Abstract
The influence of lithotrophic Fe(II)-oxidizing bacteria on patterns of ferric oxide deposition in opposing gradients of Fe(II) and O2 was examined at submillimeter resolution by use of an O2 microelectrode and diffusion microprobes for iron. In cultures inoculated with lithotrophic Fe(II)-oxidizing bacteria, the majority of Fe(III) deposition occurred below the depth of O2 penetration. In contrast, Fe(III) deposition in abiotic control cultures occurred entirely within the aerobic zone. The diffusion microprobes revealed the formation of soluble or colloidal Fe(III) compounds during biological Fe(II) oxidation. The presence of mobile Fe(III) in diffusion probes from live cultures was verified by washing the probes in anoxic water, which removed ca. 70% of the Fe(III) content of probes from live cultures but did not alter the Fe(III) content of probes from abiotic controls. Measurements of the amount of Fe(III) oxide deposited in the medium versus the probes indicated that ca. 90% of the Fe(III) deposited in live cultures was formed biologically. Our findings show that bacterial Fe(II) oxidation is likely to generate reactive Fe(III) compounds that can be immediately available for use as electron acceptors for anaerobic respiration and that biological Fe(II) oxidation may thereby promote rapid microscale Fe redox cycling at aerobic-anaerobic interfaces.
Neutrophilic bacteria associated with Fe(III) oxide precipitation have been known for a long time (26). Some species were shown to precipitate Fe(III) oxides during heterotrophic metabolism (6, 7). However, neutrophilic autotrophic iron oxidation has only recently been reliably demonstrated (8, 10). The role of these bacteria in biogeochemical iron cycling, however, has not been extensively studied. Such lack of study can be attributed to the fact that Fe(II) is highly unstable in oxic environments at circumneutral pH, resulting in the widely held belief that Fe(II) will be rapidly oxidized regardless of the presence or absence of microbial catalysis (24). Furthermore, Emerson and Moyer (8) have convincingly demonstrated that Fe(II)-oxidizing bacteria do not alter the rate of Fe(III) oxide accumulation in diffusion-limited opposing-gradient systems.
Regardless of the possible influence of Fe(II)-oxidizing bacteria on rates of Fe(II) oxidation, these organisms have the potential to affect Fe(III) oxide precipitation processes by altering the spatial relationship between O2 and Fe(II) gradients. Emerson and Moyer (8) suggested that bacterial Fe(II) oxidation might promote coupling between iron oxidation and reduction by producing amorphous (9) or poorly crystalline Fe(III) oxides which are readily available for Fe(III)-reducing bacteria. The potential for a tight, microbially mediated coupling between iron oxidation and reduction has important environmental implications, given the critical influence which iron cycling exerts on the behavior of various organic and inorganic compounds in aquatic systems (24).
In this study we examined O2 and Fe(II) gradients together with bacterial numbers and patterns of Fe(III) oxide deposition at submillimeter resolution in Fe(II)-oxidizing gradient cultures, using organisms enriched from iron-rich freshwater wetland sediments. The goal was to determine the positioning of Fe(II)-oxidizing bacteria with respect to Fe(II) and O2 gradients and to examine how these organisms influence patterns of Fe(III) oxide deposition within these gradients.
MATERIALS AND METHODS
Enrichment and isolation.
A neutrophilic Fe(II)-oxidizing enrichment culture (TW1) was obtained from iron-rich surficial sediments of a freshwater wetland in the Talladega National Forest in north central Alabama by use of gradient cultures (15) with FeS as an Fe(II) source. After several passages on FeS (low iron) medium, the culture was transferred to medium with 50 mM PIPES [piperazine-N,N′-bis(2-ethenesulfonic acid)]-buffered FeCl2 · 2H2O as an iron source (high iron), the same medium used in the experiments reported here. The culture was shown to contain heterotrophic satellite bacteria capable of growth on rich medium (50% strength tryptic soy agar [TSA]). PCR-denaturing gradient gel electrophoresis (DGGE) analysis of ca. 200-bp 16S rRNA gene fragments was performed as described elsewhere (18) and revealed two strains, one presumably the lithotrophic Fe(II) oxidizer, and the other presumably a heterotrophic satellite. However, as described below, we found no evidence that the latter organism had a major impact on the results of our Fe(II) oxidation experiments. Another, apparently pure culture (TW2) used in this study was enriched and isolated by repeated passages on high-iron medium. This culture failed to grow on rich medium, and DGGE analysis revealed only a single genome. A BLAST search (2) on a 1,485-bp fragment of the 16S rRNA gene sequenced suggested that our organism is closely related (94% similarity) to Dechlorisoma suilla, a dissimilatory perchlorate-reducing bacterium (1, 4). Further molecular and physiological characterization of TW2 is under way.
Gradient cultures.
The gradient culture system consisted of two layers in 250-ml beakers: a bottom layer containing the Fe(II) source (50 mM FeCl2 in anaerobic 10 mM PIPES buffer with 2% [wt/vol] Noble agar [pH 7.0]), and a top layer consisting of mineral medium (NaHCO3, 30 mM; NH4Cl, 10 mM; KH2PO4, 1 mM) supplemented with vitamins and minerals (17) and stabilized with 0.25% Noble agar. Layer volumes were 25 ml (bottom) and 125 ml (top), resulting in layer depths of 12 and 60 mm, respectively. This concentration of Fe(II) was necessary to sustain Fe(II) flux over the course of the experiment. Although the Fe(II) concentration in the bottom layer was unrealistically high relative to natural systems, diffusion within the agar column resulted in environmentally relevant Fe(II) concentrations of 1 to 2 mM (22) close to the oxic-anoxic boundary. Prior to initiation of the experiment, beakers were covered with aluminum foil and autoclaved. The foil cover was kept on all the time except when components were added. After the bottom layer was poured, probes for Fe(II) and bacterial numbers (see below) were inserted, and the agar was allowed to solidify under an anaerobic atmosphere (about an hour under approximately 95:5 [vol/vol] N2-H2). The top layer, which had been degassed with 80:20 (vol/vol) N2-CO2, autoclaved in crimp-sealed bottles, and cooled to approximately 30°C, was then added to the system. The beakers were incubated overnight in an anaerobic chamber in order to allow a supply of Fe(II) to diffuse into the top layer prior to inoculation. Inoculation was achieved by inserting a pipette into the top layer and ejecting about 0.1 ml of the microaerobic inoculum (surface layer of a high-iron culture of TW1 or TW2) as the pipette was withdrawn; this procedure was repeated four to six times per culture. Although this procedure could have enhanced O2 penetration into the surface layer, our “time zero” O2 measurements revealed no difference between O2 profiles in inoculated and uninoculated cultures. Additionally, in later experiments with TW2, possible O2 introduction was controlled for by stabbing uninoculated controls with a sterile pipette in the manner similar to the inoculation procedure.
Fe(II) measurements.
We employed a diffusion microprobe technique modified from that of Davison et al. (5) to determine Fe(II) concentrations at submillimeter resolution in the gradient cultures. The probe consisted of a 0.1-mm-thick 5% (vol/vol) agar film attached to a glass microscope slide. The film was cast between hot (ca. 70°C) glass slides autoclaved in an aluminum foil boat, and the agar was allowed to solidify. Excess agar was trimmed from the sides of the slides, and the slides were separated, leaving the film attached to one slide. The probe was inserted into the culture beaker with the agar film facing the center. All of the above procedures were accomplished under aseptic conditions. Probes were retrieved immediately after the final O2 measurements (see below), dipped into 1% (wt/vol) potassium ferricyanide [K3Fe(CN)6] solution [which forms an insoluble blue complex with Fe(II)] for about 1 s, retrieved, and allowed to react for ca. 3 min after being removed from the solution. This fixed the Fe(II) within the agar film and provided a colored substance whose abundance could be quantified. Probes were then soaked in distilled water for ca. 5 min to remove excess potassium ferricyanide. Images of the probes were collected under a dissecting microscope with an attached camera. The images were then digitized and converted to black and white by use of Adobe PhotoShop. The optical density of the images, presumably representing the density of Fe3[Fe(CN)6]2, was measured by the NIH-Image software. For TW1 cultures and controls, three depth profiles from a single probe were collected and measured. For TW2, a single profile was recorded for each of the probes from triplicate cultures. Fe(II) concentrations in the probes were quantified against a calibration curve obtained by measuring the optical density of the agar strips of the same thickness incubated overnight in anoxic Fe(II)-EDTA solutions of known concentration. This yielded linear standard curves with an R2 of 0.8 or greater within the range from 1 to 25 mM Fe(II).
In order to validate the diffusion probe technique, culture systems were cored with a detipped 1-ml plastic syringe immediately after O2 measurements but before probe retrieval. The core was sectioned anoxically, and Fe(II) in several depth intervals was extracted with 0.5 M HCl and measured by the Ferrozine (23) method. To ensure complete extraction of Fe(II) from the agar, tightly closed vials containing 0.5 M HCl and sample were gently heated in a waterbath (ca. 80°C) until the agar dissolved.
To determine the abundance of Fe(III) in probes from control and live culture systems, high-iron cultures were set up as described above and incubated for 6 days at 20°C. Core samples of the medium which included the whole depth of the top layer were taken anaerobically and immediately placed into preweighed vials containing 0.5 M HCl. Probes were retrieved immediately after taking the core, and the agar film (exposed to the medium) was scraped off into preweighed vials containing 0.5 M HCl. The portion of the film exposed to the atmosphere above the medium dried out and was firmly attached to the slide, allowing collection of only the portion submerged in the medium. Acid extracts were analyzed using Ferrozine for Fe(II) and total Fe, from which Fe(III) content was calculated.
To estimate what percentage of the Fe(III) trapped in the probes comprised mobile (soluble and/or colloidal) compounds, triplicate sets of probes were retrieved from live and abiotic control cultures and washed three times in 100 ml of anoxic distilled water, transferring the probes each time into a fresh beaker of water. Results were compared to the Fe(III) content of triplicate unwashed probes retrieved from parallel cultures. The difference was assumed to represent the diffusionally mobile Fe(III) content.
Oxygen microelectrode measurements.
A Clark-style O2 microelectrode with guard cathode (21) (Diamond General Corp, Ann Arbor, Mich.), attached to an electronically controlled micromanipulator (National Aperture model MM33CR), was used to determine O2 profiles in the gradient cultures. The microelectrode was calibrated to indicate percent air saturation of O2, with a detection limit of approximately 0.1% saturation, which, under our conditions, was equivalent to 0.279 μM. Zero depth was set by manually lowering the electrode to the agar surface and identifying the moment of contact by the formation of a visible meniscus around the electrode tip. The electrode was raised until the tip separated from the surface and the meniscus disappeared, and slowly lowered until the meniscus formed again. At this point, the manipulator counter was set at zero. Oxygen was considered depleted when three consecutive measurements (covering a distance of 50 to 200 μm) below the detection limit (ca. 0.28 μM) were obtained.
In one experiment, O2 microprofiles were measured repeatedly over a 7-day course. In this case, uninoculated culture systems were measured first to minimize contamination. Between sampling, the electrode was allowed to stand in distilled water. The electrode was occasionally soaked in dilute HCl to remove oxide precipitates.
In some experiments, pH gradients were measured using a microcombination pH electrode (Orion) according to the manufacturer's instructions. Since this electrode has a relatively large tip (ca. 1 mm diameter), pH was measured at 1-mm intervals.
Bacterial numbers.
The Rossi-Cholodny buried slide technique (20) was employed to enumerate bacteria (in units of cells per unit area of slide) at submillimeter resolution in our cultures. This technique is based on colonization of glass slides inserted into stratified bacterial communities. After colonization, slides are retrieved, fixed, and stained, and the bacteria attached to them are enumerated. Although this technique enumerates only those bacteria which attach to the slide, it provides a better depth resolution (0.2 mm or better) than coring and slicing the culture (1 to 2.5 mm). A basic assumption of our application of this technique is that an equal percentage of bacteria attach to the slides at each depth, which is not unreasonable for a pure culture. Slides were inserted into the culture systems for the duration of the experiment, and bacterial growth was allowed to occur. Slides were then removed, fixed with 4% formaldehyde solution, and stained with acridine orange, and bacteria were counted under an epifluorescent microscope. Five fields were counted at each of the depth intervals spaced at 0.2 mm or greater. These area counts were used as a proxy for actual number of bacteria per unit volume.
RESULTS
Oxygen and Fe(III) oxide distributions.
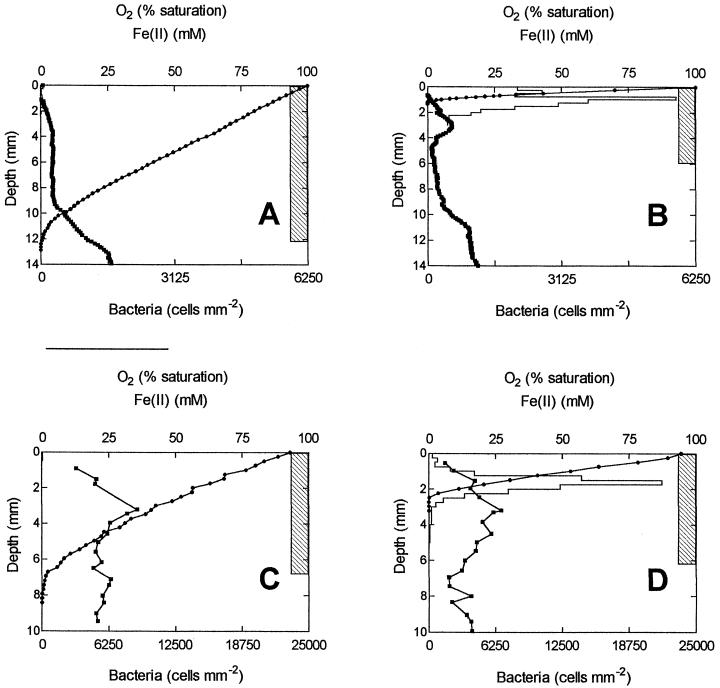
Oxygen gradients were much steeper in culture systems inoculated with Fe(II)-oxidizing bacteria than in sterile controls (Fig. 1). After 5 days of incubation, O2 penetrated to 12 mm in the controls, versus 1.5 mm in the TW1 culture (average of three separate profiles in a single system for both control and live cultures). A distinct bacterial plate formed at the oxic-anoxic interface in the live cultures, whereas in the control system only low bacterial numbers (>10-fold lower) were detected, and only at the surface (Fig. 1A and B). A similar experiment conducted with the presumably pure TW2 culture produced nearly identical results (Fig. 1C and D).
FIG. 1.
Distribution of Fe(II), particulate Fe(III) oxides, O2, and bacteria in two Fe(II)-oxidizing cultures. (B and D) TW1 and TW2 cultures, respectively. (A and C) Abiotic controls for the cultures shown in B and D, respectively. Note that Fe(II) profiles determined by densitometry in diffusion probes are confounded by the presence of Fe(III) compounds in the probe (see text). O2 profiles are averages of triplicate measurements. (A and B) Representative Fe(II) profile from triplicate profiles obtained from a single probe. (C and D) Fe(II) profiles are averages of single measurements from probes in triplicate cultures. No error bars are shown. Bacterial numbers in A and B are averages of counts on triplicate slides from a single culture (A and B) or averages of counts from a single slide from each of triplicate cultures (C and D); error bars were omitted for clarity. In control cultures, bacterial numbers were never significantly different from zero.
Fe(III) oxides were deposited below the O2 penetration zone in the bacterial cultures as opposed to the controls, in which the entire zone of oxide deposition was aerobic (Fig. 1).
Control experiments.
Since TW1 was not pure, control experiments in iron-free cultures were conducted to account for possible heterotrophic growth of the satellite organisms on impurities in the agar, because such growth could influence O2 gradients and thus confound interpretation of the oxide band data. TW1 formed a visible bacterial plate when inoculated into Fe-free gradient cultures and changed O2 profiles compared to uninoculated controls (data not shown). However, this was not the case for TW2, which failed to grow or alter the O2 profile in an identical Fe(II)-free system (data not shown).
The possible effect of the growth of heterotrophic bacteria in TW1 on the O2/Fe(II) relationship in our experimental systems was tested by inoculating a culture of satellite organisms isolated and grown on 50% strength TSA into Fe(II)-containing gradient systems. After 10 days of incubation, no significant differences between O2 profiles in inoculated and uninoculated control were detected (data not shown). These results suggest that the effect of the heterotrophic bacteria on Fe(II) oxidation, independent of their possible synergistic interaction with Fe(II)-oxidizing bacteria, was minor.
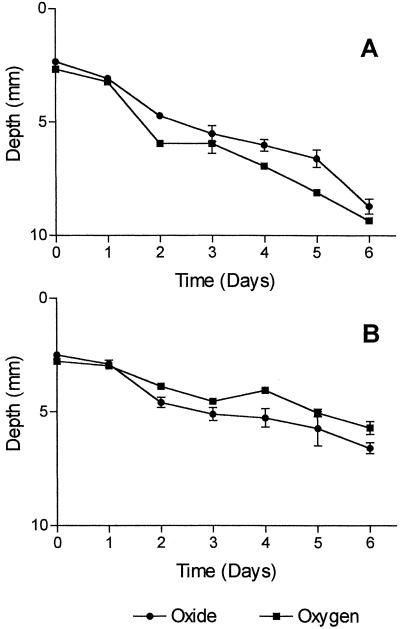
We were concerned that deposition of oxide below the depth of O2 penetration in the TW1 and TW2 cultures was an artifact caused by the existence of a deeper oxic-anoxic boundary early in the experiment. To test this possibility, measurements of O2 and Fe(III) oxide band positions were obtained at daily intervals over 6 days in cultures inoculated with TW1. In the abiotic control systems, the bottom boundary of the oxide band was always observed above the oxic-anoxic boundary. This is illustrated in Fig. 2; where the depth of the oxide band lower boundary and the depth of O2 penetration are plotted versus time; O2 always penetrated to depths below the lower boundary of the oxide band (Fig 2A) in the controls. In contrast, in the TW1-inoculated cultures, the depth of O2 penetration (as defined above) was above the bottom boundary of the oxide band at all times except for the first two measurements (Fig. 2B). In addition, the depth of O2 penetration increased steadily during the experiment, which argues against the possibility that the oxides were deposited in association with an upward-retreating O2 front.
FIG. 2.
Relationship between O2 penetration depth and the base of the oxide band in control and TW1-inoculated cultures. Data represent averages of triplicate cultures, and error bars show 95% confidence intervals.
Iron distributions.
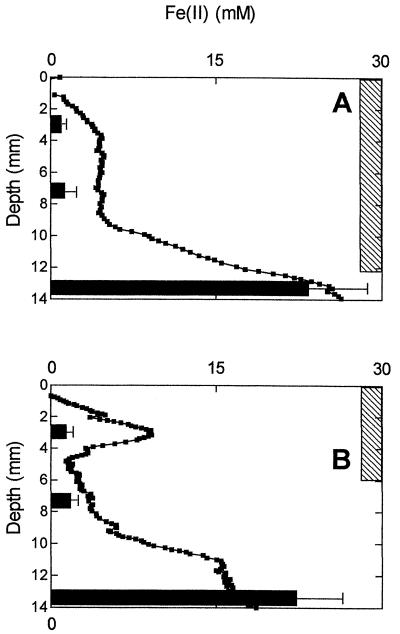
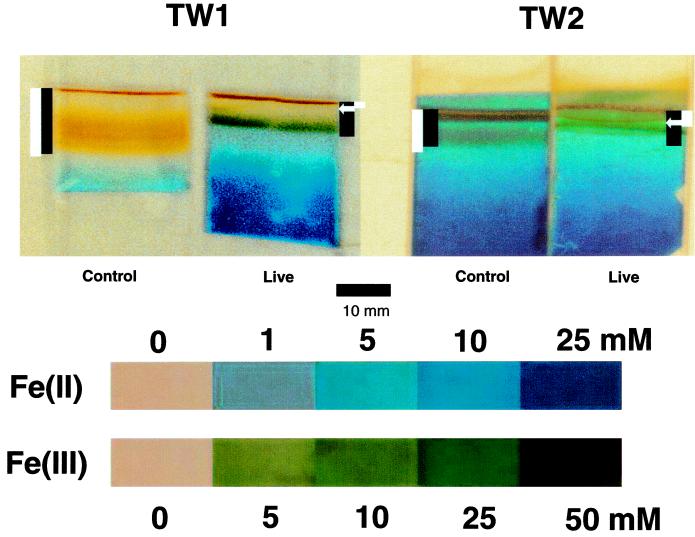
Fe(II) measurements obtained from the diffusion microprobes agreed well with Ferrozine analysis at depth in the cultures (Fig. 3). However, near the surface, the probes indicated Fe(II) concentrations several times higher than found by the Ferrozine analyses. This effect was likely due to (i) the presence of heavy oxide deposits in the probe from the control culture and to (ii) the presence of a dark green band in the probe from the live TW1 and TW2 cultures (Fig. 4). In both cases, the dark bands were detected by densitometric analysis of the images of the probes, leading to erroneously high Fe(II) concentration estimates. The green band observed in the probe from the live cultures was not observed in the untreated probes and was likely due to the presence of soluble or colloidal Fe(III) compounds which reacted with the potassium ferricyanide reagent. We infer this from the fact that potassium ferricyanide is known to form a green precipitate with Fe(III) at neutral pH (Fig. 4, standards) and that no other components in the medium were present in quantities sufficient to produce such a colored precipitate. Because no such bands were evident in the control cultures, the formation of soluble or colloidal Fe(III) can be attributed to bacterial activity.
FIG. 3.
Comparison of soluble Fe(II) concentration determined by microprobe technique and Ferrozine in control (A) and TW1-inoculated (B) cultures. Ferrozine data are averages of triplicate cores, and error bars represent the 95% confidence interval; they are omitted if smaller than the size of the symbol. For the microprobes, a single representative profile from three different measurements from a single probe is shown.
FIG. 4.
Photo of K3Fe(CN)6-fixed microprobes from control and Fe(II)-oxidizing organism-inoculated cultures shown in Fig. 1. Superimposed black bars indicate the positioning of Fe(III) oxide bands, measured independently, in the cultures; white bars denote oxic zones. Arrows indicate the approximate position of the bacterial number peaks (live cultures only). Standards were photographed under different light conditions and may not be directly comparable with the microprobes.
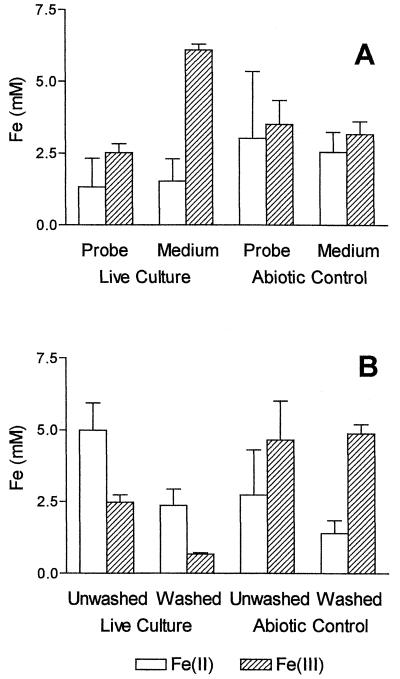
Although copious amounts of Fe(III) oxide deposits, detected by their brownish color, were observed in probes from the sterile control cultures, much smaller quantities of such oxides were evident in the probes from the live cultures (Fig. 4). Similar observations were obtained consistently with both the mixed (TW1) and pure (TW2) cultures. As discussed further below, the lower abundance of particulate oxide deposits in live than in control cultures indicates that bacterial catalysis was the dominant mechanism for Fe(II) oxidation in the live cultures. In order to verify quantitatively the lower abundance of particulate oxides in probes from the live cultures, we measured the concentrations of Fe(III) in probes as well as in cores of whole medium in three replicate live and control cultures. We also examined how washing affected Fe(III) concentrations in probes from live cultures, which, as mentioned above, appeared to contain soluble and/or colloidal Fe(III). The concentration of Fe(II) was nearly identical in probes and whole medium in both culture systems (Fig. 5A). The same was true for Fe(III) concentrations in the control systems. In contrast, the concentration of Fe(III) was about threefold higher in whole medium than in probes from the live culture. Since oxides from the control culture did not dissolve completely in 0.5 M HCl (discussed below), the actual ratio is probably higher. These findings quantitatively confirm our consistent visual observation of lower Fe(III) oxide abundance in probes from live versus control cultures.
FIG. 5.
Fe(II) and Fe(III) in whole medium samples and diffusion probes. (A) Comparison among probes and the medium in TW2 and control cultures; (B) comparison between washed and unwashed probes from TW2 and control cultures. Data represent averages of triplicate cultures, and error bars show 95% confidence intervals.
Washing of probes from the live culture resulted in removal of approximately 70% of their Fe(III) content (Fig. 5B, right). These results verified the presence of soluble Fe(III), as indicated by the green band in potassium ferricyanide-fixed diffusion probes. In contrast, identical washing of probes from the abiotic control cultures resulted in no change in Fe(III) content, whereas the amount of Fe(II) was significantly decreased (Fig. 5B, left). This observation is consistent with the potassium ferricyanide analysis and further indicates that the presence of mobile Fe(III) forms is a biologically mediated phenomenon. The concentration of Fe(III) in whole medium from live cultures was more than twice as high as in probes from the same cultures (Fig. 5A). In addition, we can attribute most of the Fe(III) present in the probe to soluble or colloidal Fe(III) compounds (detected visually with potassium ferricyanide) (Fig. 4), since washing with anoxic distilled water removed a large portion (ca. 70%) of the Fe(III) from these probes (Fig. 5B).
DISCUSSION
Oxygen distribution and locus of oxide deposition.
One of the most important observations in this study is the inversion of the locus of oxide deposition in relation to the depth of O2 penetration in biotic and abiotic opposing-gradient systems. In the abiotic systems, the whole oxide band resided within the oxic zone (Fig. 1A and C). However, when Fe(II)-oxidizing bacteria were present, a significant part of the band was located below the depth of O2 penetration, which was substantially shallower than in abiotic control cultures (Fig. 1B and D). This observation held true for both mixed and pure cultures. The control experiments demonstrated that the satellite bacteria present in TW1 did not alter O2 gradients in the presence of Fe(II). Since TW2 failed to grow or alter O2 gradients in the absence of Fe(II), we can safely assume that in all our experiments, O2 gradients were controlled by bacterially mediated Fe(II) oxidation rather than heterotrophic metabolism.
A certain amount of Fe(III) oxide could have been deposited below the apparent O2 penetration boundary by means of Fe(II) reacting with O2 at concentrations below the detection limit of our electrode. However, several lines of evidence argue that this phenomenon had no significant confounding effect on our observations. First, the pronounced suboxic Fe(III) oxide deposition observed in the live cultures (up to 60% of the oxide band width, or up to approximately 4 mm, was found below the O2 penetration boundary), together with our conservative estimate of the O2 depletion depth (see Materials and Methods), suggests that even if O2 was present at concentrations below the detection limit (<1 μM), it would have been depleted before reaching the bottom of the oxide band. In addition, no suboxic deposition was detected in abiotic cultures, in which gradients were not as sharp as in the live system, which suggests that interaction between Fe(II) and O2 at subdetectable concentration did not play a significant role in suboxic deposition of Fe(III) compounds. Finally, the postulated presence of mobile forms of Fe(III) (discussed below) suggests a plausible mechanism responsible for the observed effects.
Previous studies in opposing-gradient systems similar to our low-iron system demonstrated decreased O2 penetration depth in biotic versus abiotic cultures (8). However, no inversion of the oxide band relative to O2 gradient position was reported. In the study of Emerson and Moyer (8), the use of FeS as an Fe(II) source provided a submillimolar equilibrium concentration of Fe2+ at circumneutral pH (24), far lower than the concentration of Fe(II) present in our culture systems. This low iron content resulted in lesser oxide deposition over the course of the experiment relative to that in the experiments presented here. Consequently, there was less material to be observed, so oxide deposition below the O2 penetration zone (if any) would probably not have been as apparent as in our study. A recent study of bacterial Fe(II) oxidation by Benz et al. (3), in which millimolar concentrations of soluble Fe(II) were employed in an opposing-gradient system, failed to demonstrate suboxic deposition of Fe(III) alone or in the presence of microaerophilic nitrate-reducing, Fe(II)-oxidizing bacteria. The contrast between these findings and our own suggests that suboxic Fe(III) deposition may depend on the type of bacteria involved in Fe(II) oxidation—specifically, perhaps, on the organism's ability to produce a mobile form of Fe(III) as the initial end product of Fe(II) oxidation (see below).
Biotic and abiotic oxidation.
Our original intent in deploying the diffusion probes was to try to detect the influence of bacterial activities on Fe(II) microgradients at the aerobic-anaerobic interface, in a manner analogous to the analysis of the influence of Beggiatoa spp. on H2S oxidation done by Nelson et al. (19). Unfortunately, due to Fe(III) interference with the densitometric measurements, the diffusion probes did not provide satisfactory measurements of dissolved Fe(II) microgradients at the aerobic-anaerobic interface. However, they revealed several effects of biologically catalyzed Fe(II) oxidation which have not been described previously.
The relative scarcity of particulate oxides in the diffusion probes from the live cultures suggests that Fe(II) oxidation was dominated by biological processes. Since the 5% agar content of the probe would be expected to effectively exclude bacteria, only chemical oxidation would be expected to occur within the agar film. Equilibrium is achieved very rapidly in films as thin as our probes (h = 0.1 mm). Assuming a diffusion coefficient (D) of 10−5 cm2 s−1 for Fe(II) (5), the characteristic diffusion time (h2/D) is only 10 s, which is essentially instantaneous on the time scale of a week-long experiment. The low abundance of oxide deposits in probes from the live cultures therefore suggests that the Fe(II)-oxidizing bacteria scavenged Fe(II) rapidly enough to strongly depress Fe(II) diffusion into the probe and subsequent abiotic oxidation and oxide precipitation within the film.
The above findings suggest that the diffusion probes may provide a tool for distinguishing quantitatively between biological and abiotic oxidation of Fe(II) in opposing-gradient systems. Simply comparing total Fe(III) oxide deposition in biotic and abiotic systems will not be adequate, because the limiting step in Fe(II) oxidation is often diffusional transport of the reduced compounds rather then the reaction itself (8). However, by applying a diffusion probe to the system and quantifying the amount of ferric oxide deposited per unit volume of the probe [corrected for the presence of soluble Fe(III)], it should be possible to estimate the amount of oxide which was deposited abiotically. The difference between the concentration of oxide accumulated within the probe and that in the system as a whole will reflect the amount of oxide deposition that was biologically catalyzed. Applying this approach to our live cultures, we can estimate the percentage of Fe(III) oxide deposited abiotically as (Fe(III)Pprobe)/Fe(III)Tmedium, where Fe(III)Pprobe is the Fe(III) remaining in the probe after anoxic wash and Fe(III)Tmedium is the total Fe(III) in the medium. Since Fig. 5B indicates that ca. 70% of the Fe(III) present in the probe from the live culture is lost during wash and Fig. 5A shows that there was ca. 2.5 times more total Fe(III) in the live culture medium than in the probe, we can estimate that ca. 90% of total Fe(III) in the live cultures was generated via biological processes. In fact, this fraction might be higher in steady-state systems, since in our situation some Fe(II) oxidation inevitably occurred abiotically before a plate of Fe(II)-oxidizing bacteria was established. The presence of similar Fe(III) oxide concentrations in the probes and the whole medium in control cultures suggested that Fe(II) oxidation and Fe(III) oxide deposition proceeded at similar rates and by the same mechanism in both the medium and the probes.
Soluble Fe(III) formation.
The observation of a green band in the live system (Fig. 4) suggested the presence of soluble and/or colloidal forms of Fe(III). The presence of such compounds was verified by analysis of washed and unwashed probes (Fig. 5B). Although the pH decrease associated with Fe(II) oxidation at the O2-Fe(II) boundary (8) might potentially account for the Fe(III) remaining in solution, in our experiments such a decrease was far less than would be required to stabilize any significant amount of Fe(III) (the lowest pH value observed was 6.6 in the zone of oxide deposition, compared to 7.2 at the surface; data not shown). It is possible that the Fe(III) was kept in solution by a chelator excreted by the bacteria specifically for the purpose of retarding or at least delaying cell surface encrustation with oxide precipitates. Encrustation of the bacterial cells with particulate oxides, leading to their eventual entombment, has been suggested as one of the possible environmental challenges which gradient-dwelling solid-phase oxide-producing organisms have to overcome (8). Formation of soluble Fe(III) compounds and eventual remote deposition of the oxides may reduce or delay such encrustation. We hypothesize that, as soluble ferric iron complexes diffuse away from the Fe(II)-oxidizing bacteria, they become destabilized, resulting in precipitation of Fe(III) oxides within as well as below the zone of O2 penetration.
Davison et al. (5), using diffusion microprobe techniques in lake surface sediments, identified a soluble iron peak in their gels at a depth of approximately 8 mm beneath the sediment-water interface. The authors hypothesized that this peak represented accumulation of Fe(II) as a result of localized Fe(III) oxide reduction activity. However, their analysis could not distinguish between mobile forms of Fe(III) and Fe(II). Our results suggest a possible alternative explanation, i.e., that the activity of the iron-oxidizing bacteria caused a local accumulation of soluble Fe(III). Interestingly, a green band was observed at a depth of ca. 2 mm when a diffusion probe was applied to a sediment core from the freshwater wetland from which the organisms used in this study were obtained (not shown). A recent study, employing voltammetric electrodes, demonstrated the presence of soluble organic complexes of Fe(III) in marine surface sediments (25). These observations suggest that the presence of bacterially generated dissolved or colloidal Fe(III) might be more widespread than has previously been recognized.
Biogeochemical implications.
Measurements of Fe(III) abundance in the diffusion probes and the whole medium showed that the vast majority of Fe(II) was oxidized biologically in the presence of Fe(II)-oxidizing bacteria. These results suggest that bacteria can compete successfully with the abiotic oxidation process and that the biological oxidation of Fe(II) might be the predominant process leading to the formation of Fe(III) oxides in surficial aquatic sediments. Emerson and Revsbech (10) found that organisms from a natural Fe(III)-depositing bacterial mat accelerated Fe(II) oxidation in a reactor system designed to simulate the in situ conditions. Since their system was not diffusionally limited, acceleration of Fe(II) oxidation when bacteria were present suggested a significant involvement of those organisms in the process. Our experiments show that Fe(II)-oxidizing bacteria could be similarly involved in Fe(III) generation in a diffusion-limited system.
Our results indicate that Fe(II)-oxidizing bacterial activity can lead to suboxic deposition of reactive Fe(III) oxides. An important implication of these findings is that they suggest the possibility for a rapid coupling between Fe(II) oxidation and Fe(III) oxide reduction within millimeters of the oxic-anoxic interface. Furthermore, Emerson and Revsbech (9) found active Fe(III)-reducing bacteria in a natural Fe(II)-oxidizing mat in an iron seep, indicating tight coupling of Fe(II) oxidation and Fe(III) reduction.
Fe(III) produced by Fe(II)-oxidizing bacteria has traditionally been considered to be represented by immobile, solid-phase compounds. Our findings suggest, however, that, at least immediately after formation, Fe(III) compounds can be treated as soluble ions which are subject to diffusive transport. As mobile Fe(III) compounds diffuse away from the bacterial plate, they are likely to become destabilized, resulting in hydrolysis and precipitation of amorphous Fe(III) oxides. Formed in the anoxic zone, these oxides would be immediately available for reduction by Fe(III)-reducing bacteria, completing the microscale Fe redox cycle. In addition to sediment-water interface environments, other environments where microscale bacterial Fe redox coupling might occur include subsurface sediments, where Fe(II) and O2 may coexist in “patchy” redox environments, forming a dynamic network of oxic-anoxic interfaces (14), and the rhizosphere of aquatic plants, in which rapid Fe cycling is known to occur (12, 13, 22) and in which the presence of both Fe(II)-oxidizing and Fe(III)-reducing bacteria in close association with plant roots has recently been demonstrated (11, 16).
ACKNOWLEDGMENTS
This research was supported by grants from the National Science Foundation (DEB 94-7233), the U.S. Department of Energy, Office of Energy Research, Environmental Management Science Program (DE-FG07-96ER62321), and the School of Mines and Energy Development, University of Alabama.
We thank D. Emerson, W. C. Ghiorse, and R. G. Wetzel for review of an earlier version of the manuscript.
REFERENCES
- 1.Achenbach, L. A., U. Michaelidou, R. A. Bruce, J. Fryman, and J. D. Coates. Dechlorimonas agitata n. n. gen., sp. nov., and Dechlorosoma suillum n. n. gen., sp. nov. Two novel environmentally dominant (per) chlorate-reducing bacteria and their phylogenetic position. Int. J. Syst. Evol. Microbiol., in press. [DOI] [PubMed]
- 2.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benz M, Brune A, Schink B. Anaerobic and aerobic oxidation of ferrous iron at neutral pH by chemoheterotrophic nitrate-reducing bacteria. Arch Microbiol. 1998;169:159–165. doi: 10.1007/s002030050555. [DOI] [PubMed] [Google Scholar]
- 4.Coates J D, Michaelidou U, Bruce R A, O'Connor S M, Crespi J N, Achenbach L A. Ubiquity and diversity of dissimilatory (per)chlorate-reducing bacteria. Appl Environ Microbiol. 1999;65:5234–5241. doi: 10.1128/aem.65.12.5234-5241.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davison W, Grime G W, Morgan J A W, Clarke K. Distribution of dissolved iron in sediment pore waters at submillimeter resolution. Nature. 1991;352:323–325. [Google Scholar]
- 6.Dubinina G A. Functional role of bivalent iron and manganese oxidation in Leptothrix pseudoochracea. Mikrobiologiya. 1978;47:783–789. [PubMed] [Google Scholar]
- 7.Dubinina G A. Mechanism of the oxidation of the divalent iron and manganese by iron bacteria growing at a neutral pH of the medium. Mikrobiologiya. 1978;47:591–599. [PubMed] [Google Scholar]
- 8.Emerson D, Moyer C. Isolation and characterization of novel iron-oxidizing bacteria that grow at circumneutral pH. Appl Environ Microbiol. 1997;63:4784–4792. doi: 10.1128/aem.63.12.4784-4792.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emerson D, Revsbech N P. Investigation of an iron-oxidizing microbial mat community located near Aarhus, Denmark: field studies. Appl Environ Microbiol. 1994;60:4022–4031. doi: 10.1128/aem.60.11.4022-4031.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emerson D, Revsbech N P. Investigation of an iron-oxidizing microbial mat community located near Aarhus, Denmark: laboratory studies. Appl Environ Microbiol. 1994;60:4032–4038. doi: 10.1128/aem.60.11.4032-4038.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emerson D, Weiss J V, Megonigal J P. Iron-oxidizing bacteria are associated with ferric hydroxide precipitate (Fe-plaque) on the roots of wetland plants. Appl Environ Microbiol. 1999;65:2758–2761. doi: 10.1128/aem.65.6.2758-2761.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frenzel P, Bosse U, Janssen P H. Rice roots and methanogenesis in a paddy soil: ferric iron as an alternative electron acceptor in the rooted soil. Soil Biol Biochem. 1999;31:421–430. [Google Scholar]
- 13.Giblin A E, Howarth R W. Porewater evidence for a dynamic sedimentary iron cycling in salt marshes. Limnol Oceanogr. 1984;29:47–63. [Google Scholar]
- 14.Hunter K S, Wang Y, Van Cappelen P. Kinetic modelling of microbially-driven redox chemistry of the subsurface environment: coupling transport, microbial metabolism and geochemistry. J Hydrol. 1998;209:53–80. doi: 10.1016/s0169-7722(00)00158-3. [DOI] [PubMed] [Google Scholar]
- 15.Jones J G. A note on isolation and enumeration of bacteria which deposit and reduce ferric iron. J Appl Bacteriol. 1983;54:305–310. [Google Scholar]
- 16.King G M, Garey M A. Ferric iron reduction by bacteria associated with the roots of freshwater and marine macrophytes. Appl Environ Microbiol. 1999;65:4393–4398. doi: 10.1128/aem.65.10.4393-4398.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lovley D R, Phillips E J P. Availability of ferric iron for microbial reduction in bottom sediments of the freshwater tidal Potomac River. Appl Environ Microbiol. 1986;52:751–757. doi: 10.1128/aem.52.4.751-757.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muyzer G, Waal E C D, Uitterlinden A G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol. 1993;59:695–700. doi: 10.1128/aem.59.3.695-700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson D C, Revsbech N P, Jorgensen B B. Microoxic-anoxic niche of Beggiatoa spp.: microelectrode survey of marine and freshwater strains. Appl Environ Microbiol. 1986;52:161–168. doi: 10.1128/aem.52.1.161-168.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perfil'ev B V, Gabe D R. Capillary methods of investigating micro-organisms. Toronto, Canada: University of Toronto Press; 1969. [Google Scholar]
- 21.Revsbech N P. An oxygen microelectrode with guard cathode. Limnol Oceanogr. 1989;34:472–476. [Google Scholar]
- 22.Roden E E, Wetzel R G. Organic carbon oxidation and suppression of methane production by microbial Fe(III) oxide reduction in vegetated and unvegetated freshwater wetland sediments. Limnol Oceanogr. 1996;4:1733–1748. [Google Scholar]
- 23.Stookey L L. Ferrozine—a new spectrophotometric reagent for iron. Anal Chem. 1970;42:779–781. [Google Scholar]
- 24.Stumm W, Morgan J J. Aquatic chemistry: chemical equilibria and rates in natural water. 2nd ed. New York, N.Y: Wiley-Interscience; 1996. [Google Scholar]
- 25.Taillefert M, B. B A, Luther G W., III Reactivity of freshly formed Fe(III) in synthetic solutions and (pore) water: voltammetric evidence of an aging process. Environ Sci Technol. 2000;34:2169–2177. [Google Scholar]
- 26.Winogradski S. Über Eisenbakterien. Bot Ztg. 1888;46:263–270. [Google Scholar]