Abstract
Acute Kaposi sarcoma herpes virus (KSHV)-associated inflammatory diseases are under-recognized. We describe a woman with HIV presenting with disseminated KS and refractory shock, concerning for KSHV-associated inflammatory cytokine syndrome (KICS) versus multicentric Castelman’s disease (MCD). High-quality research and clinician education are needed to improve prognosis of patients with KSHV-associated diseases.
Keywords: Kaposi Sarcoma, KICS, HHV8, KSHV
Introduction
Kaposi Sarcoma Herpes Virus (KSHV; also known as Human Herpes Virus-8 [HHV8]), causes a spectrum of diseases, including Kaposi sarcoma (KS), primary effusion lymphoma (PEL), multicentric Castleman disease (MCD), and KSHV-associated inflammatory cytokine syndrome (KICS).1 KICS is a rare and aggressive disorder, typically reported in persons with HIV (PWH). It is a syndrome triggered by interleukin-6 (IL6) dysregulation, which leads to symptoms including anasarca, effusions, circulatory shock, and multi-organ failure. Delayed diagnosis and the lack of rapidly effective therapeutic options contribute to a high mortality (approximately 60%).2 It is critical to rule out concomitant MCD and PEL in patients with suspected KICS, as management strategies differ. Here we discuss the data guiding KICS diagnosis and therapy via a recent patient’s case, with the objective of improving the medical community’s recognition and management of KSHV-related diseases.
Case
A 45-year-old Nigerian woman presented to the emergency department with three days of severe fatigue and hematemesis. She reported being in her usual state of health until six months prior, when she noticed a dark rash over her face, chest, and inner thighs. She also described a progressive dry cough and dyspnea with exertion. Initial vital signs revealed tachycardia, hypotension, and hypoxemia (requiring 6 liters per minute of oxygen by nasal cannula). On physical exam she had bilateral rales and wheezes as well as hyperpigmented papules over her face, gums, trunk, and bilateral extremities (Figure 1A–C).
Figure 1:
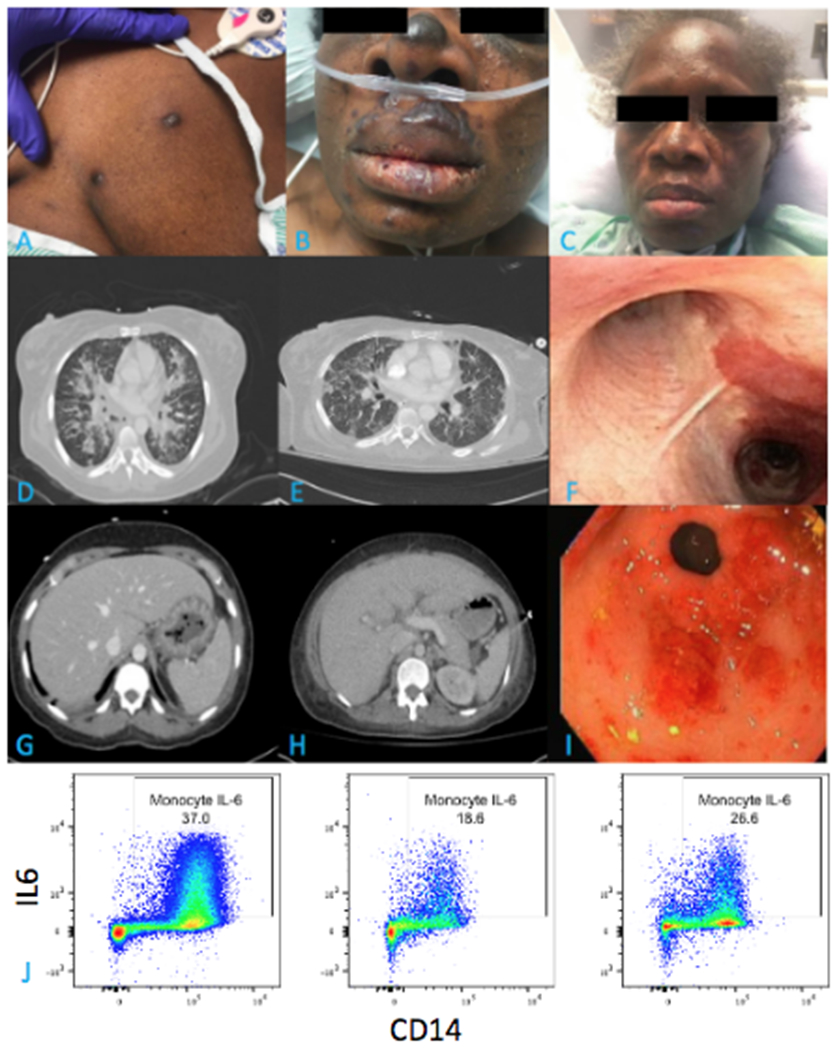
A. Violaceous papules on chest wall, biopsy confirmed Kaposi Sarcoma (KS). B. Facial involvement of KS on admission. C. Interval improvement in facial KS involvement after 6 months of therapy. D. Admission CT axial cross section demonstrating diffuse, centrally-predominant ground glass and consolidative opacities with nodular components. E. Interval improvement in ground glass and nodular opacities after 7 weeks of therapy. F. Bronchoscopic view of the carina with violaceous KS nodule. G. Admission CT axial cross section demonstrating gastric wall thickening. H. Interval improvement in gastric wall thickening after 7 weeks of therapy. I. Endoscopic view of gastric antrum / pylorus with friable erythematous nodular lesions, biopsy confirmed KS. J. Peripheral blood flow cytometry plot of monocyte hIL6 expression from the patient prior to therapy for KICS (left) and one week after initiation of therapy (middle) compared to a healthy control (right).
Admission laboratory evaluation showed elevated venous lactic acid (3.1 mmol/L), elevated white blood cell count (13,600 cells/μL) with neutrophilic predominance (83%), microcytic anemia (hemoglobin 6.5 g/dL), and marked thrombocytopenia (31,000 cells/μL). She was hyponatremic (130 mg/dL) and hypoalbuminemic (1.8 mg/dL) with normal renal and liver function tests. Further evaluation revealed elevated lactate dehydrogenase (329 U/L), ferritin (467 ng/ml), and c-reactive protein (CRP) (>80 mg/L) with normal random serum cortisol (27 ug/dL). Fourth generation HIV testing was positive; CD4+ cell count was 32 cells/μL with an HIV viral load of 136,000 copies/mL. Computed tomography (CT) of the chest, abdomen, and pelvis demonstrated diffuse bilateral airspace opacities and peri-bronchial flame-shaped nodules (Figure 1D), small bilateral pleural effusions, gastric wall thickening and colitis (Figure 1G), trace ascites, and bilateral pelvic lymphadenopathy.
Due to concern for septic shock, the patient was rapidly resuscitated with intravenous fluids; initiated on vancomycin, azithromycin, ceftriaxone, and trimethoprim-sulfamethoxazole; and moved to the intensive care unit (ICU). Additional testing revealed three negative acid-fast bacilli sputum smears and a negative Mycobacterium tuberculosis PCR. Nasopharyngeal molecular testing for common respiratory viruses, Mycoplasma, Chlamydia, and SARS-CoV-2 were negative. Urine antigen testing for Streptococcus pneumoniae, Legionella pneumophila, and Histoplasma were negative, as was a serum antigen test for Cryptococcus. Bronchoscopy with bronchoalveolar lavage (BAL) on hospital day (HD) 2 demonstrated violaceous lesions throughout the upper airways (Figure 1F). BAL cytology was negative for Pneumocystis, and routine bacterial, mycobacterial, and fungal stains and cultures were negative. She was intubated for respiratory failure on hospital day HD-3 and subsequently developed severe acute respiratory distress syndrome, acute renal failure requiring renal replacement therapy, and refractory shock requiring vasopressor support and hydrocortisone. Esophagogastroduodenoscopy on HD-3 revealed nodular lesions of the stomach wall (Figure 1I). Biopsies of skin lesions, gastric body, and an inguinal lymph node uniformly showed KSHV-expressing spindle cells, consistent with disseminated KS. Importantly, lymph node histopathology revealed no evidence for KSHV-MCD or extra-cavitary PEL.
KICS was suspected early given characteristic KS lesions, multi-organ dysfunction, and lack of initial improvement despite aggressive resuscitation and broad anti-microbial coverage. Peripheral blood flow cytometry revealed elevated IL6 (Figure 1J) and tumor necrosis factor-α (TNFα) production by CD14+ monocytes. Thoracentesis was not performed to rule out concomitant PEL, as pleural effusions were too small to safely sample. Anti-retroviral therapy (ART; specifically, dolutegravir, abacavir, and renally-dosed lamivudine) was started on HD-6 and liposomal doxorubicin and rituximab were given on HD-7 and 8, respectively. Her fevers resolved by HD-9, vasopressors were stopped by HD-14, and hemodialysis was discontinued on HD-28. Repeat flow cytometry on HD-10 demonstrated decreased CD14+ monocyte IL6 production (Figure 1J) and TNFα post-chemotherapy. She continued to receive liposomal doxorubicin every three weeks, three total doses of weekly rituximab, daily ART, and supportive care. Repeat imaging on HD-48 demonstrated improvement in lung nodules (Figure 1E), intestinal wall thickening (Figure 1H), and inguinal lymphadenopathy.
Discussion
KSHV-associated diseases can have devastating consequences if not appropriately diagnosed and treated, and KICS is no exception. KICS was first described in 2010 in a series of six PWH with laboratory and clinical signs of KSHV-mediated inflammation but without evidence of MCD.1 As more data accumulated characterizing KICS, the case definition expanded to require the presence of clinical symptoms (fever, fatigue, edema, cachexia, respiratory manifestations, gastrointestinal disturbance, arthralgia, myalgia, altered mental state, or neuropathy), laboratory abnormalities (anemia, thrombocytopenia, hypoalbuminemia, or hyponatremia), and radiological findings (hepatosplenomegaly, lymphadenopathy, or body cavity effusions), in addition to evidence of systemic inflammation (e.g., CRP >3g/dL), KSHV viremia, and exclusion of concomitant MCD. The prognosis is usually poor.2
Our patient met the current KICS definition, with the following limitations: (1) inability to measure KSHV level (due to hospital resource limitations), (2) delay in obtaining lymph node biopsy to evaluate for MCD (due to initial clinical instability), and (3) inability to obtain pleural fluid to evaluate for PEL (due to small effusion size). While nearly all patients with effusions in the setting of KS have KICS,3 when effusions are present in this scenario it is important to rule out PEL, which requires distinct combination chemotherapy. Use of advanced imaging studies such as FDG-PET may be helpful in identifying lymphadenopathy and body cavity effusions that suggest MCD or PEL, respectively.
KICS treatment approaches resemble those of KSHV-MCD. Our patient was treated with a combination of medications from each of the three core KICS therapy strategies, including (1) effective ART to control HIV and restore T-cell function, (2) chemotherapy to eliminate KS spindle cells, and (3) immunotherapy to attenuate the proinflammatory response underlying KSHV-MCD and KICS.4 Each of these is discussed below.
ART is a mainstay of therapy for all KSHV-related diseases in PWH, and our patient likely benefited from rapid ART initiation. In a retrospective study of PWH with KS, an undetectable HIV viral load was the best predictor of KS remission, independent of the patient’s CD4 cell count.5 As an aside, patients suspected to have concomitant KS-associated immune reconstitution inflammatory syndrome and KICS should start chemotherapy as early as possible (while continuing ART), especially if there is visceral involvement, and corticosteroid treatment should be avoided as it stimulates KS spindle cell proliferation.6 Though our patient transiently received hydrocortisone for refractory shock, she was not otherwise treated with corticosteroids.
Chemotherapy is a critical component of KICS treatment as it targets the underlying KS spindle cells. Both liposomal doxorubicin and paclitaxel are FDA-approved for advanced or rapidly progressive KS. Several studies demonstrate that treatment with liposomal anthracyclines results in better treatment response and more favorable side effect profiles compared to non-liposomal doxorubicin, bleomycin, and vincristine.7
Regarding immunotherapy, the CD20 receptor inhibitor rituximab is commonly used in combination with ART and liposomal doxorubicin as first line treatment in KSHV-MCD with KS and therefore is a rational choice for KICS. Importantly, rituximab alone is associated with worsening of KS and therefore must be given concomitantly with a chemotherapeutic agent active against KS.4 Multiple studies of adjunctive or alternative immunotherapies are currently underway, including a phase 1 trial of pomalidomide plus liposomal doxorubicin for patients with advanced KS, KS and concomitant KSHV-MCD, and KICS.8 The severe systemic inflammation that defines KICS is thought due to exuberant production of pro-inflammatory cytokines induced by KSHV, including both human IL6 derived from myeloid cells (hIL6) and a functionally active viral homolog of IL6 derived from KSHV-infected spindle cells (vIL6).9 This was demonstrated by our patient, who had elevated pre-treatment pro-inflammatory cytokine levels (specifically, hIL-6 [Figure 1J] and TNF-α) that improved post-treatment. Accordingly, treatment with hIL6 inhibition (e.g., tocilizumab [an IL6 receptor inhibitor] or siltuximab [an anti-hIL6 antibody]) is of theoretical interest.9 However, a recent study demonstrated that, while tocilizumab was safe and had activity against KSHV-MCD, patients’ progression-free survival was shorter than those treated with rituximab, likely related to rituximab’s longer half-life.10
Finally, anti-herpesvirus drugs such as ganciclovir derivatives have been used in KS and other KSHV-related diseases, but efficacy data are limited.11 In one pilot study of PWH with KSHV-MCD, those given high-dose zidovudine plus valganciclovir showed significant improvements in CRP, albumin, platelets, hIL6, IL10, and KSHV viral load, though hematological toxicities were common.12
KICS remains an inadequately studied pathologic process on the spectrum of KSHV-associated diseases and carries a high mortality rate. While data guiding management are relatively sparse, disease regression can occur, as in our patient, if KICS is recognized early and treated with combination therapy including ART, liposomal doxorubicin, and rituximab. Given the complexity and rarity of KICS, it is essential to refer suspected cases to clinical trials at specialist centers to (1) improve our understanding of the disease process and its relationship with other KSHV-related entities, and (2) systematically develop optimal therapeutic strategies.
Footnotes
Conflict of Interest Statement: No authors report any conflicts of interest.
References
- 1.Uldrick TS, Wang V, O’Mahony D, et al. An interleukin-6-related systemic inflammatory syndrome in patients co-infected with Kaposi sarcoma-associated herpesvirus and HIV but without Multicentric Castleman disease. Clin Infect Dis. Aug 1 2010;51(3):350–8. doi: 10.1086/654798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polizzotto MN, Uldrick TS, Wyvill KM, et al. Clinical Features and Outcomes of Patients With Symptomatic Kaposi Sarcoma Herpesvirus (KSHV)-associated Inflammation: Prospective Characterization of KSHV Inflammatory Cytokine Syndrome (KICS). Clin Infect Dis. Mar 15 2016;62(6):730–738. doi: 10.1093/cid/civ996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramaswami R, Lurain K, Marshall VA, et al. Elevated IL-13 in effusions of patients with HIV and primary effusion lymphoma as compared with other Kaposi sarcoma herpesvirus-associated disorders. AIDS. Jan 1 2021;35(1):53–62. doi: 10.1097/QAD.0000000000002692 [DOI] [PMC free article] [PubMed] [Google Scholar]; AIDS. Jun 13 2003;17(9):1409–10. doi: 10.1097/00002030-200306130-00023 [DOI] [PubMed] [Google Scholar]
- 4.Gerard L, Berezne A, Galicier L, et al. Prospective study of rituximab in chemotherapy-dependent human immunodeficiency virus associated multicentric Castleman’s disease: ANRS 117 CastlemaB Trial. J Clin Oncol. Aug 1 2007;25(22):3350–6. doi: 10.1200/JCO.2007.10.6732 [DOI] [PubMed] [Google Scholar]
- 5.Martinez V, Caumes E, Gambotti L, et al. Remission from Kaposi’s sarcoma on HAART is associated with suppression of HIV replication and is independent of protease inhibitor therapy. Br J Cancer. Apr 10 2006;94(7):1000–6. doi: 10.1038/sj.bjc.6603056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leidner RS, Aboulafia DM. Recrudescent Kaposi’s sarcoma after initiation of HAART: a manifestation of immune reconstitution syndrome. AIDS Patient Care STDS. Oct 2005;19(10):635–44. doi: 10.1089/apc.2005.19.635 [DOI] [PubMed] [Google Scholar]
- 7.Northfelt DW, Dezube BJ, Thommes JA, et al. Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi’s sarcoma: results of a randomized phase III clinical trial. J Clin Oncol. Jul 1998;16(7):2445–51. doi: 10.1200/JCO.1998.16.7.2445 [DOI] [PubMed] [Google Scholar]
- 8.Ramaswami R, Lurain KA, Widell A, et al. A phase I trial of pomalidomide in combination with liposomal doxorubicin in patients with Kaposi sarcoma with or without other KSHV-associated diseases. Journal of Clinical Oncology. 2020;38(15_suppl):11552–11552. doi: 10.1200/JCO.2020.38.15_suppl.11552 [DOI] [Google Scholar]
- 9.Polizzotto MN, Uldrick TS, Wang V, et al. Human and viral interleukin-6 and other cytokines in Kaposi sarcoma herpesvirus-associated multicentric Castleman disease. Blood. Dec 19 2013;122(26):4189–98. doi: 10.1182/blood-2013-08-519959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramaswami R, Lurain K, Peer CJ, et al. Tocilizumab in patients with symptomatic Kaposi sarcoma herpesvirus–associated multicentric Castleman disease. Blood. 2020;135(25):2316–2319. doi: 10.1182/blood.2019004602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krown SE, Dittmer DP, Cesarman E. Pilot study of oral valganciclovir therapy in patients with classic Kaposi sarcoma. J Infect Dis. Apr 15 2011;203(8):1082–6. doi: 10.1093/infdis/jiq177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uldrick TS, Polizzotto MN, Aleman K, et al. High-dose zidovudine plus valganciclovir for Kaposi sarcoma herpesvirus-associated multicentric Castleman disease: a pilot study of virus-activated cytotoxic therapy. Blood. Jun 30 2011;117(26):6977–86. doi: 10.1182/blood-2010-11-317610 [DOI] [PMC free article] [PubMed] [Google Scholar]


