Abstract
The brain and spinal cord constitute the central nervous system (CNS), which when injured, can be exceedingly devastating. The mechanistic roles of proteoglycans (PGs) and their glycosaminoglycan (GAG) side chains in such injuries have been extensively studied. CNS injury immediately alters endothelial and extracellular matrix (ECM) PGs and GAGs. Subsequently, these alterations contribute to acute injury, postinjury fibrosis, and postinjury repair. These effects are central to the pathophysiology of CNS injury. This review focuses on the importance of PGs and GAGs in multiple forms of injury including traumatic brain injury, spinal cord injury, and stroke. We highlight the causes and consequences of degradation of the PG and GAG-enriched endothelial glycocalyx in early injury and discuss the pleiotropic roles of PGs in neuroinflammation. We subsequently evaluate the dualistic effects of PGs on recovery: both PG/GAG-mediated inhibition and facilitation of repair. We then report promising therapeutic strategies that may prove effective for repair of CNS injury including PG receptor inhibition, delivery of endogenous, pro-repair PGs and GAGs, and direct degradation of pathological GAGs. Finally, we discuss the importance of two PG- and GAG-containing ECM structures (synapses and perineuronal nets) in CNS injury and recovery.
Keywords: central nervous system injury, glycobiology, glycosaminoglycans, proteoglycans
INTRODUCTION
The glycobiology of the central nervous system (CNS; consisting of the brain and spinal cord) is critical to its form, function, and dysfunction. It is integral to communication between cells and the maintenance of both structural and functional integrity in the CNS. Glycobiology includes the study of a subset of proteins called proteoglycans (PGs), which are abundant in the extracellular matrix (ECM) of the CNS. PGs have received considerable attention for their roles in CNS development, homeostasis, and injury. Consisting of a core protein to which glycosaminoglycan (GAG) chains are linked, PGs are a family of highly bioactive molecules that affect many CNS functions including neuronal growth (1), synaptic plasticity (2), and blood-brain barrier (BBB) integrity (3).
The bioactivity attributed to PGs is primarily a function of their covalently bound GAG chains, which are a family of linear polysaccharides. Many GAG polysaccharides carry significant negative charge due to variable patterns of sulfation (4). Structural and functional heterogeneity of GAGs is driven by the diversity of sulfation patterns and chain lengths of these polysaccharides, which tailors how individual PGs modulate their environment (5).
PGs and GAGs have emerged as mediators of CNS injury through their involvement in myriad cellular processes within the brain and spinal cord. They are central to BBB permeability (6–8), neuroinflammation (9–12), glial scar formation (10, 13), and CNS regeneration (14), thus presenting promising new therapeutic targets for CNS injury. This review seeks to highlight a subset of the biological contributions of PGs and their side-chain GAGs to endothelial dysfunction, neuroinflammation, glial scar dynamics, and repair of CNS injury.
PGs AND GAGs
Although found in all cellular domains of the CNS, nearly all PGs are localized to the cell surface or the extracellular matrix (ECM) (15). The core protein not only dictates subcellular localization, but also the types of GAGs to which they attach (16, 17). PGs are categorized by the predominant GAG that they contain, making four general classes (15): heparan sulfate proteoglycans (HSPGs), chondroitin sulfate proteoglycans (CSPGs), dermatan sulfate proteoglycans (DSPGs), and keratan sulfate proteoglycans (KSPGs).
GAGs are linear polysaccharides comprised of repeating disaccharide units that include one amino sugar (e.g., N-acetylgalactosamine, d-glucosamine) linked to a second sugar (e.g., iduronic acid, glucuronic acid). These unique polysaccharides can be composed of hundreds of disaccharide units with molecular weights ranging from several kDa to upward of 1,000 kDa (18, 19). GAG type is dictated by the precise pattern of constituent disaccharides. Excluding hyaluronic acid (HA), sulfation can occur in several locations on every monosaccharide in a GAG chain. These sulfation groups, when present, impart negative charge and thereby allow for precise electrostatic interactions with positively charged ligands. HA is a unique GAG as it lacks sulfation and is not covalently bound to a core protein (20). It interacts with and regulates the activity of many proteins while constituting the structural backbone of the ECM.
PGs AND THEIR GAG SIDECHAINS IN THE CNS
Based upon recent proteomics analysis, CSPGs and HSPGs are highly prevalent in the adult mammalian CNS (21). The lectican family—aggrecan, brevican, versican, and neurocan—are extracellular CSPGs that populate the CNS ECM, of which brevican is exclusively expressed within the CNS. Neuron-glial antigen 2 (NG2)/chondroitin sulfate proteoglycan 4 (CSPG4), phosphacan, and CD44 are cell-surface CSPGs. The neurexin (NRXN), syndecan (SDC), and glypican families (particularly NRXN-1, NRXN-3, SDC-3, and glypican-1) are cell surface HSPGs found in the CNS. Notably, the CNS is highly enriched in brevican and NRXN-3 when compared with other tissue types (21). Keratan and dermatan sulfate (KS/DS) are attached to a handful of CS-predominant core proteins including members of the lectican and small leucine-rich proteoglycan (SLRP) families. Within the CNS, KS is bound to lecticans, phosphacan, aggrecan, and two SLRPs (fibromodulin and lumican), whereas DS is bound to CD44, NG2/CSPG4, several lecticans (neurocan, versican, and brevican), and two SLRPs (decorin and biglycan) (15, 17). The CSPG, sushi-repeat-containing protein, X-linked 2 (SRPX2), which has been implicated in epilepsy and language disorders while expressed widely during development, appears to be restricted to the hypothalamopituitary axis in the mature CNS (22–24). Glypican-3, betaglycan, thrombomodulin, and perlecan are not expressed significantly within the CNS (21). For excellent reviews of PG expression, localization, and homeostatic activity in the normal CNS refer to Schwartz and Domowic (25) and Hayes and Melrose (1).
PGs AND GAGs IN THE ACUTE PHASE OF CNS INJURY
The early phase of most forms of CNS injury [e.g., stroke, traumatic brain injury (TBI), traumatic spinal cord injury (SCI)] is characterized by vascular compromise with accompanying endothelial leak and initiation of a neuroinflammatory cascade. PGs and their sidechain GAGs are involved in these complex processes. They contribute to early BBB compromise due to their presence in the CNS endothelial glycocalyx (EG; Fig. 1) and are key mediators and modulators of many inflammatory pathways (Fig. 2).
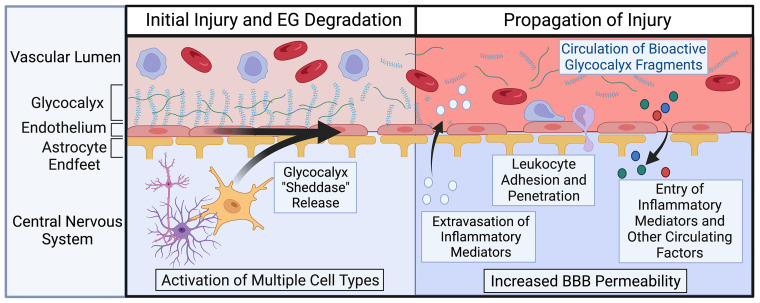
Figure 1.
Role of endothelial glycocalyx degradation in the propagation of central nervous system (CNS) injury. The endothelial glycocalyx (EG), a proteoglycan and glycosaminoglycan-enriched endothelial surface layer, is compromised early in CNS injury due to the release of “sheddases” by multiple cell types including endothelial cells, astrocytes, glia, and neurons. Loss of this critical barrier structure leads to compromise of the blood brain barrier (BBB), resulting in increases in vascular leak manifesting as edema, penetration of circulating molecules, and invasion of peripheral leukocytes. Image created with BioRender.com and published with permission.
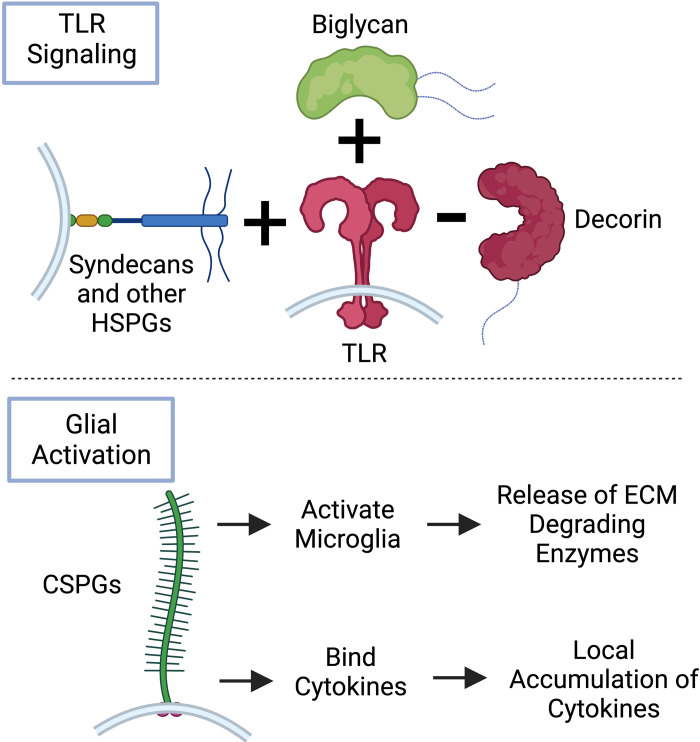
Figure 2.
Proteoglycans as mediators of early inflammation during central nervous system (CNS) injury. Proteoglycans (PGs) have significant roles in the early stages of CNS injury through their involvement in regulation of neuroinflammation. Many heparan sulfate-PGs and chondroitin sulfate-PGs upregulated early after CNS injury are proinflammatory through their actions on the Toll-like receptor (TLR) cascade and via direct interactions with microglia. Decorin, a chondroitin sulfate/dermatan sulfate-PG that is also upregulated early in injury, uniquely acts to downregulate TLR signaling and dampen inflammation. Image created with BioRender.com and published with permission. CSPG, chondroitin sulfate proteoglycans; ECM, extracellular matrix.
Endothelial Glycocalyx Degradation in CNS Injury
BBB compromise occurs early in the course of CNS injury, which augments local neuroinflammation that promotes secondary injury. HSPGs and hyaluronan are major constituents of the EG, a PG-rich nanolayer on the luminal endothelial surface of the vasculature, that in concert with the endothelial cell layer, abluminal basement membrane, and astrocyte endplates, is critical to BBB integrity (6). The intact EG helps prevent cerebral edema formation and regulates the entry of many circulating factors into the CNS through its network of selectively permeable GAGs and helps maintain a continuous endothelial cell layer via mechanotransduction at cell junctions (26). Degradation of this endothelial surface layer is known to occur in many diseases ranging from those associated with vascular malfunction like stroke to those associated with severe systemic inflammation like sepsis (27). In addition, EG damage is known to initiate a robust inflammatory cascade through increased vascular permeability and immune cell recruitment (8, 28, 29).
EG damage can be driven by several glycocalyx “sheddases” including heparanase-1 (HPSE), hyaluronidase-1 (HYAL1), hyalouronidase-2 (HYAL2), and reactive oxygen species (ROS) that directly cleave GAGs, and matrix-metalloproteinases (MMPs) and a disintegrin and metalloproteinases (ADAMs) that degrade PG core proteins (27). In CNS injury, these “sheddases” are known to be released by various cell types including endothelial cells, neurons, glia, astrocytes, and infiltrating neutrophils (30–37). Clinical studies have indirectly provided evidence for EG degradation in patients with traumatic brain injury (TBI), stroke, and subarachnoid hemorrhage (SAH, a form of stroke characterized by bleeding into the subarachnoid space) based upon increased levels of circulating EG constituents (38, 39). Consistent with these clinical observations, TBI, focal ischemia, SAH, and SCI models result in significant EG damage and vascular barrier compromise (7, 33–35, 40).
The precise mechanisms responsible for EG degradation in different types of CNS injury are incompletely understood and may diverge by disease process (Table 1). After TBI, it has been found that S100β/RAGE-mediated upregulation of ADAM17 promotes EG shedding (34). Furthermore, in a porcine model of TBI, EG constituents circulate within 15 min of injury without preceding nor coincident rises in circulating inflammatory markers, indicating early TBI-mediated EG degradation occurs independently of systemic inflammation (41). After global CNS ischemia (i.e., cardiac arrest), EG degradation is accompanied by MMP9 upregulation (7). In models of focal ischemic stroke, HYAL1, HYAL2, HPSE, and MMP9 have all been found to be upregulated within 24 h of injury, consistent with mechanistic redundancy for EG destruction in the disease (33, 36, 37). Acrolein, a reactive aldehyde generated at the site of injury after ischemia, may be specifically responsible for activation of proHPSE to HPSE (33). In SCI, local increases in MMP3 activate MMP9, which may directly account for observed EG loss (30, 31, 40). Increases in both MMP3 and MMP9 after SCI are driven by upregulation of the histone demethylase, Jumonji domain-containing protein-3 (Jmjd3) (32). After SAH, HPSE causes endothelial GAG degradation, which initiates inflammation at the site of injury and facilitates leukocyte migration into the brain parenchyma (35). In contrast, genetic overexpression of HPSE reduces neuroinflammation after endotoxin injection (42), suggesting that its activity may influence CNS inflammation in an insult-dependent manner. Although never specifically evaluated for their role in CNS-injury-related EG loss, increases in ROS in multiple forms of injury indicate they may contribute to degradation (43, 44). The central role of other “sheddases” in other conditions (e.g., ADAM15 in systemic inflammation and atrial natriuretic peptide in hypervolemia) suggests that alternative mechanisms for EG destruction may also be involved (45, 46). Further elucidating the complex cell biology of EG “sheddases” and the clinical consequences of EG degradation are important focuses of ongoing CNS injury research with clear therapeutic implications.
Table 1.
Endothelial glycocalyx “sheddase” mechanisms implicated in CNS injury
| CNS Injury | Sheddase(s) Implicated | Upstream Mediator(s) | Reference(s) |
|---|---|---|---|
| Traumatic brain injury | ADAM17 | S100β/RAGE | (34) |
| Global ischemia | MMP9 | Unknown | (7) |
| Focal ischemia | HYAL1, HYAL2, MMP9, HPSE | HPSE release from immune cells Acrolein-mediated activation of ProHPSE to generate HPSE | (33, 36, 37) |
| Spinal cord injury | MMP9 | MMP3, Jmjd3 | (31, 32, 40) |
| Subarachnoid hemorrhage | HPSE | Unknown | (35) |
ADAM17, a disintegrin and metalloproteinase 17; CNS, central nervous system; HPSE, heparanase-1; HYAL, hyaluronidase; MMP, matrix-metalloproteinase.
PGs and GAGs as Modulators and Mediators of Neuroinflammation
Increased BBB permeability caused by EG degradation exacerbates local neuroinflammation by facilitating infiltration of circulating leukocytes and cytokines at the injury site (28, 29). Once activated by infiltrating leukocytes and cytokines, resident microglia undergo rapid proliferation and begin releasing more inflammatory cytokines and structural proteins—resulting in a positive feedback loop of glial activation and local ECM remodeling (9).
HSPGs and CSPGs are known to be critical in neuroinflammation due to their ability to modulate cytokine activity via direct binding (47), form complexes with MMPs (48), and regulate inflammatory cascades like Toll-like receptor (TLR) and leukocyte common antigen-related phosphatase (LAR) signaling (49–51). In vitro, HSPGs have been found to facilitate glial activation after lipopolysaccharide exposure through interaction with TLR4 (50). Further in vitro evidence suggests HS oligosaccharides can activate transforming growth factor β (TGF-β) (52), which stimulates microglial (53) and astrocytic (54) production of CSPGs. CSPGs are purported to promote glial activation and bind cytokines, which localizes them to the site of injury (51, 55). Activation of microglia by CSPGs promotes production of ECM degrading MMPs through interactions with CD44 (55, 56).
The SLRPs biglycan and decorin also modulate neuroinflammation via TLR signaling. Biglycan has been found to induce microglial activation through TLR4 after SAH in mice (11) and via TLR2 in vitro (57), whereas decorin dampens neuroinflammation through inhibition of TLR signaling, which may be mediated by its inhibitory effects on TGF-β (12). Finally, HA is believed to have effects on neuroinflammation that are polysaccharide chain-length dependent. Like decorin, the presence of high-molecular-weight (HMW, full-length) HA is generally considered to be anti-inflammatory (58). Conversely, low-molecular-weight (LMW) HA fragments, generated by reactive oxygen-species-induced degradation of HMWHA at injury sites, have many proinflammatory effects including stimulation of apoptosis, activation of astrocytes, and propagation of cytokine release (10).
PGs AND GAGs IN THE GLIAL SCAR
Activation of astrocytes, microglia, oligodendrocytes, and oligodendrocyte precursor cells (OPCs) early in CNS injury results in their proliferation and migration toward the injury core. The consequence of this carefully orchestrated cellular migration is the formation of a glial scar, a structure that assumes a dichotomous role in CNS injury: adaptively preventing the spread of neuronal injury from the insult site and pathologically acting as a barrier to the regeneration of damaged neurons. Although highly cellular, the glial scar is also enriched in CSPGs, KSPGs, and GAGs (10, 13). The temporospatial dynamics and functional consequences of these unique molecules in multiple forms of CNS injury have been thoroughly elucidated.
PG Constituents of the Glial Scar
The glycobiology of the early glial scar is primarily characterized by changes in CSPGs. Increased expression of lecticans, NG2/CSPG4, phosphacan, biglycan, and decorin have all been observed across several models of CNS injury (14, 59). TBI in rodent models elicits consistent upregulation of CSPGs bordering the glial scar—a phenomenon that has also been observed in cortical stab wounds, SCI, and stroke models (60). The area directly surrounding the injury core, densely populated by scar-forming astrocytes and OPCs, expresses high levels of aggrecan, neurocan, brevican, versican, phosphocan, and NG2/CSPG4 in rodents with TBI (10, 61) and SCI (62). In humans, phosphacan, and NG2/CSPG4 are constituents of the post-SCI glial scar (63). KSPGs are also upregulated following TBI (64) and SCI (65) in rodents and work in tandem with CSPGs to form a mechanical scar (9). Based upon local upregulation of hyaluronan synthase after CNS injury, it has been proposed that HA may also be increased within the glial scar (66). Less is known about the dynamics of HSPGs and DSPGs in the glial scar.
CSPGs as Inhibitors of CNS Repair
Upregulation of CSPGs within the glial scar peak between 2 and 4 wk after TBI (67) and SCI (62, 65), marking a transition from a subacute to chronic phase that rarely fully resolves. It is during this chronic phase, once cell death driven by inflammation, infiltrating leukocytes, and oxidative stress has diminished, that the inhibitory nature of the CSPG-rich lesion border becomes detrimental to injury repair and contributes to persistent neurologic dysfunction. In a classic example, it was found that if a spinal cord nerve root called the dorsal root ganglion is surgically attached to a separate, damaged region of the spinal cord, dorsal root ganglion neurons are able to grow until reaching a CSPG-rich region surrounding the injured zone (68). As these axons extended readily through areas heavily populated by reactive astrocytes, but devoid of CSPGs, it was hypothesized that CSPGs may be a primary inhibitor of neural regeneration after injury. Since this foundational discovery, a large body of evidence now supports this compelling hypothesis (13, 14). The inhibitory effects of CSPGs [extensively reviewed by Tran et al. (69) and Sami et al. (70)] are believed to be primarily mediated by their interactions with and activation of Nogo receptors 1 and 3 (NGr1 and NGr3) and two members of the LAR family (RPTPσ and LAR). Activation of these cell surface receptors by CSPGs causes convergent, augmented RhoA/Rock signaling and divergent effects on several other intracellular signaling pathways, all of which have effects on cell physiology that account for their deleterious effects on injury repair. These effects include worsening of cell survival, pathological effects on cytoskeletal structure, and blockade of adaptive autophagy necessary for cellular and axonal regeneration.
Structural Characteristics of CS That Inhibit CNS Repair
Improvements in functional outcomes after CNS injury after degradation of CS sidechains with chondroitinase ABC (ChABC) suggest that GAGs may be primarily responsible for the inhibitory effects of CSPGs on CNS repair (71–73). Given this, understanding the precise structural characteristics of CS (i.e., sulfation, length, and sequence) that are responsible for its inhibitory effects on regenerative mechanisms has become an exciting new area of investigation (51). CS enriched in 4-O sulfation (CS-A) upregulated after SCI has been posited to mediate inhibition of CNS repair (74). Although local CS-A upregulation may be driven by TGF-β (74), the downstream signaling cascade responsible for the inhibitory effects of CS-A remains unknown. In addition to CS-A, CS enriched in -4S-6S sulfation (CS-E), which is also upregulated in the glial scar, directly inhibits neuronal repair (75). This effect may be mediated by the activation of RPTPσ by CS-E-containing CSPGs (76). Discovery of such sulfation-dependent determinants of protein modulation by CS supports that a highly regulated process of GAG chain patterning during CNS injury may be a major contributor to its pathology and resolution (or lack thereof).
THERAPEUTIC IMPLICATIONS OF PGs AND GAGs FOR CNS INJURY
Reversing Inhibitory Actions of CSPGs
Reversing the generally inhibitory effects of CSPGs is a promising therapeutic strategy for CNS injury. RPTPσ, LAR, and combined NgR1/NgR3 knockout mice have all demonstrated significant neuronal regeneration after CNS injury, suggesting that targeting these mechanisms may be a viable therapeutic option (69). A novel molecule, intracellular sigma peptide (ISP), which targets the RPTPσ pathway by disrupting CS-RPTPσ interactions, significantly improves locomotion in rats following SCI. Like RPTPσ knockout mice, ISP augmented protease secretion, which degraded CSPGs, thus reducing the inhibitory milieu at the site of injury (77). A novel LAR inhibitory peptide has also been shown to improve functional recovery after SCI in rodents (78). Analogous to the effects of ISP and LAR inhibitory peptides, a recent study in nonhuman primates found that NgR1 inhibition by a soluble decoy molecule improved both axon regeneration and locomotor function following SCI (79).
Directly targeting GAGs may be another potential therapeutic strategy for CNS injury. Treatment with ChABC, which specifically degrades CS chains, promotes axonal regeneration and functional recovery in models of rodent (72) and primate SCI (73). ChABC has also been shown to reduce cerebral edema after TBI and improve outcomes after stroke (80). Targeting KSPGs has shown similarly promising results. Both genetic knockout of an enzyme critical to KS assembly and targeted degradation of KS chains by keratanase II decreased scarring and improved axon regeneration after TBI and SCI (81, 82). Despite these promising results, nonspecific degradation of GAGs may compromise normal function in healthy tissue. This concern has spurred interest in sulfation-specific targeting of CS to promote recovery. Treatment with chondro-4-sulfatase (removing 4-O sulfation from CS, thus decreasing CS-A content) promoted axon growth across aggrecan dense regions in vitro (74). Similarly, enzymatic degradation of CS-A with a single injection of human arylsulfatase B (ARSB) also stimulated axon mobility across the injury site and improved functional recovery after SCI (83).
HSPGs, Highly Sulfated HS, and Decorin Promote CNS Repair
HSPGs, in contrast to CSPGs, are generally thought to facilitate rather than inhibit repair after injury. For example, SDC-1 is upregulated around the site of injury after cortical stab wounds (84). This increase in SDC-1 provides a microenvironment that supports regeneration through its interaction with and docking of pro-repair growth factors including FGFs and pleiotrophin (85). In addition, highly sulfated-HS can bind the same RPTPσ domain as CS-E, but in contrast to CS-E, inactivates the receptor and facilitate axonal growth in vitro (86). These results are supplemented by in vivo data showing that treatment with enoxaparin, a mixture of highly sulfated HS oligosaccharides, can enhance axon regeneration and motor function recovery after SCI in rodents via RPTPσ inhibition (87). In humans, a small clinical trial using enoxaparin (a mix of highly sulfated HS oligosaccharides) for TBI showed favorable neurological outcomes (88). The opposing effects of CSPGs and HSPGs on RPTPσ signaling have led to a “traffic light” hypothesis for the effects of HS(PGs) and CS(PGs) on axon growth and support ongoing efforts to fully elucidate how HS and HSPGs can be utilized to “green light” axonal regeneration in fibrotic regions heavily populated by inhibitory CS/CSPGs following brain injury (89).
Breaking the mold of most CSPGs, decorin is upregulated around the injury site after TBI, but is believed to facilitate injury repair (10). Decorin is an antagonist of TGF-β, a growth factor that promotes glial activation, postinjury fibrosis, and inhibitory CSPG production. In line with this, rats with both TBI and SCI have decreased CSPG expression and glial scarring following decorin treatment (90, 91). Suggesting therapeutic relevance, intraperitoneal decorin supplementation after TBI has been found to decrease ROS levels and reduce neuronal degeneration in rats, thus curtailing injury (90). A study in SCI utilizing decorin at the site of injury has shown similar improvements in axonal regeneration and functional outcomes (91).
PGs IN SYNAPSES AND PERINEURONAL NETS IN CNS INJURY
Synapses and perineuronal nets (PNNs), structures intrinsic to normal neurologic function in the healthy CNS, are disrupted in multiple forms of CNS injury (92–96). Accordingly, the dynamics of these structures likely play critical roles in recovery after CNS injury. Notably, both are dependent upon PGs and their GAG side chains for their functions.
It has recently been discovered that synapse formation and stability depend upon the interaction of HS sidechains of the presynaptic HSPG NRXN with postsynaptic LRTTM and neuroligin (97). Consistent with a potential role for NRXN in recovery after injury, its overexpression has been found to augment synapse formation and improve functional outcomes in models of SAH (98). In contrast, depletion of NRXN-neuroligin interaction by hypoxia treatment preceding injury in a focal ischemia model has been found to be neuroprotective (99). Based upon these conflicting results and the general paucity of data on their roles in functional recovery, further exploration of NRXNs and their interaction with postsynaptic ligands before, during, and after CNS injury should be pursued.
PNNs, which are found throughout the CNS, are netlike ECM structures highly enriched in HA and CSPGs including brevican, neurocan, versican, aggrecan, and potentially CD44. They play central physiologic roles in plasticity and modulation of ion channel activity and have been implicated as mediators of learning and memory, cognitive domains frequently impaired after CNS injury (2, 100, 101). PNNs are significantly diminished after focal ischemia (94, 102, 103), SCI (95, 104), and TBI (105). In focal ischemia, it has been found that PNNs are disrupted within 24 h of injury with complete dissolution at the injury core and partial loss in surrounding, peri-infarct tissue. There is an initial loss of GAG side chains and subsequent removal of PG core proteins. This sequential degradation is thought to be mediated by activated microglia and macrophages and lasts for at least 5 wk (94). Intuitively, the degree of PNN loss after focal ischemia may be dependent upon insult severity (103). Like focal ischemia, TBI leads to complete PNN loss at the injury core (at the site of the acellular scar). In addition, remote tissues (e.g., amygdala) demonstrate a loss of PNNs, which may be mediated by upregulation of decorin (105). Similar losses of PNNs have been observed in SCI at the injury site with decreases rostral to injury (below) and increases caudal to injury (above) (104). Although local activation of CNS immune cells resulting in release of GAG- and PG-degrading enzymes may account for PNN loss during CNS injury, it has also been observed that albumin extravasation into CNS tissue as a result of BBB compromise from injury can activate TGF-β and contribute to PNN degradation (106). Although PNNs are generally considered critical to homeostasis in health (2), observed losses of PNNs after CNS injury may in fact be adaptive as this may allow for renewed neural plasticity facilitating functional recovery. In fact, there is substantial evidence that direct degradation of PNNs through administration of ChABC in multiple forms of injury can improve functional outcomes (72, 102, 107). Concordantly, exercise and enriched environments that lead to improved outcomes after SCI have been associated with augmented loss of PNNs (95).
Conclusions
In the past several decades, many crucial responses to CNS injury have been found to have significant glycobiological contributions. BBB permeability, initiation and propagation of neuroinflammation, and postinjury repair (both adaptive and maladaptive) are modulated by myriad PGs and their sidechain GAGs (Table 2). Injury- and perhaps patient-specific GAG structural characteristics including chain length and sulfation refine the effects of PGs as exemplified by the opposing actions of 1) HMW and LMWHA in neuroinflammation and 2) highly sulfated CS-E and highly sulfated HS in postinjury repair. Discovery of these PG and GAG-specific mechanisms of CNS injury has led to the development of several promising therapeutic approaches: targeting inhibitory CSPGs (ISP, LAR inhibitory peptide, and Ngr1 decoys), repurposing endogenous PGs (decorin), and established glycosaminoglycan-based therapies to augment repair (enoxaparin), and even specifically degrading GAGs or GAG sulfation groups enzymatically (ChABC, keratanase II, chondro-4-sulfatase, ARSB). Further elucidating the precise determinants of GAG-protein interactions and their downstream effects on cell physiology is certain to reveal additional PG- and GAG-specific pathological mechanisms in CNS injury and facilitate the development of novel therapeutic strategies.
Table 2.
Key mechanisms involving proteoglycans that affect the endothelial glycocalyx, neuroinflammation, and glial scar activity after CNS injury
| Mechanism | Effects | Reference(s) |
|---|---|---|
| Endothelial glycocalyx (EG) | ||
| EG heparan sulfate (HS) degradation by multiple mechanisms (see Table 1) | Increased blood-brain barrier (BBB) permeability evidenced by increased edema and leukocyte infiltration of brain interstitium | (7, 31–37, 40) |
| Neuroinflammation | ||
| HPSE overexpression | Attenuates endotoxin-induced neuroinflammation | (42) |
| Activation of TLR pathway by HSPGs and biglycan | Stimulates microglial activation and their production of proinflammatory cytokines TNFα and IL-1β in culture and rodent subarachnoid hemorrhage (SAH) | (11, 50, 56, 57) |
| Binding of LAR and CD44 by CSPGs | Facilitates microglial activation and upregulates their expression of MMPs in vitro | (55, 108) |
| HS activation of TGF-β | Induces microglial activation and increased CSPG production by activated microglia and astrocytes in vitro | (52, 53, 109) |
| Glial scar | ||
| Binding of LAR and RPTPσ by CSPGs | Facilitates inhibition of axon growth through several common signaling pathways including Rho/ROCK, mTOR and Erk, as well as distinct effects through cofilin and PKC. Disruption ofcytoskeletal assembly and autophagic flux near growth cones are a few of the downstream effects that inhibit axon regrowth | (69, 70, 108) |
| Binding of NgR1 and NgR3 by CSPGs | Inhibits axon outgrowth presumably by enhancing myelin-associated inhibitor activity through Rho/ROCK mechanisms | (70) |
| Binding of RPTPσ to HSPGs | Competes with CSPGs for the binding domain and promotes axon extension by oligomerizing RPTPσ and inhibiting its downstream effects | (70, 86) |
| Antagonism of TGF-β by decorin | Decreases fibrosis, CSPG expression and promotes neural regeneration both in culture and across in vivo models of TBI, SCI, and SAH | (57, 90, 91) |
CNS, central nervous system; CSPG, chondroitin sulfate proteoglycan; HSPG, heparan sulfate proteoglycan; LAR, leukocyte common antigen-related phosphatase; MMP, matrix-metalloproteinase; mTOR, mammalian target of rapamycin; SCI, spinal cord injury; TBI, traumatic brain injury; TLR, Toll-like receptor.
GRANTS
This work was supported by the National Institutes of Health Grants K08HL1593520 and R03AG074056 (to J.A.H.).
AUTHOR CONTRIBUTIONS
N.S., K.O., and J.A.H. conceived and designed research; N.S. and J.A.H. prepared figures; N.S., K.O., and J.A.H. drafted manuscript; N.S., K.O., and J.A.H. edited and revised manuscript; N.S., K.O., and J.A.H. approved final version of manuscript.
ACKNOWLEDGMENTS
This article is part of the special collection “Deciphering the Role of Proteoglycans and Glycosaminoglycans in Health and Disease.” Liliana Schaefer, MD, served as Guest Editor of this collection. No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Hayes AJ, Melrose J. Neural tissue homeostasis and repair is regulated via CS and DS proteoglycan motifs. Front Cell Dev Biol 9: 696640, 2021. doi: 10.3389/fcell.2021.696640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fawcett JW, Oohashi T, Pizzorusso T. The roles of perineuronal nets and the perinodal extracellular matrix in neuronal function. Nat Rev Neurosci 20: 451–465, 2019. doi: 10.1038/s41583-019-0196-3. [DOI] [PubMed] [Google Scholar]
- 3.Yang T, Dai Y, Chen G, Cui S. Dissecting the dual role of the glial scar and scar-forming astrocytes in spinal cord injury. Front Cell Neurosci 14: 78, 2020. [Erratum in Front Cell Neurosci 14: 270, 2020]. doi: 10.3389/fncel.2020.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarrazin S, Lamanna WC, Esko JD. Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol 3: a004952, 2011. doi: 10.1101/cshperspect.a004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vallet SD, Clerc O, Ricard-Blum S. Glycosaminoglycan-protein interactions: the first draft of the glycosaminoglycan interactome. J Histochem Cytochem 69: 93–104, 2021. doi: 10.1369/0022155420946403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kutuzov N, Flyvbjerg H, Lauritzen M. Contributions of the glycocalyx, endothelium, and extravascular compartment to the blood-brain barrier. Proc Natl Acad Sci USA 115: E9429–E9438, 2018. doi: 10.1073/pnas.1802155115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu J, Li X, Yin J, Hu Y, Gu Y, Pan S. Glycocalyx degradation leads to blood-brain barrier dysfunction and brain edema after asphyxia cardiac arrest in rats. J Cereb Blood Flow Metab 38: 1979–1992, 2018. doi: 10.1177/0271678X17726062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ando Y, Okada H, Takemura G, Suzuki K, Takada C, Tomita H, Zaikokuji R, Hotta Y, Miyazaki N, Yano H, Muraki I, Kuroda A, Fukuda H, Kawasaki Y, Okamoto H, Kawaguchi T, Watanabe T, Doi T, Yoshida T, Ushikoshi H, Yoshida S, Ogura S. Brain-specific ultrastructure of capillary endothelial glycocalyx and its possible contribution for blood brain barrier. Sci Rep 8: 17523, 2018. doi: 10.1038/s41598-018-35976-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heindryckx F, Li JP. Role of proteoglycans in neuro-inflammation and central nervous system fibrosis. Matrix Biol 68–69: 589–601, 2018. doi: 10.1016/j.matbio.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 10.George N, Geller HM. Extracellular matrix and traumatic brain injury. J Neurosci Res 96: 573–588, 2018. doi: 10.1002/jnr.24151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie Y, Peng J, Pang J, Guo K, Zhang L, Yin S, Zhou J, Gu L, Tu T, Mu Q, Liao Y, Zhang X, Chen L, Jiang Y. Biglycan regulates neuroinflammation by promoting M1 microglial activation in early brain injury after experimental subarachnoid hemorrhage. J Neurochem 152: 368–380, 2020. doi: 10.1111/jnc.14926. [DOI] [PubMed] [Google Scholar]
- 12.Yan H, Chen Y, Li L, Jiang J, Wu G, Zuo Y, Zhang JH, Feng H, Yan X, Liu F. Decorin alleviated chronic hydrocephalus via inhibiting TGF-β1/Smad/CTGF pathway after subarachnoid hemorrhage in rats. Brain Res 1630: 241–253, 2016. doi: 10.1016/j.brainres.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci 5: 146–156, 2004. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 14.Galtrey CM, Fawcett JW. The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res Rev 54: 1–18, 2007. doi: 10.1016/j.brainresrev.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Iozzo RV, Schaefer L. Proteoglycan form and function: a comprehensive nomenclature of proteoglycans. Matrix Biol 42: 11–55, 2015. doi: 10.1016/j.matbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindahl U. A personal voyage through the proteoglycan field. Matrix Biol 35: 3–7, 2014. doi: 10.1016/j.matbio.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Lindahl U, Couchman J, Kimata K, Esko JD. Proteoglycans and sulfated glycosaminoglycans. In: Essentials of Glycobiology, edited by Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, Schnaar RL, Seeberger PH.. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 2015, p. 207–221. [Google Scholar]
- 18.Marcellin E, Steen JA, Nielsen LK. Insight into hyaluronic acid molecular weight control. Appl Microbiol Biotechnol 98: 6947–6956, 2014. doi: 10.1007/s00253-014-5853-x. [DOI] [PubMed] [Google Scholar]
- 19.Linhardt RJ, Gunay NS. Production and chemical processing of low molecular weight heparins. Semin Thromb Hemost 25, Suppl 3: 5–16, 1999. [PubMed] [Google Scholar]
- 20.Hascall V, Esko JD. Hyaluronan. In: Essentials of Glycobiology, edited by Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, Schnaar RL, Seeberger PH.. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 2015, p. 197–206. [PubMed] [Google Scholar]
- 21.Sharma K, Schmitt S, Bergner CG, Tyanova S, Kannaiyan N, Manrique-Hoyos N, Kongi K, Cantuti L, Hanisch UK, Philips MA, Rossner MJ, Mann M, Simons M. Cell type- and brain region-resolved mouse brain proteome. Nat Neurosci 18: 1819–1831, 2015. doi: 10.1038/nn.4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka K, Arao T, Tamura D, Aomatsu K, Furuta K, Matsumoto K, Kaneda H, Kudo K, Fujita Y, Kimura H, Yanagihara K, Yamada Y, Okamoto I, Nakagawa K, Nishio K. SRPX2 is a novel chondroitin sulfate proteoglycan that is overexpressed in gastrointestinal cancer. PLoS One 7: e27922, 2012. doi: 10.1371/journal.pone.0027922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anwer M, Bolkvadze T, Ndode-Ekane XE, Puhakka N, Rauramaa T, Leinonen V, van Vliet EA, Swaab DF, Haapasalo A, Leskelä S, Bister N, Malm T, Carlson S, Aronica E, Pitkänen A. Sushi repeat-containing protein X-linked 2: a novel phylogenetically conserved hypothalamo-pituitary protein. J Comp Neurol 526: 1806–1819, 2018. doi: 10.1002/cne.24449. [DOI] [PubMed] [Google Scholar]
- 24.Roll P, Rudolf G, Pereira S, Royer B, Scheffer IE, Massacrier A, Valenti MP, Roeckel-Trevisiol N, Jamali S, Beclin C, Seegmuller C, Metz-Lutz MN, Lemainque A, Delepine M, Caloustian C, de Saint Martin A, Bruneau N, Depétris D, Mattéi MG, Flori E, Robaglia-Schlupp A, Lévy N, Neubauer BA, Ravid R, Marescaux C, Berkovic SF, Hirsch E, Lathrop M, Cau P, Szepetowski P. SRPX2 mutations in disorders of language cortex and cognition. Hum Mol Genet 15: 1195–1207, 2006. doi: 10.1093/hmg/ddl035. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz NB, Domowicz MS. Proteoglycans in brain development and pathogenesis. FEBS Lett 592: 3791–3805, 2018. doi: 10.1002/1873-3468.13026. [DOI] [PubMed] [Google Scholar]
- 26.Nian K, Harding IC, Herman IM, Ebong EE. Blood-brain barrier damage in ischemic stroke and its regulation by endothelial mechanotransduction. Front Physiol 11: 605398, 2020. doi: 10.3389/fphys.2020.605398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sullivan RC, Rockstrom MD, Schmidt EP, Hippensteel JA. Endothelial glycocalyx degradation during sepsis: causes and consequences. Matrix Biol Plus 12: 100094, 2021. doi: 10.1016/j.mbplus.2021.100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soares HD, Hicks RR, Smith D, McIntosh TK. Inflammatory leukocytic recruitment and diffuse neuronal degeneration are separate pathological processes resulting from traumatic brain injury. J Neurosci 15: 8223–8233, 1995. doi: 10.1523/JNEUROSCI.15-12-08223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banks WA. Blood-brain barrier transport of cytokines: a mechanism for neuropathology. Curr Pharm Des 11: 973–984, 2005. doi: 10.2174/1381612053381684. [DOI] [PubMed] [Google Scholar]
- 30.Noble LJ, Donovan F, Igarashi T, Goussev S, Werb Z. Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J Neurosci 22: 7526–7535, 2002. doi: 10.1523/JNEUROSCI.22-17-07526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JY, Choi HY, Ahn HJ, Ju BG, Yune TY. Matrix metalloproteinase-3 promotes early blood-spinal cord barrier disruption and hemorrhage and impairs long-term neurological recovery after spinal cord injury. Am J Pathol 184: 2985–3000, 2014. doi: 10.1016/j.ajpath.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 32.Lee JY, Na WH, Choi HY, Lee KH, Ju BG, Yune TY. Jmjd3 mediates blood-spinal cord barrier disruption after spinal cord injury by regulating MMP-3 and MMP-9 expressions. Neurobiol Dis 95: 66–81, 2016. doi: 10.1016/j.nbd.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 33.Ko K, Suzuki T, Ishikawa R, Hattori N, Ito R, Umehara K, Furihata T, Dohmae N, Linhardt RJ, Igarashi K, Toida T, Higashi K. Ischemic stroke disrupts the endothelial glycocalyx through activation of proHPSE via acrolein exposure. J Biol Chem 295: 18614–18624, 2020. doi: 10.1074/jbc.RA120.015105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou Z, Li L, Li Q, Zhao P, Zhang K, Liu C, Cai D, Maegele M, Gu Z, Huang Q. The role of S100B/RAGE-enhanced ADAM17 activation in endothelial glycocalyx shedding after traumatic brain injury. J Neuroinflammation 19: 46, 2022. doi: 10.1186/s12974-022-02412-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Changyaleket B, Chong ZZ, Dull RO, Nanegrungsunk D, Xu H. Heparanase promotes neuroinflammatory response during subarachnoid hemorrhage in rats. J Neuroinflammation 14: 137, 2017. doi: 10.1186/s12974-017-0912-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katarzyna Greda A, Nowicka D. Hyaluronidase inhibition accelerates functional recovery from stroke in the mouse brain. J Neurochem 157: 781–801, 2021. doi: 10.1111/jnc.15279. [DOI] [PubMed] [Google Scholar]
- 37.Al Qteishat A, Gaffney JJ, Krupinski J, Slevin M. Hyaluronan expression following middle cerebral artery occlusion in the rat. Neuroreport 17: 1111–1114, 2006. doi: 10.1097/01.wnr.0000227986.69680.20. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez Rodriguez E, Cardenas JC, Cox CS, Kitagawa RS, Stensballe J, Holcomb JB, Johansson PI, Wade CE. Traumatic brain injury is associated with increased syndecan-1 shedding in severely injured patients. Scand J Trauma Resusc Emerg Med 26: 102, 2018. doi: 10.1186/s13049-018-0565-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DellaValle B, Hasseldam H, Johansen FF, Iversen HK, Rungby J, Hempel C. Multiple soluble components of the glycocalyx are increased in patient plasma after ischemic stroke. Stroke 50: 2948–2951, 2019. doi: 10.1161/STROKEAHA.119.025953. [DOI] [PubMed] [Google Scholar]
- 40.Noble LJ, Mautes AE, Hall JJ. Characterization of the microvascular glycocalyx in normal and injured spinal cord in the rat. J Comp Neurol 376: 542–556, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 41.Sillesen M, Rasmussen LS, Jin G, Jepsen CH, Imam A, Hwabejire JO, Halaweish I, DeMoya M, Velmahos G, Johansson PI, Alam HB. Assessment of coagulopathy, endothelial injury, and inflammation after traumatic brain injury and hemorrhage in a porcine model. J Trauma Acute Care Surg 76: 12–19, 2014. doi: 10.1097/TA.0b013e3182aaa675. [DOI] [PubMed] [Google Scholar]
- 42.Zhang X, Wang B, Li JP. Implications of heparan sulfate and heparanase in neuroinflammation. Matrix Biol 35: 174–181, 2014. doi: 10.1016/j.matbio.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 43.Baker K, Marcus CB, Huffman K, Kruk H, Malfroy B, Doctrow SR. Synthetic combined superoxide dismutase/catalase mimetics are protective as a delayed treatment in a rat stroke model: a key role for reactive oxygen species in ischemic brain injury. J Pharmacol Exp Ther 284: 215–221, 1998. [PubMed] [Google Scholar]
- 44.Corps KN, Roth TL, McGavern DB. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol 72: 355–362, 2015. doi: 10.1001/jamaneurol.2014.3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang X, Meegan JE, Jannaway M, Coleman DC, Yuan SY. A disintegrin and metalloproteinase 15-mediated glycocalyx shedding contributes to vascular leakage during inflammation. Cardiovasc Res 114: 1752–1763, 2018. doi: 10.1093/cvr/cvy167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chappell D, Bruegger D, Potzel J, Jacob M, Brettner F, Vogeser M, Conzen P, Becker BF, Rehm M. Hypervolemia increases release of atrial natriuretic peptide and shedding of the endothelial glycocalyx. Crit Care 18: 538, 2014. doi: 10.1186/s13054-014-0538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mulloy B, Rider CC. Cytokines and proteoglycans: an introductory overview. Biochem Soc Trans 34: 409–413, 2006. doi: 10.1042/BST0340409. [DOI] [PubMed] [Google Scholar]
- 48.Dear ML, Shilts J, Broadie K. Neuronal activity drives FMRP- and HSPG-dependent matrix metalloproteinase function required for rapid synaptogenesis. Sci Signal 10: eaan3181, 2017. doi: 10.1126/scisignal.aan3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dyck SM, Alizadeh A, Santhosh KT, Proulx EH, Wu CL, Karimi-Abdolrezaee S. Chondroitin sulfate proteoglycans negatively modulate spinal cord neural precursor cells by signaling through LAR and RPTPσ and modulation of the Rho/ROCK pathway. Stem Cells 33: 2550–2563, 2015. doi: 10.1002/stem.1979. [DOI] [PubMed] [Google Scholar]
- 50.O’Callaghan P, Li JP, Lannfelt L, Lindahl U, Zhang X. Microglial heparan sulfate proteoglycans facilitate the cluster-of-differentiation 14 (CD14)/Toll-like receptor 4 (TLR4)-dependent inflammatory response. J Biol Chem 290: 14904–14914, 2015. doi: 10.1074/jbc.M114.634337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hussein RK, Mencio CP, Katagiri Y, Brake AM, Geller HM. Role of chondroitin sulfation following spinal cord injury. Front Cell Neurosci 14: 208, 2020. doi: 10.3389/fncel.2020.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee J, Wee S, Gunaratne J, Chua RJ, Smith RA, Ling L, Fernig DG, Swaminathan K, Nurcombe V, Cool SM. Structural determinants of heparin-transforming growth factor-β1 interactions and their effects on signaling. Glycobiology 25: 1491–1504, 2015. doi: 10.1093/glycob/cwv064. [DOI] [PubMed] [Google Scholar]
- 53.Sugimoto K, Nishioka R, Ikeda A, Mise A, Takahashi H, Yano H, Kumon Y, Ohnishi T, Tanaka J. Activated microglia in a rat stroke model express NG2 proteoglycan in peri-infarct tissue through the involvement of TGF-β1. Glia 62: 185–198, 2014. doi: 10.1002/glia.22598. [DOI] [PubMed] [Google Scholar]
- 54.Asher RA, Morgenstern DA, Fidler PS, Adcock KH, Oohira A, Braistead JE, Levine JM, Margolis RU, Rogers JH, Fawcett JW. Neurocan is upregulated in injured brain and in cytokine-treated astrocytes. J Neurosci 20: 2427–2438, 2000. doi: 10.1523/JNEUROSCI.20-07-02427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stephenson EL, Yong VW. Pro-inflammatory roles of chondroitin sulfate proteoglycans in disorders of the central nervous system. Matrix Biol 71-72: 432–442, 2018. doi: 10.1016/j.matbio.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 56.Rolls A, Shechter R, London A, Segev Y, Jacob-Hirsch J, Amariglio N, Rechavi G, Schwartz M. Two faces of chondroitin sulfate proteoglycan in spinal cord repair: a role in microglia/macrophage activation. PLoS Med 5: e171, 2008. doi: 10.1371/journal.pmed.0050171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duan W, Zou J, Chen X, Xiao C, Jiang W. Biglycan expression promotes β-amyloid-induced microglial activation via TLR2 in mouse cell culture model. Clin Lab 67, 2021. doi: 10.7754/Clin.Lab.2020.200252. [DOI] [PubMed] [Google Scholar]
- 58.Smith PD, Coulson-Thomas VJ, Foscarin S, Kwok JC, Fawcett JW. GAG-ing with the neuron: the role of glycosaminoglycan patterning in the central nervous system. Exp Neurol 274: 100–114, 2015. doi: 10.1016/j.expneurol.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 59.Burnside ER, Bradbury EJ. Manipulating the extracellular matrix and its role in brain and spinal cord plasticity and repair. Neuropathol Appl Neurobiol 40: 26–59, 2014. doi: 10.1111/nan.12114. [DOI] [PubMed] [Google Scholar]
- 60.Harris NG, Carmichael ST, Hovda DA, Sutton RL. Traumatic brain injury results in disparate regions of chondroitin sulfate proteoglycan expression that are temporally limited. J Neurosci Res 87: 2937–2950, 2009. doi: 10.1002/jnr.22115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yi JH, Katagiri Y, Susarla B, Figge D, Symes AJ, Geller HM. Alterations in sulfated chondroitin glycosaminoglycans following controlled cortical impact injury in mice. J Comp Neurol 520: 3295–3313, 2012. doi: 10.1002/cne.23156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tang X, Davies JE, Davies SJ. Changes in distribution, cell associations, and protein expression levels of NG2, neurocan, phosphacan, brevican, versican V2, and tenascin-C during acute to chronic maturation of spinal cord scar tissue. J Neurosci Res 71: 427–444, 2003. doi: 10.1002/jnr.10523. [DOI] [PubMed] [Google Scholar]
- 63.Buss A, Pech K, Kakulas BA, Martin D, Schoenen J, Noth J, Brook GA. NG2 and phosphacan are present in the astroglial scar after human traumatic spinal cord injury. BMC Neurol 9: 32, 2009. doi: 10.1186/1471-2377-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Geisert EE Jr, Bidanset DJ, Del Mar N, Robson JA. Up-regulation of a keratan sulfate proteoglycan following cortical injury in neonatal rats. Int J Dev Neurosci 14: 257–267, 1996. doi: 10.1016/0736-5748(96)00012-3. [DOI] [PubMed] [Google Scholar]
- 65.Jones LL, Tuszynski MH. Spinal cord injury elicits expression of keratan sulfate proteoglycans by macrophages, reactive microglia, and oligodendrocyte progenitors. J Neurosci 22: 4611–4624, 2002. doi: 10.1523/JNEUROSCI.22-11-04611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xing G, Ren M, Verma A. Divergent temporal expression of hyaluronan metabolizing enzymes and receptors with craniotomy vs. controlled-cortical impact injury in rat brain: a pilot study. Front Neurol 5: 173, 2014. doi: 10.3389/fneur.2014.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Asher RA, Morgenstern DA, Shearer MC, Adcock KH, Pesheva P, Fawcett JW. Versican is upregulated in CNS injury and is a product of oligodendrocyte lineage cells. J Neurosci 22: 2225–2236, 2002. doi: 10.1523/JNEUROSCI.22-06-02225.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Davies SJ, Fitch MT, Memberg SP, Hall AK, Raisman G, Silver J. Regeneration of adult axons in white matter tracts of the central nervous system. Nature 390: 680–683, 1997. doi: 10.1038/37776. [DOI] [PubMed] [Google Scholar]
- 69.Tran AP, Warren PM, Silver J. The biology of regeneration failure and success after spinal cord injury. Physiol Rev 98: 881–917, 2018. doi: 10.1152/physrev.00017.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sami A, Selzer ME, Li S. Advances in the signaling pathways downstream of glial-scar axon growth inhibitors. Front Cell Neurosci 14: 174, 2020. doi: 10.3389/fncel.2020.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koh CH, Pronin S, Hughes M. Chondroitinase ABC for neurological recovery after acute brain injury: systematic review and meta-analyses of preclinical studies. Brain Inj 32: 715–729, 2018. doi: 10.1080/02699052.2018.1438665. [DOI] [PubMed] [Google Scholar]
- 72.Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416: 636–640, 2002. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- 73.Rosenzweig ES, Salegio EA, Liang JJ, Weber JL, Weinholtz CA, Brock JH, Moseanko R, Hawbecker S, Pender R, Cruzen CL, Iaci JF, Caggiano AO, Blight AR, Haenzi B, Huie JR, Havton LA, Nout-Lomas YS, Fawcett JW, Ferguson AR, Beattie MS, Bresnahan JC, Tuszynski MH. Chondroitinase improves anatomical and functional outcomes after primate spinal cord injury. Nat Neurosci 22: 1269–1275, 2019. doi: 10.1038/s41593-019-0424-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang H, Katagiri Y, McCann TE, Unsworth E, Goldsmith P, Yu ZX, Tan F, Santiago L, Mills EM, Wang Y, Symes AJ, Geller HM. Chondroitin-4-sulfation negatively regulates axonal guidance and growth. J Cell Sci 121: 3083–3091, 2008. doi: 10.1242/jcs.032649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gilbert RJ, McKeon RJ, Darr A, Calabro A, Hascall VC, Bellamkonda RC. 4,6 is differentially upregulated in glial scar and is a potent inhibitor of neurite extension. Mol Cell Neurosci 29: 545–558, 2005. doi: 10.1016/j.mcn.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 76.Brown JM, Xia J, Zhuang B, Cho KS, Rogers CJ, Gama CI, Rawat M, Tully SE, Uetani N, Mason DE, Tremblay ML, Peters EC, Habuchi O, Chen DF, Hsieh-Wilson LC. A sulfated carbohydrate epitope inhibits axon regeneration after injury. Proc Natl Acad Sci USA 109: 4768–4773, 2012. doi: 10.1073/pnas.1121318109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lang BT, Cregg JM, DePaul MA, Tran AP, Xu K, Dyck SM, Madalena KM, Brown BP, Weng YL, Li S, Karimi-Abdolrezaee S, Busch SA, Shen Y, Silver J. Modulation of the proteoglycan receptor PTPσ promotes recovery after spinal cord injury. Nature 518: 404–408, 2015. doi: 10.1038/nature13974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cheng L, Sami A, Ghosh B, Urban MW, Heinsinger NM, Liang SS, Smith GM, Wright MC, Li S, Lepore AC. LAR inhibitory peptide promotes recovery of diaphragm function and multiple forms of respiratory neural circuit plasticity after cervical spinal cord injury. Neurobiol Dis 147: 105153, 2021. doi: 10.1016/j.nbd.2020.105153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang X, Zhou T, Maynard GD, Terse PS, Cafferty WB, Kocsis JD, Strittmatter SM. Nogo receptor decoy promotes recovery and corticospinal growth in non-human primate spinal cord injury. Brain 143: 1697–1713, 2020. doi: 10.1093/brain/awaa116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hill JJ, Jin K, Mao XO, Xie L, Greenberg DA. Intracerebral chondroitinase ABC and heparan sulfate proteoglycan glypican improve outcome from chronic stroke in rats. Proc Natl Acad Sci USA 109: 9155–9160, 2012. doi: 10.1073/pnas.1205697109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ishikawa Y, Imagama S, Ohgomori T, Ishiguro N, Kadomatsu K. A combination of keratan sulfate digestion and rehabilitation promotes anatomical plasticity after rat spinal cord injury. Neurosci Lett 593: 13–18, 2015. doi: 10.1016/j.neulet.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 82.Ito Z, Sakamoto K, Imagama S, Matsuyama Y, Zhang H, Hirano K, Ando K, Yamashita T, Ishiguro N, Kadomatsu K. N-acetylglucosamine 6-O-sulfotransferase-1-deficient mice show better functional recovery after spinal cord injury. J Neurosci 30: 5937–5947, 2010. doi: 10.1523/JNEUROSCI.2570-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yoo M, Khaled M, Gibbs KM, Kim J, Kowalewski B, Dierks T, Schachner M. Arylsulfatase B improves locomotor function after mouse spinal cord injury. PLoS One 8: e57415, 2013. doi: 10.1371/journal.pone.0057415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Properzi F, Lin R, Kwok J, Naidu M, van Kuppevelt TH, Ten Dam GB, Camargo LM, Raha-Chowdhury R, Furukawa Y, Mikami T, Sugahara K, Fawcett JW. Heparan sulphate proteoglycans in glia and in the normal and injured CNS: expression of sulphotransferases and changes in sulphation. Eur J Neurosci 27: 593–604, 2008. doi: 10.1111/j.1460-9568.2008.06042.x. [DOI] [PubMed] [Google Scholar]
- 85.Iseki K, Hagino S, Mori T, Zhang Y, Yokoya S, Takaki H, Tase C, Murakawa M, Wanaka A. Increased syndecan expression by pleiotrophin and FGF receptor-expressing astrocytes in injured brain tissue. Glia 39: 1–9, 2002. doi: 10.1002/glia.10078. [DOI] [PubMed] [Google Scholar]
- 86.Coles CH, Shen Y, Tenney AP, Siebold C, Sutton GC, Lu W, Gallagher JT, Jones EY, Flanagan JG, Aricescu AR. Proteoglycan-specific molecular switch for RPTPsigma clustering and neuronal extension. Science 332: 484–488, 2011. doi: 10.1126/science.1200840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ito S, Ozaki T, Morozumi M, Imagama S, Kadomatsu K, Sakamoto K. Enoxaparin promotes functional recovery after spinal cord injury by antagonizing PTPRsigma. Exp Neurol 340: 113679, 2021. doi: 10.1016/j.expneurol.2021.113679. [DOI] [PubMed] [Google Scholar]
- 88.Baharvahdat H, Ganjeifar B, Etemadrezaie H, Farajirad M, Zabihyan S, Mowla A. Enoxaparin in the treatment of severe traumatic brain injury: a randomized clinical trial. Surg Neurol Int 10: 10, 2019. doi: 10.4103/sni.sni_112_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lindsay SL, McCanney GA, Willison AG, Barnett SC. Multi-target approaches to CNS repair: olfactory mucosa-derived cells and heparan sulfates. Nat Rev Neurol 16: 229–240, 2020. doi: 10.1038/s41582-020-0311-0. [DOI] [PubMed] [Google Scholar]
- 90.Özay R, Türkoğlu E, Gürer B, Dolgun H, Evirgen O, Ergüder Bİ, Hayırlı N, Gürses L, Şekerci Z, Yılmaz ER. Does decorin protect neuronal tissue via its antioxidant and antiinflammatory activity from traumatic brain injury? An experimental study. World Neurosurg 97: 407–415, 2017. doi: 10.1016/j.wneu.2016.09.115. [DOI] [PubMed] [Google Scholar]
- 91.Matthews J, Surey S, Grover LM, Logan A, Ahmed Z. Thermosensitive collagen/fibrinogen gels loaded with decorin suppress lesion site cavitation and promote functional recovery after spinal cord injury. Sci Rep 11: 18124, 2021. doi: 10.1038/s41598-021-97604-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jamjoom AAB, Rhodes J, Andrews PJD, Grant SGN. The synapse in traumatic brain injury. Brain 144: 18–31, 2021. doi: 10.1093/brain/awaa321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shi X, Luo L, Wang J, Shen H, Li Y, Mamtilahun M, Liu C, Shi R, Lee JH, Tian H, Zhang Z, Wang Y, Chung WS, Tang Y, Yang GY. Stroke subtype-dependent synapse elimination by reactive gliosis in mice. Nat Commun 12: 6943, 2021. doi: 10.1038/s41467-021-27248-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hobohm C, Günther A, Grosche J, Rossner S, Schneider D, Brückner G. Decomposition and long-lasting downregulation of extracellular matrix in perineuronal nets induced by focal cerebral ischemia in rats. J Neurosci Res 80: 539–548, 2005. doi: 10.1002/jnr.20459. [DOI] [PubMed] [Google Scholar]
- 95.Sánchez-Ventura J, Giménez-Llort L, Penas C, Udina E. Voluntary wheel running preserves lumbar perineuronal nets, enhances motor functions and prevents hyperreflexia after spinal cord injury. Exp Neurol 336: 113533, 2021. doi: 10.1016/j.expneurol.2020.113533. [DOI] [PubMed] [Google Scholar]
- 96.Vita SM, Grayson BE, Grill RJ. Acute damage to the blood–brain barrier and perineuronal net integrity in a clinically-relevant rat model of traumatic brain injury. Neuroreport 31: 1167–1174, 2020. doi: 10.1097/WNR.0000000000001531. [DOI] [PubMed] [Google Scholar]
- 97.Zhang P, Lu H, Peixoto RT, Pines MK, Ge Y, Oku S, Siddiqui TJ, Xie Y, Wu W, Archer-Hartmann S, Yoshida K, Tanaka KF, Aricescu AR, Azadi P, Gordon MD, Sabatini BL, Wong ROL, Craig AM. Heparan sulfate organizes neuronal synapses through neurexin partnerships. Cell 174: 1450–1464.e3, 2018. doi: 10.1016/j.cell.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shen H, Chen Z, Wang Y, Gao A, Li H, Cui Y, Zhang L, Xu X, Wang Z, Chen G. Role of neurexin-1β and neuroligin-1 in cognitive dysfunction after subarachnoid hemorrhage in rats. Stroke 46: 2607–2615, 2015. doi: 10.1161/STROKEAHA.115.009729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li C, Han D, Zhang F, Zhou C, Yu HM, Zhang GY. Preconditioning ischemia attenuates increased neurexin-neuroligin1-PSD-95 interaction after transient cerebral ischemia in rat hippocampus. Neurosci Lett 426: 192–197, 2007. doi: 10.1016/j.neulet.2007.08.065. [DOI] [PubMed] [Google Scholar]
- 100.Tsien RY. Very long-term memories may be stored in the pattern of holes in the perineuronal net. Proc Natl Acad Sci USA 110: 12456–12461, 2013. doi: 10.1073/pnas.1310158110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mirzadeh Z, Alonge KM, Cabrales E, Herranz-Pérez V, Scarlett JM, Brown JM, Hassouna R, Matsen ME, Nguyen HT, Garcia-Verdugo JM, Zeltser LM, Schwartz MW. Perineuronal net formation during the critical period for neuronal maturation in the hypothalamic arcuate nucleus. Nat Metab 1: 212–221, 2019. doi: 10.1038/s42255-018-0029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Soleman S, Yip PK, Duricki DA, Moon LD. Delayed treatment with chondroitinase ABC promotes sensorimotor recovery and plasticity after stroke in aged rats. Brain 135: 1210–1223, 2012. doi: 10.1093/brain/aws027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dzyubenko E, Manrique-Castano D, Kleinschnitz C, Faissner A, Hermann DM. Topological remodeling of cortical perineuronal nets in focal cerebral ischemia and mild hypoperfusion. Matrix Biol 74: 121–132, 2018. doi: 10.1016/j.matbio.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 104.Lipachev N, Arnst N, Melnikova A, Jäälinoja H, Kochneva A, Zhigalov A, Kulesskaya N, Aganov AV, Mavlikeev M, Rauvala H, Kiyasov AP, Paveliev M. Quantitative changes in perineuronal nets in development and posttraumatic condition. J Mol Histol 50: 203–216, 2019. doi: 10.1007/s10735-019-09818-y. [DOI] [PubMed] [Google Scholar]
- 105.Shi Y, Wu X, Zhou J, Cui W, Wang J, Hu Q, Zhang S, Han L, Zhou M, Luo J, Wang Q, Liu H, Feng D, Ge S, Qu Y. Single-nucleus RNA sequencing reveals that decorin expression in the amygdala regulates perineuronal nets expression and fear conditioning response after traumatic brain injury. Adv Sci (Weinh) 9: e2104112, 2022. doi: 10.1002/advs.202104112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim SY, Senatorov VV Jr, Morrissey CS, Lippmann K, Vazquez O, Milikovsky DZ, Gu F, Parada I, Prince DA, Becker AJ, Heinemann U, Friedman A, Kaufer D. TGFβ signaling is associated with changes in inflammatory gene expression and perineuronal net degradation around inhibitory neurons following various neurological insults. Sci Rep 7: 7711, 2017. doi: 10.1038/s41598-017-07394-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Finan JD, Cho FS, Kernie SG, Morrison B 3rd.. Intracerebroventricular administration of chondroitinase ABC reduces acute edema after traumatic brain injury in mice. BMC Res Notes 9: 160, 2016. doi: 10.1186/s13104-016-1968-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method: a new method for detection of delirium. Ann Intern Med 113: 941–948, 1990. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 109.Properzi F, Carulli D, Asher RA, Muir E, Camargo LM, van Kuppevelt TH, ten Dam GB, Furukawa Y, Mikami T, Sugahara K, Toida T, Geller HM, Fawcett JW. Chondroitin 6‐sulphate synthesis is up‐regulated in injured CNS, induced by injury‐related cytokines and enhanced in axon‐growth inhibitory glia. Eur J Neurosci 21: 378–390, 2005. doi: 10.1111/j.1460-9568.2005.03876.x. [DOI] [PubMed] [Google Scholar]