POINT (RPG and JOA)
Hodgkin Lymphoma is a Non-Hodgkin Lymphoma: Mutatis mutandis
If Lincoln were alive today he’d be turning over in his grave.
President Gerald R. Ford
The disease we called Hodgkin lymphoma was first described by Sir Thomas Hodgkin in 1832 noting a possible 1666 reference by Marcello Malpighi. [1,2] At the time Hodgkin was museum curator (morbid anatomist) at Guy’s Hospital, London where he studied 7 autopsy cases of people with massive lymph node enlargement and severe symptoms. Interestingly, the 7 were prior patients of other famous English or Scottish physicians including Richard Bright (acute and chronic nephritis), Thomas Addison (adrenal insufficiency and pernicious anaemia) and Robert Carswell (probably multiple sclerosis). Hodgkin’s report, On some morbid appearances of the absorbent glands and spleen, was published in Medical-Chirurgical Society Transactions but went largely unnoticed. Hodgkin is said not to have viewed his contribution as particularly important and certainly did not suggest it be called Hodgkin disease. In 1856, Samuel Wilks, succeeding Hodgkin as museum curator, reported 13 seemingly similar cases. [3] In his initial report Wilks failed to mention Hodgkin. [4] However, Bright advised him of Hodgkin’s work and in a second publication Wilks named the illness Hodgkin’s disease [sic; Hodgkin did not have it]. [5] The name stuck. At that time it was common practice to name a disease after the physician first describing it. Wilks however was less lucky than Hodgkin, Bright and Addison. Although he was the first to describe ulcerative colitis, myasthenia gravis and Korsakoff syndrome, none are named for him showing the importance of engaging a good public relations firm for academic success.
In 1872 and 1876, 40 years after Hodgkin’s observation, Langhans and Greenfield independently described histologic features of Hodgkin lymphoma and in 1898 and 1902 Carl Sternberg and Dorothy Reed independently described the multi-nucleated cell considered a hallmark of Hodgkin lymphoma. [2,6,7]
Why we say Reed-Sternberg rather than Sternberg-Reed cell is unclear and again speaks to the importance of engaging a publicist. Interestingly, Reed was a medical student at Johns Hopkins at the time of her report who as a female had been discouraged from attending medical school by Sir William Osler.
Beginning with Wilks naming the disorder Hodgkin disease, lymphomas have been classified as Hodgkin and non-Hodgkin lymphomas. Why is unclear. The clinical features of Hodgkin’s cases were not unique and most were later determined to not have Hodgkin lymphoma (vide infra). We now know lymphomas are cancers of B- and T- lymphocytes and that Hodgkin lymphoma arises in a B-lymphocyte like other B-lymphocyte lymphomas such as diffuse large B-cell lymphoma (DLBCL), follicular lymphoma, mantle cell lymphoma (MCL), small cell lymphoma (SCL) and others.
These considerations prompt the question whether it is time to reject the dichotomization of lymphomas into Hodgkin and non-Hodgkin lymphomas? Put otherwise, isn’t Hodgkin lymphoma simply a sub-type of non-Hodgkin lymphomas? Using an exclusionary designator for a disease or group of diseases lacks precision and courts confusion. One is reminded acute myeloid leukaemia was once referred to acute non-lymphocytic leukaemia ignoring the dozens of other non-lymphocytic leukaemias. We emphasize our proposal is not to abolish the designator Hodgkin lymphoma as a specific lymphoma sub-type but rather the clinical and biologically irrational dichotomization of lymphomas. Into Hodgkin and non-Hodgkin lymphomas.
But the plot thickens. The 2016 revision of the World Health Organization classification of lymphoid neoplasms divides Hodgkin lymphoma into classical and nodular lymphocyte predominant sub-types. [8] Nodular lymphocyte predominant Hodgkin lymphoma is strongly CD20-positive and behaves more like a typical indolent non-Hodgkin lymphoma including transforming into DLBCL.
Previously some argued Hodgkin lymphoma was really 2 diseases, one, a real cancer with widespread disease, systemic symptoms and a poor survival and the other probably infection-related. Although this notion is wrong one can argue for at least 2 forms of classical Hodgkin lymphoma. Most cases occur in young to middle-aged persons who generally have a favorable prognosis and a seemingly similar lymphoma in older persons with a worse prognosis despite similar therapies.
Can it be reasonably argued therapy of Hodgkin lymphoma with regimens such a ABVD and BEACOPP justifies distinguishing it from all other lymphomas. We think no. For example, the combination of bendamustine and rituximab is widely used to treat mantle cell lymphoma but would be an inappropriate as initial therapy for DLBCL. CHOP-R has remained the therapy choice for most DLBCLs, but EPOCH-R is favored for high-grade B-cell double-hit lymphomas. CHOP-R would be inappropriate for Burkitt lymphoma and classical Hodgkin lymphoma. Consequently, we reject the argument the therapy of Hodgkin lymphoma justifies dichotomization of lymphomas into Hodgkin and non-Hodgkin types.
The practice of dividing lymphomas into Hodgkin and non-Hodgkin lymphomas was useful and important in advancing our understanding of the biology and therapy of lymphomas. However, given knowledge gained since Hodgkin description of his cases it has become clear the distinction between Hodgkin lymphoma and other lymphomas prolongs an artificial clinical and biologic dichotomy. What better support for this opinion is results of a review of tissue specimens from Hodgkin’s 7 cases nearly 100 years later. [9] Hodgkin lymphoma was confirmed in only 3 the other diagnoses included non-Hodgkin lymphoma, tuberculosis and syphilis. Put otherwise, more than one-half of the cases Hodgkin described bearing his name did not have Hodgkin disease. A similar retrospective review of Wilks 13 cases reported Hodgkin lymphoma in only about one-half. [9]
Dividing lymphomas into Hodgkin and non-Hodgkin is a dichotomization, namely a partition of a whole into two parts or categories. These categories must be jointly exhaustive: (1) everything must belong to one category or the other; and (2) mutually exclusive meaning nothing can belong simultaneously to both categories. Dichotiztion reflects a propensity to think in binary terms which our brains favour because it is an efficient way to categorize complex information. Dichotomization allows us to better understand something and to make rapid decision, a process related to thin slicing which we discuss elsewhere. [10] But there are obvious problems with and limitations to dichotomization. First, often something complex does not easily fall into mutually exclusive categories. For example, both Hodgkin lymphoma and non-Hodgkin lymphomas are B-cell cancers and nodular lymphocyte predominant Hodgkin lymphoma frequently transforms into a DLBCL. A study by MacCallum and co-workers tested the tested benefits and detriments of dichotomous thinking in science. [11] They found comparison of the results of analyses before an after dichotomization showed a loss of discrimination. Simply put, when you dichotomize a complex topic, issue or biologic function you lose considerable important information.
Our bottom line is that dividing lymphomas into Hodgkin and non-Hodgkin lymphomas is no longer conceptually useful, makes little biologic sense and results in a loss of valuable information. We suggest lymphomas no longer be dichotomized into Hodgkin and non-Hodgkin lymphomas. The effect is to make Hodgkin lymphoma, seemingly paradoxically, into a non-Hodgkin lymphoma. For those who consider this an oxymoron, a figure of speech juxtaposing concepts with opposing meanings creating an ostensible self-contradiction, we refer to Shakespeare: A rose by any other name would smell as sweet. Were Sir Thomas Hodgkin alive today we suspect he would be turning over in his grave to know haematologists divide lymphomas into Hodgkin and non-Hodgkin lymphomas. Mutatis mutandis.
Lest readers think we are demoting Hodgkin we hasten to point out he was greatly respected as a physician, scientist and teacher. Something never change in Academy and despite his achievements he was not appointed Physician to Guy’s Hospital. Thereafter he devoted his time to philanthropy, denounced conditions in London including poor sanitation, over-crowding, poor air quality and childrens employment as chimney sweeps and in factories and promoted smallpox vaccination. We need him today. Hodgkin was a linguist, ethnologist, anthropologist, geologist, one of the leaders of the Royal Geographical Society and a founder of the University of London. Sir Thomas Hodgkin died of typhus in Yaffo, Israel in 1866 whislt on a humanitarian mission with Sir Moses Montefiore to help the poor of Jerusalem during the Ottoman occupation.
COUNTERPOINT (ESJ)
Hodgkin Lymphomas: Let me count the ways
Armitage and Gale call for the elimination of the term “Non-Hodgkin Lymphoma” in medical practice, rejecting the arbitrary dichotomization of all lymphoma subtypes into these two broad categories as being clinically and biologically irrational. They state, the point is not to eliminate “Hodgkin lymphoma” as a term, but to eliminate non-Hodgkin lymphoma as a category, since in the modern era it is meaningless. I concur in their arguments and conclusions. A PUBMED search of the term “Non-Hodgkin Lymphoma” for 2021 identified nearly 350 articles using the phrase in either the title or keywords. A review of the article contents indicated that in virtually all instances the term was used to encompass various forms of B-cell lymphoma, as distinct from Hodgkin lymphoma, also a B-cell neoplasm. However, in this day greater precision is required, given the huge diversity of lymphoid neoplasms, in both lineage (B-cell, T-cell, NK-cell) and pathobiology. More than 75 different subtypes of lymphoma have been recognized.(1)
Armitage and Gale review for us the history of the term “Hodgkin Disease”. Since its initial description, there were arguments regarding the nature of Hodgkin’s disease. In 1898 Sternberg proposed that it was a chronic inflammatory process related to tuberculosis. However, Dorothy Reed in 1902 argued for its neoplastic nature.(2) She also made several other prescient observations: 1) that the general health of the patient before the onset of disease was usually excellent; 2) that there was an early peak in incidence among children and young adults; 3) there was evidence for anergy or risk of infection; 4) patients had progressive cervical lymph node enlargement without leukemia; and 5) the pathological picture was sufficient for diagnosis. Unfortunately her career in pathology was cut short when she failed to obtain a faculty position at Johns Hopkins.(2) Her departmental chair, William Welch, noted to her that “no women had ever held a teaching position in the school, and there would be great opposition to it”.
The term Hodgkin’s disease officially persisted until 2001, based on uncertainty regarding its etiology and cell of origin. However, in the 1990’s the B-cell origin of Sternberg-Reed cell was proven following single cell microdissection of the diagnostic cells and evidence of monoclonal immunoglobulin gene rearrangement by PCR amplification.(3, 4) Based on these and other data, in 2001 the WHO classification recommended the use of the term “Hodgkin lymphoma”,(5) a proposal that received widespread acceptance and gradual adoption in clinical use, although some habits are hard to break. “Hodgkin’s disease” still crops up in common conversation.
The distinctive biological and clinical features of nodular lymphocyte predominant Hodgkin lymphoma (NLPHL) have been appreciated for many years,(6) and many have suggested that the term “Hodgkin” be excised from the diagnostic term. Arguments can be made on both sides of the issue. CHL and NLPHL share a common cell lineage (B-cell) and both are formed by a minority of neoplastic cells in a rich inflammatory background. However, the strongest argument in favor of a change in nomenclature is that the management and therapeutic options employed for NLPHL are very different from those of CHL, but the use of the common modifier “Hodgkin” in some cases leads to less than optimal clinical care.
In their perspective the authors a hint at a diversity of CHL, but do not spell out the various subtypes and their distinctive features. An argument can be made that even CHL is not a single disease, but likely consists of two or more entities.(7) These constitute 1) nodular sclerosis CHL (NSCHL) and 2) the spectrum encompassing mixed cellularity (MC) and lymphocyte-depleted (LD) CHL. The latter two histological variants represent a continuum, with a diminishing host reaction, a gradual depletion of infiltrating T-cells and a relative increase in tissue-based macrophages. Lymphocyte-rich CHL is still an enigma in terms of its biology and clinical features, and in some cases even shows overlap with NLPHL.(8, 9)
The diversity of CHL was first suggested by MacMahon in 1966(10), when based on epidemiological data he recognized three distinct age peaks. CHL in young children is mainly Epstein-Barr virus (EBV)-positive and largely constitutes the mixed cellularity subtype. A second peak identified CHL in young adults; it largely comprises nodular sclerosis (NS) CHL in young adults (Females> Males) most often presenting with mediastinal masses. It is infrequently positive for EBV. The third peak is CHL in the elderly or immune suppressed. This consists of the MC and LD subtypes, and is nearly always positive for EBV.(7) (Table 1)
Table 1:
Clinical features of the most common Hodgkin lymphoma subtypes
| Main Features | Nodular Sclerosis | MC/LD | Nodular LP |
|---|---|---|---|
| Risk factors | High socio-economic status | Lower socio-economic status, HIV+, immune suppression Developing world |
No risk factors |
| Demographics | F> M, young adults | M > F, children or elderly 2 peaks |
M> F, Young adult May occur in children |
| Clinical presentation | Mediastinal, axial lymph nodes May have thymic involvement |
Generalized lymphadenopathy | Peripheral lymph nodes Peri-Parotid, Axillary Mesenteric (infrequent) |
| Histological progression | May progress to PMBL | Uncommon progression to other B-cell lymphomas | May progress to DLBCL, T-cell/histiocyte-rich large B-cell lymphoma |
| EBV (malignant cells) | EBV-negative (< 25% +) | EBV positive (75% +) | EBV negative (<5% +) |
Abbreviations: MC, mixed cellularity; LD, lymphocyte depleted; LP, lymphocyte predominant; PMBL, primary mediastinal large B-cell lymphoma; DLBCL, diffuse large B-cell lymphoma
In recent years NSCHL has emerged as an entity distinct from other forms of CHL. It shares many common features with primary mediastinal (thymic) large B-cell lymphoma (PMBL). Both neoplasms are common in young women, exhibit common cytogenetic and molecular alterations, and share a similar gene expression profile.(11, 12) Both are thought to arise from a thymic B-cell. NSCHL and PMBL can occur sequentially in the same patient, can present as “composite” lymphoma at a single point in time, or in some cases as “mediastinal grey zone lymphoma”, exhibiting a hybrid morphology and phenotype that is a hodgepodge of both lymphoma variants.
Unfortunately, the scientific literature exploring the pathogenetic basis of CHL often fails to specify either the specific subtype of CHL, or the presence or absence of EBV. This fact has impaired our opportunity to advance knowledge regarding the spectrum of lymphoma subtypes that constitute CHL.
Figure 1.
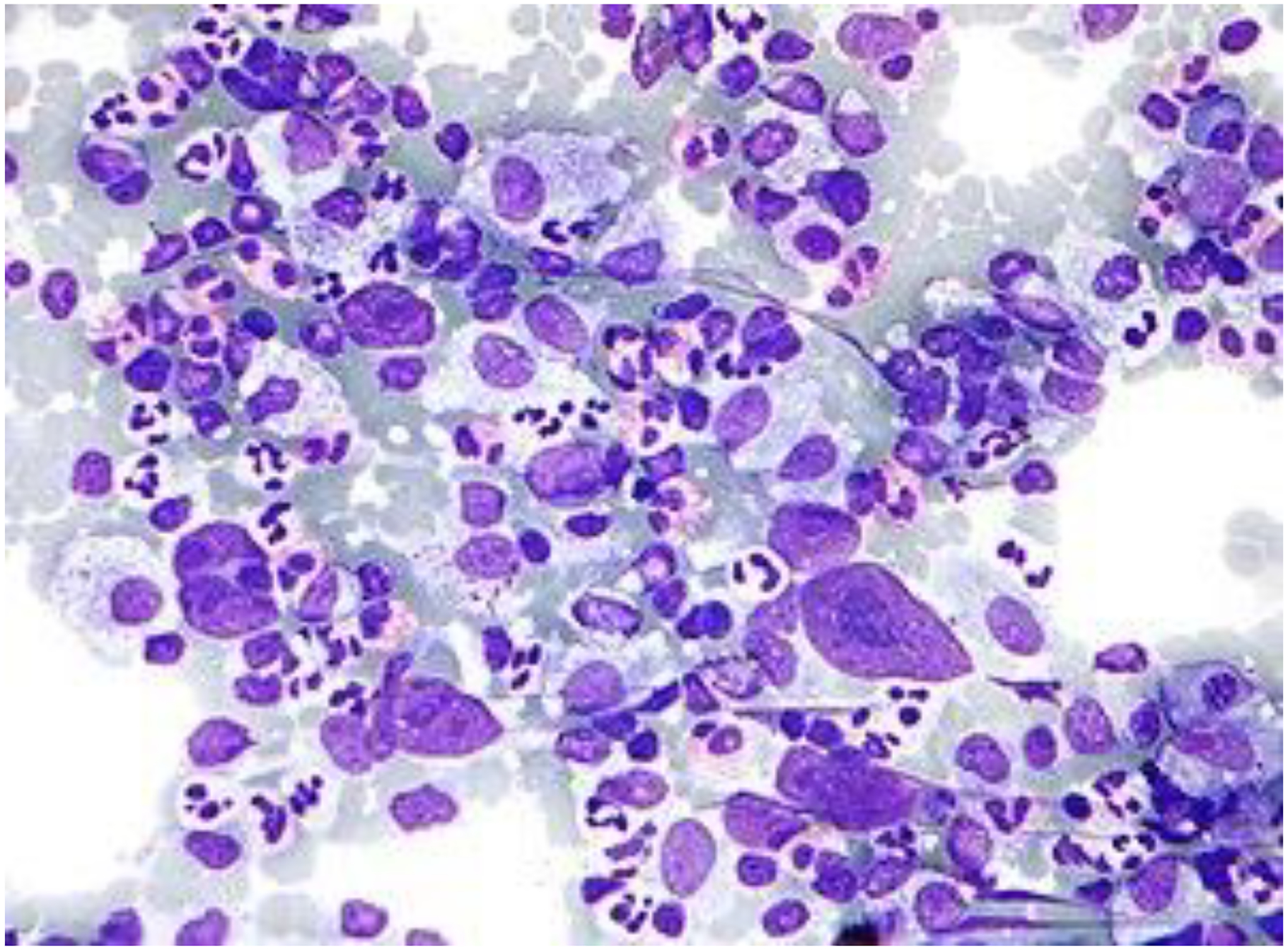
Hodgkin lymphoma.
Figure 2,

Hodgkin’s grave in Yaffo, Israel. Monument erected by the Israel Haematology Society.
Acknowledgement [Point]
RPG acknowledges support from the National Institute of Health Research (NIHR) Biomedical Research Centre funding scheme.
Acknowledgements [Counter-Point]
This work was supported by the Intramural Research Budget of the Center for Cancer Research, NCI, NIH. The author has no disclosures. No Conflict of interest to report
Footnotes
Conflict-of-Interest
RPG is a consultant to BeiGene Ltd., Fusion Pharma LLC, LaJolla NanoMedical Inc., Mingsight Pharmaceuticals Inc. CStone Pharmaceuticals, NexImmune Inc. and Prolacta Bioscience; advisor to Antengene Biotech LLC, Medical Director, FFF Enterprises Inc.; partner, AZAC Inc.; Board of Directors, Russian Foundation for Cancer Research Support; and Scientific Advisory Board: StemRad Ltd.
References [Point]
- 1.Hodgkin. On some Morbid Appearances of the Absorbent Glands and Spleen. Med Chir Trans. 1832;17:68–114. doi: 10.1177/095952873201700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hellman S. A brief consideration of Thomas Hodgkin and his times. Hodgkin’s Disease. Philadelphia: Lippincott, Williams & Wilkins. 1999:3–7. [Google Scholar]
- 3.Geller SA. Comments on the anniversary of the description of Hodgkin’s disease. J Natl Med Assoc. 1984. Aug;76(8):815–7. [PMC free article] [PubMed] [Google Scholar]
- 4.Wilks S. Cases of lardaceous disease. Guy’s Hosp Rep 3rd Series. 1856;2:103–32. [Google Scholar]
- 5.Wilks S. Cases of enlargement of the lymphatic glands and spleen (or, Hodgkin’s disease) with remarks. Guy’s Hosp Rep (3rd series) 1865; 11:56–67. [Google Scholar]
- 6.Sternberg C. Ueber eine eigenartige unter dem Bilde der pseudoleukemic verlaufende Tuberkulose des lymphatischen Apparatus. Ztschr f. Heilk. 1898;19:21–90. [Google Scholar]
- 7.Reed DM. On the pathological changes in Hodgkin’s disease with special reference to its relation to tuberculosis. Johns Hopkins Hosp Rep. 1902;10:133–98. [Google Scholar]
- 8.Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016. May 19;127(20):2375–90. doi: 10.1182/blood-2016-01-643569. Epub 2016 Mar 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox H Remarks on the Presentation of Microscopical Preparations Made from Some of the Original Tissue Described by Thomas Hodgkin, 1832. Ann Med Hist. 1926. Winter;8(4):370–374. [PMC free article] [PubMed] [Google Scholar]
- 10.Gale RP. Being certain even when you’re wrong: heuristics and thin slicing in haematopoietic cell transplantation. Bone Marrow Transplant. 2021. Jun;56(6):1223–1226. doi: 10.1038/s41409-020-01167-9. Epub 2020 Dec 8. [DOI] [PubMed] [Google Scholar]
- 11.MacCallum RC, Zhang S, Preacher KJ, Rucker DD. On the practice of dichotomization of quantitative variables. Psychol Methods. 2002. Mar;7(1):19–40. doi: 10.1037/1082-989x.7.1.19. [DOI] [PubMed] [Google Scholar]
References [Counter-Point]
- 1.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. , editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues Revised 4th Edition ed. Lyon, France: International Agency for Research on Cancer (IARC) 2017. [Google Scholar]
- 2.Dawson PJ. Whatever happened to Dorothy Reed? Ann Diagn Pathol. 2003;7(3):195–203. [DOI] [PubMed] [Google Scholar]
- 3.Kuppers R, Rajewsky K, Zhao M, Simons G, Laumann R, Fischer R, et al. Hodgkin disease: Hodgkin and Reed-Sternberg cells picked from histological sections show clonal immunoglobulin gene rearrangements and appear to be derived from B cells at various stages of development. Proc Natl Acad Sci U S A. 1994;91(23):10962–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamaru J, Hummel M, Zemlin M, Kalvelage B, Stein H. Hodgkin’s disease with a B-cell phenotype often shows a VDJ rearrangement and somatic mutations in the VH genes. Blood. 1994;84(3):708–15. [PubMed] [Google Scholar]
- 5.Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mason D, Banks P, Chan J, Cleary M, Delsol G, de Wolf-Peeters C, et al. Nodular lymphocyte predominance Hodgkin’s disease: a distinct clinico-pathological entity. Am J Surg Pathol. 1994;18:528–30. [DOI] [PubMed] [Google Scholar]
- 7.Wang HW, Balakrishna JP, Pittaluga S, Jaffe ES. Diagnosis of Hodgkin lymphoma in the modern era. Br J Haematol. 2019;184(1):45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anagnostopoulos I, Hansmann ML, Franssila K, Harris M, Harris NL, Jaffe ES, et al. European Task Force on Lymphoma project on lymphocyte predominance Hodgkin disease: histologic and immunohistologic analysis of submitted cases reveals 2 types of Hodgkin disease with a nodular growth pattern and abundant lymphocytes. Blood. 2000;96(5):1889–99. [PubMed] [Google Scholar]
- 9.Nam-Cha SH, Montes-Moreno S, Salcedo MT, Sanjuan J, Garcia JF, Piris MA. Lymphocyte-rich classical Hodgkin’s lymphoma: distinctive tumor and microenvironment markers. Mod Pathol. 2009;22(8):1006–15. [DOI] [PubMed] [Google Scholar]
- 10.MacMahon B Epidemiology of Hodgkin’s disease. Cancer Res. 1966;26(6):1189–201. [PubMed] [Google Scholar]
- 11.Traverse-Glehen A, Pittaluga S, Gaulard P, Sorbara L, Alonso MA, Raffeld M, et al. Mediastinal Gray Zone Lymphoma: The Missing Link Between Classic Hodgkin’s Lymphoma and Mediastinal Large B-Cell Lymphoma. Am J Surg Pathol. 2005;29(11):1411–21. [DOI] [PubMed] [Google Scholar]
- 12.Pittaluga S, Nicolae A, Wright GW, Melani C, Roschewski M, Steinberg S, et al. Gene Expression Profiling of Mediastinal Gray Zone Lymphoma and Its Relationship to Primary Mediastinal B-cell Lymphoma and Classical Hodgkin Lymphoma. Blood Cancer Discov. 2020;1(2):155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]


