW, & Miller BL (2004). Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology, 55(3), 335–346. 10.1002/ana.10825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez MF, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, Manes F, Dronkers NF, Vandenberghe R, Rascovsky K, Patterson K, Miller BL, Knopman DS, Hodges JR, Mesulam MM, & Grossman M (2011). Classification of primary progressive aphasia and its variants. Neurology, 76(11), 1006–1014. 10.1212/WNL.0b013e31821103e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halai AD, Woollams AM, & Lambon Ralph MA (2017). Using principal component analysis to capture individual differences within a unified neuropsychological model of chronic post-stroke aphasia: Revealing the unique neural correlates of speech fluency, phonology and semantics. Cortex, 86, 275–289. 10.1016/j.cortex.2016.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart J, & Gordon B (1990). Delineation of single-word semantic comprehension deficits in aphasia, with anatomical correlation. Annals of Neurology, 27(3), 226–231. 10.1002/ana.410270303 [DOI] [PubMed] [Google Scholar]
- Henry ML, & Grasso SM (2018). Assessment of Individuals with Primary Progressive Aphasia. Seminars in Speech and Language, 39(3), 231–241. 10.1055/s-0038-1660782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis AE, Beh YY, Sebastian R, Breining BL, Tippett DC, Wright A, Saxena S, Rorden C, Bonilha L, Basilakos A, Yourganov G, & Fridriksson J (2018). Predicting recovery in acute poststroke aphasia. Annals of Neurology, 83(3), 612–622. 10.1002/ana.25184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis AE, Heidler-Gary J, Newhart M, Chang S, Ken L, & Bak TH (2006). Naming and comprehension in primary progressive aphasia: The influence of grammatical word class. Aphasiology, 20(2–4), 246–256. 10.1080/02687030500473262 [DOI] [Google Scholar]
- Hillis AE, Oh S, & Ken L (2004). Deterioration of naming nouns versus verbs in primary progressive aphasia. Annals of Neurology, 55(2), 268–275. 10.1002/ana.10812 [DOI] [PubMed] [Google Scholar]
- Hillis AE, Rorden C, & Fridriksson J (2017). Brain regions essential for word comprehension: Drawing inferences from patients. Annals of Neurology, 81(6), 759–768. 10.1002/ana.24941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis AE, Tuffiash E, & Caramazza A (2002). Modality-specific deterioration in naming verbs in nonfluent primary progressive aphasia. Journal of Cognitive Neuroscience, 14(7), 1099–1108. 10.1162/089892902320474544 [DOI] [PubMed] [Google Scholar]
- Hillis AE, Tuffiash E, Wityk RJ, & Barker PB (2002). Regions of neural dysfunction associated with impaired naming of actions and objects in acute stroke. Cognitive Neuropsychology, 19(6), 523–534. 10.1080/02643290244000077 [DOI] [PubMed] [Google Scholar]
- Hope TMH, Leff AP, & Price CJ (2018). Predicting language outcomes after stroke: Is structural disconnection a useful predictor? NeuroImage: Clinical, 19(March), 22–29. 10.1016/j.nicl.2018.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RS, Paller KA, Rogalski EJ, & Mesulam MM (2012). Neural Mechanisms of Object Naming and Word Comprehension in Primary Progressive Aphasia. Journal of Neuroscience, 32(14), 4848–4855. 10.1523/jneurosci.5984-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, Laakso MP, & Pitkänen A (1998). MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. AJNR. American Journal of Neuroradiology, 19(4), 659–671. http://www.ncbi.nlm.nih.gov/pubmed/9576651 [PMC free article] [PubMed] [Google Scholar]
- Kaplan D, Goodglass H, & Weintraub S (1983). Boston Naming Test. Lea & Febige. [Google Scholar]
- Keator LM, Wright AE, Saxena S, Kim K, Demsky C, Sebastian R, Sheppard SM, Breining B, Hillis AE, & Tippett DC (2019). Distinguishing logopenic from semantic & nonfluent variant primary progressive aphasia: Patterns of linguistic and behavioral correlations. Neurocase, 25(3–4). 10.1080/13554794.2019.1625929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keator LM, Faria AV, Kim KT, Saxena S, Wright AE, Sheppard SM, Breining BL, Goldberg E, Tippett DC, Meier E, & Hillis AE (2020). An Efficient Bedside Measure Yields Prognostic Implications for Language Recovery in Acute Stroke Patients. Cognitive and Behavioral Neurology, 33(3), 192–200. 10.1097/WNN.0000000000000238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmerer D, Rudrauf D, Manzel K, & Tranel D (2012). Behavioral patterns and lesion sites associated with impaired processing of lexical and conceptual knowledge of actions. Cortex, 48(7), 826–848. 10.1016/j.cortex.2010.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodumuri N, Sebastian R, Davis C, Posner J, Kim EH, Tippett DC, Wright A, & Hillis AE (2016). The association of insular stroke with lesion volume. NeuroImage: Clinical, 11, 41–45. 10.1016/j.nicl.2016.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo H, Saleem KS, & Price JL (2003). Differential connections of the temporal pole with the orbital and medial prefrontal networks in macaque monkeys. The Journal of Comparative Neurology, 465(4), 499–523. 10.1002/cne.10842 [DOI] [PubMed] [Google Scholar]
- Kondo H, Saleem KS, & Price JL (2005). Differential connections of the perirhinal and parahippocampal cortex with the orbital and medial prefrontal networks in macaque monkeys. The Journal of Comparative Neurology, 493(4), 479–509. 10.1002/cne.20796 [DOI] [PubMed] [Google Scholar]
- Kučera H, & Francis WN (1967). Computational analysis of present-day American English. Providence, R.I.: Brown University Press. [Google Scholar]
- Lacey EH, Skipper-Kallal LM, Xing S, Fama ME, & Turkeltaub PE (2017). Mapping Common Aphasia Assessments to Underlying Cognitive Processes and Their Neural Substrates. Neurorehabilitation and Neural Repair, 31(5), 442–450. 10.1177/1545968316688797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph MA, Jefferies E, Patterson KE, & Rogers TT (2016). The neural and computational bases of semantic cognition. Nature Reviews Neuroscience, 18(1), 42–55. 10.1038/nrn.2016.150 [DOI] [PubMed] [Google Scholar]
- Leff AP, Schofield TM, Crinion JT, Seghier ML, Grogan A, Green DW, & Price CJ (2009). The left superior temporal gyrus is a shared substrate for auditory short-term memory and speech comprehension: evidence from 210 patients with stroke. Brain : A Journal of Neurology, 132(Pt 12), 3401–3410. 10.1093/brain/awp273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton CE, Hodges JR, Piguet O, & Ballard KJ (2017). Common and divergent neural correlates of anomia in amnestic and logopenic presentations of Alzheimer’s disease. Cortex, 86, 45–54. 10.1016/j.cortex.2016.10.019 [DOI] [PubMed] [Google Scholar]
- Long C, Sebastian R, Faria AV, & Hillis AE (2018). Longitudinal imaging of reading and naming recovery after stroke. Aphasiology, 32(7), 839–854. 10.1080/02687038.2017.1417538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack WJ, Freed DM, Williams BW, & Henderson VW (1992). Boston Naming Test: Shortened versions for use in Alzheimer’s disease. Journals of Gerontology, 47(3), 154–158. 10.1093/geronj/47.3.P154 [DOI] [PubMed] [Google Scholar]
- Marcotte K, Graham NL, Black SE, Tang-Wai D, Chow TW, Freedman M, Rochon E, & Leonard C (2014). Verb production in the nonfluent and semantic variants of primary progressive aphasia: The influence of lexical and semantic factors. Cognitive Neuropsychology, 31, 565–583. 10.1080/02643294.2014.970154 [DOI] [PubMed] [Google Scholar]
- Martin A, Haxby JV, Lalonde FM, Wiggs CL, & Ungerleider LG (1995). Discrete cortical regions associated with knowledge of color and knowledge of action. Science (New York, N.Y.), 270(5233), 102–105. 10.1126/science.270.5233.102 [DOI] [PubMed] [Google Scholar]
- McKinnon ET, Fridriksson J, Basilakos A, Hickok G, Hillis AE, Spampinato MV, Gleichgerrcht E, Rorden C, Jensen JH, Helpern JA, & Bonilha L (2018). Types of naming errors in chronic post-stroke aphasia are dissociated by dual stream axonal loss. Scientific Reports, 8(1), 1–12. 10.1038/s41598-018-32457-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinshausen N, & Yu B (2009). Lasso-type recovery of sparse representations for high-dimensional data. Annals of Statistics, 37(1), 246–270. 10.1214/07-AOS582 [DOI] [Google Scholar]
- Mesulam MM (1982). Slowly progressive aphasia without generalized dementia. Annals of Neurology, 11(6), 592–598. 10.1002/ana.410110607 [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Wieneke C, Hurley R, Rademaker A, Thompson CK, Weintraub S, & Rogalski EJ (2013). Words and objects at the tip of the left temporal lobe in primary progressive aphasia. Brain, 136(2), 601–618. 10.1093/brain/aws336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AM, Faria AV, Tippett DC, Hillis AE, & Friedman RB (2017). The relationship between baseline volume in temporal areas and post-treatment naming accuracy in primary progressive aphasia. Aphasiology, 31(9), 1059–1077. 10.1080/02687038.2017.1296557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miceli G, & Capasso R (1997). Semantic Errors as Neuropsychological Evidence for the Independence and the Interaction of Orthographic and Phonological Word Forms. Language and Cognitive Processes, 12(5–6), 733–764. 10.1080/016909697386673 [DOI] [Google Scholar]
- Migliaccio R, Boutet C, Valabregue R, Ferrieux S, Nogues M, Lehéricy S, Dormont D, Levy R, Dubois B, & Teichmann M (2016). The brain network of naming: A lesson from primary progressive aphasia. PLoS ONE, 11(2), 1–17. 10.1371/journal.pone.0148707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mion M, Patterson K, Acosta-Cabronero J, Pengas G, Izquierdo-Garcia D, Hong YT, Fryer TD, Williams GB, Hodges JR, & Nestor PJ (2010). What the left and right anterior fusiform gyri tell us about semantic memory. Brain, 133(11), 3256–3268. 10.1093/brain/awq272 [DOI] [PubMed] [Google Scholar]
- Mirman D, Chen Q, Zhang Y, Wang Z, Faseyitan OK, Coslett HB, & Schwartz MF (2015). Neural organization of spoken language revealed by lesion-symptom mapping. Nature Communications, 6, 1–9. 10.1038/ncomms7762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhart M, Ken L, Kleinman JT, Heidler-Gary J, & Hillis AE (2007). Neural networks essential for naming and word comprehension. Cognitive and Behavioral Neurology, 20(1), 25–30. 10.1097/WNN.0b013e31802dc4a7 [DOI] [PubMed] [Google Scholar]
- Odolil A, Wright AE, Keator LM, Sheppard SM, Breining B, Tippett DC, & Hillis AE (2020). Leukoaraiosis severity predicts rate of decline in primary progressive aphasia. Aphasiology, 34(3). 10.1080/02687038.2019.1594152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson RB, Raymer A, Chang YL, FitzGerald DB, & Crosson B (2009). Lesion characteristics related to treatment improvement in object and action naming for patients with chronic aphasia. Brain and Language, 110(2), 61–70. 10.1016/j.bandl.2009.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piras F, & Marangolo P (2007). Noun-verb naming in aphasia: A voxel-based lesion-symptom mapping study. NeuroReport, 18(14), 1455–1458. 10.1097/WNR.0b013e3282ef6fc9 [DOI] [PubMed] [Google Scholar]
- Purcell J, Sebastian R, Leigh R, Jarso S, Davis C, Posner J, Wright A, & Hillis AE (2017). Recovery of orthographic processing after stroke: A longitudinal fMRI study. Cortex, 92, 103–118. 10.1016/j.cortex.2017.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Python G, Glize B, & Laganaro M (2018). The involvement of left inferior frontal and middle temporal cortices in word production unveiled by greater facilitation effects following brain damage. Neuropsychologia, 121(November), 122–134. 10.1016/j.neuropsychologia.2018.10.026 [DOI] [PubMed] [Google Scholar]
- Race DS, Tsapkini K, Crinion JT, Newhart M, Davis C, Gomez Y, Hillis AE, & Faria AV (2013). An area essential for linking word meanings to word forms: Evidence from primary progressive aphasia. Brain and Language, 127(2), 167–176. 10.1016/j.bandl.2013.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp BC, Benzing L, & Caramazza A (1997). The Autonomy of Lexical Orthography. Cognitive Neuropsychology, 14(1), 71–104. 10.1080/026432997381628 [DOI] [Google Scholar]
- Raymer AM, Foundas AL, Maher LM, Greenwald ML, Morris M, Rothi LJG, & Heilman KM (1997). Cognitive neuropsychological analysis and neuroanatomic correlates in a case of acute anomia. Brain and Language, 58(1), 137–156. 10.1006/brln.1997.1786 [DOI] [PubMed] [Google Scholar]
- Riello M, Faria AV, Ficek B, Webster K, Onyike CU, Desmond J, Frangakis C, & Tsapkini K (2018). The Role of Language Severity and Education in Explaining Performance on Object and Action Naming in Primary Progressive Aphasia. Frontiers in Aging Neuroscience, 10(October), 1–10. 10.3389/fnagi.2018.00346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rofes A, De Aguiar V, Ficek B, Wendt H, Webster K, & Tsapkini K (2019). The role of word properties in performance on fluency tasks in people with primary progressive aphasia. Journal of Alzheimer’s Disease, 68(4), 1521–1534. 10.3233/JAD-180990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Caso F, Mahoney C, Henry M, Rosen HJ, Rabinovici G, Rossor MN, Miller B, Warren JD, Fox NC, Ridgway GR, & Gorno-tempini ML (2013). Patterns of longitudinal brain atrophy in the logopenic variant of primary progressive aphasia. Brain and Language, 127(2), 121–126. 10.1016/j.bandl.2012.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MF, Faseyitan O, Kim J, & Coslett HB (2012). The dorsal stream contribution to phonological retrieval in object naming. Brain, 135(12), 3799–3814. 10.1093/brain/aws300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MF, Kimberg DY, Walker GM, Faseyitan O, Brecher A, Dell GS, & Coslett HB (2009). Anterior temporal involvement in semantic word retrieval: Voxel-based lesion-symptom mapping evidence from aphasia. Brain, 132(12), 3411–3427. 10.1093/brain/awp284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian R, Long C, Purcell JJ, Faria AV, Lindquist M, Jarso S, Race D, Davis C, Posner J, Wright A, & Hillis AE (2016). Imaging network level language recovery after left PCA stroke. Restorative Neurology and Neuroscience, 34(4), 473–489. 10.3233/RNN-150621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian R, Thompson CB, Wang NY, Wright A, Meyer A, Friedman RB, Hillis AE, & Tippett DC (2018). Patterns of decline in naming and semantic knowledge in primary progressive aphasia. Aphasiology, 32(9), 1010–1030. 10.1080/02687038.2018.1490388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden JS, Harris JM, Thompson JC, Kobylecki C, Jones M, Richardson AM, & Neary D (2018). Semantic dementia and the left and right temporal lobes. Cortex, 107, 188–203. 10.1016/j.cortex.2017.08.024 [DOI] [PubMed] [Google Scholar]
- Stefanacci L, Suzuki WA, & Amaral DG (1996). Organization of connections between the amygdaloid complex and the perirhinal and parahippocampal cortices in macaque monkeys. The Journal of Comparative Neurology, 375(4), 552–582. [DOI] [PubMed] [Google Scholar]
- Tainturier M-J, Moreaud O, David D, Leek EC, & Pellat J (2001). Superior written over spoken picture naming in a case of frontotemporal dementia. Neurocase, 7(1), 89–96. 10.1093/neucas/7.1.89 [DOI] [PubMed] [Google Scholar]
- Thompson CK, Lukic S, King MC, Mesulam MM, & Weintraub S (2012). Verb and noun deficits in stroke-induced and primary progressive aphasia: The Northwestern Naming Battery. Aphasiology, 26(5), 632–655. 10.1080/02687038.2012.676852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorndike EL and Lorge I (1944). The Teacher’s Word Book of 30,000 Words. New York: Teachers College, Columbia University. [Google Scholar]
- Thye M, & Mirman D (2018). Relative contributions of lesion location and lesion size to predictions of varied language deficits in post-stroke aphasia. NeuroImage: Clinical, 20(October), 1129–1138. 10.1016/j.nicl.2018.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibshirani R (1996). Regression Shrinkage and Selection Via the Lasso. Journal of the Royal Statistical Society: Series B (Methodological), 58(1), 267–288. 10.1111/j.2517-6161.1996.tb02080.x [DOI] [Google Scholar]
- Tippett DC, Breining B, Goldberg E, Meier E, Sheppard SM, Sherry E, Stockbridge M, Suarez A, Wright AE, & Hillis AE (2020). Visuomotor figure construction and visual figure delayed recall and recognition in primary progressive aphasia. Aphasiology, 34(12), 1456–1470. 10.1080/02687038.2019.1670330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tochadse M, Halai AD, Lambon Ralph MA, & Abel S (2018). Unification of behavioural, computational and neural accounts of word production errors in post-stroke aphasia. NeuroImage: Clinical, 18(August 2017), 952–962. 10.1016/j.nicl.2018.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel D, Adolphs R, Damasio H, & Damasio AR (2001). A Neural Basis for the Retrieval of Words for Actions. Cognitive Neuropsychology, 18(7), 655–674. 10.1080/02643290143000015 [DOI] [PubMed] [Google Scholar]
- Tranel D, Kemmerer D, Adolphs R, Damasio H, & Damasio AR (2003). Neural Correlates of Conceptual Knowledge for Actions. Cognitive Neuropsychology, 20(3–6), 409–432. 10.1080/02643290244000248 [DOI] [PubMed] [Google Scholar]
- Tranel D, Manzel K, Asp E, & Kemmerer D (2008). Naming dynamic and static actions: neuropsychological evidence. Journal of Physiology, Paris, 102(1–3), 80–94. 10.1016/j.jphysparis.2008.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsapkini K, Frangakis CE, & Hillis AE (2011). The function of the left anterior temporal pole: Evidence from acute stroke and infarct volume. Brain, 134(10), 3094–3105. 10.1093/brain/awr050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsapkini K, Webster KT, Ficek BN, Desmond JE, Onyike CU, Rapp B, Frangakis CE, & Hillis AE (2018). Electrical brain stimulation in different variants of primary progressive aphasia: A randomized clinical trial. Alzheimer’s and Dementia: Translational Research and Clinical Interventions, 4, 461–472. 10.1016/j.trci.2018.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler LK, Russell RP, Fadili J, & Moss HE (2001). The neural representation of nouns and verbs: PET studies. Brain : A Journal of Neurology, 124(Pt 8), 1619–1634. http://www.ncbi.nlm.nih.gov/pubmed/11459753 [DOI] [PubMed] [Google Scholar]
- Unal G, Ficek B, Webster K, Shahabuddin S, Truong D, Hampstead B, Bikson M, & Tsapkini K (2020). Impact of brain atrophy on tDCS and HD-tDCS current flow: a modeling study in three variants of primary progressive aphasia. Neurological Sciences, 41(7), 1781–1789. 10.1007/s10072-019-04229-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker GM, Schwartz MF, Kimberg DY, Faseyitan O, Brecher A, Dell GS, & Coslett HB (2011). Support for anterior temporal involvement in semantic error production in aphasia: New evidence from VLSM. Brain and Language, 117(3), 110–122. 10.1016/j.bandl.2010.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss PH, Ubben SD, Kaesberg S, Kalbe E, Kessler J, Liebig T, & Fink GR (2016). Where language meets meaningful action: a combined behavior and lesion analysis of aphasia and apraxia. Brain Structure and Function, 221(1), 563–576. 10.1007/s00429-014-0925-3 [DOI] [PubMed] [Google Scholar]
- Zingeser LB, & Berndt RS (1990). Retrieval of nouns and verbs in agrammatism and anomia. Brain and Language, 39(1), 14–32. 10.1016/0093-934X(90)90002-X [DOI] [PubMed] [Google Scholar]