Abstract
Inward-rectifier potassium channel 7.1 (Kir7.1) is present in the polarized epithelium, including the retinal pigmented epithelium. A single amino acid change at position 153 in the KCNJ13 gene, a substitution of threonine to isoleucine in the Kir7.1 protein, causes blindness. We hypothesized that the disease caused by this single amino acid substitution within the transmembrane protein domain could alter the translation, localization, or ion transport properties. We assessed the effects of amino acid side-chain length, arrangement, and polarity on channel structure and function. We showed that the T153I mutation yielded a full-length protein localized to the cell membrane. Whole cell patch-clamp recordings and chord conductance analyses revealed that the T153I mutant channel had negligible K+ conductance and failed to hyperpolarize the membrane potential. However, the mutant channel exhibited enhanced inward current when rubidium was used as a charge carrier, suggesting that an inner pore had formed and the channel was dysfunctional. Substituting with a polar, nonpolar, or short side-chain amino acid did not affect the localization of the protein. Still, it had an altered channel function due to differences in pore radius. Polar side chains (cysteine and serine) with inner pore radii comparable to wildtype exhibited normal inward K+ conductance. Short side chains (glycine and alanine) produced a channel with wider than expected inner pore size and lacked the biophysical characteristics of the wild-type channel. Leucine substitution produced results similar to the T153I mutant channel. This study provides direct electrophysiological evidence for the structure and function of the Kir7.1 channel’s narrow inner pore in regulating conductance.
Keywords: electrophysiology, innerpore structure, Kir7.1, pediatric blindness, potassium channels
INTRODUCTION
Inward rectifier potassium channel 7.1 (Kir7.1), a weak inward rectifier, is expressed in the brain, kidney, uterus, and eye. In the eye, it is expressed in the retinal pigmented epithelium (RPE) and exhibits a polarized membrane distribution (1). In the RPE cells, it is present in the apical processes and is responsible for maintaining membrane potential and potassium (K+) concentration in the subretinal space (2, 3). Kir7.1 is a vital component of the visual cycle as it supports the photoreceptor function within the retina (2). The channel is regulated by phosphatidylinositol 4,5-bisphosphate (PIP2) hydrolysis within the cell membrane milieu (4, 5) and protein kinase C in the cytoplasmic domain (6). Kir7.1 is a homotetramer with two transmembrane domains, two cytoplasmic domains, and an extracellular selectivity loop in each subunit (7). The extracellular loop is the primary regulator of ion permeability (8), with a characteristic narrow inner pore. Kir7.1 channel’s inner pore is composed of the second transmembrane domain of each subunit and narrows at the interface between the transmembrane and cytoplasmic domains (9, 10), which is conserved across species. Its inward conductance is inversely dependent on extracellular K+ concentration (8) and has a unique characteristic of high rubidium (Rb+) conductance relative to K+ conductance (8).
Leber’s congenital amaurosis (LCA16), a rare form of pediatric blindness, is caused by mutations in the KCNJ13 gene that are either nonsense or missense (11) and is characterized by photophobia, nystagmus, pigmentation, and retinal detachment from a very early age (12). A missense mutation in this gene at amino acid position 153 where a hydrophilic threonine (T) is changed to hydrophobic isoleucine (I) leads to LCA16. This mutation carries the LCA16 phenotype in humans and zebrafish (11). A homology search across Kir channels indicated that a hydrophilic amino acid is required for normal weak inwardly rectifying function. In contrast, a hydrophobic amino acid at the homologous position is necessary for normal function in strong inward rectifiers such as Kir2.1 (13).
The crystal structure of Kir7.1 is yet to be resolved, and current knowledge relies on homology modeling based on the structure of Kir2.2 (14). The disease-causing T153I mutation provides a unique opportunity to elucidate the structural and functional implications of changes in Kir7.1’s inner pore region as an experiment of nature. T153I is acutely positioned in the inner pore region at the second transmembrane and cytoplasmic domain interface, where regulation is unknown (1). Hydrophobicity of 153 amino acid position might be critical for interactions between the channel subunits or protein-lipid interactions, causing the channel to be more tightly integrated into the cell membrane (8), constricting the inner pore region. The constriction of the inner pore could be one of the reasons for the reduced K+ conductance. We substituted the 153 amino acid with either polar, nonpolar, or short side-chain amino acid to address these questions and elucidate the inner pore structure. We hypothesized that the polar amino acid at the transmembrane-cytoplasmic interface is critical for the stability of the protein-protein interactions, the integrity of the inner pore region, and the normal inwardly rectifying function of the Kir7.1 channel.
MATERIALS AND METHODS
Site-Directed Mutagenesis
Wild-type Kir7.1 plasmid fused with green fluorescent protein (GFP) at the N-terminus and driven by human elongation factor-1alpha promoter was used as a template to create a mutant variant T153I (ACA changed to ATA) by replacing a single base. PCR-based Agilent Quick-Change XLII (Agilent, Santa Clara, CA) site-directed mutagenesis kit was used to substitute the base following the manufacturer’s protocol. Five more mutant variants were created independently using the newly created T153I plasmid, similarly to the desired amino acid substitution in the 153 position. The changes are as follows: T153L ATA to TTA, T153C ATA to TGC, T153A ATA to GCA, T153G ATA to GGA, and lastly, T153S ATA to TCA to assess the effects of polar, nonpolar, and short side chains. Oligonucleotides were designed using the tool from Agilent’s website, and their sequences are available in Table 1. Sanger sequencing was performed on all newly created plasmids to verify the desired substitution without identifying any off-target amino acid mutations. Plasmids were transformed into the Escherichia coli DH5 α competent cells.
Table 1.
Site-directed mutagenesis primer sequences
| Mutations | Forward Primer | Reverse Primer |
|---|---|---|
| T153I | 5′GGC CTC ATG CTA GAG GCT TTT ATC ATA GGT GCT TTT GTG3′ | 5′CAC AAA AGC ACC TAT GAT AAA AGC CTC TAG CAT GAG GCC3′ |
| T153L | 5′GGC CTC ATG CTA GAG GCT TTT ATC TTA GGT GCT TTT GTG3′ | 5′CAC AAA AGC ACC TAA GAT AAA AGC CTC TAG CAT GAG GCC3′ |
| T153C | 5′AAT CTT CGC CAC AAA AGC ACC GCA GAT AAA AGC CTC TAG CAT GAG G3′ | 5′CCT CAT GCT AGA GGC TAT CTG CGG TGC TTT TGT GGC GAA GAT T3′ |
| T153A | 5′CTT CGC CAC AAA AGC ACC TGC GAT AAA AGC CTC TAG CAT GAG3′ | 5′CTC ATG CTA GAG GCT TTT ATC GCA GGT GCT TTT GTG GCG AAG3′ |
| T153G | 5′CTT CGC CAC AAA AGC ACC TCC GAT AAA AGC CTC TAG CAT GAG3′ | 5′CTC ATG CTA GAG GCT TTT ATC GGA GGT GCT TTT GTG GCG AAG3′ |
| T153S | 5′GCC ACA AAA GCA CCT GAG ATA AAA GCC TCT AGC AT3′ | 5′ATG CTA GAG GCT TTT ATC TCA GGT GCT TTT GTG GC3′ |
Mass Spectrometry
Mass spectrometry analysis was performed to determine whether the T153I mutant subunit yielded a full-length protein product. HEK-293T cells were cultured to 60% confluency before being transiently transfected with GFP-Kir7.1 or GFP-T153I plasmids using the LT1 transfection reagent (Mirus, Madison, WI). After 48 h, the transfected cells expressing GFP fluorescence were harvested using the scraper and washed with PBS. The pelleted cells were then resuspended in radio immuno precipitation assay buffer (RIPA) lysis buffer containing 1% protease inhibitor (15) and subjected to sonication. The protein lysate was subjected to the GFP pull-down. We used a GFP trap [rabbit polyclonal GFP antibody (Cat. No. 50430-2-AP, Proteintech, Rosemont, IL) fused to Dynabeads (Thermo Fisher, Waltham, MA)] for three subsequent 1-h incubations at 4°C. Protein was denatured in Laemli sample buffer (BioRad, Hercules, CA) with β-mercapto-ethanol (BME) at 70°C for 20 min. MS/MS mass spectrometry was conducted with a combination of trypsin and AspN enzymes. In brief, protein samples were deglycosylated with PNGase. Deglycosylated protein was incubated in 200 ng of trypsin for 2 h at 42°C. An additional 100 ng of trypsin (Promega, Madison. WI) was added. Protein was incubated overnight at 37°C, followed by 75 ng of AspN (Promega, Madison, WI), and continued incubation for 4 h. Digested samples were desalted using ZipTip C18 SPE cartridges (Millipore Sigma, Burlington, MA). HPLC was performed using PepMap C18, 3 µM, 100 Å, 150 × 0.075 mm (Thermo Fisher, Waltham, MA). NanoLC-MS/MS was performed using Agilent 1100 nanoflow system (Agilent, Santa Clara, CA) and acquired in the LTQ-Orbitrap Elite (Thermo Fisher, Waltham, MA) with a resolution of 120,000, followed by fragmentation of the 30 most intense peptides present in the scan from 350 to 1,800 m/z. Mascot (Matrix Science, Mount Prospect, IL) analysis was performed using a previously published GFP-Kir7.1 wildtype (2) or GFP-T153I sequence, respectively, to determine which protein domains were present in the digested samples. Protein identification was accepted with a 99% probability and contained at least four identified peptides (16) using the Protein Prophet (17) algorithm.
Immunocytochemistry
HEK293 cells were transfected with either GFP-tagged wild-type Kir7.1 or T153I at 60% confluency. After 48 h, cells were fixed on coverslips in 4% paraformaldehyde for 10 min at room temperature, followed by three PBS washes. Cells were permeabilized in 0.5% TritonX 100 at room temperature for 5 min. Blocking was at room temperature for 2 h in 2% BSA with 0.25% TritonX 100. Coverslips were incubated in primary antibody overnight at 4°C for Kir7.1 C-12 mouse monoclonal (1:300) (sc-398810, Santa Cruz Biotechnology, Santa Cruz, CA) (18). Coverslips were washed three times in PBS with Tween-20 for 5 min each and incubated in secondary antibody goat anti-mouse AlexaFluor-594 (1:3,000) (SA00006-3, Proteintech, Rosemont, IL). Following incubation, coverslips were washed three times with PBS with Tween-20 for 5 min each and stained with DAPI (1:1,000) for 10 min. Control experiments were untransfected cells in the field that did not express Kir and were not detected by the primary antibody. Coverslips were mounted onto microscope slides. Images were acquired using the Nikon-C2 confocal microscope with a wavelength of 488 nm for GFP, 568 nm for AlexaFluor-594, and 405 nm for DAPI. Offline analysis was conducted with NIS Elements (Nikon, Melville, NY) software.
Live-Cell Imaging
Human embryonic kidney (HEK) cells were plated on the poly-d-lysine-coated glass-bottom dishes to reach 60% confluency and transfected with GFP-tagged wild-type Kir7.1, T153I, T153L, T153G, T153A, T153C, or T153S. After 48 h in culture, cells were washed with PBS, stained with wheat germ agglutinin/Alexa594 conjugate (WGA594) (Thermo Fisher, Waltham, MA) at a concentration of 2 µg/mL, and incubated for 10 min at room temperature. Hoescht stain (Thermo Fisher, Waltham, MA) was used to label the nucleus. Controls were untransfected cells in the image labeled with Hoescht and WGA594. Images were acquired using the Nikon-C2 confocal microscope with a wavelength of 488 nm for GFP, 568 nm for WGA-594, and 405 nm for Hoescht with consistent laser settings across all experiments. Offline analysis was conducted with NIS Elements (Nikon, Melville, NY) software.
Whole Cell Patch Clamp
Whole cell patch-clamp recordings were performed on transfected HEK-293 cells to elucidate the functional effects of single amino acid substitutions at position 153 in Kir7.1 protein at the single-cell level. Negative controls were untransfected HEK293 cells. As previously published, cells were plated on glass coverslips at low density and subjected to a whole cell patch clamp (5). Pipette solution contained (in mM) 30 KCl, 83 K-gluconate, 5.5 EGTA-KOH, 0.5 CaCl2, 4 MgCl2, 10 HEPES, and 4 ATP with a pH of 7.2 using KOH. Extracellular Ringer’s solution (HR) contained (in mM) 135 NaCl, 1 MgCl2, 10 HEPES, 1.8 CaCl2, 10 glucose, 5 KCl with pH of 7.4 using NaOH. For experiments using high K+ (10, 50, 100 mM) or 135 mM Rb+, equimolar Na+ in Ringer’s solution was replaced. Voltage-clamp data were acquired as ramps from −150 to 50 mV with a pipette resistance of 3–5 MΩ and gigaseals, using Axopatch200B, Digidata1550, Clampex10, and analyzed using Clampfit (Molecular Devices, CA). We expressed the extent of inward current increase by Rb+ current amplitude at −150 mV (19). Chord conductance analysis was completed as previously published (8) using the following equation:
And results are presented as normalized g/g(−150 mV) and g/g(100 mM K+).
Structural and Stability Analysis
We used in silico methods to determine the hydrophobicity, Gibbs free energy, and structural effects of single amino acid substitutions at position 153. Membrane protein explorer software (MPex) was used to assess the stability of the Kir7.1 ion channel (20) and selected amino acid position 153 mutant channels based on hydrophobicity and Gibbs free energy. To determine the effect of single amino acid substitutions at position 153 on pore size, we used Protean 3D (DNASTAR, Madison, WI) (21) with the previously developed Kir2.2-based homology model of wild-type Kir7.1 (Uniprot ID: 060928) (22). The site-directed mutagenesis feature was used to generate energy-minimized (with free rotamers and protein backbone) models of mutant channels of interest. The inner pore size was measured as the distance between the ends of the R-groups at amino acid position 153 across the inner pore region.
Statistical Analysis
To assess the statistical differences in Kir7.1 channel function between the mutations at amino acid position 153, we used a one-way analysis of variance (ANOVA) with pairwise comparison and the Tukey test. Logarithmic transformations were performed on the maximum K+ current amplitude and Rb+ current amplitude data to fulfill the data normality assumptions of the ANOVA. Full statistical analysis is provided in Table 2.
Table 2.
Statistical analysis table
| Outcome | Original | Transformation | Overall Difference | vs. Kir7.1 | vs. T153A | vs. T153C | vs. T153G | vs. T153I | vs. T153L | vs. T153S | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rb+ current amplitude (n = 50) | Overall | −2,317.64 (412.63) | 6.60 (0.25) | <0.0001 | |||||||
| Log(x) | Kir7.1 | −5,417.37 (909.22) | 8.50 (0.16) | <0.0001 | 0.9973 | <0.0001 | <0.0001 | <0.0001 | 0.0782 | ||
| T153A | −77.91 (21.11) | 4.19 (0.22) | <0.0001 | <0.0001 | 0.0345 | 0.0019 | <0.0001 | <0.0001 | |||
| T153C | −6,349.12 (850.16) | 8.68 (0.15) | 0.9973 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.021 | |||
| T153G | −212.06 (31.07) | 5.30 (0.14) | <0.0001 | 0.0345 | <0.0001 | 0.9798 | 0.2274 | <0.0001 | |||
| T153I | −430.52 (188.77) | 5.56 (0.33) | <0.0001 | 0.0019 | <0.0001 | 0.9798 | 0.5839 | <0.0001 | |||
| T153L | −506.13 (67.44) | 6.18 (0.15) | <0.0001 | <0.0001 | <0.0001 | 0.2274 | 0.5839 | 0.0209 | |||
| T153S | −2219.13 (450.86) | 7.51 (0.34) | 0.0782 | <0.0001 | 0.021 | <0.0001 | <0.0001 | 0.0209 | |||
| K+ current amplitude (n = 51) | Overall | −408.87(75.10) | 5.16 (0.19) | <0.0001 | |||||||
| log(−x) | Kir7.1 | −863.67 (142.44) | 6.68 (0.15) | <0.0001 | 0.973 | <0.0001 | <0.0001 | <0.0001 | 0.0138 | ||
| T153A | −63.06 (22.48) | 3.90 (0.25) | <0.0001 | <0.0001 | 0.4688 | 0.9832 | 1.0000 | <0.0001 | |||
| T153C | −1,218.03 (229.94) | 6.93 (0.25) | 0.973 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0011 | |||
| T153G | −104.72 (24.80) | 4.48 (0.24) | <0.0001 | 0.4688 | <0.0001 | 0.8712 | 0.4630 | 0.0059 | |||
| T153I | −68.54 (10.49) | 4.13 (0.16) | <0.0001 | 0.9832 | <0.0001 | 0.8712 | 0.9683 | <0.0001 | |||
| T153L | −48.83 (7.40) | 3.84 (0.15) | <0.0001 | 1.0000 | <0.0001 | 0.4630 | 0.9683 | <0.0001 | |||
| T153S | −309.07 (46.69) | 5.64 (0.19) | 0.0138 | <0.0001 | 0.0011 | 0.0059 | <0.0001 | <0.0001 | |||
| Zero-current potential (n = 50) | Overall | −27.03 (3.69) | <0.0001 | ||||||||
| Kir7.1 | −57.75 (3.23) | <0.0001 | 0.9983 | <0.0001 | <0.0001 | <0.0001 | 0.0015 | ||||
| T153A | 0.21 (3.00) | <0.0001 | <0.0001 | 0.3133 | 0.9460 | 0.2298 | <0.0001 | ||||
| T153C | −60.65 (0.85) | 0.9983 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0003 | ||||
| T153G | −12.78 (3.14) | <0.0001 | 0.3133 | <0.0001 | 0.8396 | 0.9998 | 0.0472 | ||||
| T153I | −5.46 (6.01) | <0.0001 | 0.9460 | <0.0001 | 0.8396 | 0.6953 | 0.0008 | ||||
| T153L | −15.17 (5.49) | <0.0001 | 0.2298 | <0.0001 | 0.9998 | 0.6953 | 0.1833 | ||||
| T153S | −31.86 (4.45) | 0.0015 | <0.0001 | 0.0003 | 0.0472 | 0.0008 | 0.1833 |
RESULTS
Functional Consequences of the T153I Mutation
Our objective was to elucidate the molecular mechanism of channel dysfunction caused by the T153I mutation in Kir7.1. For ion channels to function, full-length protein subunits need to be assembled. Here, we assessed the mutant channel’s translation, localization, and function compared with the wild-type channel. Mass spectrometry analysis confirmed that T153I produced a full-length protein product (Table 3). After enzymatic digestion targeted for the membrane protein, sequence alignment of the membrane protein from Kir7.1 wildtype and T153I protein products revealed both the N-terminal and C-terminal domains of the Kir7.1 protein subunit (Table 3). Mascot analysis values of >1,000 indicated high integrity of the sequence alignment and protein identity (Table 3). Immunocytochemistry for C-terminal Kir7.1 indicates that the Kir7.1 wild-type channel had full-length protein subunits localized to the cell membrane in transfected HEK293 cells (Fig. 1, A–C). Immunocytochemistry further revealed that the T153I mutation did not affect the translation or membrane localization of the protein (Fig. 1, D–F). Live-cell confocal imaging of transfected HEK293T cells also confirmed the wild-type Kir7.1 channel localization to the cell membrane and colocalized with the membrane marker WGA-594 (data not shown). Furthermore, colocalization of the T153I mutant channel with the membrane marker WGA-594 (data not shown) confirmed that T153I mutation did not interfere either in protein translation or trafficking to the membrane.
Table 3.
Mass spectrometry data
| Analysis Type | Kir7.1 % Match | Kir7.1 Quality Score | T153I % Match | T153I Quality Score |
|---|---|---|---|---|
| Mascot GFP Kir7.1 Trypsin/AspN | 42% | 1,137 | 34% | 1,958 |
| Mass spectrometry sequence alignment | GFPKir7.1 MVSKGEELFTGVVPILVELDGDVNGHKFSVSGEGEGDATYGKLTLKFICTTGKLPVPWPTLVTTLTYGVQCFSRYPDHMKQHDFFKSAMPEGYVQERTIFFKDDGNYKTRAEVKFEGDTLVNRIELKGIDFKEDGNILGHKLEYNYNSHNVYIMADKQKNGIKVNFKIRHNIEDGSVQLADHYQQNTPIGDGPVLLPDNHYLSTQSALSKDPNEKRDHMVLLEFVTAAGITLGMDELYKMDSSNCKVIAPLLSQRYRRMVTKDGHSTLQMDGAQRGLAYLRDAWGILMDMRWRWMMLVFSASFVVHWLVFAVLWYVLAEMNGDLELDHDAPPENHTICVKYITSFTAAFSFSLETQLTIGYGTMFPSGDCPSAIALLAIQMLLGLMLEAFITGAFVAKIARPKNRAFSIRFTDTAVVAHMDGKPNLIFQVANTRPSPLTSVRVSAVLYQERENGKLYQTSVDFHLDGISSDECPFFIFPLTYYHSITPSSPLATLLQHENPSHFELVVFLSAMQEGTGEICQRRTSYLPSEIMLHHCFASLLTRGSKGEYQIKMENFDKTVPEFPTPLVSKSPNRTDLDIHINGQSIDNFQISETGLTE |
GFPT153I MVSKGEELFTGVVPILVELDGDVNGHKFSVSGEGEGDATYGKLTLKFICTTGKLPVPWPTLVTTLTYGVQCFSRYPDHMKQHDFFKSAMPEGYVQERTIFFKDDGNYKTRAEVKFEGDTLVNRIELKGIDFKEDGNILGHKLEYNYNSHNVYIMADKQKNGIKVNFKIRHNIEDGSVQLADHYQQNTPIGDGPVLLPDNHYLSTQSALSKDPNEKRDHMVLLEFVTAAGITLGMDELYKMDSSNCKVIAPLLSQRYRRMVTKDGHSTLQMDGAQRGLAYLRDAWGILMDMRWRWMMLVFSASFVVHWLVFAVLWYVLAEMNGDLELDHDAPPENHTICVKYITSFTAAFSFSLETQLTIGYGTMFPSGDCPSAIALLAIQMLLGLMLEAFIIGAFVAKIARPKNRAFSIRFTDTAVVAHMDGKPNLIFQVANTRPSPLTSVRVSAVLYQERENGKLYQTSVDFHLDGISSDECPFFIFPLTYYHSITPSSPLATLLQHENPSHFELVVFLSAMQEGTGEICQRRTSYLPSEIMLHHCFASLLTRGSKGEYQIKMENFDKTVPEFPTPLVSKSPNRTDLDIHINGQSIDNFQISETGLTE |
||
Mascot analysis was conducted to determine the percent match and the quality score (greater than 1,000) of the mass spectrometry protein samples. Trypsin/AspN were the enzymes used to digest the protein sample where both N-terminal and C-terminal domains were identified. Underlined regions were identified in the sample analysis. Italicized letters indicate GFP domains, and black letters indicate Kir domains.
Figure 1.
T153I mutation primarily alters Kir7.1 function. HEK293 cells were transfected with either GFP-Kir7.1 WT (A) or T153I (D). After fixation, the cells were stained with a C-terminal Kir7.1 antibody (C-12) (B and E). Merged images (C and F) signify the expression of Kir7.1 protein on the cell membrane. G: Kir7.1 WT (n = 8) current trace average from −150 to 50 mV voltage ramp for 5 mM K+ (black) and 135 mM Rb+ (dashed), the time-course plot of current amplitude at −150 mV from a single cell is in the insert. H: T153I (n = 9) current trace average from voltage ramp for 5 mM K+ (gray) and 135 mM Rb+ (dashed), the time-course plot of inward current amplitude at −150 mV from a single cell is in the insert. I: K+ current amplitude at −150 mV compares Kir7.1 wildtype and T153I (P < 0.0001). *Significance ≤ 0.05. Current from untransfected cells is included as the negative control in I and J. J: zero-current potential comparison between Kir7.1 wildtype and T153I (P < 0.0001) calculated from G and H. K: Rb+ current amplitude at −150 mV for Kir7.1 wildtype and T153I. HEK293, human embryonic kidney cells; Kir7.1, inward-rectifier potassium channel; K+, potassium; Rb+, rubidium; WT, wildtype.
We then compared the biophysical properties of the T153I mutant channel with the Kir7.1 wild-type channel to understand why the channel has altered function in patients with LCA16. Mild inwardly rectifying current-voltage (I-V) plot, K+ inward current amplitude at hyperpolarized membrane potentials, and an enhanced inward current in extracellular Rb+ as a charge carrier are all defining characteristics of the wild-type channel function. Figure 1G depicts the average I-V plot from current traces for the Kir7.1 wild-type channel in the presence of HR or 135 mM Rb+ in the extracellular solution. In comparison, current recordings of T153I mutant protein demonstrated a linear I-V plot in HR (Fig. 1H, solid line). Time-course plots for single cells expressing Kir7.1 wildtype and T153I are included as insets in Fig. 1, G and H. The maximum K+ inward current amplitude measured at −150 mV for the T153I channel was −0.07 ± 0.015 nA (n = 9) compared with −0.9 ± 0.1 nA (n = 8) (P < 0.0001) for the wild-type Kir7.1 channel (Fig. 1I). The maximum inward current amplitude for untransfected HEK293 cells was −0.09 ± 0.02 nA (n = 5). Cells expressing T153I had a zero-current potential of −5.5 ± 6.0 mV, which was significantly depolarized compared with −57.8 ± 3.2 mV (P < 0.0001) for the wild-type Kir7.1 channel (Fig. 1J). The zero-current potential for untransfected HEK293 cells was 1.3 ± 4.6 mV (n = 5). It was assumed that T153I was nonfunctional based on the inward current amplitude and the zero-current potential findings. Surprisingly, the substitution of Na+ ion with Rb+ ion slightly enhanced the inward current of T153I with an amplitude of −0.4 ± 0.2 nA compared with −5.4 ± 0.9 nA for the wild-type Kir7.1 channel (Fig. 1K).
Does T153I Mutation Alter the Kir7.1 Channel’s Extracellular K+ Dependence?
A fundamental biophysical property of the Kir7.1 channel is that conductance is independent of extracellular K+ concentration even as current amplitude increases (23). To determine if this holds for the T153I mutant channel, we recorded current sweeps from −150 to +50 mV in the presence of increasing concentrations of extracellular K+ (5, 10, 50, and 100 mM) in cells expressing wild-type Kir7.1 (Fig. 2A), T153I (Fig. 2B), or untransfected (Fig. 2C). Figure 2A demonstrates that increased extracellular K+concentration led to the expected positive shift in Erev for wild-type Kir7.1. Alternatively, Fig. 2B illustrates no shift in the current trace for the T153I mutant channel, which was similar to the nontransfected control (Fig. 2C). We calculated normalized chord conductance [g/g(−150 mV)] in 100 mM K+ for Kir7.1 wildtype (Fig. 2D, black line), T153I (Fig. 2D, gray line), or untransfected (Fig. 2D, dashed line) using the current recordings between −20 to −150 mV, and the results are displayed as an exponential fit. We observed a progressive increase in g/g(−150 mV) as the membrane potential became more negative in cells expressing the wild-type channel (Fig. 2D, black line). Alternatively, untransfected cells (Fig. 2D, dashed line) or cells expressing the T153I mutant channel exhibited a progressive decrease in g/g(−150 mV) as the membrane potential became more negative (Fig. 2D, gray line). We then evaluated the effects of increasing extracellular K+ concentrations and applied a linear fit model to channel chord conductance. As expected, the wild-type channel did not exhibit a change in g/g(−150 mV) in the presence of increasing extracellular K+ (Fig. 2E, black line). However, for the untransfected cells or T153I mutant channel, we observed slightly decreased g/g(−150 mV) to increase extracellular K+ concentrations (Fig. 2E, gray line, dashed line). To assess membrane depolarization of cells expressing wild-type Kir7.1 or T153I, we calculated the zero-current potentials from Fig. 2, A, B, and C and plotted linear regression as a function of extracellular K+ concentration (Fig. 2F). Cells expressing the wild-type Kir7.1 channel exhibited concentration-dependent depolarization of the cell membrane (increasing zero-current potential) with a slope of 0.6362 (Fig. 2F, black line) compared with cells expressing the T153I mutant channel that did not exhibit concentration-dependent depolarization, slope −0.0044 (Fig. 2F, gray line). Untransfected cells did not exhibit concentration-dependent depolarization, slope 0.0953 (Fig. 2F, dashed line). Our results indicated that the T153I mutant channel does not permeate K+.
Figure 2.
Mutant channel does not show extracellular K+ dependence. A: Kir7.1 K+ current from −150 to 50 mV voltage ramp in 5 mM (n = 9), 10 mM (n = 7), 50 mM (n = 8), and 100 mM (n = 8) external K+. Colors and symbols remain the same for A–E. B: T153I K+ current (gray) voltage ramp in 5 mM (n = 6), 10 mM (n = 2), 50 mM (n = 6), and 100 mM (n = 6) extracellular K+. C: untransfected cell K+ current voltage ramp in 5 mM (n = 5), 10 mM (n = 5), 50 mM (n = 4), and 100 mM (n = 4) extracellular K+. D: normalized chord conductance in 100 mM K+ [g/g(−150 mV)] from A and B comparing wild-type Kir7.1 and T153I (n ≥ 3). Dashed line indicates untransfected cells (n ≥ 4) in C–E. E: plot of g/g(100 mM K+) at −150 mV across external K+ concentration calculated from A and B (n ≥ 3). F: plot of zero-current potential relative to external K+ concentration for wild-type Kir7.1 (black) and T153I (gray) from A and B. Kir7.1, inward-rectifier potassium channel; K+, potassium.
Single Amino Acid Substitution to Probe the Specific Role of the 153 Position
The amino acid substitution at position 153 altered channel function compared with the wild-type channel. As 153 is positioned in the inner pore region of the channels, we sought to determine whether the change in polarity of the substituted amino acid was the primary cause for this observation. To test this, we examined the effects of nonpolar (T153L, Fig. 3A), short side-chain [T153G (Fig. 3B) and T153A (Fig. 3C)], and polar [T153C (Fig. 3D) and T153S (Fig. 3E)] amino acids at position 153 on membrane localization and Kir7.1 channel function. Confocal imaging indicated that single amino acid substitutions at position 153 did not affect the localization of the Kir7.1 channel to the cell membrane in HEK293 cells (Fig. 3). Using the whole cell patch-clamp technique, we determined the effects of polarity and side-chain length at amino acid position 153 on the Kir7.1 channel function at a single-cell level in extracellular HR (Fig. 3F) or extracellular Rb+ (Fig. 3G). One-way ANOVA indicated that single amino acid substitutions at position 153 had an overall statistically significant effect (P < 0.0001) on maximum inward current amplitude at −150 mV (Fig. 3H), zero-current potential (Fig. 3I), and Rb+ inward current amplitude (Fig. 3J). These findings indicate that side-chain length and/or polarity at amino acid position 153 directly affects the Kir7.1 channel function.
Figure 3.
Effect of amino acid side chains on channel localization and K+ current. A: live-cell image of HEK293 cells expressing T153L, the inset is an intensity plot (A–E), and I-V plot showing average current recordings from 5 cells in HR (solid line) and Rb+ (dashed line) (n = 5). For all live-cell images, green is GFP, red is WGA-594 membrane stain, and blue is Hoescht nuclear stain. All intensity plots show fluorescence arbitrary unit (Fl. AU) vs. distance (Dist.) B: live-cell image of HEK293 cells expressing T153G, and average (n = 7) I-V plot in HR (solid line) and Rb+ (dashed line). C: live-cell image of HEK293 cells expressing T153A, and average (n = 7) I-V plot in HR (solid line) and Rb+ (dashed line). D: live-cell image of HEK293 cells expressing T153C, and average (n = 8) I-V plot in HR (solid line) and Rb+ (dashed line). E: live-cell image of HEK293 cells expressing T153S and average (n = 7) I-V plot in HR (solid line) and Rb+ (dashed line). F: average I-V plot as current traces in response to voltage ramp from −150 to 50 mV in 5 mM K+ of Kir7.1, T153I, T153L, T153G, T153A, T153C, and T153S. G: average I-V plot responses in 135 mM Rb+ for the same cells as in F. H: inward-current amplitude measured at −150 mV for alternate side-chain mutants compared with wildtype and T153I. *P value ≤ 0.05 compared with wildtype. I: zero-current potential calculated from F of alternate side-chain mutants compared with wildtype and T153I. J: Rb+ current amplitude at −150 mV from G. GFP, green fluorescent protein; HEK293, human embryonic kidney cells; HR, extracellular Ringer’s solution; Kir7.1, inward-rectifier potassium channel; K+, potassium; Rb+, rubidium; WGA594, wheat germ agglutinin/Alexa594 conjugate; WT, wildtype.
As leucine is an isomer of isoleucine, the biophysical effects of nonpolar amino acid side-chain branching at position 153 were examined using the T153L mutant channel. The I-V plot for the T153L mutant channel was linear in HR (Fig. 3A) with a maximum inward current amplitude of −0.05 ± 0.007 nA (n = 5) (Fig. 3H), significantly less than Kir7.1 wildtype; P < 0.0001. Cells expressing T153L had depolarized cell membranes with a zero-current potential of −15.2 ± 5.5 mV (Fig. 3I) compared with cells expressing the wild-type Kir7.1 channel; P < 0.0001. Extracellular Rb+-dependent inward conductance was enhanced (Fig. 3, A and G) compared with HR with an amplitude of −0.5 ± 0.07 nA at −150 mV for T153L (Fig. 3J), which is slightly higher than T153I. These biophysical properties indicate that nonpolar amino acids isoleucine and leucine at position 153 yield dysfunctional Kir7.1 channels.
Contribution of Amino Acid Side-Chain Length
We substituted glycine (T153G) and alanine (T153A) at position 153. Compared with wildtype, the average current trace I-V plot from cells expressing T153G was linear (Fig. 3B) with a relatively small K+ inward current amplitude of −0.1 ± 0.02 nA (n = 7) (Fig. 3H); P < 0.0001. Current recordings from cells expressing T153A likewise had a linear I-V plot in HR (Fig. 3C,) with a K+ inward current amplitude of −0.06 ± 0.02 nA (n = 7) (Fig. 3H), significantly lower than wildtype; P < 0.0001. Cells expressing either T153G or T153A exhibited membrane depolarization compared with cells expressing the wild-type Kir7.1 channel with zero-current potentials of −12.8 ± 3.1 mV (Fig. 3I); P < 0.0001 and 0.2 ± 3.0 mV (Fig. 3I); P < 0.0001, respectively. The inward current was not enhanced in the presence of extracellular Rb+ (Fig. 3, B, C, and G) in cells expressing T153G or T153A with Rb+ current amplitudes at −150 mV of −0.2 ± 0.03 nA (Fig. 3J) and −0.08 ± 0.02 nA (Fig. 3J), respectively. The Rb+ current amplitude is significantly reduced compared with the wild-type channel current profile. These results imply that a channel with short side chains at amino acid position 153 is nonfunctional.
Reliance of Kir7.1 Function on Amino Acid 153 Side-Chain Polarity
We assessed cysteine (T153C) and serine (T153S) substitutions at position 153 to determine whether a polar amino acid is essential for normal Kir7.1 channel function. Current recordings from cells expressing T153C showed mild inward rectification (Fig. 3D), compared with that of wild-type Kir7.1 −0.9 ± 0.1 nA (n = 8), with a maximum inward current amplitude at −150 mV measuring −1.2 ± 0.2 nA (n = 8) (Fig. 3H); P = 0.9730. T153C maintained hyperpolarized cell membranes like wild-type Kir7.1 with a measured zero-current potential of −60.7 ± 0.9 mV (Fig. 3I); P = 0.9983. The inward current from T153C-expressing cells was enhanced in extracellular Rb+ (Fig. 3, D and G) with an inward current amplitude of −6.3 ± 0.9 nA (Fig. 3J), compared with the wildtype channel. These data indicated that T153C yielded a functional Kir7.1 channel. Alternatively, the I-V plot from cells expressing T153S mutant channel had reduced inward rectification in HR (Fig. 3D) with a maximum inward current amplitude of −0.3 ± 0.04 nA (n = 7) (Fig. 3H), P = 0.0138, compared with wildtype P = 0.0011 compared with T153C. Cells expressing the T153S mutant channel exhibited slight membrane depolarization with a zero-current potential of −31.86 ± 4.45 mV (Fig. 3I) compared with wildtype (P < 0.0001) and T153C (P = 0.0003). In the presence of extracellular Rb+, T153S enhanced inward current (Fig. 3, E and G) with an inward current amplitude of −2.2 ± 0.5 nA (Fig. 3J) compared with wildtype. These data indicated that T153S formed a channel with reduced or altered function, which we evaluated further in increasing extracellular K+ concentrations.
Interdependence of Amino Acid 153 Polarities, Side-Chain Length, and Extracellular K+ Concentration
We examined the effect of increasing extracellular K+ concentrations on T153A, T153C, and T153S further to evaluate the biophysical impacts of polarity and side-chain length. We recorded current sweeps from −150 to +50 mV from cells expressing T153A, T153C, or T153S in 5, 10, 50, and 100 mM extracellular K+ (Fig. 4, A–C). We calculated g/g(−150 mV) in 100 mM extracellular K+ using current recordings from −20 mV to −150 mV and displayed the results as an exponential fit (Fig. 4D). T153C (Fig. 4D) and T153S (Fig. 4D) exhibited a progressive increase in g/g(−150 mV) as the membrane potentials became more negative similar to Kir7.1 wildtype (Fig. 4D, black line). T153A (Fig. 4D) exhibited a progressive decrease in g/g(−150 mV) as the membrane potential became more negative similar to T153I (Fig. 4D, gray line). We next evaluated chord conductance as a function of increasing extracellular K+ concentrations and displayed a linear fit. We found that T153C (Fig. 4E, gray symbols) did not exhibit a change in g/g(−150 mV) in increasing extracellular K+ concentrations, similar to the wild-type channel (Fig. 4E, black line). The g/g(−150 mV) of T153A (Fig. 4E, open symbols) decreased like T153I (Fig. 4E, gray line) with increasing extracellular K+ concentrations. However, T153S (Fig. 4E, half-filled symbols) showed a gradual increase in g/g(−150 mV) with increasing extracellular K+ concentrations. We then evaluated the effects of T153A (Fig. 4F, open symbols), T153C (Fig. 4F, gray symbols), and T153S (Fig. 4F, half-filled symbols) mutant channels on concentration-dependent membrane depolarization. Cells expressing T153A (Fig. 4F, open symbols) lacked concentration-dependent depolarization of the cell membrane with a slope of 0.0511, similar to T153I (Fig. 4F, gray line). Cells expressing T153C (Fig. 4F, gray symbols) exhibited concentration-dependent depolarization of the cell membrane with a slope of 0.5148. In contrast, cells expressing T153S (Fig. 4F, half-filled symbols) had diminished concentration-dependent depolarization of the cell membrane with a slope of 0.3306. T153I and T153A mutant channels lacked the characteristic inverse dependence on extracellular K+ of the wild-type channel. T153C maintained wildtype inverse dependence on extracellular K+ and provided further evidence that T153C mutation yielded a functional Kir7.1 channel. T153S mutant channels K+ conductance showed a positive trend upon increasing extracellular K+ concentration.
Figure 4.
Functional correlation of amino acid polarity and size with extracellular K+. A: average current trace for T153A channel (voltage ramp −150 to 50 mV) in 5 mM (n = 4), 10 mM (n = 4), 50 mM (n = 3), and 100 mM (n = 3) external K+. Symbols by concentration are the same for A–F. B: average current trace for T153C channel in 5 mM (n = 6), 10 mM (n = 6), 50 mM (n = 6), and 100 mM (n = 6) external K+. C: average current trace for T153S channel in 5 mM (n = 6), 10 mM (n = 6), 50 mM (n = 6), and 100 mM (n = 6) external K+. D: normalized chord conductance to 100 mM K+ [g/g(−150 mV)] from A–C compared with WT and T153I across voltages (n ≥ 3). E: plot of g/g(100 mM K+) at −150 mV across external K+ concentration from A and B compared with WT and T153I (n ≥ 3). F: plot of zero-current potential relative to external K+ concentration for polar and short side-chain T153 mutants compared with wildtype and T153I channel from A–C. K+, potassium; WT, wildtype.
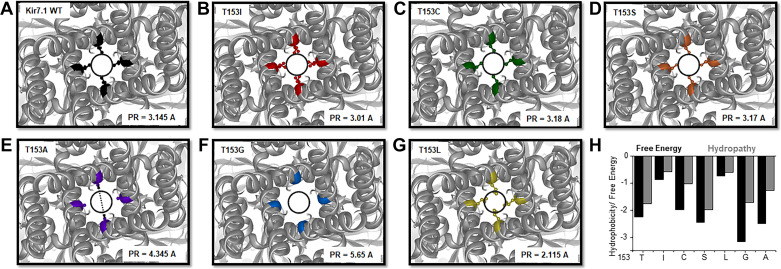
Molecular Models Predicted Kir7.1 Structural Alterations
We used in silico methods to determine if the inner pore size, polarity, or both were associated with the Kir7.1 channel function. Figure 5 shows molecular models and inner pore size measurements from Kir7.1 wildtype, T153I, T153C, T153S, T153A, T153G, and T153L (A–G) channels. Wild-type Kir7.1 (3.145 Å), T153C (3.18 Å), and T153S (3.17 Å) have similar inner pore radii. T153L (2.115 Å) and T153I (3.01 Å) have smaller inner pore radii. T153G (5.65 Å) and T153A (4.345 Å) have larger inner pore radii yet lack Kir7.1 channel function. Polarity and Gibb’s free energy (Fig. 5F) were somewhat predictive of Kir7.1 channel function as threonine, cysteine, and serine all had polar side chains and demonstrated characteristic K+ current and enhanced Rb+ current. Glycine and alanine are nonpolar R-groups with negative hydrophobicity scores (Fig. 5H) and did not demonstrate normal Kir7.1 channel function.
Figure 5.
Molecular models of altered pore structure and size. A: top view of the Kir7.1 WT homology model with amino acid 153 in black. The black circle represents the hydrated K+ ion in A–E. The dashed arrow (shown in E) indicates the distance between the side chains used to measure the pore radius (PR). B: top view of the T153I mutant with amino acid 153 in red. C: top view of T153C with amino acid 153 in green. D: top view of T153S with amino acid 153 in orange. E: top view of T153A with amino acid 153 in purple. F: top view of T153G with amino acid 153 in blue. G: top view of T153L with amino acid 153 in yellow. H: membrane protein explorer (MPex) data from both Gibbs free energy (black) and hydropathy (gray) calculations for the wildtype Kir7.1, T153I, T153C, T153S, T153L, T153G, and T153A channels. Kir7.1, inward-rectifier potassium channel; WT, wildtype.
DISCUSSION
Changing hydrophilic threonine to hydrophobic isoleucine at amino acid position 153 (T153I) causes LCA16 blindness in humans and zebrafish (11). We ruled out any effects of T153I on protein translation and membrane localization by determining that the mutant protein was a full-length protein product localized to the cell membrane. The mutant protein did not make a functional channel as the I-V plot using physiological K+ Ringer’s was non-inward rectifying. However, we observed inward rectification using Rb+ as a charge carrier consistent with wildtype. Substituting the 153 position with a nonpolar amino acid leucine, T153L, did not alter the biophysical signature of the T153I mutant channel. Short side-chain amino acid substitutions, T153G and T153A, lacked normal channel function. Cells expressing T153A also lost extracellular K+ dependence similar to T153I. Substituting polar amino acids, T153C and T153S, restored mild inward rectification to the T153I channel. Cells expressing T153C had wildtype extracellular K+ dependence. Alternatively, cells expressing T153S exhibited a slight inward conductance increase upon increasing extracellular K+, which is not observed in cells expressing the wild-type channel. Molecular modeling indicated the effects of single amino acid changes on the inner pore size of the Kir7.1 channel.
T153I Disease Mutation in KCNJ13 Leads to a Dysfunctional Kir7.1 Channel
Amino acid 153 is not within the signaling sequence responsible for trafficking, i.e., 323–360 (24) and does not perturb protein translation or membrane localization. Vera et al. (19) demonstrated that heterotetramers of R166X nonsense mutant subunits with wild-type subunits were subjected to degradation by the proteosome due to improper tetramer assembly. If the T153I tetramer were misassembled and degraded by the proteosome, we would have visualized GFP-tagged protein throughout the cell, not just the membrane. These findings suggested that a functional defect, rather than a protein translation or membrane localization defect, leads to LCA16 blindness caused by the T153I mutation.
The T153I mutant channel is dysfunctional rather than nonfunctional, as Rb+ inward current is maintained in the mutant channel. Rb+ permeability has previously been attributed to amino acid M125 (23) in the extracellular selectivity loop of Kir7.1, which could explain why it is retained in the T153I disease mutant channel where the extracellular domain is unaffected. Our findings suggested that the inner pore ion conduction path of Kir7.1 is critical and independent of the extracellular loop. The dysfunctional nature of the T153I mutant channel creates an opportunity to develop small molecule activators of the Kir7.1 channel targeted to the inner pore region, specifically, to stretch open the 153 residues in the ion conduction pathway.
The 153 amino acid position makes the narrowest opening within the inner pore of Kir7.1 (25) and is further constricted in the T153I mutant channel relative to wildtype. The alphafold structural prediction platform (26, 27) shows that in the homology model for Kir7.1 (Uniprot ID: 060928), threonine at position 153 forms two hydrogen bonds with glutamate 149 (E149) backbone within the second transmembrane domain of the inner pore. One of these bonds is formed between the hydroxyl group of the threonine R-group and the E149 backbone. The other is formed between the backbone carbonyl group of T153 and E149 backbone. T153I mutant subunits only form one hydrogen bond with E149 because isoleucine lacks a hydroxyl group in its side chain. Altered hydrogen bonding within the binding pocket and constricted pore size could lead to the negligible K+ inward conductance of the T153I channel while maintaining enhanced inward current in extracellular Rb+.
Single Amino Acid Substitutions Alter Kir7.1 Pore Size beyond Simple Replacement
After identifying the T153I disease mutation in Kir7.1, we determined which amino acid attributes in the inner pore region are critical for the normal functioning of the Kir7.1 channel. The factors considered were side-chain polarity, length, and structure (branching). Amino acid 153 mutant channel structural changes were analyzed using a previously published homology model of Kir7.1 based on Kir2.2 (19) to determine each channel’s inner pore size and molecular structure. We used a similar approach to Lam et al. (28) to evaluate the neck structure and size measurements of TMEM16A to determine the relationship between inner pore size and ion conductance in the Kir7.1 channel. The inner pore size changes between the amino acid substitutions are more substantial than the difference in size between the amino acid R-groups. These observations suggested that changes in inner pore size resulted from alterations in protein-protein interactions within the Kir7.1 channel inner pore. Cysteine and serine, like wildtype, form two hydrogen bonds with E149 based on hydrophobicity and amino acid R-group structure. However, isoleucine, leucine, glycine, and alanine only form a single hydrogen bond between E149 and the threonine backbone carbonyl group. These amino acids do not have hydroxyl in their side chain. Structural and hydrophobicity data provided a unique understanding of the characteristics of the inner pore region of the Kir7.1 channel.
The Characteristic Narrow Inner Pore of Kir7.1 Is Essential for Ion Conductance
The importance of the narrow inner pore for normal Kir7.1 biophysical function was evaluated. The presence of a narrow inner pore is critical for ion selectivity and conductance of the Kir7.1 channel. When relating ion conductance to inner pore size, we consider K+ and Rb+ ions and their hydration shells to determine the steric and biochemical relationship between the hydrated ion and the position of the ion within the tunnel pore-binding pockets within the inner pore of the channel. K+ has a large hydration shell (28) that must fit tightly into the binding pockets of the Kir7.1 channel inner pore and interact with the polar side chains present there. A constricted inner pore region limits K+ conductance by altering critical protein-protein interactions. Rb+ has a slender hydration shell and can pass through channels that K+ ions cannot (29). T153G and T153A channels have a similar pore radius of 6 Å (30), whereas K+ channels have a pore radius of 3.2 Å (31). The lack of K+ and Rb+ inward current in these channels could be due to the absence of a narrow inner pore.
Inner pore size is critical for Rb+ and K+ conductance in Kir7.1 channels. Previous studies indicate that the K+ ion is hydrated as it enters the Kir conduction pathway. However, the hydration shell undergoes controlled replacement as the ion passes through the pathway. As it reaches the inner pore and the amino acid at position 153, it may have a smaller hydration shell than in the outer pore (32, 33). Regardless, both Rb+ and K+ likely remain hydrated as they pass through the channel. The hydrated Rb+ ion radius is 2.87 Å (34), 0.7 Å greater than the pore size of T153L. Similarly, the hydrated K+ ion has a radius of 3.8 Å, and the inner pore of a standard K+ channel is 3.2 Å (35). Rb+ would fit well into the smaller binding pockets and interact with the polar amino acid side chains. The larger K+ hydrated ion fits well into the wild-type Kir7.1-binding pockets. Another possibility is that leucine in the inner pore has an additional exposed carbon compared with isoleucine that likely narrows the inner pore and increases the Rb+ conductance while maintaining negligible K+ conductance. These findings suggest that, in addition to the M125 amino acid in the extracellular domain (7), Kir7.1 inner pore size is essential for Rb+ conductance.
Side-Chain Polarity Is Critical for Kir7.1 Ion Conductance
We predicted that polar amino acid at position 153 is required for the normal function of the Kir7.1 channel as threonine at that position is a polar amino acid. When the inner pore size of T153C and T153S was measured, their pore radii were near the ideal inner pore size for K+ channels at a 3.2 Å radius. We anticipated that with identical polarity and pore size, T153C and T153S would have a similar biophysical function as the wild-type Kir7.1 channel and both exhibit K+ and Rb+ conductance. T153C had slightly enhanced channel function relative to wildtype. These findings are supported by the study by Kharade et al. (10), who demonstrated that T153C was inhibited more efficiently by the Kir7.1 pharmacological inhibitor VU590, which binds to the channel inner pore, than the wild-type Kir7.1 channel independent of the M125R mutation. Because cysteine can readily form hydrogen bonds similar to threonine, the biochemistry of the inner pore is likely unaffected by the T153C mutation. We predicted that cysteine might form a disulfide bond across the inner pore region, blocking ion conductance (25), which did not occur because the inner pore radius would need to be 2 Å or smaller (36).
T153S introduces an exposed hydroxyl group to the inner pore, unlike the wild-type Kir7.1 channel. Altered biophysical characteristics may result from hydrogen bonding between the T153S hydroxyl group and the hydrated K+ ion. Furthermore, the exposed hydroxyl group may increase hydrogen bonding between amino acid side chains within the inner pore and the two hydrogen bonds in the wild-type channel (37). Previous studies in Kir2.1 indicate that Ser165 has a higher binding affinity for magnesium and polyamine block in the inner pore region (38) relative to other amino acid side chains that lack the exposed hydroxyl group. We hypothesized that higher extracellular K+ concentrations increased chord conductance because they can overcome the magnesium and/or polyamine block, characteristic of strong inwardly rectifying Kir channels. Moreover, strong inwardly rectifying K+ channels (Kir2.1) exhibit similar concentration-dependent K+ conductance to T153S (37), indicating that Ser165 or other serine side chains in the inner pore of Kir channels may alter the K+ binding pockets and be responsible for concentration-dependent K+ conductance.
Inner Pore Size and Biochemistry Are Critical Determinants of K+ and Rb+ Permeability Independent of the Extracellular Selectivity Loop
Figure 6 summarizes the relationship between inner pore size, biomolecular interactions, and Kir7.1 channel function. We demonstrated that a polar amino acid side chain is required at position 153 for Kir7.1 channel function. It has been previously found that amino acid M125 in the extracellular loop is responsible for ion conductance, with the narrow inner pore being a secondary factor (23). Although we only modified the amino acid position 153 in the inner pore lining, our work supports the finding that the extracellular loop is responsible for ion selectivity. Still, the inner pore contributes to ion conductance. In this study, through biophysical assays and energy-minimized modeling, we demonstrated critical structural features, including polarity and side chain length, in the inner pore region of Kir7.1.
Figure 6.
Kir7.1 structure-function correlation. Functional channels form a narrow inner pore with two hydrogen bonds (dashed lines) between the amino acid side chains at position 153 and the backbone of amino acid 149, conducting both K+ and Rb+. Dysfunctional channels form a constricted inner pore with one hydrogen bond between the amino acid side chain at position 153 and the backbone of amino acid 149 that selectively restricts K+ conductance. Nonfunctional channels form a wide inner pore with one hydrogen bond between the amino acid side chain at position 153 and the backbone of amino acid 149. Channels with altered functions have limited K+ and Rb+ conductance. Kir7.1, inward-rectifier potassium channel; K+, potassium; Rb+, rubidium.
GRANTS
This work was supported by Endocrinology and Reproductive Physiology NICHD T32HD041921 (to K.M.B.). The work was also supported by NIH R01 EY024995 and R24 EY032434 and Retina Research Foundation M. D. Matthews Research Professorship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
This article is part of the special collection “Inward Rectifying K+ Channels.” Jerod Denton, PhD, and Eric Delpire, PhD, served as Guest Editors of this collection.
AUTHOR CONTRIBUTIONS
K.M.B. and B.R.P. conceived and designed research; K.M.B., P.K.S., M.K., J.H., and J.S. performed experiments; K.M.B., P.K.S., Q.Z., and B.R.P. analyzed data; K.M.B., P.K.S., and B.R.P. interpreted results of experiments; K.M.B. and B.R.P. prepared figures; K.M.B. and B.R.P. drafted manuscript; K.M.B., P.K.S., M.K., Q.Z., and B.R.P. edited and revised manuscript; K.M.B., P.K.S., M.K., Q.Z., J.H., J.S., and B.R.P. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge all past and present members of the Pattnaik lab for their insights and support of the work presented in this manuscript. The authors also acknowledge Dr. Jonathan Makielski for critical manuscript review.
REFERENCES
- 1.Kumar M, Pattnaik BR. Focus on Kir7.1: physiology and channelopathy. Channels (Austin) 8: 488–495, 2014. doi: 10.4161/19336950.2014.959809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shahi PK, Liu X, Aul B, Moyer A, Pattnaik A, Denton J, Pillers DM, Pattnaik BR. Abnormal electroretinogram after Kir7.1 channel suppression suggests role in retinal electrophysiology. Sci Rep 7: 10651, 2017. doi: 10.1038/s41598-017-11034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakamura N, Suzuki Y, Sakuta H, Ookata K, Kawahara K, Hirose S. Inwardly rectifying K+ channel Kir7.1 is highly expressed in thyroid follicular cells, intestinal epithelial cells and choroid plexus epithelial cells: implication for a functional coupling with Na+,K+-ATPase. Biochem J 342: 329–336, 1999. doi: 10.1038/s41598-017-11034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pattnaik BR, Hughes BA. Regulation of Kir channels in bovine retinal pigment epithelial cells by phosphatidylinositol 4,5-bisphosphate. Am J Physiol Cell Physiol 297: C1001–C1011, 2009. doi: 10.1152/ajpcell.00250.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.York N, Halbach P, Chiu MA, Bird IM, Pillers DM, Pattnaik BR. Oxytocin (OXT)-stimulated inhibition of Kir7.1 activity is through PIP2-dependent Ca(2+) response of the oxytocin receptor in the retinal pigment epithelium in vitro. Cell Signal 37: 93–102, 2017. doi: 10.1016/j.cellsig.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang W, Zitron E, Bloehs R, Muller-Krebs S, Scholz E, Zeier M, Katus H, Karle C, Schwenger V. Dual regulation of renal Kir7.1 potassium channels by protein Kinase A and protein kinase C. Biochem Biophys Res Commun 377: 981–986, 2008. doi: 10.1016/j.bbrc.2008.10.110. [DOI] [PubMed] [Google Scholar]
- 7.Raphemot R, Lonergan DF, Nguyen TT, Utley T, Lewis LM, Kadakia R, Weaver CD, Gogliotti R, Hopkins C, Lindsley CW, Denton JS. Discovery, characterization, and structure-activity relationships of an inhibitor of inward rectifier potassium (Kir) channels with preference for Kir2.3, Kir3.x, and Kir7.1. Front Pharmacol 2: 75, 2011. doi: 10.3389/fphar.2011.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimura M, Yuan Y, Chang JT, Zhang S, Campochiaro PA, Zack DJ, Hughes BA. Expression and permeation properties of the K(+) channel Kir7.1 in the retinal pigment epithelium. J Physiol 531: 329–346, 2001. doi: 10.1111/j.1469-7793.2001.0329i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev 90: 291–366, 2010. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- 10.Kharade SV, Sheehan JH, Figueroa EE, Meiler J, Denton JS. Pore polarity and charge determine differential block of Kir1.1 and Kir7.1 potassium channels by small-molecule inhibitor VU590. Mol Pharmacol 92: 338–346, 2017. doi: 10.1124/mol.117.108472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toms M, Dubis AM, Lim WS, Webster AR, Gorin MB, Moosajee M. Missense variants in the conserved transmembrane M2 protein domain of KCNJ13 associated with retinovascular changes in humans and zebrafish. Exp Eye Res 189: 107852, 2019. doi: 10.1016/j.exer.2019.107852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pattnaik BR, Shahi PK, Marino MJ, Liu X, York N, Brar S, Chiang J, Pillers DA, Traboulsi EI. A novel KCNJ13 nonsense mutation and loss of Kir7.1 channel function causes leber congenital amaurosis (LCA16). Hum Mutat 36: 720–727, 2015. doi: 10.1002/humu.22807. [DOI] [PubMed] [Google Scholar]
- 13.Levitan I. Cholesterol and Kir channels. IUBMB Life 61: 781–790, 2009. doi: 10.1002/iub.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansen SB, Tao X, MacKinnon R. Structural basis of PIP2 activation of the classical inward rectifier K+ channel Kir2.2. Nature 477: 495–498, 2011. doi: 10.1038/nature10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Subedi P, Schneider M, Philipp J, Azimzadeh O, Metzger F, Moertl S, Atkinson MJ, Tapio S. Comparison of methods to isolate proteins from extracellular vesicles for mass spectrometry-based proteomic analyses. Anal Biochem 584: 113390, 2019. doi: 10.1016/j.ab.2019.113390. [DOI] [PubMed] [Google Scholar]
- 16.Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem 74: 5383–5392, 2002. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 17.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem 75: 4646–4658, 2003. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 18.Bjorkgren I, Mendoza S, Chung DH, Haoui M, Petersen NT, Pv L. The epithelial potassium channel Kir7.1 is stimulated by progesterone. J Gen Physiol 153: e202112924, 2021. doi: 10.1085/jgp.202112924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vera E, Cornejo I, Burgos J, Niemeyer MI, Sepulveda FV, Cid LP. A novel Kir7.1 splice variant expressed in various mouse tissues shares organisational and functional properties with human Leber amaurosis-causing mutations of this K+ channel. Biochem Biophys Res Commun 514: 574–579, 2019. doi: 10.1016/j.bbrc.2019.04.169. [DOI] [PubMed] [Google Scholar]
- 20.Shirani A, Mojarrad JS, Farkhani SM, Khosroshahi AY, Zakeri-Milani P, Samadi N, Sharifi S, Mohammadi S, Valizadeh H. The relation between thermodynamic and structural properties and cellular uptake of peptides containing tryptophan and arginine. Adv Pharm Bull 5: 161–168, 2015. doi: 10.15171/apb.2015.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donato L, Scimone C, Alibrandi S, Abdalla EM, Nabil KM, D'Angelo R, Sidoti A. New omics-derived perspectives on retinal dystrophies: could ion channels-encoding or related genes act as modifier of pathological phenotype? Int J Mol Sci 22: 70, 2020. doi: 10.3390/ijms22010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pattnaik BR, Tokarz S, Asuma MP, Schroeder T, Sharma A, Mitchell JC, Edwards AO, Pillers DA. Snowflake vitreoretinal degeneration (SVD) mutation R162W provides new insights into Kir7.1 ion channel structure and function. PLoS One 8: e71744, 2013. doi: 10.1371/journal.pone.0071744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doring F, Derst C, Wischmeyer E, Karschin C, Schneggenburger R, Daut J, Karschin A. The epithelial inward rectifier channel Kir7.1 displays unusual K+ permeation properties. J Neurosci 18: 8625–8636, 1998. doi: 10.1523/JNEUROSCI.18-21-08625.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tateno T, Nakamura N, Hirata Y, Hirose S. Role of C-terminus of Kir7.1 potassium channel in cell-surface expression. Cell Biol Int 30: 270–277, 2006. doi: 10.1016/j.cellbi.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Denton JS, Kharade SV. Plight of the pore polar bar(rier). Channels (Austin) 11: 502–503, 2017. doi: 10.1080/19336950.2017.1367234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Zidek A, Potapenko A, Bridgland A, Meyer C, Kohl SAA, Ballard AJ, Cowie A, Romera-Paredes B, Nikolov S, Jain R, Adler J, Back T, Petersen S, Reiman D, Clancy E, Zielinski M, Steinegger M, Pacholska M, Berghammer T, Silver D, Vinyals O, Senior AW, Kavukcuoglu K, Kohli P, Hassabis D. Applying and improving AlphaFold at CASP14. Proteins 89: 1711–1721, 2021. doi: 10.1002/prot.26257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varadi M, Anyango S, Deshpande M, Nair S, Natassia C, Yordanova G, Yuan D, Stroe O, Wood G, Laydon A, Zidek A, Green T, Tunyasuvunakool K, Petersen S, Jumper J, Clancy E, Green R, Vora A, Lutfi M, Figurnov M, Cowie A, Hobbs N, Kohli P, Kleywegt G, Birney E, Hassabis D, Velankar S. AlphaFold protein structure database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res 50: D439–D444, 2022. doi: 10.1093/nar/gkab1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lam AKM, Rheinberger J, Paulino C, Dutzler R. Gating the pore of the calcium-activated chloride channel TMEM16A. Nat Commun 12: 785, 2021. doi: 10.1038/s41467-020-20787-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sushko ML, Thomas DG, Pabit SA, Pollack L, Onufriev AV, Baker NA. The role of correlation and solvation in ion interactions with B-DNA. Biophys J 110: 315–326, 2016. doi: 10.1016/j.bpj.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato C, Sato M, Iwasaki A, Doi T, Engel A. The sodium channel has four domains surrounding a central pore. J Struct Biol 121: 314–325, 1998. doi: 10.1006/jsbi.1998.3990. [DOI] [PubMed] [Google Scholar]
- 31.Duignan TT, Schenter GK, Fulton JL, Huthwelker T, Balasubramanian M, Galib M, Baer MD, Wilhelm J, Hutter J, Del Ben M, Zhao XS, Mundy CJ. Quantifying the hydration structure of sodium and potassium ions: taking additional steps on Jacob's Ladder. Phys Chem Chem Phys 22: 10641–10652, 2020. [DOI] [PubMed] [Google Scholar]
- 32.Black KA, He S, Jin R, Miller DM, Bolla JR, Clarke OB, Johnson P, Windley M, Burns CJ, Hill AP, Laver D, Robinson CV, Smith BJ, Gulbis JM. A constricted opening in Kir channels does not impede potassium conduction. Nat Commun 11: 3024, 2020. doi: 10.1038/s41467-020-16842-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin R, He S, Black KA, Clarke OB, Wu D, Bolla JR, Johnson P, Periasamy A, Wardak A, Czabotar P, Colman PM, Robinson CV, Laver D, Smith BJ, Gulbis JM. Ion currents through Kir potassium channels are gated by anionic lipids. Nat Commun 13: 490, 2022. doi: 10.1038/s41467-022-28148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caralampio DZ, Martinez JM, Pappalardo RR, Marcos ES. The hydration structure of the heavy-alkalines Rb+ and Cs+ through molecular dynamics and X-ray absorption spectroscopy: surface clusters and eccentricity. Phys Chem Chem Phys 19: 28993–29004, 2017. doi: 10.1039/c7cp05346k. [DOI] [PubMed] [Google Scholar]
- 35.Li H, Francisco JS, Zeng XC. Unraveling the mechanism of selective ion transport in hydrophobic subnanometer channels. Proc Natl Acad Sci USA 112: 10851–10856, 2015. doi: 10.1073/pnas.1513718112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ainavarapu SRK, Brujic J, Huang HH, Wiita AP, Lu H, Li L, Walther KA, Carrion-Vazquez M, Li H, Fernandez JM. Contour length and refolding rate of a small protein controlled by engineered disulfide bonds. Biophys J 92: 225–233, 2007. doi: 10.1529/biophysj.106.091561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopatin AN, Nichols CG. [K+] dependence of open-channel conductance in cloned inward rectifier potassium channels (IRK1, Kir2.1). Biophys J 71: 682–694, 1996. doi: 10.1016/S0006-3495(96)79268-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujiwara Y, Kubo Y. Ser165 in the second transmembrane region of the Kir2.1 channel determines its susceptibility to blockade by intracellular Mg2+. J Gen Physiol 120: 677–693, 2002. doi: 10.1085/jgp.20028663. [DOI] [PMC free article] [PubMed] [Google Scholar]