Abstract
The incidence of diastolic dysfunction increases with age in both humans and mice. This is characterized by increased passive stiffness and slower relaxation of the left ventricle. The stiffness arises at least partially from progressively increased interstitial collagen deposition because of highly secretory fibroblasts. In the past, we demonstrated that AMPK activation via the drug 5-aminoimidazole-4-carboxamide riboside (AICAR) in middle-aged mice reduced adverse remodeling after myocardial infarction. Therefore, as an attempt to normalize the fibroblast phenotype, we used 21-mo-old male and female mice and treated them with AICAR (0.166 mg/g body wt) where each mouse was followed in a functional study over a 3-mo period. We found sex-related differences in extracellular matrix (ECM) composition as well as heart function indices at baseline, which were further accentuated by AICAR treatment. AICAR attenuated the age-related increase in left atrial volume (LAV, an indicator of diastolic dysfunction) in female but not in male hearts, which was associated with reduced collagen deposition in the old female heart, and reduced the transcription factor Gli1 expression in cardiac fibroblasts. We further demonstrated that collagen synthesis was dependent on Gli1, which is a target of AMPK-mediated degradation. By contrast, AICAR had a minor impact on cardiac fibroblasts in the old male heart because of blunted AMPK phosphorylation. Hence, it did not significantly improve old male heart function indices. In conclusion, we demonstrated that male and female hearts are phenotypically different, and sex-specific differences need to be considered when analyzing the response to pharmacological intervention.
NEW & NOTEWORTHY The aging heart develops diastolic dysfunction because of increased collagen deposition. We attempted to reduce collagen expression in the old heart by activating AMPK using AICAR. An improvement of diastolic function and reduction of cardiac fibrosis was found only in the female heart and correlated with decreased procollagen expression and increased degradation of the transcription factor Gli1. Male hearts display blunted AICAR-dependent AMPK activation and therefore this treatment had no benefits for the male mice.
Keywords: aging, extracellular matrix, fibroblast, heart, sex differences
INTRODUCTION
Recent observations support the idea that sexual dimorphism occurs in the heart, leading to sex-specific age-related cardiac defects (1, 2). In fact, while men are more susceptible to cardiovascular diseases and heart failure (HF) in their adulthood and early elder years (70s), women older than 80 years constitute a large fraction of patients suffering from HF (3, 4). Older women more frequently manifest a form of HF with preserved ejection fraction (HFpEF) (4, 5) that develops from progressive diastolic dysfunction, whereas men are more prone to develop HF with reduced ejection fraction (HFrEF), regardless of their age (3, 6). Moreover, the cardiac disease of the older women is not only less responsive to current HF treatments, but it also causes greater morbidity and an overall worse outcome following the diagnosis (3, 6).
The aging human male heart loses up to 0.95 g of mass per year, primarily because of cardiomyocyte loss (estimated at 1% of cells per year), which may be compensated by an increase in remaining cardiomyocyte size (7). By contrast, the aging of the female heart is associated with a thickening of the left ventricular wall and increased interstitial fibrosis in both humans and rodents (8–10), both contributing to higher stiffness of the myocardium and impaired passive ventricular filling. Therefore, in the absence of underlying conditions, the aging process of the male heart is mainly associated with an impairment of cardiomyocyte function, whereas ventricular stiffness is more characteristic of the older female heart. A common feature of both male and female aged hearts is the progressive decline in diastolic function (with increasing left atrial dimensions), as reported in both humans, and mice (11–13). Our own studies in old C57BL6/J mice have shown that left atrial volume (LAV) nearly triples at 29–31 mo of age compared with younger mice and that this change correlates with traditional measures of diastolic function (12).
Previously, we identified the dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN) ligand [DCSL1 (14)] as beneficial only to old female mice, by promoting a shift in macrophage polarization, normalizing fibroblast activation and collagen synthesis. Those changes stabilized the diastolic function in the treated female, but not in male, mice, and suggested important sex differences in the profibrotic/proinflammatory processes contributing to cardiac aging (14).
In the past, we also demonstrated that the short-term administration of 5-aminoimidazole-4-carboxamide riboside (AICAR), an AMPK agonist, reduced adverse scarring in an acute model of ischemia/reperfusion performed in old male mice (15). Therefore, we hypothesized that chronic administration of AICAR in old male and female mice may delay the development of age-related features of diastolic dysfunction by promoting an optimal fibroblast phenotype and extracellular matrix (ECM) turnover.
In the present article, we used aged, intervention-free male and female C57BL/6J mice in a longitudinal study. Although numerous studies have been conducted using shorter treatments (15–17), to our knowledge, this is the first attempt to chronically stimulate AMPK for 12 wk, which is equivalent to 1–5 years in humans (18, 19), to monitor changes in an organ as well as at the cellular level.
Our study demonstrates that the progressive changes in the aging heart are characterized by sex-specific pathophysiological pathways. Surprisingly, AICAR treatment specifically influenced fibroblasts and ECM in the old female heart but not in the aging male heart. We identified Gli1 as a transcription factor involved in collagen expression that was a target of the AICAR-AMPK axis. Gli1 is a crucial transcriptional activator factor that controls cell proliferation, migration, and differentiation. Its connection to the fibroblast activation program had been notably reported in the acute model of fibrosis (in lung, liver, and heart) (20–22). However, its involvement in the aging-associated cardiac fibrosis remains uncertain. Moreover, others have shown that AMPK activation may promote Gli1 phosphorylation and subsequent proteasomal degradation, impairing its transcriptional activity (23, 24). Therefore, AMPK activation should alleviate age-related cardiac defects by affecting Gli1-dependent profibrotic axis. However, AMPK phosphorylation in the male hearts in response to AICAR was markedly reduced, and procollagen type I synthesis was not significantly altered in response to the treatment. Interestingly, the treatment with GANT61 (a Gli inhibitor) showed a reduction in procollagen type I synthesis in both male and female fibroblasts, suggesting that Gli1 is a transcription factor that controls collagen synthesis in cardiac fibroblasts.
The distinctive sex-specific observations add to our previously reported sex-dependent mechanisms associated with the aging heart and may further suggest that therapeutic approaches may need to be adapted to the aging male or female heart to prevent diastolic dysfunction.
MATERIALS AND METHODS
Animals
Old female and male C57BL/6J mice were obtained from the National Institute on Aging and aged until they reached 20–21 mo. The mice were housed at the Center of Comparative Medicine of Baylor College of Medicine, under a 12-h:12-h day/night cycle, with food and water provided ad libitum. Sterilized huts were also provided as an enrichment. Mice were randomly assigned to their group of study (simple randomization). They were then injected intraperitoneally (0.166 mg/g body wt, 3 times/wk) with AICAR (Toronto Research Chemicals, Cat. No. A611700) or the same volume of saline for 12 wk. All animals were treated following the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals [DHHS publication (NIH) 85-23, revised 1996] and approved by the Baylor College of Medicine Institutional Animal Care and Use Committee (AN-5433). Baylor College of Medicine is certified by the United States Department of Agriculture (USDA) (Animal Welfare Act, Certification No. 74-R-0018). At the endpoint of the study, mice were deeply anesthetized (5% isoflurane) and directly euthanized by cervical dislocation. The absence of toe reflex was verified before the bilateral opening of the thorax and sample collection.
Heart Function
Noninvasive measurements were performed on anesthetized mice under 1.5% isoflurane gas in oxygen on a preheated ECG board. Two-dimensional (2-D) and M-mode images to assess left atrial and ventricular anatomy and function were obtained using a Vevo 770 system (VisualSonics, Toronto, ON, Canada) as we have previously described. Left atrial dimensions were collected to obtain the chamber volume using the ellipse formula: LA volume = (4π × D1 × D2 × L)/(3 × 2 × 2 × 2) with anteroposterior (D1), mediolateral (D2), and superoinferior (L) left atrial dimensions. Mitral inflow and aortic outflow Doppler measurements were recorded using a 10-MHz-pulsed probe and Doppler Signal Processing Workstation (DSPW; Indus Instruments, Houston, TX) as described previously (12, 25).
Cell Isolation
Twenty-four hours after the last AICAR injection, animals were euthanized and hearts were excised. Hearts were cut into 1-mm3 pieces and placed into a digestion buffer consisting of 2 mg/mL of collagenase type 4 (Worthington, Cat. No. LS004188) and 2 mg/mL of dispase II (Millipore Sigma, Cat. No. D4693) in DPBS with calcium and magnesium (Thermo Fisher Scientific, Cat. No. 14040133). Heart tissue was incubated in a 37°C shaking water bath with trituration until a single-cell suspension was obtained. Cells were then filtered through a 40-µm strainer into a quenching buffer containing 2% FBS (Atlanta Biologicals, Cat. No. S11550) in Dulbecco’s phosphate-buffered saline (DPBS) without calcium and magnesium (Thermo Fisher Scientific, Cat. No. 14190144) and 7.4 U/mL of DNase (Sigma Aldrich, Cat. No. D4513). During this digestion, the myocytes disintegrate and are removed, so the remaining cells are a nonmyocyte population. The cells then are either cultured or used for flow cytometry.
Flow Cytometry
After isolation, cardiac cells were washed, erythrocytes were removed using Red Cell Lysis buffer (BioLegend, Cat. No. 420301), and then the remaining cells were centrifuged. Cells were stained with Zombie Yellow (BioLegend, Cat. No. 423104) to discriminate between dead and live cells following blocking Fc receptors (using anti-CD16/CD32 antibody, BioLegend, Cat. No. 156604, RRID:AB_2783138) were then stained for specific markers. For fixation and permeabilization, Foxp3/Transcription Factor Staining Buffer Set (eBioscience, Cat. No. 005523-00) was used for internal antigens. Antibodies against the following markers were used: CD45 (BioLegend, Cat. No. 109828, RRID:AB_893350), CD31 (BioLegend, Cat. No. 102524, RRID:AB_2572182), PDGFRα (CD140a, BD Biosciences Cat. No. 562777, RRID:AB_2737788), procollagen type I (Rockland, Cat. No. 600-401-D19, RRID:AB_11181573), Gli1 (Novus, Cat. No. NB 600-600, RRID:AB_531016), fibroblast activation protein (FAP, R&D Systems, Cat. No. MAB9727), and periostin (R and D Systems, Cat. No. AF2955, RRID:AB_664123). The following secondary antibodies were purchased from Jackson ImmunoResearch Labs: BV421 anti-rabbit (Cat. No. 711-675-152, RRID:AB_2651108), PE anti-rat (Cat. No. 712-116-153, RRID:AB_2340657), and FITC anti-goat (Cat. No. 705-096-147, RRID:AB_2340402). A fluorescence minus one approach and UltraComp Beads (Thermo Fisher Scientific, Cat. No. 01-2222-42) were used to determine the positive populations and determine color compensation. Cytometry was performed on a Beckman Coulter CytoFLEX Flow Cytometer (RRID:SCR_019627) using the CytExpert software (RRID:SCR_017217) for analysis.
Immunofluorescence Staining
Paraffin-embedded zinc-Tris-fixed mouse-heart sections were prepared at the same time and stained with antibodies against the following markers: Gli1 (R and D Systems, Cat. No. AF3455, RRID:AB_2247710), periostin (R and D Systems, Cat. No. AF2955, RRID:AB_664123), collagen type VI (Proteintech Cat. No. 17023-1-AP, RRID:AB_2229737), vitronectin (Biorbyt, orb44435), anti-procollagen type Iα1 (Sigma Aldrich, Cat. No. ABT257, RRID:AB_2890134), or a reagent detecting degraded collagen (3Helix, Cat. No. BIO300), followed by the secondary antibodies (Cat. No. 705-096-147, RRID:AB_2340402 and Cat. No. 711-116-152, RRID:AB_2340599, both from Jackson ImmunoResearch).
Picture acquisition was performed using an Olympus Provis-AX70 microscope and a QImaging Retina 2000 R camera (×10 and ×20 objective lens) on QCapture Pro 6.0 software. RGB stacking and channel merging (from grayscale originals) was conducted using ImageJ 1.52a version. The experimenter was blinded during an image acquisition. In total, three to four hearts per group, and at least five fields per heart (midmyocardium cross section at the area of the papillary muscles) were photographed.
Tissue Culture in 2-D Settings
Cells were cultured in complete medium [DMEM/F12 (1:1); Thermo Fisher Scientific, 11330-032] supplemented with 10% FBS (Atlanta Biologicals, Cat. No. S11550) and 1% antibiotic-antimycotic (Thermo Fisher Scientific, Cat. No. 15240062). We used cells at passages 4–6 for the experiments.
Tissue Culture in 3-D Settings
Cross sections (1 mm thick) of the aged mouse heart were decellularized using the following protocol. First, heart pieces were snap-frozen in liquid nitrogen. After thawing, tissue was incubated overnight in sterile water at +4°C. Samples were next incubated in decellularization buffer (2% Triton X-100 and 0.8% ammonium hydroxide in sterile deionized water) (26) for 24 h at +4°C. Samples were then washed in sterile deionized water for 48 h. Finally, samples were incubated with 7.4 U/mL of DNase (Millipore Sigma, Cat. No. D4513) in PBS.
Bioreactor vessels (Synthecon, Cat. No. RCCS-4DQ) were incubated with complete medium, which was then removed before use. Primary fibroblasts were added (5 × 105) to tissue pieces in a conical tube and allowed to attach for 2 h in the medium. The tissue pieces and cells were placed into the vessels that were then completely filled with fresh medium. Rotation speed was adjusted so that the tissue pieces remained in freefall. The next day, 0.5 mM AICAR (Millipore Sigma, Cat. No. A9978) or an equal volume of DMSO was added to the vessels. Medium supplemented with AICAR or DMSO was changed every 2 days. Matrices invaded with cells were then fixed and processed for histology. For this study, we used cells and matrices of the same age and sex.
Scanning Electron Microscopy
Cross sections (1-mm thick) of the hearts were fixed in 4% glutaraldehyde for 1 h and then rinsed with water and subsequently decellularized in 10% aqueous NaOH for 6 days (27). Tissue was next rinsed again with water, then immersed in 1% tannic acid for 4 h, washed with water overnight, and proceeded to osmication and dehydration. The matrices were then point dried by the CO2 method. After the drying process, they were coated with 5 nm of platinum in an argon ion beam sputter coater. Ultrahigh resolution scanning microscopy was performed using a FEI Nova NanoSEM 230 at the Houston Methodist Electron Core Microscopy.
Mass Spectrometry
The sample was dissolved in 50 mM ammonium bicarbonate solution and digested using LysC enzyme for 2 h at room temperature followed by trypsin enzyme at 37°C overnight. The enzyme reaction was neutralized using 10% formic acid and the peptides were measured using the Pierce Quantitative Colorimetric Peptide Assay (Thermo Scientific, Cat. No. 23275). The tryptic peptides were subjected to a simple C18 cleanup using a C18 disk plug (3M Empore C18) and dried in a speed vac. LC-MS/MS analysis was carried out using an EASY-nLC 1200 system (Thermo Fisher Scientific, San Jose, CA) coupled to Orbitrap Lumos ETD mass spectrometer (Thermo Fisher Scientific, San Jose, CA). Peptide (1 µg) was loaded on a precolumn of 2 cm × 100 µm internal diameter (ID) switched inline with an inhoused 20 cm × 75 µm ID. Column (Reprosil-Pur Basic C18, 1.9 µm, Dr. Maisch, Germany) was equilibrated in 0.1% formic acid-water. The peptides were eluted using a 110-min gradient of 2%–30% acetonitrile-0.1% formic acid at a flow rate of 200 nL/min. The mass spectrometer was operated in the data-dependent acquisition mode with 2.5-s cycle time. MS1 was acquired in Orbitrap (120,000 resolution, 350–1,400 m/z) followed by MS2 in IonTrap (HCD 32%, AGC 2E4, 100-ms ion injection) with 15-s dynamic exclusion time. Obtained MS/MS spectra were searched against target-decoy Mus musculus National Center for Biotechnology Information (NCBI) RefSeq protein database (updated on March 24, 2020) in the Proteome Discoverer (PD2.1, Thermo Fisher) with Mascot algorithm (Mascot 2.4, Matrix Science). Dynamic modification of oxidation and protein NH2-terminal acetylation was allowed. The precursor mass tolerance was confined within 20 ppm with fragment mass tolerance of 0.5 Da, and a maximum of two missed cleavages were allowed. The peptides identified from the mascot result file were validated with 5% false discovery rate (FDR). The gene product inference and iBAQ-based quantification was carried out using the gpGrouper algorithm (28). The median normalized iBAQ values were used for data analysis. The differentially expressed proteins were calculated using the moderated t test to calculate P values and log2 fold changes in the R package limma. The false discovery rate-corrected P value was calculated using the Benjamini–Hochberg procedure.
Preparation of Cytosolic/Nuclear Fractions
Cultured primary cardiac fibroblasts (8–10 × 106 per condition) were synchronized in serum-free medium [DMEM-Low Glucose-GlutaMAX Supplement, pyruvate (Thermo Fisher Scientific, Cat. No. 10567014), 1% antibiotics-antimycotic] for 12 h and then incubated in complete medium [DMEM/F12 (1:1) (Thermo Fisher Scientific, Cat. No. 11330-032)–10% fetal bovine serum (Atlanta Biologicals, Cat. No. S11550)–1% antibiotics-antimycotic (Thermo Fisher Scientific, Cat. No. 15240062)] containing 0.5 mM AICAR or an equivalent volume of DMSO for 1 h. Cells were then washed once with ice-cold D-PBS (4°C) (Thermo Fisher Scientific, Cat. No. 14190144), dissociated using TrypLE Express Enzyme (Gibco, Cat. No. 12604013), and spun at 350 g for 5 min. Cell pellets were resuspended in 500 µL of ice-cold DPBS and 400 µL was directly processed for cell fractionation.
Cell suspension was spun at 350 g for 5 min (4°C). Cell pellet was then resuspended in 100 µL of cell lysis buffer (10 mM HEPES, 10 mM KCl, 0.1 mM EDTA, and 0.5% NP-40), containing 1× antiproteases/antiphosphatases (cOmplete, Mini, EDTA-free Protease Inhibitor Cocktail 11836170001 and PhosSTOP 4906845001, both from Roche). After 5-min incubation on ice, centrifugation was performed at 350 g for 5 min. The supernatant was collected (cytosolic fraction). The pellet was resuspended in cell lysis buffer, vortexed 15 s, incubated for 5 min, and spun down at 8,000 g for 5 min. The supernatant was collected (membrane and organelle fraction), and the final pellet was directly resuspended in 50 µL nuclear extraction buffer (20 mM HEPES, 0.4 M NaCl, and 0.1 mM EDTA), supplemented with 1× antiproteases/antiphosphatases (nuclear fraction). The respective purity of the nuclear and cytosolic fractions was ascertained by blotting cell extracts with antibody targeting SP-1 (Proteintech, Cat. No. 21962-1-AP, RRID:AB_10898171), a transcription factor used as a canonical nuclear marker.
Western Blot Analysis
Proteins of total heart lysate (20 µg) or cultured cell lysate (5–10 µg) or cytosolic/nuclear fractions (7 µg) were loaded in the wells of Bio-Rad 4%–15% Mini-Protean TGX precast gels (Bio-Rad, Cat. No. 4561081). Migration and transfer were performed under denaturing conditions following the manufacturer’s recommendation (migration buffer: 25 mM Tris, 192 mM glycine, and 0.1% SDS; and transfer buffer: 25 mM Tris, 192 mM glycine, and 20% methanol). The nitrocellulose membranes (Bio-Rad, Cat. No. 1620145) were blocked for 1 h with Intercept Blocking Buffer (LI-COR Biosciences, Cat. No. 927-60001) and blotted overnight (at +4°C) with the following antibodies diluted at 1:1,000 in Intercept T20 Antibody Diluent (LI-COR Biosciences, Cat. No. 927–75001): anti-phospho-AMPKα (Thr172) (Cell Signaling Technology, Cat. No. 2535, RRID:AB_331250), anti-AMPKα (Cell Signaling Technology, Cat. No. 2793, RRID:AB_915794), anti-procollagen type Iα (Sigma Aldrich, Cat. No. ABT257, RRID:AB_2890134), anti-Gli1 (Proteintech, Cat. No. 66905-1-Ig, RRID:AB_2882232), and anti-SP-1 (Proteintech, Cat. No. 21962-1-AP, RRID:AB_10898171). Secondary antibodies [anti-rabbit IgG 800 nm (Cat. No. 926-32211, RRID:AB_621843) and anti-mouse IgG 680 nm (Cat. No. 926-68072, RRID:AB_10953628), both from LI-COR Biosciences] were diluted at 1:20,000 and incubated for 1 h. Washing steps were performed at room temperature using PBS-Tween-0.1% or PBS. Infrared detection was performed using an Odyssey Imager (LI-COR Biosciences). Quantification was done by densitometry using Image Studio Lite (v. 5.2) and normalized to the signal for total protein staining [performed before blocking using Revert 700 nm Total Protein staining from LI-COR Biosciences (Cat. No. 926-11021)].
Hydroxyproline Assay
The collagen content in the heart was measured by using a colorimetric hydroxyproline assay kit (Millipore-Sigma, MAK008), following the manufacturer’s recommendation. Briefly, after homogenization, cardiac samples (∼20 mg of both ventricles) were weighed and resuspended in a volume of 50 µL H2O per 10 mg of tissue. Acid hydrolysis was then done in 6 N HCl (vol/vol) for 180 min at 120°C. The lysate was cooled and centrifuged for 3 min at 10,000 g. The supernatant was collected, and 25 µL was added to a 96-well plate for the assay (in duplicate for each sample). Drying was then performed in an oven at 60°C overnight. The next day, hydroxyproline was first oxidized with 7% chloramine T in oxidation buffer and then Ehrlich’s solution was added in the wells (1.4 M 4-dimethylaminobenzaldehyde in 20% perchloric acid and 67% isopropanol), and the plate was incubated at 60°C for 90 min. After cooling, absorbance was read at 560 nm. The hydroxyproline content was calculated using a standard curve of high purity hydroxyproline and finally reported per mg of the heart weight.
RT-qPCR
Total RNAs were extracted using TRIzol (Thermo Fisher, Cat. No. 15596026), followed by DNase treatment and RNeasy kit (Qiagen Cat. No. 74004). cDNA was generated from 1 µg of resulting mRNA using Verso cDNA Synthesis kit (Thermo Fisher, Cat. No. AB1453A). RT-qPCR for the genes of interest (see Supplemental Table S3; all Supplemental Tables are available at https://doi.org/10.6084/m9.figshare.20110514) was conducted with a CFX Opus 96 instrument (Bio-Rad, Cat. No. 12011319) using SYBR Green Master Mix (Bio-Rad, Cat. No. 1725271). Specific primers (500 nM final concentration) were designed to generate an exon-spanning product. Expression was calculated using the ΔΔCt method (CFX Maestro Software, Bio-Rad, Cat. No. 12013758). Relative expression was normalized to 18 s and Hprt1.
Antibodies Validation
As a reference, RRIDs were provided for the antibodies used in the experiments.
Statistical Analysis
Data are presented as means ± SE. For the comparison of two groups, Student’s t test was performed, followed by the Mann–Whitney test if the distribution was not normal. Nonparametric tests were used when normal distribution was not present. Analyses were all performed using GraphPad Prism version 8.0 (GraphPad Prism RRID:SCR_002798).
For more than two groups, a one-way or two-way ANOVA was applied with Kruskal–Wallis correction. When one-way ANOVA was run, a post hoc analysis was performed to identify the significant differences among the groups of the studies, and the P values were adjusted for multiple comparisons. For all the other analyses, including more than two groups, Brown–Forsythe and Welch ANOVA tests were run. P values were always adjusted for multiple comparison.
RESULTS
AICAR Prevents the Age-Related Increase of LAV Only in Old Female Mice
The aging heart undergoes changes that affect matrix stiffness and its hemodynamic properties (1, 10). To investigate the responses of the old male and the old female heart, we used the AMPK activator AICAR, because of its previously identified role in affecting the fibroblast phenotype (15). For this study, we used 21-mo-old mice. AICAR or saline was administered three times/wk for 12 wk by intraperitoneal injection. Cardiac function was measured at the beginning (to establish a baseline), at the midpoint (6 wk), and at the end point of the study (12 wk) (Fig. 1A). Baseline heart function studies from the control male and female mice showed that old mice exhibit a higher isovolumic relaxation time (IVRT) and a lower E/A ratio compared with standard values (14, 29, 30), which may reflect an impaired left ventricular relaxation (Supplemental Table S1, A and B).
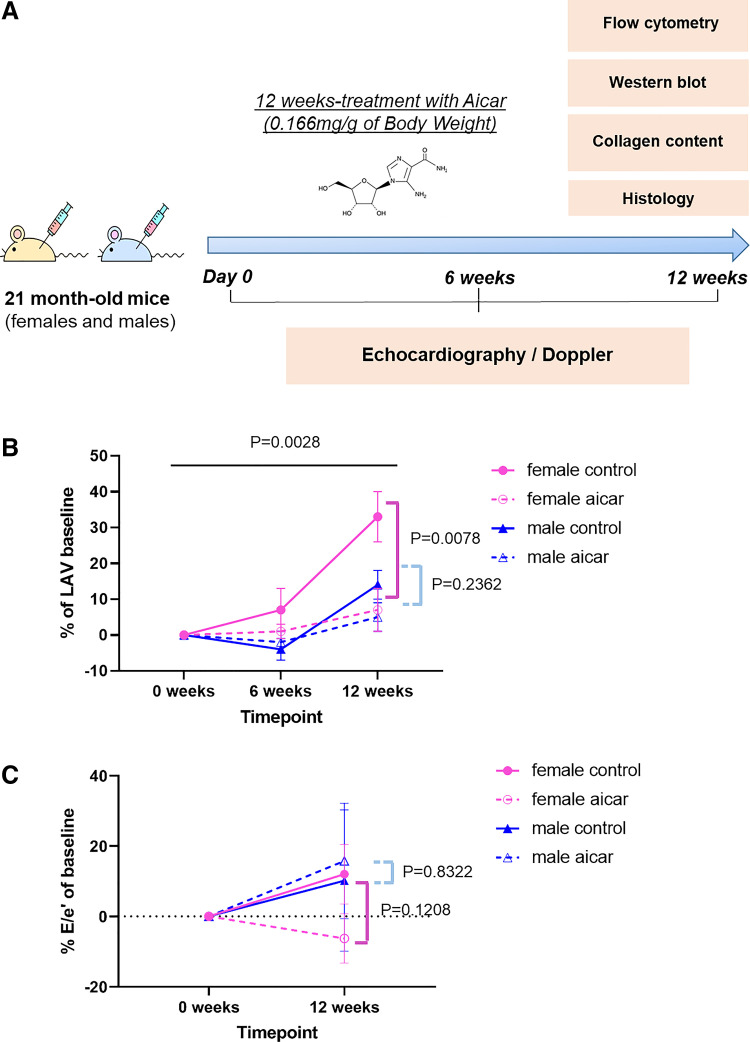
Figure 1.
Age-related increase of left atrial volume (LAV) in control vs. 5-aminoimidazole-4-carboxamide riboside (AICAR)-treated old female and old male mice. A: summary of the experimental in vivo procedure. Twenty-one-month-old male and female mice were treated with AICAR three times/wk for 12 wk. Cardiac function was assessed by echocardiography and Doppler at weeks 0 (baseline), 6, and 12 (end point) of the study. B: LAV progression in AICAR-treated (14 males, 13 females) and control mice (11 males, 12 females). C: progression of E/e′ ratio in AICAR-treated (9 males, 7 females) and control mice (6 animals/group) at baseline and 12 wk. Statistical analysis was conducted using repeated-measures (RM) two-way ANOVA test between AICAR-treated and saline mice. One-way ANOVA was used to evaluate statistical analysis in all four experimental groups.
For echocardiography and Doppler, a longitudinal functional study was done such that each animal was used as its own control throughout the experiment. Each parameter’s relative change is hence estimated as the percentage of baseline values for these cardiac indices (Supplemental Table S2, A and B).
The baseline LAV in old female mice is 18.0 ± 0.6 mm3 and 24.8 ± 1 mm3 for males. The LAV is larger in male mice, as are the aortic diameters, total body weights, and body surface areas, suggesting that body size should be integrated into the data interpretation (Supplemental Table S1, A and B). Among diastolic function parameters, the LAV remains the one that exhibits the most definitive difference between the study groups (Fig. 1B). AICAR-treated females had a significant attenuation of LAV increases compared with their controls (+7 ± 6 vs. +33 ± 7%) over the 12 wk of the treatment. However, no significant differences were found between treated and control males (+5 ± 4% in AICAR, +14 ± 4% in controls) (Fig. 1B).
Because the E/e′ ratio usually correlates with the ventricular filling pressure, we calculated the E/e′ ratio from the measurements of early mitral inflow and the mitral annular early diastolic velocity values from each animal (Fig. 1C). Although the E/e′ ratio remained nonsignificantly changed within the 12 wk of treatment among the groups, there was a notable trend to decrease (compared with baseline) in the AICAR-treated female group, which also might suggest reduced LV filling pressures and improved diastolic function.
Differences in ECM Composition between the Aging Male and Female Hearts
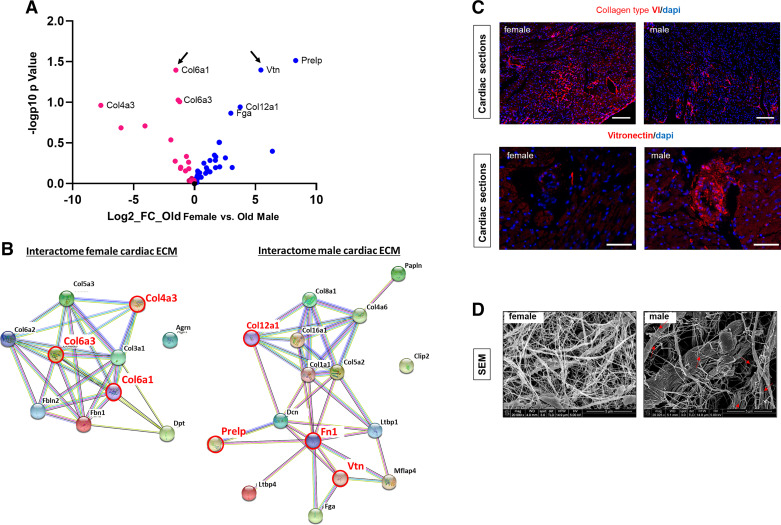
LAV is an indirect indicator of the relative left ventricle compliance and stiffness (25). We previously found that the old female mouse heart exhibits increased total collagen synthesis (as increased expression of procollagen type I in nonmyeloid and nonmyocyte cells) (14) and deposition into the matrix. In the absence of an AICAR effect on ventricular relaxation, we hypothesized that the difference in the AICAR response between old male and old female mice may reside within the ventricular filling properties derived from the ECM composition. Therefore, decellularized cardiac ECMs from old male and old female mice were analyzed by mass spectrometry (Fig. 2A). The old female cardiac ECM exhibited a higher relative enrichment in collagen type VI than other collagens (especially collagen type IV). On the other hand, we found that the old male cardiac ECM was especially enriched in vitronectin, collagen type XII, and Prelp (proline/arginine-rich end leucine-rich repeat protein). A model of protein interaction networks was created using STRING online interactome database (Fig. 2B). Interestingly, the network of the old female cardiac ECM was strongly focused on collagen fibrillogenesis, whereas the male ECM network included proteins that may facilitate the connection between the ECM, the fibroblasts, and the basal lamina (Fig. 2B). In accordance with the mass spectrometry data, we found a striking increased immunofluorescence staining for collagen type VI in the old female heart and a more intense signal for vitronectin in the old male heart (Fig. 2C). Then, we investigated the tridimensional organization of the cardiac matrix by scanning electron microscopy (Fig. 2D). We observed that the ECM of the old female heart appeared as an organized framework of fibrils, whereas the old male cardiac ECM was disorganized and exhibited clear signs of cleavage along the thinner fibrils (Fig. 2D, red arrows) that was consistently absent in the female ECM.
Figure 2.
Differences in extracellular matrix (ECM) composition and architecture of the aging female and male mouse hearts. A: mass spectrometry analysis. Volcano plot showing the log2 fold change (FC) against the log10 adjusted P value comparing proteins that are highly expressed in male (blue circle) vs. female (red circle) cardiac matrices. Arrows indicate proteins that have been chosen to validate mass spectrometry data by immunofluorescence staining (n = 4). B: protein-to-protein networks generated with STRING for cardiac female (left) and male (right) matrices. Red circle and red fonts depict proteins that are the most significantly different in the female and male cardiac matrices, respectively. C: representative pictures of immunostaining on cardiac cross sections from old male and female mice for collagen type VI α1 (top, Scale bar = 200 µm) and vitronectin (bottom, Scale bar = 100 μm). Nucleus counterstaining was performed with DAPI (n = 4). D: decellularized cardiac matrices prepared from old male and old female mice and visualized by scanning electron microscopy. Red arrows point to broken fibrils that are consistently found in male matrices. Scale bar = 5 μm (n = 3) N, number of animals/group..
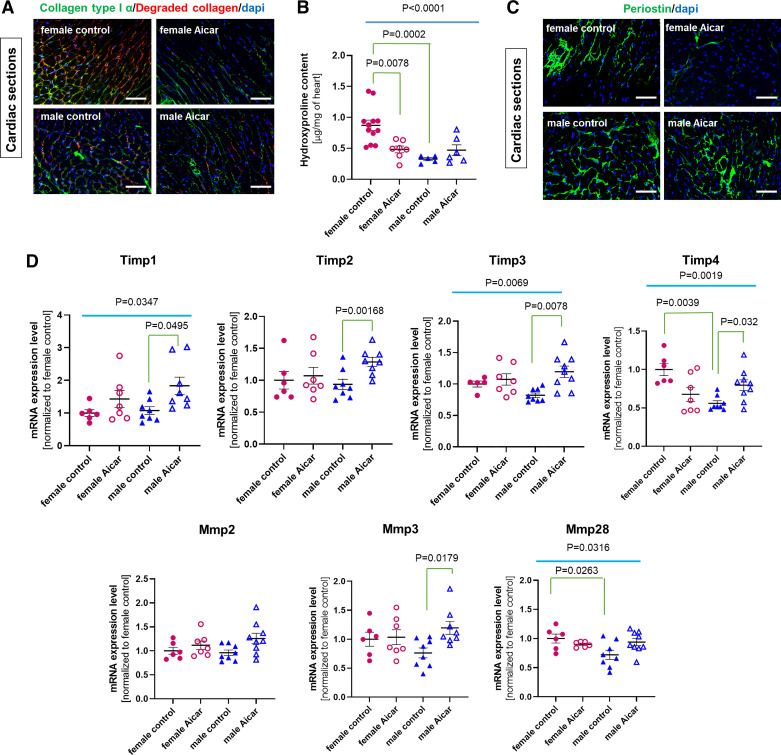
We then examined the effect of AICAR on collagen turnover by immunofluorescence staining on cardiac cross sections (Fig. 3A). This staining experiment depicted clear differences between old male and old female control hearts and different responses to AICAR between males and females. Signal intensities were high for both intact and degraded collagen in the old control female hearts. By contrast, they were barely detectable in the old male mouse heart (using the same acquisition settings) (Fig. 3A). AICAR treatment was associated with a clear reduction of the staining intensity for both total and degraded collagen in cardiac sections from old female mice, whereas AICAR-treated male hearts were not distinguishable from their age- and sex-matched controls (Fig. 3A).
Figure 3.
Sex-specific phenotype of the cardiac fibroblast population in response to 5-aminoimidazole-4-carboxamide riboside (AICAR) treatment. A: staining of total collagen type I α (green) or degraded collagen (red) was performed on cardiac sections from controls and AICAR-treated male and female old mice. Nuclear counterstaining with DAPI (blue) (n = 3). Scale bar = 100 μm. B: graph shows level of hydroxyproline in the myocardium of control or AICAR-treated female and male mice (n = 6–13 samples/group). C: immunostaining for periostin (green) performed on cardiac sections prepared from control and AICAR-treated old female and male mice. Scale bar = 100 μm. D: diagram representing relative level of mRNA for Mmp2, Mmp3, and Mmp28 and Timp1, Timp2, Timp3, and Timp4 as estimated by RT-qPCR in AICAR- or saline-treated female and male hearts (n = 6–9, normalized to the female control group). P value was calculated by a one-way ANOVA among the four cell groups followed by a post hoc analysis (nonparametric Tukey correction). P values were adjusted for multiple comparisons.
Myocardial total collagen content was also assayed by the detection of hydroxyproline. We found that the old female heart was significantly enriched in hydroxyproline compared with the old male mouse heart (0.87 ± 0.09 mg of myocardium in the female heart, vs. 0.33 ± 0.03 µg in the old male heart) (Fig. 3B, P = 0.0002). AICAR treatment led to a 50% reduction of the myocardial hydroxyproline content in the old female heart, whereas it had no significant impact on hydroxyproline in the old male heart (Fig. 3B).
We also analyzed the expression of periostin on cardiac cross sections from control and treated mice (Fig. 3C), since periostin expression is upregulated in various fibrosis models (31). There was a lower signal intensity for periostin in the female control heart compared with the male control heart (Fig. 3C), and that was further attenuated by AICAR treatment. AICAR did not have an obvious effect on periostin expression in the male heart.
We then ascertained the collagen turnover by estimating the level of mRNAs for the main matrix metalloproteinases (MMPs), which were indistinguishable between the male and the female old hearts, regardless of the treatment (Fig. 3D). However, the group of AICAR-treated male mice exhibited a significant increase in tissue inhibitor of metalloproteinase 3 (Timp3) and Mmp3 mRNA levels, which was not found in the old female hearts (Fig. 3D). By contrast, the AICAR-treated female hearts showed a significant decrease in Timp4 expression (Fig. 3D) and no change in MMPs RNA levels. It is worth noting that overall Timp4 and Mmp28 expression levels were reduced in control males versus control females, suggesting sex-dependent differences in ECM remodeling.
AICAR Treatment Affects the Phenotype of CD45negCD31negPDGFRα+ Fibroblasts
Collagen turnover and ECM remodeling are coordinated by fibroblasts. Since ECM composition and organization were clearly different between male and female hearts, we hypothesized that this difference may derive from the fibroblasts that are involved in ECM production and remodeling. Therefore, the phenotype of these cells might exhibit sex specificity in baseline features and in response to AICAR.
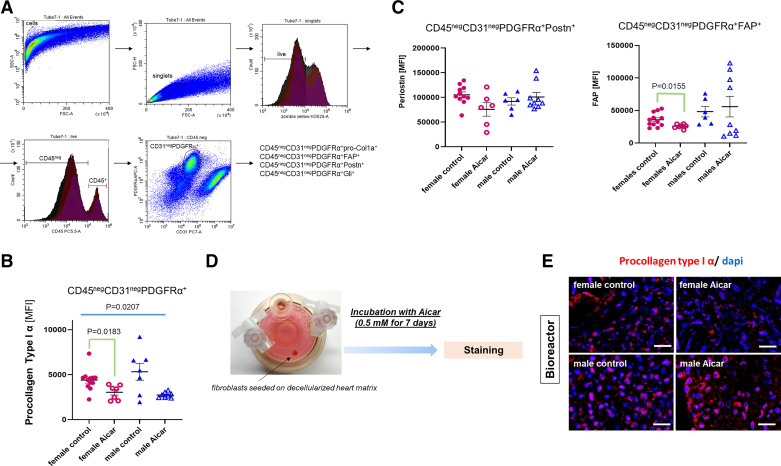
At the end of the 12-wk treatment, nonmyocyte cells were isolated from the heart of the control and AICAR-treated groups and immediately (without culture) analyzed by flow cytometry for a CD45negCD31negPDGFRα+ cell population, which includes resident cardiac fibroblasts and excludes endothelial cells and leukocytes (Fig. 4A depicts the gating strategy). The mean fluorescence intensity (MFI) for procollagen type I α1 was not different between the fibroblasts from control old male or old female hearts (suggesting that type I collagen synthesis was similar between male and female), but was significantly decreased in the cell population from the AICAR-treated female hearts (Fig. 4B). By contrast, only a trend to reduction was observed for procollagen type I α1 synthesis in AICAR-treated males. We primarily focused on periostin and fibroblast activation protein (FAP) because these proteins are involved in the ECM-fibroblast pathway/cross talk. Similar to our findings on procollagen type I α1 (Fig. 4B), the MFI for both periostin and FAP did not exhibit significant differences between fibroblasts from control female and male hearts and the control and AICAR male hearts (Fig. 4C). However, again, cells from the AICAR-treated female mouse heart had a significant reduction of MFI for FAP, suggesting a decreased protein expression (Fig. 4C).
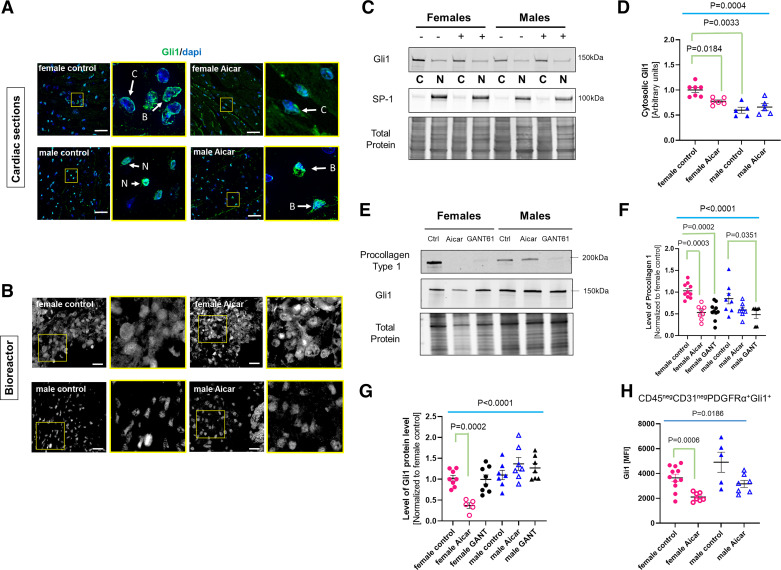
Figure 4.
Collagen deposition and turnover in the old female and old male mouse heart in response to 5-aminoimidazole-4-carboxamide riboside (AICAR) treatment. A: gating strategy to identify the nonmyocyte/nonmyeloid/nonendothelial-cell cardiac fibroblast population (CD45negCD31negPDGFRα+) that was analyzed for expression of procollagen type I α, fibroblast-activated protein (FAP), periostin (Postn), and Gli1. B: mean fluorescence intensity (MFI) of procollagen type I α in CD45negCD31negPDGFRα+ cells isolated from control or AICAR-treated old female and male mouse hearts. C: graph depicting the MFI for periostin (left) and FAP (right) in the CD45negCD31negPDGFRα+ cells (n = 6–12/group). D: summary of the ex vivo experiments performed using bioreactor vessels. Fibroblasts were seeded on sex-matched matrices isolated from old mouse hearts. They were then incubated with 0.5 mM AICAR for 7 days. E: immunostaining for procollagen type 1α in cardiac fibroblasts seeded on sex-matched matrices in the bioreactor. Scale bar = 50 μm (n = 3). Brown–Forsythe and Welch one-way ANOVA was used to test the significant difference among the four groups, to determine the significant difference among the groups for B and C (n = 7–12).
To examine the direct impact of AICAR on fibroblasts, we cultured fibroblasts on decellularized cardiac matrices in three-dimensional (3-D) culture using bioreactor vessels. This approach, in which cells are seeded on matrices in simulated microgravity, provides more physiological growth conditions than 2-D plastic dishes and an opportunity to investigate ECM-to-cell cross talk (Fig. 4D). We used age-matched and sex-matched decellularized cardiac matrices. Cells in the 3-D setting were cultured in a medium containing 0.5 mM AICAR (or DMSO for control cells) for 7 days (Fig. 4E). Cell-containing matrices were then collected and processed for immunostaining. As shown in Fig. 4E, both male and female control cells exhibit a strong signal for procollagen type I α1, and a weeklong incubation with AICAR was associated with a noticeable decrease in the procollagen type I α1 signal in fibroblasts populating female matrices.
Gli1 Orchestrates the Fibroblast Phenotype in an AMPK-Dependent and Sex-Specific Manner
Procollagen I α1 has been reported to be controlled by Gli1 (20). Therefore, we hypothesized that an AMPK/Gli1/procollagen Iα1 axis may be involved in the control of the profibrotic fibroblast phenotype.
The cardiac distribution of Gli1 was first investigated on cardiac cross sections prepared from control or AICAR-treated mice (Fig. 5A). Although Gli1+ cells are usually reported to be localized to perivascular regions, we found that the cells expressing Gli1 are more uniformly distributed throughout the old male and female myocardium. The intracellular localization of Gli1 is an indicator of its potential transcriptional status, with cytosolic Gli1 being inactive (undergoing proteasomal degradation), and the nuclear Gli1 active (32). The nuclear/cytosolic distribution of Gli1 in the old mouse heart exhibited a difference between the female and the male controls. The Gli1 signal in female Gli1+ cells appeared primarily perinuclear with only a modest nuclear signal, whereas Gli1 was mainly nuclear in the cells from the old male heart (Fig. 5A). AICAR was also associated with a less intense Gli1 signal in treated females, suggesting a decreased expression in addition to a greater cytosolic restriction (Fig. 5A). A similar trend was also found when cells were cultured in age-matched decellularized cardiac 3-D matrices for 7 days in the presence of AICAR (Fig. 5B) with increased cytosolic Gli1 expression in AICAR-treated female fibroblasts and a robust nuclear staining of Gli1 in control and AICAR-treated male fibroblasts. To clearly investigate and quantify the cellular distribution of Gli1, nuclear and cytosolic fractions were prepared from cells treated for 1 h with 0.5 mM AICAR or DMSO (untreated) and blotted for Gli1 and SP1 (nuclear marker). As shown in Fig. 5C, incubation with AICAR did not, in fact, drastically affect the localization of Gli1. The nuclear portion of Gli1 also remained unaffected, regardless of the treatment and the origin of the cells. However, the Gli1 from the cytosolic fraction of the female cells exhibited a significant decrease (1.00 ± 0.05 vs. 0.78 ± 0.028, P = 0.0184), which was not observed in male cells (0.60 ± 0.06 vs. 0.66 ± 0.07). Noteworthy, the level of cytosolic Gli1 was significantly lower in the male cells compared with the female cells (1.00 ± 0.05 vs. 0.60 ± 0.06, P = 0.0033) (Fig. 5D), which may explain why the nuclear signal for Gli1 appears stronger in male cells in both cardiac sections (Fig. 5A) and 3-D matrices (Fig. 5B).
Figure 5.
Differential activation pattern of Gli1 in fibroblasts of 5-aminoimidazole-4-carboxamide riboside (AICAR)-treated old female and male mouse hearts. A: representative immunostaining for Gli1 on cardiac cross sections prepared from control or AICAR-treated old female or male mice. Scale bar = 25 μm. Each picture is paired with a magnified image from the area marked by a yellow square. Arrows indicate the specific cellular localization of Gli1 staining: B, both; C, cytosolic; N, nuclear. Scale bar = 50 μm (n = 3). B: Gli1 staining of cardiac fibroblasts seeded in a bioreactor on age-matched/sex-matched cardiac matrices after 7 days of incubation with 0.5 mM AICAR or DMSO (control). Nonspecific autofluorescence of the matrix was indicated by “N.S.” Scale bar = 50 μm (n = 3). C: representative Western blot showing the distribution of Gli1 between the cytosolic (C) and the nuclear (N) fractions in female and male cells in response to AICAR (+). D: quantification of cytosolic Gli1 from C. E: representative Western blot depicting the change in procollagen type I a1 and Gli1 protein levels in response to 72-h incubation of the fibroblasts with 0.5 mM AICAR or 10 µM GANT61. Ctrl, control cells. Quantification of E for both procollagen (F) type I and Gli1 (G) protein levels (n = 7 or 8 samples/group). H: graph depicting MFI values for Gli1 in cardiac CD45negCD31negPDGFRα+ cell populations in control or AICAR-treated mice analyzed by flow cytometry. Each circle/triangle represents a unique biological replicate (n = 6–12/group). For D, F, and G, P value was calculated by a one-way ANOVA among the four cell groups followed by a post hoc analysis with Tukey correction. For H, Brown–Forsythe and Welch one-way ANOVA was used to test the significant difference among the four groups. P values were adjusted for multiple comparisons.
To ascertain the link between Gli1 activity and procollagen Iα1 expression in response to AICAR, cardiac fibroblasts were cultured in 2-D settings for 72 h in the presence of 0.5 mM AICAR or 10 µM of GANT61, an inhibitor of Gli1 transcriptional activity that impairs Gli1 DNA binding capacity, yet not its stability or its nuclear translocation. Cells were then collected for the detection of procollagen type Iα1 and Gli1 by Western blot (Fig. 5E). Consistent with previous findings from flow cytometry (Fig. 4B), incubation with AICAR was associated with a decrease in procollagen synthesis only in the old female cells (1.00 ± 0.05 vs. 0.53 ± 0.06, P = 0.0002) (Fig. 5, Eand F). However, procollagen I α1 was significantly reduced in response to treatment with GANT61, in fibroblasts isolated from both male and female mouse hearts (0.48 ± 0.08 and 0.56 ± 0.06, respectively) (Fig. 5, Eand F). The Gli1 protein level was not significantly affected by GANT61 treatment in fibroblasts from either male or female hearts. By contrast, AICAR-treated female but not male cells exhibited a reduction in Gli1 protein level compared with control cells.
To measure the change in Gli1 expression, we investigated the MFI for Gli1 on the CD45negCD31negPDGFRα+ cell population (Fig. 5H). By flow cytometry, we detected no significant difference in Gli1 MFI between the groups of age-matched controls, but the signal was significantly reduced only in cells from AICAR-treated old female mouse hearts (Fig. 5H). By contrast, AICAR did not affect Gli1 signal intensity in the cell populations isolated from the old male hearts (Fig. 5G).
Linked together, this data set suggests that Gli1 is a regulator of collagen synthesis in the aging cardiac fibroblast and a potential therapeutic target.
In Vivo AMPK Activation
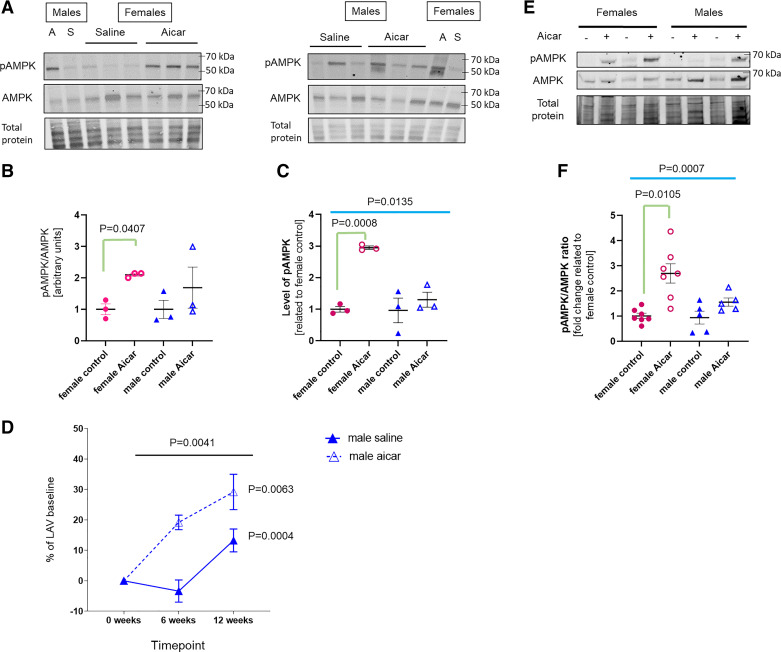
As we looked for an explanation for the blunted AICAR responses in male hearts, we evaluated the phosphorylation of AMPK 3 h after AICAR injection (saline was used as a control). We found that AICAR at the dose of 0.166 mg/g body wt stimulated AMPK phosphorylation to a higher degree in female than in male hearts (Fig. 6, A–C), which may help explain why we did not see significant improvement in males. The blunted responses may also suggest that the AICAR dose was inadequate for old males. However, when we used AICAR at three times higher dose (0.5 mg/g body wt), we found that heart function worsened with time (the percentage of change of LAV in AICAR-treated males was significantly increased when compared with saline controls), suggesting that the chronic (3 mo long) use of AICAR at this dose was not beneficial (Fig. 6D). Others have reported that the use of the same higher dose was beneficial in short-term studies (33, 34).
Figure 6.
The relative impact of 5-aminoimidazole-4-carboxamide riboside (AICAR) on AMPK activation in the old mouse heart. A: representative Western blots for phosphorylated AMPK (pAMPK) and total AMPK in the cardiac lysate obtained from 21-mo-old female and male mice. Hearts were excised 3 h after a single injection with AICAR (0.166 mg/g body wt) or saline. Each blot contains a sex-unmatched reference: S, saline; A, AICAR, for the sex-unmatched lanes. Diagrams represent pAMPK/AMPK ratio (B) and pAMPK level (C); each circle or triangle depicts a unique replicate (n = 3 samples/group). Relative normalization of the two separated blots was ensured by using the sex-unmatched references loaded on each blot. P value was calculated using a one-way ANOVA test, followed by a post hoc study to determine the significant differences among the groups. P values were then adjusted for multiple parameters. D: impact of higher dose of AICAR (0.5 mg/g body wt) on old male heart function. Effect of AICAR treatment on the LAV changes was determined by repeated-measures (RM) one-way ANOVA test/each group (n = per group). Overall significance between two groups along the time of treatment was assessed using RM two-way ANOVA test. E: representative Western blot for pAMPK and total AMPK in lysates of cardiac fibroblasts (from male and female old mice) preincubated with 0.5 mM AICAR (+) or DMSO (−) for 30 min. F: diagram representing the change in pAMPK/AMPK ratio in cardiac fibroblasts (n = 5–7 samples/group). P value was calculated using an one-way ANOVA test, followed by a post hoc study with Tukey correction to determine the significant differences among the groups. P values were adjusted for multiple parameters.
In addition, we also incubated cardiac fibroblasts from old male and old female mice with 0.5 mM AICAR for 30 min (Fig. 6, Eand F). Similarly, we found that AMPK phosphorylation was strongly and significantly induced in the female cells in response to AICAR (2.70 ± 0.38 vs. 1.00 ± 0.11, P = 0.0105). In the old male cells, the pAMPK/AMPK level was lower and more variable than in female cells (1.56 ± 0.16 in AICAR-treated male cells vs. 2.70 ± 0.38 in AICAR-treated female cells) and did not reach significance (1.56 ± 0.16 in AICAR-treated male cells vs. 0.94 ± 0.26 in control male cells).
DISCUSSION
Over the past decade, we have primarily examined the influence of aging in the absence of any surgical intervention, but our studies were done almost exclusively in male mice (15, 17, 35). In these studies, the heterogeneity within a population of aging male and female mice led to our developing techniques by which physiological changes in each animal were serially monitored to allow the assessment of an intervention (12). As presented here, this allows each mouse to be examined “clinically” for progressive dysfunction. This approach reduced the number of mice required for individual experiments, thus reducing both statistical error and animal cost and supporting ethical considerations. It is also aligned with the current recommendation to include both sexes in cardiovascular research (36). In this paper, we have confirmed striking differences between the male and female mice in the ventricular response to aging.
Diastolic Function
Clinically, left atrial volume (LAV) is a useful tool for diagnosing heart failure with preserved ejection fraction (HFpEF) and estimation of cardiovascular outcomes (25, 37). Our previous work has demonstrated that LAV is an indicator of diastolic dysfunction in mice, as age-related increases in LAV correlate with increases in left ventricular filling pressures (12, 25). In this study, we found that AICAR forestalled LAV increase over 12 wk of treatment when compared with control mice, however, the treatment was only effective in female mice. Because the E/e′ ratio usually correlates with the LA pressure and the ventricular filling pressure (30, 38), we followed this parameter longitudinally but found nonsignificant changes within 12 wk of treatment. The fact that the changes in E/e′ ratio did not fully coincide with the changes in LAV might be caused by the effect of anesthesia or other factors.
ECM
Here, we demonstrated that a distinct pattern of glycoproteins exists in the old male and female ECM. The old male cardiac ECM is enriched in vitronectin, a glycoprotein that is usually expressed during the development of the fetal heart or the healing processes of the adult heart (39–41). Once secreted within the ECM, vitronectin has the ability to sequester growth factors and to bridge ECM components, thereby coordinating mechanical signals to tissue integrity and biological activities (41–43). In addition, it can also promote fibroblast and endothelial cell migration and proliferation through an α5β1/α5β3-integrin signaling pathway (44). By contrast, ECM of the old female mouse heart shows an abundance of collagen type VI (COL6A1, 6A3), an atypical member of the collagen family that forms a unique beaded network of 100 nm thin filaments. Collagen VI is more abundant in the failing heart (45), promoting deleterious fibrosis and inflammation, but its role in aging, and especially in the female heart aging, is less well established. Collagen VI interacts with collagen IV and fibrillary collagens, such as collagen III, that are also preferentially enriched in the old female ECM. An increased proportion of collagen IV and collagen VI might explain the detection of thinner “pearled” fibrils in the matrix of the old female heart as shown in the SEM.
In the literature, MMPs/TIMPs secretion is reported to be indirectly linked to AMPK phosphorylation, both in the cardiac remodeling process or in several models of the acute inflammatory response (46). Induced expression of MMPs and TIMPs is promoted by oxidative stress (47–49) and, in the myocardium, AMPK activation usually alleviates this reactive oxygen species (ROS)-induced MMPs expression (50). This may suggest that MMPs and TIMPs expression are usually regulated by AMPK phosphorylation.
In our study, we show that, despite a significant impact on the fibrotic pathway and significant activation of pAMPK, significant change can be found only in Timp4 expression in the old female heart, which may dampen TIMP-dependent MMP inhibition and increase collagen turnover. The increase in MMPs and TIMPs in the old treated male heart is surprising. If AMPK phosphorylation affected the male ECM, there should be a reduction of these genes, instead. However, we did not examine the macrophage population in this study, and macrophages are a known source of MMPs (51). Another possibility is that AICAR may favor the glycolytic switch and dependence in the cardiomyocytes (52), which may increase ROS production and amplify the ROS/MMP axis, instead (47–49). This may also explain why a chronic injection of a higher dose of AICAR is even more deleterious to the old male mouse heart.
Identification of Cardiac Fibroblasts and the Role of Gli1 in Cardiac Fibrosis
PDGFRα has been identified by Muhl et al. (53) as a specific cardiac fibroblast marker that is not present on other cells such as pericytes. Others have shown as well that PDGFRα labels the cardiac fibroblast population (54, 55). We, therefore, use this marker to analyze cardiac fibroblasts. We found that the CD45negCD31negPDGFRα+ population expresses periostin, collagen, and FAP. All these proteins have been shown to be upregulated in cardiac fibroblasts during stress or injury and periostin and FAP promote collagen crosslinking (56, 57). Likewise, this fibroblast population expresses Gli1. Gli1 is a critical transcription factor that controls cell proliferation and migration in not only normal tissue development and homeostasis but also in cancer progression and metastasis. A combination of canonical (Hedgehog) or noncanonical pathways can favor Gli1 stability, which may later influence its transcriptional activity (58–61). In the young healthy heart, Gli1+ responsive cells are usually pericytes and perivascular fibroblasts (62), but the Gli1 staining in the old mouse heart exhibits a diffuse, interstitial pattern, no longer restricted to the perivascular areas. Gli1 cellular localization also shows some sex-specific differences, the subcellular pattern being more nuclear in the old male myocardium, but mainly cytosolic in the old female mouse heart. This might suggest a dimorphic Gli1 activation or distinct turnover dynamics in the fibroblasts of the aging male and female myocardium. Regardless of the experimental approach, treatment with AICAR was consistently associated with a decreased expression of Gli1 in the fibroblasts of the female heart, but not in the cells from the male heart. This change in Gli1 protein level in response to AICAR also correlates with a downstream decrease in procollagen type I α1 synthesis, but only in the female cardiac fibroblasts. Yet, pharmacological application of GANT61, a Gli inhibitor, led to a prompt decrease in procollagen type I α1 expression, in cells from both male and female origins. The failure of AICAR to act on male fibroblasts in the same manner as GANT61 may be due to the blunted AMPK phosphorylation by AICAR in males. Linked together, these data reinforce a potential role for Gli1 on the progression of the age-related interstitial cardiac fibrosis.
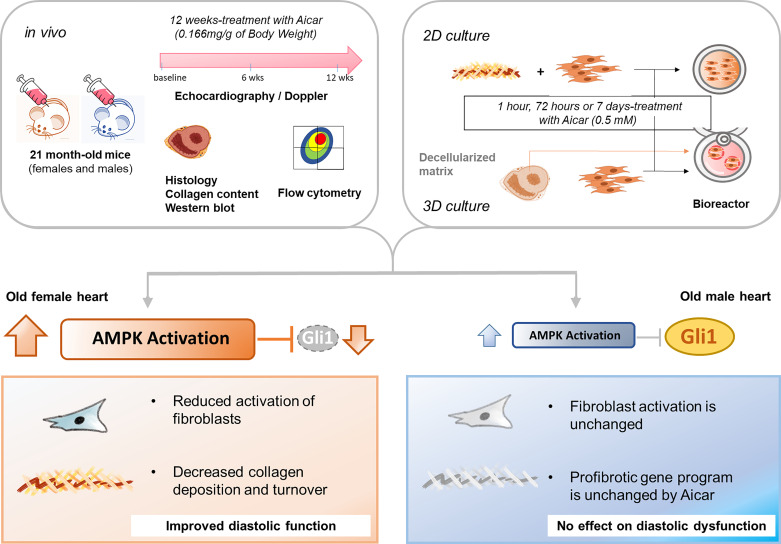
SUMMARY
Our study provides evidence of unique sex-specific features that influence the aging process of the heart. The old female heart undergoes constant remodeling, and because it produces more collagen than is removed by degradation, that results in fibrosis. The altered composition of the ECM in the female heart may signal fibroblasts to increase their secretion of matrix proteins. Furthermore, our study suggests that activation of AMPK by AICAR was able to attenuate the fibroblast stimulation in the female heart via the downregulation of Gli1-dependent collagen expression, delaying the age-related diastolic dysfunction. By contrast, the same drug and dose in the old male heart had a less significant impact on fibroblast phenotype or ECM, leading to no specific attenuation of the age-related change in diastolic function due to blunted AMPK phosphorylation in response to AICAR (Fig. 7).
Figure 7.
Graphical presentation of methodology and main findings. To evaluate the 5-aminoimidazole-4-carboxamide riboside (AICAR)-dependent effect on the aging male and female hearts, animals were treated with AICAR injections for 12 wk. Heart function was evaluated at time 0, 6, and 12 wk posttreatment, and then hearts were analyzed by histology and flow cytometry. In parallel experiments, AICAR effects on fibroblasts isolated from male and female hearts were assessed using two-dimensional (2-D) and three-dimensional (3-D) tissue culture. AICAR can reduce collagen turnover in the ECM and quench cardiac fibroblast activation through a Gli1-dependent pathway, improving age-related diastolic dysfunction in the female heart. By contrast, AICAR does not significantly improve fibroblast activation or cardiac function in the old male heart because of the blunted AICAR-dependent activation of AMPK.
DATA AVAILABILITY
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium (https://www.ebi.ac.uk/pride/) via the PRIDE (63) partner repository with the data set identifier PXD031223. Data are available via ProteomeXchange with identifier PXD031223.
SUPPLEMENTAL DATA
Supplemental Tables S1–S3: 10.6084/m9.figshare.20110514
GRANTS
Imaging for this project was supported by the Integrated Microscopy Core at Baylor College of Medicine and the Center for Advanced Microscopy and Image Informatics (CAMII) with funding from the National Institutes of Health (NIH) under Grants DK56338, CA125123, and ES030285, Cancer Prevention and Research Institute of Texas (CPRIT) Grants RP150578 and RP170719, the Dan L. Duncan Comprehensive Cancer Center, and the John S. Dunn Gulf Coast Consortium for Chemical Genomics. This work was supported by the NIH Grants AG059599 (to K. A. Cieslik and M. L. Entman), P30 CA125123 (Mass Spectrometry Proteomics Core), CPRIT Core Facility Award Grants RP170005 and RP210227 (Mass Spectrometry Proteomics Core), and the Medallion Foundation (to K. A. Cieslik).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.A.C. conceived and designed research; A.A., J.O.-U., J.T., A.K.R., A.M., A.J., and K.A.C. performed experiments; A.A., J.O.-U., J.T., A.K.R., A.M., G.E.T., and K.A.C. analyzed data; A.A., J.T., A.M., G.E.T., and K.A.C. interpreted results of experiments; A.A. and K.A.C. prepared figures; A.A. and K.A.C. drafted manuscript; A.A., J.T., M.L.E., G.E.T., and K.A.C. edited and revised manuscript; A.A., J.O.-U., J.T., A.K.R., A.M., A.J., M.L.E., G.E.T., and K.A.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the Electron Microscopy Core at Houston Methodist Hospital for providing access to the scanning electron microscope, and Huie Wang for technical assistance. Heart function analysis (Doppler) was performed with the assistance of Thuy Pham. We also thank Dorellyn Blanchette Lee for Western blot analysis, Amanda Flores Yanke and Isaac Karim for help in data analysis, and Sharon Malinowski for editorial assistance.
REFERENCES
- 1.Grilo GA, Shaver PR, Stoffel HJ, Morrow CA, Johnson OT, Iyer RP, de Castro Bras LE. Age- and sex-dependent differences in extracellular matrix metabolism associate with cardiac functional and structural changes. J Mol Cell Cardiol 139: 62–74, 2020. doi: 10.1016/j.yjmcc.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hagstrom L, Henein MY, Karp K, Waldenstrom A, Lindqvist P. Impact of age and sex on normal left heart structure and function. Clin Physiol Funct Imaging 37: 759–766, 2017. doi: 10.1111/cpf.12371. [DOI] [PubMed] [Google Scholar]
- 3.Dewan P, Jackson A, Jhund PS, Shen L, Ferreira JP, Petrie MC, Abraham WT, Desai AS, Dickstein K, Kober L, Packer M, Rouleau JL, Solomon SD, Swedberg K, Zile MR, McMurray JJV. The prevalence and importance of frailty in heart failure with reduced ejection fraction—an analysis of PARADIGM-HF and ATMOSPHERE. Eur J Heart Fail 22: 2123–2133, 2020. doi: 10.1002/ejhf.1832. [DOI] [PubMed] [Google Scholar]
- 4.Beale AL, Meyer P, Marwick TH, Lam CSP, Kaye DM. Sex differences in cardiovascular pathophysiology: why women are overrepresented in heart failure with preserved ejection fraction. Circulation 138: 198–205, 2018. doi: 10.1161/CIRCULATIONAHA.118.034271. [DOI] [PubMed] [Google Scholar]
- 5.Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 14: 591–602, 2017. doi: 10.1038/nrcardio.2017.65. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Savarese G, Dahlstrom U, Lund LH, Fu M. Age-dependent differences in clinical phenotype and prognosis in heart failure with mid-range ejection compared with heart failure with reduced or preserved ejection fraction. Clin Res Cardiol 108: 1394–1405, 2019. doi: 10.1007/s00392-019-01477-z. [DOI] [PubMed] [Google Scholar]
- 7.Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, Sjostrom SL, Szewczykowska M, Jackowska T, Dos Remedios C, Malm T, Andra M, Jashari R, Nyengaard JR, Possnert G, Jovinge S, Druid H, Frisen J. Dynamics of cell generation and turnover in the human heart. Cell 161: 1566–1575, 2015. doi: 10.1016/j.cell.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 8.Liu CY, Liu YC, Wu C, Armstrong A, Volpe GJ, van der Geest RJ, Liu Y, Hundley WG, Gomes AS, Liu S, Nacif M, Bluemke DA, Lima JAC. Evaluation of age-related interstitial myocardial fibrosis with cardiac magnetic resonance contrast-enhanced T1 mapping: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 62: 1280–1287, 2013. doi: 10.1016/j.jacc.2013.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kane AE, Bisset ES, Heinze-Milne S, Keller KM, Grandy SA, Howlett SE. Maladaptive changes associated with cardiac aging are sex-specific and graded by frailty and inflammation in C57BL/6 mice. J Gerontol A Biol Sci Med Sci 76: 233–243, 2021. doi: 10.1093/gerona/glaa212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zile MR, Baicu CF, Ikonomidis JS, Stroud RE, Nietert PJ, Bradshaw AD, Slater R, Palmer BM, Van Buren P, Meyer M, Redfield MM, Bull DA, Granzier HL, LeWinter MM. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation 131: 1247–1259, 2015. doi: 10.1161/CIRCULATIONAHA.114.013215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai DF, Chen T, Johnson SC, Szeto H, Rabinovitch PS. Cardiac aging: from molecular mechanisms to significance in human health and disease. Antioxid Redox Signal 16: 1492–1526, 2012. doi: 10.1089/ars.2011.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medrano G, Hermosillo-Rodriguez J, Pham T, Granillo A, Hartley CJ, Reddy A, Osuna PM, Entman ML, Taffet GE. Left atrial volume and pulmonary artery diameter are noninvasive measures of age-related diastolic dysfunction in mice. J Gerontol A Biol Sci Med Sci 71: 1141–1150, 2016. doi: 10.1093/gerona/glv143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiao YA, Zhang H, Sweetwyne M, Whitson J, Ting YS, Basisty N, Pino LK, Quarles E, Nguyen NH, Campbell MD, Zhang T, Gaffrey MJ, Merrihew G, Wang L, Yue Y, Duan D, Granzier HL, Szeto HH, Qian WJ, Marcinek D, MacCoss MJ, Rabinovitch P. Late-life restoration of mitochondrial function reverses cardiac dysfunction in old mice. eLife 9: e55513, 2020. doi: 10.7554/eLife.55513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trial J, Diaz Lankenau R, Angelini A, Tovar Perez JE, Taffet GE, Entman ML, Cieslik KA. Treatment with a DC-SIGN ligand reduces macrophage polarization and diastolic dysfunction in the aging female but not male mouse hearts. Geroscience 43: 881–899, 2021. doi: 10.1007/s11357-020-00255-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cieslik KA, Taffet GE, Crawford JR, Trial J, Mejia Osuna P, Entman ML. AICAR-dependent AMPK activation improves scar formation in the aged heart in a murine model of reperfused myocardial infarction. J Mol Cell Cardiol 63: 26–36, 2013. doi: 10.1016/j.yjmcc.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem 229: 558–565, 1995. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- 17.Cieslik KA, Trial J, Entman ML. AICAR treatment reduces interstitial fibrosis in aging mice: suppression of the inflammatory fibroblast. J Mol Cell Cardiol 111: 81–85, 2017. doi: 10.1016/j.yjmcc.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang S, Lai X, Deng Y, Song Y. Correlation between mouse age and human age in anti-tumor research: significance and method establishment. Life Sci 242: 117242, 2020. doi: 10.1016/j.lfs.2019.117242. [DOI] [PubMed] [Google Scholar]
- 19.Fox JG. The Mouse in Biomedical Research. Amsterdam; Boston: Academic Press, 2007. [Google Scholar]
- 20.Cao H, Chen X, Hou J, Wang C, Xiang Z, Shen Y, Han X. The Shh/Gli signaling cascade regulates myofibroblastic activation of lung-resident mesenchymal stem cells via the modulation of Wnt10a expression during pulmonary fibrogenesis. Lab Invest 100: 363–377, 2020. doi: 10.1038/s41374-019-0316-8. [DOI] [PubMed] [Google Scholar]
- 21.Ke B, Wang XN, Liu N, Li B, Wang XJ, Zhang RP, Liang H. Sonic hedgehog/gli1 signaling pathway regulates cell migration and invasion via induction of epithelial-to-mesenchymal transition in gastric cancer. J Cancer 11: 3932–3943, 2020. doi: 10.7150/jca.42900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kramann R, Wongboonsin J, Chang-Panesso M, Machado FG, Humphreys BD. Gli1(+) pericyte loss induces capillary rarefaction and proximal tubular injury. J Am Soc Nephrol 28: 776–784, 2017. doi: 10.1681/ASN.2016030297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li YH, Luo J, Mosley YY, Hedrick VE, Paul LN, Chang J, Zhang G, Wang YK, Banko MR, Brunet A, Kuang S, Wu JL, Chang CJ, Scott MP, Yang JY. AMP-activated protein kinase directly phosphorylates and destabilizes hedgehog pathway transcription factor GLI1 in medulloblastoma. Cell Rep 12: 599–609, 2015. doi: 10.1016/j.celrep.2015.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang R, Huang SY, Ka-Wai Li K, Li YH, Hsu WH, Zhang GJ, Chang CJ, Yang JY. Dual degradation signals destruct GLI1: AMPK inhibits GLI1 through β-TrCP-mediated proteasome degradation. Oncotarget 8: 49869–49881, 2017. doi: 10.18632/oncotarget.17769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Granillo A, Pena CA, Pham T, Pandit LM, Taffet GE. Murine echocardiography of left atrium, aorta, and pulmonary artery. J Vis Exp (120): 55214, 2017. doi: 10.3791/55214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheridan WS, Duffy GP, Murphy BP. Mechanical characterization of a customized decellularized scaffold for vascular tissue engineering. J Mech Behav Biomed Mater 8: 58–70, 2012. doi: 10.1016/j.jmbbm.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Stephenson MK, Lenihan S, Covarrubias R, Huttinger RM, Gumina RJ, Sawyer DB, Galindo CL. Scanning electron microscopy of macerated tissue to visualize the extracellular matrix. J Vis Exp (112): 54005, 2016. doi: 10.3791/54005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saltzman AB, Leng M, Bhatt B, Singh P, Chan DW, Dobrolecki L, Chandrasekaran H, Choi JM, Jain A, Jung SY, Lewis MT, Ellis MJ, Malovannaya A. gpGrouper: a peptide grouping algorithm for gene-centric inference and quantitation of bottom-up proteomics data. Mol Cell Proteomics 17: 2270–2283, 2018. doi: 10.1074/mcp.TIR118.000850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horgan S, Watson C, Glezeva N, Baugh J. Murine models of diastolic dysfunction and heart failure with preserved ejection fraction. J Card Fail 20: 984–995, 2014. doi: 10.1016/j.cardfail.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Schaefer A, Klein G, Brand B, Lippolt P, Drexler H, Meyer GP. Evaluation of left ventricular diastolic function by pulsed Doppler tissue imaging in mice. J Am Soc Echocardiogr 16: 1144–1149, 2003. doi: 10.1067/S0894-7317(03)00679-5. [DOI] [PubMed] [Google Scholar]
- 31.Lorts A, Schwanekamp JA, Baudino TA, McNally EM, Molkentin JD. Deletion of periostin reduces muscular dystrophy and fibrosis in mice by modulating the transforming growth factor-β pathway. Proc Natl Acad Sci USA 109: 10978–10983, 2012. doi: 10.1073/pnas.1204708109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jia J, Amanai K, Wang G, Tang J, Wang B, Jiang J. Shaggy/GSK3 antagonizes hedgehog signalling by regulating cubitus interruptus. Nature 416: 548–552, 2002. doi: 10.1038/nature733. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Wang Y, Zou M, Chen C, Chen Y, Xue R, Dong Y, Liu C. AMPK blunts chronic heart failure by inhibiting autophagy. Biosci Rep 38: BSR20170982, 2018. doi: 10.1042/BSR20170982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sasaki H, Asanuma H, Fujita M, Takahama H, Wakeno M, Ito S, Ogai A, Asakura M, Kim J, Minamino T, Takashima S, Sanada S, Sugimachi M, Komamura K, Mochizuki N, Kitakaze M. Metformin prevents progression of heart failure in dogs: role of AMP-activated protein kinase. Circulation 119: 2568–2577, 2009. doi: 10.1161/CIRCULATIONAHA.108.798561. [DOI] [PubMed] [Google Scholar]
- 35.Cieslik KA, Trial J, Carlson S, Taffet GE, Entman ML. Aberrant differentiation of fibroblast progenitors contributes to fibrosis in the aged murine heart: role of elevated circulating insulin levels. FASEB J 27: 1761–1771, 2013. doi: 10.1096/fj.12-220145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindsey ML, LeBlanc AJ, Ripplinger CM, Carter JR, Kirk JA, Hansell Keehan K, Brunt KR, Kleinbongard P, Kassiri Z. Reinforcing rigor and reproducibility expectations for use of sex and gender in cardiovascular research. Am J Physiol Heart Circ Physiol 321: H819–H824, 2021. doi: 10.1152/ajpheart.00418.2021. [DOI] [PubMed] [Google Scholar]
- 37.Santos AB, Roca GQ, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Fang JC, Zile MR, Pitt B, Solomon SD, Shah AM. Prognostic relevance of left atrial dysfunction in heart failure with preserved ejection fraction. Circ Heart Fail 9: e002763, 2016. doi: 10.1161/CIRCHEARTFAILURE.115.002763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sohn DW, Chai IH, Lee DJ, Kim HC, Kim HS, Oh BH, Lee MM, Park YB, Choi YS, Seo JD, Lee YW. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol 30: 474–480, 1997. doi: 10.1016/S0735-1097(97)88335-0. [DOI] [PubMed] [Google Scholar]
- 39.Bouchey D, Argraves WS, Little CD. Fibulin-1, vitronectin, and fibronectin expression during avian cardiac valve and septa development. Anat Rec 244: 540–551, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 40.Newby AC. Vitronectin is implicated as the matrix takes control of neointima formation. Cardiovasc Res 53: 779–781, 2002. doi: 10.1016/s0008-6363(02)00235-3. [DOI] [PubMed] [Google Scholar]
- 41.Patten J, Wang K. Fibronectin in development and wound healing. Adv Drug Deliv Rev 170: 353–368, 2021. doi: 10.1016/j.addr.2020.09.005. [DOI] [PubMed] [Google Scholar]
- 42.Zhong J, Yang HC, Kon V, Fogo AB, Lawrence DA, Ma J. Vitronectin-binding PAI-1 protects against the development of cardiac fibrosis through interaction with fibroblasts. Lab Invest 94: 633–644, 2014. doi: 10.1038/labinvest.2014.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valiente-Alandi I, Potter SJ, Salvador AM, Schafer AE, Schips T, Carrillo-Salinas F, Gibson AM, Nieman ML, Perkins C, Sargent MA, Huo J, Lorenz JN, DeFalco T, Molkentin JD, Alcaide P, Blaxall BC. Inhibiting fibronectin attenuates fibrosis and improves cardiac function in a model of heart failure. Circulation 138: 1236–1252, 2018. doi: 10.1161/CIRCULATIONAHA.118.034609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maile LA, Aday AW, Busby WH, Sanghani R, Veluvolu U, Clemmons DR. Modulation of integrin antagonist signaling by ligand binding of the heparin-binding domain of vitronectin to the αVβ3 integrin. J Cell Biochem 105: 437–446, 2008. doi: 10.1002/jcb.21841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mollnau H, Munkel B, Schaper J. Collagen VI in the extracellular matrix of normal and failing human myocardium. Herz 20: 89–94, 1995. [PubMed] [Google Scholar]
- 46.Ong CW, Elkington PT, Brilha S, Ugarte-Gil C, Tome-Esteban MT, Tezera LB, Pabisiak PJ, Moores RC, Sathyamoorthy T, Patel V, Gilman RH, Porter JC, Friedland JS. Neutrophil-Derived MMP-8 drives AMPK-dependent matrix destruction in human pulmonary tuberculosis. PLoS Pathog 11: e1004917, 2015. doi: 10.1371/journal.ppat.1004917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valentin F, Bueb JL, Kieffer P, Tschirhart E, Atkinson J. Oxidative stress activates MMP-2 in cultured human coronary smooth muscle cells. Fundam Clin Pharmacol 19: 661–667, 2005. doi: 10.1111/j.1472-8206.2005.00371.x. [DOI] [PubMed] [Google Scholar]
- 48.Alge-Priglinger CS, Kreutzer T, Obholzer K, Wolf A, Mempel M, Kernt M, Kampik A, Priglinger SG. Oxidative stress-mediated induction of MMP-1 and MMP-3 in human RPE cells. Invest Ophthalmol Vis Sci 50: 5495–5503, 2009. doi: 10.1167/iovs.08-3193. [DOI] [PubMed] [Google Scholar]
- 49.Haorah J, Ramirez SH, Schall K, Smith D, Pandya R, Persidsky Y. Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood-brain barrier dysfunction. J Neurochem 101: 566–576, 2007. doi: 10.1111/j.1471-4159.2006.04393.x. [DOI] [PubMed] [Google Scholar]
- 50.Essick EE, Ouchi N, Wilson RM, Ohashi K, Ghobrial J, Shibata R, Pimentel DR, Sam F. Adiponectin mediates cardioprotection in oxidative stress-induced cardiac myocyte remodeling. Am J Physiol Heart Circ Physiol 301: H984–H993, 2011. doi: 10.1152/ajpheart.00428.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elkington PT, Green JA, Friedland JS. Analysis of matrix metalloproteinase secretion by macrophages. Methods Mol Biol 531: 253–265, 2009. doi: 10.1007/978-1-59745-396-7_16. [DOI] [PubMed] [Google Scholar]
- 52.Tepp K, Puurand M, Timohhina N, Adamson J, Klepinin A, Truu L, Shevchuk I, Chekulayev V, Kaambre T. Changes in the mitochondrial function and in the efficiency of energy transfer pathways during cardiomyocyte aging. Mol Cell Biochem 432: 141–158, 2017. doi: 10.1007/s11010-017-3005-1. [DOI] [PubMed] [Google Scholar]
- 53.Muhl L, Genove G, Leptidis S, Liu J, He L, Mocci G, Sun Y, Gustafsson S, Buyandelger B, Chivukula IV, Segerstolpe A, Raschperger E, Hansson EM, Bjorkegren JLM, Peng XR, Vanlandewijck M, Lendahl U, Betsholtz C. Single-cell analysis uncovers fibroblast heterogeneity and criteria for fibroblast and mural cell identification and discrimination. Nat Commun 11: 3953, 2020[Erratum inNat Commun11: 4493, 2020]. doi: 10.1038/s41467-020-17740-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D’Antoni ML, Debuque R, Chandran A, Wang L, Arora K, Rosenthal NA, Tallquist MD. Revisiting cardiac cellular composition. Circ Res 118: 400–409, 2016. doi: 10.1161/CIRCRESAHA.115.307778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moore-Morris T, Tallquist MD, Evans SM. Sorting out where fibroblasts come from. Circ Res 115: 602–604, 2014. doi: 10.1161/CIRCRESAHA.114.304854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kanisicak O, Khalil H, Ivey MJ, Karch J, Maliken BD, Correll RN, Brody MJ, J Lin S-C, Aronow BJ, Tallquist MD, Molkentin JD. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat Commun 7: 12260, 2016. doi: 10.1038/ncomms12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tillmanns J, Hoffmann D, Habbaba Y, Schmitto JD, Sedding D, Fraccarollo D, Galuppo P, Bauersachs J. Fibroblast activation protein α expression identifies activated fibroblasts after myocardial infarction. J Mol Cell Cardiol 87: 194–203, 2015. doi: 10.1016/j.yjmcc.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 58.Avery JT, Zhang R, Boohaker RJ. GLI1: a therapeutic target for cancer. Front Oncol 11: 673154, 2021. doi: 10.3389/fonc.2021.673154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shimokawa T, Rahman MF, Tostar U, Sonkoly E, Stahle M, Pivarcsi A, Palaniswamy R, Zaphiropoulos PG. RNA editing of the GLI1 transcription factor modulates the output of Hedgehog signaling. RNA Biol 10: 321–333, 2013. doi: 10.4161/rna.23343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tolosa EJ, Fernandez-Barrena MG, Iguchi E, McCleary-Wheeler AL, Carr RM, Almada LL, Flores LF, Vera RE, Alfonse GW, Marks DL, Hogenson TL, Vrabel AM, Horn IP, Koenig AN, Safgren SL, Sigafoos AN, Erkan M, Romecin-Duran PA, Sarabia Gonzalez A, Zhou B, Javelaud D, Marsaud V, Graham RP, Mauviel A, Elsawa SF, Fernandez-Zapico ME. GLI1/GLI2 functional interplay is required to control Hedgehog/GLI targets gene expression. Biochem J 477: 3131–3145, 2020. doi: 10.1042/BCJ20200335. [DOI] [PubMed] [Google Scholar]
- 61.Yoon JW, Kita Y, Frank DJ, Majewski RR, Konicek BA, Nobrega MA, Jacob H, Walterhouse D, Iannaccone P. Gene expression profiling leads to identification of GLI1-binding elements in target genes and a role for multiple downstream pathways in GLI1-induced cell transformation. J Biol Chem 277: 5548–5555, 2002. doi: 10.1074/jbc.M105708200. [DOI] [PubMed] [Google Scholar]
- 62.Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, Henderson JM, Ebert BL, Humphreys BD. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 16: 51–66, 2015. doi: 10.1016/j.stem.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perez-Riverol Y, Bai J, Bandla C, Garcia-Seisdedos D, Hewapathirana S, Kamatchinathan S, Kundu DJ, Prakash A, Frericks-Zipper A, Eisenacher M, Walzer M, Wang S, Brazma A, Vizcaino JA. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res 50: D543–D552, 2022. doi: 10.1093/nar/gkab1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Tables S1–S3: 10.6084/m9.figshare.20110514
Data Availability Statement
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium (https://www.ebi.ac.uk/pride/) via the PRIDE (63) partner repository with the data set identifier PXD031223. Data are available via ProteomeXchange with identifier PXD031223.