Abstract
Ferroptosis is a form of regulated cell death characterized by the accumulation of lipid peroxides in an iron-dependent manner. Ferroptotic cell death is modulated by many metabolic pathways, such as pathways governing the metabolism of sugars, lipids, amino acids, and iron, as well as mitochondrial activity and redox homeostasis. Tumor metastasis and therapy resistance are the main obstacles to curing cancers. Because tumor cells usually exhibit higher iron dependence than normal cells, they may be more susceptible to ferroptosis despite being resistant to other forms of cell death. Moreover, recent evidence has suggested that ferroptosis is involved in tumor-host interactions, modulates the tumor microenvironment, and serves as an antimetastatic mechanism. Thus, inducing ferroptosis in tumor cells has the potential to improve cancer treatment. Here, we review ferroptosis-regulating mechanisms and the roles of ferroptosis in malignant progression, including the tumor-host interactions, metastasis, and cancer therapy response.
Keywords: ferroptosis, metastasis, therapy response, tumor-host interactions
INTRODUCTION
Cell death is classified as accidental cell death (ACD) and regulated cell death (RCD; 1). RCD includes apoptosis, necroptosis, pyroptosis, autophagic cell death, entosis, anoikis, and ferroptosis. Ferroptosis was first reported in 2012 by Stockwell and colleagues (2) as a novel iron-dependent form of RCD with morphological, biochemical, and molecular characteristics distinct from other forms of RCD. Ferroptotic cell death can be induced by small molecules such as erastin and can be inhibited by ferrostatin-1 (2).
The essence of ferroptosis is the reaction between oxidative free radicals and membrane lipid polyunsaturated fatty acids (PUFAs), which generates excessive lipid peroxides leading to membrane damage and cell death (3, 4). Ferroptosis can be inhibited by small-molecule lipophilic antioxidants such as ferrostatin-1 (and its analogs, ferrostatins) and liproxstatin-1, which act through the inhibition of lipid peroxidation (5, 6). Due to higher iron dependence, cancer cells are likely to be more vulnerable to ferroptosis than normal cells (7). Since the discovery of ferroptosis, accumulating evidence has indicated that ferroptosis plays an important role in cancer (7, 8). For instance, loss of leukemia inhibitory factor receptor (LIFR) has been found to promote liver tumorigenesis and confer resistance to ferroptosis-inducing drugs (9). Intriguingly, the tumor microenvironment (TME) contains a large amount of reactive oxidative species (ROS) produced by various types of cells, which on one hand can facilitate malignant progression (10); on the other hand, however, when the capacity of redox-labile iron to generate lipid peroxides through the Fenton reaction exceeds the cell’s capacity to detoxify lipid peroxides, ferroptotic cell death occurs (11), serving as a tumor-suppressive mechanism. In addition to oxidative stress, tumor-infiltrating CD8+ T cells have been shown to trigger ferroptosis in cancer cells through interferon-gamma (IFNγ)-mediated downregulation of the two subunits of the glutamate-cystine antiporter (system xc−), SLC3A2 and SLC7A11 (12), underscoring the importance of tumor-host interactions in regulating tumor cell ferroptosis.
Drug-resistant tumor cells (which are the source of local and metastatic recurrences) and toxicities are common problems in cancer treatment. Several characteristics of cancer cells, such as abnormal lipid metabolism, ROS accumulation, and iron dependence, make them susceptible to ferroptosis, which provides a new strategy for cancer therapy (7, 13). In preclinical studies, the combination of ferroptosis inducers and other agents is effective in treating certain therapy-resistant tumors (9, 14, 15). In this review, we summarize the molecular mechanisms of ferroptosis and discuss its roles in cancer, including tumor-host interactions, metastasis, and therapy response.
FEATURES AND MOLECULAR REGULATION OF FERROPTOSIS
Morphological Features of Ferroptosis
The characteristic morphological change of cells that undergo ferroptosis is mitochondria condensation. Mitochondria are the main organelle for metabolic processes that are implicated in ferroptosis (16). When cells are undergoing ferroptosis, mitochondria shrink, the mitochondria membrane becomes thicker and more compact, the number of cristae is decreased, and the structural integrity of the mitochondria is impaired (2, 16). Unlike apoptotic or necroptotic cells, the nucleus is largely unchanged with no signs of nuclear fragmentation or chromatin condensation (2, 17).
Lipid Peroxidation: The Hallmark of Ferroptosis
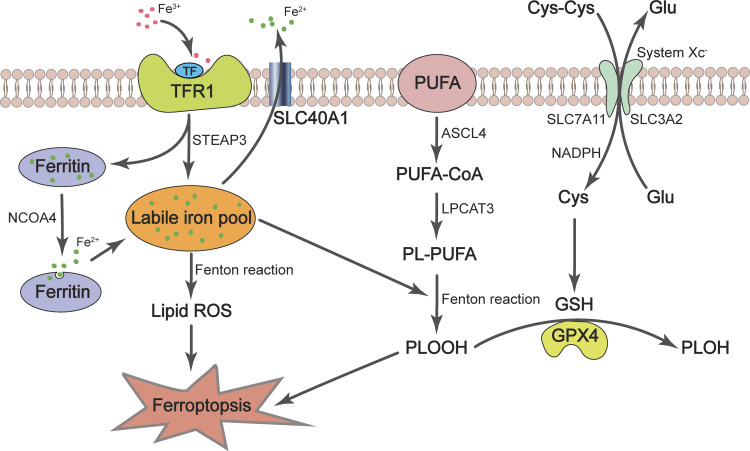
The phospholipids containing PUFA side chains on cell membranes are amenable to reactions with ROS derived from superoxide radicals, leading to lipid peroxidation. Acyl-CoA synthase long-chain family member 4 (ACSL4) is a rate-limiting enzyme, which catalyzes the conversion of long-chain PUFAs to PUFA-CoAs (18). Subsequently, PUFA-CoAs are re-esterified and incorporated into membrane phospholipids by lysophosphatidylcholine acyltransferase 3 (LPCAT3) to form phospholipids containing polyunsaturated fatty acid chain (PUFA-PLs; 18), which then undergo peroxidation to promote ferroptosis (3; Fig. 1). This process is dependent on free ferrous iron (Fe2+) in the cell, which reacts with ROS to generate hydroxyl radicals with strong oxidative power through Fenton reactions (3, 4, 19). When the lipid peroxide production rate exceeds its detoxification rate, lipid peroxides will propagate and destroy nucleic acid, proteins, and lipids, ultimately causing the breakdown of membrane integrity, rupture of the cell membrane, and cell death (4, 11).
Figure 1.
The essence of ferroptosis is iron-dependent lipid peroxidation. Fe3+ circulating in the bloodstream is endocytosed as the Fe3+-TF-TFR1 complex. In the cell, Fe3+ is dissociated from TF and is reduced to Fe2+ by STEAP3, and Fe2+ is stored in ferritin. NCOA4 promotes Fe2+ to be released from ferritin and enters the labile iron pool, which participates in multiple cellular processes and the Fenton reaction. The phospholipids containing PUFA chain are generated from PUFAs by ACSL4 and LPCAT3, which are peroxidized through the Fenton reaction to form toxic lipid hydroperoxides. The process of lipid peroxidation is antagonized by the GSH-GPX4 pathway. System xc− transports cystine into the cell, which is reduced to cysteine, the rate-limiting precursor of GSH. GPX4 uses GSH as a cofactor to convert lipid hydroperoxides to nontoxic lipid alcohols, thereby inhibiting ferroptosis. ACSL4, acyl-CoA synthetase long-chain family member 4; Fe3+, ferric iron; Fe2+, ferrous iron; GPX4, glutathione peroxidase 4; GSH, glutathione; LPCAT3, lysophosphatidylcholine acyltransferase 3; NCOA4, nuclear receptor coactivator 4; PLOOH, phospholipid hydroperoxides; PL-PUFA, phospholipid containing polyunsaturated fatty acid chain; PUFA, polyunsaturated fatty acid; SLC40A1, solute carrier family 40 member 1 (also known as ferroportin); STEAP3, six-transmembrane epithelial antigen of prostate 3; TF, transferrin; TFR1, TF receptor 1.
Regulation of Ferroptosis by the Glutathione-GPX4 Pathway
Glutathione (GSH), the most abundant cellular antioxidant, is made from the amino acids glycine, cysteine, and glutamate (20). The rate-limiting precursor, cysteine, is usually oxidized to form the dimer, cystine, in the extracellular space, due to the oxidative extracellular environment. Cells take up cystine via the cystine-glutamate antiporter (system xc−) consisting of two subunits, SLC7A11 and SLC3A2, followed by reduction of cystine to cysteine and synthesis of GSH in the cytoplasm (21). GSH is a cofactor of glutathione peroxidase 4 (GPX4), which uses GSH to detoxify lipid hydroperoxides to nontoxic lipid alcohols (6; Fig. 1). Erastin, a ferroptosis-inducing small molecule, restricts cystine uptake and GSH synthesis by inhibiting system xc− (2, 22). As a result, the antioxidant capacity of the cells is reduced, and the cells become more sensitive to oxidative stress and vulnerable to ferroptotic cell death (23). In addition to inactivation by erastin, the expression of SLC7A11 can be repressed by tumor suppressors, p53 and BAP1, leading to a reduction of cystine uptake and induction of lipid peroxide accumulation and ferroptosis (24–26). Another ferroptosis inducer, Ras-selective lethal small molecule 3 (RSL3), acts by binding and inactivating GPX4 (21, 27).
Regulation of Ferroptosis by Glutathione-GPX4-Independent Pathways
Besides the GSH-GPX4 pathway, recent studies have revealed additional pathways that protect cells from ferroptosis. One GPX4-independent ferroptosis defense mechanism relies on ferroptosis suppressor protein 1 (FSP1), which is recruited to the plasma membrane to reduce ubiquinone (also known as CoQ) to ubiquinol (also known as CoQH2; 28, 29); CoQH2, in turn, inhibits lipid peroxidation by trapping lipid peroxyl radicals in the cell membrane. CoQH2 also counteracts lipid peroxidation in the mitochondrial membrane. A recent study showed that dihydroorotate dehydrogenase (DHODH) detoxifies mitochondrial lipid peroxides by reducing CoQ to CoQH2 in the inner mitochondrial membrane (30). Notably, in preclinical models, inhibition of both DHODH and GPX4 induced the accumulation of lipid peroxides and ferroptotic cell death, leading to tumor suppression (30).
Metabolites and metabolic modifiers have been found to regulate ferroptosis. Recently, metabolic studies revealed that squalene, a lipophilic metabolite that accumulates in cellular membranes and lipid droplets, protects cancer cells from ferroptosis under oxidative stress (31). From genetic screens, tetrahydrobiopterin (BH4) biosynthesis was identified as an essential metabolic pathway upon the inactivation of GPX4 (27). BH4 protects lipid membranes by acting as a radical-trapping antioxidant. Dihydrofolate reductase (DHFR) regenerates BH4, and its inhibition by methotrexate acts synergistically with GPX4 inhibition to induce ferroptosis (27).
Regulation of Ferroptosis by Intracellular Iron
Iron is required to sustain life and plays vital roles in many physiological processes, but excessive iron causes toxicity. Dysregulated iron homeostasis (either iron deficiency or overload) is a harbinger of pathological conditions. Ferrous iron (Fe2+) from the intestine or red blood cells is oxidized by ceruloplasmin to ferric iron (Fe3+), and Fe3+ circulating in the bloodstream binds transferrin (TF) on the cell membrane to form TF-Fe3+. Cells take up TF-Fe3+ via TF receptor 1 (TFR1)-mediated endocytosis (32). After entering the cells, Fe3+ is released from TF and reduced to Fe2+ through the ferrireductase activity of six-transmembrane epithelial antigen of prostate 3 (STEAP3), and then Fe2+ enters the metabolically active pool of iron, also known as the labile iron pool (LIP; Fig. 1). Fe2+ is used in vital cellular processes such as the synthesis of heme (a precursor of hemoglobin) and biogenesis of iron-sulfur clusters, and extra iron is stored in ferritin, an iron storage protein (33). Fe2+ exits the cell mainly through the activity of ferroportin, an iron-efflux pump that is also known as SLC40A1 (33; Fig. 1); sometimes, Fe2+ can also be released through exosomes.
When iron homeostasis is disrupted, excessive cellular Fe2+ generates ROS and activates iron-containing enzymes (e.g., lipoxygenase) through the iron-dependent Fenton reaction, leading to increased ferroptosis sensitivity. For example, autophagy-induced lysosomal degradation of ferritin, which is mediated by NCOA4, promotes the release of Fe2+ into the LIP and increases the susceptibility to ferroptosis (34, 35). Moreover, depletion of ferroportin inhibits Fe2+ export, elevates intracellular Fe2+ levels, and promotes ferroptosis (36, 37), whereas stimulation of iron export leads to ferroptosis resistance (38). In addition, the mitochondrial protein cysteine desulfurase (NFS1) and the iron-sulfur (Fe-S) proteins anchored in the mitochondrial outer membrane, such as CISD1 and CISD2, inhibit ferroptosis by boosting Fe-S cluster biosynthesis and reducing active iron (39, 40).
FERROPTOSIS AND TUMOR-HOST INTERACTIONS
Tumors contain cancer cells as well as many different types of cells that interact with one another. The term “tumor microenvironment (TME)” refers to a host environment surrounding cancer cells, which consists of the extracellular matrix, blood vessels, signaling molecules, and various types of stromal cells and immune cells. The TME not only modulates carcinogenesis but also regulates tumor progression, metastasis, and therapy resistance (41).
T cells, macrophages, myeloid-derived suppressor cells, dendritic cells, B cells, and natural killer cells are among the immune cells found in the TME (42). On one hand, immune cells in the TME have been reported to trigger ferroptosis in tumor cells. On the other hand, ferroptotic tumor cells can modulate the tumor immune microenvironment. Here, we discuss some recent findings on the relationship between ferroptosis and tumor-host interactions.
Cross Talk between Ferroptotic Tumor Cells and the Tumor Immune Microenvironment
Recent studies have shown that ferroptotic tumor cells can trigger antitumor immune responses, indicating that ferroptosis may be a type of immunogenic cell death (ICD). ICD is characterized by the release of immunogenic damage-associated molecular pattern (DAMP) signals, which leads to robust and long-lasting activation of the adaptive immune system, allowing tumor cells to be eliminated (43). In vitro, fibrosarcoma and glioma cells treated with RSL3 exhibited ICD-like features, including the production of DAMP, ATP, and HMGB1, as well as phagocytosis of ferroptotic tumor cells by bone marrow-derived dendritic cells (BMDCs) and induction of BMDC maturation in coculture experiments (44). The immunogenic property of ferroptotic tumor cells was further demonstrated using a prophylactic tumor vaccination model, in which subcutaneous inoculation of the flank of immunocompetent mice with ferroptotic cancer cells elicited an antitumor immune response to cancer cells injected into the opposite flank 1 wk later (44). It should be noted that only early ferroptotic, but not late ferroptotic cancer cells, exhibited immunogenicity (44), which is different from previous studies in which both early and late apoptotic cancer cells showed immunogenic potential (45). The underlying mechanisms warrant further investigation.
Although early ferroptotic tumor cells can stimulate anticancer immunity, they also have the potential to “educate” immune cells in the TME, such as macrophages, to adopt a tumor-promoting phenotype in certain circumstances. For instance, in K-Ras-driven pancreatic cancer, ferroptotic stimuli cause tumor cells to release 8-hydroxy-2'-deoxyguanosine (8-OHdG), a major product of oxidative DNA damage, which in turn activates the stimulator of interferon genes (STING)-dependent DNA-sensing pathway in tumor-associated macrophages (TAMs), resulting in macrophage infiltration and protumor M2 polarization (46). In addition, autophagy-dependent ferroptosis causes pancreatic tumor cells to secrete oncogenic K-Ras via exosomes, which are taken up by TAMs and stimulate them to switch to an M2 phenotype, boosting pancreatic tumor growth (47). Ferroptotic tumor cells can also secrete immunosuppressants, such as prostaglandin E2 (PGE2), which suppresses the activity of natural killer cells, dendritic cells, and cytotoxic T cells (48, 49). In fact, it has been reported that cells undergoing ferroptosis release substantial amounts of oxidized lipid mediators (49), which could exert immunomodulatory functions. For example, oxidized phosphatidylcholine inhibits the maturation and function of dendritic cells by activating the transcription factor NRF2 (50). Also, oxidized lipids in the TME block antigen cross-presentation by dendritic cells, resulting in impaired antitumor immunity (51, 52).
Ferroptosis Involving Tumor-Infiltrating T Cells
Cytotoxic T cells play a pivotal role in tumor immune surveillance and immunotherapy response (53). The tumor-infiltrating CD8+ lymphocytes kill tumor cells through perforins, granzymes, and Fas ligands. Recently, activated CD8+ T cells were reported to induce lipid peroxidation and ferroptosis in tumor cells (12). Mechanistically, IFNγ secreted by CD8+ T cells activates the JAK-STAT1 pathway to repress system xc− expression in tumor cells, resulting in GSH deficiency and increased sensitivity to ferroptosis (12).
Whereas T-cell-mediated tumor cell ferroptosis contributes to enhanced immunotherapy efficacy, cytotoxic T cells in the TME can also accumulate lipid ROS and undergo ferroptosis, resulting in impaired T-cell effector function. It has been shown that high levels of cholesterol in the TME cause CD8+ T-cell depletion (54). CD36 is a fatty acid translocase and its upregulation in CD8+ T cells promotes fatty acid uptake, lipid peroxidation, and ferroptosis, which dampens antitumor immunity (55). Notably, genetic ablation of CD36 or inhibition of ferroptosis in CD8+ T cells can restore tumor-killing activity and improve the efficacy of immunotherapy (56). Taken together, these findings suggest that ferroptosis is a double-edged sword in the TME.
FERROPTOSIS AS A BARRIER TO METASTATIC PROGRESSION
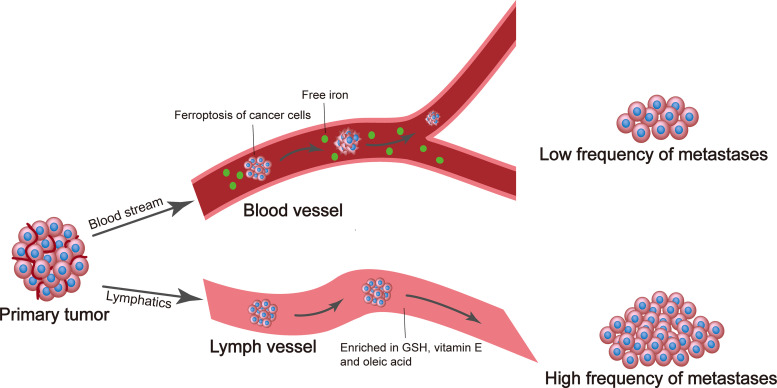
During metastatic dissemination, tumor cells tend to spread to the lymphatic system and then enter the blood circulation (57–59), and yet the advantage of this detour via lymph was not clearly understood. Recently, a study by Morrison and colleagues (60) revealed that in melanoma models, if cancer cells exit the primary tumor site and directly enter the blood circulation, the oxidative stress and high levels of iron present in the blood make the metastasizing cells prone to undergo ferroptotic cell death. In contrast, if cancer cells exit the primary tumor site via the lymphatic system, they are exposed to the lymphatic fluid that contains higher antioxidant levels and lower iron levels than the blood (60; Fig. 2). In addition, these cancer cells take up lipids containing oleic acid, a monounsaturated fatty acid (MUFA), from the lymphatic fluid. When such cancer cells subsequently enter the bloodstream, MUFA protects these cells from ferroptosis, thereby promoting the formation of distant metastases (60).
Figure 2.
The lymphatic system provides an antiferroptotic environment for metastatic tumor cells. The amounts of glutathione (GSH), vitamin E, and oleic acid are greater in lymphatic fluid than in blood. This antioxidant-rich lymphatic milieu reduces oxidative stress and the formation of lipid-reactive oxygen species, protects tumor cells from ferroptosis, and facilitates metastatic dissemination to lymph nodes and distant sites, ultimately leading to more metastases.
Besides a detour, cancer stem cells (CSCs) have recently been reported to protect metastasized breast cancer cells from ferroptosis (61). Mechanistically, breast CSCs secrete DKK1, which on one hand promotes differentiation of breast cancer cells seeded in the lung, which is required for the metastatic outgrowth of disseminated tumor cells; on the other hand, DKK1 upregulates the expression of SLC7A11 to inhibit lipid peroxidation and ferroptosis (61). Notably, combination treatment with a DKK1 inhibitor and a ferroptosis inducer exhibited a synergistic antimetastatic effect in preclinical models of breast cancer (61).
Collectively, these studies provide evidence for ferroptotic cell death serving as a barrier to metastatic progression and demonstrate how metastasizing cells exploit the microenvironment or secreted factors to break this barrier. Future work is warranted to develop safe and effective treatments to resensitize metastatic cancer cells to ferroptosis.
INDUCING FERROPTOSIS TO OVERCOME CANCER THERAPY RESISTANCE AND RELAPSE
Therapy resistance remains a major challenge in cancer treatment and correlates with metastatic relapse. Most drugs used in chemotherapy or targeted therapy have limited efficacy due to intrinsic and/or acquired drug resistance of tumor cells. Most cancer drugs used in the clinic kill tumor cells through controlled cell death, such as autophagy-dependent cell death (62, 63), apoptosis (64), and necroptosis (65). Drug-resistant tumor cells activate survival signaling that blocks one or more cell death pathways. Because cancer cells often exhibit abnormal lipid metabolism, ROS accumulation, and iron dependence, ferroptosis may represent an opportunity to overcome cancer drug resistance. Thus far, ferroptosis has been identified in multiple types of cancers, including breast cancer (66), renal cell carcinoma (67), lung cancer (68), pancreatic cancer (69), diffuse large B-cell lymphoma (70), and hepatocellular carcinoma (HCC; 9). Interestingly, the ferroptosis inducer erastin has been shown to enhance the anticancer activity of various chemotherapeutic drugs, including cytarabine (Ara-C), cisplatin, doxorubicin (Adriamycin), and temozolomide (71).
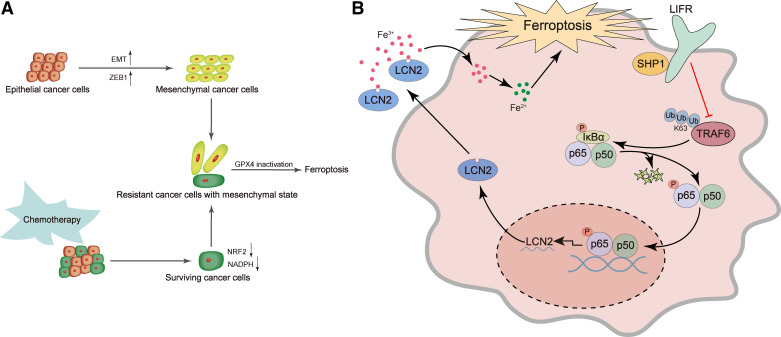
Ferroptosis also has the potential to overcome resistance to small-molecule inhibitor drugs. Accumulating evidence has suggested that cancer cells in the highly mesenchymal state are more likely to be resistant to therapies, such as kinase inhibitors (72, 73). Recently, Schreiber and colleagues (74) analyzed >500 cancer cell lines by correlating their mesenchymal scores to drug sensitivity, and this analysis showed that two groups of drugs, ferroptosis inducers and statins, could selectively kill cancer cells with high mesenchymal scores through inhibition of GPX4. Further studies revealed that ZEB1, an epithelial-mesenchymal transition (EMT)-inducing and metastasis-promoting transcription factor that is highly expressed in mesenchymal-like cancer cells, acts as a lipogenic factor and that high ZEB1 expression confers vulnerability to ferroptosis induced by inactivation of GPX4 (74, 75; Fig. 3A). Interestingly, therapy-resistant mesenchymal-like cancer cell lines, such as epidermal growth factor receptor (EGFR) inhibitor-resistant nonsmall-cell lung cancer cells and BRAF inhibitor-resistant melanoma cells, show higher dependency on GPX4 than nonresistant parental cell lines (74). Similarly, GPX4 is a prerequisite for the survival of drug-tolerant persister cancer cells and tumor relapse (15). Therefore, GPX4 represents a promising therapeutic target (76) for overcoming cancer therapy resistance and recurrence.
Figure 3.
Examples of inducing ferroptosis to overcome cancer therapy resistance. A: activated expression of the transcription factor ZEB1 can induce epithelial cancer cells to undergo EMT and convert to a more mesenchymal, therapy-resistant state. Also, after many rounds of chemotherapy, the surviving cells can acquire mesenchymal characteristics. These mesenchymal cancer cells exhibit downregulation of NRF2 target genes, lower levels of NADPH and GSH, and higher dependence on GPX4, which are prone to ferroptosis induced by inactivation of GPX4. B: in normal hepatocytes, LIFR facilitates SHP1’s ability to inhibit the K63-linked polyubiquitination and activity of TRAF6, thereby inactivating NF-κB. In liver cancer cells, loss of LIFR leads to activation of NF-κB signaling and overproduction of the NF-κB target LCN2, which is a secreted iron-sequestering cytokine. This lowers free Fe2+ levels in liver cancer cells and renders them resistant to ferroptosis-inducing drugs (e.g., sorafenib), which can be targeted by an LCN2-neutralizing antibody. EMT, epithelial-mesenchymal transition; Fe3+, ferric iron; Fe2+, ferrous iron; GPX4, glutathione peroxidase 4; GSH, glutathione; LCN2, lipocalin 2; LIFR, leukemia inhibitory factor receptor; TRAF6, tumor necrosis factor receptor-associated factor 6.
The current front-line standard of care for hepatocellular carcinoma (HCC), atezolizumab (anti-PD-L1) plus bevacizumab (anti-VEGF; 77, 78), was the first systemic therapy demonstrating an overall survival benefit over sorafenib in unresectable HCC (79). However, only 25% of HCC patients respond, and no predictive biomarkers exist (77). Furthermore, many patients cannot tolerate, or progress on the atezolizumab plus bevacizumab combination, and thus require the use of kinase inhibitors such as sorafenib. Sorafenib prolongs patient with HCC survival by up to 3 mo (80, 81). As a weak inducer of apoptosis (82), sorafenib has been reported by many groups to induce ferroptosis (83–85), but one study showed that sorafenib failed to trigger ferroptosis in multiple cancer cell lines (86). Recently, we found that genetic deletion of leukemia inhibitory factor receptor (LIFR), which is commonly downregulated in HCC, promoted liver tumorigenesis and conferred resistance to sorafenib-induced ferroptosis in mice through NF-κB-mediated upregulation of the iron-sequestering cytokine lipocalin 2 (LCN2; 9; Fig. 3B). Importantly, in HCC patient-derived xenograft (PDX) tumors expressing low levels of LIFR and high levels of LCN2, treatment with the LCN2-neutralizing antibody significantly enhanced the ferroptosis-inducing and tumor-killing effect of sorafenib (which had a modest effect when treated alone); in contrast, in PDX tumors expressing high levels of LIFR and low levels of LCN2, treatment with sorafenib alone was effective (9). Similarly, LCN2 has also been shown to promote chemoresistance through inhibition of ferroptosis in colorectal cancer, and neutralization of LCN2 with a monoclonal antibody reversed chemoresistance in preclinical models (87). Taken together, these findings suggest that combination treatment with anti-LCN2 and kinase inhibitors or chemotherapeutic agents may improve cancer therapy through ferroptosis.
CONCLUDING REMARKS
Since the Stockwell Lab defined the concept of ferroptosis in 2012 (2), the field has evolved rapidly, and the knowledge of the regulators and regulations of ferroptotic cell death has been growing. Ferroptosis is implicated in various pathological conditions, such as ischemic organ injuries, cancer, and neurodegeneration (4). However, the existence of a physiological stimulus for ferroptosis, as well as whether ferroptosis is involved in physiological processes such as organ formation, cellular homeostasis, and immunity, are largely unknown.
In the cancer field, many results about the role of ferroptosis in tumor cell biology and therapy response are based on cell-line models, which may or may not recapitulate autochthonous tumors and clinical cancers, whose behavior, aggressiveness, metastatic ability, and sensitivity to ferroptosis-inducing agents and other drugs are influenced by the tumor-host interactions. For instance, pancreatic ductal adenocarcinoma (PDAC)-derived cancer-associated fibroblasts (CAFs) are dependent on SLC7A11 to take up cystine, synthesize GSH, resist oxidative stress, and remodel collagen to support PDAC growth (88). Consequently, specific ablation of SLC7A11 in PDAC cells did not affect tumor growth, whereas depletion of SLC7A11 in both PDAC cells and CAFs reduced tumor growth and metastasis (88). Moreover, the unique composition of lymph (higher antioxidant levels and lower iron levels compared with blood) can protect melanoma cells from ferroptotic cell death, thereby increasing the efficiency of distant metastasis formation (60; Fig. 2). It would be of high interest to know whether lymph exerts long-term protective effects by inducing durable epigenetic changes or metabolic reprogramming in metastasizing tumor cells.
In fact, many types of tumor-host interactions and cross talks occur at the primary tumor site, in the lymph, in the blood circulation, and at distant anatomic sites, which could substantially influence metastatic behavior and therapy response of tumor cells. Therefore, when discovering or developing strategies for inducing ferroptosis to improve cancer therapy, we recommend the use of cancer models that recapitulate the natural TME and tumor-host interactions.
GRANTS
F.Y. is supported by the National Natural Science Foundation of China (Grant No.: 31900935). L.M. is supported by a US National Institutes of Health (NIH) Grant R01CA166051 and a Cancer Prevention and Research Institute of Texas (CPRIT) Grant RP190029.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.Y. and Z.G. prepared figures; Y.Y. and Y. Shi drafted manuscript; Y. Shi, Y. Sun, F.Y., and L.M. edited and revised manuscript; Y.Y., Y. Shi, Z.G., Y. Sun, F.Y., and L.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to members of the Ma Lab and the Yao Lab for the discussion.
This article is part of the special collection “Tumor Host Interactions in Metastasis.” Mythreye Karthikeyan, PhD, and Nadine Hempel, PhD, served as Guest Editors of this collection.
REFERENCES
- 1.Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry WS, Fulda S, Gottlieb E, Green DR, Hengartner MO, Kepp O, Knight RA, Kumar S, Lipton SA, Lu X, Madeo F, Malorni W, Mehlen P, Nunez G, Peter ME, Piacentini M, Rubinsztein DC, Shi Y, Simon HU, Vandenabeele P, White E, Yuan J, Zhivotovsky B, Melino G, Kroemer G. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ 19: 107–120, 2012. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149: 1060–1072, 2012. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conrad M, Pratt DA. The chemical basis of ferroptosis. Nat Chem Biol 15: 1137–1147, 2019. [Erratum in Nat Chem Biol 16: 223–224, 2020]. doi: 10.1038/s41589-019-0408-1. [DOI] [PubMed] [Google Scholar]
- 4.Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol 22: 266–282, 2021. doi: 10.1038/s41580-020-00324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skouta R, Dixon SJ, Wang J, Dunn DE, Orman M, Shimada K, Rosenberg PA, Lo DC, Weinberg JM, Linkermann A, Stockwell BR. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J Am Chem Soc 136: 4551–4556, 2014. doi: 10.1021/ja411006a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch A, Eggenhofer E, Basavarajappa D, Rådmark O, Kobayashi S, Seibt T, Beck H, Neff F, Esposito I, Wanke R, Förster H, Yefremova O, Heinrichmeyer M, Bornkamm GW, Geissler EK, Thomas SB, Stockwell BR, O'Donnell VB, Kagan VE, Schick JA, Conrad M. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol 16: 1180–1191, 2014. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hassannia B, Vandenabeele P, Vanden Berghe T. Targeting ferroptosis to iron out cancer. Cancer Cell 35: 830–849, 2019. doi: 10.1016/j.ccell.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascon S, Hatzios SK, Kagan VE, Noel K, Jiang X, Linkermann A, Murphy ME, Overholtzer M, Oyagi A, Pagnussat GC, Park J, Ran Q, Rosenfeld CS, Salnikow K, Tang D, Torti FM, Torti SV, Toyokuni S, Woerpel KA, Zhang DD. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171: 273–285, 2017. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao F, Deng Y, Zhao Y, Mei Y, Zhang Y, Liu X, Martinez C, Su X, Rosato RR, Teng H, Hang Q, Yap S, Chen D, Wang Y, Chen MM, Zhang M, Liang H, Xie D, Chen X, Zhu H, Chang JC, You MJ, Sun Y, Gan B, Ma L. A targetable LIFR-NF-κB-LCN2 axis controls liver tumorigenesis and vulnerability to ferroptosis. Nat Commun 12: 7333, 2021. doi: 10.1038/s41467-021-27452-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liao Z, Chua D, Tan NS. Reactive oxygen species: a volatile driver of field cancerization and metastasis. Mol Cancer 18: 65, 2019. doi: 10.1186/s12943-019-0961-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li D, Li Y. The interaction between ferroptosis and lipid metabolism in cancer. Signal Transduct Target Ther 5: 108, 2020. doi: 10.1038/s41392-020-00216-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W, Green M, Choi JE, Gijón M, Kennedy PD, Johnson JK, Liao P, Lang X, Kryczek I, Sell A, Xia H, Zhou J, Li G, Li J, Li W, Wei S, Vatan L, Zhang H, Szeliga W, Gu W, Liu R, Lawrence TS, Lamb C, Tanno Y, Cieslik M, Stone E, Georgiou G, Chan TA, Chinnaiyan A, Zou W. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 569: 270–274, 2019. doi: 10.1038/s41586-019-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zou Y, Henry WS, Ricq EL, Graham ET, Phadnis VV, Maretich P, Paradkar S, Boehnke N, Deik AA, Reinhardt F, Eaton JK, Ferguson B, Wang W, Fairman J, Keys HR, Dančík V, Clish CB, Clemons PA, Hammond PT, Boyer LA, Weinberg RA, Schreiber SL. Plasticity of ether lipids promotes ferroptosis susceptibility and evasion. Nature 585: 603–608, 2020. doi: 10.1038/s41586-020-2732-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H, Deng T, Liu R, Ning T, Yang H, Liu D, Zhang Q, Lin D, Ge S, Bai M, Wang X, Zhang L, Li H, Yang Y, Ji Z, Wang H, Ying G, Ba Y. CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Mol Cancer 19: 43, 2020. doi: 10.1186/s12943-020-01168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hangauer MJ, Viswanathan VS, Ryan MJ, Bole D, Eaton JK, Matov A, Galeas J, Dhruv HD, Berens ME, Schreiber SL, McCormick F, McManus MT. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature 551: 247–250, 2017. doi: 10.1038/nature24297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gan B. Mitochondrial regulation of ferroptosis. J Cell Biol 220: e202105043, 2021. doi: 10.1083/jcb.202105043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yagoda N, von Rechenberg M, Zaganjor E, Bauer AJ, Yang WS, Fridman DJ, Wolpaw AJ, Smukste I, Peltier JM, Boniface JJ, Smith R, Lessnick SL, Sahasrabudhe S, Stockwell BR. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature 447: 864–868, 2007. doi: 10.1038/nature05859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS, Dar HH, Liu B, Tyurin VA, Ritov VB, Kapralov AA, Amoscato AA, Jiang J, Anthonymuthu T, Mohammadyani D, Yang Q, Proneth B, Klein-Seetharaman J, Watkins S, Bahar I, Greenberger J, Mallampalli RK, Stockwell BR, Tyurina YY, Conrad M, Bayir H. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol 13: 81–90, 2017. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fenton HJH. LXXIII.—Oxidation of tartaric acid in presence of iron. J Chem Soc Trans 65: 899–910, 1894. doi: 10.1039/CT8946500899. [DOI] [Google Scholar]
- 20.Winterbourn CC, Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic Biol Med 45: 549–561, 2008. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci USA 113: E4966–E4975, 2016. doi: 10.1073/pnas.1603244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, Brown LM, Girotti AW, Cornish VW, Schreiber SL, Stockwell BR. Regulation of ferroptotic cancer cell death by GPX4. Cell 156: 317–331, 2014. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao JY, Dixon SJ. Mechanisms of ferroptosis. Cell Mol Life Sci 73: 2195–2209, 2016. doi: 10.1007/s00018-016-2194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang L, Hickman JH, Wang SJ, Gu W. Dynamic roles of p53-mediated metabolic activities in ROS-induced stress responses. Cell Cycle 14: 2881–2885, 2015. doi: 10.1080/15384101.2015.1068479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H, Baer R, Gu W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 520: 57–62, 2015. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Shi J, Liu X, Feng L, Gong Z, Koppula P, Sirohi K, Li X, Wei Y, Lee H, Zhuang L, Chen G, Xiao ZD, Hung MC, Chen J, Huang P, Li W, Gan B. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat Cell Biol 20: 1181–1192, 2018. doi: 10.1038/s41556-018-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soula M, Weber RA, Zilka O, Alwaseem H, La K, Yen F, Molina H, Garcia-Bermudez J, Pratt DA, Birsoy K. Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nat Chem Biol 16: 1351–1360, 2020. doi: 10.1038/s41589-020-0613-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, Bassik MC, Nomura DK, Dixon SJ, Olzmann JA. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575: 688–692, 2019. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I, Goya Grocin A, Xavier da Silva TN, Panzilius E, Scheel CH, Mourão A, Buday K, Sato M, Wanninger J, Vignane T, Mohana V, Rehberg M, Flatley A, Schepers A, Kurz A, White D, Sauer M, Sattler M, Tate EW, Schmitz W, Schulze A, O'Donnell V, Proneth B, Popowicz GM, Pratt DA, Angeli JPF, Conrad M. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 575: 693–698, 2019. doi: 10.1038/s41586-019-1707-0. [DOI] [PubMed] [Google Scholar]
- 30.Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee H, Koppula P, Wu S, Zhuang L, Fang B, Poyurovsky MV, Olszewski K, Gan B. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature 593: 586–590, 2021. [Erratum in Nature 596: E13, 2021] doi: 10.1038/s41586-021-03539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Bermudez J, Baudrier L, Bayraktar EC, Shen Y, La K, Guarecuco R, Yucel B, Fiore D, Tavora B, Freinkman E, Chan SH, Lewis C, Min W, Inghirami G, Sabatini DM, Birsoy K. Squalene accumulation in cholesterol auxotrophic lymphomas prevents oxidative cell death. Nature 567: 118–122, 2019. doi: 10.1038/s41586-019-0945-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Domenico I, McVey Ward D, Kaplan J. Regulation of iron acquisition and storage: consequences for iron-linked disorders. Nat Rev Mol Cell Biol 9: 72–81, 2008. doi: 10.1038/nrm2295. [DOI] [PubMed] [Google Scholar]
- 33.Andrews NC, Schmidt PJ. Iron homeostasis. Annu Rev Physiol 69: 69–85, 2007. doi: 10.1146/annurev.physiol.69.031905.164337. [DOI] [PubMed] [Google Scholar]
- 34.Mancias JD, Wang X, Gygi SP, Harper JW, Kimmelman AC. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature 509: 105–109, 2014. doi: 10.1038/nature13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao M, Monian P, Pan Q, Zhang W, Xiang J, Jiang X. Ferroptosis is an autophagic cell death process. Cell Res 26: 1021–1032, 2016. doi: 10.1038/cr.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, Andrews NC. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab 1: 191–200, 2005. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Geng N, Shi BJ, Li SL, Zhong ZY, Li YC, Xua WL, Zhou H, Cai JH. Knockdown of ferroportin accelerates erastin-induced ferroptosis in neuroblastoma cells. Eur Rev Med Pharmacol Sci 22: 3826–3836, 2018. doi: 10.26355/eurrev_201806_15267. [DOI] [PubMed] [Google Scholar]
- 38.Brown CW, Amante JJ, Chhoy P, Elaimy AL, Liu H, Zhu LJ, Baer CE, Dixon SJ, Mercurio AM. Prominin2 drives ferroptosis resistance by stimulating iron export. Dev Cell 51: 575–586.e4, 2019. doi: 10.1016/j.devcel.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan H, Li X, Zhang X, Kang R, Tang D. CISD1 inhibits ferroptosis by protection against mitochondrial lipid peroxidation. Biochem Biophys Res Commun 478: 838–844, 2016. doi: 10.1016/j.bbrc.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 40.Kim EH, Shin D, Lee J, Jung AR, Roh JL. CISD2 inhibition overcomes resistance to sulfasalazine-induced ferroptotic cell death in head and neck cancer. Cancer Lett 432: 180–190, 2018. doi: 10.1016/j.canlet.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 41.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 144: 646–674, 2011. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 42.Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI, Ostrand-Rosenberg S, Hedrick CC, Vonderheide RH, Pittet MJ, Jain RK, Zou W, Howcroft TK, Woodhouse EC, Weinberg RA, Krummel MF. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med 24: 541–550, 2018. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol 31: 51–72, 2013. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 44.Efimova I, Catanzaro E, Van der Meeren L, Turubanova VD, Hammad H, Mishchenko TA, Vedunova MV, Fimognari C, Bachert C, Coppieters F, Lefever S, Skirtach AG, Krysko O, Krysko DV. Vaccination with early ferroptotic cancer cells induces efficient antitumor immunity. J Immunother Cancer 8: e001369, 2020. doi: 10.1136/jitc-2020-001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou J, Wang G, Chen Y, Wang H, Hua Y, Cai Z. Immunogenic cell death in cancer therapy: Present and emerging inducers. J Cell Mol Med 23: 4854–4865, 2019. doi: 10.1111/jcmm.14356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dai E, Han L, Liu J, Xie Y, Zeh HJ, Kang R, Bai L, Tang D. Ferroptotic damage promotes pancreatic tumorigenesis through a TMEM173/STING-dependent DNA sensor pathway. Nat Commun 11: 6339, 2020. doi: 10.1038/s41467-020-20154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dai E, Han L, Liu J, Xie Y, Kroemer G, Klionsky DJ, Zeh HJ, Kang R, Wang J, Tang D. Autophagy-dependent ferroptosis drives tumor-associated macrophage polarization via release and uptake of oncogenic KRAS protein. Autophagy 16: 2069–2083, 2020. doi: 10.1080/15548627.2020.1714209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnson AM, Kleczko EK, Nemenoff RA. Eicosanoids in cancer: new roles in immunoregulation. Front Pharmacol 11: 595498, 2020. doi: 10.3389/fphar.2020.595498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Friedmann Angeli JP, Krysko DV, Conrad M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat Rev Cancer 19: 405–414, 2019. doi: 10.1038/s41568-019-0149-1. [DOI] [PubMed] [Google Scholar]
- 50.Rothe T, Gruber F, Uderhardt S, Ipseiz N, Rössner S, Oskolkova O, Blüml S, Leitinger N, Bicker W, Bochkov VN, Yamamoto M, Steinkasserer A, Schett G, Zinser E, Krönke G. 12/15-Lipoxygenase-mediated enzymatic lipid oxidation regulates DC maturation and function. J Clin Invest 125: 1944–1954, 2015. doi: 10.1172/JCI78490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Veglia F, Tyurin VA, Mohammadyani D, Blasi M, Duperret EK, Donthireddy L, Hashimoto A, Kapralov A, Amoscato A, Angelini R, Patel S, Alicea-Torres K, Weiner D, Murphy ME, Klein-Seetharaman J, Celis E, Kagan VE, Gabrilovich DI. Lipid bodies containing oxidatively truncated lipids block antigen cross-presentation by dendritic cells in cancer. Nat Commun 8: 2122, 2017. doi: 10.1038/s41467-017-02186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramakrishnan R, Tyurin VA, Veglia F, Condamine T, Amoscato A, Mohammadyani D, Johnson JJ, Zhang LM, Klein-Seetharaman J, Celis E, Kagan VE, Gabrilovich DI. Oxidized lipids block antigen cross-presentation by dendritic cells in cancer. J Immunol 192: 2920–2931, 2014. [Erratum in J Immunol 192: 4935, 2014]. doi: 10.4049/jimmunol.1302801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharma P, Siddiqui BA, Anandhan S, Yadav SS, Subudhi SK, Gao J, Goswami S, Allison JP. The next decade of immune checkpoint therapy. Cancer Discov 11: 838–857, 2021. doi: 10.1158/2159-8290.CD-20-1680. [DOI] [PubMed] [Google Scholar]
- 54.Ma X, Bi E, Lu Y, Su P, Huang C, Liu L, Wang Q, Yang M, Kalady MF, Qian J, Zhang A, Gupte AA, Hamilton DJ, Zheng C, Yi Q. Cholesterol Induces CD8+ T cell exhaustion in the tumor microenvironment. Cell Metab 30: 143–156.e5, 2019.doi: 10.1016/j.cmet.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma X, Xiao L, Liu L, Ye L, Su P, Bi E, Wang Q, Yang M, Qian J, Yi Q. CD36-mediated ferroptosis dampens intratumoral CD8+ T cell effector function and impairs their antitumor ability. Cell Metab 33: 1001–1012.e5, 2021. doi: 10.1016/j.cmet.2021.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu S, Chaudhary O, Rodríguez-Morales P, Sun X, Chen D, Zappasodi R, Xu Z, Pinto AFM, Williams A, Schulze I, Farsakoglu Y, Varanasi SK, Low JS, Tang W, Wang H, McDonald B, Tripple V, Downes M, Evans RM, Abumrad NA, Merghoub T, Wolchok JD, Shokhirev MN, Ho PC, Witztum JL, Emu B, Cui G, Kaech SM. Uptake of oxidized lipids by the scavenger receptor CD36 promotes lipid peroxidation and dysfunction in CD8+ T cells in tumors. Immunity 54: 1561–1577.e7, 2021. doi: 10.1016/j.immuni.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sleeman J, Schmid A, Thiele W. Tumor lymphatics. Semin Cancer Biol 19: 285–297, 2009. doi: 10.1016/j.semcancer.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 58.Alitalo A, Detmar M. Interaction of tumor cells and lymphatic vessels in cancer progression. Oncogene 31: 4499–4508, 2012. doi: 10.1038/onc.2011.602. [DOI] [PubMed] [Google Scholar]
- 59.Leong SP, Gershenwald JE, Soong SJ, Schadendorf D, Tarhini AA, Agarwala S, Hauschild A, Soon CW, Daud A, Kashani-Sabet M. Cutaneous melanoma: a model to study cancer metastasis. J Surg Oncol 103: 538–549, 2011. doi: 10.1002/jso.21816. [DOI] [PubMed] [Google Scholar]
- 60.Ubellacker JM, Tasdogan A, Ramesh V, Shen B, Mitchell EC, Martin-Sandoval MS, Gu Z, McCormick ML, Durham AB, Spitz DR, Zhao Z, Mathews TP, Morrison SJ. Lymph protects metastasizing melanoma cells from ferroptosis. Nature 585: 113–118, 2020. doi: 10.1038/s41586-020-2623-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu M, Zhang X, Zhang W, Chiou YS, Qian W, Liu X, Zhang M, Yan H, Li S, Li T, Han X, Qian P, Liu S, Pan Y, Lobie PE, Zhu T. Cancer stem cell regulated phenotypic plasticity protects metastasized cancer cells from ferroptosis. Nat Commun 13: 1371, 2022. doi: 10.1038/s41467-022-29018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.White E, Mehnert JM, Chan CS. Autophagy, metabolism, and cancer. Clin Cancer Res 21: 5037–5046, 2015. doi: 10.1158/1078-0432.CCR-15-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang T, Song X, Yang Y, Wan X, Alvarez AA, Sastry N, Feng H, Hu B, Cheng SY. Autophagy and Hallmarks of cancer. Crit Rev Oncog 23: 247–267, 2018. doi: 10.1615/CritRevOncog.2018027913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wong RS. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res 30: 87, 2011. doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Najafov A, Chen H, Yuan J. Necroptosis and cancer. Trends Cancer 3: 294–301, 2017. doi: 10.1016/j.trecan.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang HL, Hu BX, Li ZL, Du T, Shan JL, Ye ZP, Peng XD, Li X, Huang Y, Zhu XY, Chen YH, Feng GK, Yang D, Deng R, Zhu XF. PKCβII phosphorylates ACSL4 to amplify lipid peroxidation to induce ferroptosis. Nat Cell Biol 24: 88–98, 2022. doi: 10.1038/s41556-021-00818-3. [DOI] [PubMed] [Google Scholar]
- 67.Tan SK, Mahmud I, Fontanesi F, Puchowicz M, Neumann CKA, Griswold AJ, Patel R, Dispagna M, Ahmed HH, Gonzalgo ML, Brown JM, Garrett TJ, Welford SM. Obesity-dependent adipokine chemerin suppresses fatty acid oxidation to confer ferroptosis resistance. Cancer Discov 11: 2072–2093, 2021. doi: 10.1158/2159-8290.CD-20-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alvarez SW, Sviderskiy VO, Terzi EM, Papagiannakopoulos T, Moreira AL, Adams S, Sabatini DM, Birsoy K, Possemato R. NFS1 undergoes positive selection in lung tumours and protects cells from ferroptosis. Nature 551: 639–643, 2017. doi: 10.1038/nature24637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Badgley MA, Kremer DM, Maurer HC, DelGiorno KE, Lee HJ, Purohit V, Sagalovskiy IR, Ma A, Kapilian J, Firl CEM, Decker AR, Sastra SA, Palermo CF, Andrade LR, Sajjakulnukit P, Zhang L, Tolstyka ZP, Hirschhorn T, Lamb C, Liu T, Gu W, Seeley ES, Stone E, Georgiou G, Manor U, Iuga A, Wahl GM, Stockwell BR, Lyssiotis CA, Olive KP. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science 368: 85–89, 2020. doi: 10.1126/science.aaw9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schmitt A, Xu W, Bucher P, Grimm M, Konantz M, Horn H, Zapukhlyak M, Berning P, Brändle M, Jarboui MA, Schönfeld C, Boldt K, Rosenwald A, Ott G, Grau M, Klener P, Vockova P, Lengerke C, Lenz G, Schulze-Osthoff K, Hailfinger S. Dimethyl fumarate induces ferroptosis and impairs NF-κB/STAT3 signaling in DLBCL. Blood 138: 871–884, 2021. doi: 10.1182/blood.2020009404. [DOI] [PubMed] [Google Scholar]
- 71.Mou Y, Wang J, Wu J, He D, Zhang C, Duan C, Li B. Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J Hematol Oncol 12: 34, 2019. doi: 10.1186/s13045-019-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger S, Cosper AK, Akhavanfard S, Heist RS, Temel J, Christensen JG, Wain JC, Lynch TJ, Vernovsky K, Mark EJ, Lanuti M, Iafrate AJ, Mino-Kenudson M, Engelman JA. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 3: 75ra26, 2011. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Uramoto H, Shimokawa H, Hanagiri T, Kuwano M, Ono M. Expression of selected gene for acquired drug resistance to EGFR-TKI in lung adenocarcinoma. Lung Cancer 73: 361–365, 2011. doi: 10.1016/j.lungcan.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 74.Viswanathan VS, Ryan MJ, Dhruv HD, Gill S, Eichhoff OM, Seashore-Ludlow B, et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature 547: 453–457, 2017. doi: 10.1038/nature23007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gubelmann C, Schwalie PC, Raghav SK, Röder E, Delessa T, Kiehlmann E, Waszak SM, Corsinotti A, Udin G, Holcombe W, Rudofsky G, Trono D, Wolfrum C, Deplancke B. Identification of the transcription factor ZEB1 as a central component of the adipogenic gene regulatory network. eLife 3: e03346, 2014. doi: 10.7554/eLife.03346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eaton JK, Furst L, Ruberto RA, Moosmayer D, Hilpmann A, Ryan MJ, Zimmermann K, Cai LL, Niehues M, Badock V, Kramm A, Chen S, Hillig RC, Clemons PA, Gradl S, Montagnon C, Lazarski KE, Christian S, Bajrami B, Neuhaus R, Eheim AL, Viswanathan VS, Schreiber SL. Selective covalent targeting of GPX4 using masked nitrile-oxide electrophiles. Nat Chem Biol 16: 497–506, 2020. doi: 10.1038/s41589-020-0501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers 7: 6, 2021. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 78.Sangro B, Sarobe P, Hervás-Stubbs S, Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 18: 525–543, 2021. doi: 10.1038/s41575-021-00438-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 382: 1894–1905, 2020. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 80.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359: 378–390, 2008. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 81.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10: 25–34, 2009. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 82.Galmiche A, Chauffert B, Barbare JC. New biological perspectives for the improvement of the efficacy of sorafenib in hepatocellular carcinoma. Cancer Lett 346: 159–162, 2014. doi: 10.1016/j.canlet.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 83.Louandre C, Ezzoukhry Z, Godin C, Barbare JC, Mazière JC, Chauffert B, Galmiche A. Iron-dependent cell death of hepatocellular carcinoma cells exposed to sorafenib. Int J Cancer 133: 1732–1742, 2013. doi: 10.1002/ijc.28159. [DOI] [PubMed] [Google Scholar]
- 84.Dixon SJ, Patel DN, Welsch M, Skouta R, Lee ED, Hayano M, Thomas AG, Gleason CE, Tatonetti NP, Slusher BS, Stockwell BR. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife 3: e02523, 2014. doi: 10.7554/eLife.02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lachaier E, Louandre C, Godin C, Saidak Z, Baert M, Diouf M, Chauffert B, Galmiche A. Sorafenib induces ferroptosis in human cancer cell lines originating from different solid tumors. Anticancer Res 34: 6417–6422, 2014. [PubMed] [Google Scholar]
- 86.Zheng J, Sato M, Mishima E, Sato H, Proneth B, Conrad M. Sorafenib fails to trigger ferroptosis across a wide range of cancer cell lines. Cell Death Dis 12: 698, 2021. doi: 10.1038/s41419-021-03998-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chaudhary N, Choudhary BS, Shah SG, Khapare N, Dwivedi N, Gaikwad A, Joshi N, Raichanna J, Basu S, Gurjar M, P KS, Saklani A, Gera P, Ramadwar M, Patil P, Thorat R, Gota V, Dhar SK, Gupta S, Das M, Dalal SN. Lipocalin 2 expression promotes tumor progression and therapy resistance by inhibiting ferroptosis in colorectal cancer. Int J Cancer 149: 1495–1511, 2021. doi: 10.1002/ijc.33711. [DOI] [PubMed] [Google Scholar]
- 88.Sharbeen G, McCarroll JA, Akerman A, Kopecky C, Youkhana J, Kokkinos J, et al. Cancer-associated fibroblasts in pancreatic ductal adenocarcinoma determine response to SLC7A11 inhibition. Cancer Res 81: 3461–3479, 2021. doi: 10.1158/0008-5472.CAN-20-2496. [DOI] [PubMed] [Google Scholar]