Abstract
Excessive bleeding—or hemorrhage—causes millions of civilian and non-civilian casualties every year. Additionally, wound sequelae, such as infections, are a significant source of chronic morbidity, even if the initial bleeding is successfully stopped. To treat acute and chronic wounds, numerous wound healing materials have been identified, tested, and adopted. Among them are topical dressings, such as gauzes, as well as natural and biomimetic materials. However, none of these materials successfully mimic the complex and dynamic properties of the body’s own wound healing material: the blood clot. Specifically, blood clots exhibit complex mechanical and biochemical properties that vary across spatial and temporal scales to guide the wound healing response, which make them the ideal wound healing material. In this manuscript, we review blood clots’ complex mechanical and biochemical properties, review current wound healing materials, and identify opportunities where new materials can provide additional functionality, with a specific focus on hydrogels. We highlight recent developments in synthetic hydrogels that make them capable of mimicking a larger subset of blood clot features: as plugs and as stimuli for tissue repair. We conclude that future hydrogel materials designed to mimic blood clot biochemistry, mechanics, and architecture can be combined with exciting platelet-like particles to serve as hemostats that also promote the biological wound healing response. Thus, we believe synthetic hydrogels are ideal candidates to address the clear need for better wound healing materials.
Keywords: blood clot, hydrogel, wound healing
1. Introduction
Hemorrhage or excessive bleeding after vascular injury may be caused during surgical procedures, follow blunt injury, or result from penetrating wounds [1]. Regardless of the mechanism, severe blood loss following vascular trauma leads to millions of casualties every year [2]. For all but small injuries, the body’s native sealant, the blood clot, is quickly overwhelmed. Thus, medical intervention is needed to stop significant blood flow [3]. While vascular trauma may be effectively treated within the hospital setting through surgical intervention, management of severe vascular injuries in the pre-hospital setting—i.e. in the field—is more challenging and, perhaps predictably, less effective [4]. Field responses generally include volume infusion, tourniquet application, and use of topical hemostatic agents [5]. Hemostatic agents comprise both passive agents that provide a physical barrier to blood flow at the injury site as well as biologically active agents that promote faster blood coagulation and wound site occlusion through direct interaction with the coagulation cascade [6]. The classic example of a passive agent is surgical gauze [7], while active agents often include molecular therapies such as thrombin [8]. Whether passive or active, many, if not all, of today’s hemostatic agents were designed for the acute setting [9]. However, cessation of bleeding is only the first step in a complex and long cascade of events that ultimately lead to wound healing [10] (figures 1(A)–(C)).
Figure 1.
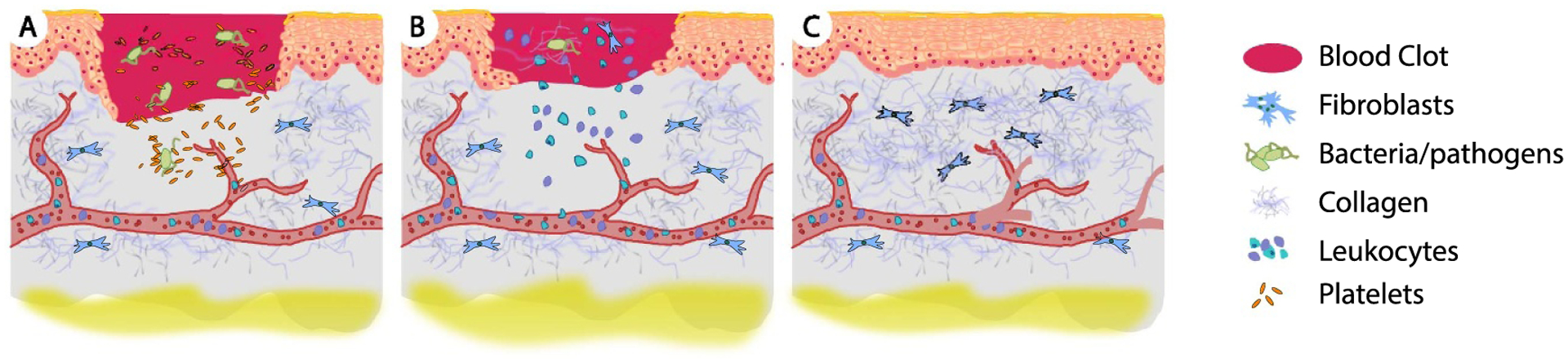
The diverse and multi-fold roles of blood clots. Among them, (A) blood clots occlude the initial wound site to stop hemorrhage. Additionally, blood clot constituents, such as platelets, release chemokines that attract immune cells. (B) As wound healing progresses, blood clots also provide a substrate for migrating matrix-synthesizing cells to infiltrate the clot and remodel the clot’s fibrin mesh with collagen. (C) Eventually, the blood clot is replaced by the repaired tissue. Reprinted by permission from Springer Nature Customer Service Centre GmbH: Springer Nature, Pediatric Research [10], Copyright © 2013, International Pediatric Research Foundation, Inc.
The natural wound healing cascade centers on blood clot formation, a hemostatic plug with unique material properties that evolve over time. Upon vascular injury, blood coagulates into a blood clot and (for non-severe injuries) occludes the initial wound site (figure 1(A)). This original clot acts as a host for immune cells—such as neutrophils and macrophages—into which matrix-synthesizing cells, such as fibroblasts that synthesize extracellular matrix proteins, arrive (figure 1(B)). The orchestrated interplay between immune and matrix-synthesizing cells eventually transforms the blood clot into collageneous scar tissue, which permanently seals the wound (figure 1(C)). As the body’s native wound healing material, the blood clot has evolved to optimally support the wound healing response along its full temporal evolution and on all critical scales ranging across many orders of magnitude. Thus, optimal, synthetic wound healing materials would mimic blood clot’s biophysical properties across all temporal and spatial scales. The current lack of such an optimal material creates an opportunity for novel, synthetic materials to fill this gap. Hydrogels are an example of such a material due to their biocompatibility, mechanical properties, and customizability.
The goals of this review are: (a) to reflect on blood clot’s dynamic biophysical and biochemical properties, (b) to determine the extent to which current wound healing materials mimic blood clot’s properties, and (c) to highlight how hydrogel materials could potentially fill the gap that exists with current wound healing materials. In section 2, we discuss the salient physical and biochemical features of the prototypical wound sealant material: our bodies’ blood clots. This allows us, as we review current wound healing materials in section 3, to carefully reflect on where the biophysical properties of state-of-the-art materials diverge from those of the ideal wound sealant. Finally, in section 4, we identify potential synthetic building blocks upon which future wound healing materials could be built to embody the biophysical features of blood clots, with a specific focus on hydrogel-based materials.
2. Blood clot: the prototypical wound healing material
Blood clots play a critical role in wound healing [11]. That is, blood clots provide an initial scaffold that, during the wound healing response, is eventually replaced with scar tissue [12]. The functions of this initial scaffold are many-fold, but can be broadly categorized into mechanical and biochemical in nature. Here, we briefly describe blood clot formation, composition, and structure before summarizing mechanical and biochemical clot functions and properties. Additionally, we identify additional physical properties of blood clots that play important but secondary roles during wound healing.
2.1. Blood clot formation and composition
In response to injury, a blood clot forms in a cascade-like chain-reaction known as coagulation. This clotting cascade starts as platelets—cell-like blood constituents—are activated upon contact with newly exposed tissue extracellular matrix. They activate and transform into sticky elements that form an initial ‘plug’ [13]. Secretion of additional factors subsequently leads to positive-feedback amplification that, in turn, activates more platelets. The exact details of the clotting cascade and different blood factor secretion can be found in other excellent reviews of blood clotting [14, 15]. For the purposes of this section on clot formation, it suffices to say that tissue factor release or exposure drives the conversion of the zymogen prothrombin to thrombin [16]. Thrombin ultimately cleaves fibrinogen, a soluble blood protein, into fibrin by enzymatic release of fibrinopeptides A and B (figure 2(A)). Cleavage of these peptides results in self-assembly of fibrin molecules, each with a symmetric E–D–E structure, into double-stranded protofibrils that are stabilized via factor XIIIa mediated cross-linking (figure 2(B)). Together, cross-linked fibrin protofibrils bundle into fibers and ultimately entangle into a hierarchical, three-dimensional network, which entraps platelets and red blood cells, becoming a functional clot (figure 2(C)) [17, 18].
Figure 2.
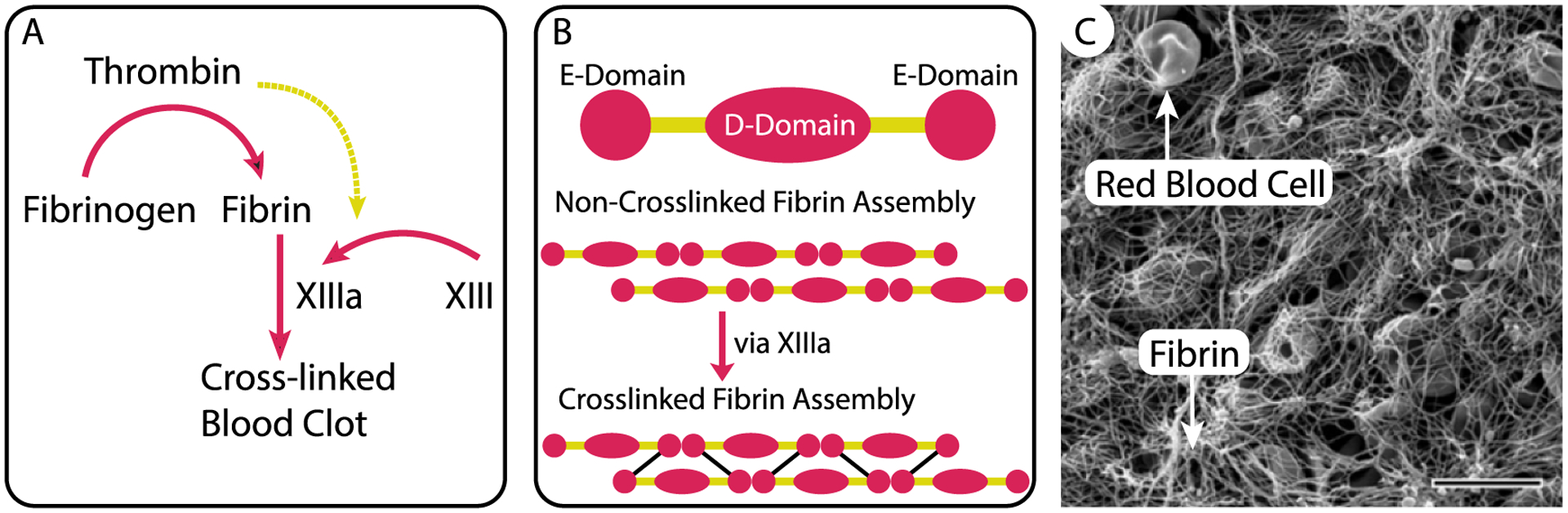
Blood clots are biocomposites in which platelets and red blood cells are entrapped in a semi-flexible biopolymer network of fibrin fibers. The fibrin network arises following a complex cascade of reactions among coagulation factors that lead to (A) cleavage of fibrinogen into fibrin, (B) the assembly of cross-linked fibrin molecules into fibers, and (C) network formation that entraps red blood cells and other blood-borne elements. Scale bar is 10 μm. Republished with permission of American Society of Hematology Publications, from [19], 2016; permission conveyed through Copyright Clearance Center, Inc..
2.2. Mechanical properties
Being comprised of formed elements, red blood cells and platelets, as well as a semi-flexible fibrin biopolymer network, blood clots exhibit highly nonlinear, viscoelastic properties and may undergo very large deformations [20, 21] (figure 3). Ostensibly, each of these properties have a teleological origin and thus should be considered critical to blood clot’s function [22]. Below, we focus our review of these properties on whole blood clot studies rather than fibrin-only investigations, which have been reviewed before in excellent work by others [17, 18, 23].
Figure 3.
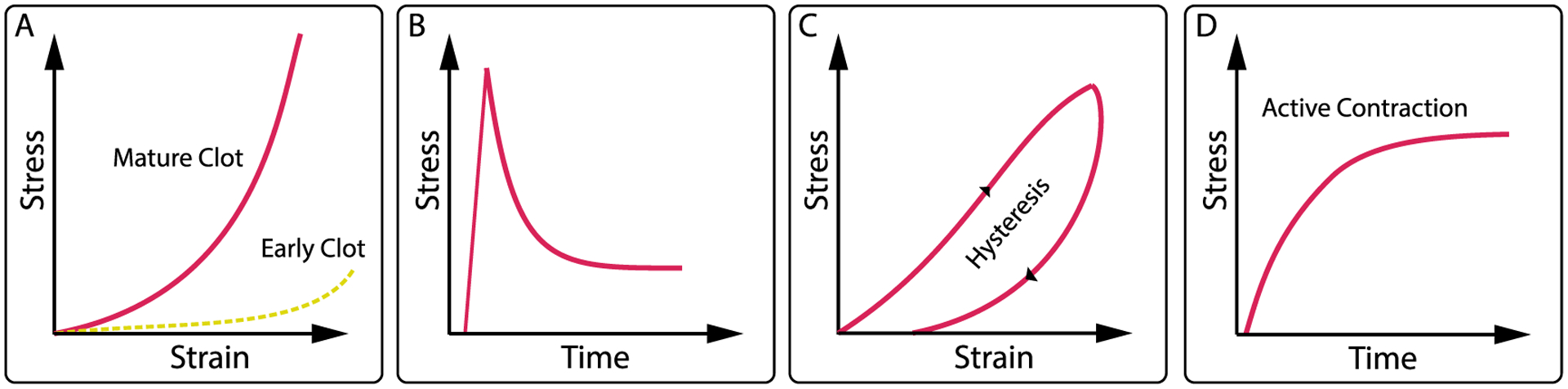
Blood clots show highly nonlinear, time-dependent mechanics. (A) Strain-stiffening behavior that evolves during thrombus maturation with more mature thrombus being stiffer, ((B) and (C)) viscoelastic behavior, including stress-relaxation and creep, hysteresis, and set, and finally (D) active stress due to platelet activity and fibrin contraction.
2.2.1. Stiffness
Stiffness is an important measure of material behavior and quantifies a material’s resistance to deformation. The stiffer a material, the more force is required to deform it. It is also an important measure as it regulates cell fate by modulating fundamental cellular processes, such as cell spreading, cell growth, cell proliferation, cell migration, and cell differentiation [24–27]. Interestingly, in the case of blood clots, there is disagreement over to what extent the material exhibits strain-stiffening behavior during its deformation. Some studies show that a blood clot increases in stiffness with increasing strain [28–30] (figure 3(A)), while other studies show that the stiffness remains approximately constant [31–33], similar to a linearly elastic spring. The origin of this discrepancy has yet to be identified. Regardless of the exact origin of the disagreement, this uncertainty sets whole blood clots apart from fibrin meshes that have, without exception, been shown to demonstrate strain-stiffening behavior [34–36]. Of course, the difference between whole blood clots and fibrin gels is significant, with the former being a heterogeneous composite material with multiple structural elements—both active (platelets) and passive (red blood cell)- in addition to a fibrin polymer scaffold [37]. This also serves as an important reminder of the limitations of fibrin gels as blood clot mimics.
Quantitatively, the stiffness of a blood clot varies based on the origin of the material, i.e. whether it was formed in-vivo or in-vitro [30], based on the anatomic location from which samples were taken [38], based on the flow conditions under which it was formed [39], and based on the duration for which it has resided within the body [40]. Generally speaking, early blood clots may be described as ‘very soft’ with elastic moduli of 0.1 – 0.6 kPa [41–44], which is comparable to other soft tissues such as those in the liver or brain [45]. Fully formed clots (within hours) show an elasticity of ~10 kPa [30, 37]. Importantly, more ‘mature’ blood clots (those that have resided longer within the host tissue, i.e. weeks) are much stiffer, having moduli of ~500 kPa [40]. This apparent stiffening coincides with the replacement of fibrin by a dense collagen matrix and infiltration of fibroblast cells into the clot [46, 47]. Similar to fibrin, collagen is a hierarchical polymer; however, it has individual fibers 10–100× stiffer than fibrin [48–51]. Once fully transformed, blood clots may be orders of magnitude stiffer and, owing to the highly collageneous nature, comparably stiff to connective tissues such as ligaments or muscle [52].
2.2.2. Viscoelastic properties
Blood clots also exhibit signs of viscoelasticity. That is, a blood clot’s mechanical response to a deformation is time dependent and shows characteristics of both elastic materials, such as rubber, and viscous materials, such as water [20, 53] (figures 3(B) and (C)). Importantly, viscoelastic behaviors such as strain-rate dependent stiffness, hysteresis, creep, and stress-relaxation are associated with energy dissipation. For example, stress-relaxation rates in blood clots increase by ~50% with strain, and permanent set can be ~15% [20]. This is contrasted with purely elastic materials, where both stress relaxation and permanent set are essentially non-existent. Viscoelasticity lends an ability to dissipate externally applied loads and may thus support its ability to resist failure—a leading cause of deadly thromboses [54]. Interestingly, viscoelasticity has also been recognized as a critical mediator of cell function similar to stiffness [55]. The source of blood clot viscoelasticity has not yet been fully identified but is likely related to either or both of two disparate mechanisms: (a) solid-phase viscoelasticity, and (b) poroelasticity [20]. The former mechanism arises from the inherent viscoelasticity of the constituents and likely stems from the dissipative nature of mechanical and chemical interactions in the fibrin backbone and of its formed elements [56, 57]. The latter mechanism arises from the momentum exchange between the fluid-phase, the interstitial fluid of this highly hydrated material, and its solid-phase [58]. This momentum exchange follows from internal pressure gradients that drive fluid within the material and cause dissipative losses as fluid-phase and solid-phase move relative to each other.
2.3. Active contraction
Wound contraction is a critical element in the wound healing response [10, 59]. The initial wound contraction is driven by a blood clot’s contractile function (figure 3(D)). Both platelets and the fibrin polymer network apply internal tension that leads to active stress production and contraction [60, 61]. Platelets’ ability to contract has long been known and is today well understood. Specifically, platelets possess an actin-myosin contractile apparatus that enables production of significant forces when pulling against each other and against substrates, including blood clot’s fibrin network [62]. Their active function is not only critical to wound contraction, but also to regulation of other critical clot functions by changing clot stiffness, clot visoelastic properties, and (as discussed below) clot transport properties [63]. Interestingly, fibrin itself also contributes to clot contraction. Specifically, as fibrinogen polymerizes into its network form, it builds prestresses within its fibers [64]. Cumulatively, these stresses give rise to an internally stressed material. Fibrin-based prestress and contraction have also been attributed to important functions, such as aiding clot clearance [65]. The latter phenomenon is likely a result of strain-enhanced fibrinolysis.
2.4. Biochemical properties
2.4.1. Cell recruitment and angiogenesis
Wound healing is a diverse process, involving various blood cells, mesenchymal cells, skin cells, and muscle cells. The interplay between these cells during wound healing is regulated by the biological wound healing process and has been exhaustively reviewed elsewhere [66]. As blood clots are at the center of the wound healing process, different cell types enter and exit the clot environment throughout healing. Immediately following wound formation, platelets are the primary cells involved in the blood clot, with red blood cells being entrapped by the formation of the fibrin scaffold. Upon the secondary activation of platelets, via the the thrombin pathway described above, platelets release platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), and a host of cyto- and chemokines. With platelets secreting so many factors, the initial blood clot environment becomes somewhat inflammatory by infiltration of monocytes and neutrophils to clean the wound area from bacteria and debris. Mast and dendritic cells also play important roles in this inflammatory phase.
Following the inflammatory phase, blood clots enter a scarring and neovascularization or angiogenesis phase. The formation or granulation phase is mediated primarily by fibroblasts, which are known to engage with the fibrin scaffold via integrin receptors. These cells deposit collagen that forms the basis of the longer-term tissue repair. While fibroblasts secrete the collagenous matrix, microvascular and endothelial cells prepare the wound site for neovascularization. The environment containing PDGF and fibroblast growth factor secreted by fibroblasts encourage endothelization of the clot and angiogenesis. During the angiogenesis process, other cells including smooth muscle cells and pericytes, contribute to rebuilding the vessel structure. As healing progresses, epithelial cells and kerantinocytes eventually rebuild the dermal and epidermal layers to complete the wound healing process.
2.4.2. Mechano-chemical regulation
During the wound healing response, blood clots interact with several soluble blood factors and processing enzymes. Two well-studied enzymes are plasmin, the activated form of plasminogen that catalyzes fibrin lysis, and the serine protease tissue plasminogen activator (tPA), which coincidentally activates plasminogen [67]. The degradation of fibrin by plasmin has been studied at the single fiber and whole clot level, with somewhat contradictory results showing both impeded and accelerated lysis of fibrin under tensile deformation [64, 68, 69]. Recent data shows that the fibrin-tPA interaction is down-regulated by increasing tensile deformation of fibrin at the single fiber and hydrogel levels [70]. These studies suggest that the molecular structure of fibrin—at least for the strain-sensitive αC domains and the coiled-coil connectors—has a functional consequence for biochemistry and clot stability. In other words, a blood clot, through its structural constituent fibrin, can regulate enzymatic activity and control its own lysis during the wound healing response in which it is ultimately replaced with collagenous scar tissue.
The various cells within blood clots also play a role in its mechano-chemical regulation. Platelets and fibroblasts affect clot mechanics as fillers, similar to red blood cells; however, they also play an active role in blood clot remodeling. Platelet contraction stiffens blood clots and further regulates blood clot signaling [60, 62] while fibroblasts deposit collagen within the clot to promote scar formation. Both cell types interact with blood clots through highly specific intergrin-matrix interactions. Platelets bind to the fibrin scaffold via the αIIbβ3 integrin receptor that binds to multiple motifs along fibrin molecules [23]. Fibroblasts bind to fibrin via (αvβ3) integrins through RGD sequences [71]. Analogous to the regulation of remodeling enzymes, the cellular binding motifs in blood clots also exhibit mechano-chemical regulation [72, 73]. For example, platelet binding is substantially reduced on strained fibrin compared to relaxed fibrin (peripherally shown by [74]), which can be linked to reduced integrin-fibrin interaction [70]. Thereby, blood clot biochemistry regulates interaction with cells, which in turn further modulates blood clot biochemistry. Interestingly, as the clot becomes more scar-like with increased collagen deposition over days to weeks, its biochemistry also changes due to increased collagen and decreased fibrin content. The gradual increase in collagen in the clot demonstrates that blood clot biochemical signaling is also temporally evolving.
2.5. Transport properties
A blood clot has also non-mechanical and non-mechano-chemical physical functions, such as regulating transport. Being a highly porous material with ~5% of the material being solid, this porosity is not only important to cell migration, but also to various transport phenomena that may be critical to both mechanical and mechano-chemical functions [75, 76]. Here we differentiate between diffusion-based transport that may be quantified by the materials diffusivity and advective transport along pressure gradients that may be quantified by the material’s hydraulic permeability [75]. Because of the very large pore sizes on the order of 1–5 μm, small molecule diffusivity in blood clots is close to free, or unhindered, and may thus be successfully approximated by the diffusivity of water [77]. On the other hand, hydraulic permeability is finite and a likely critical contributor of blood clots viscoelasticity [20]. Importantly, both measures of transport are mechanically-sensitive. In other words, as clot mechanically deforms and the pore-space anisotropically collapses, transport properties may change in magnitude and direction [47]. In turn, this directionally-dependent change to transport properties may also alter blood clot’s mechanical behavior in a directionally-dependent manner, further highlighting the complexity of blood clot mechanics and its coupled, multi-physical nature. Additionally, blood clots may act not only as physical plugs against blood leakage but also as superficial barriers to microbial invasion into the wound site [78]. This barrier was described as resembling bio-film. The sheet-like bio-films composed of fibrinogen and fibrin monomers self-assemble at the blood-air interfaces, resulting in Langmuir-like films. Structurally different than fibrin fiber networks, these thin smooth surface layers accommodate very small pores (few tens of nanometers in diameter) and are connected to the interior of a blood clot through fiber linkages [78, 79]. Formation of these layers was shown to be delayed using surfactants, leading to microbial invasion or out-leakages of red blood cells [80].
3. The temporo-spatial plane of blood clot function and current wound healing materials
3.1. The temporo-spatial plane of blood clot properties and function
Blood clots’ mechanical and biochemical properties and functions span several spatial scales and evolve over time [10, 59]. The temporo-spatial plane of blood clot function and properties is depicted in figure 4. On the smallest scale, the key property is the composition of a blood clot’s structural constituents. During initial formation, fibrin fibers are a blood clot’s structural basis and govern its mechanics [40]. As a blood clot matures, fibrin fibers are replaced by stiffer collagen fibers [81]. The transformation of a fibrin rich clot to a collageneous scar tissue, which ultimately replaces the clot, is driven by immune cells and, later, matrix-synthesizing cells such as fibroblasts [82, 83]. As different types of cells inhabit the clot, they also contribute to its mechanics. On a larger scale, a clot also provides a bed for sprouting blood vessels that supply the newly formed tissue at the site of injury [11]. Ultimately, a blood clot orchestrates an intricate interplay between spatial and temporal scales, between biopolymer matrix remodeling and cell infiltration, over hours to weeks. At the conclusion of the wound healing response, a regulated transformation of the blood clot results in a functional, neovascularized tissue at the wound site [10]. Many current wound healing materials interact with wounds at one or more of these length scales, but few demonstrate the temporal changes of blood clots during healing.
Figure 4.
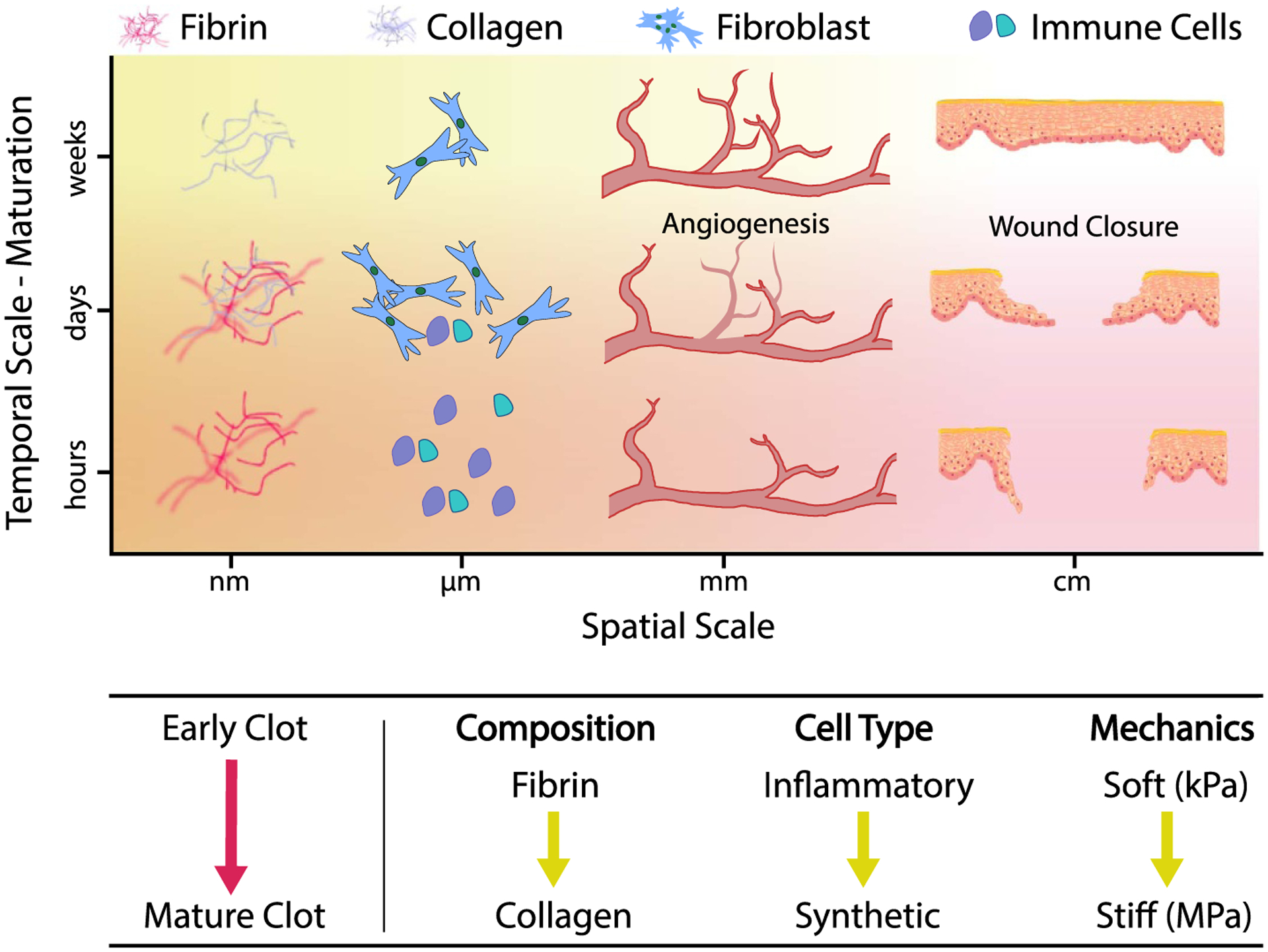
During wound healing a blood clot performs diverse roles that span multiple orders of spatial and temporal scales. Among them, a blood clot provides a fibrinous and—in the course of maturation—collageneous scaffold that acts as a substrate for immune and matrix-synthesizing cells as well as a bed for newly sprouting blood vessels. Finally, a blood clot evolves into scar tissue that seals the injury site upon conclusion of the wound healing response. Reprinted by permission from Springer Nature Customer Service Centre GmbH: Springer Nature, Pediatric Research [10], Copyright © 2013, International Pediatric Research Foundation, Inc.
3.2. Current wound healing materials
Numerous materials have been investigated as topical dressings, gauzes, or sprays that actively or passively contribute to wound healing, see figure 5. Dozens of such materials are commercially marketed and used in a clinical setting, primarily for hemostasis—stabilizing the wound against bleeding out. Among these materials, polymeric hydrogels possess several desirable properties for going beyond hemostasis by providing cues similar to those in the natural wound healing response.
Figure 5.
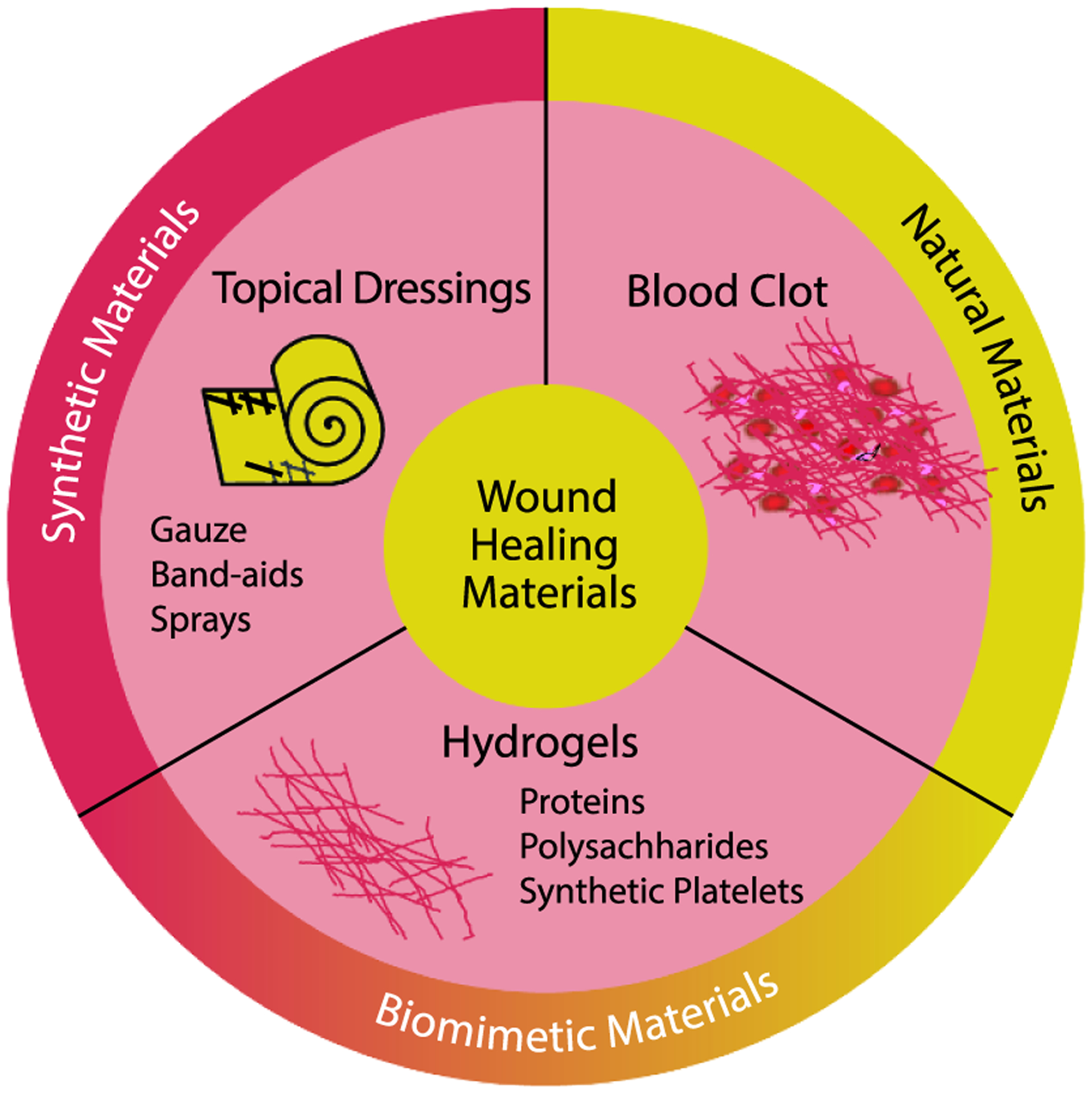
Wound healing hydrogels offer the potential for mimicking blood clots, bridging the gap between synthetic and natural materials.
Polymeric hydrogels are water-swollen networks with similar physicochemical properties to the native blood clot matrix. They have a high water content, have demonstrated good biocompatibility with a plethora of cell types, and can encapsulate therapeutic or antibiotic molecules for controlled release. In addition, several hydrogel formulations are injectable, which enables them to conform to irregular wound sites and access deep wounds below the skin [84–86]. Importantly, hydrogels may be composed of either naturally derived biopolymers or synthetic polymers, which enables access to a range of mechanical or biochemical properties by tuning the chemical composition. With regard to wound healing hydrogels in particular, most systems have been composed of naturally derived biopolymers, such as proteins and polysaccharides.
Because fibrin is a major component of blood clots, it is perhaps no surprise that fibrin is the most studied hydrogel platform for wound healing. Fibrin is readily available, with human fibrinogen and thrombin sourced from commercial vendors. Forming hydrogels in vitro offers control over polymerization conditions to modify hydrogel properties via fibrinogen, thrombin, calcium, and salt concentrations [87]. Variation in thrombin and salt concentrations modulate fibrin fiber thickness and therefore mechanics [88]. Fibrinogen concentration modulates porosity and overall gel stiffness to an even greater degree, with high concentrations forming very dense networks that hinder cellular infiltration (e.g. use as sealant). While fibrin itself possesses clear advantageous characteristics, such as natural biodegradability and ideal individual fiber mechanics, fibrin hydrogels alone are not mechanically robust enough for long term cell culture or implantation [89]. Thus, a number of studies have increased the robustness of fibrin by forming composites with other proteins or synthetic polymers, or by using covalent crosslinking methods [87].
Alternative protein-based hydrogels for wound healing include gelatin/collagen (e.g. Costasis) and silk fibroin. These proteinaceous materials are of interest because they are widely available, possess inherent biocompatibility, and have been shown to stimulate hemostasis by activating the coagulation cascade [90, 91]. However, gelatin on its own is a denatured form of collagen with poor mechanical robustness, particularly at body temperature, and is easily degraded by proteases. Moreover, its adhesion to wet surfaces is sub-optimal. Structured protein materials, such as silk fibroin, vastly improve mechanics yet still fall short on adhesion to wet tissues and stimulating downstream wound healing behaviors. To address the adhesion issue, catechol-containing molecules have been incorporated into protein-based hydrogels. For example, one strategy is to oxidize the tyrosine residues in gelatin to 3,4-dihydroxyphenol-L-alanine (DOPA) [92]. Another strategy is to incorporate polydopamine [93] or tannic acid [94–96], which contains pyrogallol and catechol groups for adhesion. These strategies have significantly increased wet tissue adhesion, allowing for significantly enhanced hemostasis and wound sealing.
In addition to protein-based hydrogels, polysaccharides have also been used as hemostatic materials. Chitosan (e.g. HemCon, TraumaStat, Vetigel), alginate, cellulose, and hyaluronic acid have all been used as the polymers for wound healing materials. Compared to protein-based materials, polysaccharides can be cheaper and are available from a broader set of sources, including both animals and plants. As these polymers are often polyelectrolytes, their electrostatic interactions can also promote activation of different parts of the coagulation cascade, such as erythrocyte agglutination by positively charged chitosan [97, 98] or clotting factor recruitment by negatively charged alginate [99]. The electrostatic properties of the polysaccharides also lead to high water uptake, in general, which can then absorb much of the liquid in blood and concentrate platelets and other coagulation factors. In the case of alginate, the local release of CaCl2 quickly leads to polymerization and cross-linking, thereby enhancing function as a topical hemostat, in addition to its role in platelet and coagulation factor concentration [100]. Finally, many polysaccharide materials can be spun into nanofibers, enabling fabrication of mats or dressings that can be marketed for wound healing [101].
3.3. Filling the gap between current wound healing materials and blood clot
Hydrogel materials clearly offer strong potential as multifunctional materials that can function as hemostats, as well as additional biochemical and biophysical support to the wound site. However, current hydrogel wound sealants do not yet span the temporospatial plane of ideal blood clots. The primary limitations are: (a) providing the appropriate fibrillar architecture seen in fibrin-based blood clots, (b) activation of platelets, and (c) challenges with degradability, which can limit fibroblast infiltration or possibly lead to premature failure. Nevertheless, the outstanding potential to customize hydrogels with numerous chemical functionalities and recent work advancing synthetic hydrogel architectures make it very promising to design multifunctional hydrogels that better mimic a broad array of blood clot functions.
4. Focus on temporospatial properties of synthetic materials and the future of wound healing materials
Moving toward advanced wound healing materials will require special attention to material architecture, mechanical properties, and composition. While much of the current commercially available wound healing materials rely on natural polymers, ongoing challenges with batch-to-batch variability, immunogenicity, and limited functionality merit further research into synthetic materials. The majority of synthetic hydrogels, however, are developed from flexible polymer chains and covalent crosslinking methods, leading to elastic mechanical properties and mesh sizes of ~10 nm that significantly deviate from that of fibrillar biopolymer networks found in blood clots. Coincident with this change in architecture is a mismatch in viscoelastic properties compared to hydrogels composed of natural biopolymers, leading to decreased cell-matrix remodeling and biochemical signaling [89]. To address the issues of polymer architecture and mechanics, a number of innovative approaches have recently emerged to induce dynamic and active properties into synthetic polymer hydrogels, see figure 6.
Figure 6.
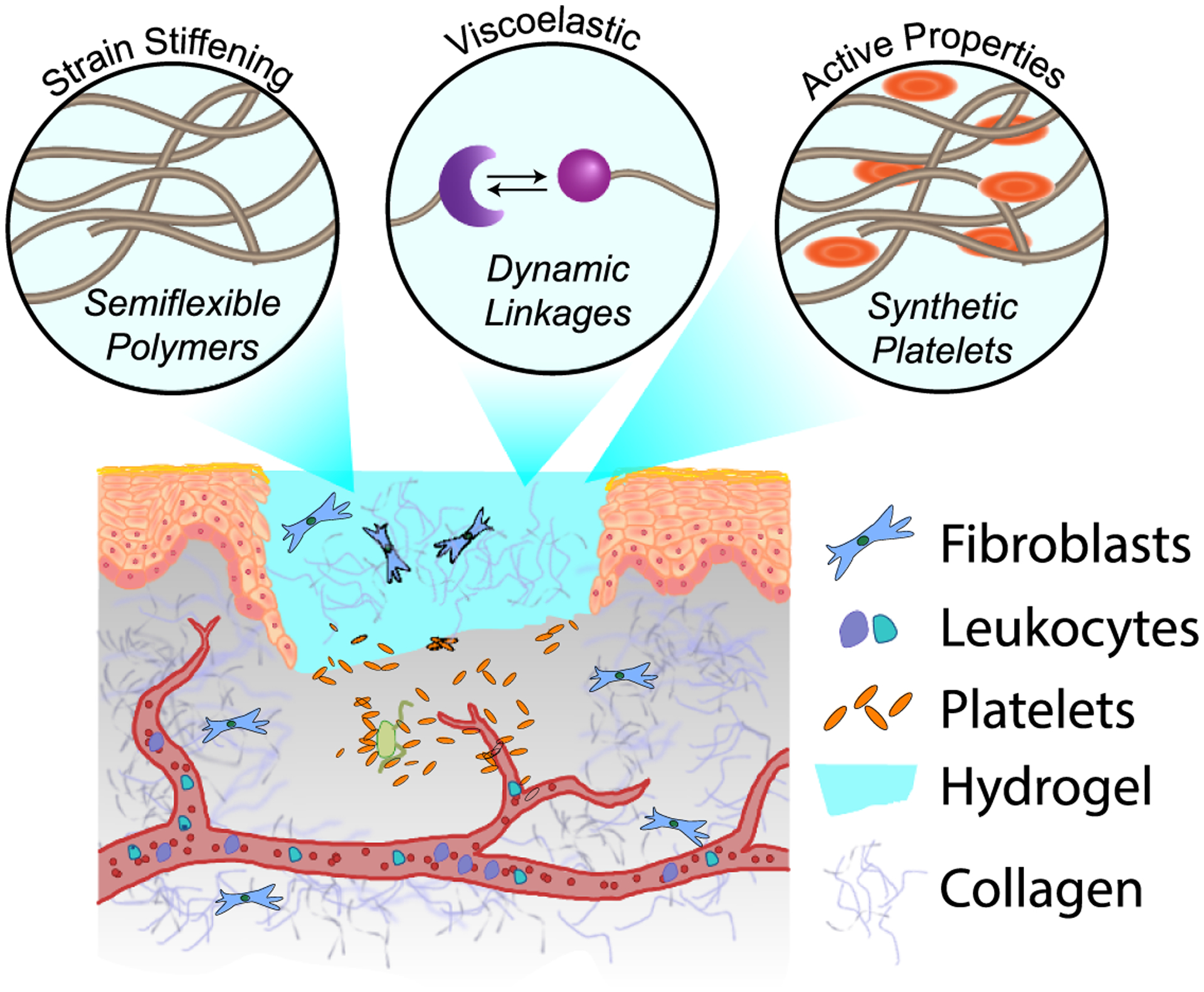
Synthetic hydrogels can mimic the properties of blood clots through the use of structural, chemical, and biological strategies. These strategies include the use of semiflexible polymers to induce strain-stiffening behavior, dynamic crosslinks to elicit viscoelasticity, and inclusion of synthetic platelets to generate active contraction.
4.1. Fibrillar architecture and stiffness
Recognition of the nonlinear elastic properties of tissues, including blood clots, has led to a number of synthetic approaches to stress-(or strain-) stiffening systems. One approach involves the synthesis of semiflexible polymers, such as through self-assembly into rodlike micelles [102] and fibers [103], or via helical chain conformation [104]. For example, ethyleneglycol-functionalized polyisocyanopeptides (PICs) are semiflexible, helical polymers that form hydrogels upon heating past a lower critical solution temperature [104, 105]. Due to physical interactions such as hydrophobic effects and chain entanglement, these semiflexible networks exhibit stress-stiffening behavior that is highly tunable with polymer length, enabling high stiffness gels at low concentrations, similar to that seen in fibrin scaffolds of blood clots. The tunability of this platform has made it attractive for use as a cell scaffold for tissue engineering [106] and organoid formation [107]. Furthermore, recent application as a wound healing material in full thickness dorsal skin wounds in mice demonstrated no foreign body response and similar closure properties as Matrigel controls [108]. The PIC hydrogels also decreased immune cell populations in the wound (e.g. granulocytes including neutrophils), showing promise as barriers to foreign bacteria and infection. While the precise contribution of the nonlinear mechanics of these materials in wound healing is yet unknown, these materials offer a facile route to tune this parameter.
Other approaches to engineer strain-stiffening behavior in synthetic hydrogels include strain-induced molecular aggregation and dynamic crosslinking chemistries. In the first approach, strain was shown to induce aggregation of specific functional groups, such as ionic clusters in polyelectrolyte hydrogels [109] and ‘movable’ beta-cyclodextrin crosslinkers in polyrotaxane hydrogels [110]. In these cases, the stiffening behavior was reversible, although the lifetime of the aggregates largely depended on both polymer architecture and charge screening conditions. In the second approach, strain was used to physically bring polymer chains together, enabling latent crosslinking of functional moieties within the hydrogel [111, 112]. This mechanism led to a permanent, rather than transient, increase in the storage modulus with strain, transforming the material from viscoelastic to more elastic in nature. This mechanism may be one way to mimic the temporal evolution in mechanics that occurs from early clot to more mature clot.
Bulk matrix stiffening at longer timescales has also been demonstrated with many other stimuli-responsive chemistries in synthetic hydrogels. For example, photo-mediated crosslinking can be used to increase the bulk elasticity over many stages for a gradual increase in stiffness [113, 114]. The use of light as a stimulus offers very high spatiotemporal control. In another approach, enzymes, such as tyrosinase, have also been used to introduce additional crosslinks and stiffen synthetic hydrogels in the presence of adhered cells [115]. While in situ stiffening via enzymatic crosslinking relies on diffusion throughout the hydrogel, advantages of this approach include enzyme substrate specificity and well-controlled reaction kinetics. In an example related to blood clots and wound healing, enzymatic crosslinking was demonstrated for poly(ethylene glycol) (PEG) hydrogels containing peptides specific for thrombin-activated transglutaminase factor XIII (FXIIIa) [116]. These specific interactions are promising for in situ stiffening of hydrogels in the wound healing environment. Overall, a combination of the reversible crosslinking for strain-stiffening behavior and irreversible stiffening mechanisms over time offer great potential for mimicry of changes in bulk stiffness between early clot and mature clot.
4.2. Viscoelasticity
In contrast to strain-stiffening behavior, synthetic hydrogels have seen much recent activity regarding strategies to engineer viscoelastic behavior. Several dynamic crosslinking mechanisms have been incorporated into synthetic hydrogel networks, including guest-host linkages [117–119], dynamic covalent chemistry [120–123], and hydrogen bonding moieties [124, 125]. Because these types of bonds are amenable to exchange on a fast timescale (similar to that of cell-mediated forces), these synthetic networks exhibit time-dependent mechanics, such as stress relaxation, that is tunable over several orders of magnitude. Excitingly, the tunability of these viscoelastic properties has been shown to impact a number of biological outcomes, including stem cell differentiation [126], vasculogenesis [127], and extracellular matrix remodeling [128]. This toolbox of dynamic hydrogel chemistries will be important to shaping wound healing materials and synthetic blood clots in the future.
Like blood clots, synthetic hydrogel viscoelasticity has contributions from both solid-phase viscoelasticity and poroelasticity. As mentioned above, the relative contributions of solid-phase viscoelasticity and poroelasticity to blood clot function is unknown, and it is further complicated by measurement techniques that span long length and time scales where these contributions are confounded [129]. Many recent efforts have advanced the characterization methods of poroelastic and viscoelastic deformation in soft synthetic hydrogels, especially using polyacrylamide hydrogels as a model system [130–134]. From these efforts and others, key differences in the poroelasticity of semiflexible biopolymer and flexible polymer networks have emerged. For one, it is known that the normal stress is negative in semiflexible bipolymer networks, such as fibrin, whereas it is positive in flexible polymer networks, such as polyacrylamide [135]. Further work is needed to characterize normal stress and poroelasticity in semiflexible, synthetic networks, such as those mentioned above, to examine whether they replicate the behavior of biopolymer networks. Furthermore, research on the poroelasticity of blood clots is an ongoing area of research [136], and an increased understanding of its properties will enable researchers to better design hydrogel systems with fluid flow timescales relevant to in vivo systems.
4.3. Composites and active properties
In addition to elastic and viscoelastic mechanical behavior, other properties of blood clots arise from their composite nature, especially from the presence of cells and activated platelets. Platelets actively contract the fibrin scaffold, ultimately guiding wound healing via plug formation. To boost clotting in a clinical setting, platelet transfusion has been used as a therapy; however, stored platelets have a limited shelf life, and donor sourcing inherently includes the risk of immunogenic response [137]. To address these issues, biomimetic platelets have been developed from a variety of synthetic materials, including lipid-based constructs [138], polymeric particles [139, 140], and soft microgels [141]. Typically, these materials are functionalized with a biological binding motif to target their interaction with elements in the wound site, especially fibrin. Recent research efforts have further focused on the deformability of these particles to mimic the shape change that occurs in contractile natural platelets, as well as their interaction with synthetic hydrogels. In one example, Brown et al functionalized low-crosslinked polymeric microgel particles with fibrin binding motifs (sdFvs) identified through phage display [141]. Excitingly, these platelet-like particles induced fibrin network collapse in vitro and reduced bleeding times in vivo compared to particles without sdFvs. It is thought that the high affinity and multivalent display of the fibrin binding motif, coupled with the high deformability of the particle, enabled the clot collapse. More recent work also demonstrated that the collapse led to stiffening of fibrin networks in vitro, thereby encouraging fibroblast migration in early wound healing models [142].
In another approach to synthetic multicomponent hydrogels, Wang et al recently developed supramolecular, strain-stiffening hydrogels that retain their dynamic properties when embedded with micrometer-sized liposomes [143]. Interestingly, these networks indicated a higher density of fibers when higher lipid concentrations were present due to local catalysis of fiber formation provided by the phospholipid bilayers [144]. This strategy potentially enables hydrogel networks with local variation in structure that mimics that of the contracted blood clot, which is promising for future applications. In particular, this system provides a modular route to introducing functional handles on either the supramolecular fibers or the liposomes to engineer bioactivity in the wound healing environment.
5. Conclusion
Wound healing is a critical process to stem uncontrolled bleeding and reduce infections that affect millions of people each year. In many cases, natural wound healing is too slow and additional support is required. Natural wound healing is highly dynamic with intertwined physical and biochemical processes that are tightly regulated within blood clots. As such, mimicking the functional and material properties of blood clots are a great starting point for development of artificial wound healing materials. From many potential options, we find that hydrogel materials offer an attractive combination of dynamically tunable biochemical functionality, mechanics, and biocompatibility that maps well onto the temporospatial plane of blood clots. We believe that future hydrogel materials designed to mimic blood clot biochemistry, mechanics, and architecture can be combined with exciting platelet-like particles to serve as hemostats and also promote the biological wound healing response.
Acknowledgments
We appreciate funding through the National Science Foundation: Grants #1916663 (MKR), #2046148 (MKR), #2105175 (SHP, MKR), and the Welch Foundation F-2008-20190330 (SHP). We also appreciate funding through the National Institutes of Health: R35GM138193 (AMR).
Data availability statement
No new data were created or analysed in this study.
References
- [1].Kauvar DS and Wade CE 2005. Critical Care 9 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Schöchl H, Grassetto A and Schlimp CJ 2013. J. Cardiothorac. Vascular Anesthesia 27 S35–43 [DOI] [PubMed] [Google Scholar]
- [3].Curry N, Hopewell S, Dorée C, Hyde C, Brohi K and Stanworth S 2011. Critical care 15 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Johansson PI, Stensballe J and Ostrowski SR 2012. Scand. J. Trauma Resuscitation Emergency Med 20 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mabry R and Mcmanus J 2008. Critical Care Med 36 S258. [DOI] [PubMed] [Google Scholar]
- [6].Samudrala S 2008. AORN J 88 S2–11 [DOI] [PubMed] [Google Scholar]
- [7].Huang L, Liu GL, Kaye AD and Liu H 2020. Adv. Ther 37 4132–48 [DOI] [PubMed] [Google Scholar]
- [8].Spotnitz WD 2007. Surgery 142 S34–8 [DOI] [PubMed] [Google Scholar]
- [9].Murphy PS and Evans GR 2012. Plastic Surgery Int. 2012 190436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tepole AB and Kuhl E 2016. Comput. Methods Biomech. Biomed. Eng 19 13–30 [DOI] [PubMed] [Google Scholar]
- [11].Singh S, Young A and McNaught CE 2017. Surgery (Oxford) 35 473–7 [Google Scholar]
- [12].Beldon P 2010. Surgery (Oxford) 28 409–12 [Google Scholar]
- [13].Heemskerk JWM, Bevers E and Lindhout T 2002. J. Thrombosis Haemostasis 88 186–93 [PubMed] [Google Scholar]
- [14].Smith SA, Travers RJ and Morrissey JH 2015. Critical Rev. Biochem. Mol. Biol 50 326–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Palta S, Saroa R and Palta A 2014. Indian J. Anaesthesia 58 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mackman N 2004. Arteriosclerosis Thrombosis Vascular Biol. 24 1015–22 [DOI] [PubMed] [Google Scholar]
- [17].Weisel JW and Litvinov RI 2017. Fibrin Formation, Structure and Properties (Singapore: Springer International Publishing; ) pp 405–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Undas A and Ariëns RA 2011. Arteriosclerosis Thrombosis Vascular Biol 31 e88–99 [DOI] [PubMed] [Google Scholar]
- [19].Tutwiler V, Litvinov RI, Lozhkin AP, Peshkova AD, Lebedeva T, Ataullakhanov FI, Spiller KL, Cines DB and Weisel JW 2016. Blood 127 149–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sugerman GP, Parekh SH and Rausch MK 2020. Soft Matter 16 9908–16 [DOI] [PubMed] [Google Scholar]
- [21].Rausch MK, Sugerman GP, Kakaletsis S and Dortdivanlioglu B 2021. Biomech. Model. Mechanobiol 20 1–13 [DOI] [PubMed] [Google Scholar]
- [22].Qiu Y, Myers DR and Lam WA 2019. Nat. Rev. Mater 4 294–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Litvinov RI and Weisel JW 2017. Matrix Biol. 60 110–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pelham RJ and Wang Y l 1997. Proc. Natl Acad. Sci 94 13661–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ulrich TA, de Juan Pardo EM and Kumar S 2009. Cancer Res. 69 4167–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Discher DE, Janmey P and Wang Y l 2005. Science 310 1139–43 [DOI] [PubMed] [Google Scholar]
- [27].Engler AJ, Sen S, Sweeney HL and Discher DE 2006. Cell 126 677–89 [DOI] [PubMed] [Google Scholar]
- [28].Rausch MK and Humphrey JD 2016. J. Mech. Behav. Biomed. Mater 55 12–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Teng Z, Feng J, Zhang Y, Huang Y, Sutcliffe MP, Brown AJ, Jing Z, Gillard JH and Lu Q 2015. Ann. Biomed. Eng 43 2745–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sugerman GP, Kakaletsis S, Thakkar P, Chokshi A, Parekh SH and Rausch MK 2021. J. Mech. Behav. Biomed. Mater 115 104216. [DOI] [PubMed] [Google Scholar]
- [31].Di Martino E, Mantero S, Inzoli F, Melissano G, Astore D, Chiesa R and Fumero R 1998. Eur. J. Vascular Endovascular Surg 15 290–9 [DOI] [PubMed] [Google Scholar]
- [32].Gasser TC, Görgülü G, Folkesson M and Swedenborg J 2008. J. Vascular Surg 48 179–88 [DOI] [PubMed] [Google Scholar]
- [33].Geest JPV, Sacks MS and Vorp DA 2006. J. Biomech 39 2347–54 [DOI] [PubMed] [Google Scholar]
- [34].Münster S, Jawerth LM, Leslie BA, Weitz JI, Fabry B and Weitz DA 2013. Proc. Natl Acad. Sci 110 12197–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Storm C, Pastore JJ, MacKintosh FC, Lubensky TC and Janmey PA 2005. Nature 435 191–4 [DOI] [PubMed] [Google Scholar]
- [36].Piechocka IK, Jansen KA, Broedersz CP, Kurniawan NA, MacKintosh FC and Koenderink GH 2016. Soft Matter 12 2145–56 [DOI] [PubMed] [Google Scholar]
- [37].van Oosten AS, Chen X, Chin L, Cruz K, Patteson AE, Pogoda K, Shenoy VB and Janmey PA 2019. Nature 573 96–101 [DOI] [PubMed] [Google Scholar]
- [38].Chernysh IN, Nagaswami C, Kosolapova S, Peshkova AD, Cuker A, Cines DB, Cambor CL, Litvinov RI and Weisel JW 2020. Sci. Rep 10 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gersh K, Edmondson K and Weisel J 2010. J. Thrombosis Haemostasis: JTH 8 2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lee YU, Lee A, Humphrey J and Rausch M 2015. Biorheology 52 235–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ryan EA, Mockros LF, Weisel JW and Lorand L 1999. Biophys. J 77 2813–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mockros L, Roberts W and Lorand L 1974. Biophys. Chem 2 164–9 [DOI] [PubMed] [Google Scholar]
- [43].Fukada E and Kaibara M 1973. Biorheology 10 129–38 [DOI] [PubMed] [Google Scholar]
- [44].Shen L and Lorand L et al. 1983. J. Clin. Invest 71 1336–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Weickenmeier J, de Rooij R, Budday S, Steinmann P, Ovaert TC and Kuhl E 2016. Acta Biomaterialia 42 265–72 [DOI] [PubMed] [Google Scholar]
- [46].Bainbridge P 2013. J. Wound Care 22 407–8, 410–12 [DOI] [PubMed] [Google Scholar]
- [47].Rausch MK and Humphrey JD 2017. J. Elast 129 125–44 [Google Scholar]
- [48].Helms CC, Ariëns RA, Uitte de Willige S, Standeven KF and Guthold M 2012. Biophys. J 102 168–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].McDaniel DP, Shaw GA, Elliott JT, Bhadriraju K, Meuse C, Chung KH and Plant AL 2007. Biophys. J 92 1759–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wenger MPE, Bozec L, Horton MA and Mesquida P 2007. Biophys. J 93 1255–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Li W, Sigley J, Pieters M, Helms CC, Nagaswami C, Weisel JW and Guthold M 2016. Biophys. J 110 1400–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kakaletsis S, Meador WD, Mathur M, Sugerman GP, Jazwiec T, Malinowski M, Lejeune E, Timek TA and Rausch MK 2021. Acta Biomaterialia 123 154–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].van Kempen TH, Donders WP, van de Vosse FN and Peters GW 2016. Biomech. Model. Mechanobiol 15 279–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].White RH 2003. Circulation 107 I–4 [DOI] [PubMed] [Google Scholar]
- [55].Chaudhuri O, Cooper-White J, Janmey PA, Mooney DJ and Shenoy VB 2020. Nature 584 535–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Weisel JW 2004. Biophys. Chem 112 267–76 [DOI] [PubMed] [Google Scholar]
- [57].Mills J, Qie L, Dao M, Lim C and Suresh S 2004. Mol. Cellular Biomech 1 169–80 [PubMed] [Google Scholar]
- [58].Hu Y and Suo Z 2012. Acta Mech. Solida Sin 25 441–58 [Google Scholar]
- [59].Tepole AB and Kuhl E 2013. Pediatric Res 73 553–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kim OV, Litvinov RI, Alber MS and Weisel JW 2017. Nat. Commun 8 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Weisel JW, Nagaswami C and Makowski L 1987. Proc. Natl Acad. Sci 84 8991–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Lam WA, Chaudhuri O, Crow A, Webster KD, Kita A, Huang J and Fletcher DA et al. 2011. Nat. Mater 10 61–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Sun Y. et al. Biomaterials. 2021;274:120828. doi: 10.1016/j.biomaterials.2021.120828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Bucay I, O’Brien ET III, Wulfe SD, Superfine R, Wolberg AS, Falvo MR and Hudson NE 2015. PLoS One 10 e0116350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Cone SJ, Fuquay AT, Litofsky JM, Dement TC, Carolan CA and Hudson NE 2020. Acta Biomaterialia 107 164–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Rodrigues M, Kosaric N, Bonham CA and Gurtner GC 2019. Physiol. Rev 99 665–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Nieuwenhuizen W 2001. Ann. New York Acad. Sci 936 237–46 [DOI] [PubMed] [Google Scholar]
- [68].Li W, Lucioni T, Li R, Bonin K, Cho SS and Guthold M 2017. Acta Biomaterialia 60 264–74 [DOI] [PubMed] [Google Scholar]
- [69].Hudson NE 2017. BioMed Research Int. 2017 2748340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kumar S, Wang Y, Rausch MK and Parekh SH 2020. bioRxiv ( 10.1101/2020.08.24.265611) [DOI] [Google Scholar]
- [71].Gailit J, Clarke C, Newman D, Tonnesen MG, Mosesson MW and Clark RA 1997. Exp. Cell Res 232 118–26 [DOI] [PubMed] [Google Scholar]
- [72].Zhang Y, Qiu Y, Blanchard AT, Chang Y, Brockman JM, Ma VPY, Lam WA and Salaita K 2018. Proc. Natl Acad. Sci 115 325–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Bachmann M et al. 2020. J. Cell. Sci 133 jcs24240432193334 [Google Scholar]
- [74].Lishko V, Yermolenko I and Ugarova T 2010. J. Thrombosis Haemostasis 8 799–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Wufsus AR, Macera NE and Neeves KB 2013. Biophys. J 104 1812–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].de Cagny HCG, Vos BE, Vahabi M, Kurniawan NA, Doi M, Koenderink GH, MacKintosh FC and Bonn D 2016. Phys. Rev. Lett 117 217802. [DOI] [PubMed] [Google Scholar]
- [77].Spero RC, Sircar RK, Schubert R, Taylor RM, Wolberg AS and Superfine R 2011. Biophys. J 101 943–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Macrae FL et al. 2018. J. Clin. Invest 128 3356–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Gu SX and Lentz SR 2018. J. Clin. Invest 128 3243–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Punter MT, Vos BE, Mulder BM and Koenderink GH 2020. Soft Matter 16 1298–305 [DOI] [PubMed] [Google Scholar]
- [81].Schriefl AJ, Collins MJ, Pierce D, Holzapfel GA, Niklason LE and Humphrey JD 2012. Thrombosis Res 130 e139–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Fineschi V, Turillazzi E, Neri M, Pomara C and Riezzo I 2009. Forensic Sci. Int 186 22–8 [DOI] [PubMed] [Google Scholar]
- [83].Nosaka M, Ishida Y, Kimura A and Kondo T 2010. Forensic Science Int 195 143–7 [DOI] [PubMed] [Google Scholar]
- [84].Cheng L. et al. Adv. Funct. Mater. 2020;30:2001196. [Google Scholar]
- [85].Lokhande G, Carrow JK, Thakur T, Xavier JR, Parani M, Bayless KJ and Gaharwar AK 2018. Acta Biomaterialia 70 35–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Gaharwar AK, Avery RK, Assmann A, Paul A, McKinley GH, Khademhosseini A and Olsen BD 2014. ACS Nano 8 9833–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Brown AC and Barker TH 2014. Acta Biomaterialia (Biological Materials) 10 1502–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Potier E, Noailly J, Sprecher CM and Ito K 2010. J. Mater. Sci 45 2494–503 [Google Scholar]
- [89].Caliari SR and Burdick JA 2016. Nat. Methods 13 405–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Hickman DA, Pawlowski CL, Sekhon UDS, Marks J and Gupta AS 2018. Adv. Mater 30 1700859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Lei C, Zhu H, Li J, Feng X and Chen J 2016. J. Biomater. Sci. Edn 27 403–18 [DOI] [PubMed] [Google Scholar]
- [92].Chan Choi Y, Choi JS, Jung YJ and Cho YW 2014. J. Mater. Chem. B 2 201–9 [DOI] [PubMed] [Google Scholar]
- [93].Rajabi N, Kharaziha M, Emadi R, Zarrabi A, Mokhtari H and Salehi S 2020. J. Colloid Interface Sci 564 155–69 [DOI] [PubMed] [Google Scholar]
- [94].Guo J et al. 2018. Acta Biomaterialia 72 35–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Fan H, Wang J, Zhang Q and Jin Z 2017. ACS Omega 2 6668–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Bai S et al. 2019. Nanoscale Horiz 4 1333–41 [Google Scholar]
- [97].Okamoto Y, Yano R, Miyatake K, Tomohiro I, Shigemasa Y and Minami S 2003. Carbohydrate Polym 53 337–42 [Google Scholar]
- [98].Rao SB and Sharma CP 1997. J. Biomed. Mater. Res 34 21–8 [DOI] [PubMed] [Google Scholar]
- [99].Lee KY and Mooney DJ 2012. Prog. Polym. Sci 37 106–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Aderibigbe BA and Buyana B 2018. Pharmaceutics 10 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Pourshahrestani S, Zeimaran E, Kadri NA, Mutlu N and Boccaccini AR 2020. Adv. Healthcare Mater 9 2000905. [DOI] [PubMed] [Google Scholar]
- [102].Fernàndez-Castaño RM, Lafleur RPM, Guibert C, Voets IK, Storm C and Sijbesma RP 2017. Angew. Chem., Int. Ed 56 8771–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Fernàndez-Castaño RM, Lou X, Schill J, ter Huurne G, Fransen PPKH, Voets IK, Storm C and Sijbesma RP 2018. J. Am. Chem. Soc 140 17547–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Kouwer PHJ et al. 2013. Nature 493 651–5 [DOI] [PubMed] [Google Scholar]
- [105].Jaspers M, Dennison M, Mabesoone MFJ, MacKintosh FC, Rowan AE and Kouwer PHJ 2014. Nat. Commun 5 5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Das RK, Gocheva V, Hammink R, Zouani OF and Rowan AE 2016. Nat. Mater 15 318–25 [DOI] [PubMed] [Google Scholar]
- [107].Zhang Y. et al. Adv. Sci. 2020;7:2001797. doi: 10.1002/advs.202001797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].op ‘t Veld RC et al. 2018. Biomaterials 181 392–401 [DOI] [PubMed] [Google Scholar]
- [109].Miquelard-Garnier G, Creton C and Hourdet D 2008. Soft Matter 4 1011–23 [DOI] [PubMed] [Google Scholar]
- [110].Cui Y, Tan M, Zhu A and Guo M 2014. RSC Adv 4 56791–7 [Google Scholar]
- [111].Zheng J, Smith Callahan LA, Hao J, Guo K, Wesdemiotis C, Weiss RA and Becker ML 2012. ACS Macro Lett 1 1071–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Tran YH, Rasmuson MJ, Emrick T, Klier J and Peyton SR 2017. Soft Matter 13 9007–14 [DOI] [PubMed] [Google Scholar]
- [113].Guvendiren M and Burdick JA 2012. Nat. Commun 3 792. [DOI] [PubMed] [Google Scholar]
- [114].Mabry KM, Lawrence RL and Anseth KS 2015. Biomaterials 49 47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Liu HY, Greene T, Lin TY, Dawes CS, Korc M and Lin CC 2017. Acta Biomaterialia 48 258–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Anjum F, Lienemann PS, Metzger S, Biernaskie J, Kallos MS and Ehrbar M 2016. Biomaterials 87 104–17 [DOI] [PubMed] [Google Scholar]
- [117].Sinawang G, Osaki M, Takashima Y, Yamaguchi H and Harada A 2020. Polym. J 52 839–59 [Google Scholar]
- [118].Mantooth SM, Munoz-Robles BG and Webber MJ 2019. Macromol. Biosci 19 1800281. [DOI] [PubMed] [Google Scholar]
- [119].Jin J, Cai L, Jia YG, Liu S, Chen Y and Ren L 2019. J. Mater. Chem. B 7 1637–51 [DOI] [PubMed] [Google Scholar]
- [120].Tang S, Richardson BM and Anseth KS 2021. Prog. Mater. Sci 120 100738 [Google Scholar]
- [121].Tong Z, Jin L, Oliveira JM, Reis RL, Zhong Q, Mao Z and Gao C 2021. Bioactive Mater 6 1375–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Lou J, Stowers R, Nam S, Xia Y and Chaudhuri O 2018. Biomaterials 154 213–22 [DOI] [PubMed] [Google Scholar]
- [123].FitzSimons TM, Oentoro F, Shanbhag TV, Anslyn EV and Rosales AM 2020. Macromolecules 53 3738–46 [Google Scholar]
- [124].Vereroudakis E, Bantawa M, Lafleur RPM, Parisi D, Matsumoto NM, Peeters JW, Del Gado E, Meijer EW and Vlassopoulos D 2020. ACS Central Sci 6 1401–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Ren Z, Zhang Y, Li Y, Xu B and Liu W 2015. J. Mater. Chem. B 3 6347–54 [DOI] [PubMed] [Google Scholar]
- [126].Chaudhuri O et al. 2016. Nat. Mater 15 326–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Wei Z, Schnellmann R, Pruitt HC and Gerecht S 2020. Cell Stem Cell 27 798–812.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Loebel C, Mauck RL and Burdick JA 2019. Nat. Mater 18 883–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Wang QM, Mohan AC, Oyen ML and Zhao XH 2014. Acta Mech. Sin 30 20–7 [Google Scholar]
- [130].Kalcioglu ZI, Mahmoodian R, Hu Y, Suo Z and Van Vliet KJ 2012. Soft Matter 8 3393–8 [Google Scholar]
- [131].Berry JD, Biviano M and Dagastine RR 2020. Soft Matter 16 5314–24 [DOI] [PubMed] [Google Scholar]
- [132].Esteki MH, Alemrajabi AA, Hall CM, Sheridan GK, Azadi M and Moeendarbary E 2020. Acta Biomaterialia 102 138–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Islam MR and Oyen ML 2021. J. Mater. Res 36 2582–90 [Google Scholar]
- [134].Nam S, Hu KH, Butte MJ and Chaudhuri O 2016. Proc. Natl Acad. Sci 113 5492–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Janmey PA, McCormick ME, Rammensee S, Leight JL, Georges PC and MacKintosh FC 2007. Nat. Mater 6 48–51 [DOI] [PubMed] [Google Scholar]
- [136].Ehret AE, Bircher K, Stracuzzi A, Marina V, Zündel M and Mazza E 2017. Nat. Commun 8 1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Nandi S and Brown AC 2016. Exp. Biol. Med 241 1138–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Haji-Valizadeh H, Modery-Pawlowski CL and Gupta AS 2014. Nanoscale 6 4765–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Doshi N, Orje J, Molins B, Smith J, Mitragotri S and Ruggeri Z 2012. Adv. Mater 24 3864–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Anselmo AC et al. 2014. ACS Nano 8 11243–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Brown AC et al. 2014. Nat. Mater 13 1108–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Nandi S, Sproul EP, Nellenbach K, Erb M, Gaffney L, Freytes DO and Brown AC 2019. Biomater. Sci 7 669–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Wang Y, Xu Z, Lovrak M, le Sage VAA, Zhang K, Guo X, Eelkema R, Mendes E and Esch JH 2020. Angew. Chem., Int. Ed 59 4830–4 [DOI] [PubMed] [Google Scholar]
- [144].Versluis F et al. 2016. J. Am. Chem. Soc 138 8670–3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analysed in this study.


