Abstract
Background and Aims
Symptomatic uncomplicated diverticular disease is a controversial diagnosis defined as chronic gastrointestinal symptoms in patients with diverticulosis. We assessed whether individuals with diverticulosis had an increased risk of abdominal pain, irritable bowel syndrome, or altered bowel habits.
Methods
We performed a prospective cohort study of participants who had a first-time screening colonoscopy at the University of North Carolina between 2013 and 2015. The colonoscopy included a detailed assessment for diverticulosis. Participants completed a follow-up interview between 2019 and 2020 to measure bowel habits and gastrointestinal symptoms. Poisson regression was used to estimate relative risk and 95% confidence intervals (CIs).
Results
Among the 310 participants, 128 (41%) had diverticulosis at baseline. Follow-up interviews were performed a mean of 6.8 years after the baseline colonoscopy. After adjustment for confounders, there was no association between diverticulosis and abdominal pain lasting >24 hours (relative risk [RR], 0.40; 95% CI, 0.05–3.45) or symptoms of irritable bowel syndrome (RR, 1.30; 95% CI, 0.69–2.42) at the time of follow-up. Compared to those with no diverticulosis, participants with diverticulosis were more likely to have more frequent bowel movements per day (RR, 1.60; 95% CI, 1.05–2.44). The association was stronger in participants with >10 diverticula (RR, 2.03; 95% CI, 1.19–3.48). Diverticulosis was not associated with altered stool consistency.
Conclusion
These findings suggest that diverticulosis is associated with more frequent bowel movements contrary to the widespread belief that patients with diverticulosis are constipated. Diverticulosis was not associated with abdominal pain or symptoms of irritable bowel syndrome. The diagnosis of symptomatic uncomplicated diverticular disease must be reconsidered.
Keywords: Symptomatic Uncomplicated Diverticular Disease, Painful Diverticular Disease, Symptomatic Diverticulosis, Irritable Bowel Syndrome
Introduction
Colonic diverticulosis is a common condition in the United States. Most diverticula are acquired with age. Diverticula form when mucosa and submucosa prolapse through natural weak points in the muscle wall where blood vessels penetrate. Recent genome-wide association studies suggest that genes associated with neuromuscular function and connective tissues play a role in diverticulosis.1 The number, size, and distribution of colonic diverticula increase with age.2 More than 70% of individuals over the age of 80 years have diverticulosis on colonoscopy.3 Despite limited evidence, diverticulosis has been associated with chronic gastrointestinal symptoms also known as symptomatic uncomplicated diverticular disease (SUDD).4
SUDD is a controversial diagnosis sometimes defined as bloating, abdominal pain, or altered bowel movements and is reportedly indistinguishable from the symptoms of irritable bowel syndrome.5 In one report, as many as 25% of individuals with diverticulosis will develop SUDD.5 On the other hand, a cross-sectional study found no association between diverticulosis and symptoms of irritable bowel syndrome.6 SUDD has also been defined as moderate to severe left lower quadrant pain that lasts more than 24 hours in individuals with diverticulosis.7 While there is a growing literature on SUDD, inadequate evidence supports this diagnosis, particularly in the United States. Ongoing gastrointestinal symptoms after an acute episode of diverticulitis (incidence: 180/100,000 persons per year) are also common and should not be confused with SUDD.8,9
We performed a prospective cohort study to determine whether individuals with diverticulosis are at increased risk of experiencing protracted abdominal pain, symptoms of irritable bowel syndrome, or altered bowel habits compared to individuals without diverticulosis. Our study included participants who had a screening colonoscopy at baseline with a standard assessment for diverticulosis and, years later, a structured interview for chronic gastrointestinal symptoms. We excluded participants with a history of acute diverticulitis because ongoing gastrointestinal symptoms are common after recovery from an acute episode of diverticulitis.10
Methods
We performed a prospective cohort study using data collected at the University of North Carolina Hospital (UNC) in Chapel Hill, North Carolina. The first phase of the study recruited 619 outpatients undergoing a first-time screening colonoscopy between 2013 and 2015 at the UNC. UNC is a not-for-profit medical system and a safety net institution for the state of North Carolina. The UNC patient population is diverse with respect to socioeconomic status, race/ethnicity, and religion. Screening colonoscopy in the UNC system is largely opportunistic and generally representative of the US screening population. The study included patients 30 years of age and older with at least a satisfactory preparation and a complete examination to the cecum. A research assistant measured the participant’s height and weight on the day of the colonoscopy. The research assistant was present during the entire colonoscopy. The gastroenterologist counted the number of diverticula in each segment of the colon. The research assistant recorded information from the procedure on a data-collection form. Prior to the colonoscopy, each participant was invited to complete a detailed interview to obtain information on demographics, past medical history, and lifestyle factors.
In the second phase of the study, 420 participants who completed baseline telephone surveys were invited to complete a second, follow-up phone interview. Participants were interviewed between 2019 and 2020 about bowel habits, laxative use, and gastrointestinal symptoms over the last 3 months. Bowel movement frequency and stool form using the Bristol Stool Scale were reported.11 Gastrointestinal symptoms were assessed using Rome III diagnostic criteria questions for irritable bowel syndrome. Abdominal pain from SUDD was defined as pain in the left side of the abdomen lasting at least 24 consecutive hours.7 Participants reported all health-care utilization for diverticular diseases and any interval colonoscopies. The results from interval colonoscopies were obtained and data extracted. As part of the interview, participants were asked if they had ever been diagnosed with diverticulosis. Self-report was not used to classify cases and controls but to understand whether patients were aware of the diagnosis. Participants were also asked if and how they changed their diet because of a diverticulosis diagnosis. Informed consent was obtained from all participants. The University of North Carolina School of Medicine Institutional Review Board approved this study.
Means and standard deviations were calculated for continuous variables and proportions for categorical data. Cases were defined as participants with diverticulosis on the baseline colonoscopy. Controls had no diverticulosis on the baseline colonoscopy. We excluded participants (n = 7) with a self-reported history of diverticulitis. To determine the association between colonic diverticulosis and prevalent gastrointestinal symptoms, modified Poisson regression with robust error variance was used to estimate relative risk and 95% confidence intervals (CIs).12 The 10% change-in-estimate approach was used to identify confounding variables.13 The final models were adjusted for age, sex, and daily total fiber intake. We assessed for effect measure modification by sex. We performed analyses with all diverticulosis cases and by number of diverticula in categories (1–3, 4–10, >10). All tests of significance were 2-tailed, and P values <.05 were considered significant. The analysis was performed using SAS 9.4 (SAS, Cary, NC). All authors had access to the study data and reviewed and approved the final manuscript.
Results
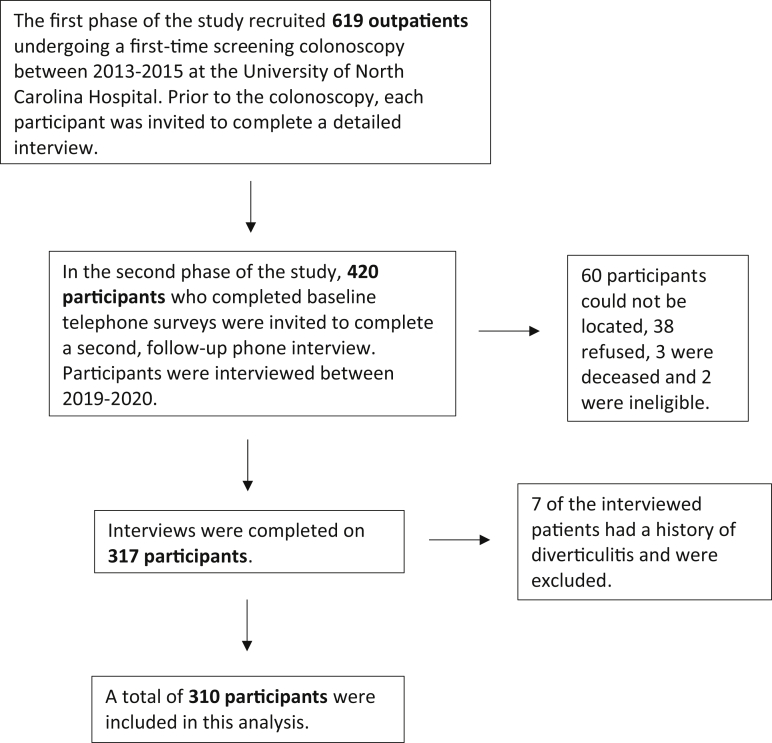
A total of 420 patients were invited for interview between March 2019 and May 2020. Interviews were completed on 317, 60 could not be located, 38 refused, 3 were deceased, and 2 were ineligible. Seven of the interviewed patients had a history of diverticulitis and were excluded (Figure). The follow-up interview was performed a mean of 6.8 years (standard deviation 0.2 years) after the baseline colonoscopy. All 310 participants had a baseline colonoscopy as part of our research study, and 82 participants had at least 1 subsequent colonoscopy. The subsequent colonoscopies were performed for any clinical indication and were not performed as part of our research study. Among those who had diverticulosis on the baseline colonoscopy, 69% had diverticulosis reported on a subsequent colonoscopy (kappa, 0.5; 95% CI, 0.3–0.7). For those with unreported diverticulosis on the subsequent exam, the mean number of diverticula on the baseline colonoscopy was 10 compared to 18 for those with diverticulosis reported on follow-up. Among those who did not have diverticulosis on the baseline colonoscopy, 18% had diverticulosis recorded on the subsequent colonoscopy. Participants who were found to have diverticulosis on the subsequent colonoscopy were more likely to be older, male, and to have an overweight or obese body mass index than those who did not develop diverticulosis.
Figure.
Flow diagram of participants included in our study.
Among the 310 participants, 128 (41%) had diverticulosis on the baseline colonoscopy. Participants with diverticulosis were more likely to be older, male, and to have an overweight or obese body mass index than those without diverticulosis (Table 1). Among participants with diverticulosis, 23% had 1–3 diverticula, 41% had 4–10 diverticula, and 34% had more than 10 diverticula. With regards to distribution, 62% of participants with diverticulosis had only distal diverticula, 32% had diverticula in the distal and proximal colon, and 6% had only proximal diverticula. Among those with diverticulosis, 82% did not recall being given a diagnosis of diverticulosis. Among those who recalled the diagnosis, 17% (n = 4) reported that they changed their diet. Among those who changed their diet, 50% (n = 2) started a high-fiber diet, and 25% (n = 1) began avoiding nuts, seeds, and popcorn.
Table 1.
Baseline Characteristics
| Participant characteristics | Diverticulosis |
No diverticulosis |
|---|---|---|
| n = 128 | n = 182 | |
| Age at follow-up interview, y | 63 ± 7 | 60 ± 6 |
| Sex | ||
| Male | 51% | 36% |
| Female | 49% | 64% |
| Race | ||
| White | 80% | 83% |
| Black | 19% | 15% |
| Other | 2% | 2% |
| Smoking status | ||
| Never | 51% | 65% |
| Former | 42% | 27% |
| Current | 7% | 8% |
| Body mass index, kg/m2 | ||
| Underweight (<18.5) | 2% | 3% |
| Normal (18.5–25) | 30% | 38% |
| Overweight (25–30) | 33% | 27% |
| Obese (>30) | 35% | 32% |
| Physical activity per d, metabolic equivalent of task, min | 1776 ± 278 | 1758 ± 220 |
| Daily dietary intake | ||
| Total energy intake, kilocalories | 2131 ± 795 | 1996 ± 755 |
| Total fiber, grams | 21 ± 9 | 20 ± 10 |
| Nonsteroidal anti-inflammatory drug use per mo | ||
| Never | 75% | 63% |
| 1–4 Times | 6% | 18% |
| >4 Times | 19% | 19% |
| Aspirin use per mo | ||
| Never | 66% | 74% |
| 1–4 Times | 2% | 2% |
| >4 Times | 31% | 24% |
| Alcohol use, drinks per mo | 12 ± 22 | 10 ± 15 |
Data are given as n (%) or mean ± standard deviation.
In our cohort, the prevalence of left-sided abdominal pain lasting at least 24 consecutive hours was 1% in cases and 2% in controls (Table 2). After adjustment for potential confounders, there was no association between diverticulosis and abdominal pain lasting >24 consecutive hours (relative risk [RR], 0.40; 95% CI, 0.05–3.45). Among participants, 13% of cases and 11% of controls met the criteria for Rome III irritable bowel syndrome. There was no association between diverticulosis and irritable bowel syndrome (RR, 1.30; 95% CI, 0.69–2.42).
Table 2.
Colonic Diverticulosis and Risk of Gastrointestinal Symptoms and Altered Bowel Habits
| Gastrointestinal symptoms and bowel habits | All cases (n = 128) |
Controls (n = 182) |
Adjusted risk ratioa | 95% CI | ||
|---|---|---|---|---|---|---|
| No. | (%) | No. | (%) | |||
| Left-sided abdominal pain > 24 h | 1 | 1% | 4 | 2% | 0.40– | 0.05–3.45 |
| Irritable bowel syndrome | 16 | 13% | 20 | 11% | 1.30– | 0.69–2.42 |
| Bowel movement frequency | ||||||
| <1 Bowel movement daily | 19 | 15% | 27 | 15% | 1.26– | 0.75–2.12 |
| 1 Bowel movement daily | 64 | 50% | 109 | 60% | Reference | – |
| >1 And <3 bowel movements daily | 34 | 27% | 32 | 18% | 1.60– | 1.05–2.44 |
| ≥3 Bowel movements daily | 11 | 9% | 12 | 7% | 1.46– | 0.68–3.17 |
| Bristol Stool Scaleb | ||||||
| Types 1 & 2 | 7 | 5% | 17 | 9% | 0.84– | 0.37–1.89 |
| Types 3, 4, & 5 | 90 | 70% | 139 | 76% | Reference | – |
| Types 6 & 7 | 9 | 7% | 8 | 4% | 1.75– | 0.71–4.31 |
| Regular or occasional laxative use | 19 | 15% | 33 | 18% | 0.79– | 0.47–1.35 |
Adjusted for age, sex, and dietary fiber intake.
Category does not add up to 100% because participants could answer that stool form was varied day to day.
After adjustment for potential confounders, participants with diverticulosis were more likely to have greater than 1 but less than 3 bowel movements per day (RR, 1.60; 95% CI, 1.05–2.44) than have 1 bowel movement per day. Because diverticulosis is thought to be associated with constipation, we examined laxative use as a marker of constipation. The prevalence of regular or occasional laxative use was 15% in cases and 18% in controls. Compared to controls without diverticulosis, there was no association between diverticulosis and laxative use (RR, 0.79; 95% CI, 0.47–1.35) (Table 2). Diverticulosis was not associated with stool consistency, either hard stool (Bristol types 1 & 2) (RR, 0.84; 95% CI, 0.37–1.89) or loose stool (Bristol types 6 & 7) (RR, 1.75; 95% CI, 0.71–4.31) compared to regular stool consistency (Bristol types 3, 4, & 5). The results were unchanged in analyses stratified by sex. There was no significant association between diverticulosis and having 3 or more bowel movements per day (RR, 1.46; 95% CI, 0.68–3.17).
We next stratified by number of diverticula to determine whether effects might be stronger or more apparent among participants with more numerous diverticula. The outcome of left-sided abdominal pain was too rare to model with diverticula in categories by number. The association between diverticula and bowel frequency was stronger in participants with >10 diverticula (RR, 2.03; 95% CI, 1.19–3.48) (Table 3).
Table 3.
Colonic Diverticulosis in Categories by Number and Risk of Gastrointestinal Symptoms and Bowel Habits
| Gastrointestinal symptoms and bowel habits | Cases 1–3 diverticula (n = 29) |
Cases 4–10 diverticula (n = 52) |
Cases >10 diverticula (n = 43) |
|||
|---|---|---|---|---|---|---|
| Adjusteda risk ratio | 95% CI | Adjusteda risk ratio | 95% CI | Adjusteda risk ratio | 95% CI | |
| Irritable bowel syndrome | 1.39 | 0.50–3.82 | 1.58 | 0.75–3.36 | 0.72 | 0.24–2.13 |
| Bowel movement frequency | ||||||
| <1 Bowel movement daily | 1.53 | 0.75–3.16 | 1.15 | 0.56–2.37 | 1.09 | 0.45–2.62 |
| 1 Bowel movement daily | Reference | – | Reference | – | Reference | – |
| >1 And <3 bowel movements daily | 1.33 | 0.58–3.04 | 1.35 | 0.76–2.39 | 2.03 | 1.19–3.48 |
| ≥3 Bowel movements daily | 1.56 | 0.55–4.44 | 1.45 | 0.53–3.92 | 1.44 | 0.38–5.43 |
| Bristol Stool Scale | ||||||
| Types 1 & 2 | 0.80 | 0.20–3.13 | 1.35 | 0.50–3.67 | 0.38 | 0.05–2.56 |
| Types 3, 4, & 5 | Reference | – | Reference | – | Reference | – |
| Types 6 & 7 | 0.82 | 0.10–6.50 | 2.58 | 0.88–7.53 | 1.72 | 0.46–6.41 |
| Regular or occasional laxative use | 0.60 | 0.19–1.84 | 0.92 | 0.47–1.81 | 0.60 | 0.25–1.45 |
Adjusted for age, sex, and dietary fiber intake.
Discussion
In this prospective cohort study with a baseline exam for diverticulosis, we found that participants with diverticulosis are not at increased risk for symptoms of irritable bowel syndrome or the protracted left lower quadrant abdominal pain associated with SUDD. There was no association between a higher burden of colonic diverticula and painful abdominal symptoms. Participants with diverticulosis were not more likely to take laxatives or to have less-frequent bowel movements. Instead, participants with diverticulosis were more likely to have >1 bowel movement per day, and the association was stronger in participants with >10 colonic diverticula. There was no significant association between diverticulosis and altered bowel consistency.
It has been estimated that as many as 25% of individuals with diverticulosis will develop SUDD based on studies from Italy.5 SUDD has been defined as abdominal pain and changes in bowel habits attributed to diverticula in the absence of alternate etiologies.14, 15, 16 SUDD has also been defined as abdominal pain in patients with diverticulosis in the absence of any complications (stenosis, abscess, fistulas) and in the left lower quadrant lasting for more than 24 hours.7,17,18 The pain is thought to arise from muscular contractions and/or chronic low-grade inflammation. Recent colonoscopy-based studies have found no association between diverticulosis and mucosal inflammation.6,19 In a 24-hour manometry study, patients with diverticulosis had a significant increase in regular patterns of phasic pressure activity compared to controls, and 30% reported cramping and lower abdominal pain during colonic contractions.20 The study was based on small numbers (12 patients), and the contractions lasted for 5–10 minutes while SUDD has been defined as pain lasting for 24 hours.
In a prior cross-sectional study, we found no association between diverticulosis and irritable bowel syndrome or nonspecific chronic abdominal pain.6 Irritable bowel syndrome (IBS) symptoms were measured in the months before the colonoscopy, not years later when symptoms may have developed. A colonoscopy-based study in Sweden found that diverticulosis was associated with diarrhea-predominant IBS, but only in participants over the age of 60 years (odds ratio, 9.66; CI, 1.08–84.08).14 The estimate was imprecise, confounding variables were not considered, symptoms were assessed at some time before the colonoscopy, and the colonoscopy was not performed with a standard assessment for diverticulosis. In contrast, the present study included a colonoscopy with assessment for diverticulosis and then followed up patients prospectively and systematically determined whether they had the abdominal pain associated with SUDD or symptoms associated with IBS. We found that participants with colonic diverticulosis are not at increased risk for symptoms of irritable bowel syndrome or the abdominal pain associated with SUDD. This suggests that diverticulosis is not commonly associated with painful symptoms, if at all.
Because diverticula are herniations through the colon wall, physicians historically assumed that high pressure in the colon caused the mucosa to herniate through the muscularis. A low-fiber diet and constipation were thought to contribute to the high pressure.21,22 In clinical practice, fiber supplements were recommended to patients with diverticulosis because these patients were thought to be constipated. Contrary to these beliefs, recent cross-sectional studies have found that constipation and a low-fiber diet were not associated with diverticulosis.23, 24, 25 A cross-sectional study also found no association between diverticulosis and straining or incomplete bowel movements.24 Instead, individuals with more frequent bowel movements were more likely to have diverticulosis.23,24 The finding of more frequent bowel movements in individuals with diverticulosis was also found in the colonoscopy-based study from Sweden.14 In the Swedish study, diverticulosis was associated with mushy stool, high-frequency defecation, urgency, and passing mucus.14 Again, all these studies assessed bowel habits prior to colonoscopy.
In contrast with prior cross-sectional studies, we performed a prospective study with a standardized assessment for diverticulosis in all participants. We found that individuals with diverticulosis had more frequent bowel movements, and the association was stronger in participants with more diverticula. Evidence from genome-wide association studies suggests that loci associated with the colonic neuromuscular function play a role in diverticulosis pathogenesis.1 Evidence from histopathology studies suggests enteric myopathies and neuropathies in the bowel wall of patients with a history of diverticular disease (diverticulosis and diverticulitis).26 There is also some evidence to suggest that patients with diverticulosis have altered neuropeptides and response to electrical stimulation which could drive faster colonic transit and more frequent bowel movements.27,28 Altogether, this suggests that individuals with diverticulosis may have altered colonic motility but not slow transit as previously assumed. High-quality studies of colonic motility in patients with diverticulosis have not yet been performed and will be an important next step in understanding this disease.29
In our cohort, most (82%) participants with diverticulosis did not recall being given a diagnosis. Among the few who recalled the diagnosis, very few changed their diet, with only 1 participant avoiding nuts, seeds, and popcorn. These findings may be specific to our institution. At our institution, we educate our patients that while diverticulosis is common, most patients never develop a complication, and there is no reason to avoid nuts, seeds, and popcorn.30
This study has notable strengths. We performed a prospective cohort study, and every participant had a baseline colonoscopy that included a standardized assessment for diverticulosis with a research assistant present. Because of the uniform assessment in a screening population, there was no risk of participants being diagnosed with diverticulosis because of a history of chronic gastrointestinal symptoms. UNC is a not-for-profit medical system, and our screening population is diverse with respect to socioeconomic status, race/ethnicity, and religion. We had extensive baseline clinical information on the cohort including demographics, medical history, and medication use and were able to account for confounding variables in our analyses. We excluded participants with a history of diverticulitis because ongoing gastrointestinal symptoms are common after recovery from an acute episode of diverticulitis.10 This work has limitations. Our cohort is small, and some of the controls may have developed diverticulosis since the baseline colonoscopy which would bias our results towards the null. Some of our sub-analyses may be underpowered. Among those who did not have diverticulosis on the baseline colonoscopy, 18% had diverticulosis recorded on the subsequent colonoscopy. We performed a sensitivity analysis in those with diverticulosis on the baseline or subsequent colonoscopy, and our results were unchanged. While we do not know the reproducibility of diverticulosis on colonoscopy, a research assistant was present for the duration of each exam to capture the number, size, and location of diverticulosis in each colon segment.
In conclusion, providers should hesitate to make a diagnosis of SUDD in patients with diverticulosis and chronic, painful abdominal symptoms. Instead, an alternative diagnosis should be sought. Diverticulosis is associated with more frequent bowel movements, not infrequent bowel movements. Research using high-quality assessments of colonic motility will be an important next step in understanding this common condition.
Acknowledgments
Authors' Contributions:
Anne F. Peery: Study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis. Robert S. Sandler: Study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and obtained funding. Temitope O. Keku: Analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and obtained funding. Joseph A. Galanko: Analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and statistical analysis.
Footnotes
Conflicts of Interest: The authors disclose no conflicts.
Funding: This study was funded in full by the National Institutes of Health (NIH) through grant award numbers P30 DK034987 and R01 DK094738. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Ethical Statement: The corresponding author, on behalf of all authors, jointly and severally, certifies that their institution has approved the protocol for any investigation involving humans or animals and that all experimentation was conducted in conformity with ethical and humane principles of research.
Data Transparency Statement: The data will not be made available to other researchers.
References
- 1.Camilleri M., Sandler R.S., Peery A.F. Etiopathogenetic mechanisms in diverticular disease of the colon. Cell Mol Gastroenterol Hepatol. 2020;9:15–32. doi: 10.1016/j.jcmgh.2019.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peery A.F., Keku T.O., Martin C.F., et al. Distribution and characteristics of colonic diverticula in a United States screening population. Clin Gastroenterol Hepatol. 2016;14:980–985.e1. doi: 10.1016/j.cgh.2016.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peery A.F., Keku T.O., Galanko J.A., et al. Sex and race disparities in diverticulosis prevalence. Clin Gastroenterol Hepatol. 2019;18:1980–1986. doi: 10.1016/j.cgh.2019.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma W., Chan A.T. Does subclinical inflammation play a role in the pathogenesis of diverticulosis? Clin Gastroenterol Hepatol. 2018;16:817–818. doi: 10.1016/j.cgh.2017.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tursi A., Scarpignato C., Strate L.L., et al. Colonic diverticular disease. Nat Rev Dis Primers. 2020;6:20. doi: 10.1038/s41572-020-0153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peery A.F., Keku T.O., Addamo C., et al. Colonic diverticula are not associated with mucosal inflammation or chronic gastrointestinal symptoms. Clin Gastroenterol Hepatol. 2018;16:884–891.e1. doi: 10.1016/j.cgh.2017.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tursi A., Elisei W., Picchio M., et al. Moderate to severe and prolonged left lower-abdominal pain is the best symptom characterizing symptomatic uncomplicated diverticular disease of the colon: a comparison with fecal calprotectin in clinical setting. J Clin Gastroenterol. 2015;49:218–221. doi: 10.1097/MCG.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 8.Peery A.F., Shaukat A., Strate L.L. AGA clinical practice update on medical management of colonic diverticulitis: expert review. Gastroenterology. 2021;160:906–911.e1. doi: 10.1053/j.gastro.2020.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bharucha A.E., Parthasarathy G., Ditah I., et al. Temporal trends in the incidence and natural history of diverticulitis: a population-based study. Am J Gastroenterol. 2015;110:1589–1596. doi: 10.1038/ajg.2015.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen E., Fuller G., Bolus R., et al. Increased risk for irritable bowel syndrome after acute diverticulitis. Clin Gastroenterol Hepatol. 2013;11:1614–1619. doi: 10.1016/j.cgh.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis S.J., Heaton K.W. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920–924. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 12.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 13.Mickey R.M., Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129:125–137. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 14.Jarbrink-Sehgal M.E., Andreasson A., Talley N.J., et al. Symptomatic diverticulosis is characterized by loose stools. Clin Gastroenterol Hepatol. 2016;14:1763–1770.e1. doi: 10.1016/j.cgh.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 15.Kohler L., Sauerland S., Neugebauer E. Diagnosis and treatment of diverticular disease: results of a consensus development conference. The Scientific Committee of the European Association for Endoscopic Surgery. Surg Endosc. 1999;13:430–436. doi: 10.1007/s004649901007. [DOI] [PubMed] [Google Scholar]
- 16.Annibale B., Lahner E., Maconi G., et al. Clinical features of symptomatic uncomplicated diverticular disease: a multicenter Italian survey. Int J Colorectal Dis. 2012;27:1151–1159. doi: 10.1007/s00384-012-1488-5. [DOI] [PubMed] [Google Scholar]
- 17.Spiller R. Is it diverticular disease or is it irritable bowel syndrome? Dig Dis. 2012;30:64–69. doi: 10.1159/000335721. [DOI] [PubMed] [Google Scholar]
- 18.Cuomo R., Barbara G., Andreozzi P., et al. Symptom patterns can distinguish diverticular disease from irritable bowel syndrome. Eur J Clin Invest. 2013;43:1147–1155. doi: 10.1111/eci.12152. [DOI] [PubMed] [Google Scholar]
- 19.Jarbrink-Sehgal M.E., Rassam L., Jasim A., et al. Diverticulosis, symptoms and colonic inflammation: a population-based colonoscopy study. Am J Gastroenterol. 2019;114:500–510. doi: 10.14309/ajg.0000000000000113. [DOI] [PubMed] [Google Scholar]
- 20.Bassotti G., Battaglia E., De Roberto G., et al. Alterations in colonic motility and relationship to pain in colonic diverticulosis. Clin Gastroenterol Hepatol. 2005;3:248–253. doi: 10.1016/s1542-3565(04)00614-7. [DOI] [PubMed] [Google Scholar]
- 21.Painter N.S. Diverticular disease of the colon—a disease of western civilisation. Dis Mon. 1970;16:1–57. [PubMed] [Google Scholar]
- 22.Painter N.S., Burkitt D.P. Diverticular disease of the colon: a deficiency disease of western civilization. BMJ. 1971;2:450–454. doi: 10.1136/bmj.2.5759.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peery A.F., Barrett P.R., Park D., et al. A high-fiber diet does not protect against asymptomatic diverticulosis. Gastroenterology. 2012;142:266–272.e1. doi: 10.1053/j.gastro.2011.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peery A.F., Sandler R.S., Ahnen D.J., et al. Constipation and a low-fiber diet are not associated with diverticulosis. Clin Gastroenterol Hepatol. 2013;11:1622–1627. doi: 10.1016/j.cgh.2013.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higashimori A., Nakatani M., Jinnai K., et al. Chronic constipation is negatively associated with colonic diverticula. Scand J Gastroenterol. 2021;56:1264–1270. doi: 10.1080/00365521.2021.1961307. [DOI] [PubMed] [Google Scholar]
- 26.Wedel T., Barrenschee M., Lange C., et al. Morphologic basis for developing diverticular disease, diverticulitis, and diverticular bleeding. Viszeralmedizin. 2015;31:76–82. doi: 10.1159/000381431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Espin F., Rofes L., Ortega O., et al. Nitrergic neuro-muscular transmission is up-regulated in patients with diverticulosis. Neurogastroenterol Motil. 2014;26:1458–1468. doi: 10.1111/nmo.12407. [DOI] [PubMed] [Google Scholar]
- 28.Tomita R. Are there any functional differences of the enteric nervous system between the right-sided diverticular colon and the left-sided diverticular colon? An in vitro study. Int J Colorectal Dis. 2014;29:571–577. doi: 10.1007/s00384-014-1837-7. [DOI] [PubMed] [Google Scholar]
- 29.Jaung R., Robertson J., O'Grady G., et al. Limited evidence of abnormal intra-colonic pressure profiles in diverticular disease - a systematic review. Colorectal Dis. 2017;19:O168–O176. doi: 10.1111/codi.13692. [DOI] [PubMed] [Google Scholar]
- 30.Strate L.L., Liu Y.L., Syngal S., et al. Nut, corn, and popcorn consumption and the incidence of diverticular disease. JAMA. 2008;300:907–914. doi: 10.1001/jama.300.8.907. [DOI] [PMC free article] [PubMed] [Google Scholar]