Abstract
Objectives:
Urine propylene glycol (PG) and vegetable glycerin (VG) were evaluated as potential markers for discriminating ECIG users from non-users and verifying ECIG abstinence.
Methods:
Urine samples from 51 ECIG users (collected pre/post 12-hours ECIG abstinence), and 50 controls (who do not use nicotine/tobacco) were analyzed for urine cotinine, PG, and VG concentration.
Results:
Of 42 ECIG users with pre-abstinence urine cotinine indicating nicotine use, mean (SD) urine cotinine concentration was 1053.7 ng/ml (874.5) and for controls was 1.93 ng/ml (0.4); after abstinence, ECIG users’ mean cotinine decreased to 615.4 ng/ml (753.0). For ECIG users, mean urine PG pre-abstinence was 25.6 mcg/ml (20.0) and was 9.8 mcg/ml (13.5) for controls; after abstinence, ECIG users’ mean urine PG decreased to 9.7 mcg/ml (15.0; ps < .05). For ECIG users, mean urine VG pre-abstinence was 7.5 mcg/ml (7.1) and was 13.2 mcg/ml (25.0) for controls; after abstinence, ECIG users’ mean VG decreased to 5.0 mcg/ml (4.4; ps < .05).
Conclusions:
ECIG users’ mean urine PG was greater than controls and decreased after 12-hours ECIG abstinence suggesting urine PG may be useful for discriminating ECIG users from non-users and verifying short-term abstinence.
Keywords: electronic cigarette, e-cigarette, propylene glycol, vegetable glycerin, biomarker
Electronic cigarettes (ECIGs) heat a liquid solution to produce an aerosol for users to inhale for users to inhale. ECIGs share common features including a battery, heating element, and a liquid comprised of propylene glycol (PG) and vegetable glycerin (VG), flavorants, and nicotine. Biomarkers of ECIG exposure would have utility for verifying ECIG use status in clinical and research settings and to inform policymaking and regulation, though no such biomarkers have been identified1,2.
Biomarkers are used in research and clinical settings to determine tobacco use status3,4. For example, biomarkers of cigarette smoking, such as cotinine, tobacco specific nitrosamines (TSNAs), and expired air carbon monoxide (CO) can distinguish smokers from non-smokers, verify self-reported tobacco use status, assess abstinence status3–8, and facilitate smoking cessation9. However, these biomarkers are not found in exclusive ECIG users and biomarkers of exclusive ECIG use and abstinence have not been identified10. Nicotine exposure can be detected following ECIG use through plasma nicotine or saliva/urine cotinine; however, nicotine is not specific to ECIG use3,5.
Two ECIG-liquid constituents, PG and VG, can comprise up to 95% of ECIG-liquid11, and these solvents may be useful markers to discriminate ECIG users from non-users and to determine short-term ECIG abstinence. However, because PG and VG also are contained in many commercially available products (eg, foods, beverages, and personal care products), determining whether ECIG users have higher exposure to PG and VG relative to individuals who do not use ECIGs is important. The purpose of this study was to examine if urine PG and/or VG concentrations are greater in ECIG users relative to individuals who do not use ECIGs/tobacco and assess whether urine PG and/or VG can be used as a biomarker for recent (ie, past 12–24 hour) ECIG use or abstinence.
METHOD
Participants & Study Procedures
Data were collected as a part of a broader study assessing nicotine and toxicant exposure in ECIG users conducted from Fall of 2016- Spring 2019. Experienced ECIG users and individuals who reported no use of ECIGs/tobacco-containing products (controls) were eligible to participate if they reported being healthy and between 18–55 years of age.
ECIG users.
ECIG users were eligible if they reported using: an ECIG daily for ≥1 month, ≥1 ml of ECIG-liquid/cartridge daily, and a liquid containing ≥3 mg/ml nicotine. Exclusion criteria included use of: other tobacco products >3 times weekly, NRT in the past 7 days, marijuana ≥10 days in the past 30 days, or illicit drugs in the past 30 days. Self-reported chronic disease was also exclusionary. Eligible ECIG users provided a urine sample on 2 separate visits completed in a fixed order. Visit 1 was preceded by participants’ use of their own ECIG/liquid (ie, normal use visit) and visit 2 was preceded by ≥12 hours abstinence from ECIG/tobacco use. Prior to Visit 1, ECIG users were instructed to use their ECIG/liquid as they normally do; however, the time course of use was not controlled. During each visit, abstinence from combustible tobacco was verified via participants’ expired air CO (<10 ppm; BreathCO monitor; Vitalograph, Lenexa, KS) and during visit 2 a bogus pipeline saliva test was administered12 to improve participant compliance with ECIG abstinence requirements.
Controls:
Controls were eligible if they reported no tobacco use (ie, cigarettes, ECIGs, smokeless, little cigars) in the past year and reported lifetime use of ≤100 cigarettes and ≤100 ECIGs. Exclusion criteria related to marijuana, other illicit drugs, and chronic disease were the same as for ECIG users. Eligible controls completed one visit in which they provided a urine sample. A semi-quantitative test for urine cotinine (NicAlert; had to be <3; ie, 0–200 ng/ml) and an expired air CO sample (<3 ppm) were used to confirm non-ECIG/tobacco use status.
Outcome Measures and Sample Analysis
All urine samples were stored at −80° C until analysis. Urine concentrations of cotinine (to verify tobacco use status), PG, and VG were determined using liquid chromatography tandem mass spectrometry (LC-MS/MS); limit of quantitation for PG and VG was 1.0 mcg/ml. A method for extracting and measuring PG and VG in urine was developed and validated using a subset of participants from this study (without regard to normal ECIG use or abstinence status when reporting results) and is detailed elsewhere13. Urine PG and VG concentrations were adjusted for creatinine.
Statistical Analyses
Differences in urine cotinine, PG, and VG concentration between ECIG users and controls were assessed using independent samples t-tests. For ECIG users, within-participant differences in urine cotinine, PG, and VG concentrations pre- and post-12 hours ECIG abstinence were assessed using paired samples t-tests. For ECIG users, who were the only participants to provide data for an abstinence condition, difference scores were generated by subtracting post-abstinence from pre-abstinence concentrations for cotinine, PG and VG.
Prior to conducting analyses, urine cotinine concentration for 51 ECIG users was inspected to confirm ECIG use status using an inclusion criterion of urine cotinine ≥200 ng/ml (following normal ECIG use for visit 1); nine had urine cotinine <200 ng/ml) and their data were therefore excluded.
RESULTS
Participant Characteristics
ECIG users.
The 42 confirmed ECIG users’ had a mean age of 27.8 (SD=7.9) and 24% were women; 73.8% self-identified as White/Caucasian, 10.5% as African-American/Black (AA), 10.5% as Asian, and the remaining 5.2% as more than one race or ‘other.’ On average, they had been using their ECIG for 1.8 years (SD=1.2), used an ECIG of 58.4 watts (SD=44.9), and used 6.5 ml of ECIG-liquid daily (SD=5.3) with a mean nicotine concentration of 8.2 mg/ml (SD=7.7). Average expired air CO was 2.1 ppm (SD=1.6), consistent with no recent combustible tobacco use, although 4 ECIG users reported occasional smoking, averaging 0.2 cigarettes/week (SD=1.6).
Controls.
The 50 controls had a mean age of 26.0 (SD=9.9) and 78% were women; 58% self-identified as White/Caucasian, 18% as Asian, 12% as AA/Black, 10% as Hispanic, and 2% as ‘other.’ Average expired air CO was 1.5 ppm (SD=0.5).
Urine Cotinine
Mean urine cotinine concentration for ECIG users’, following regular ECIG use, was 1053.7 ng/ml (SD=874.5), consistent with recent nicotine/tobacco use. Relative to ECIG users, controls had significantly lower mean urine cotinine concentration of 1.93 ng/ml (SD=0.4; p < .05), consistent with no nicotine/tobacco use. Following 12 hours of self-reported abstinence, ECIG users’ mean cotinine concentration decreased significantly to 615.4 ng/ml (SD=753.0; p < .05). ECIG users’ mean decrease in urine cotinine pre-to-post abstinence was 467.7 ng/ml (SD=459.6).
Urine Propylene Glycol (PG)
Results for urine PG are presented in Table 1. For ECIG users’, mean urine PG concentration, following normal ECIG use, was 25.6 mcg/ml (SD=20.0) and was significantly lower for controls whose mean urine PG was 9.8 mcg/ml (SD=13.5; p < .05; see Figure 1). Following 12 hours self-reported abstinence, ECIG users’ mean urine PG concentration decreased significantly to 9.7 mcg/ml (SD=15.0; p < .05) and no longer differed significantly from controls. ECIG users’ mean decrease in urine PG pre-to-post abstinence was 16.7 mcg/ml (SD=20.8).
Table 1.
Mean (SD) and Median PG and VG Concentrations for Daily ECIG Users and Controls
| ECIG users (N = 42) | Controls (N = 50) | |
|---|---|---|
| PG concentration (mcg/ml) | ||
| Mean (SD) | 25.6 (20.0) | 9.8 (13.5) |
| Median | 20.9 | 3.2 |
| PG concentration abstinent (mcg/ml) | ||
| Mean (SD) | 9.7 (15.0) | n.a |
| Median | 4.9 | n.a. |
| VG concentration (mcg/ml) | ||
| Mean (SD) | 7.5 (7.1) | 13.2 (25.0) |
| Median | 5.1 | 4.4 |
| VG concentration abstinent (mcg/ml) | ||
| Mean (SD) | 5.0 (4.4) | n.a. |
| Median | 4.1 | n.a. |
Mean (SD) and median values for urine propylene glycol (PG) and vegetable glycerin (VG) concentration for daily ECIG users pre and post 12-hour ECIG/nicotine abstinence and for controls. Urine PG and VG normalized for creatinine. n.a. = not applicable/not measured; control group did not undergo an abstinence period as they were not users of ECIGs or nicotine/tobacco products. For controls, urine VG concentrations are presented with two outliers included whose urine VG concentrations were 5–6 times greater than the mean, possibly due to greater than average exposures to VG-containing food and/or products. Upon removal of these two outliers, mean urine VG concentration was 8.1 mcg/ml (SD=10.9) for controls (N=48).
Figure 1. Mean (+SEM) Urine PG and VG Concentrations for Daily ECIG Users and Controls.
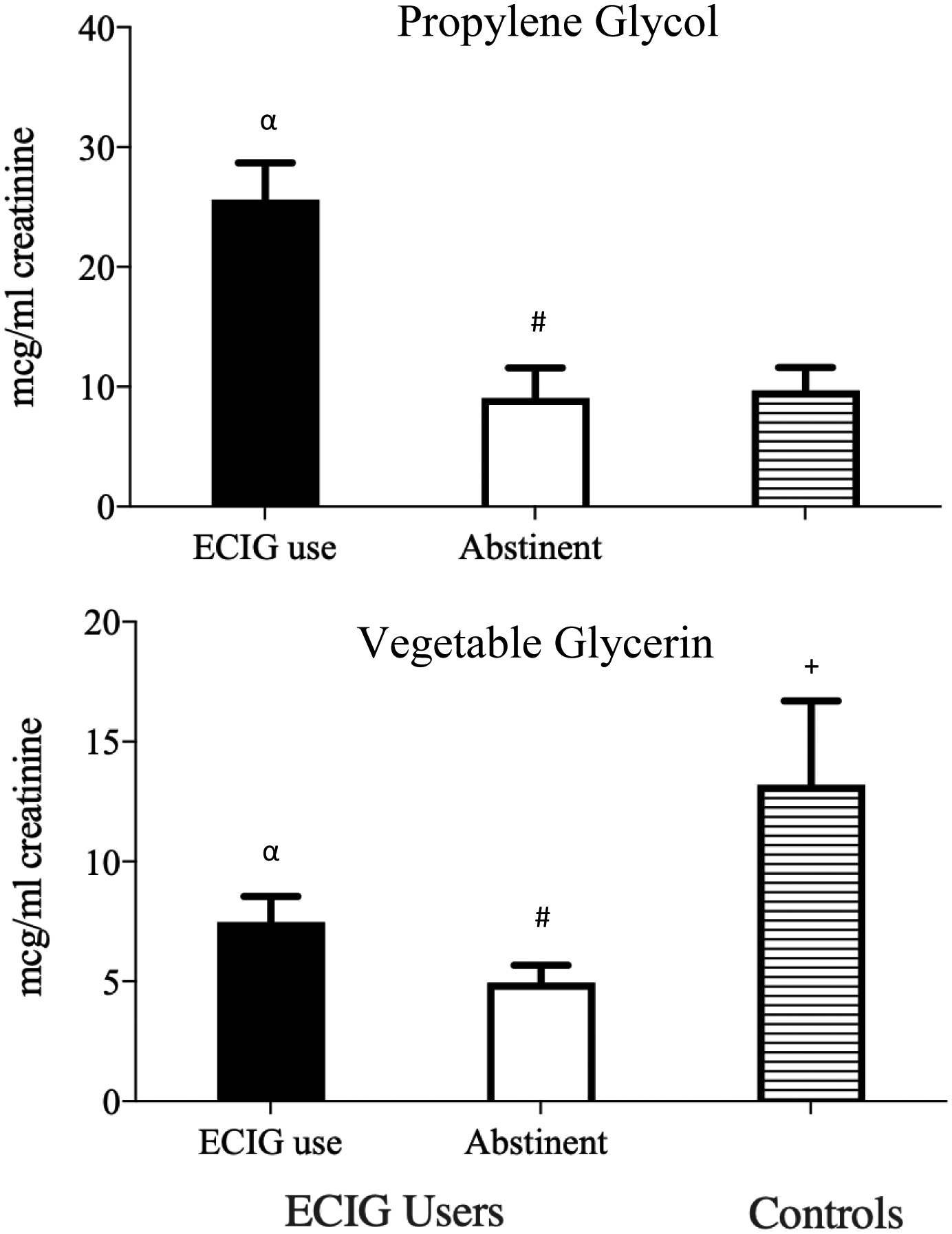
Mean (+SEM) values for urine propylene glycol (PG) and vegetable glycerin concentration for experienced ECIG users pre- and post- 12-hours ECIG/nicotine abstinence and for controls. Among ECIG users, pound symbols (#) indicate significant differences pre- and post-ECIG/nicotine abstinence. Alpha symbols (α) indicate significant differences between non-abstinent ECIG users and controls; crosses (+) indicate significant differences between 12-hour abstinent ECIG users and controls. Note that, for controls, data for urine VG are presented with two outliers included whose urine VG concentrations were 5–6 times greater than the mean, possibly due to greater than average exposures to VG-containing food and/or products.
Urine Vegetable Glycerin (VG)
ECIG users’ mean urine VG concentration, following normal ECIG use, was 7.5 mcg/ml (SD=7.1). Controls’ mean urine VG concentration was significantly higher: 13.2 mcg/ml (SD=25.0; p<0.05, see Figure 1). Following 12 hours of self-reported abstinence, ECIG users’ mean urine VG concentration decreased significantly to 5.0 mcg/ml (SD=4.4; p< 0.05). ECIG user’s mean decrease in urine VG pre-to-post abstinence was 2.9 mcg/ml (SD=6.5).
There were 2 outliers among controls with urine VG concentrations 5–6 times greater than the mean, possibly due to greater than average exposures to VG-containing food and/or products. Upon removal of these 2 outliers, mean urine VG concentration was 8.1 mcg/ml (SD=10.9) for controls (N = 48) and was no longer significantly different compared to non-abstinent ECIG users.
CONCLUSIONS
This is the first observational study to characterize urinary PG and VG concentration in daily ECIG users compared to non-ECIG/tobacco using controls. Daily ECIG users, following normal use of their own ECIG and liquid, had higher concentrations of urinary PG relative to controls. Median urine PG concentration was almost 7 times greater in ECIG users (median=20.9 mcg/ml) relative to controls (median=3.2 mcg/ml), suggesting that urine PG may be useful for discriminating ECIG users from non-users. Following ≥12 hours of self-reported ECIG abstinence, ECIG users’ mean urine PG concentration decreased such that it did not differ from controls, highlighting the possible utility of urine PG as a marker for verifying short-term ECIG abstinence. In contrast, compared with ECIG users, urinary VG concentration was higher in controls, suggesting that urine VG concentration may be ineffective at distinguishing ECIG users from non-users. These results confirm and expand upon those found in a controlled inpatient industry-sponsored study in which ECIG users used an ECIG that contained liquid spiked with a stable-isotope-labeled tracker14. Results showed that increased concentrations of urinary and plasma PG were associated with ECIG use while VG concentrations were not14.
These findings may have implications for research and clinical settings. For example, urine PG could potentially be used as an ECIG biomarker to improve experimental rigor. Because researchers are currently unable to verify short-term abstinence from ECIGs, some ECIG users may not comply with protocol-mandated overnight ECIG abstinence15,16,17. Further, PG may be used to confirm current ECIG-use status, particularly in instances where individuals may be motivated to conceal their ECIG/tobacco use. Moreover, because most current ECIG users aged 18–34 want to quit18 and thousands aged 13–24 have enrolled in ECIG-specific cessation programs19, a biomarker, such as urine PG that can verify ECIG use and/or ECIG abstinence may be useful in facilitating ECIG cessation. If a more rapid urine PG test than the one used here were to be developed and validated (eg, a urine test strip comparable to that used to assess cotinine), it likely would have application in various research and clinical settings; though, given PG’s short half-life (approximately 4 hours20), it likely would be used as a short-term marker in conjunction with other biomarkers, such as urine cotinine.
Findings from this study should be considered in the context of several limitations. These results were obtained from a small, predominantly Caucasian sample, thereby limiting generalizability. The uneven distribution of men and women across the ECIG and control groups raises concerns regarding possible sex differences in metabolism rates for PG and VG; future studies should include age- and sex-matched comparison groups21. Furthermore, the study did not include a comparison group of cigarette smokers and/or dual users (ie, cigarettes and ECIGs). Although previous studies have not detected increases in PG and VG in the urine and blood plasma of cigarette smokers14, the majority of adult ECIG users are dual users and the results herein should be replicated in this population. Because these data were collected as a part of a broader study, analysis of PG and VG was limited to urine, 2 measurement timepoints (for ECIG users), and no control for non-ECIG sources of exposure to PG and VG (eg, foods, beverages, and personal care products). Future studies should consider use of inpatient facilities to control dietary sources of PG and VG and employ controlled time-course of ECIG use to discern the level of exposure to PG and VG from ECIGs relative to other sources. Despite these limitations, the results from this study show that, under naturalistic use conditions, ECIG users’ mean urine PG concentration was significantly higher relative to non-ECIG/tobacco using controls and ECIG user’s urinary PG decreased after 12-hours ECIG abstinence.
IMPLICATIONS FOR TOBACCO REGULATION.
The scarcity of ECIG-specific biomarkers challenges assessment of ECIG use status. These results show that daily ECIG users, following normal use of their own ECIG/liquid, have higher concentrations of urine PG relative to non-ECIG-using controls. Following 12-hours ECIG abstinence, ECIG users’ mean urinary PG concentration decreased and was lower than controls, suggesting PG’s utility for verifying short-term ECIG abstinence. Discriminating ECIG users from non-users is important in a variety of research settings and can inform regulation of these products.
Acknowledgements
This research was supported by the National Institute on Drug Abuse of the National Institutes of Health (NIH) under Award Number U54/P50DA036105 and F31DA044707 and the Center for Tobacco Products of the U.S. Food and Drug Administration and by NIH grant P30DA033934. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration.
The authors thank Dr. Maciej Goniewicz, Dr. Matthew Halquist, Dr. Leon Kosmider, Dr. Neal Benowitz, Melanie Crabtree and Lauren Ratliff for their contribution to this study.
Footnotes
Human Subjects Statement
This study was approved by Virginia Commonwealth University’s Institutional Review Board.
Conflict of Interest Statement
Dr. Eissenberg is a paid consultant in litigation against the tobacco industry and electronic cigarette industry and is named on a patent application for a device that measures the puffing behavior of electronic cigarette users. All other authors have no disclosures.
Contributor Information
Marzena Hiler, Virginia Commonwealth University, Department of Psychology, Center for the Study of Tobacco Products, Richmond, VA..
Alison Breland, Virginia Commonwealth University, Department of Psychology, Center for the Study of Tobacco Products, Richmond, VA..
Carl E. Wolf, Department of Pathology, Health Systems, Virginia Commonwealth University, Richmond, VA..
Justin L. Poklis, Department of Pharmacology and Toxicology, Virginia Commonwealth University, Richmond, VA..
Carrol R. Nanco, Department of Pharmacology and Toxicology, Virginia Commonwealth University, Richmond, VA..
Thomas Eissenberg, Virginia Commonwealth University, Department of Psychology, Center for the Study of Tobacco Products, Richmond, VA..
References
- 1.Gentzke AS, Creamer M, Cullen KA, et al. Vital signs: tobacco product use among middle and high school students—United States, 2011–2018. MMWR. 2019; 68(6): 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang TW, Asman K, Gentzke AS, et al. Tobacco product use among adults — United States, 2017. MMWR. 2018; 67:1225–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benowitz NL, Jacob P III, Ahijevych K, et al. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002; 4(2):149–159. [DOI] [PubMed] [Google Scholar]
- 4.Kim S Overview of cotinine cutoff values for smoking status classification: a review. Int. J. Environ Res Public Health 2016; 13(12): E1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benowitz NL, Hukkanen J, Jacob P. Nicotine chemistry, metabolism, kinetics and biomarkers. InNicotine Psychopharmacology. Springer, Berlin, Heidelberg. 2009, pp 29–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connor Gorber S, Schofield-Hurwitz S, Hardt J, Levasseur G, Tremblay M. The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob Res. 2009;11(1):12–24. [DOI] [PubMed] [Google Scholar]
- 7.Perkins KA, Karelitz JL, & Jao NC. Optimal carbon monoxide criteria to confirm 24-hr smoking abstinence. Nicotine Tob Res. 2013; 15(5): 978–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goniewicz ML, Eisner MD, Lazcano-Ponce E, et al. Comparison of urine cotinine and the tobacco-specific nitrosamine metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and their ratio to discriminate active from passive smoking. Nicotine Tob Res. 2011; 13(3): 202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schepis TS, Duhig AM, Liss T, et al. Contingency management for smoking cessation: enhancing feasibility through use of immunoassay test strips measuring cotinine. Nicotine Tob Res. 2008;10(9): 1495–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schick SF, Blount BC Jacob P 3rd, et al. Biomarkers of exposure to new and emerging tobacco delivery products. Am J Physiol Lung Cell Mol Physiol. 2017; 313(3): 425–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han S, Chen H, Zhang X, Liu T, & Fu Y Levels of selected groups of compounds in refill solutions for electronic cigarettes. Nicotine Tob Res. 2015; 18: 708–714. [DOI] [PubMed] [Google Scholar]
- 12.Rose JE, & Behm FM. Physiological interactions between caffeine and nicotine. Pharmacol Biochem Beh. 1991; 38: 333–337. [DOI] [PubMed] [Google Scholar]
- 13.Nanco CR, Poklis JL, Hiler MM, et al. An ultra-high pressure liquid chromatographic tandem mass spectrometry method for the analysis of benzoyl ester derivatized glycols and glycerol. J. Anal Toxicol 2019; 43(9):720–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landmesser A, Scherer M, Pluym N, Sarkar M et al. Biomarkers of exposure specific to e-vapor products based on stable-isotope labeled ingredients. Nicotine Tob Res. 2019; 21(3): 314–322. [DOI] [PubMed] [Google Scholar]
- 15.Breland A, Maloney SF, Soule EK, et al. Abuse liability of electronic cigarettes in men who are experienced electronic cigarette users. Exp Clin Psychopharm. 2019; 28(2):235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blank MD, Breland AB, Cobb CO, et al. Clinical laboratory evaluation of electronic cigarettes: methodological challenges. Tob Regul Sci. 2016; 2(4): 426–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiler M, Breland A, Spindle T, et al. Electronic cigarette user plasma nicotine concentration, puff topography, heart rate, and subjective effects: influence of liquid nicotine concentration and user experience. Exp Clin Psychopharm. 2017; 25(5):380–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosen RL, & Steinberg ML. Interest in quitting e-cigarettes among adults in the United States. Nicotine Tob Res. 2019; 22(5):857–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graham AL, Jacobs MA, Amato MS. Engagement and 3-month outcomes from a digital e-cigarette cessation program in a cohort of 27,000 teens and young adults. Nicotine Tob Res. 2019; 22(5):859–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu DK, Elmquist W, & Sawchuk RJ. Pharmacokinetics of propylene glycol in humans during multiple dosing regimens. J Pharm Sci. 1985; 74(8): 876–879. [DOI] [PubMed] [Google Scholar]
- 21.Tomicic C, & Vernez D. Sex differences in urinary levels of several biological indicators of exposure: a simulation study using a compartmental-based toxicokinetic model. J Occup Environ Hyg. 2014; 11(6): 377–387. [DOI] [PubMed] [Google Scholar]


