Abstract
Background
Stroke is the second main cause of mortality and the third leading cause of mortality and permanent disability combined. Many potential biomarkers have been described to contribute to the diagnosis, prognosis of outcomes, and risk stratification after stroke. Copeptin is an inactive peptide that is produced in an equimolar ratio to arginine vasopressin in response to the activation of the endogenous stress system.
Methods
The present study is a systematic review and meta-analysis to assess plasma copeptin concentrations, diagnostic and prognostic values for risk stratification after acute ischemic stroke and transient ischemic attack.
Results
Mean copeptin level in stroke vs. non-stroke groups varied and amounted to 19.8 ± 17.4 vs. 9.7 ± 6.6 pmol/L, respectively (mean differences [MD]: 12.75; 95% confidence interval [CI]: 5.00 to 20.49; p < 0.001), in good vs. poor outcome 12.0 ± 3.6 vs. 29.4 ± 14.5 (MD: −8.13; 95% CI: −8.37 to −7.88; p < 0.001) and in survive vs. non-survive stroke patients: 13.4 ± 3.2 vs. 33.0 ± 12.3, respectively (MD: −13.43; 95% CI: −17.82 to −9.05; p < 0.001).
Conclusions
The above systematic review and meta-analysis suggests that monitoring the copeptin levels may help predict the long-term prognosis of ischemic stroke efficiently. Determining the copeptin level may help individualize the management of ischemic stroke patients, keep stroke risk lower, reduce post-stroke complications, including patient death, and minimize healthcare costs.
Keywords: copeptin, C-terminal (pre)pro-vasopressin, prognostic biomarker, acute ischemic stroke, systematic review, meta-analysis
Introduction
Stroke is the second main cause of mortality and the third leading cause of mortality and permanent disability combined [1]. Unfortunately, despite significant progress in the clinical management of stroke patients and the invaluable role of imaging studies, there is still a lack of reliable blood biomarkers for use in diagnosis and prognosis of outcome in this patient population [2]. Many potential biomarkers have been described to contribute to risk stratification after stroke [3]. Of these, markers of inflammation (procalcitonin and mannose-binding lectin), atherogenesis (adipocyte fatty acid-binding protein), stress response (copeptin and cortisol), and the natriuretic peptide should be mentioned. These markers were most consistently associated with poorer outcomes after stroke and added a prognostic value to the established prognostic factors. However, there are some concerns about the methodological or statistical quality of these studies, thus limiting the applicability of this data to clinical practice [3]. It raises the need for further research into the most promising markers.
A transient ischemic attack (TIA) is a strong predictor of stroke. Survivors often require long-term care and are at high risk of recurrent stroke [4]. Early assessment of the risk of stroke recurrence is critical in determining a patient’s prognosis. Rapidly measurable biomarkers may play a role in helping to predict the development and consequences of stroke, which is significant in optimal differentiation of patient care and allocation of healthcare resources [5].
Arginine vasopressin (AVP) is a non-cardiac plasma marker of cardiovascular disease. It is secreted from the posterior pituitary gland in response to changes in plasma osmolality and co-stimulates adrenocorticotropin along with corticotropin-releasing hormone, thereby influencing the stress response [6]. This non-osmotic pathway is likely how AVP and copeptin can be used as predictive markers [7]. However, the challenge with AVP is its instability outside the human body and challenges in measurements. Copeptin, the C-terminal part of (pre)pro-vasopressin, is a surrogate marker for AVP. It is more stable at room temperature and easier to measure [8]. Elevated copeptin concentration was associated with higher mortality in patients with heart failure and poorer prognosis in patients after acute myocardial infarction [9, 10]. It was also described to have clinical implications in non-cardiovascular diseases such as polydipsia-polyuria syndrome, multiple sclerosis, sepsis, or preeclampsia [11–15]. Due to the positive relationship of increases in the copeptin level in patients with acute ischemic stroke and TIA, it is assumed that copeptin is a good marker for differential diagnosis between stroke, TIA, and stroke-mimics diseases [16]. Moreover, an elevated copeptin concentration was related to worse prognosis in patients after stroke and to a higher incidence of recurrent TIA or stroke after a TIA event [2]. However, some studies demonstrated the lack of any significant association between the copeptin concentrations and stroke incidence [17].
Therefore, the present systematic review and meta-analysis was performed to assess the diagnosis and prognostic value of plasma copeptin concentrations for risk stratification after acute ischemic stroke and TIA.
Methods
This systematic review and meta-analysis were done according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement [18]. All analyses were based on previously published studies; thus, ethical approval or patient consent was unsuitable for this meta-analysis.
Literature search and selection
Comprehensive systematic searches of online electronic databases, including PubMed, Scopus, Web of Science, Cochrane Library, and Google Scholar from the databases inception to November 21, 2021, were performed. The literature was searched using the following keywords: “C-terminal pro-vasopressin” OR “copeptin” AND “stroke” OR “ischemic attack” OR “TIA” OR “transient ischemic stroke” OR “recurrent cerebrovascular event”. All records were searched by two researchers (P.S. and N.B.) separately. They solved disagreements through discussion with a third researcher (L.S.). The search of databases was limited to English publications. No limitation was set for the age of participants in the searched articles. Reference lists in each publication involved were also manually checked to identify eligible studies.
The inclusion criteria were: (1) studies focused on the value of copeptin in predicting mortality in patients with stroke or studies focused on the value of copeptin in: (a) stroke vs. non-stroke patients; (b) re-events TIA vs. non-re-events TIA; (c) ischemic vs. hemorrhagic stroke; (d) stroke/TIA vs. mimic; (3) randomized controlled trials or non-randomized trials. Studies were excluded if: (1) they did not present a comparator group; (2) references were in the form of reviews, letters, editorials, conference articles, or duplicated publications.
Quality assessment and data extraction
Two authors (N.B. and L.S.) independently extracted data from relevant articles: first author name, year of publication, region of the cohort, patient characteristics (i.e., no. of patients, age, sex), type of cerebrovascular event, and copeptin levels. They resolved discrepancies through discussion with the third researcher (A.G.). Data were recorded from included studies using a Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) specific predefined report form. When data about the primary outcomes were missing, the plan was to contact the corresponding author of the original study.
Data items, outcomes, design strengths, and weaknesses across the studies were compared. The risk of bias at the study level was assessed for each study using the Cochrane ROBINS-I bias assessment tool [19]. The ROBINS-I tool examines seven bias domains due to: (1) confounders; (2) selection of participants; (3) classification of interventions; (4) deviations from intended interventions; (5) missing data; (6) measurements of outcomes; (7) selection of the reported results. The Robvis application was used to visualize the risk of bias assessments [20].
Statistical analysis
Mean differences (MD) with 95% confidence intervals (CIs) for continuous data were used. When the continuous outcome was reported in a study as median, range, and interquartile range, means and standard deviations were estimated using the formula described by Hozo et al. [21]. For dichotomous data, odds ratios (OR) as the effect measure with 95% CI were utilized. We assessed heterogeneity statistically using I2 (no heterogeneity, I2: 0–25%; moderate heterogeneity, I2: 25–50%; large heterogeneity, I2: 50–75%; extreme heterogeneity, I2: 5–100%). The randomeffects model was used for I2 > 50%; otherwise, the fixed effects model was employed. Potential publication bias was sought using a funnel plot if over 10 trials were included for an outcome. For continuous outcomes, the Egger test was used to detect funnel plot asymmetry [22]. All analyses were performed using Stata software, version 15.0 (College Station, TX, USA) as well as with the Review Manager software version 5.4 (Nordic Cochrane Center, Cochrane Collaboration). P < 0.05 (two-tailed) was considered significant.
Results
Characteristics of the articles
A flowchart of the publication selection process is presented in Figure 1. The database searches and citation tracking yielded 1273 hits. After a title review and removal of duplicate studies, screening excluded 934 articles and 48 full-text articles remained. Some articles did not meet the inclusion criteria; on this basis, 31 full-text papers with insufficient data for extraction were excluded. After screening for all the probable factors, 5057 patients were finally included from 17 studies [2, 5, 16, 17, 23–35].
Figure 1.
Database search and selection of studies according to Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines.
Table 1 summarizes the 17 articles included in the systemic review and their methodologies. All studies were conducted between 2009 and 2019 in China [5, 16, 26, 30, 31, 34, 35], Switzerland [17, 27, 28, 32], Germany [23, 33], Switzerland and Germany [2, 24], France [25], and Croatia [29]. The risk of bias of these studies was low (n = 14) or moderate (n = 3) (Suppl. Figs. S1 and S2).
Table 1.
Characteristics of included trials.
| Study | Country | Study design | No. of patients | Age | Sex, female |
|---|---|---|---|---|---|
| De Marchis et al. 2013 [24] | Switzerland/Germany | Prospective, multicenter, cohort study | 783 | 70.6 ± 3.3 | 298 (38.1%) |
| De Marchis et al. 2014 [2] | Switzerland/Germany | Prospective, multicenter, cohort study | 302 | 68.8 ± 3.2 | 112 (37.1%) |
| Deboevere et al. 2019 [25] | France | Prospective, observational, monocenter study | 135 | 59.4 ± 5.9 | 79 (58.5%) |
| Dong et al. 2013 [26] | China | Prospective, observational cohort study | 125 | 71 ± 4 | 56 (44.8%) |
| Katan et al. 2009 [27] | Switzerland | Prospective observational study | 359 | 74 ± 3.3 | 149 (41.5%) |
| Katan et al. 2011 [28] | Switzerland | Prospective observational study | 107 | 70.3 ± 3.2 | 60 (56.1%) |
| Katan et al. 2016 [17] | Switzerland | Nested case-control study | 516 | 69.5 ± 3.1 | 326 (63.2%) |
| Perovic et al. 2017 [29] | Croatia | Case-control study | 172 | 76.2 ± 2.7 | 100 ((58.1%) |
| Sun et al. 2018 [30] | China | Case-control study | 238 | 61.5 ± 2.7 | 92 (38.7%) |
| Tang et al. 2017 [5] | China | Post hoc analysis | 405 | Not specified | Not specified |
| Tu et al. 2013 [31] | China | Prospective cohort study | 189 | 66.5 ± 4.7 | 72 (55.0%) |
| Urwyler et al. 2010 [32] | Switzerland | Prospective cohort study | 362 | 74.5 ± 3 | 145 (40.1%) |
| von Recum et al. 2015 [33] | Germany | Prospective cohort study | 36 | 68 ± 6.3 | 16 (44.4%) |
| Wang et al. 2014 [16] | China | Prospective cohort study | 275 | 68.8 ± 3.2 | 135 (49.1%) |
| Wang et al. 2016 [34] | China | Prospective cohort study | 247 | 65.3 ± 3.8 | 108 (43.7%) |
| Wendt et al. 2015 [23] | Germany | Prospective cohort study | 561 | 72.7 ± 13.7 | 302 (53.8%) |
| Zhang et al. 2013 [35] | China | Prospective cohort study | 245 | 73 ± 64.8 | 103 (42.0%) |
Search results
Five trials reported copeptin levels in stroke vs. non-stroke groups. In most of the studies, the non-stroke patients group consisted of healthy subjects, except for studies conducted by Deboevere et al. [25] where the non-stroke group, contained patients visiting emergency department for a new episode of dizziness with the exclusion of stroke diagnosis based on brain imaging, and DeMarchis et al. [2] in which the patients who did not experience a stroke within 3 months after the index TIA were investigated. Mean copeptin level in stroke vs. non-stroke groups varied and amounted to 19.8 ± 17.4 vs. 9.7 ± 6.6 pmol/L, respectively (MD: 12.75; 95% CI: 5.00 to 20.49; p < 0.001; Fig. 2).
Figure 2.
Forest plot of copeptin levels in stroke and non-stroke groups. The center of each square represents the weighted mean differences for individual trials, and the corresponding horizontal line stands for a 95% confidence interval (CI). The diamond represents pooled results; SD — standard deviation.
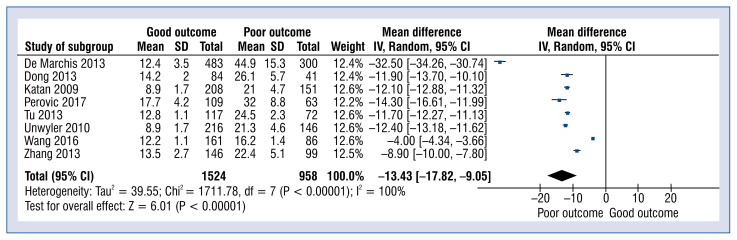
Eight studies reported copeptin levels in good vs. poor outcomes. The definitions used by each study were utilized to classify neurologic outcomes. This categorization incorporated modified Rankin Scale (classified as good: 0 to 2, poor: > 2) and Barthel Index (good: 60 or more, poor: less than 60) outcome scales. The result was assessed after 1 year/3 months (90 days) or when the patients were discharged from the baseline. Pooled analysis showed that the copeptin level in the good outcome group was 12.0 ± 3.6 pmol/L and was statistically significantly lower than in the poor outcome group 29.4 ± 14.5 pmol/L (MD: −8.13; 95% CI: −8.37 to −7.88; p < 0.001; Fig. 3).
Figure 3.
Forest plot of copeptin levels in good and poor outcome groups. The center of each square represents the weighted mean differences for individual trials, and the corresponding horizontal line stands for a 95% confidence interval (CI). The diamond represents pooled results; SD — standard deviation.
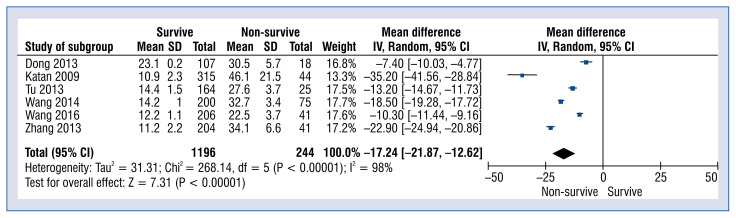
Six studies reported copeptin levels in survive vs. non-survive stroke patients which was 13.4 ± 3.2 vs. 33.0 ± 12.3 pmol/L, respectively (MD: −13.43; 95% CI: −17.82 to −9.05; p < 0.001; Fig. 4).
Figure 4.
Forest plot of copeptin levels in survive vs. non-survive groups. The center of each square represents the weighted mean differences for individual trials, and the corresponding horizontal line stands for a 95% confidence interval (CI). The diamond represents pooled results; SD — standard deviation.
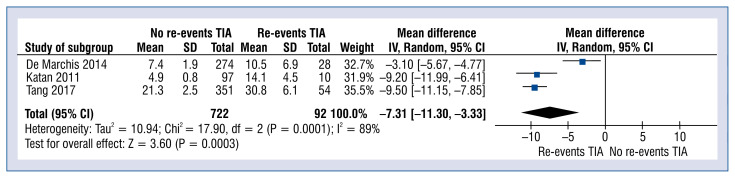
Copeptin levels in no-re-events vs. re-events TIA varied and amounted to 13.8 ± 7.6 vs. 22.8 ± 11.4 pmol/L, respectively (MD: −7.31; 95% CI: −11.30 to −3.33; p < 0.001; Fig. 5).
Figure 5.
Forest plot of copeptin levels in no re-events transient ischemic attack (TIA) vs. re-events TIA groups. The center of each square represents the weighted mean differences for individual trials, and the corresponding horizontal line stands for a 95% confidence interval (CI). The diamond represents pooled results; SD — standard deviation.
Additional analysis showed that two studies [23, 33] reported copeptin levels between the stroke/TIA group and the mimic group. Pooled analysis showed that lower copeptin levels were observed in stroke/TIA group compared to mimic group (14.8 ± 5.1 vs. 18.1 ± 25.9, respectively; MD: 1.27; 95% CI: 0.18 to 2.36; p = 0.02).
Discussion
The main finding of the meta-analysis was that the level of copeptin was significantly higher in groups with stroke as compared to the groups in which stroke did not occur (MD: 12.75; 95% CI: 5.00 to 20.49; p < 0.001). Furthermore, copeptin concentration analyzed in relation to good or poor outcomes was statistically significantly lower in the group with good results than in the group with poor results. On this basis, it was found that a higher blood biomarker level contributed to the poor results (MD: −8.13; 95% CI: −8.37 to 7.88; p < 0.001). The findings of the meta-analysis are in line with the conclusions of the first published meta-analysis assessing the prognostic value of copeptin in acute stroke [36]. Thirteen relevant studies involving 2746 patients included in the meta-analysis showed that increased plasma copeptin levels have been associated with an increased risk of adverse outcomes and mortality after stroke. The relationship between copeptin concentrations and survival in patients after stroke was also evaluated in our meta-analysis. Based on the data embodied in the included studies, it was concluded that an increase in the level of this biomarker in plasma, reduces the chances of patients’ survival after stroke (MD: −13.43; 95% CI: − −17.82 to −9.05; p < 0.001). The studies included in the meta-analysis examined all-cause mortality, while it would be ideal to consider cause-specific mortality. However, it is difficult in clinical practice to obtain reliable data about the cause of death.
From the pathophysiologic viewpoint, the AVP works through the V1a, V1b, and V2 receptors. The influence on V1a receptors is associated with vasoconstriction. Copeptin is found in the circulation in equimolar amounts to AVP. It is a very stable peptide, and it is easy to estimate [37]. Copeptin correlates positively with the initial infarct volume measured in the brain by computed tomography (CT) or brain magnetic resonance imaging (MRI). AVP stimulates V1a and V2 receptors, which trigger platelet aggregation, vasoconstriction, and water retention. The above process results, are hypovolemic or normovolemic hyponatremia, and low plasma osmolality may occur [38]. Hyponatremia is a common condition in patients after stroke. It is estimated that 40–45% of stroke patients develop hyponatremia during hospitalization. This electrolyte disturbance is associated with severe complications, such as cerebral edema, which may increase the risk of poor outcomes and death in post-stroke patients. However, it is still unclear whether the appropriate restoration of sodium levels improves outcomes in patients after stroke [39]. Likewise, there is a close relationship between copeptin levels and cerebral edema, which develops early after the focal ischemia onset and is correlated with infarct volume. The AVP V1a receptor is involved in the pathogenesis of secondary brain injury following acute ischemia by exacerbating cerebral edema. The relationship between the copeptin concentration in serum and AVP level and cerebral edema development. The blocking of AVP receptors reduces cerebral edema with ischemia and trauma.
It is essential to mention that one study, which was not included in the analysis, sought to assess the temporal profile of copeptin in relation to revascularization techniques and the development of cerebral edema and hemorrhagic transformation by evaluation upon admission, at 24 hours, and between the third and fifth day of hospitalization. Initial copeptin rise was substantially associated with stroke severity. Copeptin decremental course was noticeably steeper in patients receiving a combined reperfusion strategy, than in patients receiving single reperfusion therapy or a conservative approach in the following days [40].
The concentration of copeptin were further analyzed in patients with a recurrent TIA and in patients who experienced a TIA once. In the studies by De Marchis et al. [2, 24], TIA was defined as a neurological dysfunction caused by focal cerebral ischemia that lasts less than 24 hours, regardless of whether diffusion-weighted MRI revealed an ischemic lesion. On the contrary, von Recum et al. [33] introduced the term of transient symptoms with infarction in the case of visible lesions in brain imaging with resolving symptoms within 24 hours, following the criteria of the World Health Organization for TIA definition. Previous studies have indicated that copeptin levels can differentiate patients with TIA after the first episode into patients with high or low risk for stroke recurrence. This could allow appropriate treatment to be tailored for particular groups of patients [41]. The level of copeptin in the present meta-analysis was lower in the group of subjects without a recurring event of a TIA (MD: −7.31; 95% CI: −11.30 to −3.33; p < 0.001). Based on this, it can be assumed that this biomarker can predict a TIA recurrence. Further studies are required to adjudicate these data’s clinical utility and find cut-off points for different treatment approaches. The exact mechanism behind the association between copeptin levels and the recurrence of cerebrovascular events, remains unknown. However, several hypotheses have been presented. Copeptin appeared to capture unknown risk variables in addition to the ABCD2 score. Additionally, the activation of the stress axis was more apparent in patients with a more severe “ischemic danger” (as indicated by a diffusion-weighted imaging and/or patients with longer-lasting and more severe symptoms) [2]. These patient groups were known to be at a higher risk of recurrent cerebrovascular incidents [28]. Copeptin levels that are high in patients with significant artery atherosclerosis may also indicate unstable vascular plaques [41].
Copeptin may aid in predicting ischemic stroke and TIA outcomes, however, its utility in distinguishing between cerebral ischemia and stroke mimics has not been proven. However, researchers demonstrated that prospective biomarker research is feasible in a prehospital setting [23] without causing time delays in patient care and thus providing valuable recommendations for future studies of noninvasive tests, aimed at quickly distinguishing stroke from stroke mimics.
There are some limitations of this meta-analysis that have to be considered. First, observational studies are always characterized by some degree of risk of bias that cannot be entirely eliminated. Second, the methods of measuring copeptin concentration could have affected the results of the current meta-analysis. The measuring method was specified in only 7 out of 17 studies eligible for analysis. Three of them used KRYPTOR test, which is the most appropriate according to the study conducted by Sailer et al. [42] because it is highly accurate in non-healthy subjects. The sandwich-type immunoassay (ELISA) was used in 3 studies, and the CT-proAVP-luminescence-immunoassay was used in 1 study.
Contrary to the KRYPTOR test, sandwich-type immunoassay (ELISA) and the CT-proAVP-luminescence-immunoassay have poorer diagnostic values in detecting copeptin levels. Moreover, included studies did not provide serial measurements of copeptin; thus, further studies need to evaluate whether serial copeptin measurements will bring additional benefits in stratifying the risk of acute stroke patients. Finally, other potential biases and confounders could not be entirely excluded in the present meta-analysis since the outcomes may also have depended on the severity and etiology of the cerebrovascular event, its treatment, how the comorbidities were managed, and the professionalism and experience in the centers where the patients were treated. Thus, despite results consistent with others in the literature and including a large group of patients, the current analysis should be treated with caution because all possible confounding variables could be not accounted for.
Copeptin measurement is still not used in the routine care of post-stroke patients despite years of increasing evidence on the association of copeptin with unfavorable outcomes after stroke. Studies that reported an association of copeptin with post-stroke outcomes, tended to include a small study population, which decreased the significance of the results. In addition, copeptin is also elevated in other diseases, such as heart failure and infections, acting as a body stress marker [17]. Researchers do not always consider all potential factors that may affect copeptin levels, which increases the risk of bias. Cut-off points for copeptin are necessary for clinical utility and have not been well established to date. Current studies suggest that copeptin could play a subsidiary role to other current prognostic factors or as a panel with other biomarkers [31]. However, this requires further large-scale, well-designed studies that consider multiple confounding factors and aim to establish the actual clinical utility of copeptin in stroke patients.
Conclusions
The above systematic review and meta-analysis suggest that monitoring copeptin concentrations, may help predict long-term prognosis of TIA and ischemic stroke efficiently. Thus, copeptin is a prospective blood biomarker that could be determined along with other established risk factors in patients with stroke or TIA. Therefore, it can reduce post-stroke complications by identifying patients requiring more intensive care. Furthermore, individualization of stroke treatment based on copeptin concentration, may reduce mortality after stroke and healthcare costs associated with stroke patient management. Nevertheless, more studies with better data reliability are needed before copeptin measurements may be used in routine clinical practice.
Supplementary Information
Acknowledgments
The study was supported by the Polish Society of Disaster Medicine.
Footnotes
This paper was guest edited by Prof. Togay Evrin
Conflict of interest: None declared
References
- 1.Feigin VL, Stark BA, Johnson CO, et al. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20(10):795–820. doi: 10.1016/S1474-4422(21)00252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Marchis GM, Weck A, Audebert H, et al. Copeptin for the prediction of recurrent cerebrovascular events after transient ischemic attack: results from the CoRisk study. Stroke. 2014;45(10):2918–2923. doi: 10.1161/STROKEAHA.114.005584. [DOI] [PubMed] [Google Scholar]
- 3.Montellano FA, Ungethüm K, Ramiro L, et al. Role of blood-based biomarkers in ischemic stroke prognosis: a systematic review. Stroke. 2021;52(2):543–551. doi: 10.1161/STROKEAHA.120.029232. [DOI] [PubMed] [Google Scholar]
- 4.Coutts SB. Diagnosis and management of transient ischemic attack. Continuum (Minneap Minn) 2017;23(1, Cerebrovascular Disease):82–92. doi: 10.1212/CON.0000000000000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang WZ, Wang XB, Li HT, et al. Serum copeptin predicts severity and recurrent stroke in ischemic stroke patients. Neurotox Res. 2017;32(3):420–425. doi: 10.1007/s12640-017-9754-5. [DOI] [PubMed] [Google Scholar]
- 6.Frank E, Landgraf R. The vasopressin system — from antidiuresis to psychopathology. Eur J Pharmacol. 2008;583(2–3):226–242. doi: 10.1016/j.ejphar.2007.11.063. [DOI] [PubMed] [Google Scholar]
- 7.Katan M, Morgenthaler N, Widmer I, et al. Copeptin, a stable peptide derived from the vasopressin precursor, correlates with the individual stress level. Neuro Endocrinol Lett. 2008;29(3):341–346. [PubMed] [Google Scholar]
- 8.Morgenthaler NG, Struck J, Alonso C, et al. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem. 2006;52(1):112–119. doi: 10.1373/clinchem.2005.060038. [DOI] [PubMed] [Google Scholar]
- 9.Zhong Y, Wang R, Yan L, et al. Copeptin in heart failure: review and meta-analysis. Clin Chim Acta. 2017;475:36–43. doi: 10.1016/j.cca.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Yalta K, Yalta T, Sivri N, et al. Copeptin and cardiovascular disease: a review of a novel neurohormone. Int J Cardiol. 2013;167(5):1750–1759. doi: 10.1016/j.ijcard.2012.12.039. [DOI] [PubMed] [Google Scholar]
- 11.Fenske W, Refardt J, Chifu I, et al. A copeptin-based approach in the diagnosis of diabetes insipidus. N Engl J Med. 2018;379(5):428–439. doi: 10.1056/NEJMoa1803760. [DOI] [PubMed] [Google Scholar]
- 12.Winzeler B, Christ-Crain M. Use of coetin in the diagnosis of olyuria-olydisia syndrome - Authors’ reply. Lancet (London, England) 2020;395:267–268. doi: 10.1016/S0140-6736(19)32964-2. [DOI] [PubMed] [Google Scholar]
- 13.Koseoglu M, Ozben S, Gozubatik-Celik G, et al. Plasma copeptin levels in patients with multiple sclerosis. J Clin Neurosci. 2020;78:143–146. doi: 10.1016/j.jocn.2020.04.095. [DOI] [PubMed] [Google Scholar]
- 14.Gomes DA, de Almei da Beltrão RL, de Oliveira FM, Junior, et al. Vasopressin and copeptin release during sepsis and septic shock. Peptides. 2021;136:170437. doi: 10.1016/j.peptides.2020.170437. [DOI] [PubMed] [Google Scholar]
- 15.Bellos I, Pergialiotis V, Papapanagiotou A, et al. Association between serum copeptin levels and preeclampsia risk: A meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020;250:66–73. doi: 10.1016/j.ejogrb.2020.04.051. [DOI] [PubMed] [Google Scholar]
- 16.Wang CW, Wang JL, Zhang Yi, et al. Plasma levels of copeptin predict 1-year mortality in patients with acute ischemic stroke. Neuroreport. 2014;25(18):1447–1452. doi: 10.1097/WNR.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 17.Katan M, Moon YP, Paik MC, et al. Procalcitonin and midregional proatrial natriuretic peptide as markers of ischemic stroke: The Northern Manhattan study. Stroke. 2016;47(7):1714–1719. doi: 10.1161/STROKEAHA.115.011392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. doi: 10.1016/j.ijsu.2021.105906. [DOI] [PubMed] [Google Scholar]
- 19.Sterne JAc, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021;12(1):55–61. doi: 10.1002/jrsm.1411. [DOI] [PubMed] [Google Scholar]
- 21.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wendt M, Ebinger M, Kunz A, et al. Copeptin levels in patients with acute ischemic stroke and stroke mimics. Stroke. 2015;46(9):2426–2431. doi: 10.1161/STROKEAHA.115.009877. [DOI] [PubMed] [Google Scholar]
- 24.De Marchis GM, Katan M, Weck A, et al. Copeptin adds prognostic information after ischemic stroke: results from the CoRisk study. Neurology. 2013;80(14):1278–1286. doi: 10.1212/WNL.0b013e3182887944. [DOI] [PubMed] [Google Scholar]
- 25.Deboevere N, Marjanovic N, Sierecki M, et al. Value of copeptin and the S-100b protein assay in ruling out the diagnosis of stroke-induced dizziness pattern in emergency departments. Scand J Trauma Resusc Emerg Med. 2019;27(1):72. doi: 10.1186/s13049-019-0651-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong X, Tao DB, Wang YX, et al. Plasma copeptin levels in Chinese patients with acute ischemic stroke: a preliminary study. Neurol Sci. 2013;34(9):1591–1595. doi: 10.1007/s10072-013-1291-2. [DOI] [PubMed] [Google Scholar]
- 27.Katan M, Fluri F, Morgenthaler NG, et al. Copeptin: a novel, independent prognostic marker in patients with ischemic stroke. Ann Neurol. 2009;66(6):799–808. doi: 10.1002/ana.21783. [DOI] [PubMed] [Google Scholar]
- 28.Katan M, Nigro N, Fluri F, et al. Stress hormones predict cerebrovascular re-events after transient ischemic attacks. Neurology. 2011;76(6):563–566. doi: 10.1212/WNL.0b013e31820b75e6. [DOI] [PubMed] [Google Scholar]
- 29.Perovic E, Mrdjen A, Harapin M, et al. Diagnostic and prognostic role of resistin and copeptin in acute ischemic stroke. Top Stroke Rehabil. 2017;24(8):614–618. doi: 10.1080/10749357.2017.1367454. [DOI] [PubMed] [Google Scholar]
- 30.Sun H, Huang D, Wang H, et al. Association between serum copeptin and stroke in rural areas of Northern China: a matched case-control study. Dis Markers. 2018;2018:9316162. doi: 10.1155/2018/9316162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tu WJ, Dong X, Zhao SJ, et al. Prognostic value of plasma neuroendocrine biomarkers in patients with acute ischaemic stroke. J Neuroendocrinol. 2013;25(9):771–778. doi: 10.1111/jne.12052. [DOI] [PubMed] [Google Scholar]
- 32.Urwyler SA, Schuetz P, Fluri F, et al. Prognostic value of copeptin: one-year outcome in patients with acute stroke. Stroke. 2010;41(7):1564–1567. doi: 10.1161/STROKEAHA.110.584649. [DOI] [PubMed] [Google Scholar]
- 33.von Recum J, Searle J, Slagman A, et al. Copeptin: limited use-fulness in early stroke differentiation? Stroke Res Treat. 2015;2015:768401. doi: 10.1155/2015/768401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang CB, Zong M, Lu SQ, et al. Plasma copeptin and functional outcome in patients with ischemic stroke and type 2 diabetes. J Diabetes Complications. 2016;30(8):1532–1536. doi: 10.1016/j.jdiacomp.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 35.Zhang JL, Yin CH, Zhang Y, et al. Plasma copeptin and long-term outcomes in acute ischemic stroke. Acta Neurol Scand. 2013;128(6):372–380. doi: 10.1111/ane.12132. [DOI] [PubMed] [Google Scholar]
- 36.Choi KS, Kim HJ, Chun HJ, et al. Prognostic role of copeptin after stroke: a systematic review and meta-analysis of observational studies. Sci Rep. 2015;5:11665. doi: 10.1038/srep11665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baranowska B, Kochanowski J. Copeptin: a new diagnostic and prognostic biomarker in neurological and cardiovascular diseases. Neuro Endocrinol Lett. 2019;40(5):207–214. [PubMed] [Google Scholar]
- 38.Oraby M, Soliman R, Elkareem RA, et al. Copeptin: a potential blood biomarker for acute ischemic stroke. Egypt J Neurol Psychiatry Neurosurg. 2021;57(1) doi: 10.1186/s41983-021-00393-2. [DOI] [Google Scholar]
- 39.Liamis G, Barkas F, Megapanou E, et al. Hyponatremia in acute stroke patients: pathophysiology, clinical significance, and management options. Eur Neurol. 2019;82(1–3):32–40. doi: 10.1159/000504475. [DOI] [PubMed] [Google Scholar]
- 40.Spagnolello O, De Michele M, Lorenzano S, et al. Copeptin kinetics in acute ischemic stroke may differ according to revascularization strategies: pilot data. Stroke. 2019;50(12):3632–3635. doi: 10.1161/STROKEAHA.119.025433. [DOI] [PubMed] [Google Scholar]
- 41.Purroy F, Suárez-Luis I, Cambray S, et al. The determination of copeptin levels helps management decisions among transient ischaemic attack patients. Acta Neurol Scand. 2016;134(2):140–147. doi: 10.1111/ane.12523. [DOI] [PubMed] [Google Scholar]
- 42.Sailer CO, Refardt J, Blum CA, et al. Validity of different copeptin assays in the differential diagnosis of the polyuria-polydipsia syndrome. Sci Rep. 2021;11(1):10104. doi: 10.1038/s41598-021-89505-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.