Abstract
COVID-19 has different clinical stages, and effective therapy depends on the location and extent of the infection. The purpose of this review is to provide a background for understanding the progression of the disease throughout the pulmonary epithelium and discuss therapeutic options. The prime sites for infection that will be contrasted in this review are the conducting airways and the gas exchange portions of the lung. These two sites are characterised by distinct cellular composition and innate immune responses, which suggests the use of distinct therapeutic agents. In the nose, ciliated cells are the primary target cells for SARS-CoV-2 viral infection, replication and release. Infected cells shed their cilia, which disables mucociliary clearance. Evidence further points to a suppressed or incompletely activated innate immune response to SARS-CoV-2 infection in the upper airways. Asymptomatic individuals can still have a productive viral infection and infect others. In the gas exchange portion of the lung, the alveolar type II epithelial cell is the main target cell type. Cell death and marked innate immune response during infection likely contribute to alveolar damage and resultant acute respiratory distress syndrome. Alveolar infection can precipitate a hyperinflammatory state, which is the target of many therapies in severe COVID-19. Disease resolution in the lung is variable and may include scaring and long-term sequalae because the alveolar type II cells are also progenitor cells for the alveolar epithelium.
INTRODUCTION
COVID-19 is a complex disease with clinical responses varying from asymptomatic to severe with catastrophic respiratory failure and death. Some therapies are effective early in the course of the disease but appear relatively ineffective in critically ill patients. Other treatments are contraindicated early in the disease but are proven to be effective late in the disease. COVID-19 and the response to therapy can be understood from the perspective of where the infection is located and which cells are infected during the clinical course of disease. The initial site of infection and viral replication is the sinonasal airway epithelium, a patchwork of luminal ciliated and mucus secretory cells that mediate mucociliary clearance. Studies show that ciliated cells are the primary target of SARS-CoV-21 and mediate initial viral replication and luminal release. At this stage, treatment needs to be designed to block infection and limit viral propagation. As the disease spreads down along the respiratory tract to the alveolar compartment, target cells and responses change and thus treatment strategies need to adapt. It is in the alveoli where the infection can turn deadly . Here, the primary cell that is infected is the alveolar type II cell, which secretes pulmonary surfactant for effective gas exchange and acts as the progenitor cell for type II and type I epithelial cells, the latter of which cover 95% of the alveolar surface. In addition to blocking viral entry and replication, treatment for SARS-CoV-2 in the alveoli needs to focus on preventing damage to alveolar epithelial cells and the endothelial cells of the microvasculature, limiting the influx of inflammatory cells and promoting epithelial repair.
This review will focus on the target cell types that are directly infected and initiate the innate immune response, summarising knowledge gained from human patients and controls, in vivo pathogen challenge models as well as in vitro studies with SARS-CoV-2-infected human respiratory epithelial cells. More detailed reviews on different aspects of SARS-CoV-2 and COVID-19 are available elsewhere and will provide more information on the virus itself and the response of recruited inflammatory cells.2-4
THE CONDUCTING AIRWAYS
Initial infection site: the sinonasal passages
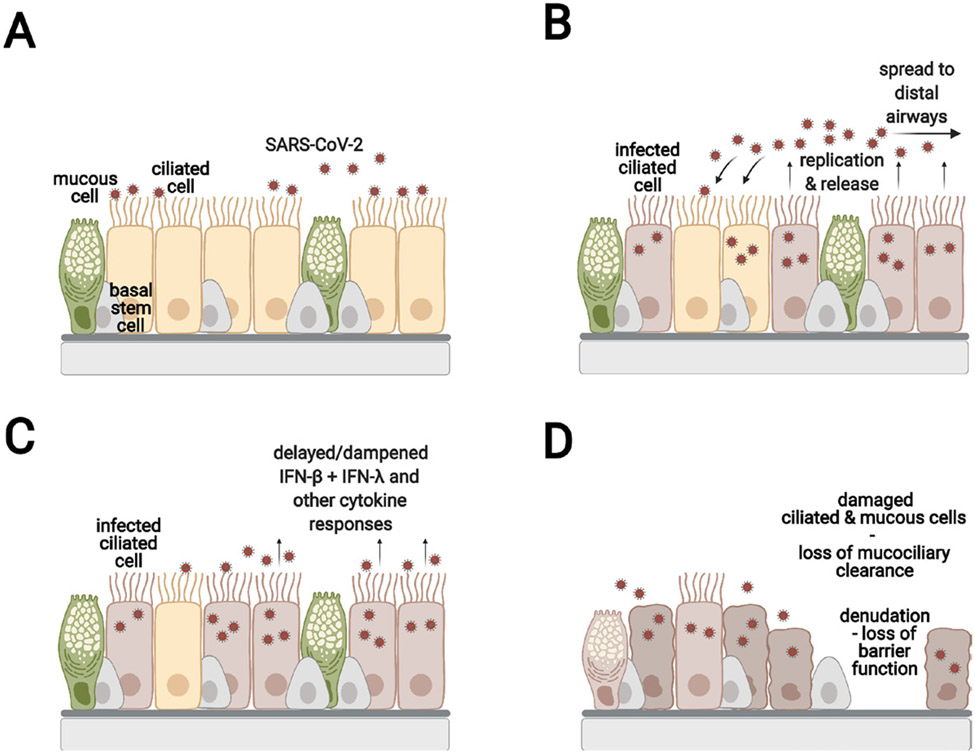
Inhaled viral particles are primarily deposited on the nasal mucosa where the virus infects, replicates within and is released from epithelial target cells.5 figure 1 These infections may be asymptomatic or may cause local symptoms. Presence of the angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2) viral entry factor transcripts in single-cell RNA sequencing (scRNAseq) data sets was used as the first approximation of the cells that are likely targets of SARS CoV-2 within a given tissue.6 7 ACE2 is the cell surface receptor recognised by the SARS-CoV-2 spike (S) protein, and TMPRSS2 is a serine protease that cleaves the S protein for viral entry. It should be noted, however, that ACE2 expression is highly variable among cells and is expressed at low levels.
Figure 1.
SARS-CoV-2 infection of the upper airway epithelium (A) SARS-CoV-2 infects the ACE2 expressing ciliated cells. (B) SARS-CoV-2 replicates, is released apically, and infects neighbouring cells. (C) The ciliated cells initiate the innate immune response by secreting type I and type III interferons and other cytokines. It should be noted that the events in (B) and (C) are shown to illustrate the infection process. However, they occur nearly simultaneously in an infected tissue. The time from infection to initial release of virus is estimated to be about 6 hours. SARS-CoV-2 has the ability to dampen or delay the interferon and cytokine response compared with similar infections with influenza. (D) The infected ciliated cells shed their cilia leading to impaired mucociliary clearance. In some areas, the epithelial barrier is severely damaged. The basal cells are spared from infection and can proliferate and restore the damaged the epithelium.
Along with patient samples, primary airway epithelial cell cultures derived from nasal brush- ings have provided much of our understanding of SARS-CoV-2 infection mechanisms and cellular responses. Primary cultured epithelia are readily infected with SARS-CoV-2 and release a substantial amount of virus from the apical cell surface.6 8 9 In the nasal epithelium, both ciliated and mucus-secreting goblet cells expressACE2 and TMPRSS2.10 ACE2 protein has only been detected on cilia and the apical ciliated cell surface.11 Tropism and viral entry likely depend on additional molecules and structures. Other proteases such as cathepsin B and L and furin may also be involved in infection. Pinto et al reported that SARS-CoV-2 attaches to the actin-rich microvilli on the ciliated cell apical surface and not the cilia themselves.12 It will be important to localise ACE2 more precisely and to better define the functions of ciliated cell structures. Importantly, the airway epithelium has altered cellular composition and contains structurally and functionally aberrant ciliated cells in chronic inflammatory airway diseases such as asthma, cystic fibrosis, chronic rhinosinusitis and chronic obstructive pulmonary disease.13-17 Furthermore, ACE2 expression is stimulated by IL-1β and IFN-β and inhibited by IL-13 in bronchial epithelial cells.9 It will be critical to understand whether this impacts infection and COVID-19 disease outcomes in patients with chronic airway diseases.
Target cells for SARS-CoV-2 infection in the upper airways
Infected ciliated cells shed their ciliary axonemes,12 18 which disables mucociliary clearance and likely enables disease progression. However, the nasal epithelium itself should be able to recover, since the basal stem cells are relatively spared from infection. Epithelial cells within the nasal submucosal glands also remain uninfected.9 The one unusual feature of COVID-19 in some patients is anosmia, the loss of sense of smell. This is likely due to infection of the epithelial cells in the olfactory bulb.5 19 There is concern that infection of the nerves in the nasal bulb may lead to retrograde passage of the virus into the central nervous system, but this requires additional study.5 We need more information on the rate of recovery of the sense of smell and whether these symptoms are associated with a distinctive clinical outcome.
Dampened innate immune response to SARS-CoV-2 infection
The innate immune response to SARS-CoV-2 in the nasal passages is complex and has recently been reviewed.20 Evidence points to a dampened interferon response, which may be related to disease severity. ScRNAseq of cells obtained from nasal brushings of COVID-19 patients10 failed to detect type I (interferon alpha or beta) or type III (interferon lambda) transcripts, although cells did express interferon responsive antiviral genes (MX1 and IFITM3). Interferon gene expression was markedly lower in patients with severe COVID-19 infection than in those with mild disease. Fiege et al used scRNAseq of cultures of human tracheal cells and not only demonstrated primary infection in ciliated cells and a reduced interferon response but also observed a markedly reduced rate of infection after treatment with interferon beta.21 Pizzorno et al showed that both nasal and bronchial primary airway epithelial cultures produced minimal interferon and interferon-regulated gene expression at 24 hours after SARS-CoV-2 infection,22 although expression rose at later time points. Gamage et al measured multiple secreted cytokines after SARS-CoV-2 infection of nasal epithelial cells, and, with the exception of CXCL10, also reported dampened cytokine secretion.8 This innate immune response was significantly reduced from what was observed with influenza A infection.8
The reason for the dampened interferon response to SARS-CoV-2 is not known and is currently being investigated. The signalling cascades in infected epithelial cells that lead to cytokine production begin with recognition of the virus by foreign pattern-recognition molecules such as Toll-like Receptor-3 (TLR-3) and TLR-7 in the endosome and RIG-I and MDA5 in the cytoplasm.23 This leads to activation of the latent transcription factors interferon regulatory factors 3 and 7 and nuclear factor-kappa β, which ultimately initiate transcription of type I and type III interferons and other cytokines.23 Interferon beta signals through a JAK/STAT pathway to stimulate hundreds of interferon stimulated genes in infected and uninfected bystander cells. While the precise cause for the reduced interferon signal is not known, viruses such as SARS-CoV-1, Middle East Respiratory Syndrome (MERS) and influenza A have viral proteins that can inhibit the interferon response.10 The NS1 protein of influenza A markedly reduces interferon-stimulated gene expression.24 Comparable candidate proteins in SARS-CoV-2 are open reading frame 3b (ORF3b) and ORF6 but defining the functions of these and other non-structural proteins requires more investigation.4 25 26 The host microbiome and other pathogen exposures may also play a role in the dampened immune response. The nose is the entry point for a variety of inhaled viruses, pollens, pollutants and other antigens. It supports an extensive microbiome-containing culturable and nonculturable bacteria. Because of ongoing exposure to microbes and their pathogen-associated molecular patterns, the innate immune response in the nose is likely dampened. Finally, the relatively lower temperature of the sinonasal airspace and epithelial surface may also contribute to the dampened immune response in cells in spite of high levels of viral shedding.27 V’kovski et al reported that SARS-CoV-2 grew to higher titres at 33°C compared with 37°C in airway epithelial cells, and this was associated with a reduced interferon transcriptomic response.27 This also provides additional rationale as to why even asymptomatic or mildly symptomatic individuals can shed a significant amount of SARS-CoV-2 virus from their nasal passages.
Therapeutic strategies for early infection in the upper airways
Treatments for early, mild infections are designed to block virus interaction with host ACE2 or to inhibit subsequent viral replication. Monoclonal antibodies to the receptor binding domain of the SARS-CoV-2 spike protein inhibit viral docking to the ACE2 receptor. This treatment is very effective if administered in the first few days of clinical infection.28 29 However, administrating these antibodies as outpatients is logistically difficult, and some of these antibodies may not recognise the new and emerging variants. Drugs that block viral replication such as remdesivir are also effective at this early stage of the disease.30 More antiviral drugs are needed and currently are being studied in clinical trials. The innate immune response is not only important for clearing the infection but also required for subsequent activation of the adaptive immune response. Although IgM, IgG and IgA are all important in protecting individuals against SARS-CoV-2 infection, IgA is especially important in mucosal immunity and is highly effective in neutralising SARS-CoV-2 in the first few weeks after the onset of symptoms.31 32 There are concerns that the reduced innate immune response in asymptomatic individuals might result in reduced antibody and T cell responses that are required for lasting acquired immunity.33
Infection of the lower conducting airways—target cells, cellular responses and therapeutic strategies
The next stage of the disease occurs after the virus enters the conducting airways, likely by microaspiration of pharyngeal secretions. This may be particularly facilitated in the elderly, who have diminished cough reflexes and reduced baseline mucociliary clearance.34 Infection in the bronchi produces epithelial injury, cellular infiltrates and in some cases marked denudation of the epithelial surface.35 36 The cells that express ACE2 and TMPRSS2 in the conducting airways are ciliated cells, mucus secreting cells and club cells.6 10 Club cells, identified by uteroglobin (SCGB1A1) as well as surfactant protein (SP)-A and SP-D expression, are present in only the very small bronchioles in humans, although they are more widespread in the airways of hamsters and mice.37 As in the nose, ciliated cells are the primary infected cell type.9 In pathologic specimens, ciliated and secretory epithelial cells appear infected, but progenitor basal cells are spared. This is supported by the primary bronchial epithelial cell culture model, where ciliated cells are the main cells infected at early times and then the infection spreads to secretory cells.1
Similar to the nasal epithelium, Belanco-Melo et al found only a modest cytokine response to SARS-CoV-2 in cultured bronchial cells in contrast to influenza A or treatment with Interferon beta (IFN-β).24 Type I and type III interferons were undetectable, and there was only a modest increase in CXCL10 expression, a marker of interferon stimulation. Ravindra et al showed that using scRNAseq type I and type III interferon gene expression was restricted to the infected ciliated cells with no detectable expression in neighbouring bystander cells.1 However, both infected and bystander cells expressed interferon responsive genes, implying a response to secreted interferon by the bystander cells. Hui et al demonstrated reduced CXCL10 and multiple other cytokines at the transcript and secreted protein38 level, respectively, in bronchial cultures compared to infections with influenza. Hence, as in the nose, the interferon and cytokine responses are only modest following SARS-CoV-2 infection. Chua et al used scRNAseq of nasopharygeal and bronchial samples from patients with COVID-19 to better define the interactions of epithelial cells and immune cells and reported that patients with critical COVID-19 had an enhanced inflammatory response and highlighted the importance of C-C Motif Chemokine Receptor 1 (CCR1) and CCR5 pathways for the recruitment of macrophages.39
As in the nose, current treatment at this stage of the infection is directed at preventing viral infection with monoclonal antibodies and limiting viral propagation with antiviral agents. Inhaled beta interferon has been tested to stimulate the innate immune response in a phase 2 clinical trial, but additional larger clinical trials are required to determine its efficacy.40 Hamsters are susceptible to SARS-CoV-2 infection, which produces extensive pulmonary infiltrates but is not lethal.41 Treatment of hamsters with interferon alpha markedly reduces the viral burden and pulmonary inflammation.42 These findings provide additional support for inhaled type I or type III interferons as possible therapeutic agents. Systemic steroids are not indicated early in the infection, but there remains an interest in the role of inhaled steroids at this stage awaiting a large, controlled clinical trial.43
THE GAS EXCHANGE UNITS
Infection of the alveolar epithelium: potential for structural and functional damage and severe illness
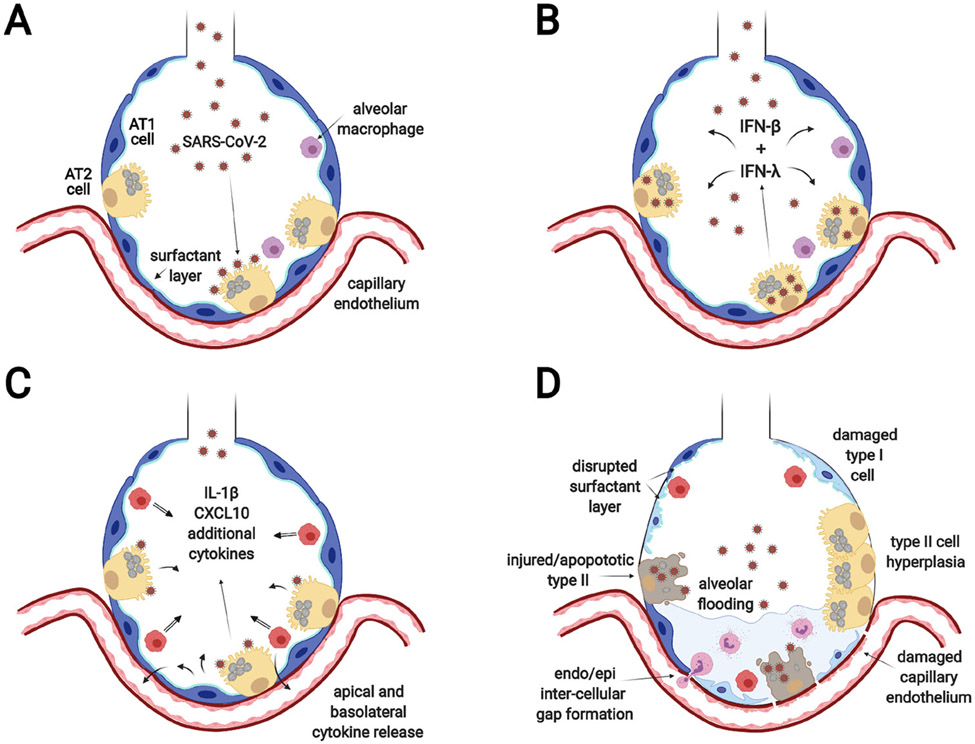
The extension of SARS-CoV-2 infection into the gas exchange portions of the lung is the primary cause of severe morbidity and mortality in patients with COVID-19 (figure 2). Infection in this region produces progressive hypoxia associated with pulmonary infiltrates. Early pathologic changes include alveolar flooding and inflammatory cell infiltrates.44 However, the disease rapidly progresses to acute respiratory distress syndrome (ARDS) with diffuse alveolar damage, hyaline membranes, epithelial and microvascular injury and, in some cases, thrombi of small and large vessels.35 36 45 The pathologic alterations are very similar to what was reported with SARS-CoV-1.46 The main cell type that expresses ACE2 and TMPRSS2 in this region is the alveolar type II cell,6 10 47 although scRNAseq shows that only a small proportion of type II cells (1%–3%) have detectable levels of ACE2 transcripts.48 Infection is demonstrated by the presence of SARS-CoV-2 antigens and mRNA within alveolar type II cells.35 Type I cells express less ACE2, but in primates, they can be infected.49 Alveolar type I-like cells (dedifferentiating type II cells in culture) are less permissive to infection with SARS-CoV-1 than well-differentiated type II cells.50 51 The widespread damage to type I cells is likely due to the robust inflammatory response following infection as opposed to direct infection. Similarly, microvascular endothelial cells do not appear to be directly infected but are damaged by the vigorous inflammatory response.9 This is also similar to the pathologic findings with SARS-CoV-1.46 Macrophages may respond to the virus through TLR signalling but do not propagate SARS-CoV-2.38
Figure 2.
SARS-CoV-2 infection of the alveolar epithelium (A) SARS-CoV-2 enters the alveoli, infects ACE2 expressing alveolar type II cells and replicates. (B) Infected AT2 cells release virus that infects bystander type II cells and type I (INFβ) and type III (INFλ) interferons are induced to initiate the innate immune response. (C) Infected and bystander cells respond to the interferons by secreting a variety of inflammatory cytokines and chemokines to recruit and activate immune cells. The illustration shows secretion into the alveolar lumen for simplicity, but there is also secretion to the basolateral side to attract inflammatory cells and affect endothelial cells. (D) The resultant effect is diffuse alveolar damage with loss of functional surfactant, alveolar flooding, influx of inflammatory cells, and damage to type I cells and endothelial cells.
Target cells for SARS-CoV-2 infection in the alveoli
The infection of and damage to type II cells by SARS-CoV-2 in pathologic reports likely have multiple physiologic consequences, which can be extrapolated from similar infection by influenza and SARS-CoV-1. Type II cells produce pulmonary surfactant, which is required to produce the low surface tension at the air–liquid interface on the alveolar surface. The low surface tension markedly decreases the work of breathing and facilitates gas exchange. Surfactant also contains SP-A and SP-D, which are multivalent lectins that are important in host defence proteins. SP-D is critical in protecting the host against influenza A infections.52 There is some evidence that SP-D may also protect against coronavirus infections, but more definitive studies need to be conducted.53 A subset of type II cells undergoes self-renewal during homeostasis and after injury and also serve as progenitor cells for type I cells.54 55 Hence, damage to type II cells can significantly impede epithelial repair mechanisms, as shown in mice infected with influenza A/PR8/1934 (H1N1).56 Tight junctions between alveolar epithelial cells form a major barrier for fluid flux from the microvasculature and interstitium into the alveolar space.57 Type II and type I cells actively transport fluid from the alveolar subphase to the interstitium driven by the basolateral Na/K ATPase.58-60 Based on the studies with SARS-CoV-1 and influenza, alveolar type II cells can also initiate the innate host response subsequent to viral infections and secrete type I and type III interferons and chemokines.50 61 These cells are among the first responders to respiratory viruses in the gas exchange portion of the lung. In addition, human alveolar type II cells express Human Leukocyte Antigen DR-isotype (HLA-DR), CD80 and CD86, which are required for antigen presentation to T cells,62 and, thus, are thought to serve as antigen-presenting cells during viral infections to initiate T cell responses but are less efficient than dendritic cells.63
Robust innate immune response to alveolar infection
There have been a number of in vitro studies of SARS-CoV-2 infection with human type II cells. For example, Huang et al used a model system of induced pluripotent stem cell-derived type II cells to study the impact of SARS-CoV-2 infection.48 In this study, there was a large increase in expression of inflammatory cytokines 24 hours after inoculation including IL-6, TNF, CXCL2 and CXCL8 and by 96 hours postinfection, type II cell differentiation genes were downregulated, including surfactant-associated genes (SFTPC, SFTPA1 and LAMP3). The interferon response was surprisingly low in this model system with no detectable interferon beta or lambda response at 1 or 4 days after inoculation and CXCL-10 expression was variable. Youk et al used alveolar organoids of expanded human type II cells and demonstrated a high level of infection associated with type I and type III interferon signals at 1 day postinoculation and induction of a broad range of interferon-stimulated genes days postinfection.64 Similarly, Katura et al used human type II cell organoids derived from primary lung tissue to detail a robust interferon-mediated inflammatory response, reduction of SFTPB and SFTPB SP expression and type II cell apoptosis in response to SARS-CoV-2 infection in vitro.65 Hence, alveolar type II cells appear to have a more rapid and robust innate response to SARS-CoV-2 infection compared with nasal or bronchial epithelial cells.
An animal model that shows the intense and catastrophic effect on type II cells would be beneficial for investigating the pathogenesis, the interaction with the immune system and response to therapy. Leist et al developed a mouse-adapted strain of SARS-CoV-2 (SARS-CoV-2 MA 10), which will be useful in defining the host response in the distal mouse lung in vivo.26 These investigators demonstrated profound damage to type II cells and bronchiolar club cells in response to infection,26 including reduced expression of Sftpb and Sftpc (SP-B and SP-C homologs), suggesting a critical loss of pulmonary surfactant. Because there are so many reagents and genetic constructs available in mice, this will serve as a powerful platform going forward.
Comparison of airway versus alveolar infection mechanisms and outcomes
Several more factors may indicate why SARS-CoV-2 infection is more devastating in the alveolar region compared with the nasal passages. In addition to robust activation of inflammatory cytokines during the ARDS phase of COVID-19, the complement cascade may also be activated, similar to what has been shown following SARS-CoV-1 infections in mice.66 Alveolar epithelial cells produce and secrete several components of the complement pathways, including C2, C3, C4 and C5.67
There is also a difference in the clearance mechanisms between the conducting airways and the alveolar region. Debris in the airways can be cleared by mucociliary clearance and coughing. Clearing debris in the alveolar compartment is more difficult, and most of the debris is phagocytosed by macrophages locally and transported to regional draining lymph nodes. Finally, major differences exist between the alveolar epithelium and the nasal and bronchial epithelium with respect to epithelial repair. Type II cells are the primary progenitor cells for the alveolar epithelium, which is a simple epithelium, and if they are damaged, the repair mechanisms are compromised. In the conducting airways, basal cells are the progenitor cells, and are not infected by SARS-CoV-2. Thus, the spared basal cells can proliferate and differentiate into secretory and ciliated cells to repair damage and reinitiate mucociliary clearance in this pseudostratified epithelium.
Mechanisms of severe disease with advanced age
For COVID-19 as well as SARS, the elderly have been particularly vulnerable to infection and have had a markedly worse clinical outcome. The reason for the susceptibility of this population in COVID-19 is not known. Some strains of SARS-CoV-1 are lethal in aged but not young mice.68 When aged mice are infected with lethal SARS-CoV-1 strains, they demonstrate an earlier and more robust interferon and acute phase response within 24 hours following infection characterised by a substantial increase in CXCL-10, IFN-β and IL-6 expression.68 The implication is that early elevation of these cytokines in the lung might indicate a worse clinical outcome in the elderly.69 Congruent with these data, ACE2 expression in alveolar type II cells has been demonstrated to increase with age.7 Furthermore, single cell analyses of airway epithelial cells from adult and paediatric patients identified reduced interferon responses in epithelial cells and a higher proportion of innate lymphoid and naive T cells in peripheral blood in COVID-19 paediatric patients compared with adults.70
Therapeutic strategies for alveolar infection
During the alveolar phase of COVID-19, the goals of treatment are to inhibit viral replication, to preserve type II cell survival and function, and to dampen the inflammatory response to maintain the architecture of the gas exchange units. Although endothelial cells are not likely to be direct targets of SARS-CoV-2, they are clearly damaged, thus treatments to prevent endothelial injury and microvascular thrombi are also very important. The two treatments that have been studied in detail are dexamethasone and anticytokine therapies. Corticosteroids are administered primarily to diminish tissue inflammation, but they also stimulate surfactant production in vivo and type II cells in vitro and induce structural maturation of the lung in the newborn.71-73 Dexamethasone is beneficial in hospitalised patients requiring mechanical ventilation or supplemental oxygen but not in those not requiring respiratory support.74 If a goal is to preserve injured type II cells, factors that stimulate cultured adult type II cells or organoids of type II cells in vitro should be considered. These factors include, but are not limited to, dexamethasone, cyclic Cyclic adenosine monophosphate (cyclic AMP) (isobutyl-methylxanthine, cholera toxin) and mitogenic growth factors (KGF, Hepatocyte growth factor (HGF), Transforming growth factor alpha (TGFa)).48 71 75 However, stimulating type II cell proliferation may be detrimental by increasing the number of ACE2 expressing cells and thereby increasing viral replication, similar to what has been reported in mice treated with KGF prior to infection with influenza.76 Type II cells are also the primary target cell of influenza infection in the alveolar region.77 There have been many attempts to inhibit individual components of the resultant hyperinflammation (previously termed “cytokine storm”), but these therapies have only been partially successful. Baricitinib, an inhibitor of Janus kinase 1 and 2, blocks several cytokines elevated in COVID-19 and is promising in patients receiving high-flow oxygen or non-invasive ventilation.78 Tocilizumab and sarilumab are monoclonal antibodies that block the IL-6 receptor. In the recent open-label Randomised, Embedded, Multi-factorial Adaptive Platform Trial for Community-Acquired Pneumonia with rapid enrollment of COVID-19 patients with ICU and greater than 80% receiving corticosteroids, tocilizumab and sarilumab increased respiratory and blood pressure support-free days to day 21 as well as survival and met the predefined criteria for efficacy.79 In an earlier standard controlled clinical trial prior to the widespread use of glucocorticoids, tocilizumab was not effective for preventing intubation or death in a cohort of moderately ill hospitalised patients with COVID-19.80 Rubin et al commented in an accompanying editorial on the differences in study design between the two trials and stressed the importance of context of the trials at different times in the COVID-19 pandemic both in terms of the concomitant use of glucocorticoids and in the evolving practice patterns in intensive care medicine for patients with COVID-19 .81
SUMMARY
Treatment of COVID-19 depends on the stage of the disease and the cell types that are infected. The mortality and long-term sequalae of SARS-CoV-2 infection depend on the extent of damage to the alveolar epithelium. One of the major differences for the long-term effects of SARS-CoV-2 infections is damage to the progenitor cells for the epithelium in the conducting airways and the alveoli. In the conducting airways, basal stem cells are spared; in the alveoli, type II cells are damaged. There remain a number of important questions to be answered. What is the mechanism for this purported dampened immune response in the nose and bronchi compared with the lower respiratory tract? At what stages of the disease would enhancing or inhibiting the innate immune system be beneficial? What treatments can preserve type II cell survival and function in the lung and enable them to fully restore the alveolar epithelium after injury? What is the cellular and molecular basis for the impaired response to the virus in the elderly? There are many unknowns in the pathogenesis of COVID-19 that are currently under study and will be reported in the near future.
Acknowledgements
This is a rapidly expanding field of research and we may have missed some critical reports. We apologise for this oversight.
Funding
The authors are supported in part from funds from the National Institutes of Health (HL131634 JPB), Cystic Fibrosis Foundation (EKV), and NeuroRx corporation (JPB and RJM). EKV is a Boettcher Early Career Investigator.
Footnotes
Competing interests None declared.
REFERENCES
- 1.Ravindra NG, Alfajaro MM, Gasque V, et al. Single-Cell longitudinal analysis of SARS-CoV-2 infection in human airway epithelium identifies target cells, alterations in gene expression, and cell state changes. PLoS Biol 2021;19:e3001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu B, Guo H, Zhou P, et al. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol 2021;19:141–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA 2020;324:782–93. [DOI] [PubMed] [Google Scholar]
- 4.V’kovski P, Kratzel A, Steiner S, et al. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol 2021;19:155–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gengler I, Wang JC, Speth MM, et al. Sinonasal pathophysiology of SARS-CoV-2 and COVID-19: a systematic review of the current evidence. Laryngoscope Investig Otolaryngol 2020;5:354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sungnak W, Huang N, Bécavin C, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med 2020;26:681–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muus C, Luecken MD, Eraslan G, et al. Single-Cell meta-analysis of SARS-CoV-2 entry genes across tissues and demographics. Nat Med 2021;27:546–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gamage AM, Tan KS, Chan WOY, et al. Infection of human nasal epithelial cells with SARS-CoV-2 and a 382-nt deletion isolate lacking ORF8 reveals similar viral kinetics and host transcriptional profiles. PLoS Pathog 2020;16:e1009130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hou YJ, Okuda K, Edwards CE, et al. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell 2020;182:429–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziegler CGK, Miao VN, Owings AH, et al. Impaired local intrinsic immunity to SARS-CoV-2 infection in severe COVID-19. bioRxiv 2021. doi: 10.1101/2021.02.20.431155. [Epub ahead of print: 20 Feb 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee IT, Nakayama T, Wu C-T, et al. Ace2 localizes to the respiratory cilia and is not increased by ACE inhibitors or Arbs. Nat Commun 2020;11:5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinto AL, Rai RK, Brown JC, et al. Ultrastructural insight into SARS-CoV-2 attachment, entry and budding in human airway epithelium. bioRxiv 2021:2021.04.10.439279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gudis D, Zhao K-qing, Cohen NA. Acquired cilia dysfunction in chronic rhinosinusitis. Am J Rhinol Allergy 2012;26:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leung HM, Birket SE, Hyun C, et al. Intranasal micro-optical coherence tomography imaging for cystic fibrosis studies. Sci Transl Med 2019;11:eaav3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas B, Rutman A, Hirst RA, et al. Ciliary dysfunction and ultrastructural abnormalities are features of severe asthma. J Allergy Clin Immunol 2010;126:722–9. [DOI] [PubMed] [Google Scholar]
- 16.Vladar EK, Nayak JV, Milla CE, et al. Airway epithelial homeostasis and planar cell polarity signaling depend on multiciliated cell differentiation. J CI Insight 2016;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yaghi A, Zaman A, Cox G, et al. Ciliary beating is depressed in nasal cilia from chronic obstructive pulmonary disease subjects. Respir Med 2012;106:1139–47. [DOI] [PubMed] [Google Scholar]
- 18.Zhu N, Wang W, Liu Z, et al. Morphogenesis and cytopathic effect of SARS-CoV-2 infection in human airway epithelial cells. Nat Commun 2020;11:3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urata S, Maruyama J, Kishimoto-Urata M, et al. Regeneration profiles of olfactory epithelium after SARS-CoV-2 infection in golden Syrian hamsters. ACS Chem Neurosci 2021;12:589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallo O, Locatello LG, Mazzoni A, et al. The central role of the nasal microenvironment in the transmission, modulation, and clinical progression of SARS-CoV-2 infection. Mucosal Immunol 2021;14:305–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiege JK, Thiede JM, Nanda HA, et al. Single cell resolution of SARS-CoV-2 tropism, antiviral responses, and susceptibility to therapies in primaiy human airway epithelium. PLoS Pathog 2021;17:e1009292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pizzorno A, Padey B, Julien T, et al. Characterization and treatment of SARS-CoV-2 in nasal and bronchial human airway epithelia. Cell Rep Med 2020;1:100059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lester SN, Li K. Toll-Like receptors in antiviral innate immunity. J Mol Biol 2014;426:1246–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blanco-Melo D, Nilsson-Payant BE, Liu W-C, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 2020;181:1036–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konno Y, Kimura I, Uriu K, et al. SARS-CoV-2 ORF3b is a potent interferon antagonist whose activity is increased by a naturally occurring elongation variant. Cell Rep 2020;32:108185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leist SR, Dinnon KH, Schäfer A, et al. A mouse-adapted SARS-CoV-2 induces acute lung injury and mortality in standard laboratory mice. Cell 2020;183:1070–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.V’kovski P Gultom M, Kelly JN, et al. Disparate temperature-dependent virus-host dynamics for SARS-CoV-2 and SARS-CoV in the human respiratory epithelium. PLoS Biol 2021;19:e3001158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gottlieb RL, Nirula A, Chen P, et al. Effect of Bamlanivimab as monotherapy or in combination with Etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA 2021;325:632–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen P, Nirula A, Heller B, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med 2021;384:229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med 2020;383:1813–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sterlin D, Mathian A, Miyara M, et al. Iga dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med 2021;13:eabd2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cervia C, Nilsson J, Zurbuchen Y, et al. Systemic and mucosal antibody responses specific to SARS-CoV-2 during mild versus severe COVID-19. J Allergy Clin Immunol 2021;147:545–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Long Q-X, Tang X-J, Shi Q-L, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med 2020;26:1200–4. [DOI] [PubMed] [Google Scholar]
- 34.Ho JC, Chan KN, Hu WH, et al. The effect of aging on nasal mucociliary clearance, beat frequency, and ultrastructure of respiratory cilia. Am J Respir Crit Care Med 2001;163:983–8. [DOI] [PubMed] [Google Scholar]
- 35.Martines RB, Ritter JM, Matkovic E, et al. Pathology and pathogenesis of SARS-CoV-2 associated with fatal coronavirus disease, United States. Emerg Infect Dis 2020;26:2005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borczuk AC, Salvatore SP, Seshan SV, et al. COVID-19 pulmonary pathology: a multi-institutional autopsy cohort from Italy and new York City. Mod Pathol 2020;33:2156–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plopper CG, Hill LH, Mariassy AT. Ultrastructure of the nonciliated bronchiolar epithelial (Clara) cell of mammalian lung. III. A study of man with comparison of 15 mammalian species. Exp Lung Res 1980;1:171–80. [DOI] [PubMed] [Google Scholar]
- 38.Hui KPY, Cheung M-C, Perera RAPM, et al. Tropism, replication competence, and innate immune responses of the coronavirus SARS-CoV-2 in human respiratory tract and conjunctiva: an analysis in ex-vivo and in-vitro cultures. Lancet Respir Med 2020;8:687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chua RL, Lukassen S, Trump S, et al. COVID-19 severity correlates with airway epithelium-immune cell interactions identified by single-cell analysis. Nat Biotechnol 2020;38:970–9. [DOI] [PubMed] [Google Scholar]
- 40.Monk PD, Marsden RJ, Tear VJ, et al. Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir Med 2021;9:196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cleary SJ, Pitchford SC, Amison RT, et al. Animal models of mechanisms of SARS-CoV-2 infection and COVID-19 pathology. Br J Pharmacol 2020;177:4851–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoagland DA, Møller R, Uhl SA, et al. Leveraging the antiviral type I interferon system as a first line of defense against SARS-CoV-2 pathogenicity. Immunity 2021;54:557–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Halpin DMG, Singh D, Hadfield RM. Inhaled corticosteroids and COVID-19: a systematic review and clinical perspective. Eur Respir J 2020;55:2001009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tian S, Hu W, Niu L, et al. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol 2020;15:700–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bösmüller H, Matter M, Fend F, et al. The pulmonary pathology of COVID-19. Virchows Arch 2021;478:137–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gu J, Korteweg C. Pathology and pathogenesis of severe acute respiratory syndrome. Am J Pathol 2007;170:1136–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao Y, Zhao Z, Wang Y, et al. Single-Cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2. Am J Respir Crit Care Med 2020;202:756–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang J, Hume AJ, Abo KM, et al. SARS-CoV-2 infection of pluripotent stem cell-derived human lung alveolar type 2 cells elicits a rapid Epithelial-Intrinsic inflammatory response. Cell Stem Cell 2020;27:962–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rockx B, Kuiken T, Herfst S, et al. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science 2020;368:1012–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qian Z, Travanty EA, Oko L, et al. Innate immune response of human alveolar type II cells infected with severe acute respiratory syndrome-coronavirus. Am J Respir Cell Mol Biol 2013;48:742–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mossel EC, Wang J, Jeffers S, et al. Sars-Cov replicates in primaiy human alveolar type II cell cultures but not in type I-like cells. Virology 2008;372:127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hartshorn KL, Crouch EC, White MR, et al. Evidence for a protective role of pulmonary surfactant protein D (SP-D) against influenza A viruses. J Clin Invest 1994;94:311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Madan T, Biswas B, Varghese PM, et al. A recombinant fragment of human surfactant protein D binds spike protein and inhibits infectivity and replication of SARS-CoV-2 in clinical samples. Am J Respir Cell Mol Biol 2021;65:41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Travaglini KJ, Nabhan AN, Penland L, et al. A molecular cell atlas of the human lung from single-cell RNA sequencing. Nature 2020;587:619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Juul NH, Stockman CA, Desai TJ. Niche cells and signals that regulate lung alveolar stem cells in vivo. Cold Spring Harb Perspect Biol 2020;12:a035717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yee M, Domm W, Gelein R, et al. Alternative progenitor lineages regenerate the adult lung depleted of alveolar epithelial type 2 cells. Am J Respir Cell Mol Biol 2017;56:453–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schneeberger EE, Karnovsky MJ. Substructure of intercellular junctions in freeze-fractured alveolar-capillary membranes of mouse lung. Circ Res 1976;38:404–11. [DOI] [PubMed] [Google Scholar]
- 58.Cott GR, Sugahara K, Mason RJ. Stimulation of net active ion transport across alveolar type II cell monolayers. Am J Physiol 1986;250:C222–7. [DOI] [PubMed] [Google Scholar]
- 59.Mason RJ, Williams MC, Widdicombe JH, et al. Transepithelial transport by pulmonary alveolar type II cells in primary culture. Proc Natl Acad Sci U S A 1982;79:6033–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kryvenko V, Vadász I. Molecular mechanisms of Na,K-ATPase dysregulation driving alveolar epithelial barrier failure in severe COVID-19. Am J Physiol Lung Cell Mol Physiol 2021;320:L1186–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang J, Nikrad MP, Phang T, et al. Innate immune response to influenza A virus in differentiated human alveolar type II cells. Am J Respir Cell Mol Biol 2011;45:582–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zissel G, Ernst M, Rabe K, et al. Human alveolar epithelial cells type II are capable of regulating T-cell activity. J Investig Med 2000;48:66–75. [PubMed] [Google Scholar]
- 63.Toulmin S, Bhadiadra C, Paris A, et al. Type II alveolar cells with constitutive expression of MHCII and limited antigen presentation capacity contribute to improved respiratory viral disease outcomes. bioRxiv 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Youk J, Kim T, Evans KV, et al. Three-Dimensional human alveolar stem cell culture models reveal infection response to SARS-CoV-2. Cell Stem Cell 2020;27:905–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Katsura H, Sontake V, Tata A, et al. Human lung stem cell-based Alveolospheres provide insights into SARS-CoV-2-Mediated interferon responses and pneumocyte dysfunction. Cell Stem Cell 2020;27:890–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gralinski LE, Sheahan TP, Morrison TE, et al. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. mBio 2018;9:e01753–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Strunk RC, Eidlen DM, Mason RJ. Pulmonary alveolar type II epithelial cells synthesize and secrete proteins of the classical and alternative complement pathways. J Clin Invest 1988;81:1419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rockx B, Baas T, Zornetzer GA, et al. Early upregulation of acute respiratory distress syndrome-associated cytokines promotes lethal disease in an aged-mouse model of severe acute respiratory syndrome coronavirus infection. J Virol 2009;83:7062–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meftahi GH, Jangravi Z, Sahraei H, et al. The possible pathophysiology mechanism of cytokine storm in elderly adults with COVID-19 infection: the contribution of “inflame-aging”.Inflamm Res 2020;69:825–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoshida M, Worlock KB, Huang N, et al. The local and systemic response to SARS-CoV-2 infection in children and adults. medRxiv 2021:2021.03.09.21253012. [Google Scholar]
- 71.Wang J, Edeen K, Manzer R, et al. Differentiated human alveolar epithelial cells and reversibility of their phenotype in vitro. Am J Respir Cell Mol Biol 2007;36:661–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bridges JP, Sudha P, Lipps D, et al. Glucocorticoid regulates mesenchymal cell differentiation required for perinatal lung morphogenesis and function. Am J Physiol Lung Cell Mol Physiol 2020;319:L239–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schmidt AF, Kannan PS, Bridges J, et al. Prenatal inflammation enhances antenatal corticosteroid-induced fetal lung maturation. JCI Insight 2020;5:e139452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021;384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lamers MM, van der Vaart J, Knoops K, et al. An organoid-derived bronchioalveolar model for SARS-CoV-2 infection of human alveolar type II-like cells. Embo J 2021;40:e105912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nikolaidis NM, Noel JG, Pitstick LB, et al. Mitogenic stimulation accelerates influenza-induced mortality by increasing susceptibility of alveolar type II cells to infection. Proc Natl Acad Sci U S A 2017;114:E6613–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weinheimer VK, Becher A, Tönnies M, et al. Influenza A viruses target type II pneumocytes in the human lung. J Infect Dis 2012;206:1685–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus Remdesivir for hospitalized adults with Covid-19. N Engl J Med 2021;384:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.REMAP-CAP Investigators, Gordon AC, Mouncey PR, et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med 2021;384:1491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Salama C, Han J, Yau L, et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med 2021;384:20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rubin EJ, Longo DL, Baden LR. Interleukin-6 Receptor Inhibition in Covid-19 - Cooling the Inflammatory Soup. N Engl J Med 2021;384:1564–5. [DOI] [PMC free article] [PubMed] [Google Scholar]