Abstract
Hilar cholangiocarcinoma (HC) is a rare and highly aggressive biliary tract neoplasm. As such, data driving management of this disease is generally not based on prospective clinical trial data but rather consists of retrospective experiences and limited level 1 data. Surgical resection offers the best chance of long-term survival, but local and distant recurrence are common. Herein, we present landmark articles that form the basis of preoperative, operative and adjuvant strategies in HC.
INTRODUCTION
Hilar cholangiocarcinoma (HC) is a biliary tract neoplasm involving the proximal extrahepatic bile ducts. It comprises 50-70% of all biliary tract neoplasms but remains a rare disease, with approximately 7,000 cases diagnosed annually in North America.1,2 The management of HC is particularly challenging given its aggressive nature and complex anatomical relationships, which have important management implications. Complete resection is the most effective therapy; however, only a small fraction of patients is eligible for resection at presentation. While the liver is the most common site of metastatic disease, HC has a propensity for axial extension along the bile duct wall and radial invasion into adjacent tissues, both of which play a role in determining resectability (i.e. achieving an R0 resection). Resected HC has a 5-year disease-specific survival of approximately 40%, and recurrence after resection is common (75% of patients).3,4 Resection alone is thus far from perfect, and further improvements in outcome will require a multidisciplinary treatment approach; more effective therapies and treatment strategies are sorely needed. In this Landmark Series review, we discuss seminal reports that shape the management of HC, including randomized controlled clinical trials that have guided preoperative management and adjuvant therapy.
MANAGEMENT PRINCIPLES
Pre-treatment biliary drainage
Most patients present with jaundice, and the degree of hyperbilirubinemia is typically higher than that seen with benign obstructing gallstone disease. Other common disease manifestations include abdominal pain, weight loss, anorexia, and pruritis.5 Initial evaluation should begin with high-quality cross-sectional imaging, either liver angiogram protocol computed tomography (CT) or magnetic resonance imaging (MRI)/magnetic resonance cholangiopancreatography, before biliary decompression is performed. In most cases, both modalities are appropriate, since they provide complementary information.
Nearly all patients will require biliary decompression before starting treatment. Adequate biliary drainage is particularly important prior to resection, given that extended partial hepatectomy is often necessary, and a healthy liver remnant is required - preoperative biliary drainage (PBD) to decompress a jaundiced liver remnant decreases the risk of post-resection morbidity and mortality by fostering postoperative liver regeneration. However, cholangitis related to biliary drainage procedures and related infections are an important source of perioperative morbidity. Positive intra-operative bile cultures correlate closely with preoperative biliary stents and are associated with increased infectious complications. Misplacement of biliary drains and ill-advised procedures are major contributing factors in this regard; accurate placement of biliary drains and stents is, therefore, critical to avoid debilitating infectious sequelae which can preclude curative resection.
Biliary drainage can be achieved through endoscopic and percutaneous approaches. Selective biliary drainage should be used to optimize outcomes; however, the indications for and the best approach to biliary drainage are widely debated.6–9 Cholangitic patients, jaundiced patients requiring systemic chemotherapy, patients with hyperbilirubinemia-induced malnutrition, hepatic insufficiency, and jaundiced patients undergoing portal vein embolization (PVE) require immediate biliary decompression. Outside of these indications, however, many have proposed routine PBD prior to resection, with a goal of obtaining a baseline bilirubin below 2-3 mg/dL.7–9 The rationale for this approach is that routine drainage allows for normalization of hepatic function and thus reduces operative morbidity and mortality.
In an attempt to analyze the impact of future liver remnant (FLR) and PBD on postoperative liver failure and mortality, Kennedy and colleagues identified 60 patients at Memorial Sloan Kettering Cancer Center (MSKCC) who underwent hepatic resection from 1997-2007, where PBD of the FLR was used selectively.10 Patients with HC undergoing liver resection with a FLR <30% of total liver volume had an increased risk of postoperative hepatic insufficiency and mortality that was strongly associated with a lack of preoperative biliary drainage of the FLR. By contrast, of the patients with FLR ≥30%, none experienced hepatic insufficiency, and PBD did not improve perioperative outcomes.10 In fact, the mortality rate in patients with an FLR exceeding 30% who had PBD was 9% compared to 0% for those that did not have preoperative PBD. The data suggests that PBD in patients with large FLR (≥30% in this study) is associated with worse outcomes, likely due to increased stent-related, post-operative infective complications. By contrast, the benefit of PBD in patients with small FLR (<30%) appeared to outweigh the infection risk and resulted in improved outcomes.10 A larger combined analysis from MSKCC and the Academic Medical Center in Amsterdam showed similar results. In this study, pre-resection cholangitis, seen only in patients subjected to biliary drainage, was a powerful predictor of post-resection mortality. In patients with a small FLR, defined as ≤50%, PBD did appear to reduce the risk of postoperative liver failure and mortality compared to non-drained patients11 By contrast, the benefit of PBD was not seen in patients with a FLR > 50% (mortality = 12%) compared to no PBD (mortality = 0%).11 Taken together, the data would suggest that a liver with a large FLR is healthy enough to support a resection without the need for PBD; in such patients, the added benefit of biliary decompression is not realized and only exposes the patient to the risks associated with biliary stents. On the other hand, PBD is needed in patients with small FLR in order to improve its regenerative capacity.
Many studies have attempted to identify a cut-off serum total bilirubin level above which decompression should be mandatory; but to date, such a value has eluded definition and likely does not exist as an absolute number appropriate for all clinical situations. Indeed, given the number of variables that contribute to perioperative outcome after resection of hilar cholangiocarcinoma, it is very unlikely that a single cut off level would be universally applicable.12 In a recent study of 90 patients from the University of Hong Kong, preoperative serum bilirubin <75umol/L (4.4mg/dL) was associated with lower blood loss, transfusion rates and perioperative mortality.13 However, this study is limited by a small sample size, with only 8 patients in the high bilirubin group, and lack of validation. Furthermore, this study, like most others of its kind, does not consider the degree of FLR hypertrophy, which likely would allow a higher bilirubin level. At present, therefore, in the absence of clear guidelines, preoperative biliary decompression is likely performed more than is necessary, particularly in the presence of significant FLR hypertrophy. Of course, the decision to proceed with biliary drainage requires mature clinical judgment and must take into account other considerations, including co-existing medical conditions, patient nutrition, the overall fitness of the patient for major surgery, and the likelihood that the procedure would have a positive impact.
There are three approaches to PBD: percutaneous biliary drainage (PTBD), endoscopic biliary drainage (EBD), and endoscopic nasobiliary drainage (ENBD). PTBD has several advantages, including precise anatomic placement of catheters and improved delineation of tumor involvement of the biliary tree. Both factors are critical when planning a resection, given the importance of adequate drainage of the future liver remnant when PBD is indicated. Endoscopic approaches are more appealing since they avoid external drains but suffer from a higher incidence of stent misplacement, which may lead to the need for additional procedures. In one study, endoscopic stenting was associated with increased time to adequate biliary drainage, and half of patients undergoing PBD via endoscopic methods will ultimately require PTBD.14 This results in delays to resection in potentially curable patients. Another reported drawback for PTBD is tract seeding with malignant cells, but this is rare and generally associated with systemic recurrence.15
In an effort to address this issue, Coelen and colleagues performed a multicenter, randomized controlled trial at four academic centers in the Netherlands evaluating PTBD versus EBD in patients with potentially resectable HC requiring major liver resection and PBD.14 The primary endpoint was the rate of severe complications between randomization and surgery in the intention-to-treat population. From 2013-2016, 54 patients (of a planned 106) were randomized to either EBD or PTBD. The study was prematurely closed due to increased mortality in the PTBD versus the EBD group (41% vs. 11%, relative risk [RR] 3.67, 95% CI 1.15-11.7, p=0.03). Of 11 deaths in the PTBD cohort, 8 patients died after laparotomy, while 3 died before surgery. The causes of death included liver failure, bile leak complications, cholangitis, and disease progression. There was no difference in the preoperative mortality rate in patients who had PTBD alone (n=3/26), EBD alone (n=0/11), or crossed over to PTBD from EBD (n=0/16) (p=0.2). A direct link to the biliary drainage procedure as the cause of death could not be clearly made. Limitations of this study include enrollment of patients who had undergone previous drainage attempts, crossover treatment, and insufficient sample size. Although this was the first prospective trial evaluating PBD approaches in potentially resectable HC, termination of the study at 50% of its intended accrual may have led to significant type I error and warrants cautious interpretation and implementation of its findings. Of note, the overall complication rate was similar between the two groups (63% vs. 67%). The primary outcome (the rate of serious preoperative complications) was similar between both groups. Moreover, 56% of patients in the EBD group required PTBD, as opposed to 4% of PTBD patients requiring EBD. Further prospective studies are warranted to address the optimal method of PBD in this patient population. In high volume centers with an experienced multidisciplinary team, PTBD remains a viable approach in patients with resectable HC.
Surgical Resection
Goals of Operation
The goals of surgery for HC are to achieve complete resection of the tumor (R0) while leaving behind a well-perfused liver remnant of adequate size with good venous and biliary drainage. To accomplish this, one must remove the extrahepatic biliary apparatus en bloc with the involved liver, which should include the caudate lobe in most cases, porta hepatis lymphadenectomy and vascular resection and reconstruction in selected patients (see below).
Historically, resection for HC has been associated with excessive morbidity and mortality. The reasons for this are multifactorial but largely result from postoperative infections arising in the setting of a major sacrifice of hepatic parenchyma. In this setting, the future liver remnant (FLR) is dysfunctional due to prolonged biliary obstruction, and the added insult of complications related to infected bile, impairs postoperative regeneration. Over the years, it has become clear that optimization of the FLR can improve post-operative outcome. This has included not only biliary decompression to improve function but also preoperative portal vein embolization (PVE) to increase parenchymal volume.
Volumetric analysis of the FLR is a key component to preoperative evaluation and determining risk for postoperative hepatic insufficiency. PVE is utilized in cases where the FLR is expected to be small and insufficient thereby decreasing the morbidity of hepatic resection. HC patients commonly require neoadjuvant chemotherapy or present with significant cholestasis, both of which can impair liver function and regeneration.3 As such, it is our practice to perform PVE in HC patients with a FLR of less than 40%. Hypertrophy is assessed 3-4 weeks after PVE for both FLR volume and kinetic growth rate (KGR). A meta-analysis of 1088 patients from 37 studies showed PVE to be both safe and effective in inducing liver hypertrophy.16 Abulkhir et al reported an overall morbidity rate of 2.2% for PVE without any mortality and percentage increase from 8-27%.16 In an analysis from MSKCC of patients undergoing major hepatectomy after PVE, no patient with a KGR greater than 2.66% experienced postoperative liver insufiiciency.17 Patients who fail to reach this threshold can still be considered for surgery, however are at increased risk of surgical complications.
Associating liver partition and portal vein embolization for staged hepatectomy (ALPPS) is a two-stage hepatectomy combined with in-situ splitting of the liver and concomitant portal ligation during the first stage that could facilitate more rapid hypertrophy of the future liver remnant (FLR) than with conventional portal vein embolization (PVE). It is associated with a 60% to 80% increase in the original FLR volume over 7 to 10 days, but with high morbidity and mortality (80% and 12%, respectively).18–20 ALPPS for HC is rare. Concerns regarding the use of ALPPS for HC include bacteria in bile inducing infective complications such as bacteremia or intra-abdominal abscesses after the ALPPS procedure resulting in high morbidity and mortality. The international ALPPS registry reported a 90 day-mortality rate of 27% for ALPPS in HC patients.18 A matched case–control study compared outcomes of HC patients resected with ALPPS from the international ALPPS registry with a cohort from Amsterdam Medical Center and Memorial Sloan Kettering Cancer Center.21 Twenty-nine ALPPs patients from the international ALPPS registry were matched to 29 standard resection patients based on future liver remnant volume. The mortality rate in the ALPPS group was twice as high as that among patients with a similar liver volume who did not undergo ALPPS (48% vs 24%).21 Median survival was just 6 months in the ALPPS group compared with 29 months in the matched control group.21 A modified ALPPS approach for PHC has been suggested where partial parenchymal transection is performed in combination with PVE instead of portal vein ligation/transection.19 This theoretically limits morbidity in the first stage by avoiding hilar dissection, however, these variations have not been convincingly tested in HC.
Distant metastatic disease precludes resection, and while surgical therapy may still be feasible in the face of regional nodal disease (N1), survival outcomes are dramatically reduced. It should be noted that transplantation is contraindicated in both settings. The liver is the most common site of HC metastasis, but lymph node and lung metastases are also not uncommon. Determining resectability involves evaluating the local extent of disease and assessing ductal involvement, hepatic atrophy, and vascular involvement. Resection is the most effective therapy for patients with resectable tumors.
Up to half of HC patients explored with curative intent may have unresectable disease.22 In order to decrease the frequency of non-curative laparotomies, staging laparoscopy has been suggested.23,24 Weber and colleagues evaluated the utility of staging laparoscopy in 56 patients with potentially resectable HC.23 The overall yield of laparoscopy in this cohort was 25%; however, this increased to 36% in patients with T2/T3 tumors. Overall, 42% of patients were deemed unresectable and thus avoided an unnecessary laparotomy, suggesting utility of staging laparoscopy in patients with locally advanced, potentially resectable HC.
Results of Resection
Reports on outcomes after surgical resection of HC are limited to single-center and a few multicenter case series accumulated over many years.22,25–27 Long-term survival is possible in well-selected patients; however, HC resection comes with significant morbidity/mortality, and most patients recur after curative resection.4 Complete (R0) resection of HC is associated with 5-year survival rates of 25-40%.22,25,26 Over time, refinements in surgical techniques and perioperative management have led to decreased rates of morbidity, higher resectability rates, and improved overall survival (Table 1).26,28 In a single-center review of 574 consecutive cases from a center in Asia, morbidity decreased to 43% from 2005-2010, and mortality decreased from 11.1% before 1990 to 1.4% in the last 5 years.26
Table 1:
Literature review of perioperative and long-term outcomes of hilar cholangiocarcinoma resection
| Study | Resections | Morbidity (%) | Mortality (%) | Survival (5-yr) |
|---|---|---|---|---|
| Nakeeb et al (1996)75 | 109 | 47 | 4 | 11 |
| Klempnauer et al (1997)43 | 151 | NR | 10 | 28 |
| Neuhaus et al (1999)30 | 80 | 55 | 8 | 22 |
| Launois et al (2000)76 | 131 | NR | 17 | NR |
| Lee et al (2000)77 | 128 | 64 | 10 | 35 |
| Gerhards et al (2000)78 | 112 | 65 | 17 | NR |
| Nishio et al (2005)79 | 301 | NR | 7.6 | 22 |
| Jarnagin et al (2005)22 | 106 | 50 | 7.5 | 40 |
| Igami et al (2010)80 | 298 | 43 | 2 | 42 |
| Unno et al (2010)81 | 125 | 49 | 8 | 35 |
| Matsuo et al (2012)25 | 157 | 59 | 7 | 32 |
| Nagino et al (2013)26 | 574 | 57 | 4.7 | 33 |
| Wiggers et al (2016)11 | 287 | NR | 14 | NR |
| Komaya et al (2018)28 | 402 | NR | NR | 43.7 |
NR, not reported;
Nearly 25% of resected patients have a component of papillary carcinoma, which is an important determinant of survival after resection of HC.22 In an analysis of 106 resected patients at MSKCC, 23.6% of resected tumors contained papillary components, and disease-specific survival after resection of papillary tumors was 55.7 months compared to 33.5 months after resection of nodular-sclerosing lesions (p=0.01).22 Papillary phenotype was an independent predictor of survival, and this benefit was more pronounced for less invasive tumors.22
Vascular Resection
The world’s first hepatectomy with portal vein resection for HC was performed by Dr. Kajitani from the Cancer Institute Hospital on August 6, 1965.29 Portal vein resection may be necessary in select cases to achieve negative surgical margins and has been utilized to broaden eligibility for surgical resection in HC. Neuhaus and colleagues advocate for a “no-touch” technique approach to resection of right-sided lesions, which includes a hilar en bloc resection and resection of the portal vein bifurcation with reconstruction.30,31 Their experience showed a 5-year survival rate of 58% with this approach versus 29% for conventional hepatectomy and bile duct resection (p=0.02).31 On multivariable analysis, the no-touch technique was an independent prognostic factor for long-term survival, but this technique was also associated with significant morbidity and mortality. The 90-day mortality after hilar en bloc resection was 12.4%. The liver insufficiency rate was 30% in the “no-touch” technique cohort versus 16% in the conventional hepatectomy cohort (p=0.07). Moreover, this technique cannot be applied to left-sided resections since the right hepatic artery has to be dissected in close vicinity to the hepatic duct, and en bloc resection requires combined portal vein and arterial resection. The morbidity associated with combined arterial and venous resection is prohibitive. Taken together, the “no-touch” technique is an aggressive surgical approach associated with significant morbidity that is best applied to a very select population. Selective portal vein resection in cases of direct tumor involvement is recommended.
Although portal vein resection for HC resection is considered safe and reasonable in select patients at high volume centers, arterial resections remain controversial. In 1983, Tsuzuki and colleagues reported two successful cases of left hepatectomy with simultaneous resection of the portal vein and hepatic artery.32 Both patients died of recurrent disease within 18 months of resection. Since this initial report, multiple groups have published small, single institution studies which have demonstrated significant morbidity with this approach and questionable clinical benefit.33,34 Nagino and colleagues reported 50 consecutive cases of patients who underwent simultaneous arterial and portal vein resection.34 Significant morbidity (54%) was seen, including a 10% reexploration rate, and 7% of patients experienced postoperative liver insufficiency. In a more recent series, Mizuno and colleagues evaluated the safety and outcomes of vascular resection in a series of 1055 consecutive HC patients from 2001-2018, including 303 cases of vascular resection, of which 100 combined portal vein and hepatic artery reconstruction.33 Although morbidity rates were similar in HC resection with and without vascular resection (48% vs. 50%, p=0.72), perioperative mortality was significantly higher in the vascular resection group (3.6% vs. 1.2%, p=0.04).33
Combined venous and arterial resection are controversial and very rarely feasible or indicated. Long-term benefits of this approach remain equivocal and likely limited. Moreover, morbidity and mortality rates are significant. If used, this approach should be applied sparingly to highly select patients in very experienced centers. At the authors’ institution (MSKCC), concomitant portal vein and arterial involvement of the FLR requiring reconstruction precludes resection, and non-operative or transplantation approaches are considered.
Resection Margin Status
Long-term survival after resection of HC can be achieved and has improved over recent years22,25,26, in large measure due to increase use of en bloc partial hepatectomy, which has increased the rate of R0 resections. This point is illustrated in a series of 269 patients accumulated over a 20-year interval demonstrating a progressive increase in the proportion of patients subjected to partial hepatectomy, with a corresponding increase in the incidence of negative histological margins and in survival.35 In a study from MSKCC reporting results of 106 consecutive HC resections, median survival was 43 months in patients who underwent an R0 resection compared to 24 months in those with an R1 resection (p=0.0003)22; concomitant hepatic resection was an independent predictor of achieving an R0 resection and of long-term survival.22
Frozen section analysis of the duct margin during operation may be helpful, but caution must be used when interpreting the results. An analysis of experience with intraoperative frozen sections found that frozen section analysis of the proximal bile duct margin was misleading in 9% of patients.36 In addition, the benefits of extending the resection with a positive frozen section result were questionable.36 By contrast, in an analysis of 53 patients who underwent additional margin resection (bile duct resection in 44 and pancreatoduodenectomy in 9), R0 resection was achieved in 30 patients (57%).37 The 44 patients with additional bile duct resection had a 5-year overall survival rate of 31%, while all 21 patients without additional resection after a frozen section positive distal bile duct margin died within 5 years. Multivariable analysis showed reresection of the margin as an independent prognostic factors for survival (bile duct reresection vs. no additional resection: hazard ratio [HR] 0.32, 0.17 to 0.60; pancreatoduodenectomy vs. no additional resection: HR 0.08, 0.02 to 0.29).37
Although ductal margin status resected HC has been extensively studied, assessment of radial margin (RM) has received less attention but has been increasingly recognized as an important prognostic factor. In a large single-center retrospective cohort analysis, 478 consecutive patients who underwent resection of HC between 2001 and 2014 were reviewed.38 Eight-five (18%) patients had positive surgical margins (R1 resection) – 37 (43%) were RM positive, 33 (39%) had positive ductal margins, and 15 (18%) had both margins positive. The RM positivity rate in the hepatoduodenal ligament was higher in left-sided hepatectomy versus right-sided hepatectomy (8.7% vs. 3.6%, p=0.03), probably related to involvement of the right hepatic artery. Survival of patients with positive RM was worse than that of R0 patients (median survival 2.1 vs. 4.9 years, p<0.001) and similar to that of patients with positive ductal margin.38 Multivariate analysis identified positive RM as an independent prognostic factor of survival. This study identified a positive RM as the most common cause of R1 resection, with similar negative effects on overall survival as a positive distal bile duct margin. Resected HC specimens are complex, and communication with the pathologist before the specimen is processed will help ensure accuracy of margin assessment.
Lymphadenectomy
The extent of lymphadenectomy has been an area of controversy. Some surgeons advocate for extended nodal dissection, as some studies have demonstrated measurable 5-year actuarial survival in the presence of metastatic disease to distant nodal basins (i.e., paraaortic).39,40 However, an analysis of studies specifically reporting 5-year survival in patients would suggest that any nodal involvement is a powerful adverse factor and that very few patients benefit from such an aggressive approach.39 Thus, while a complete porta hepatis lymphadenectomy should be routinely performed as part of a complete resection, it must be recognized that this is a staging procedure with no therapeutic benefit, and extended lymph node dissection is never indicated.
As is the case for other tumors, the clinical implication of a negative lymph node on histopathologic analysis is likely dependent on the total number of lymph nodes sampled. In a prior study investigating this issue, it was found that 7 lymph nodes seemed to be the target sampling number in order to accurately stage HC.41 This must be weighed against the reality that in most series, the median number of nodes sampled from a porta hepatis lymphadenectomy is usually around 3.
Liver Transplantation in Hilar Cholangiocarcinoma
Outcomes from reports of orthotopic liver transplantation for unresectable HC were initially prohibitive and did not justify its use.42–45 The Cincinnati Transplant Tumor Registry reported a 28% 5-year survival rate, with a 51% tumor recurrence rate, most recurring in the first 2 years after transplantation.44 Klempnauer and colleagues reported 4 long-term survivors out of 32 patients who underwent transplantation for HC.43 Comparable results were reported by Iwatsuki and colleagues.42 As a result of these early experiences, liver transplantation for HC was initially contraindicated.
Over the last 15 years, however, data have emerged suggesting good results with transplantation in highly selected patients with low volume unresectable disease when combined with an intensive pre-transplant treatment regimen.46,47 HC transplant protocols include specific entry criteria, as well as preoperative chemotherapy and radiation protocols to better select patients. Transplant is contraindicated in patients with tumors >3 cm, positive nodal or metastatic disease, previous resection attempt or transperitoneal biopsy, history of prior malignancy, and previous therapy that precludes completing neoadjuvant therapy.48,49 Eligible patients should have either a positive endoluminal biopsy, a mass lesion at the site of biliary stricture, or CA19-9 >100.48 Approximately 30-40% of patients will drop out pretransplant.50,51
The Mayo HC transplant protocol for HC is the most widely adopted and consists of neoadjuvant chemotherapy, radiation, and operative assessment of regional lymph nodes and distant metastases prior to transplant.52 In 2008, the Mayo group reported on all patients enrolled in their HC liver transplant protocol since 1993.53 One-hundred forty-eight patients enrolled in the protocol, of which 90 patients completed neoadjuvant therapy and subsequent liver transplantation, while 39 were removed from the protocol due to disease progression or death. The overall 5-year survival rate for the entire cohort was 55%, including a 71% 5-year overall survival rate in the cohort achieving orthotopic liver transplant.53
The US Extrahepatic Biliary Malignancy Consortium, a collaboration of 10 academic institutions in the United States, recently reported a retrospective review of 304 patients with suspected HC (234 resections, 70 in a transplant protocol).51 Transplant was associated with significantly improved overall survival compared to resection (5- year overall survival 64% vs. 18%, p<0.001). Subgroup analysis of patients who were node negative and whose tumors were <3 cm again showed improved survival in patients who underwent transplant versus resection. By contrast, the Mayo group evaluated patients with de novo HC treated by their liver transplant protocol (n=90) versus liver resection (n=124).50 They found no difference in overall survival after adjusting for age, lymph node status, and tumor size, and thus concluded that patients with clearly resectable de novo HC should be treated with resection.50
The lack of randomized, prospective data to evaluate resection versus transplant in resectable patients precludes a definitive conclusion. Liver transplantation for highly selected unresectable HC and HC in the setting of primary sclerosing cholangitis using standardized protocols, which include both radiation and systemic chemotherapy, is the standard of care.1 However, resection remains the standard approach when R0 resection is feasible, given limited organ availability as well as acceptable long term outcomes in node negative, margin negative resected patients. The TRANSPHIL study (NCT02232932) is a prospective, randomized multicenter study comparing liver transplantation with liver and bile duct resection in potentially resectable HC without evidence of primary sclerosing cholangitis. This study will be the first prospective randomized evaluation of transplant versus resection in resectable HC patients. The primary outcome is overall survival with a planned enrollment of 60 patients.
ADJUVANT THERAPY
Patterns of Recurrence
Recurrence after resection of HC is common (60-76% of resected patients)4,28,54,55 and remains as high as 50% at 10 years after resection.54 In a multi-institutional analysis of 306 consecutive patients with resected HC, 76% of patients recurred after resection.4 Over 25% of patients recurred after 5 years of recurrence-free survival. Moreover, node positive disease precluded recurrence-free survival beyond 7 years.
Although resection is the only modality to afford long-term survival benefit, high recurrence rates suggest that effective adjuvant therapy would improve outcomes, as is the case with other cancers. Despite a propensity for locoregional recurrence, distant metastases are more common than locoregional recurrence after R0 resection of HC, and therapeutic strategies evaluating systemic therapy are important.56
Unfortunately, the results of clinical trials evaluating adjuvant therapy for HC have been mixed. Until recently, the only phase III trial investigating adjuvant chemotherapy in HC was published in 2002 by Takada and colleagues.57 Between 1986 and 1992, 508 patients with resected bile duct tumors (n=139), gallbladder cancers (n=140), pancreatic cancers (n=173), and ampullary tumors (n=56) were randomized to surgery alone versus mitomycin C (6 mg/m2 IV at the time of surgery) and 5-fluorouracil (310 mg/m2 IV) in 2 courses of treatments for 5 consecutive days during postoperative weeks 1 and 3, followed by 5-FU (100 mg/m2 orally) daily from postoperative week 5 until disease recurrence.57 The primary endpoint was overall survival, with a plan to analyze per protocol for each disease separately (e.g., carcinoma of the pancreas, gallbladder, bile duct, or ampulla of Vater). Despite a significant improvement in disease-free survival for patients with gallbladder cancer treated with adjuvant therapy vs. observation, no significant differences in overall or disease-free survival were observed for bile duct tumors.
PRODIGE 12-Accord 18 Trial
Based on the findings of the Advanced Biliary Tract Cancer (ABC)-02 trial, gemcitabine and cisplatin are considered the standard of care for unresectable locally advanced and metastatic biliary tract cancers; however, this had never been studied in a randomized fashion in the adjuvant setting.58 The PRODIGE 12-Accord 18 trial was a multicenter randomized controlled trial in France that enrolled patients with R0/R1 resected biliary tract cancers, including gallbladder cancer, intrahepatic cholangiocarcinoma, HC, and distal bile duct cholangiocarcinoma (Table 2). Between 2004 and 2009, 196 patients were randomized to gemcitabine (1000 mg/m2 on day 1) and oxaliplatin (85 mg/m2 on day 2 of 2 week cycles) (GEMOX) or standard surveillance.59 The primary endpoint was relapse-free survival, and the study was designed to detect a 12 month difference in relapse-free survival with 80% power. Quality of life was an additional primary endpoint, and overall survival and disease-free survival were secondary endpoints.
Table 2:
Prospective Adjuvant Therapy Trials in Hilar Cholangiocarcinoma
| Study | Design | Date of Accrual | Intervention | Patient Population | Primary endpoint | Median Follow up | Outcome |
|---|---|---|---|---|---|---|---|
| PRODIGE-12/ACCORD-1859 | Randomized phase 3 | 2009-2014 | Adjuvant gemcitabine + oxaliplatin versus observation | IHC=96 HC=16 GBC=38 DC=56 |
Relapse free survival Health related quality of life |
47 months | Median RFS: 30.4 vs 18.5 months, p=0.48 Time to deterioration in HRQOL: 31.8 vs 32.1 months, p=0.48 |
| BILCAP60 | Randomized phase 3 | 2006-2014 | Adjuvant capecitabine versus observation | IHC=84 HC=128 GBC=79 DC=156 |
Overall survival | 60 months | ITT: Median OS 51.1 vs 36.4, p=0.1 PP: Median OS 53 vs 36 months, p=0.03 |
| BCAT55 | Randomized phase 3 | 2007-2011 | Adjuvant gemcitabine versus observation | DC = 123 HC = 102 |
Overall survival | 79 months | Median OS: 62.3 vs 63.8 months, HR 1.01, 95% CI 0.70, 1.45, p=0.96 |
| SWOG080969 | Single arm phase 2 | 2008-2012 | Adjuvant gemcitabine + capecitabine followed by concurrent capecitabine and radiation | DC=13 HC=38 GBC=25 |
2-year OS ≥65% for R0, ≥ 45% for R1 | 35 months | R0 resection 2 yr OS: 67% R1 resection 2 yr OS 60% |
ITT intention to treat; PP per protocol; IHC intrahepatic cholangiocarcinoma; HC hilar cholangiocarcinoma; GBC gallbladder cancer; RFS relapse free survival; HRQOL health related quality of life
Among the 196 randomized patients, 155 were evaluable for the primary endpoint. The results showed no difference in relapse-free survival (HR 0.88, 95% CI 0.62-1.25, p=0.48). Global health-related quality of life scores at 12 months were not different, and there was no difference in overall survival between the arms (HR 1.08, 95% CI 0.7-1.7, p=0.74). Of note, this study enrolled a low percentage of patients with high-risk disease who could theoretically derive more benefit from adjuvant therapies (e.g., lymph node metastases, 37% of the cohort). Moreover, only 15 HC patients were included in this study (GEMOX n=10, observation n = 5) therefore, the applicability of these findings to HC and tumors with high risk of recurrence (i.e., locally advanced disease and/or positive lymph nodes) remains unanswered.
BILCAP Trial
The BILCAP trial was a phase III multi-institutional clinical trial in the United Kingdom investigating adjuvant capecitabine (1250 mg/m2 twice a day on days 1-14 of 3 week cycle for 8 cycles) versus observation alone after R0/R1 resection of biliary tract cancer.60 The primary objective of the study was to determine whether adjuvant chemotherapy with capecitabine has any effect on length of survival compared to observation in patients who have undergone macroscopically complete surgical resection of biliary tract cancer. The study population included HC (29%), gallbladder cancer (18%), distal cholangiocarcinoma (35%), and intrahepatic cholangiocarcinoma (19%). The primary endpoint was overall survival, and the study was designed to detect an effect size of HR 0.71.
Of 447 randomized patients between 2006 and 2014, 430 were evaluable for the primary endpoint by intention-to-treat analysis. Over 50% of patients had an R1 resection, and 38% of patients had node positive disease. There was no significant difference in overall survival by intention-to-treat analysis (HR 0.81, 95% CI 0.63-1.04, p=0.097). However, a per protocol analysis which excluded 17 patients who could not receive at least one cycle of chemotherapy or could not be randomized, showed a statistically significant improvement in overall survival (median OS 53 mo vs 36 mo, HR 0.75, 95% CI 0.58-0.97, p=0.03) and recurrence free survival (HR 0.70, 95% CI 0.54-0.92, p=0.009) in the capecitabine arm. Despite a negative finding by intention-to-treat analysis, the benefit seen with per protocol analysis and the 9% improvement in OS in the BILCAP trial has prompted the adoption of 6 months of adjuvant capecitabine chemotherapy after HC resection.61
Bile Duct Cancer Adjuvant Trial
The Bile Duct Cancer Adjuvant Trial (BCAT) was a phase III randomized controlled trial in Japan evaluating adjuvant gemcitabine (1000 mg/m2 administered on days 1, 8, and 15 ever 4 weeks for 6 cycles) versus observation in patients with distal or HC who underwent macroscopically curative resection and were enrolled in the study within 10 weeks of surgery (Figure 1A).55 The primary endpoint was overall survival, and secondary endpoints included relapse-free survival and toxicity.
Figure 1:
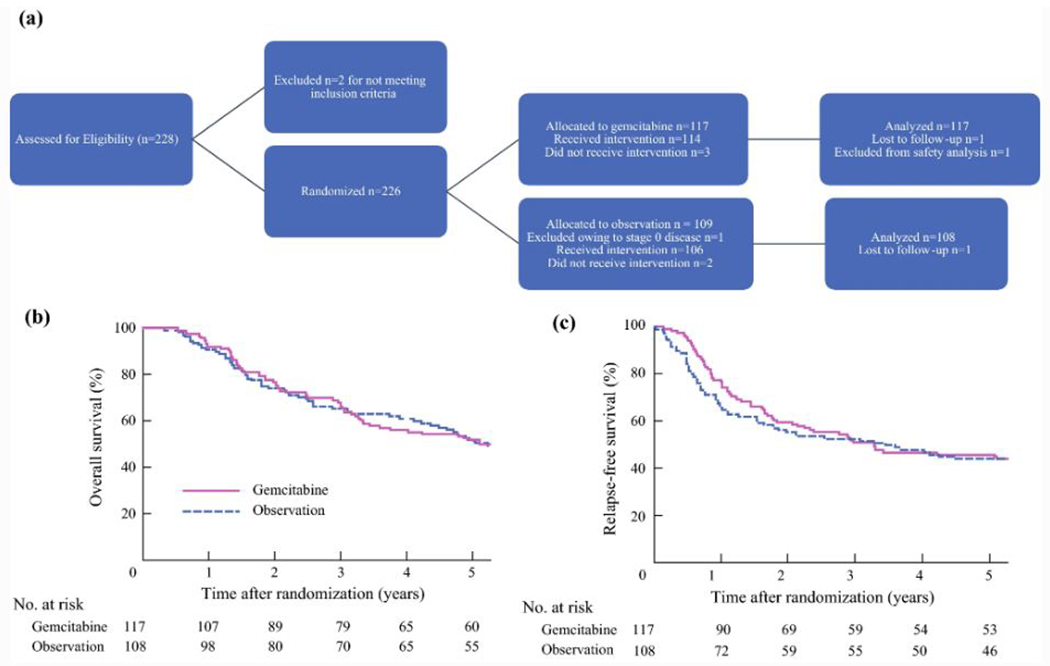
Bile duct cancer adjuvant trial (A) flow chart and Kaplan-Meier estimates of (B) overall survival (p=0.997) and (C) relapse-free survival (p=0.72)
Between 2007 and 2011, 225 patients were randomized (117 gemcitabine, 108 observation), including 102 patients with HC (51 patients in each arm). There was no significant difference in overall survival (median overall survival 62.3 months GEM vs. 63.8 months observation, HR 1.01, 95% CI 0.7-1.45) (Figure 1B). There was no difference in relapse-free survival and no differences when patients were stratified by lymph node status, margin status, or tumor location (hilar vs. distal) (Figure 1C). The median relapse-free survival in the HC cohort was 22.3 months with gemcitabine versus 30.8 months in the observation group (p=0.5). The location of first relapse was most commonly the liver in both groups (25% vs. 28%), followed by local recurrence (14% vs. 17%) and then peritoneum and abdominal lymph nodes. Given these findings, adjuvant gemcitabine monotherapy cannot be recommended after R0/R1 resection of HC.
Adjuvant Chemoradiation
Although distant recurrence in HC is commonly seen, patterns of recurrence suggest a higher likelihood of locoregional recurrence in HC compared to other biliary tract neoplasms.1,4,56,62 This has important implications for adjuvant therapy strategies and provides rationale for adjuvant therapy targeting locoregional disease. Analyses of such adjuvant strategies in HC have consisted of small, single-center reports. Two separate reports from Johns Hopkins suggest no benefit for adjuvant external beam or intraluminal radiation therapy.63,64 In contrast, other series have suggested that radiation may improve overall survival, particularly in patients with histologically positive hepatic duct margins.65–68 Importantly, however, none of these studies were randomized and most consist of a small, heterogeneous group of patients.
SWOG S0809
The SWOG S0809 trial was a phase II study of adjuvant gemcitabine (1,000 mg/m2 IV on days 1 and 8) and capecitabine (1,500 mg/m2 per day on days 1 to 14) every 21 days followed by concurrent capecitabine (1,330 mg/m2 per day) and radiotherapy in resected R0/R1 stage pT2-4 or node positive extrahepatic cholangiocarcinoma and gallbladder carcinoma patients. Radiotherapy consisted of 45 Gy to regional lymphatics and 54-59.4 Gy to the tumor bed.69 A total of 79 patients were enrolled, including 38 HC patients. The primary endpoint was 2-year overall survival after R0/R1 resection, and results would be considered promising if the 95% CI for the 2-year overall survival estimate was >45%, and R0 and R1 2-year overall survival estimates were ≥65% and 45%, respectively.
Median follow-up at the time of publication was 35 months. The 2-year overall survival rate for the entire cohort was 65% (95% CI, 53% to 74%; 67% and 60% in R0 and R1 patients, respectively). Median overall survival was 35 months, with no significant difference between those with an R0 versus R1 resection (Figure 2). Patterns of recurrence were analyzed, and there were no differences between disease subsite or within the HC cohort. Isolated local relapse versus distant relapse versus concomitant local and distant relapse were evenly distributed in HC patients who recurred (n=13).
Figure 2:
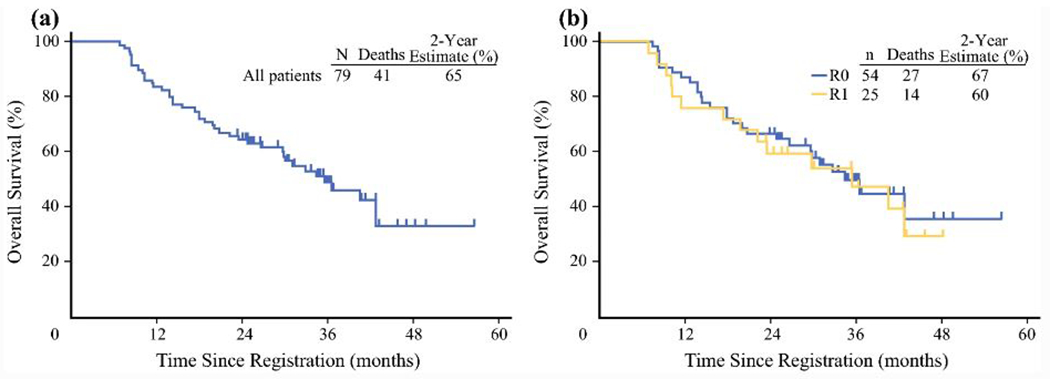
SWOG S0809 survival plots. Overall survival of the entire cohort (A) and stratified by resection margin (B) of extrahepatic bile duct cancer and gallbladder cancer patients who receive adjuvant chemotherapy and radiation.
This phase II trial met its stated goals and represents the highest level of evidence evaluating adjuvant chemoradiotherapy in HC. However, it lacked a comparison arm, and therefore results may be a function of the therapy or related to factors inherent in the patient population studied. At the present time, there are no data to support the routine use of adjuvant radiation therapy; however, patients with extrahepatic cholangiocarcinoma and positive resection margins may be offered chemoradiation.61 Data supporting specific effective protocols for this approach are limited and additional studies are needed to understand the true benefit of this adjuvant approach in HC.
Future Directions
There are several ongoing randomized studies to further address the question of the best adjuvant chemotherapy regimen for HC.70,71 The ACTICCA-1 trial is a multicenter, prospective, randomized, controlled phase III trial comparing adjuvant doublet gemcitabine and cisplatin to standard of care after curative resection of biliary tract cancer (NCT02548195).71 Patients with R0/R1 resected intrahepatic cholangiocarcinoma, HC, distal cholangiocarcinoma, or gallbladder cancer are eligible, and the primary endpoint is 2-year disease-free survival, with a target enrollment of 271 patients. Observation alone was the comparator arm through the initial phase of enrollment but was switched to capecitabine as it evolved as the new standard of care based on the BILCAP trial results.
A phase II study in Asia (NCT03079427) is currently randomizing patients with lymph node positive resected extrahepatic cholangiocarcinoma (perihilar or distal cholangiocarcinoma) to either adjuvant capecitabine (1,250 mg/m2 Day 1-14 every 3 weeks) or adjuvant doublet gemcitabine (1,000 mg/m2) plus cisplatin (25 mg/m2 day 1, 8 every 3 weeks). The primary endpoint is 2-year disease-free survival and is estimated to be complete in 2023, with a planned sample size of 100 patients.
Finally, the Adjuvant S-1 for Cholangiocarcinoma Trial (ASCOT) is a multicenter trial in Japan randomizing patients with resected biliary tract cancer to adjuvant S-1 versus observation (UMIN000011688). The primary endpoint is overall survival, and accrual began in 2013, with a planned sample size of 440 patients.70 These ongoing studies and others evaluating the utility of radiation therapy in HC and adjuvant systemic therapy strategies will provide much-needed level 1 evidence to establish the role of systemic and locoregional adjuvant therapies in HC.
Finally, genomics, immunobiology and targeted approaches represent exciting opportunities and therapeutic potential. The genomic and immunologic landscape of HC has demonstrated potential areas of exploration. For example, Lowery and colleagues demonstrated a high frequency of KRAS, TP53 or SMAD4 mutations in their single institution HC cohort.72 From an immunotherapy standpoint, high density tumor-associated macrophages have been associated with worse overall survival after HC resection73 and mismatch repair deficiency is seen in 5% of HC.2,74 Taken together, a better understanding of the genomic, epigenetic immunologic and molecular heterogeneity of this disease may lead to personalized therapeutic approaches.
CONCLUSION
Hilar cholangiocarcinoma is a highly invasive, rare disease. Most patients with unresectable HC die within 6-12 months of diagnosis, usually from liver failure or infectious complications secondary to biliary obstruction. Unfortunately, due to the rarity of these tumors and their frequently advanced stage at presentation, randomized prospective trials are rare. However, several ongoing randomized studies will help address some of the gaps in HC management. Future studies aimed at understanding the role for novel treatment modalities, such as targeted therapy and immunotherapy, are needed to continue to improve management of HC.
Acknowledgments:
The authors thank Erin Patterson for her editorial assistance.
Footnotes
Disclosures: The authors have no conflicts of interest
References
- 1.Mansour JC, Aloia TA, Crane CH, Heimbach JK, Nagino M, Vauthey JN. Hilar cholangiocarcinoma: expert consensus statement. HPB (Oxford). 2015;17(8):691–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banales JM, Marin JJG, Lamarca A, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17(9):557–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lidsky ME, Jarnagin WR. Surgical management of hilar cholangiocarcinoma at Memorial Sloan Kettering Cancer Center. Ann Gastroenterol Surg. 2018;2(4):304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Groot Koerkamp B, Wiggers JK, Allen PJ, et al. Recurrence Rate and Pattern of Perihilar Cholangiocarcinoma after Curative Intent Resection. J Am Coll Surg. 2015;221(6):1041–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakeeb A, Pitt HA. Radiation therapy, chemotherapy and chemoradiation in hilar cholangiocarcinoma. HPB (Oxford). 2005;7(4):278–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cherqui D, Benoist S, Malassagne B, Humeres R, Rodriguez V, Fagniez PL. Major liver resection for carcinoma in jaundiced patients without preoperative biliary drainage. Arch Surg. 2000;135(3):302–308. [DOI] [PubMed] [Google Scholar]
- 7.Iacono C, Ruzzenente A, Campagnaro T, Bortolasi L, Valdegamberi A, Guglielmi A. Role of preoperative biliary drainage in jaundiced patients who are candidates for pancreatoduodenectomy or hepatic resection: highlights and drawbacks. Ann Surg. 2013;257(2):191–204. [DOI] [PubMed] [Google Scholar]
- 8.Kawasaki S, Imamura H, Kobayashi A, Noike T, Miwa S, Miyagawa S. Results of surgical resection for patients with hilar bile duct cancer: application of extended hepatectomy after biliary drainage and hemihepatic portal vein embolization. Ann Surg. 2003;238(1):84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nimura Y, Kamiya J, Kondo S, et al. Aggressive preoperative management and extended surgery for hilar cholangiocarcinoma: Nagoya experience. J Hepatobiliary Pancreat Surg. 2000;7(2):155–162. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy TJ, Yopp A, Qin Y, et al. Role of preoperative biliary drainage of liver remnant prior to extended liver resection for hilar cholangiocarcinoma. HPB (Oxford). 2009;11(5):445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiggers JK, Groot Koerkamp B, Cieslak KP, et al. Postoperative Mortality after Liver Resection for Perihilar Cholangiocarcinoma: Development of a Risk Score and Importance of Biliary Drainage of the Future Liver Remnant. J Am Coll Surg. 2016;223(2):321–331 e321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaspersz MP, Buettner S, Roos E, et al. A preoperative prognostic model to predict surgical success in patients with perihilar cholangiocarcinoma. J Surg Oncol. 2018;118(3):469–476. [DOI] [PubMed] [Google Scholar]
- 13.She WH, Cheung TT, Ma KW, et al. Defining the optimal bilirubin level before hepatectomy for hilar cholangiocarcinoma. BMC Cancer. 2020;20(1):914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coelen RJS, Roos E, Wiggers JK, et al. Endoscopic versus percutaneous biliary drainage in patients with resectable perihilar cholangiocarcinoma: a multicentre, randomised controlled trial. Lancet Gastroenterol Hepatol. 2018;3(10):681–690. [DOI] [PubMed] [Google Scholar]
- 15.Kang MJ, Choi YS, Jang JY, Han IW, Kim SW. Catheter tract recurrence after percutaneous biliary drainage for hilar cholangiocarcinoma. World J Surg. 2013;37(2):437–442. [DOI] [PubMed] [Google Scholar]
- 16.Abulkhir A, Limongelli P, Healey AJ, et al. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg. 2008;247(1):49–57. [DOI] [PubMed] [Google Scholar]
- 17.Leung U, Simpson AL, Araujo RL, et al. Remnant growth rate after portal vein embolization is a good early predictor of post-hepatectomy liver failure. J Am Coll Surg. 2014;219(4):620–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lang H, de Santibanes E, Schlitt HJ, et al. 10th Anniversary of ALPPS-Lessons Learned and quo Vadis. Ann Surg. 2019;269(1):114–119. [DOI] [PubMed] [Google Scholar]
- 19.Sakamoto Y, Matsumura M, Yamashita S, Ohkura N, Hasegawa K, Kokudo N. Partial TIPE ALPPS for Perihilar Cancer. Ann Surg. 2018;267(2):e18–e20. [DOI] [PubMed] [Google Scholar]
- 20.Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255(3):405–414. [DOI] [PubMed] [Google Scholar]
- 21.Olthof PB, Miyasaka M, Koerkamp BG, et al. A comparison of treatment and outcomes of perihilar cholangiocarcinoma between Eastern and Western centers. HPB (Oxford). 2019;21(3):345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarnagin WR, Bowne W, Klimstra DS, et al. Papillary phenotype confers improved survival after resection of hilar cholangiocarcinoma. Ann Surg. 2005;241(5):703–712; discussion 712–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weber SM, DeMatteo RP, Fong Y, Blumgart LH, Jarnagin WR. Staging laparoscopy in patients with extrahepatic biliary carcinoma. Analysis of 100 patients. Ann Surg. 2002;235(3):392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vollmer CM, Drebin JA, Middleton WD, et al. Utility of staging laparoscopy in subsets of peripancreatic and biliary malignancies. Ann Surg. 2002;235(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuo K, Rocha FG, Ito K, et al. The Blumgart preoperative staging system for hilar cholangiocarcinoma: analysis of resectability and outcomes in 380 patients. J Am Coll Surg. 2012;215(3):343–355. [DOI] [PubMed] [Google Scholar]
- 26.Nagino M, Ebata T, Yokoyama Y, et al. Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann Surg. 2013;258(1):129–140. [DOI] [PubMed] [Google Scholar]
- 27.Buettner S, Margonis GA, Kim Y, et al. Conditional probability of long-term survival after resection of hilar cholangiocarcinoma. HPB (Oxford). 2016;18(6):510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Komaya K, Ebata T, Yokoyama Y, et al. Recurrence after curative-intent resection of perihilar cholangiocarcinoma: analysis of a large cohort with a close postoperative follow-up approach. Surgery. 2018;163(4):732–738. [DOI] [PubMed] [Google Scholar]
- 29.Kajitani T, Kuno K, Hishida Y, Yamanobe T. [Surgery of bile duct cancer at the porta hepatis]. Shujutsu. 1966;20(11):997–1002. [PubMed] [Google Scholar]
- 30.Neuhaus P, Jonas S, Bechstein WO, et al. Extended resections for hilar cholangiocarcinoma. Ann Surg. 1999;230(6):808–818; discussion 819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neuhaus P, Thelen A, Jonas S, et al. Oncological superiority of hilar en bloc resection for the treatment of hilar cholangiocarcinoma. Ann Surg Oncol. 2012;19(5):1602–1608. [DOI] [PubMed] [Google Scholar]
- 32.Tsuzuki T, Ogata Y, Iida S, Nakanishi I, Takenaka Y, Yoshii H. Carcinoma of the bifurcation of the hepatic ducts. Arch Surg. 1983;118(10):1147–1151. [DOI] [PubMed] [Google Scholar]
- 33.Mizuno T, Ebata T, Yokoyama Y, et al. Combined Vascular Resection for Locally Advanced Perihilar Cholangiocarcinoma. Ann Surg. 2020. [DOI] [PubMed] [Google Scholar]
- 34.Nagino M, Nimura Y, Nishio H, et al. Hepatectomy with simultaneous resection of the portal vein and hepatic artery for advanced perihilar cholangiocarcinoma: an audit of 50 consecutive cases. Ann Surg. 2010;252(1):115–123. [DOI] [PubMed] [Google Scholar]
- 35.Saldinger PF, Blumgart LH. Resection of hilar cholangiocarcinoma--a European and United States experience. J Hepatobiliary Pancreat Surg. 2000;7(2):111–114. [DOI] [PubMed] [Google Scholar]
- 36.Endo I, House MG, Klimstra DS, et al. Clinical significance of intraoperative bile duct margin assessment for hilar cholangiocarcinoma. Ann Surg Oncol. 2008;15(8):2104–2112. [DOI] [PubMed] [Google Scholar]
- 37.Otsuka S, Ebata T, Yokoyama Y, et al. Clinical value of additional resection of a margin-positive distal bile duct in perihilar cholangiocarcinoma. Br J Surg. 2019;106(6):774–782. [DOI] [PubMed] [Google Scholar]
- 38.Shinohara K, Ebata T, Shimoyama Y, et al. A Study on Radial Margin Status in Resected Perihilar Cholangiocarcinoma. Ann Surg. 2019. [DOI] [PubMed] [Google Scholar]
- 39.Kitagawa Y, Nagino M, Kamiya J, et al. Lymph node metastasis from hilar cholangiocarcinoma: audit of 110 patients who underwent regional and paraaortic node dissection. Ann Surg. 2001;233(3):385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tojima Y, Nagino M, Ebata T, Uesaka K, Kamiya J, Nimura Y. Immunohistochemically demonstrated lymph node micrometastasis and prognosis in patients with otherwise node-negative hilar cholangiocarcinoma. Ann Surg. 2003;237(2):201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ito K, Ito H, Allen PJ, et al. Adequate lymph node assessment for extrahepatic bile duct adenocarcinoma. Ann Surg. 2010;251(4):675–681. [DOI] [PubMed] [Google Scholar]
- 42.Iwatsuki S, Todo S, Marsh JW, et al. Treatment of hilar cholangiocarcinoma (Klatskin tumors) with hepatic resection or transplantation. J Am Coll Surg. 1998;187(4):358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klempnauer J, Ridder GJ, Werner M, Weimann A, Pichlmayr R. What constitutes long-term survival after surgery for hilar cholangiocarcinoma? Cancer. 1997;79(1):26–34. [PubMed] [Google Scholar]
- 44.Meyer CG, Penn I, James L. Liver transplantation for cholangiocarcinoma: results in 207 patients. Transplantation. 2000;69(8):1633–1637. [DOI] [PubMed] [Google Scholar]
- 45.Robles R, Figueras J, Turrion VS, et al. Spanish experience in liver transplantation for hilar and peripheral cholangiocarcinoma. Ann Surg. 2004;239(2):265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heimbach JK, Gores GJ, Haddock MG, et al. Liver transplantation for unresectable perihilar cholangiocarcinoma. Semin Liver Dis. 2004;24(2):201–207. [DOI] [PubMed] [Google Scholar]
- 47.Rea DJ, Heimbach JK, Rosen CB, et al. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg. 2005;242(3):451–458; discussion 458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson B, Doyle MBM. Surgical Considerations of Hilar Cholangiocarcinoma. Surg Oncol Clin N Am. 2019;28(4):601–617. [DOI] [PubMed] [Google Scholar]
- 49.Heimbach JK, Sanchez W, Rosen CB, Gores GJ. Trans-peritoneal fine needle aspiration biopsy of hilar cholangiocarcinoma is associated with disease dissemination. HPB (Oxford). 2011;13(5):356–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Croome KP, Rosen CB, Heimbach JK, Nagorney DM. Is Liver Transplantation Appropriate for Patients with Potentially Resectable De Novo Hilar Cholangiocarcinoma? J Am Coll Surg. 2015;221(1):130–139. [DOI] [PubMed] [Google Scholar]
- 51.Ethun CG, Lopez-Aguiar AG, Anderson DJ, et al. Transplantation Versus Resection for Hilar Cholangiocarcinoma: An Argument for Shifting Treatment Paradigms for Resectable Disease. Ann Surg. 2018;267(5):797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Vreede I, Steers JL, Burch PA, et al. Prolonged disease-free survival after orthotopic liver transplantation plus adjuvant chemoirradiation for cholangiocarcinoma. Liver Transpl. 2000;6(3):309–316. [DOI] [PubMed] [Google Scholar]
- 53.Rosen CB, Heimbach JK, Gores GJ. Surgery for cholangiocarcinoma: the role of liver transplantation. HPB (Oxford). 2008;10(3):186–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakahashi K, Ebata T, Yokoyama Y, et al. How long should follow-up be continued after R0 resection of perihilar cholangiocarcinoma? Surgery. 2020;168(4):617–624. [DOI] [PubMed] [Google Scholar]
- 55.Ebata T, Hirano S, Konishi M, et al. Randomized clinical trial of adjuvant gemcitabine chemotherapy versus observation in resected bile duct cancer. Br J Surg. 2018;105(3):192–202. [DOI] [PubMed] [Google Scholar]
- 56.Kobayashi A, Miwa S, Nakata T, Miyagawa S. Disease recurrence patterns after R0 resection of hilar cholangiocarcinoma. Br J Surg. 2010;97(1):56–64. [DOI] [PubMed] [Google Scholar]
- 57.Takada T, Amano H, Yasuda H, et al. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer. 2002;95(8):1685–1695. [DOI] [PubMed] [Google Scholar]
- 58.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273–1281. [DOI] [PubMed] [Google Scholar]
- 59.Edeline J, Benabdelghani M, Bertaut A, et al. Gemcitabine and Oxaliplatin Chemotherapy or Surveillance in Resected Biliary Tract Cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): A Randomized Phase III Study. J Clin Oncol. 2019;37(8):658–667. [DOI] [PubMed] [Google Scholar]
- 60.Primrose JN, Fox RP, Palmer DH, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20(5):663–673. [DOI] [PubMed] [Google Scholar]
- 61.Shroff RT, Kennedy EB, Bachini M, et al. Adjuvant Therapy for Resected Biliary Tract Cancer: ASCO Clinical Practice Guideline. J Clin Oncol. 2019;37(12):1015–1027. [DOI] [PubMed] [Google Scholar]
- 62.Jarnagin WR, Ruo L, Little SA, et al. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer. 2003;98(8):1689–1700. [DOI] [PubMed] [Google Scholar]
- 63.Cameron JL, Pitt HA, Zinner MJ, Kaufman SL, Coleman J. Management of proximal cholangiocarcinomas by surgical resection and radiotherapy. Am J Surg. 1990;159(1):91–97; discussion 97–98. [DOI] [PubMed] [Google Scholar]
- 64.Pitt HA, Nakeeb A, Abrams RA, et al. Perihilar cholangiocarcinoma. Postoperative radiotherapy does not improve survival. Ann Surg. 1995;221(6):788–797; discussion 797–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Borghero Y, Crane CH, Szklaruk J, et al. Extrahepatic bile duct adenocarcinoma: patients at high-risk for local recurrence treated with surgery and adjuvant chemoradiation have an equivalent overall survival to patients with standard-risk treated with surgery alone. Ann Surg Oncol. 2008;15(11):3147–3156. [DOI] [PubMed] [Google Scholar]
- 66.Kamada T, Saitou H, Takamura A, Nojima T, Okushiba SI. The role of radiotherapy in the management of extrahepatic bile duct cancer: an analysis of 145 consecutive patients treated with intraluminal and/or external beam radiotherapy. Int J Radiat Oncol Biol Phys. 1996;34(4):767–774. [DOI] [PubMed] [Google Scholar]
- 67.Kim TH, Han SS, Park SJ, et al. Role of adjuvant chemoradiotherapy for resected extrahepatic biliary tract cancer. Int J Radiat Oncol Biol Phys. 2011;81(5):e853–859. [DOI] [PubMed] [Google Scholar]
- 68.Nakeeb A, Comuzzie AG, Martin L, et al. Gallstones: genetics versus environment. Ann Surg. 2002;235(6):842–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ben-Josef E, Guthrie KA, El-Khoueiry AB, et al. SWOG S0809: A Phase II Intergroup Trial of Adjuvant Capecitabine and Gemcitabine Followed by Radiotherapy and Concurrent Capecitabine in Extrahepatic Cholangiocarcinoma and Gallbladder Carcinoma. J Clin Oncol. 2015;33(24):2617–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakachi K, Konishi M, Ikeda M, et al. A randomized Phase III trial of adjuvant S-1 therapy vs. observation alone in resected biliary tract cancer: Japan Clinical Oncology Group Study (JCOG1202, ASCOT). Jpn J Clin Oncol. 2018;48(4):392–395. [DOI] [PubMed] [Google Scholar]
- 71.Stein A, Arnold D, Bridgewater J, et al. Adjuvant chemotherapy with gemcitabine and cisplatin compared to observation after curative intent resection of cholangiocarcinoma and muscle invasive gallbladder carcinoma (ACTICCA-1 trial) - a randomized, multidisciplinary, multinational phase III trial. BMC Cancer. 2015;15:564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lowery MA, Ptashkin R, Jordan E, et al. Comprehensive Molecular Profiling of Intrahepatic and Extrahepatic Cholangiocarcinomas: Potential Targets for Intervention. Clin Cancer Res. 2018;24(17):4154–4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Atanasov G, Hau HM, Dietel C, et al. Prognostic significance of macrophage invasion in hilar cholangiocarcinoma. BMC Cancer. 2015;15:790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Silva VW, Askan G, Daniel TD, et al. Biliary carcinomas: pathology and the role of DNA mismatch repair deficiency. Chin Clin Oncol. 2016;5(5):62. [DOI] [PubMed] [Google Scholar]
- 75.Nakeeb A, Pitt HA, Sohn TA, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224(4):463–473; discussion 473–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Launois B, Reding R, Lebeau G, Buard JL. Surgery for hilar cholangiocarcinoma: French experience in a collective survey of 552 extrahepatic bile duct cancers. J Hepatobiliary Pancreat Surg. 2000;7(2):128–134. [DOI] [PubMed] [Google Scholar]
- 77.Lee SG, Lee YJ, Park KM, Hwang S, Min PC. One hundred and eleven liver resections for hilar bile duct cancer. J Hepatobiliary Pancreat Surg. 2000;7(2):135–141. [DOI] [PubMed] [Google Scholar]
- 78.Gerhards MF, van Gulik TM, de Wit LT, Obertop H, Gouma DJ. Evaluation of morbidity and mortality after resection for hilar cholangiocarcinoma--a single center experience. Surgery. 2000;127(4):395–404. [DOI] [PubMed] [Google Scholar]
- 79.Nishio H, Nagino M, Nimura Y. Surgical management of hilar cholangiocarcinoma: the Nagoya experience. HPB (Oxford). 2005;7(4):259–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Igami T, Nishio H, Ebata T, et al. Surgical treatment of hilar cholangiocarcinoma in the “new era”: the Nagoya University experience. J Hepatobiliary Pancreat Sci. 2010;17(4):449–454. [DOI] [PubMed] [Google Scholar]
- 81.Unno M, Katayose Y, Rikiyama T, et al. Major hepatectomy for perihilar cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2010;17(4):463–469. [DOI] [PubMed] [Google Scholar]


