Abstract
Objectives:
To test whether contractile mechanics in ischemic myocardium underlying the mitral valve impact likelihood of functional mitral regurgitation (FMR).
Background:
LV ischemia has been variably associated with FMR; Determinants of FMR in patients with ischemia are poorly understood.
Methods:
Vasodilator stress perfusion CMR was performed in multicenter CAD patients. FMR severity was confirmed quantitatively via core lab analysis. To test relationship of contractile mechanics with ischemic FMR, regional wall motion and strain were assessed in patients with inducible ischemia and minimal (≤5% LV myocardium, non-transmural) infarction.
Results:
2,647 CAD patients were studied; 34% had FMR (7% ≥moderate). FMR severity increased with presence (p<0.001) and extent (p=0.01) of sub-papillary ischemia: ≥Moderate FMR patients had more sub-papillary ischemia (OR=1.13 per 10% LV [1.05–1.21], p=0.001) independent of ischemia in remote regions (p=NS): ≥Moderate FMR prevalence increased stepwise with extent of ischemia and infarction in sub-papillary myocardium (p<0.001); stronger associations between FMR and infarction paralleled greater wall motion scores in infarct-affected territories. Among patients with inducible ischemia and minimal infarction (n=532), wall motion and radial strain analysis showed impaired sub-papillary contractile mechanics to associate with ≥moderate FMR (p<0.05) independent of remote regions (p=NS). Conversely, sub-papillary ischemia without contractile dysfunction did not augment FMR likelihood. Mitral and inter-papillary dimensions increased with sub-papillary radial strain impairment; each remodeling parameter associated with impaired sub-papillary strain (p<0.05) independent of remote strain (p=NS). Sub-papillary radial strain (OR=1.13 per 5% [CI=1.02–1.25], p=0.02) and mitral tenting area (OR=1.05 per 10mm2 [CI=1.00–1.10], p=0.04) associated with ≥moderate FMR controlling for global remodeling represented by LV end-systolic volume (p=NS): When substituting sphericity for LV volume, ≥moderate FMR remained independently associated with sub-papillary radial strain impairment (OR=1.22 per 5% [1.02–1.47], p=0.03).
Conclusions:
Among CAD patients with ischemia, FMR severity and adverse mitral apparatus remodeling increase in proportion to contractile dysfunction underlying the mitral valve.
Keywords: mitral regurgitation, cardiovascular magnetic resonance, ischemia
Introduction
Functional mitral regurgitation (FMR) commonly occurs in patients with coronary artery disease (CAD), affecting up to 3 million patients in the United States.(1) Whereas recent studies by our group and others have shown FMR to be associated with ischemic myocardium underlying the mitral valve,(2,3) these data also demonstrate that many CAD patients with ischemia in predisposing regions have minimal or no FMR. Experimental animal studies have similarly reported ischemia to be variably associated with FMR: Some have shown coronary occlusion to acutely produce FMR,(4,5) but others have not.(6) Knowledge gaps concerning links between ischemia and FMR are clinically relevant, given current limits in the ability to identify patients in whom FMR is most likely to improve after revascularization.(7) For example, studies testing impact of revascularization on FMR have yielded mixed results, with moderate or greater FMR persisting in nearly a third of CAD patients following coronary artery bypass surgery. Left untreated, FMR provides a nidus for heart failure, arrhythmia, and death.(8) In this context, further research is critical to elucidate mechanisms by which ischemia contributes to FMR, so as to better identify which CAD patients are at greatest risk for FMR and inform use of tailored therapies to address underlying causality.
Vasodilator stress perfusion cardiac magnetic resonance (CMR) provides a unique tool to differentiate ischemia from infarction, together with functional (cine) imaging with which to assess baseline contractile performance in regions with and without inducible ischemia. Advances in post-processing technology allow new insights into impact of ischemia on left ventricular (LV) function: Myocardial strain quantified via cine-CMR feature tracking has been shown capable of discerning subtle changes in contractile mechanics,(9) and to date has yet to be tested as a marker for FMR in patients with ischemia.
This study encompassed a multicenter cohort of CAD patients from the Society for Cardiovascular Magnetic Resonance (SCMR) registry undergoing vasodilator stress CMR. To systematically test myocardial contractile mechanics as a link between ischemia and FMR, regional wall motion pattern was assessed in the overall cohort and myocardial strain was quantified in sub-papillary and remote regions among patients with ischemia but minimal infarction. The goal was to test the hypothesis that contractile dysfunction in ischemic myocardium underlying the mitral valve associates with FMR independent of remote contractile function and global LV remodeling.
Methods
Study Population
The population comprised adults (≥18 years old) with clinically established CAD undergoing stress perfusion CMR at seven U.S. medical centers between May 2005 and October 2018. Patients with primary mitral pathologies (prolapse, rheumatic valve, leaflet perforation), mitral repair/replacement, or CMR-verified conditions (infiltrative/hypertrophic cardiomyopathy, congenital heart disease) that could confound impact of ischemia on LV remodeling were excluded. For patients with multiple exams, the initial CMR was used for analysis. No exams were excluded based on image quality/factors unrelated to study eligibility criteria. Data on atrial fibrillation and laboratory indices including renal function (e.g. glomerular filtration rate) were unavailable. This research was conducted using the SCMR Registry, for which details have previously been reported.(2,10) Institutional review board approval for use of data in this study was obtained at each participatory site.
De-identified CMR images were queried from the registry database and reviewed by a central core lab (Cornell) to exclude degenerative pathology and identify ≥moderate FMR using an established algorithm:(2) In brief, ≥moderate FMR was first identified based on established semi-quantitative criteria (jet >1/3 left atrial [LA] area in ≥2 long axis orientations),(11) and confirmed quantitatively (regurgitant volume ≥30ml, regurgitant fraction ≥30%, or anatomic regurgitant orifice area ≥0.2cm2).(2,12,13) Regurgitant volume and fraction were measured via differential aortic forward/LV stroke volume when aortic phase contrast imaging was available, or via differential right ventricular/LV stroke volume when ≥moderate tricuspid or aortic regurgitation were absent: Prior research has validated this multiparametric approach(2). In the current study, ≥moderate FMR was confirmed via differential aortic forward flow (phase contrast) to LV stroke volume in 30% (n=58), differential LV/RV stroke volume (cine-CMR) in 52% (n=102), and/or anatomic regurgitant orifice area in 18% (n=35) of cases.
Image Acquisition
Cine-CMR was acquired under baseline (non-stress) conditions using a steady-state free precession pulse sequence acquired in contiguous LV short, and (2-, 3-, 4-chamber) long axis orientations. Stress perfusion CMR utilized adenosine or regadenoson vasodilators concordant with established methods.(10,14) First-pass perfusion was assessed during gadolinium infusion (~4–5 short-axis images per heartbeat): Imaging was repeated 10-minutes thereafter or a second gadolinium dose was administered without imaging (total 0.13–0.20 mmol/kg). Late gadolinium enhancement (LGE)-CMR was acquired in orientations matched to cine-CMR. Phase contrast CMR was used to assess valvular flow at discretion of participatory sites.
Image Analysis
LV Geometry and Tissue Characterization
LV function and size were quantified volumetrically on cine-CMR; regional function was graded using a segmental scoring system (0=normal; 1=mild/moderate hypokinesia; 2=severe hypokinesia; 3=akinesia; 4=dyskinesia).(15) LV chamber wall ischemia was scored on perfusion CMR in a binary manner (present/absent) on a per-segment basis, as was papillary muscle ischemia (assessed using an established approach for which reproducibility has been reported).(2) Concordant with standard criteria,(2,10,14) perfusion defects were considered indicative of ischemia in absence of, or if larger than, LGE. Myocardial infarction was assessed on LGE-CMR using validated methods,(10,16,17) including segmental transmurality (0=no hyperenhancement; 1=1–25%; 2=26–50%; 3=51–75%; 4=76–100%) and global infarct size (% LV).
LV ischemia and infarction were analyzed in relation to geometric proximity to the mitral valve: An established algorithm(2,18) was used to partition segments into sub-papillary (basal-mid inferior/inferolateral | basal-mid anterior/anterolateral) and remote non-papillary (septum or distal LV) territories.
Mitral Apparatus Remodeling and Strain
To mitigate the confounding impact of concomitant infarction on the relationship between ischemia and FMR, tailored analyses were performed in all patients with inducible ischemia and minimal infarct size (≤5% LV myocardium, no transmural involvement). To assure minimal infarct size, global infarct size (initially assessed using the above scoring algorithm) was measured quantitatively; regions of visually scored hyperenhancement were measured via the full-width half maximum method as has been shown to yield good reproducibility in prior research by our group.(19) Myocardial strain was quantified in all such patients using a feature tracking algorithm(20) adapted for purposes of registry software integration. Seed points were placed around LV endocardial and epicardial borders and tracked throughout the cardiac cycle; automated outputs were manually adjusted to optimize border tracking.(21) Radial and circumferential strain analyses were performed using short axis cine-CMR datasets equidistant (base, mid, distal) throughout the LV; strain rate was obtained by indexing strain values to time to peak strain from end-diastole. To test reproducibility of strain and conventional wall motion scores, blinded repeat analyses were performed in a subgroup of 30 random patients. Within the overall population of patients with ischemia and minimal (≤5% LV) infarction, a nested case:control cohort of patients with and without ≥moderate FMR was matched (1:2) on ischemia extent and distribution, so as to further test ischemic myocardial contractile mechanics in FMR. Additional analyses in this matched cohort included quantification of longitudinal strain and mitral apparatus geometry. Longitudinal strain analyses were performed on long axis (2-, 3-, 4-chamber) datasets using methods detailed above. Mitral annular diameter, tenting height, and area were quantified at LV end-diastole in long-axis (3-chamber); inter-papillary diameters were measured in mid LV short axis concordant with established methods.(2)
Statistical Analysis
Continuous variables are summarized as means ± standard deviations when normally distributed, and otherwise as medians [interquartile range]. Categorical variables are summarized as frequencies and percents. Normally distributed continuous indices were compared via Student’s t-tests (two group comparisons) or analysis of variances with linear test of trends (multiple group comparisons); non-normally distributed indices were compared via the Mann-Whitney U test. Categorical variables were compared using Chi-square tests. Logistic regression was used to test associations between imaging parameters and FMR in the overall population; conditional logistic regression was used to evaluate univariable and multivariable associations in the matched nested case-control cohort. Linear regression was used to evaluate associations between contractile dysfunction and mitral apparatus remodeling. Multivariable models presented in text or tabular format specify all variables included in the model. Inter- and intra-observer agreement was assessed using Bland-Altman methods (including mean difference and limits of agreement) as well as intra-class correlation coefficients. A 2-sided p<0.05 was considered statistically significant. Statistical calculations were performed using SPSS version 25.0 (SPSS, Chicago IL).
Results
Population Characteristics
2,647 patients with clinically reported CAD undergoing stress perfusion CMR were studied, among whom FMR was present in 34% (899) - including 7% (195) with ≥moderate FMR (mean regurgitant fraction 39.5±10.0%, regurgitant volume 38.8±10.9ml, anatomic regurgitant orifice area 0.35±0.21cm2 [as quantified by each respective criterion]). Table 1 reports on population characteristics, including comparisons based on presence and severity of FMR. As shown, patients with FMR were older, more likely to report dyspnea, and to be treated with heart failure medications (aldosterone antagonists or loop diuretics) at time of CMR (all p≤0.001).
Table 1.
Population Characteristics
| Mitral Regurgitation | |||||
|---|---|---|---|---|---|
| Overall (n=2647) |
None (n=1748) |
Mild (n=704) |
≥Moderate (n=195) |
p | |
| CLINICAL | |||||
| Age (year) | 64.3 ± 11.7 | 63.3 ± 11.6 | 66.2 ± 11.5 | 67.0 ± 12.0 | |
| 0.001 | |||||
| Sex | <0.01 | ||||
| Male | 66% (1743) | 66% (1161) | 68% (477) | 55% (108) | |
| Female | 45% (896) | 34% (587) | 32% (227) | 45% (87) | |
| BSA (m2) | 1.82 ±0.22 | 1.83 ± 0.22 | 1.81 ± 0.23 | 1.73 ± 9.23 | <0.001 |
| Pharmacologic Stress | |||||
| Protocol | |||||
| Adenosine | 33% (876) | 34% (599) | 34% (237) | 22% (42) | |
| Regadenoson | 67% (1762) | 66% (1149) | 66% (467) | 78% (153) | <0.01 |
| Atherosclerosis Risk Factors | |||||
| Hypertension | 82% (2150) | 81% (1415) | 82% (580) | 83% (161) | 0.65 |
| Hypercholesterolemia | 78% (2064) | 78% (1367) | 80% (560) | 74% (144) | 0.23 |
| Diabetes Mellitus | 37% (978) | 36% (635) | 39% (275) | 37% (72) | 0.45 |
| Current or prior tobacco use | 40% (1055) | 40% (700) | 41% (288) | 35% (69) | 0.37 |
| Family history | 39% (1025) | 40% (692) | 39% (275) | 31% (60) | 0.02 |
| Prior myocardial infarction | 63% (1666) | 62% (1081) | 65% (457) | 66% (128) | 0.26 |
| Prior revascularization | |||||
| Percutaneous coronary intervention | 22% (588) | 24% (427) | 22% (132) | 15% (29) | <0.001 |
| Coronary artery bypass graft | 8% (214) | 8% (140) | 7% (49) | 13% (25) | 0.03 |
| Symptoms | |||||
| Chest pain | 52% (1376) | 55% (961) | 47% (329) | 47% (92) | <0.001 |
| Dyspnea | 30% (800) | 29% (502) | 31% (219) | 42% (82) | 0.001 |
| Palpitations | 3% (65) | 3% (45) | 2% (16) | 2% (4) | 0.85 |
| Cardiovascular Medications | |||||
| Aspirin | 82% (2155) | 83% (1443) | 81% (571) | 75% (147) | 0.04 |
| HMG CoA-Reductase inhibitor | 75% (1980) | 75% (1309) | 78% (546) | 67% (131) | 0.01 |
| Beta blocker | 53% (1393) | 51% (890) | 58% (405) | 51% (100) | 0.01 |
| ACE inhibitor/ARB | 61% (1615) | 61% (1061) | 62% (439) | 62% (120) | 0.74 |
| Aldosterone antagonist | 6% (152) | 4% (71) | 7% (51) | 15% (30) | <0.001 |
| Loop diuretic | 35% (919) | 32% (560) | 37% (259) | 53% (103) | <0.001 |
| CARDIAC MAGNETIC RESONANCE | |||||
| Left Ventricle Function/Geometry | |||||
| End-diastolic volume (ml/m2) | 87.9 ±34.9 | 80.5 ±29.7 | 95.1 ±34.3 | 129.6 ± 45.6 | <0.001 |
| End-systolic volume (ml/m2) | 45.7 ±32.7 | 38.6 ±26.4 | 52.6 ±33.8 | 86.2 ± 44.6 | <0.001 |
| Ejection fraction (%) | 51.8 ± 15.3 | 54.7 ± 13.9 | 48.6 ± 15.9 | 37.1 ± 14.7 | <0.001 |
| Left ventricular sphericity | 0.43 ± 0.22 | 0.42 ± 2.43 | 0.45 ±0.16 | 0.51 ±0.12 | <0.001 |
| Left atrial geometry | |||||
| Left atrial diameter (cm) | 3.7 ±0.8 | 3.5 ±0.7 | 3.8 ±0.7 | 4.5 ±0.7 | <0.001 |
| Left atrial area (cm2) | 23.1 ±6.8 | 21.5 ±5.7 | 25.1 ±6.8 | 30.6 ±8.1 | <0.001 |
| Left Ventricular Ischemia | |||||
| Sub-papillary | |||||
| Present/absent | 38% (1010) | 34% (586) | 46% (324) | 51% (100) | <0.001 |
| Ischemia extent when present (% LV) | 25 [25, 50] | 25 [25, 50] | 25 [25, 50] | 37.5 [25, 62.5] | 0.01 |
| Non-papillary | |||||
| Present/absent | 37% (968) | 32% (559) | 46% (320) | 50% (97) | <0.001 |
| Ischemia extent when present (% LV) | 37.5 [12.5, 50] | 37.5 [12.5, 50] | 37.5 [25, 59.4] | 37.5 [25, 50] | 0.03 |
| Left Ventricular Infarction | |||||
| Sub-papillary | |||||
| Present/Absent | 56% (1472) | 50% (874) | 65% (456) | 74% (145) | <0.001 |
| Infarct extent when present (% LV) | 9.4 [4.7, 20.3] | 9.4 [4.7, 18.7] | 10.9 [4.7, 21.9] | 15.6 [6.2, 26.6] | <0.001 |
| Non-papillary | |||||
| Present/absent | 63% (1673) | 58% (1016) | 71% (503) | 81% (158) | <0.001 |
| Infarct extent when present (% LV) | 9.4 [4.7, 20.3] | 9.4 [4.3, 20.3] | 10.9 [4.7, 21.91 | 12.5 [6.2, 21.91 | 0.02 |
LV Ischemia Pattern and FMR
Myocardial tissue properties in LV myocardium subtending the mitral valve, as well as in remote regions, are also detailed in Table 1: As shown, prevalence and extent of ischemia and infarction in both sub-papillary and remote (non-papillary muscle adjacent) myocardium increased in proportion to presence and magnitude of FMR (p<0.05), paralleled by decrements in LVEF (p<0.001). LV basal aneurysms were of similarly low prevalence among patients with (1.0%), and those without, ≥moderate FMR (0.8%; p=0.67).
Figure 1 provides analyses further testing associations of FMR with regional ischemia and infarction. As shown in Figure 1A, sub-papillary ischemia (OR 1.13 per 10% LV [1.05–1.21], p=0.001) was associated with ≥moderate FMR controlling for ischemia extent in remote regions (p=NS), as was sub-papillary infarction (OR 1.40 per 10% LV [1.26–1.56], p<0.001). Figure 1B demonstrates that sub-papillary infarct size and ischemia extent were synergistically associated with ≥moderate FMR, as evidenced by increased prevalence of ≥moderate MR in patients in whom both tissue abnormalities were most extensive. Data shown in Figure 1B also illustrates that for each partition, ≥moderate FMR was more common among patients with sub-papillary infarction than patients with corresponding extent of sub-papillary ischemia. Consistent with this, whereas multivariable regression showed both to be independently associated with ≥moderate FMR (both p<0.001), stronger associations were evident for sub-papillary infarction (OR 1.44 per 10% LV [1.30–1.59]) than ischemia (OR 1.13 per 10% LV [1.07–1.20]).
Figure 1. Mitral Apparatus Ischemia and Infarction in Relation to ≥Moderate FMR.
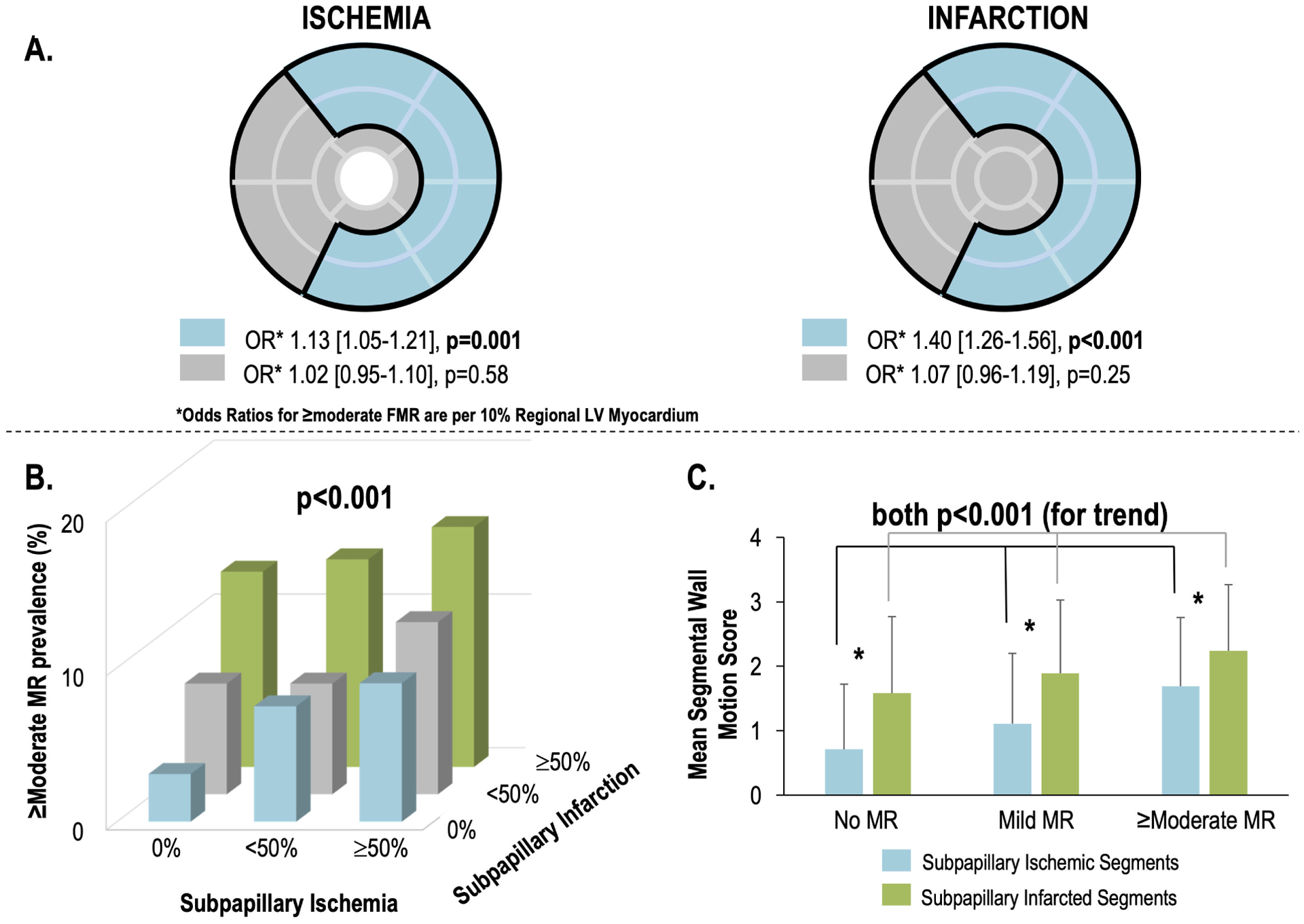
1A. Bullseye plot (17-segment model) illustrating LV myocardium subtended within the mitral apparatus, which was defined (concordant with an established algorithm)(2,18) as encompassing segments adjacent to the anterolateral and posteromedial papillary muscles (blue denotes sub-papillary regions [basal-mid anterior/anterolateral, inferior/inferolateral walls], grey denotes remote LV regions).
Note that sub-papillary ischemia (left) was associated with ≥moderate FMR (p=0.001) controlling for ischemia in remote regions (p=NS). Similarly sub-papillary infarction (right) was associated with ≥moderate FMR (p<0.001) controlling for infarction in remote regions (p=NS).
1B. Prevalence of ≥moderate FMR in relation to strata of sub-papillary ischemia (absent, <50%, ≥50% of segments with stress perfusion deficits) and infarction (absent, <50%, ≥50% of segments with late gadolinium enhancement). As shown, ≥moderate FMR prevalence increased in relation to extent of each sub-papillary tissue alteration (p<0.001 for logistic regression model).
1C. Sub-papillary mean wall motion scores (mean ± standard deviation) among ischemic (blue) and infarcted segments in patients stratified based on presence and severity of FMR (data reported as mean ± standard deviation across all LV sub-papillary segments as illustrated in 1A). Note that for both ischemia and infarction, mean sub-papillary wall motion scores increased in proportion to FMR severity (both p<0.001 for trend), but that contractile dysfunction as discerned by wall motion score was greater for infarcted compared to ischemic segments within each FMR stratum (asterisk refers to p<0.001 for each comparison). All analyses performed in full study cohort (n=2647).
FMR in Relation to Sub-Papillary Contractile Function
Wall motion in the overall population was first analyzed to explore if associations between FMR and ischemic myocardium varied in relation to systolic dysfunction, and if dysfunction similarly modified associations between FMR and sub-papillary infarction. Among patients with ≥moderate FMR, sub-papillary wall motion score was higher than non-papillary wall motion score (10.4±6.7 vs. 7.7±5.5, p<0.001). As shown in Figure 1C, among segments with sub-papillary ischemia, wall motion scores increased stepwise among patient groups partitioned by FMR grade: Similar stepwise increases in wall motion scores were evident when segments with sub-papillary infarction were compared between patients with and without FMR (both p<0.001). Paralleling above regression analyses showing FMR to be more strongly associated with sub-papillary infarction than ischemia, analyses shown in Figure 1C demonstrate that for all FMR strata, wall motion scores were higher in segments with infarction compared to those with ischemia (p<0.001).
Regression analyses provided further evidence that contractile function modified associations between sub-papillary ischemia and FMR: Patients with dysfunctional and ischemic sub-papillary myocardium were more likely to manifest ≥moderate FMR (OR 1.30 per 10% LV [1.22–1.38], p<0.001). Conversely, patients with inducible sub-papillary ischemia but preserved contractile function on cine-CMR (wall motion score = 0) were less likely to have ≥moderate FMR (OR 0.27 [0.13–0.59], p<0.001), as were patients with preserved sub-papillary contractile function and no ischemia (OR 0.18 [0.11–0.29], p<0.001). Similar associations were evident with respect to contractile function and infarction: Dysfunctional and infarcted sub-papillary myocardium was associated with ≥moderate FMR (OR 1.26 per 10% LV [1.20–1.32], p<0.001), whereas patients with infarction in sub-papillary regions but preserved contractile function on cine-CMR were less likely to have ≥moderate FMR (OR 0.43 [0.21–0.88], p=0.02), as were patients with preserved sub-papillary contractile function and no infarction (OR 0.15 [0.09–0.25], p<0.001).
Ischemic Myocardial Contractile Pattern and FMR
To explore links between contractile function and FMR in patients with ischemia while limiting the confounding impact of infarction, regional wall motion and radial strain were analyzed in all (n=532) patients with inducible ischemia on stress CMR in whom infarction was minimal (≤5% myocardium, non-transmural). Supplemental Figure 1 illustrates reproducibility of strain analyses. As shown, CMR derived radial strain analyses yielded similarly good reproducibility to circumferential strain, as evidenced by inter-reader data yielding near-equivalent intra-class correlations (e.g. global radial strain: 0.94 [0.89–0.97] | global circumferential strain: 0.92 [0.84–0.96], and mean differences (global radial strain Δ −0.4% [LOA −16.8%, 16.1%], global circumferential strain Δ 0.5% [LOA −7.4%, 8.4%]), paralleling intra-reader reproducibility (radial ICC 0.89 [0.80–0.95] / Δ −3.7% [LOA −26.2%, 18.8%] circumferential ICC 0.93 [0.85–0.97] / Δ 1.2% [LOA −6.3%, 8.7%]). Regarding wall motion scores, intra-class correlations were similar for inter-reader (0.88 [0.76–0.94]) and intra-reader (0.93 [0.85–0.96]) assessments. Table 2A details global and regional wall motion scores stratified by FMR grade. As shown, global and regional contractile dysfunction (assessed by wall motion and strain) increased stepwise with severity of FMR (p<0.001). Multivariable analyses shown in Tables 2B and 2C demonstrate that sub-papillary wall motion score and radial strain respectively were associated with both ≥moderate and mild FMR (p<0.05) independent of wall motion scores or radial strain in remote regions. Notably, associations between sub-papillary radial strain and ≥moderate FMR were consistent irrespective of method used to confirm ≥moderate FMR, as evidenced by similar odds ratios among patients in whom ≥moderate FMR was confirmed based on phase contrast/cine-CMR differential LV stroke volume (OR 1.29 per 5% strain decrement [1.14–1.47] p<0.001), cine-CMR derived differential LV/RV stroke volume (OR 1.28 per 5% strain decrement [1.13–1.42] p<0.001), and cine-CMR derived anatomic regurgitant orifice area (OR 1.25 per 5% strain decrement [1.01–1.54] p=0.04).
Table 2.
Mitral Regurgitation in Relation to Sub-papillary and Remote LV Contractile Function Among Patients with Ischemia
| Mitral Regurgitation | |||||
|---|---|---|---|---|---|
| Overall (n=532) |
None (n=348) |
Mild (n=147) |
≥Moderate (n=37) |
p | |
| Wall Motion Score | |||||
| Global | 4.5 ± 8.3 | 2.9 ± 6.5 | 6.1 ± 9.4 | 12.9 ± 12.4 | <0.001 |
| Sub-papillary | 2.5 ± 4.5 | 1.6 ± 3.5 | 3.4 ± 5.1 | 7.1 ± 6.4 | <0.001 |
| Non-papillary | 1.8 ± 3.5 | 1.2 ± 2.8 | 2.4 ± 4.0 | 4.9 ± 5.6 | <0.001 |
| Number of Dysfunctional Segments | |||||
| Global | 3.0 ± 5.0 | 2.1 ± 4.1 | 4.3 ± 5.8 | 7.4 ± 6.1 | <0.001 |
| Sub-papillary | 1.6 ± 2.5 | 1.0 ± 2.1 | 2.2 ± 2.9 | 4.0 ± 3.1 | <0.001 |
| Non-papillary | 1.5 ± 2.6 | 1.0 ± 2.2 | 2.1 ± 3.1 | 3.5 ± 3.3 | <0.001 |
| Radial Strain | |||||
| Global | 61.2 ± 24.4% | 66.0 ± 22.0% | 56.5 ±25.4% | 36.7 ± 23.1% | <0.001 |
| Sub-papillary | 62.1 ± 27.0% | 66.9 ± 24.7% | 57.8 ± 28.3% | 35.9 ± 24.6% | <0.001 |
| Non-papillary | 60.3 ± 24.9% | 65.2 ± 22.8% | 55.1 ± 25.4% | 37.5 ± 24.7% | <0.001 |
| FMR severity | Region | Multivariable OR | 95% CI | p | |
|---|---|---|---|---|---|
| Wall Motion Score | Sub-papillary | 1.24 | [1.09, 1.41] | 0.001 | |
| Non-papillary | 0.97 | [0.82, 1.14] | 0.69 | ||
| Wall Motion Score | Sub-papillary | 1.11 | [1.01, 1.21] | 0.03 | |
| Non-papillary | 1.00 | [0.89, 1.12] | 0.93 | ||
| FMR severity | Region | Multivariable OR | 95% CI | p | |
|---|---|---|---|---|---|
| Radial Strain (per 5% decrement) | Sub-papillary | 1.22 | [1.07, 1.38] | <0.01 | |
| Non-papillary | 1.12 | [0.99, 1.26] | 0.08 | ||
| Radial Strain (per 5% decrement) | Sub-papillary | 1.11 | [1.01, 1.21] | 0.03 | |
| Non-papillary | 1.00 | [0.89, 1.12] | 0.93 | ||
Analysis limited to patients with inducible perfusion defects on stress CMR and minimal infarction (≤ 5% LV myocardium, non-transmural) on LGE-CMR (n=532). All multivariable models include only the variables displayed – sub-papillary and non-papillary wall motion (2b) and radial strain (2c) respectively.
Myocardial strain analyses were further compared among patients with and without ≥moderate FMR matched (1:2) on ischemia and infarct extent and distribution (n=111): Concordant with matching, FMR patients and controls had equivalent ischemia extent (34.0 ± 22.1% vs. 33.8 ± 21.5% LV, p=0.92), and equivalently small infarcts (1.76 ± 1.90 vs. 1.61 ± 1.72 % LV, p=0.68). Despite matching on ischemia extent and distribution, papillary muscle ischemia was seen more frequently in patients with ≥moderate FMR (78% vs 50%, p<0.01), whereas papillary muscle infarction (in absence of ischemia) was equivalent between groups (5.4% vs. 1.4%, p=0.26). Figure 2 provides a representative example of strain analyses in ischemia-matched patients with and without ≥moderate FMR: As shown, despite similar ischemia distribution, greater sub-papillary radial strain impairment was evident with ≥moderate FMR. These patterns paralleled analyses shown in Table 3A; greatest relative difference in sub-papillary deformation between patients with and without ≥moderate FMR was discerned via radial strain: Consistent with this, multivariable analysis shown in Table 3B demonstrates that sub-papillary radial strain impairment was associated with FMR (p<0.05) independent of contractile function in remote regions (p=NS) – a similar trend was evident for circumferential strain (p=0.06). Figure 3 reports prevalence of ≥moderate FMR across quartiles of radial and circumferential strain indices, demonstrating prevalence of FMR to increase stepwise in parallel with increasing severity of sub-papillary contractile dysfunction. Additionally, the above noted association between papillary ischemia and ≥moderate FMR was attenuated (OR 3.54 [CI 0.96–12.96] p=0.06) when controlling for sub-papillary strain impairment (OR 1.25 per 5% decrement in radial strain [CI 1.10–1.43] p<0.001).
Figure 2. Typical Examples of ≥Moderate FMR in Relation to Sub-Papillary Ischemia and Strain.
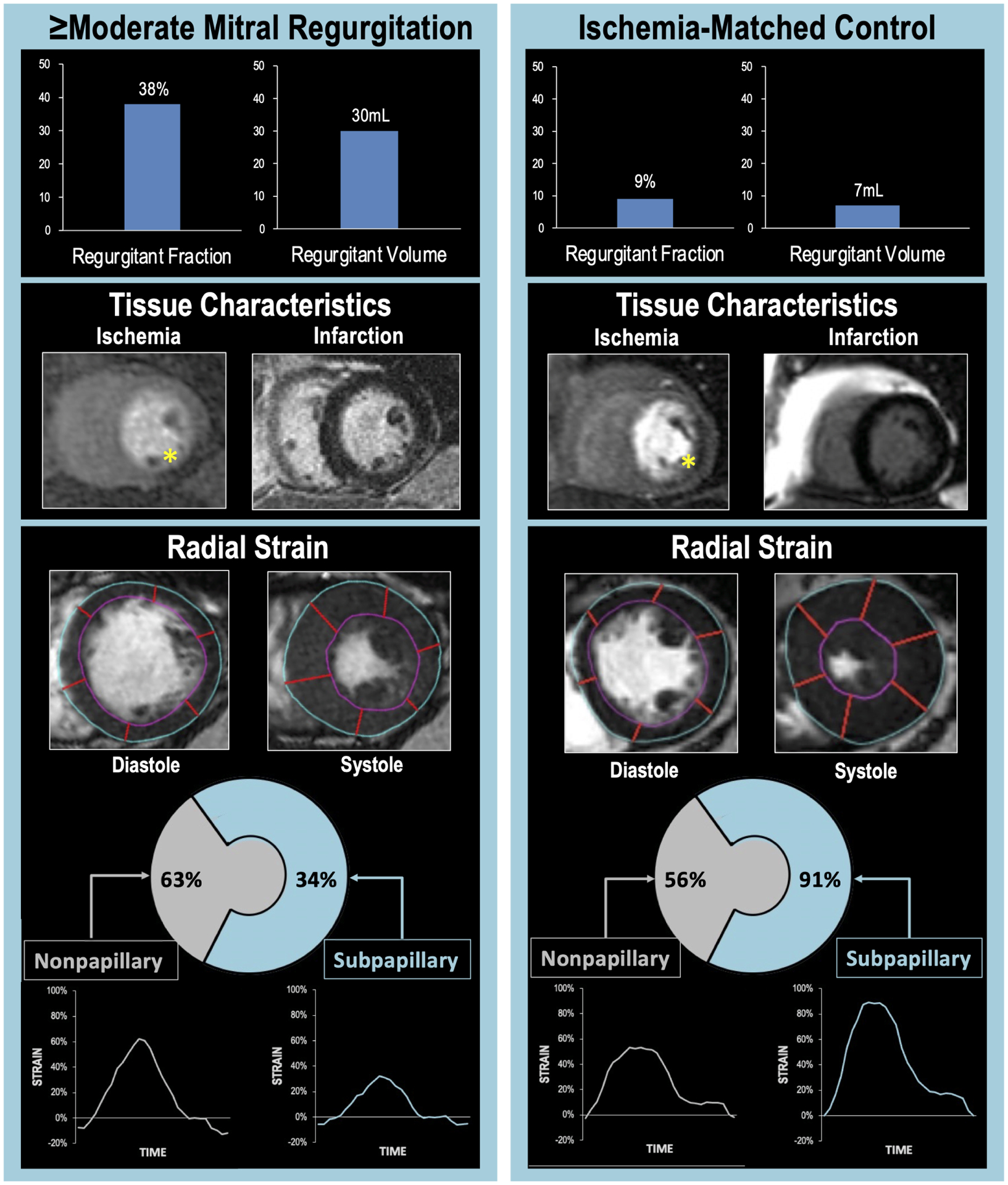
Representative examples of ischemic CAD patients with (left) and without (right) ≥moderate FMR. Note that despite marked differences in quantitative severity of FMR (top), both patients manifested perfusion deficits in mitral valve adjacent (inferior/lateral) LV myocardium on stress perfusion CMR (middle left, yellow asterisks) and absence of infarction on late gadolinium enhancement CMR (middle right). Regional strain analysis (bottom) demonstrated FMR differences to parallel differential pattern and severity of impaired contractile mechanics: Despite similar strain in remote LV regions, sub-papillary radial strain (calculated as mean strain within sub-papillary segments illustrated in Figure 1A) was lower in the patient with ≥moderate FMR.
Table 3.
Myocardial Strain in Sub-papillary and Remote LV Territories Among Ischemia Matched Patients with and without FMR
| ≥Moderate FMR Present (n=37) |
Absent (n=74) | Absolute Difference | Relative Difference | p | |
|---|---|---|---|---|---|
| Radial | |||||
| Global | 35.2±21.0% | 60.3±25.7% | 25.1% | 41.6% | <0.001 |
| Sub-papillary | 34.1±22.2% | 62.9±28.9% | 28.8% | 46.2% | <0.001 |
| Non-papillary | 36.3±22.8% | 57.6±26.0% | 22.7% | 39.7% | <0.001 |
| Circumferential | |||||
| Global | −19.4±9.1% | −32.2±10.7% | 12.8% | 40.0% | <0.001 |
| Sub-papillary | −18.5±8.7% | −30.8±10.4% | 12.3% | 40.0% | <0.001 |
| Non-papillary | −20.3±10.1% | −33.7±12.3% | 13.4% | 40.0% | <0.001 |
| Longitudinal | |||||
| Global | −16.1±6.1% | −22.6±4.6% | 6.5% | 28.8% | <0.01 |
| Sub-papillary | −18.0±6.3% | −24.2±5.2% | 6.2% | 25.6% | <0.01 |
| Non-papillary | −14.2±6.5% | −21.1±5.1% | 6.9% | 32.7% | <0.01 |
| Region | Multivariable OR | 95% CI | p | |
|---|---|---|---|---|
| Radial Strain (per % decrement) | Sub-papillary | 1.05 | [1.01, 1.08] | <0.01 |
| Non-papillary | 1.00 | [0.97, 1.03] | 0.92 | |
| Circumferential Strain (per % increment) | Sub-papillary | 1.11 | [0.97, 1.24] | 0.06 |
| Non-papillary | 1.05 | [0.96, 1.15] | 0.31 | |
| Longitudinal Strain (per % increment) | Sub-papillary | 1.12 | [0.96–1.31] | 0.16 |
| Non-papillary | 1.16 | [1.00, 1.34] | 0.053 |
Analysis encompassed patients with inducible perfusion defects on stress CMR and minimal infarction (≤5% LV myocardium, non-transmural) on LGE-CMR, with and without ≥moderate FMR, matched on ischemia extent and distribution (nested case:control cohort, n=111). All multivariable models (3b) include only the variables displayed – sub-papillary and non-papillary radial, circumferential and longitudinal strain values for each respective model. Odds ratios are for ≥moderate FMR.
Figure 3. ≥Moderate FMR in relation to Sub-Papillary Strain Impairment.
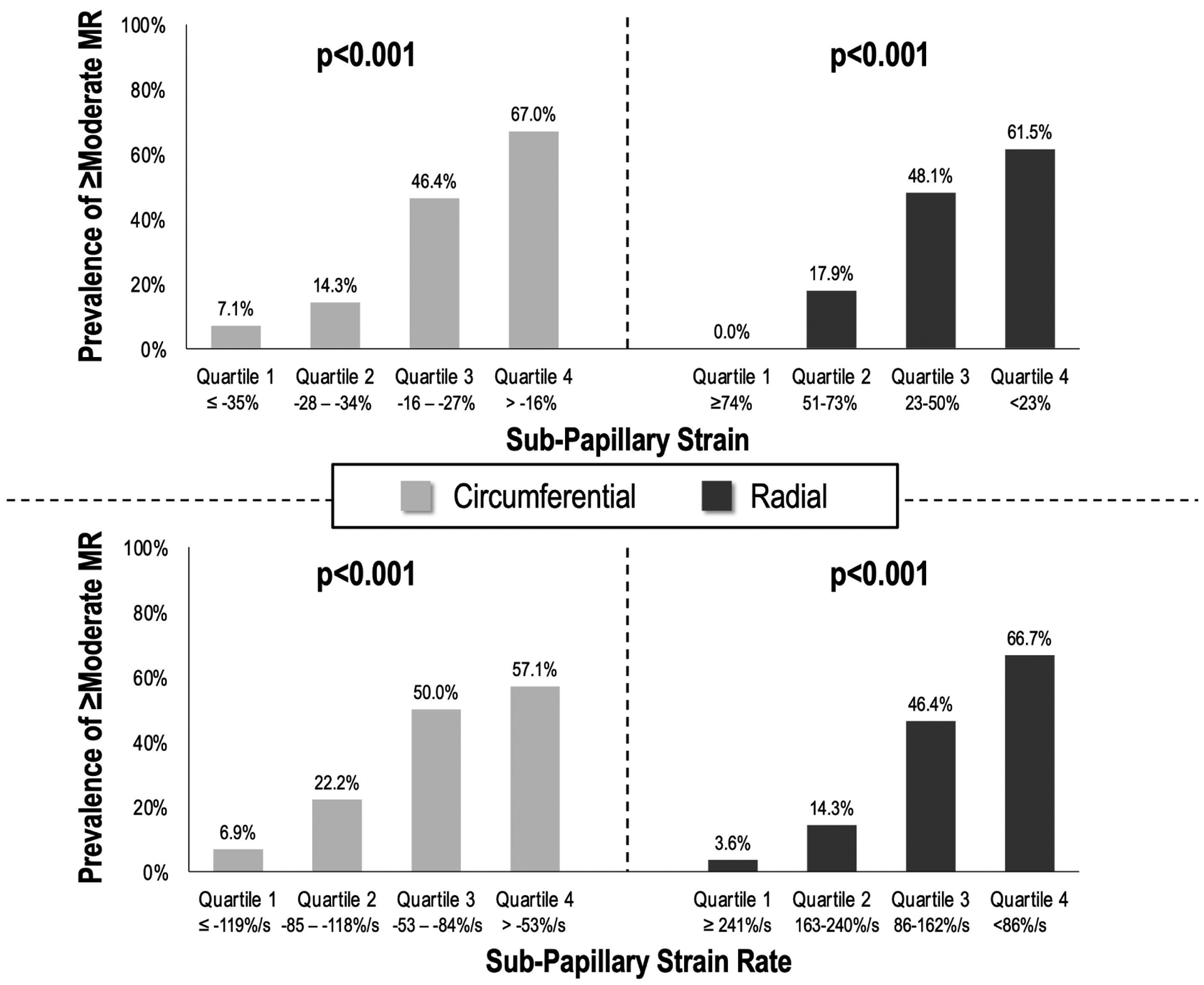
Prevalence of ≥moderate FMR (% patients affected) stratified in relation to cohort-based quartiles of circumferential (left) and radial (right) strain impairment within sub-papillary LV segments (strain impairment calculated as means within sub-papillary segments depicted in Figure 1A; strain analysis and quartile-based partitions in nested case-control sub-study of patients with LV ischemia with and without FMR [n=111]). Note that for both circumferential and radial strain, prevalence of ≥moderate FMR increased.
Mitral apparatus geometric analyses also supported a link between sub-papillary contractile dysfunction and adverse remodeling associated with FMR. As shown in Table 4A, annular diameter and tenting increased stepwise in relation to strata of impaired radial strain in both sub-papillary myocardium and remote LV regions (all p<0.05), paralleled by decreased papillary muscle fractional shortening (p<0.001). However, paralleling analyses testing radial strain in relation to FMR itself, Table 4B reports multivariable analyses showing sub-papillary radial strain to be independently associated with each mitral remodeling parameter (p<0.05) when controlling for strain in remote LV regions (p=NS). Further analyses tested whether impaired sub-papillary strain was associated with FMR independent of regional and global LV geometry: As shown in Table 5a, sub-papillary radial strain (OR 1.13 per 5% decrement [1.02–1.25] p=0.02) and mitral tenting area (OR 1.05 per 10mm2 increment [CI 1.00–1.10] p=0.04) were each significantly associated with ≥moderate FMR controlling for LV end-systolic volume (p=NS). When substituting LV sphericity for LV end-systolic volume (Table 5b), ≥moderate FMR remained associated with sub-papillary radial strain impairment (OR=1.22 per 5% decrement [1.02–1.47], p=0.03) even after controlling for regional and global geometry as represented by mitral tenting area and LV sphericity (both p<0.05).
Table 4.
Sub-papillary and Remote LV Strain in Relation to Mitral Apparatus Remodeling
| Sub-papillary Radial Strain Quartiles | |||||
|---|---|---|---|---|---|
| 1 (≥74%) |
2 (51–73%) |
3 (23–50%) |
4 (<23%) |
p | |
| Mitral Annular Diameter (cm) | 29.7±3.7 | 32.8±5.2 | 33.0±4.4 | 34.0±4.9 | <0.01 |
| Mitral Leaflet Tenting Height (cm) | 7.3±1.8 | 7.1±2.8 | 9.1±2.3 | 11.2±3.1 | <0.001 |
| Mitral Valve Tenting Area (cm2) | 1.14±0.39 | 1.32±0.83 | 1.73±0.49 | 2.47±1.00 | <0.001 |
| Interpapillary Fractional Shortening (%) | 61.1±10.5 | 53.7±9.2 | 43.2±10.6 | 27.3±10.1 | <0.001 |
| Non-papillary Radial Strain Quartiles | |||||
| 1 (≥69%) |
2 (49–68%) |
3 (31–49%) |
4 (<31%) |
p | |
| Mitral Annular Diameter (cm) | 30.1±3.2 | 32.6±6.1 | 33.1±4.1 | 33.3±4.7 | 0.02 |
| Mitral Leaflet Tenting Height (cm) | 6.9±2.1 | 8.1±2.2 | 8.6±23.0 | 10.5±3.0 | <0.001 |
| Mitral Valve Tenting Area (cm2) | 1.08±0.41 | 1.40±0.44 | 1.74±0.88 | 2.24±0.93 | <0.001 |
| Interpapillary Fractional Shortening (%) | 59.9±11.5 | 51.9±11.7 | 46.7±11.9 | 30.8±11.3 | <0.001 |
| Region | Multivariable β | 95% CI | p | |
|---|---|---|---|---|
| Mitral Annular Diameter (cm) | Sub-papillary radial strain | −0.06 | [−0.11, −0.01] | 0.02 |
| Non-papillary radial strain | 0.01 | [−0.04, 0.06] | 0.87 | |
| Mitral Leaflet Tenting Height (cm) | Sub-papillary radial strain | −0.04 | [−0.07, −0.02] | 0.001 |
| Non-papillary radial strain | −0.01 | [−0.04, 0.02] | 0.49 | |
| Mitral Valve Tenting Area (cm2) | Sub-papillary radial strain | −0.01 | [−0.02, −0.01] | 0.001 |
| Non-papillary radial strain | −0.006 | [−0.013, 0.002] | 0.14 | |
| Interpapillary Fractional Shortening (%) | Sub-papillary radial strain | 0.36 | [0.26–0.46] | <0.001 |
| Non-papillary radial strain | 0.07 | [−0.05, 0.17] | 0.22 |
Analysis encompassed patients with inducible perfusion defects on stress CMR and minimal infarction (≤ 5% LV myocardium, non-transmural) on LGE-CMR, with and without ≥moderate FMR, matched on ischemia extent and distribution (nested case:control cohort, n=111). All multivariable models (4b) include only the variables displayed – sub-papillary and non-papillary radial strain for each respective model.
Table 5.
Sub-papillary Strain and Mitral Apparatus Remodeling in Relation to ≥ Moderate Functional Mitral Regurgitation
| Variable | Univariable Odds Ratio | 95% CI | P | Multivariable Odds Ratio | 95% CI | P |
|---|---|---|---|---|---|---|
| Sub-papillary radial strain (per 5% decrement) | 1.15 | 1.07–1.24 | <0.001 | 1.13 | 1.02–1.25 | 0.02 |
| Mitral tenting area (per 10 mm2 increment) | 1.06 | 1.03–1.10 | <0.001 | 1.05 | 1.00–1.10 | 0.04 |
| LV End-Systolic Volume (per 10ml increment) | 1.06 | 1.03–1.10 | <0.001 | 0.98 | 0.91–1.04 | 0.45 |
| Variable | Univariable Odds Ratio | 95% CI | p | Multivariable Odds Ratio | 95% CI | p |
|---|---|---|---|---|---|---|
| Sub-papillary radial strain (per 5% decrement) | 1.15 | 1.07–1.24 | <0.001 | 1.22 | 1.02–1.47 | 0.03 |
| Mitral tenting area (per 10 mm2 increment) | 1.06 | 1.03–1.10 | <0.001 | 1.16 | 1.02–1.32 | 0.02 |
| LV Sphericity index (per 10% increment) | 2.32 | 1.38–3.91 | <0.001 | 2.19 | 1.06–14.50 | 0.03 |
Analysis encompassed patients with inducible perfusion defects on stress CMR and minimal infarction (≤ 5% LV myocardium, non-transmural) on LGE-CMR, with and without ≥moderate FMR, matched on ischemia extent and distribution (nested case:control cohort, n=111). Each multivariable model includes only the variables displayed.
Discussion
This study provides new insights regarding ischemic myocardial contractile mechanics in FMR. Key findings are as follows: First, presence and severity of FMR paralleled extent of stress CMR evidenced ischemia in regions underlying the mitral valve: ≥Moderate FMR associated with sub-papillary ischemia (p=0.001) when controlling for ischemia in remote regions, paralleling similar associations for sub-papillary and remote infarction: Second, whereas prevalence of ≥moderate FMR was independently associated with both sub-papillary ischemia and infarction (p=0.001), stronger associations were evident between infarction and FMR - paralleled by increased dysfunction in infarcted segments (p<0.05). Third, wall motion and quantitative strain analyses among patients with minimal LV infarction (≤5% LV myocardium, no transmural involvement), showed that whereas patients with ≥moderate FMR had greater wall motion scores and strain impairments in sub-papillary and remote regions – sub-papillary contractile dysfunction remained associated with FMR (p<0.05) when controlling for remote regions (p=NS). Paralleling associations between sub-papillary strain and FMR itself, mitral adverse remodeling indices were similarly associated with impaired sub-papillary strain (p<0.001); multivariable analysis demonstrated impaired sub-papillary radial strain and mitral tenting area were each associated with ≥moderate FMR (p<0.05) controlling for global LV remodeling as represented by LV end-systolic volume or LV sphericity. Taken together, findings support the concept that among CAD patients with ischemia, FMR severity and adverse mitral apparatus remodeling increase in proportion to contractile dysfunction underlying the mitral valve.
Regarding mechanism, our findings are consistent with the notion that FMR stems from alterations in sub-valvular myocardium (22) as can be produced by both acute injury and chronic LV ischemia – each of which impairs contractility, alters geometry, and augments mitral valve tethering forces (23,24), resulting in a deleterious cycle that ultimately impedes mitral valve coaptation. Notably, our observed associations between ischemia and FMR paralleled those of infarction – which, in the absence of contractile dysfunction, demonstrated similar lack of association with FMR. These data support the notion that for both ischemia and infarction, contractile dysfunction provides a common link by which altered myocardial tissue substrate provides a nidus for FMR.
Applied clinically, our results support a role for assessment of both myocardial tissue substrate (to delineate ischemia from [non-viable] infarction) and contractile function (by methods such as strain quantification) in patients with CAD and FMR, so as to identify regions of ischemia with dysfunction in which targeted revascularization would be most likely to reduce FMR. Whereas our study did not include data regarding coronary anatomy (which cannot be definitively established based on perfusion pattern) or test FMR response to therapeutic interventions, it should be noted that prior studies have reported that patients in whom FMR improves after coronary revascularization manifest improved contractile function in mitral valve adjacent regions.(7) It is also known that myocardial perfusion deficits are reversible with restoration of blood flow to ischemic regions, and that improved perfusion parallels increased contractile function. Given that both ischemia and infarction have been associated with poor prognosis in FMR(2,8) and that ischemia is potentially reversible, our data support a construct by which stress CMR (inclusive of functional, as well as ischemia and infarction based tissue characterization) can be used to identify patient-specific mechanisms for FMR and guide therapeutic interventions most likely to address underlying causality for mitral valve incompetence.
It is important to recognize that while some studies have reported associations between ischemia and FMR to parallel localized dysfunction and adverse remodeling in sub-papillary myocardium(4,5) results on this issue have been mixed(6,7) – resulting in a clinical knowledge gap and rationale for the current study. To address this, we analyzed multicenter stress CMR data to test ischemia and contractile pattern in relation to FMR: While this aspect of our study enabled findings broadly generalizable to real world clinical practice, variability in acquisition parameters (e.g. pulse sequences, contrast infusion rate) prohibited quantitative perfusion assessment - highlighting the need for future studies (using modalities such as CMR or PET) to test incremental utility of quantitative rest and stress perfusion evidenced ischemia severity as a determinant of FMR.
Several limitations should be noted. First, this study focused on links between ischemia-associated contractile dysfunction and FMR, but did not test FMR response to a standardized intervention, serial imaging (for change in FMR, ischemia, and/or function), modifiers such as atrial fibrillation, renal insufficiency, or clinical outcomes. However, regarding the latter, prior studies have shown CMR-evidenced ischemia to augment mortality among patients with and without FMR(2,10). Another limitation relates to selection bias, given that patients clinically referred for stress CMR (as are encompassed in the SCMR registry from which study data are derived) would be expected to differ from unselected CAD cohorts including patients with acute coronary syndromes (ACS). As FMR can be dynamic in ACS (e.g. improve with contractile recovery), our findings are most relevant to patients with known or suspected chronic CAD being considered for stress testing or elective revascularization. Regarding imaging, our CMR protocol used conventional LGE pulse sequences which are validated,(10,19) but can be limited for papillary muscle imaging. Also, contractile dysfunction was assessed during baseline conditions rather than during stress and rest – which could have been useful to assess whether ischemia mediated changes in LV function parallel changes in FMR: While it is possible that patients with ischemia can develop contractile dysfunction and FMR during dynamic provocation, an array of prior studies – including studies testing outcomes and therapeutic response in FMR(2,8,25) – have assessed MR under baseline conditions, consistent with our approach. Regarding strain, it should be noted that whereas sub-papillary strain differed between patients with and without FMR, within group variance was substantial, suggesting that current strain approaches may be useful for population risk stratification but suboptimal for individual patient decision-making. It should also be noted that strain and wall motion can be impacted by loading conditions, and that unrecognized differences in preload and/or afterload could have impacted our results. Regarding study design, it is important to recognize that strain was measured in only 37 patients with ≥moderate MR, that this group likely drove relationships between sub-papillary strain and MR severity, and that the limited size of this group constrained scope of strain analyses. However, our protocol design specifically used strain to quantitatively assess contractile mechanics in patients with ischemia and minimal infarction, so as to further explore wall motion score associations in the overall cohort. In this context, our finding that strain and wall motion yielded consistent findings – with both approaches demonstrating good reproducibility and associations between contractile dysfunction and FMR in patients with ischemia – adds to the rigor of our results. Last, while ≥moderate FMR was confirmed quantitatively, a multiparametric approach was used to do so. On the other hand, FMR was confirmed using established cutoffs(2,12,13) and this same approach has been validated in prior research by our group,(2) for which MR quantification by each algorithmic component predicted mortality, providing support to our approach.
In conclusion, this study demonstrates contractile function in ischemic sub-papillary myocardium to associate with both FMR and adverse mitral remodeling. Further studies are warranted to test if targeted therapies to abrogate ischemia and augment contractile function in mitral adjacent regions improve outcomes for CAD patients at risk for FMR and its serious clinical consequences.
Supplementary Material
Central Illustration. Myocardial Contractile Mechanics in Ischemic Mitral Regurgitation.
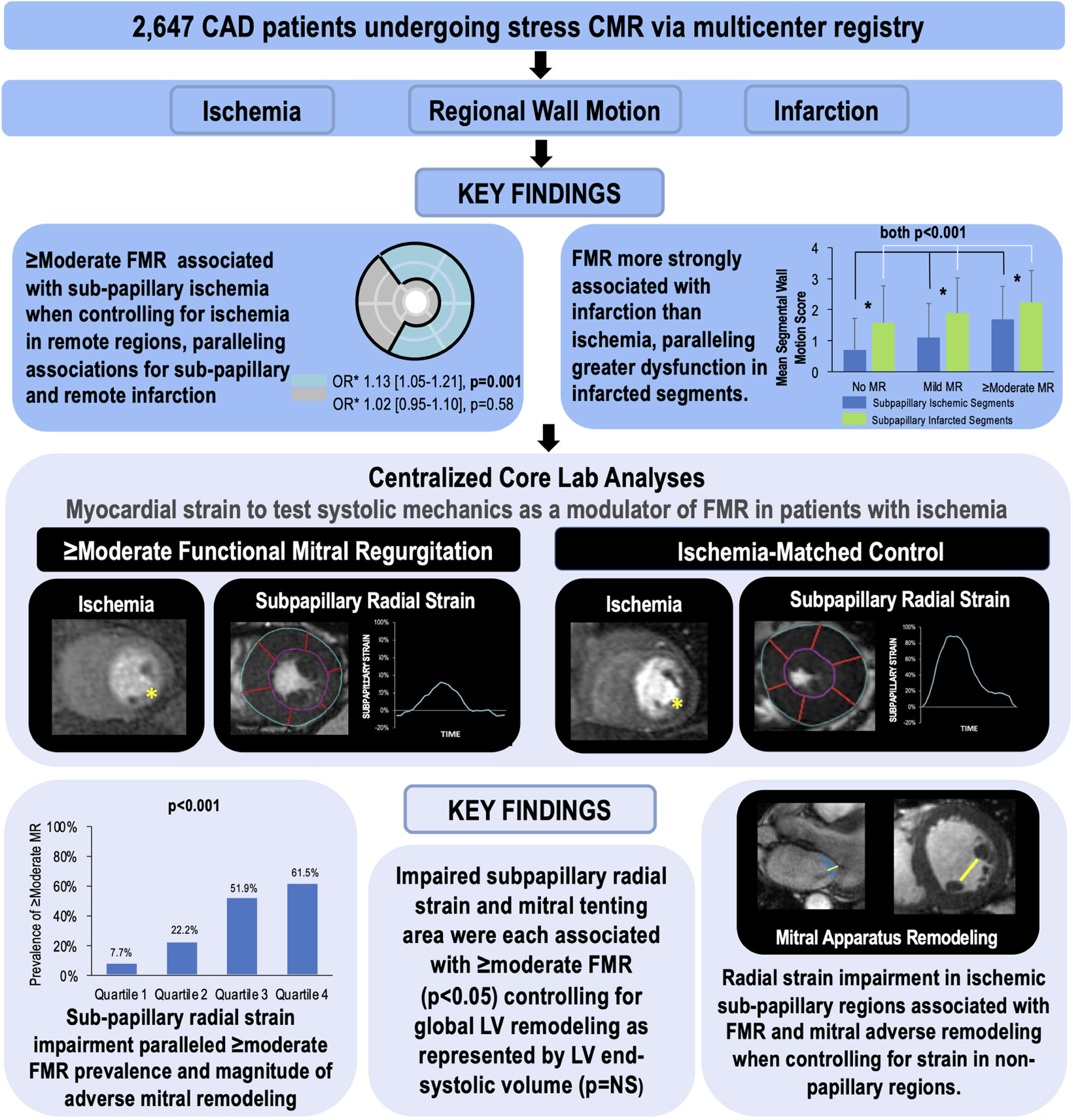
Multicenter stress CMR data from the SCMR registry demonstrated LV ischemia with contractile dysfunction in mitral valve adjacent (sub-papillary) territories to associate with FMR: Sub-papillary ischemia was associated with ≥moderate FMR independent of ischemia in remote regions. Associations between sub-papillary ischemia and FMR were weaker than for sub-papillary infarction, paralleled by greater dysfunction in infarcted segments – for both ischemia and infarction, only affected segments with contractile dysfunction conferred increased likelihood of FMR. Strain analysis in patients with and without ≥moderate FMR matched on ischemia distribution/extent demonstrated sub-papillary radial strain impairment to be associated with ≥moderate FMR and mitral apparatus remodeling independent of impaired strain in remote LV regions. Findings support a key role for ischemia-mediated contractile dysfunction in LV myocardium underlying the mitral valve in FMR.
Perspectives: Core Clinical Competencies and Translational Implications.
Competency in Medical Knowledge 1:
Among CAD patients with ischemia, pattern and functional sequelae of impaired myocardial perfusion are key risk factors for functional mitral regurgitation (FMR): Sub-papillary ischemia with contractile dysfunction confers increased likelihood of FMR, independent of ischemia or contractile dysfunction in remote myocardial regions.
Competency in Medical Knowledge 2:
Sub-papillary myocardial strain impairment increases in proportion to FMR as well as adverse mitral valve geometric remodeling; both sub-papillary radial strain and adverse mitral apparatus remodeling are associated with FMR independent of global LV dilation and regional mitral apparatus remodeling.
Translational Outlook 1:
Future research is warranted to test whether stress CMR can be used to identify FMR patients in whom mitral valve incompetence will resolve via targeted coronary revascularization of ischemic and dysfunctional sub-papillary myocardium, and whether such revascularization-induced decrements in FMR are accompanied by improved clinical prognosis.
Acknowledgements:
This research has been conducted using the SCMR Registry Resource. The authors thank Dr. Seth Uretsky for valuable review and suggestions regarding initial study design.
Funding Sources:
National Institutes of Health [Bethesda, MA] grants R01 HL128278 (JWW, RAL, JK), R01 HL128099 & R01 HL141917 (RAL), K23 HL140092 (JK), K23 HL132011 (CS), T32 HL7854-23 (JDK), Glorney-Raisbeck Fellowship / NY Academy of Medicine [New York, NY] (JDK).
Conflicts of Interests / Disclosures:
Dr. Weinsaft has received a speaker honorarium from General Electric Healthcare and is a consultant for Lexeo Therapeutics. Dr. Judd has an equity interest, Dr. Raymond Kim serves on the Board of Directors, and Kevin Judd is a consultant of Heart Imaging Technologies. Dr. Klem is a consultant and a speaker for Bayer, and receives funding from Medtronic. Dr. Leon receives funding from Abbott Vascular, Boston Scientific, and Medtronic.
Abbreviations:
- ACS
Acute coronary syndromes
- CAD
Coronary artery disease
- CMR
Cardiovascular magnetic resonance
- FMR
Functional mitral regurgitation
- LA
Left atrium
- LGE
Late gadolinium enhancement
- LOA
Limits of agreement
- LV
Left ventricle
- LVEF
Left ventricular ejection fraction
- PET
Positron emission tomography
- SCMR
Society for Cardiovascular Magnetic Resonance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Groarke JD CB, O’Gara PT, Fuster V, Harrington RA, Narula J, Eapen ZJ. Ischemic Mitral Regurgitation. In: JW H, editor Hurst’s The Heart. 14 ed. New York, NY: McGraw-Hill Education, 2017. [Google Scholar]
- 2.Kochav JD, Kim J, Judd R et al. Ischemia-Mediated Dysfunction in Subpapillary Myocardium as a Marker of Functional Mitral Regurgitation. JACC Cardiovasc Imaging 2021;14:826–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volo SC, Kim J, Gurevich S et al. Effect of myocardial perfusion pattern on frequency and severity of mitral regurgitation in patients with known or suspected coronary artery disease. Am J Cardiol 2014;114:355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kono T, Sabbah HN, Rosman H et al. Mechanism of functional mitral regurgitation during acute myocardial ischemia. J Am Coll Cardiol 1992;19:1101–5. [DOI] [PubMed] [Google Scholar]
- 5.Messas E, Guerrero JL, Handschumacher MD et al. Paradoxic decrease in ischemic mitral regurgitation with papillary muscle dysfunction: insights from three-dimensional and contrast echocardiography with strain rate measurement. Circulation 2001;104:1952–7. [DOI] [PubMed] [Google Scholar]
- 6.Kaul S, Spotnitz WD, Glasheen WP, Touchstone DA. Mechanism of ischemic mitral regurgitation. An experimental evaluation. Circulation 1991;84:2167–80. [DOI] [PubMed] [Google Scholar]
- 7.Michler RE, Smith PK, Parides MK et al. Two-Year Outcomes of Surgical Treatment of Moderate Ischemic Mitral Regurgitation. N Engl J Med 2016;374:1932–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavalcante JL, Kusunose K, Obuchowski NA et al. Prognostic Impact of Ischemic Mitral Regurgitation Severity and Myocardial Infarct Quantification by Cardiovascular Magnetic Resonance. JACC Cardiovasc Imaging 2019. [DOI] [PubMed] [Google Scholar]
- 9.Romano S, Judd RM, Kim RJ et al. Feature-Tracking Global Longitudinal Strain Predicts Death in a Multicenter Population of Patients With Ischemic and Nonischemic Dilated Cardiomyopathy Incremental to Ejection Fraction and Late Gadolinium Enhancement. JACC Cardiovasc Imaging 2018;11:1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heitner JF, Kim RJ, Kim HW et al. Prognostic Value of Vasodilator Stress Cardiac Magnetic Resonance Imaging: A Multicenter Study With 48000 Patient-Years of Follow-up. JAMA Cardiol 2019;4:256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heitner J, Bhumireddy GP, Crowley AL et al. Clinical application of cine-MRI in the visual assessment of mitral regurgitation compared to echocardiography and cardiac catheterization. PLoS One 2012;7:e40491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buchner S, Debl K, Poschenrieder F et al. Cardiovascular magnetic resonance for direct assessment of anatomic regurgitant orifice in mitral regurgitation. Circ Cardiovasc Imaging 2008;1:148–55. [DOI] [PubMed] [Google Scholar]
- 13.Zoghbi WA, Adams D, Bonow RO et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017;30:303–371. [DOI] [PubMed] [Google Scholar]
- 14.Klem I, Heitner JF, Shah DJ et al. Improved detection of coronary artery disease by stress perfusion cardiovascular magnetic resonance with the use of delayed enhancement infarction imaging. J Am Coll Cardiol 2006;47:1630–8. [DOI] [PubMed] [Google Scholar]
- 15.Weinsaft JW, Kim HW, Shah DJ et al. Detection of left ventricular thrombus by delayed-enhancement cardiovascular magnetic resonance prevalence and markers in patients with systolic dysfunction. J Am Coll Cardiol 2008;52:148–57. [DOI] [PubMed] [Google Scholar]
- 16.Sievers B, Elliott MD, Hurwitz LM et al. Rapid detection of myocardial infarction by subsecond, free-breathing delayed contrast-enhancement cardiovascular magnetic resonance. Circulation 2007;115:236–44. [DOI] [PubMed] [Google Scholar]
- 17.Kim RJ, Wu E, Rafael A et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 2000;343:1445–53. [DOI] [PubMed] [Google Scholar]
- 18.Kampaktsis PN, Albert BJ, Kim J et al. Impact of Mitral Regurgitation Severity and Cause on Effort Tolerance-Integrated Stress Myocardial Perfusion Imaging and Echocardiographic Assessment of Patients With Known or Suspected Coronary Artery Disease Undergoing Exercise Treadmill Testing. J Am Heart Assoc 2019;8:e010974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chinitz JS, Chen D, Goyal P et al. Mitral apparatus assessment by delayed enhancement CMR: relative impact of infarct distribution on mitral regurgitation. JACC Cardiovasc Imaging 2013;6:220–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bai W, Sinclair M, Tarroni G et al. Automated cardiovascular magnetic resonance image analysis with fully convolutional networks. J Cardiovasc Magn Reson 2018;20:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palumbo MC, Rong LQ, Kim J et al. Prosthetic aortic graft replacement of the ascending thoracic aorta alters biomechanics of the native descending aorta as assessed by transthoracic echocardiography. PLoS One 2020;15:e0230208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otsuji Y, Handschumacher MD, Schwammenthal E et al. Insights from three-dimensional echocardiography into the mechanism of functional mitral regurgitation: direct in vivo demonstration of altered leaflet tethering geometry. Circulation 1997;96:1999–2008. [DOI] [PubMed] [Google Scholar]
- 23.Schwammenthal E, Chen C, Benning F, Block M, Breithardt G, Levine RA. Dynamics of mitral regurgitant flow and orifice area. Physiologic application of the proximal flow convergence method: clinical data and experimental testing. Circulation 1994;90:307–22. [DOI] [PubMed] [Google Scholar]
- 24.He S, Fontaine AA, Schwammenthal E, Yoganathan AP, Levine RA. Integrated mechanism for functional mitral regurgitation: leaflet restriction versus coapting force: in vitro studies. Circulation 1997;96:1826–34. [DOI] [PubMed] [Google Scholar]
- 25.Uretsky S, Gillam L, Lang R et al. Discordance between echocardiography and MRI in the assessment of mitral regurgitation severity: a prospective multicenter trial. J Am Coll Cardiol 2015;65:1078–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


