Abstract
Diabetes is a major risk factor for cardiovascular diseases, including diabetic cardiomyopathy, atherosclerosis, myocardial infarction, and heart failure. As cardiovascular disease represents the number one cause of death in people with diabetes, there has been a major emphasis on understanding the mechanisms by which diabetes promotes cardiovascular disease, and how antidiabetic therapies impact diabetic heart disease. With a wide array of models to study diabetes (both type 1 and type 2), the field has made major progress in answering these questions. However, each model has its own inherent limitations. Therefore, the purpose of this guidelines document is to provide the field with information on which aspects of cardiovascular disease in the human diabetic population are most accurately reproduced by the available models. This review aims to emphasize the advantages and disadvantages of each model, and to highlight the practical challenges and technical considerations involved. We will review the preclinical animal models of diabetes (based on their method of induction), appraise models of diabetes-related atherosclerosis and heart failure, and discuss in vitro models of diabetic heart disease. These guidelines will allow researchers to select the appropriate model of diabetic heart disease, depending on the specific research question being addressed.
Keywords: cardiac function, diabetic cardiomyopathy, obesity, type 1 diabetes, type 2 diabetes
INTRODUCTION
Diabetes continues to increase at an alarming rate, with current estimates now indicating that there will be ∼700 million people worldwide living with diabetes by 2045 (1). Despite effective control of glycemia (targeting glycated hemoglobin <7.0%) being positively associated with reduced risk for microvascular complications (2), the majority of deaths in individuals with diabetes are due to macrovascular complications (3–5). Big data studies totaling over 1.9 million people showed that diabetes increases the risk of angina and myocardial infarction, with peripheral arterial disease and heart failure being the most common initial manifestations of cardiovascular disease in type 2 diabetes (T2D) (6). Therefore, there is currently an unmet need for cardiovascular therapies for patients with diabetes. Furthermore, major health regulatory agencies (e.g., US Food and Drug Administration and European Medicines Agency) have mandated that all new therapies in development for diabetes undergo rigorous assessment of cardiovascular risk through large-scale cardiovascular outcome trials before approval. This has resulted in a greater demand for better preclinical models of diabetes not only for the development of cardiac therapies but also for the early identification of deleterious cardiac side effects.
In the past 10–20 years, the field has made great strides in identifying key mechanisms driving diabetes-related heart disease (extensively reviewed in Refs. 4 and 7), which has been greatly aided by the development and improved characterization of models of diabetes, primarily in animals. The requirements of a model of diabetic heart disease depend upon the specific scientific question being asked, but broadly the model needs to replicate the human condition, replicate the mechanistic changes occurring within the heart of a person with diabetes, or replicate the drivers of diabetic myocardial dysfunction. The model must be reproducible, easily accessible, and fall within the remit of animal guidelines within the country of research. Although there is currently no specific model of diabetes whose associated cardiac dysfunction perfectly models the human disease, the purpose of this guidelines document is to provide the field with information on which aspects of cardiovascular disease are best represented by the available models. This review aims to emphasize the advantages and disadvantages of using these models to investigate mechanisms and potential treatments of cardiovascular diseases, and to highlight the practical challenges and technical considerations involved. We will herein review the preclinical models of diabetes according to their method of induction—dietary, pharmacological, and genetic—focusing first on T2D and then on type 1 diabetes (T1D). A discussion of models of diabetes-related atherosclerosis and heart failure is also included, as well as in vitro models of diabetes.
THE CLINICAL PICTURE OF DIABETIC HEART DISEASE
Diabetes is a major risk factor for vascular disease including both microvascular (retinopathy, nephropathy, coronary microvascular, and neuropathy) and macrovascular manifestations (peripheral vascular disease, cerebrovascular, and coronary artery disease). Patients with diabetes have a two- to fourfold increased risk of coronary heart disease and ischemic stroke, and a 1.5- to 3.6-fold increase in mortality. As such, diabetes is a major risk for adverse cardiovascular events, and is such a powerful risk factor that it has been considered a “cardiovascular risk equivalent” (i.e., patients with diabetes but without coronary heart disease have a similar coronary mortality to patients without diabetes who had a previous coronary event) (8).
The term diabetic cardiomyopathy refers in the broadest sense to cardiac morphological and functional changes that occur because of diabetes, and importantly in the absence of other etiologies that exert their own independent effects, for example, independent of coronary artery disease, hypertension, valvular, or congenital heart disorders. One of the major challenges is the lack of a universally accepted and consistently applied definition of diabetic cardiomyopathy, with several definitions being used that cover the whole spectrum of diabetic heart disease from subclinical changes to overt heart failure. It is further complicated by the bidirectional link between diabetes and heart failure in humans, where diabetes increases the risk of heart failure, and heart failure itself increases the risk of T2D. With no universally accepted definition, it is difficult to assess the true incidence of diabetic cardiomyopathy.
Although they may vary in severity, it is now widely accepted that several common cardiac structural changes are seen in humans with diabetes. Left ventricular (LV) hypertrophy (defined for the purposes of this review as elevated total LV mass) is commonly seen in adults with diabetes, with around 70% showing some form of hypertrophy (9). Although both eccentric hypertrophy (elevated LV cavity size and preserved wall thickness) and concentric hypertrophy (normal or reduced cavity size and elevated wall thickness) are both reported, it is now generally accepted that reduced LV cavity size and concentric LV hypertrophy represent the main structural characteristics of diabetic heart disease (10). However, any cardiac hypertrophic remodeling must be taken in the context of the patients’ sex (11), ethnicity (12), body habitus (13), and arterial blood pressure (14). Patients with diabetes have evidence of diffuse myocardial fibrosis and expanded extracellular volume (15), as detected using magnetic resonance imaging (MRI) techniques.
Diastolic dysfunction is the earliest functional change in diabetic cardiomyopathy. Observational studies have found an increased frequency of diastolic dysfunction in T2D with prevalence varying from 20% to 78% depending on the criteria used (16, 17). Heart failure with preserved ejection fraction (HFpEF) is emerging as a major complication for patients with diabetes, with 30%–40% of patients with HFpEF also having diabetes (18). However, the mechanisms linking diabetes to this growing prevalence of HFpEF are currently undefined. There is a clear epidemiological relationship between T2D and heart failure with reduced ejection fraction (HFrEF), however, there are very few, if any, studies linking diabetes per se, in the absence of myocardial infarction, to progressive systolic decline. Most of the human studies reporting systolic dysfunction describe subclinical changes in systolic strain (12), rather than global changes in LV ejection fraction (LVEF).
Traditionally, cardiac dysfunction in diabetes is thought to progress along a spectrum from subclinical diastolic dysfunction to subclinical systolic dysfunction and then to overt systolic dysfunction and HFrEF. Although all are seen in diabetes, the evidence for this progression, in humans, is weak. Rather than being successive stages of diabetic cardiomyopathy, it is now thought that the HFrEF (dilated cavity and reduced systolic function) and HFpEF (reduced cavity size, concentric LV hypertrophy, and diastolic dysfunction) are not successive stages, but rather develop as separate phenotypes. The latest theories suggest that in the HFpEF phenotype, the central pathology is concentric LV remodeling resulting from coronary microvascular dysfunction, myocardial fibrosis, and metabolic dysregulation, whereas in HFrEF eccentric LV remodeling results from cardiomyocyte cell death, fibrosis, and microvascular rarefaction (19). What determines whether someone with diabetes develops HFpEF or HFrEF is unknown but is likely multifactorial and influenced by both genes and the environment.
Cardiac metabolic changes are almost universally reported in human studies and involve a metabolic shift away from utilization of glucose toward a greater utilization of fatty acids. Using positron emission tomography, diabetes has been shown to be associated with increased myocardial fatty acid utilization, increased oxygen consumption, and reduced cardiac efficiency (20, 21). When coupled with the evidence of reduced carbohydrate uptake and metabolism and the recent hyperpolarized 13C study showing reduced pyruvate dehydrogenase flux (21–23), this suggests a shift in cardiac energy substrate utilization from carbohydrate to lipids in humans. Although lipid accumulation is commonly reported in diabetes (24, 25) and is linked to diastolic dysfunction (26), the evidence for lipotoxicity as the causative mechanism is not well developed in humans. Similarly, glucotoxicity, as shown by changes in the receptor for advanced glycation end products, is associated with cardiovascular disease and mortality in people with diabetes. However, mechanistic studies showing a causative relationship are lacking (27, 28). With 31P magnetic resonance spectroscopy, a decreased myocardial phosphocreatine (PCr)-to-adenosine triphosphate (ATP) ratio has been described (23, 24), suggesting that myocardial energetic impairment is a key component in the pathophysiology of human diabetic heart disease. In addition, impaired metabolic flexibility in response to increased cardiac workload has been demonstrated by a decreased PCr-to-ATP ratio in exercising patients with diabetes compared with when at rest (24).
MODELS OF DIABETIC HEART DISEASE
There are numerous experimental models now available for studying the heart in diabetes, from in vitro systems in isolated cells to in vivo models in small rodents. In addition, larger animal species such as pigs, dogs, and even nonhuman primates have now been characterized for their validity in modeling diabetes. There are important differences between these models in terms of their ability to reproduce key features of diabetic heart disease. Although our primary focus will be on rodent models of diabetes, we will begin by discussing the growing development of more humanized translational cell models using inducible pluripotent stem cell-derived cardiomyocytes.
IN VITRO MODELS OF INSULIN RESISTANCE AND TYPE 2 DIABETES
Human Inducible Pluripotent Stem Cell-Derived Cardiomyocytes
Cell models offer valuable tools to study the underlying molecular mechanisms of insulin resistance and T2D in greater detail. In 2006, Takahashi and Yamanaka (29) demonstrated that human somatic cells can be reprogrammed into a developmental “ground state” (i.e., induced pluripotent stem cell, iPSC), before being differentiated and matured into human-iPSC-derived cardiomyocytes (hiPSC-CMs) (30). This exciting breakthrough in cell research has opened new avenues for translational studies, investigating disease mechanisms, high-throughput drug testing, and advancing the potential for personalized medicine.
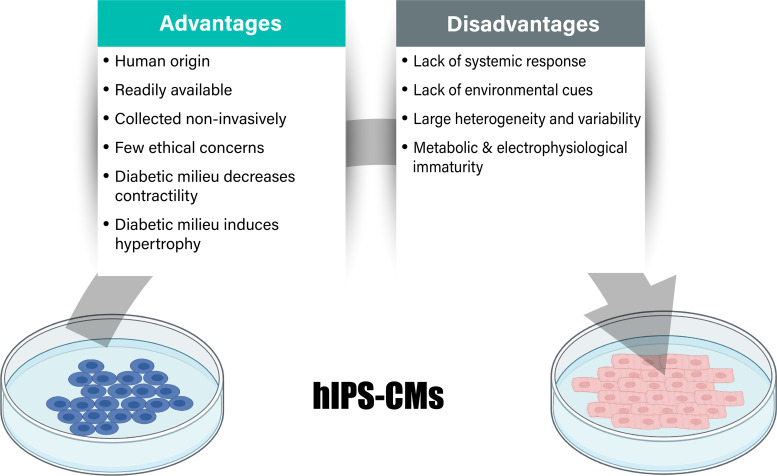
The use of hiPSC-CMs to study complex multifactorial, lifestyle-related disorders like diabetes has been minimally explored when compared with monogenic diseases and regenerative medicine. The few available studies primarily make use of healthy donor-derived hiPSC-CMs and expose these to a diabetic-like environment, by mimicking the hyperglycemia, hyperlipidemia, hyperinsulinemia, or other circulating factors including cortisol and endothelin-1 (31–37). An advantage of this approach is that different developmental stages within the diabetes pathogenesis can be mimicked, and the different circulating factors can be independently manipulated (Fig. 1). hiPSC-CMs exposed to high palmitate conditions showed reduced expression of proteins involved in insulin signaling, and reduced insulin-stimulated glucose uptake, together with increased non-insulin stimulated fatty acid uptake (31–33, 37). hiPSC-CMs exposed to a diabetic-like media showed increased hypertrophic markers, cellular hypertrophy, and reduced contractility (35–37). This experimental approach has been used to screen for new therapeutic compounds as well as to investigate the mechanisms of action of existing drugs used in the treatment of diabetes (34–36).
Figure 1.
In vitro models of type 2 diabetes (T2D). Advantages and disadvantages of human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) with details on key aspects relating to phenotypic features of diabetic heart disease.
A few studies have generated iPSC-CMs directly from patients with T2D (T2D hiPSC-CMs) (36, 38). One study used skin biopsies from two patients with diabetes, one patient with slow disease progression without cardiovascular disease, and one patient with fast disease progression with cardiovascular disease (36). Both studies showed that iPSC-CMs obtained from patients with diabetes contained features similar to that seen in the diabetic heart, suggesting genomic or epigenomic predisposition to the disorder that is retained during the reprogramming/maturation protocol. T2D hiPSC-CMs exhibited a hypertrophic phenotype, increased brain natriuretic peptide release, and sarcomeric disarray (37, 38). In addition, the patient-derived cells showed increased lipid accumulation and peroxidation, reduced mitochondrial number, abnormal mitochondrial structure, and decreased oxygen consumption rates (38). The cellular changes seem to correspond to the clinical status of the donor (36), although the disease phenotype at the cellular level seems to precede clinical manifestations (38). Therefore, both healthy hiPSC-CMs that are exposed to a diabetogenic environment and iPSC-CMs from patients with T2D show some features like those seen in diabetic cardiomyopathy and therefore may be valuable models to study its pathology.
The advantages of hiPSCs are that they are of human origin, readily available, collected in a noninvasive manner, potentially able to form any cell type, and have relatively few ethical issues. However, there is greater heterogeneity obtained during cell culturing when compared with other widely used in vitro models of insulin resistance and diabetes, such as rodent cardiomyocytes or immortalized cardiac cell lines. Differences in individual donors, genetic stability, and experimental variability (e.g., in reprogramming, differentiation, and maturation protocols) cause variation (39). To account for this variability, the use of multiple patient and control cell lines, as well as multiple clones is recommended. Another limitation of hiPSC-CMs is their relative immaturity on a metabolic, structural, and electrophysiological level when compared with human adult cardiomyocytes. Engineered heart tissue and self-assembling organoids are now being used in the field to provide models to investigate disease pathogenesis as well as for early stage drug testing but are in the infancy of their development and utilization for diabetic heart disease (40–42). Taken together, this hiPSC-CM model approach holds exciting promise for diabetic heart disease research. Technological improvement of hiPSC-CM maturity, standardization of experimental protocols for generating hiPSC-CM models, and creation of three-dimensional engineered heart tissue will help bridge the gap between model and clinical practice. In addition, it is advised to consider the disease development stage of the hiPSC-CM model and to report information on genetic background, possible epigenetic modifiers (e.g., lifestyle), gender, and clinical phenotype of the donor whenever possible.
Cardiomyocyte Models of Animal Origin
Before the development of iPSCs, most in vitro studies on insulin resistance and diabetes have been performed using neonatal and adult rodent cardiomyocytes. Cells are typically isolated from control animals and exposed to diabetic-like conditions, or cells can be directly isolated from insulin-resistant/diabetic animal models. Alternatively, immortalized rodent cell lines, such as the mouse atrial cell line HL-1 and the rat cardiomyoblast cell line H9C2, have been used following exposure to a diabetogenic milieu.
Immortalized cell lines have unlimited cell renewal capacity and are relatively easy to transfect, enabling molecular biological manipulations. However, they present with a tumor-like metabolic phenotype with suppression of oxidative metabolism and triggered glycolytic metabolism, contrasting that of the adult heart. Approaches to promote an insulin-resistant state in primary and immortalized cardiomyocytes are similar to those used in hiPSC-CMs by exposing them to metabolic stimuli, including high amounts of palmitate (31), glucose (43), insulin (44), and uric acid (45). Furthermore, activators of the inflammatory response like lipopolysaccharide (46) and tumor necrosis factor-α (47) have been used to induce insulin resistance. Paralleling hiPSC-CMs, exposure of rodent cardiomyocytes to diabetic-like conditions generally leads to reduced insulin signaling and insulin-stimulated glucose uptake, a metabolic shift to fatty acid metabolism and lipotoxicity (48). Decreased contractile function has also been demonstrated in adult rat cardiomyocytes exposed to lipid surplus (31). Isolated rodent cardiomyocytes incubated with high concentrations of glucose have demonstrated glucotoxic phenotypes, characterized by increased apoptosis, NADPH oxidase activation, and flux through the polyol and hexosamine biosynthetic pathways (49, 50). Of note, palmitate-induced insulin resistance models of hiPSC-CMs, human embryonic SC-CMs, HL-1 cells, and primary adult rat cardiomyocytes have been compared in the literature (33). Despite their differences in degree of maturation, all model systems showed similar responses to lipid exposure regarding changes in fatty acid uptake, glucose uptake, and insulin signaling.
Primary cardiomyocytes from rodent models of diabetes maintain their diabetic-like phenotype after isolation (51–53), although it is unclear for how long. Although cells that are isolated from a streptozotocin (STZ)-induced rat model of T1D maintain a blunted insulin-stimulated glucose uptake following overnight culturing (43), cells isolated from Zucker obese (ZO) fatty rats appear to lose their metabolic phenotype toward increased fatty acid oxidation after 48 h in culture (54). Caution should therefore be taken when culturing isolated cardiomyocytes of in vivo models of (pre)diabetes, as their diabetic-like phenotype may disappear. Often neonatal cardiomyocytes are used, because of their greater cell yield, ease of transfection, and spontaneous beating when compared with isolation from adult animals (55). However, neonatal cardiomyocytes are more metabolically and functionally immature than their adult cardiomyocyte counterparts.
IN VIVO MODELS OF INSULIN RESISTANCE AND TYPE 2 DIABETES
Dietary Manipulation to Induce Insulin Resistance and Type 2 Diabetes
High-fat diet-induced obesity (prediabetes).
One of the most frequently used models of obesity/insulin resistance in rodents involves provision of a high-fat diet, with the time frame of dietary provision usually ranging anywhere from as little as 4 wk to as long as 1 year. There are a variety of commercial vendors that supply high-fat diets containing different fat percentages and sources of fat (e.g., saturated fat-based and unsaturated fat-based). One of the most popular vendors in North America is Research Diets, with their 45% kcal from lard and 60% kcal from lard high-fat diets being frequently used to study the pathology of obesity and/or prediabetes.
The diet-induced obesity (DIO) model is widely used in rodents as it recapitulates numerous features of human obesity, including a progressive weight gain involving expansion of visceral adipose tissue and whole body insulin resistance (Fig. 2) (56). However, there are also a multitude of variables one must consider when using the DIO model. Strain is highly important, as certain strains are resistant to DIO [such as the inbred SWR/J and A/J mice (56, 57)], whereas others are highly susceptible, such as the inbred C57BL/6J strain from Jackson Laboratories, which is one of the most widely used strains for DIO studies (56, 58). Yet despite being highly susceptible to DIO, only a proportion of C57BL/6J mice develop significant obesity in response to high-fat diet supplementation, with males being more susceptible than their female counterparts (59, 60). The most common rat strains used for DIO studies include Sprague–Dawley, Wistar, and Long-Evans rats, but unlike the previously described mouse strains, these rat strains are all outbred, and thus one needs to consider potential genetic variation in the strain between studies. In parallel to their mouse counterparts, Sprague–Dawley rats also demonstrate significant variability regarding the progression of obesity in response to DIO (61, 62), though their progression is much more comparable between males and females (61). Another area of consideration in DIO studies involves the control diet of comparison, as investigators frequently use animal facility standard chow for their lean control mice, which are often included within an institution’s operating animal housing costs. A commercial provider’s DIO-associated control diet will usually be matched to the high-fat diet for micronutrients and protein.
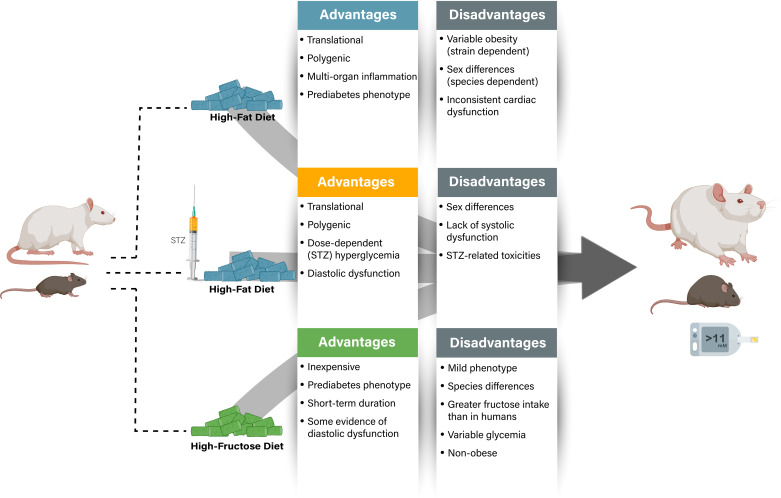
Figure 2.
Dietary models of prediabetes/type 2 diabetes (T2D). Advantages and disadvantages of the primary dietary models of prediabetes/T2D, with details on key aspects relating to phenotypic features of diabetic heart disease. While “nonobese” is listed as a disadvantage due to prediabetes/T2D often been associated with underlying obesity, the absence of obesity can also be an advantage if one needs to address whether the cardiac phenotype is independent of body weight gain. STZ, streptozotocin.
Although obesity is a clear risk factor for the progression of cardiovascular disease in humans, one of the primary concerns with DIO models in animals, particularly in mice, is that they do not consistently exhibit changes in cardiac function in vivo. Many studies have shown no changes in diastolic or systolic function in obese C57BL/6J mice, regardless of the type of diet used to induce obesity (e.g., lard-based, hydrogenated coconut oil) (63–66). Nonetheless, there are inconsistencies in the field with these diets, as others have reported that they do promote cardiac dysfunction (67), and in some cases they induce significant systolic dysfunction (e.g., decreased LVEF) reminiscent of a HFrEF phenotype (68). Similarly, some studies have suggested that high-fat-based DIO models do promote in vivo diastolic dysfunction (69, 70), but this still needs to be evaluated more extensively. It is important to consider how these findings relate to the human population being modeled, and the relationship between cardiac dysfunction and the degree of obesity in patients (71). Numerous mechanisms proposed to contribute to diabetic heart disease are also exhibited in DIO models. This includes alterations in cardiac metabolism [e.g., decreased glucose oxidation and increased fatty acid oxidation (53, 65, 72, 73)], cardiac lipotoxicity [e.g., ceramide and/or diacylglycerol (DAG) accumulation (63, 67, 74)], inflammation (75), microvascular dysfunction (76), and endoplasmic reticulum stress (77, 78) to name a few. The fact that these mechanisms are also evident in rodent models of T2D would suggest that they are induced by obesity and may be directly causal to the pathology of diabetic heart disease.
High fructose-induced insulin resistance/prediabetes.
A high-fructose dietary intervention has proven to be a useful experimental tool to generate a preclinical rodent model to study cardiac pathology associated with insulin resistance and prediabetes (Fig. 2). These dietary studies have demonstrated that dietary fructose confers adverse effects on systemic metabolism and cardiac function, even in the absence of marked hyperglycemia and obesity (for review, see Ref. 79). Tightly controlled dietary intervention studies comparing fructose, glucose, and sucrose have advanced the proposition that it is the fructose component of the sucrose dimer that mediates the adverse metabolic response to sugar-induced metabolic disease (80). The characteristics of fructose-induced systemic metabolic dysregulation in rodents may be species-specific, and caution must be taken when comparing between species, but in most settings rats are more susceptible than mice. In general, the systemic phenotype induced by a high-fructose diet in rodents most commonly includes insulin resistance and dyslipidemia (81–83), with fructose-induced hypertension and hyperinsulinemia also reported in some studies (84, 85). Blood glucose is either unchanged or mildly elevated in fructose-fed rats and mice, recapitulating the very early stages of disease progression (86, 87).
Reported methodology for fructose dietary interventions varies in diet composition (% fructose, iso- vs. hyper-caloric), administration (pellets vs. drinking water), and diet duration. Typically, studies in rats and mice use isocaloric diets containing 60%–72% of energy from fructose, for 4–12 wk duration. A custom-control diet matched to the fructose diet for energy, macronutrients and micronutrients allows for a tightly controlled dietary intervention where the effects of dietary fructose can be directly investigated. Administration of the diet using pelleted food generally provides stable food intake and ensures an isocaloric setting. Interventions that add fructose to the drinking water (usually 10% fructose) may result in higher (or variable) caloric intake, depending on whether extra calories ingested via the drinking water are offset by a reduction in food intake. Housing conditions can also have an effect, and an important consideration for fructose dietary studies is the humidity of the animal housing unit. High humidity can affect the consistency of the fructose diet pellets, and frequent replacement of food is required to maintain palatability (e.g., every 1 to 2 days). To preserve the integrity of the fructose diet, the pellets should be refrigerated (or frozen for long-term storage) in sealed containers because of the susceptibility of high sugar content to support bacterial growth.
The key practical advantages of the fructose diet model of prediabetes are that it is relatively inexpensive, short term, and can be easily applied to rodent models with underlying genetic conditions. The model recapitulates clinical features of insulin resistance and allows investigation into the impact of a relatively mild prediabetic state, in the absence of marked hyperglycemia and obesity. Despite this mild systemic phenotype, a notable cardiac phenotype has been reported. Cardiac tissue insulin resistance is evident as demonstrated by downregulation of the insulin signaling pathway in fructose-fed rodents (86). A high-fructose diet has also been shown to induce cardiac hypertrophy, oxidative stress, and apoptosis (82, 88, 89). Fructose-induced cardiac dysfunction has been characterized by alterations in cardiomyocyte excitation-contraction coupling and Ca2+ handling (90). Although not yet extensively studied, some evidence of diastolic dysfunction in fructose-fed rodents has been reported using flow-Doppler echocardiography (E/A ratio) (91, 92). However, further work is required to fully characterize the structural and functional changes that occur in response to a high-fructose diet using in vivo imaging modalities.
Combination Dietary Manipulation and Pharmacology to Induce Insulin Resistance and Type 2 Diabetes
High-fat diet/streptozotocin model of type 2 diabetes.
Although numerous dietary strategies are used to model insulin resistance and/or a prediabetic state in rodents, the addition of low-dose STZ injections (usually around 25 mg/kg in rats and 75 mg/kg in mice) to a modified diet is becoming increasingly popular. STZ is a -cell toxin, typically used at higher concentrations to produce T1D (see Streptozotocin Model of Type 1 Diabetes); however, when used at a lower dose in combination with a high-fat diet, it can induce hyperinsulinemia and hyperglycemia. This model imparts several practical advantages including its lower cost, its speed to develop the disease, and the ability to modify the STZ dose to generate a spectrum of disease severity (Fig. 2). The diet incorporated is often a high fat or a combination of high fat and high sucrose provided anywhere from 3 to 12 wk in duration, with STZ administered at ∼2 wk in rats, and at ∼4 or 5 wk in mice. Solutions of STZ should be prepared freshly in a citrate buffer at pH 4 and used immediately, because STZ rapidly precipitates out of solution (93). Fasting animals (for at least 5 h or overnight) before STZ administration increases reproducibility, by minimizing competition between STZ and blood glucose for the islet β-cell glucose transporter (GLUT) 2 (56). The amount of STZ can be titrated to induce the extent of hyperglycemia required and can either be given as a one-off bolus or on multiple occasions. For example, in Wistar rats, an STZ concentration below 30 mg/kg induces modest increases in plasma glucose while maintaining the hyperinsulinemia produced by the high-fat diet, but above 30 mg/kg the hyperglycemia becomes more severe and is accompanied by hypoinsulinemia, weight loss, and hyperketonemia (94). When one uses this model for the first time, it is recommended that a pilot study is carried out to optimize the dose of STZ needed, as strain, sex, and housing environment can influence susceptibility.
The addition of low-dose STZ to a high-fat diet elicits a highly reproducible diastolic dysfunction (4, 95–98), and manifests as a reduction in the mitral E/A and tissue Doppler e′/a′ ratios, or an elevation in the E/e′ ratio, the latter of which is often more reliable and reproducible than the former (99). Using the isolated perfused heart, diastolic dysfunction is evident in response to stress such as hypoxia, which induces an increase in end-diastolic pressure and can be reversed by treatment with metabolic therapy (100). Of interest, the diastolic dysfunction observed in males does not appear to be evident in females, though this could be due to female C57BL/6J demonstrating resistance to the actions of STZ (101, 102), and this will need to be further characterized in future studies.
Despite the dietary/low-dose STZ model producing a highly reproducible diastolic dysfunction in rodents, for the most part, LV systolic function remains normal when compared with lean, healthy controls (96, 97), but whether prolonged duration of the model yields systolic dysfunction remains to be determined. In unpublished findings by the Ussher group, male and female C57BL/6J mice following 18 wk of dietary supplementation with a high-fat diet (STZ administered at the 5-wk time point) are still devoid of any notable systolic dysfunction. Although baseline cardiac function is normal in rodents subjected to the dietary/low-dose STZ model, the response following ex vivo ischemia-reperfusion is abnormal, with decreased recovery of rate pressure product (34). However, it has not been determined how rodents subjected to this model recover following in vivo myocardial infarction or heart failure produced via surgical intervention.
Importantly, the dietary/low-dose STZ model in rodents recapitulates a number of molecular mechanisms indicative of diabetic cardiomyopathy in humans, including impaired energetics and mitochondrial dysfunction (103, 104), often accompanied by elevations and reductions in myocardial fatty acid oxidation and glucose oxidation, respectively (94–97, 100). In addition, this model presents with impaired metabolic flexibility in response to stress, including an impaired ability to upregulate glycolytic flux in response to both acute and chronic oxygen restriction (100, 105). Increases in cardiac fibrosis, inflammation, and oxidative stress, as well as impaired calcium handling are also observed in hearts of rodents subjected to dietary/low-dose STZ-induced T2D (103, 106, 107). This model is highly responsive to most major therapies used in the treatment of T2D, including the first-line therapy metformin (108), the glucagon-like peptide-1 receptor (GLP-1R) agonist liraglutide (95), and the sodium-glucose cotransporter-2 (SGLT2) inhibitor empagliflozin (109), verifying its validity as a translational model. However, a limitation with this model is the inherent toxicities associated with STZ use (e.g., hepatic genotoxicities). There is also a dearth of published information with this model in female rodents, as well as in older rodents, and it will be important for investigators to take this into consideration, given the demographic of individuals affected by T2D.
Genetic Models of Insulin Resistance and Type 2 Diabetes
ob/ob and db/db mouse models of type 2 diabetes.
There are two genetic mouse models of T2D that are frequently used to study the pathophysiology of diabetic heart disease: the obese ob/ob mouse and the diabetic db/db mouse (Fig. 3) (110). Leptin is a peptide hormone secreted by mature adipocytes that regulates food intake and energy expenditure, and these two mouse models are congenitally deficient in either leptin (ob/ob) or the leptin receptor (db/db). Consequently, both the ob/ob and the db/db mice are hyperphagic with development of severe obesity due to increased energy intake and reduced metabolic rate. The increase in body weight plateaus at ∼2 mo of age, approximately double the weight of their lean genetic controls. Although there may be some quantitative and chronological differences, the overall metabolic alterations appear very similar in both ob/ob and db/db mouse strains (111), but with the db/db being more extreme phenotypically. Both models exhibit early signs of hyperinsulinemia due to insulin resistance, euglycemia which progresses to overt hyperglycemia, and dyslipidemia which presents by ∼15 wk of age (111).
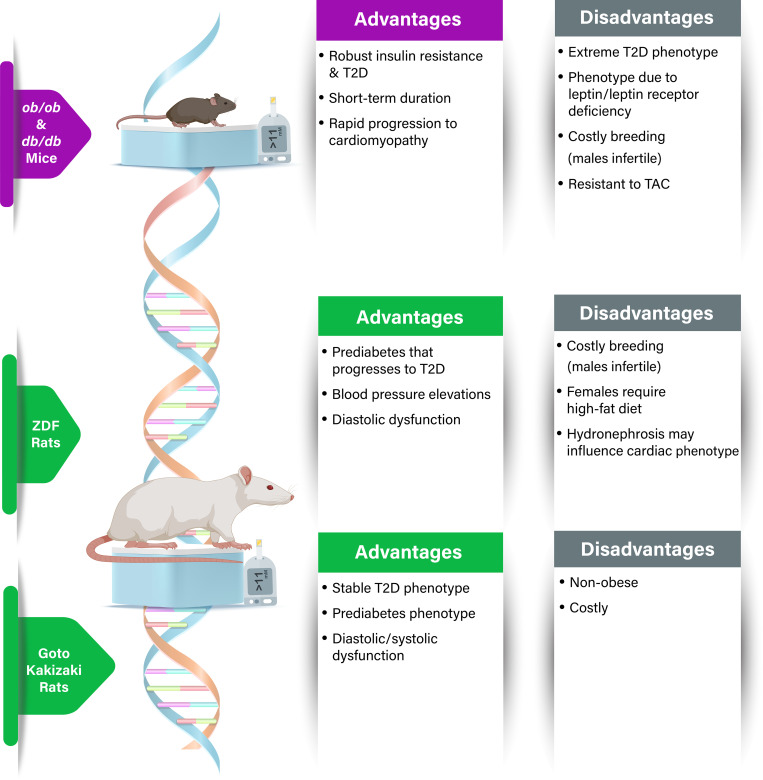
Figure 3.
Genetic models of type 2 diabetes (T2D). Advantages and disadvantages of the primary genetic models of T2D, with details on key aspects relating to phenotypic features of diabetic heart disease. While “nonobese” is listed as a disadvantage due to T2D often been associated with underlying obesity, the absence of obesity can also be an advantage if one needs to address whether the cardiac phenotype is independent of body weight gain. TAC, transverse aortic constriction.
These two models exhibit a cardiac metabolic phenotype with high reliance on myocardial fatty acid oxidation, reduced glucose oxidation rates, mitochondrial dysfunction, and impaired insulin-stimulated glucose uptake (112–114). Another consistent finding is impaired cardiac energetics with reduced energy status (115, 116). Studies addressing the progression from prediabetic state (around 4–6 wk of age) to overt insulin-resistance state (around 12–15 wk of age), suggest that the alterations in myocardial metabolism precede the development of ventricular dysfunction (111, 117, 118), findings which are concordant with observations made in clinical studies (20). Studies on cardiovascular pathophysiological processes in older db/db and ob/ob animals report common traits of diabetes-induced cardiovascular pathology such as increased vascular and myocardial oxidative stress, fibrosis, apoptosis, impaired calcium handling, endothelial dysfunction, and impaired vascular compliance (112, 119–124). As these models are quite severe, the progression to cardiac complications is rapid in these genetic models, facilitating time-effective studies when compared with less severe models of obesity-induced insulin resistance. Careful assessment of ex vivo ventricular function in older db/db mice with established hyperglycemia reports LV systolic and diastolic dysfunction with reduced cardiac output and impaired parameters of ventricular function (116, 125, 126). In vivo assessment of cardiac function with echocardiography or MRI are not conclusive, reporting both impaired (118) and unaltered in vivo cardiac function (115, 126) in db/db mice, which could be explained by systemic adaptations to sustain cardiac function.
The db/db mouse has been used extensively to study antidiabetic treatments, including established drugs used in clinical practice (e.g., biguanides, α-glucosidase inhibitors, sulfonylureas, thiazolidinediones, dipeptidyl peptidase-4 inhibitors, GLP-1R agonists, and SGLT2 inhibitors), as well as experimental compounds. Branded drugs are commonly used as positive controls and produce plasma glucose and insulin lowering effects in db/db mice (127–129). Of interest, the effects on hyperglycemia of these antidiabetic drugs are not always sufficient to preserve insulin secretory capacity in the db/db mouse (129), most likely due to the severity of this model. In terms of cardiovascular effects, many studies have shown that antidiabetic treatments attenuate cardiac pathological remodeling in db/db mice (128, 130–133), even in the absence of glucose-lowering effects (134).
The db/db and the ob/ob models are extreme models of hyperphagia, obesity, and eventually uncontrolled diabetes, and the progression from obesity to β-cell dysfunction in the db/db mouse is extremely rapid when compared with patients (and mechanistically most patients with obesity/diabetes do not have genetic mutations in leptin or its receptor). If planning longer-term treatment/aging studies in the db/db mouse, care must be taken in older mice as they develop additional systemic complications, which challenges both housing and handling of these animals. They show early signs of cold intolerance, are susceptible to several stressors, and with age they frequently develop subcutaneous inflammation, abnormal liver, kidney and spleen morphology, weight loss, lymphomas, peripheral neuropathy, and poor wound healing. This can have practical implications, for example, limiting tail vein cannulations in older mice to avoid tail necrosis and consideration of the humane endpoint for terminating experiments. There are also challenges concerning breeding as these monogenic obese animals are functionally infertile, which makes breeding between the heterozygotes costly.
Zucker diabetic fatty rat model of type 2 diabetes.
This obese T2D model originates from the identification by Theodore and Lois Zucker of a missense mutation in the leptin receptor gene in outbred Merck-M rats, leading to the development of obesity. This strain was named Zucker-Leprfa/fa and is commonly referred to as the fatty or ZO rat. Selective inbreeding of ZO rats over several generations led to the identification of a new substrain of ZO rats with a more diabetic-like phenotype referred to as the Zucker diabetic fatty (ZDF) rat. When compared with their lean equivalents, namely, lean fa/+ or lean Zucker (LZ) rats, ZO rats exhibit increased caloric intake from the age of 4 wk and progressively develop obesity (135). From 6 to 7 wk of age, sustained hyperinsulinemia is sufficient to maintain normoglycemia in most ZO rats, with some reports demonstrating a mild to moderate increase in blood glucose in some ZO rats later in age (136, 137). In contrast, ZDF rats become insulin resistant at 6 to 7 wk of age and have elevated concentrations of insulin (138, 139), which progresses to a robust hyperglycemia by 10–14 wk of age (138, 140). As ZDF rats age, an imbalance between β-cell hyperplasia and apoptosis results in the progressive decrease of insulin secretion, paralleled by an increase in glycemia that can reach >700 mg/dL at 24 wk of age (141, 142). Accordingly, ZDF rats reproduce the course of human T2D from the prediabetic to the diabetic state, as well as other key features of the human metabolic syndrome including dyslipidemia (142, 143) and a mild elevation in blood pressure (Fig. 3) (144).
Unfortunately, there has been a relative lack of comparison between male and female ZDF rats, likely because the spontaneous diabetic phenotype in males is not observed in female ZDF rats. Despite female ZDF rats being obese and hyperinsulinemic, they need to be fed an obesogenic diet to induce T2D (141, 145). The recommended obesogenic diet comprises 48% kcal from fat, which if provided to females induces T2D and if provided to male ZDF rats will further exacerbate their diabetic phenotype (145). The mechanisms explaining these sex-specific differences in diabetes susceptibility remain unknown and will need to be resolved. This is particularly important if using ZDF rats as a model for studying diabetic cardiomyopathy, as in humans diabetic cardiomyopathy and HFpEF are more prominent in women with T2D than in men (146). From 2 mo of age, male ZDF rats demonstrate decreased heart capillary density, and an increase in cardiomyocyte area by 8 mo of age (141, 144). In contrast, echocardiography analysis demonstrates that ZDF rats exhibit no changes in LV end-diastolic diameter or septal and posterior wall thickness (143), and no changes in heart weight-to-tibia length or body weight ratios have been reported in multiple studies using male ZDF rats (138, 143, 147). Millar catheter pressure/volume studies identified the presence of diastolic dysfunction in ZDF male rats at 16 wk of age, which was further exacerbated at 36 wk of age and accompanied by an increased end-diastolic volume (147). Similarly, diastolic dysfunction has also been reported in ZDF rats at 44/45 wk of age, reflected by an increase in LV end-diastolic pressure (148). Of interest, diastolic dysfunction and cardiac hypertrophy have been identified in female ZDF rats, but only when fed on an obesogenic diet (141).
ZDF male rats also recapitulate the changes in myocardial metabolism characteristic of diabetic cardiomyopathy, including decreased rates of glucose metabolism (149, 150), as well as an increase in palmitate metabolism (151). The increased palmitate oxidation in ZDF rats is unable to match the elevated rate of palmitate uptake, such that myocardial triacylglycerol (TAG) and other fatty acid intermediates such as DAG and ceramide accumulate, mediating cardiac lipotoxicity. Of clinical relevance, ZDF rats do respond to the majority of antidiabetic therapies including metformin (152), the GLP-1R agonist liraglutide (153), and the SGLT2 inhibitor empagliflozin (154), highlighting their utility as a translational model of T2D.
An important limitation in using ZDF rats relates to their cardiac phenotype being potentially dependent on their spontaneous development of hydronephrosis, which is characterized by a swelling of the kidney due to an obstructed bladder, a clinical symptom that is absent in humans with T2D (147, 155). Moreover, LV systolic wall stress positively correlates with blood urea nitrogen levels, consistent with the notion that the diabetes-related cardiac dysfunction in ZDF rats may be biased by the presence of underlying kidney disease (155). Therefore, it is recommended that users of the ZDF rat strain rigorously evaluate renal morphology and function to exclude the presence of hydronephrosis as a confounding factor when evaluating the onset of diabetic cardiomyopathy. Similar to what has been previously described for db/db mice, ZDF rats are functionally infertile, which makes breeding costly as heterozygotes (Zucker-Leprfa/+) are needed to maintain the strain.
Goto–Kakizaki rat model of type 2 diabetes.
The Goto–Kakizaki (GK) rat was generated by selective breeding of Wistar rats over numerous generations with glucose intolerance used as a selection index (156). The GK rats manifest the major features of the metabolic, hormonal, and microvascular disorders described in T2D (Fig. 3). GK rats show basal hyperglycemia, hyperinsulinemia, and impaired secretory response to glucose as early as 3 to 4 wk of age, and insulin resistance, dyslipidemia, and T2D at ∼12–16 wk of age (157–159). There are no apparent differences linked to sex regarding basal circulating glucose and insulin levels in GK rats (159), and nondiabetic Wistar rats are the optimal control group for comparison. The GK rats show β-cell dysfunction even in the absence of glucose intolerance from the first 3 wk after birth, a prediabetes period, which cannot be reversed by normal postnatal nutrition and nursing behavior, indicating a genetic basis of T2D (159, 160). They also display late diabetic complications, such as neuropathy (161), retinopathy (162), and polycystic ovary syndrome (163). Genetic linkage analysis suggests that distinct combinations of genetic loci are responsible for different physiological characteristics associated with the diabetic phenotypes, which is consistent with the features of polygenic T2D in humans (164, 165).
Cardiac morphology and function of the GK rat largely mimic those of patients with T2D, including hypertrophy, contractile dysfunction, and metabolic alterations. Cardiac hypertrophy assessed by heart weight to body weight ratio, wall thickness by echocardiography, and individual cardiomyocyte size by histology can be detected as early as at 8 wk of age (166). Functional contractile analyses have generated mixed results. Female GK rats have reduced myocardial blood flow and contractility at the age of 8 to 13 mo, as assessed by perfusion and cine MRI (167). Ultrasound echocardiography studies have demonstrated that male GK rats have preserved systolic but reduced diastolic function at 6 to 7 mo of age, indicative of a diabetic cardiomyopathy phenotype (168, 169), whereas systolic function declines at 12 mo of age (170). Other studies show preserved basal contractility in isolated perfused hearts (171) and in isolated ventricular myocytes (172). Despite this, male GK rats show increased susceptibility to ischemic injury in isolated perfused hearts (171, 173) and accelerated cardiac remodeling after myocardial infarction (174). The male GK rat shows decreased myocardial glucose utilization and nearly a twofold increase in fatty acid oxidation (169). A limitation of the GK rat is that they are costly and nonobese, with minimal fat accumulation in the liver (160, 175). This absence of obesity does not replicate the classical presentation in most patients. However, feeding the GK rat with a high-fat diet can be an alternative approach to induce obesity, which exacerbates the defects in metabolism and cardiac ultrastructure (176, 177).
Other monogenic models of type 2 diabetes.
Several other mouse models of T2D have been used over the years with various benefits and limitations, some of which have value in replicating aspects of diabetes but have not yet been examined extensively for cardiovascular phenotypes. The KKAy mouse was first described in 1970 (178) and generated from the spontaneously diabetic Kuo Konodo (KK) mouse (179), which was bred to also carry the yellow obese gene (Ay) (178). The KKAy mouse model has predominantly been used to study diabetic kidney disease (180, 181), with only a few studies fully exploring its impact on diabetic cardiomyopathy (182). This model has been shown to respond to empagliflozin treatment, thereby improving cardiac fibrosis and oxidative stress markers (182).
More recently, the TallyHo (TH) mouse strain was derived from mice showing polyuria and glucosuria, with genetic mapping identifying three quantitative trait loci focused on regions of chromosome 19 including Tanidd1, chromosome 18 Tanidd2, and a locus on chromosome 16 (183). Relatively little work has been carried out on the cardiovascular phenotype of these mice, making them another novel model for potentially studying the impact of T2D on the heart. A limitation of the TH mouse is its relatively late onset of hyperglycemia [∼26 wk of age (183)] and that only males develop overt hyperglycemia, which is not 100% penetrant (184).
Other investigators have focused on specific aspects of diabetes and have generated targeted approaches to specifically define metabolism and signaling in the heart, such as overexpression or loss of function of key metabolic regulators (e.g., peroxisome proliferator-activated receptor α, insulin receptor) and have been previously reviewed in detail (185, 186). Finally, in the attempt to address precision medicine approaches, investigators have examined common monogenic mutations that are linked to obesity and diabetes. One example is the generation of mouse models for mutations in the melanocortin 4 receptor, a G protein-coupled receptor. Specifically, the recent development of melanocortin 4 receptor hypermorphic mice revealed obesogenic and diabetogenic effects but did not characterize heart function (187).
IN VIVO MODELS OF TYPE 1 DIABETES
Streptozotocin Model of Type 1 Diabetes
STZ is derived from the Gram-positive bacterium Streptomyces achromogenes, and primarily damages insulin-producing pancreatic β-cells because of its affinity for GLUT2, producing a T1D phenotype (188, 189). As described in combination dietary manipulation and pharmacology to induce insulin resistance and type 2 diabetes, STZ must be freshly prepared in acidic citrate buffer and administered in the fasted state to increase the homogeneity of diabetes. The detailed methodology for STZ treatment for mice and rats has been elaborated in a recent publication (190). It is important to note that mice and rats have different susceptibilities to STZ-induced diabetes, with mice requiring a higher dose than rats. There are two frequently used methods of STZ administration to induce T1D, which include either a single, high-dose treatment or a multiple, lower-dose treatment protocol. In mice, a single intraperitoneal injection of 200 mg/kg STZ can induce hyperglycemia, whereas, in rats, a single dose of 65 mg/kg STZ can be sufficient (190). This method increases blood glucose levels to >500 mg/dL within 2 days through nearly complete destruction of pancreatic β-cells (191). As high-dose STZ causes massive and rapid β-cell death, the risk of mortality within the initial 24 h postadministration is high, therefore, animals must be monitored closely. In addition, 10% sucrose water should be provided following STZ administration, to help avoid hypoglycemia caused by the destruction of pancreatic β-cells. To slowly develop a T1D phenotype, which more closely mimics an autoimmune insulitis, low-dose (40–50 mg/kg body wt in mice) intraperitoneal injections of STZ for five consecutive days can be used (190). This method causes less STZ-induced toxicity and has reduced mortality risk (and supplementation of sucrose water can also be applied to this approach).
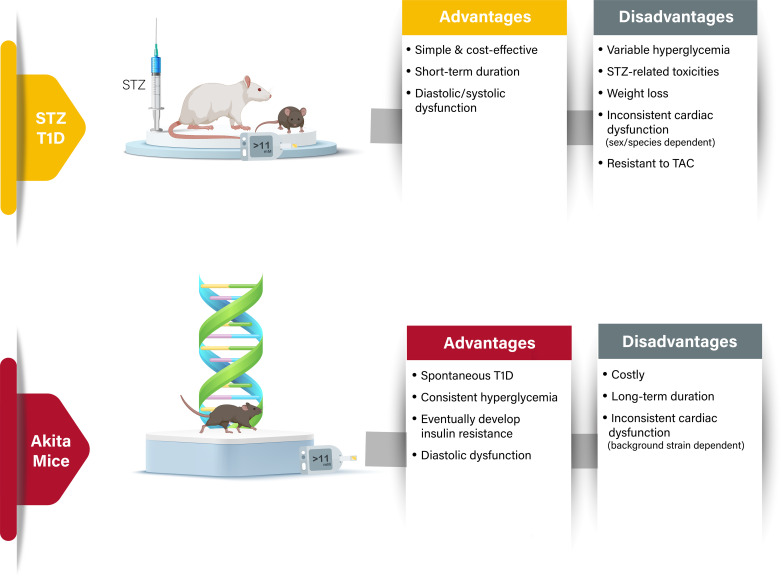
One of the major advantages of the STZ model of T1D is that it is simple, cost-effective, and expeditious (Fig. 4) (192). However, one must consider when using this model that the hyperglycemic effect of STZ can be highly variable within a group of animals, and that STZ sensitivity varies between different strains, where some are high responders and others are low responders (193). The weight loss induced by high-dose STZ can be extreme, so it may be worth using animals with a higher starting body weight, and carefully considering the window of time between STZ administration and final experiments. Although STZ predominantly affects islets of the pancreatic core, its cytotoxic effects are not restricted to pancreatic β-cells but can also affect the liver and kidney (194–196). In addition, STZ has T1D-independent cardiotoxic effects that need to be considered if using this model to study T1D-related cardiomyopathy (197). STZ can have genotoxic effects such as DNA methylation, DNA strand breaks, and inhibition of DNA synthesis (188). Another important aspect of consideration involves the timing of STZ injection, which can affect the progression of hyperglycemia in animals (strongest at 4:00 PM and weakest at 8:00 AM) (198). There also appears to be sex-specific differences regarding susceptibility to STZ, as female mice are less sensitive to STZ-induced β-cell toxicity (199–201).
Figure 4.
Models of type 1 diabetes (T1D). Advantages and disadvantages of the primary models of T1D, with details on key aspects relating to phenotypic features of diabetic heart disease. T2D, type 2 diabetes; TAC, transverse aortic constriction.
Regarding the relevance of STZ-induced T1D in modeling diabetic heart disease, it produces a cardiomyopathy with increased nuclear chromatin condensation and mitochondrial swelling in cardiomyocytes, as well as a marked increase in lipid droplets (202, 203). The metabolic profile of the heart in T1D (increased fatty acid oxidation and decreased glucose oxidation) is also evident in both mice and rats subjected to STZ-induced T1D (116, 204–207). Although female mice may be less sensitive to STZ-induced β-cell toxicity and associated hyperglycemia, the progression of both systolic dysfunction (decreased LVEF) and diastolic dysfunction (increased E/e′ ratio and decreased e′/a′ ratio) appears to be more noticeable than in their male counterparts (208). In general, the overall decrease in LVEF and ensuing systolic dysfunction observed in STZ-treated mice is relatively mild (204, 208), though another study using male Wistar rats was reminiscent of an HFrEF phenotype (209). STZ-induced T1D in male Sprague–Dawley rats also results in systolic dysfunction assessed using pressure-volume loop analysis with Millar catheters, as reflected by decreases in LV pressure and the maximal rate of LV pressure rise (210). Conversely, STZ-induced T1D in male Wistar rats did not produce any systolic dysfunction (decreased LVEF) or diastolic dysfunction (increased E/e′ ratio and decreased e′/a′ ratio) as assessed by ultrasound echocardiography (211). Similarly, ultrasound echocardiography analysis did not reveal any notable systolic dysfunction (decreased LVEF) in male and female mice subjected to STZ-induced T1D (212). Despite some of these inconsistencies in cardiac function profiles, the STZ model of T1D has been invaluable for investigations of T1D-induced structural, metabolic, and functional remodeling in the heart.
Akita Mouse Model of Type 1 Diabetes
Insulin 2 heterozygous (Ins2+/−) Akita mice are a spontaneous, genetic, and nonobese model of T1D, due to a mutation in Ins2 gene that causes a disruption in normal folding of proinsulin, thereby inducing endoplasmic reticulum stress and subsequent β-cell toxicity and loss. As such, Akita mice exhibit decreased pancreatic β-cell density and elevated blood glucose levels starting from the age of 3 to 4 wk, achieving robust (>500 mg/dL) hyperglycemia at the age of 8 wk (213). Females show milder symptoms compared with males with a marked increase in longevity (690 days of age in females vs. 305 days of age in males) (213). Akita mice are commercially available from the Jackson Laboratory, and careful monitoring of their blood glucose levels is required. Blood glucose levels often fluctuate in female Akita mice whereby transient hyperglycemia is often seen during puberty, which undergoes remission to more moderate hyperglycemia after sexual maturation (213).
Akita mice offer several advantages for studying T1D, with one of the most notable being that they require no form of pharmacological or surgical intervention to induce a T1D phenotype (Fig. 4). Moreover, homogeneity of blood glucose concentrations in Akita mice is notable, with their blood glucose levels being very consistent among different age groups, particularly in males. Akita mice also demonstrate significant relevance to human T1D, as their diabetic phenotype progresses with age in a manner that closely recapitulates the pathogenesis in humans. Therefore, Akita mice are of use to study the pathogenesis and progression of T1D in a chronic fashion in different organs (214), such as the heart (215–218), kidney (219), and liver (220, 221). They are frequently used as a model to study the effect of insulin or antidiabetic drug treatments (219, 222–225). Another advantage of the Akita mouse is that insulin resistance begins to manifest by 13 wk of age (226), suggesting that Akita mice exhibit phenotypes observed in patients with advanced-stage T1D and insulin deficiency combined with insulin resistance. When using Akita mice, it is important to take into consideration the strain. Akita mice are available in two strains, the Ins2+/− Akita mice on a C57BL/6J background, and the Ins2+/C96Y Akita mice with a C96Y mutation in the Ins2 gene on a 129/SvEv and DBA/2 background, the latter of which demonstrates enhanced kidney injury (227). As kidney function can influence heart function, it is important to put in context the results and mention the Akita strain used in experiments.
Importantly, Akita mice exhibit several features that are observed in diabetic cardiomyopathy in humans. This includes increased lipotoxicity, metabolic remodeling, and mitochondrial dysfunction leading to cardiac hypertrophy, fibrosis, and cardiac dysfunction (215, 216, 228, 229). In terms of cardiac function, 20-wk-old male Akita mice (background strain not specified) demonstrate normal systolic function (LV fractional shortening) as assessed via ultrasound echocardiography (230). Certain parameters of systolic function continue to remain normal (LVEF and stroke volume) even at 1 year of age as assessed via invasive pressure-volume conductance catheters, though reductions in cardiac output were observed (230). Similarly, 4-mo-old Akita mice (129/SvEv and DBA/2 background) also exhibit normal systolic function (LVEF and fractional shortening) (231). Conversely, 4-mo-old male Akita mice (C57BL/6J background) have decreased systolic function (LVEF) (232). These inconsistencies in cardiac function profiles once more emphasize the importance of specifying background strain when using Akita mice. Diastolic dysfunction also appears to be present in Akita mice regardless of background, as tissue Doppler assessments indicated reductions in the e′/a′ ratio at 16 wk of age in Akita mice on a C57BL/6J background (229), while the E/e′ and deceleration time were increased in 3-mo-old Akita mice on a 129/SvEv and DBA/2 background (215).
DIABETES-RELATED ATHEROSCLEROSIS FOR STUDYING DIABETIC HEART DISEASE
High-Fat/High-Cholesterol Diet in Apolipoprotein E or Low-Density Lipoprotein Receptor Knockout Mice
Although the previous sections provided key details on models of insulin resistance and diabetes that can lead to various forms of diabetic cardiomyopathy, none of these models in isolation produce an atherosclerotic-mediated cardiovascular disease. This is clinically relevant since an increased burden of atherosclerosis is recognized as an essential contributor to increased risk of cardiovascular disease in diabetes (8). Wild-type mice predominantly carry cholesterol in high-density lipoprotein (HDL) fractions and are thus protected from spontaneous atherogenesis. Genetic engineering targeted to disrupt clearance of cholesterol-rich lipoprotein particles together with a Western-style, high-cholesterol diet is needed to increase the exposure of vessels to lipid-rich particles, thereby allowing atherosclerotic lesions to reproducibly develop in the aortic sinus and throughout the aortic arch, proximal aorta, and trunk of the brachiocephalic artery (extensively reviewed in Refs. 233 and 234). Elimination of the low-density lipoprotein (LDL) receptor in mice (Ldlr−/− mice), which increases cholesterol within the LDL particles, or genetic deletion of apolipoprotein E (Apoe−/− mice), which prevents the clearance of TAG-rich postprandial lipoproteins, have both proven to be invaluable tools in the pursuit of molecular mechanisms influencing atherogenesis (235–237). Systematic evaluation of risk factors for atherosclerosis and validation of several human genome-wide association candidates involved in coronary artery disease have determined that these mice recapitulate most human-related mechanisms for atherosclerosis (238).
To study diabetes-related atherosclerosis, both Ldlr−/− and Apoe−/− mice are frequently subjected to the dietary and/or pharmacological models of diabetes described in previous sections. Although the exact composition of “Western style,” atherogenic diets often varies, atherosclerotic lesion development in the aortic arch and throughout the aorta did not differ between high-fat versus high-fat, high-sucrose dietary supplementation (239). However, the addition of sucrose increases insulin resistance, inflammation in peripheral tissues, and accelerates lesion onset (239). Ldlr−/− mice gain weight, develop adiposity, dysglycemia, and hyperinsulinemia more significantly than Apoe−/− mice when fed a high-fat, high-carbohydrate diet (240). The breeding of Apoe−/− mice with T1D Akita mice induces dysglycemia, decreases body weight (both lean and fat mass), but increases fasting and fed cholesterol levels while producing a more severe atherosclerosis (221). Similarly, male Ldlr−/− mice bred with Akita mice have elevated cholesterol and atherosclerosis relative to Ldlr−/− mice (241). Hyperglycemia can also be induced by treating Ldlr−/− mice with STZ (50 mg/kg body wt via intraperitoneal injection for 5 days), which also heightens hypercholesterolemia without significant changes to circulating TAG levels (242). Diabetes induced in Ldlr−/− mice with viral-mediated destruction of insulin-producing cells also exhibit hypercholesterolemia, accelerated lesion initiation, and advanced stages characterized by intraplaque hemorrhage (243). Studies with hyperglycemia in the absence of differences in plasma cholesterol have identified that diabetes can change the morphology of the plaque with more significant calcification in the proximal aorta (244). Glucose-oxidized LDL also influences monocyte proliferation and migration, suggesting a more complex intertwining between these risk factors (245). It is worth remembering though that atherosclerotic lesions in humans are prothrombotic and result in myocardial ischemia. However, this does not occur spontaneously in the plaques of Ldlr−/− or Apoe−/− mice without other manipulations (further reviewed in Ref. 246), such as cross-breeding Apoe−/− mice with scavenger receptor class B type 1 knockout mice, which results in spontaneous myocardial infarction (247). In addition, despite plaque development, neither Ldlr−/− nor Apoe−/− mice develop severe cardiac dysfunction nor do they exhibit abnormal hemodynamic parameters (248). Cardiac hypertrophy has been reported in some studies in aged Apoe−/− mice, which is worsened if the animals are fed a high-fat diet (249).
EVALUATION OF CURRENT LITERATURE
We have also performed an extensive evaluation of previous research, published by the American Journal of Physiology-Heart and Circulatory Physiology, relating to the use of the aforementioned rodent models of diabetes and insulin resistance. Our initial search [performed by J. R. Ussher (April 23, 2022), followed by secondary searches by L. C. Heather and E. E. Mulvihill for confirmation (April 26, 2022)] focused on articles published from 2020 to 2022 using “diabetes” as our key word on the journal website. This search yielded 256 articles, many of which were excluded for not being “original research articles,” being performed in larger animal models, humans, or other models not characterized in this review, or published before 2020. Following the application of these criteria, our search identified 24 original research articles to include in our evaluation (Table 1) (64, 106, 209, 212, 250–269).
Table 1.
Evaluation of publications on diabetes published by the American Journal of Physiology-Heart and Circulatory Physiology
| Criteria | Results and Comments |
|---|---|
| Model of diabetes | Total: 24 publications: high-fat diet alone in mice/rats (12 publications), high-fat diet plus low-dose STZ in mice/rats (2 publications), high-fructose diet in mice/rats (1 publication), db/db mice (2 publications), ZDF rat (1 publication), Apoe−/− mice plus high-fat diet (2 publications), and STZ in mice/rats (4 publications). |
| Rationale provided for selection of diabetes model | Vast majority of studies provided a rationale centered on the theme of diabetes increasing the risk for cardiovascular disease, with limited focus on why the specific model of diabetes was selected vs. other models. |
| Sex, age, strain, and sample size information | 15 studies used only young male animals (only 6 reported females, 3 did not report sex studied, 1 did not report strain studied, and 1 did not report the age of the animals). Sample sizes were always clearly provided with majority of studies using an “n” of at least 5 or greater, and some studies reporting an “n” as high as 28. |
| Glucose homeostasis assessed | 12 studies only provided data on fasting or ad libitum blood glucose and insulin levels (only 7 reported on additional indices of glucose homeostasis, e.g., glucose tolerance, insulin tolerance). A control group was included in several studies to demonstrate that the dietary intervention, genetic model, or STZ treatment exhibited the intended metabolic phenotype. |
| Cardiac physiology assessed | 13 studies did not report on parameters of cardiac function (though this is not always a relevant end point to assess, e.g., studies whose primary goal is to study indices of atherosclerotic plaque formation or vessel function). When cardiac function was assessed, the primary method was ultrasound echocardiography, which frequently measured parameters of both systolic and diastolic function (9 studies reported on parameters of diastolic function), whereas invasive hemodynamics was also used in some studies. |
| Recommendations to Improve Standardization | |
| |
| |
| |
| |
| |
STZ, streptozotocin; ZDF, Zucker diabetic fatty.
It is important to note that models of diabetic heart disease are unique as they must model disrupted metabolism consistent with that observed in clinical diabetes in humans while also producing the cardiac dysfunction consistent with clinical phenotypes. The purpose for selecting a model of diabetes may relate to studying other mechanisms of diabetes and how they may impact the cardiovascular system (e.g., inflammation, oxidative stress, and microvascular function). However, our evaluation of these 24 studies relates to their utility in studying the pathology of diabetic heart disease.
Only approximately half of the 24 studies (11 to be exact) included in our evaluation measured parameters of cardiac function. Importantly, for the 11 studies that did report on parameters of cardiac function, the majority did include an assessment of both systolic and diastolic function. Of the 24 included studies, only seven reported on parameters of glycemia beyond simple measurement of fasting or ad-libitum blood glucose and insulin levels (e.g., glucose and/or insulin tolerance testing). The most frequently used model in our 24 included studies was the use of a high-fat diet to promote weight gain and ensuing obesity. However, as discussed previously, high-fat diet-induced obesity in rodents produces an insulin resistant, prediabetes phenotype, but not a true T2D phenotype while lacking notable cardiac dysfunction (56). There was also often variation in diet composition and duration for those studies that used a high-fat diet to promote weight gain and obesity, and it is necessary for researchers to provide these details for all diets (including the control diets) used in their studies. Other relevant concerns with the 24 included studies are the reliance on primarily young male animals (only 6 reported use of females), with multiple studies not reporting sex at all. It is readily apparent that the issues we observed in the 24 included studies in our evaluation of the current literature, published by the American Journal of Physiology-Heart and Circulatory Physiology, are persistent throughout the field. Therefore, our general recommendations are that researchers using these models need to provide more details regarding the rationale for their choice of diabetes model while providing sufficient details on the strain, age, and sex of the animals studied. Furthermore, the elevation of blood glucose levels does not definitively indicate diabetes, and additional indices of glucose homeostasis need to be reported (e.g., glucose tolerance and insulin tolerance testing). Finally, if an investigation is studying diabetic heart disease per se, regardless if it be the specific mechanisms involved or assessing a potential pharmacotherapy, it is critical that parameters of both systolic and diastolic function are measured. By applying these criteria, it is the hope of all authors of this review that the details provided herein will allow researchers to select the most relevant model to address their specific questions relating to the study of diabetic heart disease.
MODELING ISCHEMIC INJURY AND HEART FAILURE IN DIABETES MODELS
Although many of the aforementioned dietary, genetic, and pharmacological-based models reproduce key features of diabetic heart disease, additional interventions are required to study myocardial infarction and heart failure in the context of diabetes. In this particular section, we will not describe in detail the methodologies behind the various experimental models of myocardial infarction and heart failure, which have already been extensively described in the following reviews (270–276). Instead, we will focus on their application to rodent models of prediabetes and diabetes, discussing in each case both their advantages and limitations.
To study acute changes in cardiac function in response to myocardial ischemia, the most frequently used techniques are the ex vivo isolated Langendorff and the isolated working heart perfusion methods. Ischemia is often implemented in these models by inducing a temporary global no-flow or low-flow ischemia for 20–30 min, following which one can assess contractility via LV-developed pressure and cardiac work/power upon reperfusion (270, 273). These approaches interrogate the response to ischemia in diabetes, but also facilitate therapeutic studies by prior treatment of T2D animals with compounds or acute administration of compounds into the perfusion apparatus. Despite diabetes increasing the risk for ischemic heart disease in humans, both poorer and improved cardiac adaptation to hemodynamic and ischemic stress have been reported in diabetic models, which may be due to variations in the severity of the diabetic phenotype and experimental conditions between studies (34, 117, 125, 151, 251, 277, 278). Isolated perfused heart studies in ob/ob mice, db/db mice, ZDF rats, mice/rats subjected to high-fat diet plus STZ-induced T2D, mice/rats with STZ-induced T1D, and Akita mice have consistently reported an elevation in myocardial fatty acid oxidation and corresponding decline in glucose oxidation (53, 95, 100, 111, 149, 215). Although increases in myocardial fatty acid oxidation are often thought to be detrimental to the pathology of diabetic cardiomyopathy, the addition of exogenous high levels of palmitate was reported to confer beneficial functional effects and improve redox balance in isolated working hearts from db/db mice under conditions of high glucose and isoprenaline stress (279). An advantage with isolated heart perfusion techniques is that they offer a highly controlled environment for one to manipulate the substrate and hormonal concentrations to which the heart is exposed. A limitation is that the metabolic milieu the heart sees in diabetes is vastly different from that of a healthy heart. However, for comparative purposes, substrate and hormonal levels are kept identical between hearts from lean versus diabetic animals. It is also important to acknowledge that these ex vivo perfusion modalities are not equivalent to in vivo measurement of cardiac function, but allow interrogation of different research questions.
To study diabetes-related myocardial infarction and heart failure, surgical procedures involving either ligation of the left anterior descending (LAD) coronary artery or transverse aortic constriction (TAC), respectively, are used with the various animal models of diabetes described earlier. Although both surgical models have been validated with their own strengths and limitations (53, 95, 100, 111, 149, 215), there are several factors one must consider when choosing which diabetes model to use in tandem. For example, halogenated anesthetics should be avoided during temporary ligation of the LAD coronary artery to model acute myocardial infarction, because of their cardioprotective effect in nondiabetic animals, which have been reported to be attenuated with diabetes (280, 281). In addition, variations in experimental parameters such as duration of the diabetic state, changes in circulating insulin concentrations, but also differences in dietary composition (e.g., dietary fat content) can markedly influence outcomes, with infarct sizes in the animal models described earlier reported to be larger, smaller, or similar to that of nondiabetic controls (282–284). Regarding TAC surgery, although most studies demonstrate an exacerbated cardiac hypertrophic response in the setting of diabetes, a major criticism is that the rapid increase in afterload created by the surgery more closely mimics aortic stenosis than the progressive rise in blood pressure associated with diabetes. Because of the relative early development of obesity and insulin resistance, LAD coronary artery ligation or TAC is often performed using db/db mice as the animal model of choice. In line with diabetes worsening myocardial infarction outcomes in patients, db/db mice exhibit a worsening of systolic function versus their nondiabetic controls in response to a 45-min LAD coronary artery occlusion followed by 28 days of reperfusion (285). Conversely, db/db mice demonstrate robust protection against TAC-induced HFrEF (115). Because the failing heart is often thought to be energy-starved and characterized by reduced fatty acid oxidation rates (286), it has been proposed that the marked elevation in myocardial fatty acid oxidation in db/db mice is responsible for their cardioprotection against TAC (115). Similarly, mice with T2D in response to a high-fat diet plus low-dose STZ do not develop systolic dysfunction following TAC surgery (287), which may also be related to T2D-mediated increases in myocardial fatty acid oxidation (95, 100). Despite T1D also being associated with increased risk for heart failure (288), mice subjected to STZ-induced T1D are also protected against TAC-induced heart failure, as the decline in systolic function and ensuing cardiac hypertrophy are not as robust as that observed in their nondiabetic counterparts (289).
In general, a major limitation with the vast majority of LAD coronary artery ligation or TAC studies in animal models of diabetes is that both female mice and aged mice are often overlooked. Furthermore, many of the aforementioned animal models of diabetes have not been extensively validated regarding their response to LAD coronary artery ligation or TAC. A reason for the lack of validation relates to the increased cost, time, and expertise involved with performing surgical interventions in animal models of diabetes, which necessitates the requirement of additional control groups. Thus, investigators will frequently not include low-fat diet-fed lean control groups and simply study cardiovascular outcomes in diabetic animals following either sham surgery or LAD coronary artery ligation/TAC surgery, or in response to vehicle control versus pharmacological intervention. Nonetheless, because of the growing appreciation of monitoring cardiovascular outcomes in diabetes, it is our hope that the next decade will lead to much-needed advancement of knowledge in this area.
LARGE ANIMAL MODELS OF DIABETES
In general, mouse models of diabetes are more frequently used to study diabetic heart disease because of their overall low-cost, ease of use due to development of specialized equipment, and ease of genetic manipulation. Nonetheless, they do have their own limitations as a model species to study, which from a diabetes standpoint was nicely highlighted in a recent study by de Cabo and coworkers (290). They observed in comparisons of >1,000 mice from the Study of Longitudinal Aging in Mice, >250 nonhuman primates (NHPs) from the National Institute of Aging and the Wisconsin National Primate Research Center, and >3,000 humans from the Baltimore Longitudinal Study of Aging, that aging-related increases in fasting plasma glucose correlate positively with risk for mortality in both NHPs and humans. In contrast, aging is associated with a reduction in fasting plasma glucose in mice. Moreover, numerous novel pharmacotherapies that show promise for the treatment of obesity and/or T2D in rodents often fail to translate to humans. Hence, it is imperative that pharmacological effectiveness and safety/toxicology also be assessed in larger animal models.
The use of NHPs and swine models have proven useful for the study of obesity and diabetes (both T1D and T2D). Rhesus and Cynomolgus macaques can be susceptible to spontaneous obesity that progresses to insulin resistance (291, 292), whereas dietary manipulations in macaques, similar to what has been described in rodents, can also be used to induce obesity and insulin resistance (293). With regard to swine, minipig species (Ossabaw, Gottingen, and Yucatan) are frequently used to study obesity/T2D in response to dietary manipulations, where they can be maintained into adulthood at somewhat reasonable costs while also being amenable to genetic manipulation (294). STZ and alloxan can also be used to induce T1D in the aforementioned models, though because of variability of response to these chemicals, surgical methods to induce partial or complete pancreatectomy have also been used (294). Despite these larger animal models having strong translational relevance to that of humans, there has been surprisingly minimal exploration of in vivo cardiac function in obese and/or diabetic NHPs and swine. Accordingly, how accurately these larger animal models recapitulate key features of diabetic heart disease (e.g., early diastolic dysfunction seen in diabetic cardiomyopathy) is underexplored.
FINAL SUMMARY AND IMPORTANT CONSIDERATIONS FOR THE FUTURE
A wide variety of models to study both the pathology, as well as the development of new therapies for both T1D and T2D, have been developed, each of which comes with their own unique set of advantages and disadvantages (summarized in Figs. 1–4). Due to the growing recognition of the importance of also managing cardiovascular disease in diabetes, it is imperative that we also validate these models of diabetes on their ability to reproduce features of diabetic heart disease in humans. Furthermore, since these models for most part do not result in atherosclerosis, myocardial infarction, and heart failure, it also necessitates validating how responsive they are to surgical models of these macrovascular cardiovascular diseases. One notable example highlighting the importance of this issue is seen with db/db mice, which appear to exhibit diastolic dysfunction in a multitude of studies, indicating that they are a valid model for studying diabetic cardiomyopathy in humans. On the contrary, due to their resistance to TAC-mediated impairments in systolic function, they are not an ideal model to study T2D-related HFrEF.
Although some models of diabetes have interrogated sex-specific differences with regard to their ability to produce diabetic heart disease, it is imperative that this be more extensively characterized in all models, considering that diabetic cardiomyopathy is more prevalent in women (295). For models where cardiac dysfunction is absent in female animals, an important aspect to consider relates to housing temperature. The vast majority of referenced literature in this review encompasses mice studied at room temperature, but thermoneutrality for mice is between 27°C and 30°C. Of relevance, female mice are highly resistant to obesity-induced nonalcoholic fatty liver disease and subsequent steatohepatitis, but this resistance is extinguished if the mice are housed at thermoneutrality (296). More recently, studies in male mice housed at either room temperature or thermoneutrality have demonstrated that thermoneutrality lowers heart rate and mean arterial pressures in lean mice, whereas these actions are blunted in mice subjected to high-fat diet-induced obesity (252). Taken together, as the field continues to collaborate and refine the current models of diabetes, while also developing new models, this should result in further improvements in our ability to clinically manage diabetes-related cardiovascular disease.
GRANTS
L. C. Heather is a British Heart Foundation Intermediate Basic Science Fellow Grant FS/17/58/33072. G. V. Halade is supported by the National Institutes of Health (NIH) Grants R01 HL132989 and R01 HL144788. R. Harmancey is supported by NIH Grants R01 HL136438, P20 GM104357, and P01 HL051971. K. M. Mellor is supported by the New Zealand Marsden Fund Grant 19-UOA-268 and the Health Research Council of New Zealand Grant 19/190. P. K. Mishra is supported by NIH Grant P20 GM104320 of the Nebraska Center for the Prevention of Obesity Diseases. E. E. Mulvihill is supported by Canadian Institutes of Health Research (CIHR) Project Grant PJT-156136 and a New Investigator Award from the Heart and Stroke Foundation of Canada. M. Nabben is supported by a Dutch Heart Foundation Dekker Senior Scientist Grant 2019T041. M. Nakamura is supported by NIH Grant R01 HL155766. M. Ruiz is an FRQS Junior 1 Research Scholar under Grant 281577. A. R. Wende is supported by NIH Grant R01 HL133011. J. R. Ussher is supported by CIHR Project Grant PJT-159648 and a Tier 2 Canada Research Chair (Pharmacotherapy of Energy Metabolism in Obesity).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.C.H., A.D.H., G.V.H., R.H., K.M.M., P.K.M., E.E.M., M.N., M.N., O.J.R., M.R., A.R.W., and J.R.U. drafted manuscript; L.C.H., A.D.H., G.V.H., R.H., K.M.M., P.K.M., E.E.M., M.N., M.N., O.J.R., M.R., A.R.W., and J.R.U., edited and revised manuscript; L.C.H., A.D.H., G.V.H., R.H., K.M.M., P.K.M., E.E.M., M.N. M.N., O.J.R., M.R., A.R.W., and J.R.U. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Dr. Seyed Amirhossein Tabatabaei Dakhili for preparing the graphical illustrations contained within this manuscript, which were created with BioRender.com. L. C. Heather also acknowledges the long-term support of Yvonne Green.
REFERENCES
- 1.Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 138: 271–281, 2018. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 2.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352: 837–853, 1998. [Erratum in Lancet 354: 602, 1999]. doi: 10.1016/S0140-6736(98)07019-6. [DOI] [PubMed] [Google Scholar]
- 3.Groop PH, Forsblom C, Thomas MC. Mechanisms of disease: pathway-selective insulin resistance and microvascular complications of diabetes. Nat Clin Pract Endocrinol Metab 1: 100–110, 2005. doi: 10.1038/ncpendmet0046. [DOI] [PubMed] [Google Scholar]
- 4.Ritchie RH, Abel ED. Basic mechanisms of diabetic heart disease. Circ Res 126: 1501–1525, 2020. doi: 10.1161/CIRCRESAHA.120.315913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skrha J, Soupal J, Skrha J Jr, Prazny M. Glucose variability, HbA1c and microvascular complications. Rev Endocr Metab Disord 17: 103–110, 2016. doi: 10.1007/s11154-016-9347-2. [DOI] [PubMed] [Google Scholar]
- 6.Shah AD, Langenberg C, Rapsomaniki E, Denaxas S, Pujades-Rodriguez M, Gale CP, Deanfield J, Smeeth L, Timmis A, Hemingway H. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol 3: 105–113, 2015. doi: 10.1016/S2213-8587(14)70219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res 122: 624–638, 2018. doi: 10.1161/CIRCRESAHA.117.311586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 339: 229–234, 1998. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 9.Dawson A, Morris AD, Struthers AD. The epidemiology of left ventricular hypertrophy in type 2 diabetes mellitus. Diabetologia 48: 1971–1979, 2005. doi: 10.1007/s00125-005-1896-y. [DOI] [PubMed] [Google Scholar]
- 10.Jensen MT, Fung K, Aung N, Sanghvi MM, Chadalavada S, Paiva JM, Khanji MY, de Knegt MC, Lukaschuk E, Lee AM, Barutcu A, Maclean E, Carapella V, Cooper J, Young A, Piechnik SK, Neubauer S, Petersen SE. Changes in cardiac morphology and function in individuals with diabetes mellitus: the UK biobank cardiovascular magnetic resonance substudy. Circ Cardiovasc Imaging 12: e009476, 2019. doi: 10.1161/CIRCIMAGING.119.009476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rider OJ, Lewandowski A, Nethononda R, Petersen SE, Francis JM, Pitcher A, Holloway CJ, Dass S, Banerjee R, Byrne JP, Leeson P, Neubauer S. Gender-specific differences in left ventricular remodelling in obesity: insights from cardiovascular magnetic resonance imaging. Eur Heart J 34: 292–299, 2013. doi: 10.1093/eurheartj/ehs341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang ZY, Leano R, Marwick TH. Relationship between longitudinal and radial contractility in subclinical diabetic heart disease. Clin Sci (Lond) 106: 53–60, 2004. doi: 10.1042/CS20030153. [DOI] [PubMed] [Google Scholar]
- 13.Rider OJ, Francis JM, Ali MK, Byrne J, Clarke K, Neubauer S, Petersen SE. Determinants of left ventricular mass in obesity; a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 11: 9, 2009. doi: 10.1186/1532-429X-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rider OJ, Nethononda R, Petersen SE, Francis JM, Byrne JP, Leeson P, Clarke K, Neubauer S. Concentric left ventricular remodeling and aortic stiffness: a comparison of obesity and hypertension. Int J Cardiol 167: 2989–2994, 2013. doi: 10.1016/j.ijcard.2012.08.049. [DOI] [PubMed] [Google Scholar]
- 15.Ng AC, Auger D, Delgado V, van Elderen SG, Bertini M, Siebelink HM, van der Geest RJ, Bonetti C, van der Velde ET, de Roos A, Smit JW, Leung DY, Bax JJ, Lamb HJ. Association between diffuse myocardial fibrosis by cardiac magnetic resonance contrast-enhanced T(1) mapping and subclinical myocardial dysfunction in diabetic patients: a pilot study. Circ Cardiovasc Imaging 5: 51–59, 2012. doi: 10.1161/CIRCIMAGING.111.965608. [DOI] [PubMed] [Google Scholar]
- 16.Yazici M, Ozdemir K, Gonen MS, Kayrak M, Ulgen MS, Duzenli MA, Yazici R, Soylu A, Gok H. Is there any relationship between metabolic parameters and left ventricular functions in type 2 diabetic patients without evident heart disease? Echocardiography 25: 675–682, 2008. doi: 10.1111/j.1540-8175.2008.00690.x. [DOI] [PubMed] [Google Scholar]
- 17.Boyer JK, Thanigaraj S, Schechtman KB, Perez JE. Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am J Cardiol 93: 870–875, 2004. doi: 10.1016/j.amjcard.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 18.Meagher P, Adam M, Civitarese R, Bugyei-Twum A, Connelly KA. Heart failure with preserved ejection fraction in diabetes: mechanisms and management. Can J Cardiol 34: 632–643, 2018. doi: 10.1016/j.cjca.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 19.Seferovic PM, Paulus WJ. Clinical diabetic cardiomyopathy: a two-faced disease with restrictive and dilated phenotypes. Eur Heart J 36: 1718–1727, 2015. doi: 10.1093/eurheartj/ehv134. [DOI] [PubMed] [Google Scholar]
- 20.Peterson LR, Herrero P, Schechtman KB, Racette SB, Waggoner AD, Kisrieva-Ware Z, Dence C, Klein S, Marsala J, Meyer T, Gropler RJ. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation 109: 2191–2196, 2004. doi: 10.1161/01.CIR.0000127959.28627.F8. [DOI] [PubMed] [Google Scholar]
- 21.McGill JB, Peterson LR, Herrero P, Saeed IM, Recklein C, Coggan AR, Demoss AJ, Schechtman KB, Dence CS, Gropler RJ. Potentiation of abnormalities in myocardial metabolism with the development of diabetes in women with obesity and insulin resistance. J Nucl Cardiol 18: 421–429, 2011. doi: 10.1007/s12350-011-9362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peterson LR, Herrero P, Coggan AR, Kisrieva-Ware Z, Saeed I, Dence C, Koudelis D, McGill JB, Lyons MR, Novak E, Davila-Roman VG, Waggoner AD, Gropler RJ. Type 2 diabetes, obesity, and sex difference affect the fate of glucose in the human heart. Am J Physiol Heart Circ Physiol 308: H1510–H1516, 2015. doi: 10.1152/ajpheart.00722.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rider OJ, Apps A, Miller J, Lau JYC, Lewis AJM, Peterzan MA, Dodd MS, Lau AZ, Trumper C, Gallagher FA, Grist JT, Brindle KM, Neubauer S, Tyler DJ. Noninvasive in vivo assessment of cardiac metabolism in the healthy and diabetic human heart using hyperpolarized (13)C MRI. Circ Res 126: 725–736, 2020. doi: 10.1161/CIRCRESAHA.119.316260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levelt E, Mahmod M, Piechnik SK, Ariga R, Francis JM, Rodgers CT, Clarke WT, Sabharwal N, Schneider JE, Karamitsos TD, Clarke K, Rider OJ, Neubauer S. Relationship between left ventricular structural and metabolic remodeling in type 2 diabetes. Diabetes 65: 44–52, 2016. doi: 10.2337/db15-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levelt E, Pavlides M, Banerjee R, Mahmod M, Kelly C, Sellwood J, Ariga R, Thomas S, Francis J, Rodgers C, Clarke W, Sabharwal N, Antoniades C, Schneider J, Robson M, Clarke K, Karamitsos T, Rider O, Neubauer S. Ectopic and visceral fat deposition in lean and obese patients with type 2 diabetes. J Am Coll Cardiol 68: 53–63, 2016. doi: 10.1016/j.jacc.2016.03.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rijzewijk LJ, van der Meer RW, Smit JW, Diamant M, Bax JJ, Hammer S, Romijn JA, de Roos A, Lamb HJ. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J Am Coll Cardiol 52: 1793–1799, 2008. doi: 10.1016/j.jacc.2008.07.062. [DOI] [PubMed] [Google Scholar]
- 27.Kopytek M, Ząbczyk M, Mazur P, Undas A, Natorska J. Accumulation of advanced glycation end products (AGEs) is associated with the severity of aortic stenosis in patients with concomitant type 2 diabetes. Cardiovasc Diabetol 19: 92, 2020. doi: 10.1186/s12933-020-01068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas MC, Soderlund J, Lehto M, Makinen VP, Moran JL, Cooper ME, Forsblom C; P-H Groop, FinnDiane Study Group. Soluble receptor for AGE (RAGE) is a novel independent predictor of all-cause and cardiovascular mortality in type 1 diabetes. Diabetologia 54: 2669–2677, 2011. doi: 10.1007/s00125-011-2186-5. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676, 2006. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 30.Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, Raval KK, Zhang J, Kamp TJ, Palecek SP. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci USA 109: E1848–E1857, 2012. doi: 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Steinbusch LKM, Nabben M, Kapsokalyvas D, van Zandvoort M, Schonleitner P, Antoons G, Simons PJ, Coumans WA, Geomini A, Chanda D, Glatz JFC, Neumann D, Luiken J. Palmitate-induced vacuolar-type H(+)-ATPase inhibition feeds forward into insulin resistance and contractile dysfunction. Diabetes 66: 1521–1534, 2017. doi: 10.2337/db16-0727. [DOI] [PubMed] [Google Scholar]
- 32.Wang S, Schianchi F, Neumann D, Wong LY, Sun A, van Nieuwenhoven FA, Zeegers MP, Strzelecka A, Col U, Glatz JFC, Nabben M, Luiken J. Specific amino acid supplementation rescues the heart from lipid overload-induced insulin resistance and contractile dysfunction by targeting the endosomal mTOR-v-ATPase axis. Mol Metab 53: 101293, 2021. doi: 10.1016/j.molmet.2021.101293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong LY, Glatz JFC, Wang S, Geraets IME, Vanherle S, Wijngaard AVD, Brunner H, Luiken J, Nabben M. Comparison of human and rodent cell models to study myocardial lipid-induced insulin resistance. Prostaglandins Leukot Essent Fatty Acids 167: 102267, 2021. doi: 10.1016/j.plefa.2021.102267. [DOI] [PubMed] [Google Scholar]
- 34.Sousa Fialho MDL, Purnama U, Dennis K, Montes Aparicio CN, Castro-Guarda M, Massourides E, Tyler DJ, Carr CA, Heather LC. Activation of HIF1α rescues the hypoxic response and reverses metabolic dysfunction in the diabetic heart. Diabetes 70: 2518–2531, 2021. doi: 10.2337/db21-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ng KM, Lau YM, Dhandhania V, Cai ZJ, Lee YK, Lai WH, Tse HF, Siu CW. Empagliflozin ammeliorates high glucose induced-cardiac dysfuntion in human iPSC-derived cardiomyocytes. Sci Rep 8: 14872, 2018. doi: 10.1038/s41598-018-33293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drawnel FM, Boccardo S, Prummer M, Delobel F, Graff A, Weber M, Gerard R, Badi L, Kam-Thong T, Bu L, Jiang X, Hoflack JC, Kiialainen A, Jeworutzki E, Aoyama N, Carlson C, Burcin M, Gromo G, Boehringer M, Stahlberg H, Hall BJ, Magnone MC, Kolaja K, Chien KR, Bailly J, Iacone R. Disease modeling and phenotypic drug screening for diabetic cardiomyopathy using human induced pluripotent stem cells. Cell Rep 9: 810–821, 2014. doi: 10.1016/j.celrep.2014.09.055. [DOI] [PubMed] [Google Scholar]
- 37.Graneli C, Hicks R, Brolen G, Synnergren J, Sartipy P. Diabetic cardiomyopathy modelling using induced pluripotent stem cell derived cardiomyocytes: recent advances and emerging models. Stem Cell Rev Rep 15: 13–22, 2019. doi: 10.1007/s12015-018-9858-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang L, Wang H, Dai B, Wang X, Zhou D, Shen J, Guo F, Wang J, Zhou J, Wang H, Wu Q, Yao H, Gong T, Su J, Meng ZX, Niu T, Zhang L, Liang P. Human induced pluripotent stem cell-derived cardiomyocytes reveal abnormal TGFβ signaling in type 2 diabetes mellitus. J Mol Cell Cardiol 142: 53–64, 2020. doi: 10.1016/j.yjmcc.2020.03.016. [DOI] [PubMed] [Google Scholar]
- 39.Volpato V, Webber C. Addressing variability in iPSC-derived models of human disease: guidelines to promote reproducibility. Dis Model Mech 13: dmm042317, 2020. doi: 10.1242/dmm.042317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopez CA, Al-Siddiqi H, Purnama U, Iftekhar S, Bruyneel AAN, Kerr M, Nazir R, da Luz Sousa Fialho M, Malandraki-Miller S, Alonaizan R, Kermani F, Heather LC, Czernuszka J, Carr CA. Physiological and pharmacological stimulation for in vitro maturation of substrate metabolism in human induced pluripotent stem cell-derived cardiomyocytes. Sci Rep 11: 7802, 2021. doi: 10.1038/s41598-021-87186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang X, Chen XX, Yu HT, Tan Y, Lin Q, Keller BB, Zheng Y, Cai L. Engineered cardiac tissues: a novel in vitro model to investigate the pathophysiology of mouse diabetic cardiomyopathy. Acta Pharmacol Sin 42: 932–941, 2021. doi: 10.1038/s41401-020-00538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wimmer RA, Leopoldi A, Aichinger M, Wick N, Hantusch B, Novatchkova M, Taubenschmid J, Hammerle M, Esk C, Bagley JA, Lindenhofer D, Chen G, Boehm M, Agu CA, Yang F, Fu B, Zuber J, Knoblich JA, Kerjaschki D, Penninger JM. Human blood vessel organoids as a model of diabetic vasculopathy. Nature 565: 505–510, 2019. doi: 10.1038/s41586-018-0858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davidoff AJ, Davidson MB, Carmody MW, Davis ME, Ren J. Diabetic cardiomyocyte dysfunction and myocyte insulin resistance: role of glucose-induced PKC activity. Mol Cell Biochem 262: 155–163, 2004. doi: 10.1023/b:mcbi.0000038231.68078.4b. [DOI] [PubMed] [Google Scholar]
- 44.Bertrand L, Ginion A, Beauloye C, Hebert AD, Guigas B, Hue L, Vanoverschelde JL. AMPK activation restores the stimulation of glucose uptake in an in vitro model of insulin-resistant cardiomyocytes via the activation of protein kinase B. Am J Physiol Heart Circ Physiol 291: H239–H250, 2006. doi: 10.1152/ajpheart.01269.2005. [DOI] [PubMed] [Google Scholar]
- 45.Zhi L, Yuzhang Z, Tianliang H, Hisatome I, Yamamoto T, Jidong C. High uric acid induces insulin resistance in cardiomyocytes in vitro and in vivo. PLoS One 11: e0147737, 2016. doi: 10.1371/journal.pone.0147737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samokhvalov V, Ussher JR, Fillmore N, Armstrong IK, Keung W, Moroz D, Lopaschuk DG, Seubert J, Lopaschuk GD. Inhibition of malonyl-CoA decarboxylase reduces the inflammatory response associated with insulin resistance. Am J Physiol Endocrinol Metab 303: E1459–E1468, 2012. doi: 10.1152/ajpendo.00018.2012. [DOI] [PubMed] [Google Scholar]
- 47.Chanda D, Oligschlaeger Y, Geraets I, Liu Y, Zhu X, Li J, Nabben M, Coumans W, Luiken J, Glatz JFC, Neumann D. 2-Arachidonoylglycerol ameliorates inflammatory stress-induced insulin resistance in cardiomyocytes. J Biol Chem 292: 7105–7114, 2017. doi: 10.1074/jbc.M116.767384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dodd MS, Sousa Fialho MDL, Montes Aparicio CN, Kerr M, Timm KN, Griffin JL, Luiken J, Glatz JFC, Tyler DJ, Heather LC. Fatty acids prevent hypoxia-inducible factor-1α signaling through decreased succinate in diabetes. JACC Basic Transl Sci 3: 485–498, 2018. doi: 10.1016/j.jacbts.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Battault S, Renguet E, Van Steenbergen A, Horman S, Beauloye C, Bertrand L. Myocardial glucotoxicity: mechanisms and potential therapeutic targets. Arch Cardiovasc Dis 113: 736–748, 2020. doi: 10.1016/j.acvd.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 50.Li Y, Li Y, Feng Q, Arnold M, Peng T. Calpain activation contributes to hyperglycaemia-induced apoptosis in cardiomyocytes. Cardiovasc Res 84: 100–110, 2009. [Erratum in Cardiovasc Res 115: 1381, 2019]. doi: 10.1093/cvr/cvp189. [DOI] [PubMed] [Google Scholar]
- 51.Eckel J, Wirdeier A, Herberg L, Reinauer H. Insulin resistance in the heart: studies on isolated cardiocytes of genetically obese Zucker rats. Endocrinology 116: 1529–1534, 1985. doi: 10.1210/endo-116-4-1529. [DOI] [PubMed] [Google Scholar]
- 52.Kolter T, Uphues I, Eckel J. Molecular analysis of insulin resistance in isolated ventricular cardiomyocytes of obese Zucker rats. Am J Physiol Endocrinol Physiol 273: E59–E67, 1997. doi: 10.1152/ajpendo.1997.273.1.E59. [DOI] [PubMed] [Google Scholar]
- 53.Wright JJ, Kim J, Buchanan J, Boudina S, Sena S, Bakirtzi K, Ilkun O, Theobald HA, Cooksey RC, Kandror KV, Abel ED. Mechanisms for increased myocardial fatty acid utilization following short-term high-fat feeding. Cardiovasc Res 82: 351–360, 2009. doi: 10.1093/cvr/cvp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rech M, Luiken J, Glatz JFC, van Bilsen M, Schroen B, Nabben M. Assessing fatty acid oxidation flux in rodent cardiomyocyte models. Sci Rep 8: 1505, 2018. doi: 10.1038/s41598-018-19478-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mitcheson JS, Hancox JC, Levi AJ. Cultured adult cardiac myocytes: future applications, culture methods, morphological and electrophysiological properties. Cardiovasc Res 39: 280–300, 1998. doi: 10.1016/s0008-6363(98)00128-x. [DOI] [PubMed] [Google Scholar]
- 56.Kleinert M, Clemmensen C, Hofmann SM, Moore MC, Renner S, Woods SC, Huypens P, Beckers J, de Angelis MH, Schurmann A, Bakhti M, Klingenspor M, Heiman M, Cherrington AD, Ristow M, Lickert H, Wolf E, Havel PJ, Muller TD, Tschop MH. Animal models of obesity and diabetes mellitus. Nat Rev Endocrinol 14: 140–162, 2018. doi: 10.1038/nrendo.2017.161. [DOI] [PubMed] [Google Scholar]
- 57.West DB, Boozer CN, Moody DL, Atkinson RL. Dietary obesity in nine inbred mouse strains. Am J Physiol Regul Integr Comp Physiol 262: R1025–R1032, 1992. doi: 10.1152/ajpregu.1992.262.6.R1025. [DOI] [PubMed] [Google Scholar]
- 58.Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes 37: 1163–1167, 1988. doi: 10.2337/diab.37.9.1163. [DOI] [PubMed] [Google Scholar]
- 59.Hong J, Stubbins RE, Smith RR, Harvey AE, Nunez NP. Differential susceptibility to obesity between male, female and ovariectomized female mice. Nutr J 8: 11, 2009. doi: 10.1186/1475-2891-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang Y, Smith DL Jr, Keating KD, Allison DB, Nagy TR. Variations in body weight, food intake and body composition after long-term high-fat diet feeding in C57BL/6J mice. Obesity (Silver Spring) 22: 2147–2155, 2014. doi: 10.1002/oby.20811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol 273: R725–R730, 1997. doi: 10.1152/ajpregu.1997.273.2.R725. [DOI] [PubMed] [Google Scholar]
- 62.Levin BE, Triscari J, Hogan S, Sullivan AC. Resistance to diet-induced obesity: food intake, pancreatic sympathetic tone, and insulin. Am J Physiol Regul Integr Comp Physiol 252: R471–R478, 1987. doi: 10.1152/ajpregu.1987.252.3.R471. [DOI] [PubMed] [Google Scholar]
- 63.Sung MM, Koonen DP, Soltys CL, Jacobs RL, Febbraio M, Dyck JR. Increased CD36 expression in middle-aged mice contributes to obesity-related cardiac hypertrophy in the absence of cardiac dysfunction. J Mol Med (Berl) 89: 459–469, 2011. doi: 10.1007/s00109-010-0720-4. [DOI] [PubMed] [Google Scholar]
- 64.Tadinada SM, Weatherford ET, Collins GV, Bhardwaj G, Cochran J, Kutschke W, Zimmerman K, Bosko A, O'Neill BT, Weiss RM, Abel ED. Functional resilience of C57BL/6J mouse heart to dietary fat overload. Am J Physiol Heart Circ Physiol 321: H850–H864, 2021. doi: 10.1152/ajpheart.00419.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ussher JR, Koves TR, Jaswal JS, Zhang L, Ilkayeva O, Dyck JR, Muoio DM, Lopaschuk GD. Insulin-stimulated cardiac glucose oxidation is increased in high-fat diet-induced obese mice lacking malonyl CoA decarboxylase. Diabetes 58: 1766–1775, 2009. doi: 10.2337/db09-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang R, Barouch LA. Leptin signaling and obesity: cardiovascular consequences. Circ Res 101: 545–559, 2007. doi: 10.1161/CIRCRESAHA.107.156596. [DOI] [PubMed] [Google Scholar]
- 67.Noyan-Ashraf MH, Shikatani EA, Schuiki I, Mukovozov I, Wu J, Li RK, Volchuk A, Robinson LA, Billia F, Drucker DJ, Husain M. A glucagon-like peptide-1 analog reverses the molecular pathology and cardiac dysfunction of a mouse model of obesity. Circulation 127: 74–85, 2013. doi: 10.1161/CIRCULATIONAHA.112.091215. [DOI] [PubMed] [Google Scholar]
- 68.Battiprolu PK, Hojayev B, Jiang N, Wang ZV, Luo X, Iglewski M, Shelton JM, Gerard RD, Rothermel BA, Gillette TG, Lavandero S, Hill JA. Metabolic stress-induced activation of FoxO1 triggers diabetic cardiomyopathy in mice. J Clin Invest 122: 1109–1118, 2012. doi: 10.1172/JCI60329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nakamura M, Liu T, Husain S, Zhai P, Warren JS, Hsu CP, Matsuda T, Phiel CJ, Cox JE, Tian B, Li H, Sadoshima J. Glycogen synthase kinase-3α promotes fatty acid uptake and lipotoxic cardiomyopathy. Cell Metab 29: 1119–1134.e2, 2019. doi: 10.1016/j.cmet.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun X, Han F, Lu Q, Li X, Ren D, Zhang J, Han Y, Xiang YK, Li J. Empagliflozin ameliorates obesity-related cardiac dysfunction by regulating sestrin2-mediated AMPK-mTOR signaling and redox homeostasis in high-fat diet-induced obese mice. Diabetes 69: 1292–1305, 2020. doi: 10.2337/db19-0991. [DOI] [PubMed] [Google Scholar]
- 71.Lewis AJM, Abdesselam I, Rayner JJ, Byrne J, Borlaug BA, Neubauer S, Rider OJ. Adverse right ventricular remodelling, function, and stress responses in obesity: insights from cardiovascular magnetic resonance. Eur Heart J Cardiovasc Imaging. jeab175, 2021. doi: 10.1093/ehjci/jeab175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aasum E, Khalid AM, Gudbrandsen OA, How OJ, Berge RK, Larsen TS. Fenofibrate modulates cardiac and hepatic metabolism and increases ischemic tolerance in diet-induced obese mice. J Mol Cell Cardiol 44: 201–209, 2008. doi: 10.1016/j.yjmcc.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 73.Jansen KM, Moreno S, Garcia-Roves PM, Larsen TS. Dietary Calanus oil recovers metabolic flexibility and rescues postischemic cardiac function in obese female mice. Am J Physiol Heart Circ Physiol 317: H290–H299, 2019. doi: 10.1152/ajpheart.00191.2019. [DOI] [PubMed] [Google Scholar]
- 74.Zhang L, Ussher JR, Oka T, Cadete VJ, Wagg C, Lopaschuk GD. Cardiac diacylglycerol accumulation in high fat-fed mice is associated with impaired insulin-stimulated glucose oxidation. Cardiovasc Res 89: 148–156, 2011. doi: 10.1093/cvr/cvq266. [DOI] [PubMed] [Google Scholar]
- 75.Kramer B, Franca LM, Zhang Y, Paes AMA, Gerdes AM, Carrillo-Sepulveda MA. Western diet triggers Toll-like receptor 4 signaling-induced endothelial dysfunction in female Wistar rats. Am J Physiol Heart Circ Physiol 315: H1735–H1747, 2018. doi: 10.1152/ajpheart.00218.2018. [DOI] [PubMed] [Google Scholar]
- 76.Candela J, Velmurugan GV, White C. Hydrogen sulfide depletion contributes to microvascular remodeling in obesity. Am J Physiol Heart Circ Physiol 310: H1071–H1080, 2016. doi: 10.1152/ajpheart.00062.2016. [DOI] [PubMed] [Google Scholar]
- 77.Pei SJ, Zhu HY, Guo JH, Zhang X, Deng ZJ. Knockout of CNR1 prevents metabolic stress-induced cardiac injury through improving insulin resistance (IR) injury and endoplasmic reticulum (ER) stress by promoting AMPK-α activation. Biochem Biophys Res Commun 503: 744–751, 2018. doi: 10.1016/j.bbrc.2018.06.070. [DOI] [PubMed] [Google Scholar]
- 78.Souza-Neto FV, Jimenez-Gonzalez S, Delgado-Valero B, Jurado-Lopez R, Genty M, Romero-Miranda A, Rodriguez C, Nieto ML, Martinez-Martinez E, Cachofeiro V. The interplay of mitochondrial oxidative stress and endoplasmic reticulum stress in cardiovascular fibrosis in obese rats. Antioxidants (Basel) 10: 1274, 2021. doi: 10.3390/antiox10081274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mellor KM, Ritchie RH, Davidoff AJ, Delbridge LMD. Elevated dietary sugar and the heart: experimental models and myocardial remodeling. Can J Physiol Pharmacol 88: 525–540, 2010. [DOI] [PubMed] [Google Scholar]
- 80.Thresher JS, Podolin DA, Wei Y, Mazzeo RS, Pagliassotti MJ. Comparison of the effects of sucrose and fructose on insulin action and glucose tolerance. Am J Physiol Regul Integr Comp Physiol 279: R1334–R1340, 2000. doi: 10.1152/ajpregu.2000.279.4.R1334. [DOI] [PubMed] [Google Scholar]
- 81.Senador D, Key M, Brosnihan KB, Irigoyen MC, Elased KM, Morris M. Cardiovascular interactions between losartan and fructose in mice. J Cardiovasc Pharmacol Ther 15: 68–77, 2010. doi: 10.1177/1074248409351409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mellor K, Ritchie RH, Meredith G, Woodman OL, Morris MJ, Delbridge LM. High-fructose diet elevates myocardial superoxide generation in mice in the absence of cardiac hypertrophy. Nutrition 26: 842–848, 2010. doi: 10.1016/j.nut.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 83.Farah V, Elased KM, Chen Y, Key MP, Cunha TS, Irigoyen MC, Morris M. Nocturnal hypertension in mice consuming a high fructose diet. Auton Neurosci 130: 41–50, 2006. doi: 10.1016/j.autneu.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 84.Anurag P, Anuradha CV. Metformin improves lipid metabolism and attenuates lipid peroxidation in high fructose-fed rats. Diabetes Obes Metab 4: 36–42, 2002. doi: 10.1046/j.1463-1326.2002.00178.x. [DOI] [PubMed] [Google Scholar]
- 85.Jiang J, Tran L, Vasudevan H, Xia Z, Yuen VG, McNeill JH. Endothelin-1 blockade prevents COX2 induction and TXA2 production in the fructose hypertensive rat. Can J Physiol Pharmacol 85: 422–429, 2007. doi: 10.1139/y06-088. [DOI] [PubMed] [Google Scholar]
- 86.Mellor KM, Bell JR, Young MJ, Ritchie RH, Delbridge LM. Myocardial autophagy activation and suppressed survival signaling is associated with insulin resistance in fructose-fed mice. J Mol Cell Cardiol 50: 1035–1043, 2011. doi: 10.1016/j.yjmcc.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 87.Chang KC, Liang JT, Tseng CD, Wu ET, Hsu KL, Wu MS, Lin YT, Tseng YZ. Aminoguanidine prevents fructose-induced deterioration in left ventricular-arterial coupling in Wistar rats. Br J Pharmacol 151: 341–346, 2007. doi: 10.1038/sj.bjp.0707223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Delbosc S, Paizanis E, Magous R, Araiz C, Dimo T, Cristol JP, Cros G, Azay J. Involvement of oxidative stress and NADPH oxidase activation in the development of cardiovascular complications in a model of insulin resistance, the fructose-fed rat. Atherosclerosis 179: 43–49, 2005. doi: 10.1016/j.atherosclerosis.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 89.Thirunavukkarasu V, Anitha Nandhini AT, Anuradha CV. Cardiac lipids and antioxidant status in high fructose rats and the effect of α-lipoic acid. Nutr Metab Cardiovasc Dis 14: 351–357, 2004. doi: 10.1016/s0939-4753(04)80025-5. [DOI] [PubMed] [Google Scholar]
- 90.Mellor KM, Wendt IR, Ritchie RH, Delbridge LM. Fructose diet treatment in mice induces fundamental disturbance of cardiomyocyte Ca2+ handling and myofilament responsiveness. Am J Physiol Heart Circ Physiol 302: H964–H972, 2012. doi: 10.1152/ajpheart.00797.2011. [DOI] [PubMed] [Google Scholar]
- 91.Mostarda C, Moraes-Silva IC, Salemi VMC, Machi JF, Rodrigues B, De Angelis K, de Moura Azevedo Farah V, Irigoyen MC. Exercise training prevents diastolic dysfunction induced by metabolic syndrome in rats. Clinics (Sao Paulo) 67: 815–820, 2012. doi: 10.6061/clinics/2012(07)18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang YB, Meng YH, Chang S, Zhang RY, Shi C. High fructose causes cardiac hypertrophy via mitochondrial signaling pathway. Am J Transl Res 8: 4869–4880, 2016. [PMC free article] [PubMed] [Google Scholar]
- 93.King AJ. The use of animal models in diabetes research. Br J Pharmacol 166: 877–894, 2012. doi: 10.1111/j.1476-5381.2012.01911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mansor LS, Gonzalez ER, Cole MA, Tyler DJ, Beeson JH, Clarke K, Carr CA, Heather LC. Cardiac metabolism in a new rat model of type 2 diabetes using high-fat diet with low dose streptozotocin. Cardiovasc Diabetol 12: 136, 2013. doi: 10.1186/1475-2840-12-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Almutairi M, Gopal K, Greenwell AA, Young A, Gill R, Aburasayn H, Al Batran R, Chahade JJ, Gandhi M, Eaton F, Mailloux RJ, Ussher JR. The GLP-1 receptor agonist liraglutide increases myocardial glucose oxidation rates via indirect mechanisms and mitigates experimental diabetic cardiomyopathy. Can J Cardiol 37: 140–150, 2021. doi: 10.1016/j.cjca.2020.02.098. [DOI] [PubMed] [Google Scholar]
- 96.Gopal K, Al Batran R, Altamimi TR, Greenwell AA, Saed CT, Tabatabaei Dakhili SA, Dimaano MTE, Zhang Y, Eaton F, Sutendra G, Ussher JR. FoxO1 inhibition alleviates type 2 diabetes-related diastolic dysfunction by increasing myocardial pyruvate dehydrogenase activity. Cell Rep 35: 108935, 2021. doi: 10.1016/j.celrep.2021.108935. [DOI] [PubMed] [Google Scholar]
- 97.Le Page LM, Rider OJ, Lewis AJ, Ball V, Clarke K, Johansson E, Carr CA, Heather LC, Tyler DJ. Increasing pyruvate dehydrogenase flux as a treatment for diabetic cardiomyopathy: a combined 13C hyperpolarized magnetic resonance and echocardiography study. Diabetes 64: 2735–2743, 2015. doi: 10.2337/db14-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Koncsos G, Varga ZV, Baranyai T, Boengler K, Rohrbach S, Li L, Schluter KD, Schreckenberg R, Radovits T, Olah A, Matyas C, Lux A, Al-Khrasani M, Komlodi T, Bukosza N, Mathe D, Deres L, Bartekova M, Rajtik T, Adameova A, Szigeti K, Hamar P, Helyes Z, Tretter L, Pacher P, Merkely B, Giricz Z, Schulz R, Ferdinandy P. Diastolic dysfunction in prediabetic male rats: role of mitochondrial oxidative stress. Am J Physiol Heart Circ Physiol 311: H927–H943, 2016. doi: 10.1152/ajpheart.00049.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Alexandru Popescu B, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 17: 1321–1360, 2016. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 100.Mansor LS, Sousa Fialho MDL, Yea G, Coumans WA, West JA, Kerr M, Carr CA, Luiken J, Glatz JFC, Evans RD, Griffin JL, Tyler DJ, Clarke K, Heather LC. Inhibition of sarcolemmal FAT/CD36 by sulfo-N-succinimidyl oleate rapidly corrects metabolism and restores function in the diabetic heart following hypoxia/reoxygenation. Cardiovasc Res 113: 737–748, 2017. doi: 10.1093/cvr/cvx045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lovegrove AS, Sun J, Gould KA, Lubahn DB, Korach KS, Lane PH. Estrogen receptor α-mediated events promote sex-specific diabetic glomerular hypertrophy. Am J Physiol Renal Physiol 287: F586–F591, 2004. doi: 10.1152/ajprenal.00414.2003. [DOI] [PubMed] [Google Scholar]
- 102.Saadane A, Lessieur EM, Du Y, Liu H, Kern TS. Successful induction of diabetes in mice demonstrates no gender difference in development of early diabetic retinopathy. PLoS One 15: e0238727, 2020. doi: 10.1371/journal.pone.0238727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shao Q, Meng L, Lee S, Tse G, Gong M, Zhang Z, Zhao J, Zhao Y, Li G, Liu T. Empagliflozin, a sodium glucose co-transporter-2 inhibitor, alleviates atrial remodeling and improves mitochondrial function in high-fat diet/streptozotocin-induced diabetic rats. Cardiovasc Diabetol 18: 165, 2019. doi: 10.1186/s12933-019-0964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kerr M, Miller JJ, Thapa D, Stiewe S, Timm KN, Aparicio CNM, Scott I, Tyler DJ, Heather LC. Rescue of myocardial energetic dysfunction in diabetes through the correction of mitochondrial hyperacetylation by honokiol. JCI Insight 5: e140326, 2020. doi: 10.1172/jci.insight.140326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mansor LS, Mehta K, Aksentijevic D, Carr CA, Lund T, Cole MA, Le Page L, Sousa Fialho M. D L, Shattock MJ, Aasum E, Clarke K, Tyler DJ, Heather LC. Increased oxidative metabolism following hypoxia in the type 2 diabetic heart, despite normal hypoxia signalling and metabolic adaptation. J Physiol 594: 307–320, 2016. doi: 10.1113/JP271242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Prakoso D, De Blasio MJ, Tate M, Kiriazis H, Donner DG, Qian H, Nash D, Deo M, Weeks KL, Parry LJ, Gregorevic P, McMullen JR, Ritchie RH. Gene therapy targeting cardiac phosphoinositide 3-kinase (p110α) attenuates cardiac remodeling in type 2 diabetes. Am J Physiol Heart Circ Physiol 318: H840–H852, 2020. doi: 10.1152/ajpheart.00632.2019. [DOI] [PubMed] [Google Scholar]
- 107.Huang X, Liu S, Wu D, Cheng Y, Han H, Wang K, Zhang G, Hu S. Facilitated Ca2+ homeostasis and attenuated myocardial autophagy contribute to alleviation of diabetic cardiomyopathy after bariatric surgery. Am J Physiol Heart Circ Physiol 315: H1258–H1268, 2018. doi: 10.1152/ajpheart.00274.2018. [DOI] [PubMed] [Google Scholar]
- 108.Tajima A, Hirata T, Taniguchi K, Kondo Y, Kato S, Saito-Hori M, Ishimoto T, Yamamoto K. Combination of TS-021 with metformin improves hyperglycemia and synergistically increases pancreatic β-cell mass in a mouse model of type 2 diabetes. Life Sci 89: 662–670, 2011. doi: 10.1016/j.lfs.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 109.Meng Z, Liu X, Li T, Fang T, Cheng Y, Han L, Sun B, Chen L. The SGLT2 inhibitor empagliflozin negatively regulates IL-17/IL-23 axis-mediated inflammatory responses in T2DM with NAFLD via the AMPK/mTOR/autophagy pathway. Int Immunopharmacol 94: 107492, 2021. doi: 10.1016/j.intimp.2021.107492. [DOI] [PubMed] [Google Scholar]
- 110.Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J Hered 41: 317–318, 1950. doi: 10.1093/oxfordjournals.jhered.a106073. [DOI] [PubMed] [Google Scholar]
- 111.Buchanan J, Mazumder PK, Hu P, Chakrabarti G, Roberts MW, Yun UJ, Cooksey RC, Litwin SE, Abel ED. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology 146: 5341–5349, 2005. doi: 10.1210/en.2005-0938. [DOI] [PubMed] [Google Scholar]
- 112.Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, Aziz S, Johnson JI, Bugger H, Zaha VG, Abel ED. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes 56: 2457–2466, 2007. doi: 10.2337/db07-0481. [DOI] [PubMed] [Google Scholar]
- 113.Carroll R, Carley AN, Dyck JR, Severson DL. Metabolic effects of insulin on cardiomyocytes from control and diabetic db/db mouse hearts. Am J Physiol Endocrinol Physiol 288: E900–E906, 2005. doi: 10.1152/ajpendo.00491.2004. [DOI] [PubMed] [Google Scholar]
- 114.Hafstad AD, Solevag GH, Severson DL, Larsen TS, Aasum E. Perfused hearts from type 2 diabetic (db/db) mice show metabolic responsiveness to insulin. Am J Physiol Heart Circ Physiol 290: H1763–H1769, 2006. doi: 10.1152/ajpheart.01063.2005. [DOI] [PubMed] [Google Scholar]
- 115.Abdurrachim D, Nabben M, Hoerr V, Kuhlmann MT, Bovenkamp P, Ciapaite J, Geraets IME, Coumans W, Luiken J, Glatz JFC, Schafers M, Nicolay K, Faber C, Hermann S, Prompers JJ. Diabetic db/db mice do not develop heart failure upon pressure overload: a longitudinal in vivo PET, MRI, and MRS study on cardiac metabolic, structural, and functional adaptations. Cardiovasc Res 113: 1148–1160, 2017. doi: 10.1093/cvr/cvx100. [DOI] [PubMed] [Google Scholar]
- 116.How OJ, Aasum E, Severson DL, Chan WY, Essop MF, Larsen TS. Increased myocardial oxygen consumption reduces cardiac efficiency in diabetic mice. Diabetes 55: 466–473, 2006. doi: 10.2337/diabetes.55.02.06.db05-1164. [DOI] [PubMed] [Google Scholar]
- 117.Aasum E, Hafstad AD, Severson DL, Larsen TS. Age-dependent changes in metabolism, contractile function, and ischemic sensitivity in hearts from db/db mice. Diabetes 52: 434–441, 2003. doi: 10.2337/diabetes.52.2.434. [DOI] [PubMed] [Google Scholar]
- 118.Semeniuk LM, Kryski AJ, Severson DL. Echocardiographic assessment of cardiac function in diabetic db/db and transgenic db/db-hGLUT4 mice. Am J Physiol Heart Circ Physiol 283: H976–H982, 2002. doi: 10.1152/ajpheart.00088.2002. [DOI] [PubMed] [Google Scholar]
- 119.Barouch LA, Gao D, Chen L, Miller KL, Xu W, Phan AC, Kittleson MM, Minhas KM, Berkowitz DE, Wei C, Hare JM. Cardiac myocyte apoptosis is associated with increased DNA damage and decreased survival in murine models of obesity. Circ Res 98: 119–124, 2006. doi: 10.1161/01.RES.0000199348.10580.1d. [DOI] [PubMed] [Google Scholar]
- 120.Belke DD, Swanson EA, Dillmann WH. Decreased sarcoplasmic reticulum activity and contractility in diabetic db/db mouse heart. Diabetes 53: 3201–3208, 2004. doi: 10.2337/diabetes.53.12.3201. [DOI] [PubMed] [Google Scholar]
- 121.Fukuda M, Nakamura T, Kataoka K, Nako H, Tokutomi Y, Dong YF, Yasuda O, Ogawa H, Kim-Mitsuyama S. Ezetimibe ameliorates cardiovascular complications and hepatic steatosis in obese and type 2 diabetic db/db mice. J Pharmacol Exp Ther 335: 70–75, 2010. doi: 10.1124/jpet.110.170373. [DOI] [PubMed] [Google Scholar]
- 122.Pereira L, Matthes J, Schuster I, Valdivia HH, Herzig S, Richard S, Gomez AM. Mechanisms of [Ca2+]i transient decrease in cardiomyopathy of db/db type 2 diabetic mice. Diabetes 55: 608–615, 2006. doi: 10.2337/diabetes.55.03.06.db05-1284. [DOI] [PubMed] [Google Scholar]
- 123.Sikka G, Yang R, Reid S, Benjo A, Koitabashi N, Camara A, Baraban E, O'Donnell CP, Berkowitz DE, Barouch LA. Leptin is essential in maintaining normal vascular compliance independent of body weight. Int J Obes (Lond) 34: 203–206, 2010. doi: 10.1038/ijo.2009.208. [DOI] [PubMed] [Google Scholar]
- 124.Alex L, Russo I, Holoborodko V, Frangogiannis NG. Characterization of a mouse model of obesity-related fibrotic cardiomyopathy that recapitulates features of human heart failure with preserved ejection fraction. Am J Physiol Heart Circ Physiol 315: H934–H949, 2018. doi: 10.1152/ajpheart.00238.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hafstad AD, Khalid AM, How OJ, Larsen TS, Aasum E. Glucose and insulin improve cardiac efficiency and postischemic functional recovery in perfused hearts from type 2 diabetic (db/db) mice. Am J Physiol Endocrinol Physiol 292: E1288–E1294, 2007. doi: 10.1152/ajpendo.00504.2006. [DOI] [PubMed] [Google Scholar]
- 126.Pedersen TM, Boardman NT, Hafstad AD, Aasum E. Isolated perfused working hearts provide valuable additional information during phenotypic assessment of the diabetic mouse heart. PLoS One 13: e0204843, 2018. doi: 10.1371/journal.pone.0204843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fujita H, Fujishima H, Koshimura J, Hosoba M, Yoshioka N, Shimotomai T, Morii T, Narita T, Kakei M, Ito S. Effects of antidiabetic treatment with metformin and insulin on serum and adipose tissue adiponectin levels in db/db mice. Endocr J 52: 427–433, 2005. doi: 10.1507/endocrj.52.427. [DOI] [PubMed] [Google Scholar]
- 128.Moellmann J, Klinkhammer BM, Droste P, Kappel B, Haj-Yehia E, Maxeiner S, Artati A, Adamski J, Boor P, Schutt K, Lopaschuk GD, Verma S, Marx N, Lehrke M. Empagliflozin improves left ventricular diastolic function of db/db mice. Biochim Biophys Acta Mol Basis Dis 1866: 165807, 2020. doi: 10.1016/j.bbadis.2020.165807. [DOI] [PubMed] [Google Scholar]
- 129.Tang T, Reed MJ. Exercise adds to metformin and acarbose efficacy in db/db mice. Metabolism 50: 1049–1053, 2001. doi: 10.1053/meta.2001.25596. [DOI] [PubMed] [Google Scholar]
- 130.Arow M, Waldman M, Yadin D, Nudelman V, Shainberg A, Abraham NG, Freimark D, Kornowski R, Aravot D, Hochhauser E, Arad M. Sodium-glucose cotransporter 2 inhibitor Dapagliflozin attenuates diabetic cardiomyopathy. Cardiovasc Diabetol 19: 7, 2020. doi: 10.1186/s12933-019-0980-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Birnbaum Y, Tran D, Bajaj M, Ye Y. DPP-4 inhibition by linagliptin prevents cardiac dysfunction and inflammation by targeting the Nlrp3/ASC inflammasome. Basic Res Cardiol 114: 35, 2019. doi: 10.1007/s00395-019-0743-0. [DOI] [PubMed] [Google Scholar]
- 132.Lenski M, Kazakov A, Marx N, Bohm M, Laufs U. Effects of DPP-4 inhibition on cardiac metabolism and function in mice. J Mol Cell Cardiol 51: 906–918, 2011. doi: 10.1016/j.yjmcc.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 133.Xue M, Li T, Wang Y, Chang Y, Cheng Y, Lu Y, Liu X, Xu L, Li X, Yu X, Sun B, Chen L. Empagliflozin prevents cardiomyopathy via sGC-cGMP-PKG pathway in type 2 diabetes mice. Clin Sci (Lond) 133: 1705–1720, 2019. doi: 10.1042/CS20190585. [DOI] [PubMed] [Google Scholar]
- 134.Hamdani N, Hervent AS, Vandekerckhove L, Matheeussen V, Demolder M, Baerts L, De Meester I, Linke WA, Paulus WJ, De Keulenaer GW. Left ventricular diastolic dysfunction and myocardial stiffness in diabetic mice is attenuated by inhibition of dipeptidyl peptidase 4. Cardiovasc Res 104: 423–431, 2014. doi: 10.1093/cvr/cvu223. [DOI] [PubMed] [Google Scholar]
- 135.Wang X, DuBois DC, Sukumaran S, Ayyar V, Jusko WJ, Almon RR. Variability in Zucker diabetic fatty rats: differences in disease progression in hyperglycemic and normoglycemic animals. Diabetes Metab Syndr Obes 7: 531–541, 2014. doi: 10.2147/DMSO.S69891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Toblli JE, DeRosa G, Cao G, Piorno P, Pagano P. ACE inhibitor and angiotensin type I receptor antagonist in combination reduce renal damage in obese Zucker rats. Kidney Int 65: 2343–2359, 2004. doi: 10.1111/j.1523-1755.2004.00661.x. [DOI] [PubMed] [Google Scholar]
- 137.Triscari J, Stern JS, Johnson PR, Sullivan AC. Carbohydrate metabolism in lean and obese Zucker rats. Metabolism 28: 183–189, 1979. doi: 10.1016/0026-0495(79)90084-2. [DOI] [PubMed] [Google Scholar]
- 138.Hjortbak MV, Hjort J, Povlsen JA, Jensen RV, Stottrup NB, Laursen MR, Jespersen NR, Lofgren B, Botker HE. Influence of diabetes mellitus duration on the efficacy of ischemic preconditioning in a Zucker diabetic fatty rat model. PLoS One 13: e0192981, 2018. doi: 10.1371/journal.pone.0192981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger RH. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci USA 97: 1784–1789, 2000. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wohlfart P, Lin J, Dietrich N, Kannt A, Elvert R, Herling AW, Hammes HP. Expression patterning reveals retinal inflammation as a minor factor in experimental retinopathy of ZDF rats. Acta Diabetol 51: 553–558, 2014. doi: 10.1007/s00592-013-0550-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lum-Naihe K, Toedebusch R, Mahmood A, Bajwa J, Carmack T, Kumar SA, Ardhanari S, DeMarco VG, Emter CA, Pulakat L. Cardiovascular disease progression in female Zucker Diabetic Fatty rats occurs via unique mechanisms compared to males. Sci Rep 7: 17823, 2017. doi: 10.1038/s41598-017-18003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang P, Chatham JC. Onset of diabetes in Zucker diabetic fatty (ZDF) rats leads to improved recovery of function after ischemia in the isolated perfused heart. Am J Physiol Endocrinol Physiol 286: E725–E736, 2004. doi: 10.1152/ajpendo.00295.2003. [DOI] [PubMed] [Google Scholar]
- 143.Zhou Y, Wang H, Man F, Guo Z, Xu J, Yan W, Li J, Pan Q, Wang W. Sitagliptin protects cardiac function by reducing nitroxidative stress and promoting autophagy in Zucker diabetic fatty (ZDF) rats. Cardiovasc Drugs Ther 32: 541–552, 2018. doi: 10.1007/s10557-018-6831-9. [DOI] [PubMed] [Google Scholar]
- 144.Siwy J, Zoja C, Klein J, Benigni A, Mullen W, Mayer B, Mischak H, Jankowski J, Stevens R, Vlahou A, Kossida S, Perco P, Bahlmann FH. Evaluation of the Zucker diabetic fatty (ZDF) rat as a model for human disease based on urinary peptidomic profiles. PLoS One 7: e51334, 2012. doi: 10.1371/journal.pone.0051334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Corsetti JP, Sparks JD, Peterson RG, Smith RL, Sparks CE. Effect of dietary fat on the development of non-insulin dependent diabetes mellitus in obese Zucker diabetic fatty male and female rats. Atherosclerosis 148: 231–241, 2000. doi: 10.1016/S0021-9150(99)00265-8. [DOI] [PubMed] [Google Scholar]
- 146.Bouthoorn S, Valstar GB, Gohar A, den Ruijter HM, Reitsma HB, Hoes AW, Rutten FH. The prevalence of left ventricular diastolic dysfunction and heart failure with preserved ejection fraction in men and women with type 2 diabetes: a systematic review and meta-analysis. Diab Vasc Dis Res 15: 477–493, 2018. doi: 10.1177/1479164118787415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Baynes J, Murray DB. Cardiac and renal function are progressively impaired with aging in Zucker diabetic fatty type II diabetic rats. Oxid Med Cell Longev 2: 328–334, 2009. doi: 10.4161/oxim.2.5.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Daniels A, Linz D, van Bilsen M, Rutten H, Sadowski T, Ruf S, Juretschke HP, Neumann-Haefelin C, Munts C, van der Vusse GJ, van Nieuwenhoven FA. Long-term severe diabetes only leads to mild cardiac diastolic dysfunction in Zucker diabetic fatty rats. Eur J Heart Fail 14: 193–201, 2012. doi: 10.1093/eurjhf/hfr166. [DOI] [PubMed] [Google Scholar]
- 149.Chatham JC, Seymour AM. Cardiac carbohydrate metabolism in Zucker diabetic fatty rats. Cardiovasc Res 55: 104–112, 2002. doi: 10.1016/S0008-6363(02)00399-1. [DOI] [PubMed] [Google Scholar]
- 150.Young ME, Guthrie PH, Razeghi P, Leighton B, Abbasi S, Patil S, Youker KA, Taegtmeyer H. Impaired long-chain fatty acid oxidation and contractile dysfunction in the obese Zucker rat heart. Diabetes 51: 2587–2595, 2002. doi: 10.2337/diabetes.51.8.2587. [DOI] [PubMed] [Google Scholar]
- 151.Wang P, Lloyd SG, Zeng H, Bonen A, Chatham JC. Impact of altered substrate utilization on cardiac function in isolated hearts from Zucker diabetic fatty rats. Am J Physiol Heart Circ Physiol 288: H2102–H2110, 2005. doi: 10.1152/ajpheart.00935.2004. [DOI] [PubMed] [Google Scholar]
- 152.Sreenan S, Sturis J, Pugh W, Burant CF, Polonsky KS. Prevention of hyperglycemia in the Zucker diabetic fatty rat by treatment with metformin or troglitazone. Am J Physiol Endocrinol Physiol 271: E742–E747, 1996. doi: 10.1152/ajpendo.1996.271.4.E742. [DOI] [PubMed] [Google Scholar]
- 153.Schwasinger-Schmidt T, Robbins DC, Williams SJ, Novikova L, Stehno-Bittel L. Long-term liraglutide treatment is associated with increased insulin content and secretion in β-cells, and a loss of α-cells in ZDF rats. Pharmacol Res 76: 58–66, 2013. doi: 10.1016/j.phrs.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 154.Steven S, Oelze M, Hanf A, Kroller-Schon S, Kashani F, Roohani S, Welschof P, Kopp M, Godtel-Armbrust U, Xia N, Li H, Schulz E, Lackner KJ, Wojnowski L, Bottari SP, Wenzel P, Mayoux E, Munzel T, Daiber A. The SGLT2 inhibitor empagliflozin improves the primary diabetic complications in ZDF rats. Redox Biol 13: 370–385, 2017. doi: 10.1016/j.redox.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Marsh SA, Powell PC, Agarwal A, Dell'Italia LJ, Chatham JC. Cardiovascular dysfunction in Zucker obese and Zucker diabetic fatty rats: role of hydronephrosis. Am J Physiol Heart Circ Physiol 293: H292–H298, 2007. doi: 10.1152/ajpheart.01362.2006. [DOI] [PubMed] [Google Scholar]
- 156.Goto Y, Kakizaki M, Masaki N. Production of spontaneous diabetic rats by repetition of selective breeding. Tohoku J Exp Med 119: 85–90, 1976. doi: 10.1620/tjem.119.85. [DOI] [PubMed] [Google Scholar]
- 157.Kimura K, Toyota T, Kakizaki M, Kudo M, Takebe K, Goto Y. Impaired insulin secretion in the spontaneous diabetes rats. Tohoku J Exp Med 137: 453–459, 1982. doi: 10.1620/tjem.137.453. [DOI] [PubMed] [Google Scholar]
- 158.Portha B, Giroix MH, Serradas P, Gangnerau MN, Movassat J, Rajas F, Bailbe D, Plachot C, Mithieux G, Marie JC. Beta-cell function and viability in the spontaneously diabetic GK rat: information from the GK/Par colony. Diabetes 50, Suppl 1: S89–93, 2001. doi: 10.2337/diabetes.50.2007.s89. [DOI] [PubMed] [Google Scholar]
- 159.Portha B, Serradas P, Bailbe D, Suzuki K, Goto Y, Giroix MH. β-cell insensitivity to glucose in the GK rat, a spontaneous nonobese model for type II diabetes. Diabetes 40: 486–491, 1991. doi: 10.2337/diabetes.40.4.486. [DOI] [PubMed] [Google Scholar]
- 160.Movassat J, Bailbe D, Lubrano-Berthelier C, Picarel-Blanchot F, Bertin E, Mourot J, Portha B. Follow-up of GK rats during prediabetes highlights increased insulin action and fat deposition despite low insulin secretion. Am J Physiol Endocrinol Metab 294: E168–E175, 2008. doi: 10.1152/ajpendo.00501.2007. [DOI] [PubMed] [Google Scholar]
- 161.Suzuki K, Yen-Chung H, Toyota T, Goto Y, Hirata Y, Okada K. The significance of nerve sugar levels for the peripheral nerve impairment of spontaneously diabetic GK (Goto-Kakizaki) rats. Diabetes Res 14: 21–25, 1990. [PubMed] [Google Scholar]
- 162.Allen RS, Feola A, Motz CT, Ottensmeyer AL, Chesler KC, Dunn R, Thule PM, Pardue MT. Retinal deficits precede cognitive and motor deficits in a rat model of type II diabetes. Invest Ophthalmol Vis Sci 60: 123–133, 2019. doi: 10.1167/iovs.18-25110. [DOI] [PubMed] [Google Scholar]
- 163.Bourgneuf C, Bailbe D, Lamaziere A, Dupont C, Moldes M, Farabos D, Roblot N, Gauthier C, Mathieu d'Argent E, Cohen-Tannoudji J, Monniaux D, Feve B, Movassat J, di Clemente N, Racine C. The Goto-Kakizaki rat is a spontaneous prototypical rodent model of polycystic ovary syndrome. Nat Commun 12: 1064, 2021. doi: 10.1038/s41467-021-21308-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gauguier D, Froguel P, Parent V, Bernard C, Bihoreau MT, Portha B, James MR, Penicaud L, Lathrop M, Ktorza A. Chromosomal mapping of genetic loci associated with non-insulin dependent diabetes in the GK rat. Nat Genet 12: 38–43, 1996. doi: 10.1038/ng0196-38. [DOI] [PubMed] [Google Scholar]
- 165.Galli J, Li LS, Glaser A, Ostenson CG, Jiao H, Fakhrai-Rad H, Jacob HJ, Lander ES, Luthman H. Genetic analysis of non-insulin dependent diabetes mellitus in the GK rat. Nat Genet 12: 31–37, 1996. doi: 10.1038/ng0196-31. [DOI] [PubMed] [Google Scholar]
- 166.D'Souza A, Howarth FC, Yanni J, Dobryznski H, Boyett MR, Adeghate E, Bidasee KR, Singh J. Left ventricle structural remodelling in the prediabetic Goto-Kakizaki rat. Exp Physiol 96: 875–888, 2011. doi: 10.1113/expphysiol.2011.058271. [DOI] [PubMed] [Google Scholar]
- 167.Iltis I, Kober F, Desrois M, Dalmasso C, Lan C, Portha B, Cozzone PJ, Bernard M. Defective myocardial blood flow and altered function of the left ventricle in type 2 diabetic rats: a noninvasive in vivo study using perfusion and cine magnetic resonance imaging. Invest Radiol 40: 19–26, 2005. [PubMed] [Google Scholar]
- 168.Picatoste B, Ramirez E, Caro-Vadillo A, Iborra C, Ares-Carrasco S, Egido J, Tunon J, Lorenzo O. Sitagliptin reduces cardiac apoptosis, hypertrophy and fibrosis primarily by insulin-dependent mechanisms in experimental type-II diabetes. Potential roles of GLP-1 isoforms. PLoS One 8: e78330, 2013. [Erratum in PLoS One 8, 2013 and in PLoS One 9: e91601, 2014]. doi: 10.1371/journal.pone.0078330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Devanathan S, Nemanich ST, Kovacs A, Fettig N, Gropler RJ, Shoghi KI. Genomic and metabolic disposition of non-obese type 2 diabetic rats to increased myocardial fatty acid metabolism. PLoS One 8: e78477, 2013. doi: 10.1371/journal.pone.0078477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Meagher P, Civitarese R, Lee X, Gordon M, Bugyei-Twum A, Desjardins JF, Kabir G, Zhang Y, Kosanam H, Visram A, Leong-Poi H, Advani A, Connelly KA. The Goto Kakizaki rat: Impact of age upon changes in cardiac and renal structure, function. PLoS One 16: e0252711, 2021. doi: 10.1371/journal.pone.0252711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.El-Omar MM, Yang ZK, Phillips AO, Shah AM. Cardiac dysfunction in the Goto-Kakizaki rat. A model of type II diabetes mellitus. Basic Res Cardiol 99: 133–141, 2004. doi: 10.1007/s00395-004-0440-4. [DOI] [PubMed] [Google Scholar]
- 172.Howarth FC, Shafiullah M, Qureshi MA. Chronic effects of type 2 diabetes mellitus on cardiac muscle contraction in the Goto-Kakizaki rat. Exp Physiol 92: 1029–1036, 2007. doi: 10.1113/expphysiol.2007.038703. [DOI] [PubMed] [Google Scholar]
- 173.Desrois M, Clarke K, Lan C, Dalmasso C, Cole M, Portha B, Cozzone PJ, Bernard M. Upregulation of eNOS and unchanged energy metabolism in increased susceptibility of the aging type 2 diabetic GK rat heart to ischemic injury. Am J Physiol Heart Circ Physiol 299: H1679–H1686, 2010. doi: 10.1152/ajpheart.00998.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chandler MP, Morgan EE, McElfresh TA, Kung TA, Rennison JH, Hoit BD, Young ME. Heart failure progression is accelerated following myocardial infarction in type 2 diabetic rats. Am J Physiol Heart Circ Physiol 293: H1609–H1616, 2007. doi: 10.1152/ajpheart.01338.2006. [DOI] [PubMed] [Google Scholar]
- 175.Kuwabara WMT, Panveloski-Costa AC, Yokota CNF, Pereira JNB, Filho JM, Torres RP, Hirabara SM, Curi R, Alba-Loureiro TC. Comparison of Goto-Kakizaki rats and high fat diet-induced obese rats: are they reliable models to study type 2 diabetes mellitus? PLoS One 12: e0189622, 2017. doi: 10.1371/journal.pone.0189622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mine T, Miura K, Kajioka T, Kitahara Y. Nateglinide prevents fatty liver through up-regulation of lipid oxidation pathway in Goto-Kakizaki rats on a high-fat diet. Metabolism 57: 140–148, 2008. doi: 10.1016/j.metabol.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 177.Howarth FC, Qureshi MA, Sobhy ZH, Parekh K, Yammahi SR, Adrian TE, Adeghate E. Structural lesions and changing pattern of expression of genes encoding cardiac muscle proteins are associated with ventricular myocyte dysfunction in type 2 diabetic Goto-Kakizaki rats fed a high-fat diet. Exp Physiol 96: 765–777, 2011. doi: 10.1113/expphysiol.2011.058446. [DOI] [PubMed] [Google Scholar]
- 178.Iwatsuka H, Shino A, Suzuoki Z. General survey of diabetic features of yellow KK mice. Endocrinol Jpn 17: 23–35, 1970. doi: 10.1507/endocrj1954.17.23. [DOI] [PubMed] [Google Scholar]
- 179.Nakamura M. A diabetic strain of the mouse. Proc Jpn Acad 38: 348–352, 1962. doi: 10.2183/pjab1945.38.348. 20522964 [DOI] [Google Scholar]
- 180.Kitada M, Ogura Y, Koya D. Rodent models of diabetic nephropathy: their utility and limitations. Int J Nephrol Renovasc Dis 9: 279–290, 2016. doi: 10.2147/IJNRD.S103784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tomino Y. Lessons from the KK-Ay mouse, a spontaneous animal model for the treatment of human type 2 diabetic nephropathy. Nephrourol Mon 4: 524–529, 2012. doi: 10.5812/numonthly.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Li C, Zhang J, Xue M, Li X, Han F, Liu X, Xu L, Lu Y, Cheng Y, Li T, Yu X, Sun B, Chen L. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc Diabetol 18: 15, 2019. doi: 10.1186/s12933-019-0816-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kim JH, Sen S, Avery CS, Simpson E, Chandler P, Nishina PM, Churchill GA, Naggert JK. Genetic analysis of a new mouse model for non-insulin-dependent diabetes. Genomics 74: 273–286, 2001. doi: 10.1006/geno.2001.6569. [DOI] [PubMed] [Google Scholar]
- 184.Ramasubramanian B, Reddy PH. Are TallyHo mice a true mouse model for type 2 diabetes and Alzheimer’s disease? J Alzheimers Dis 72: S81–S93, 2019. doi: 10.3233/JAD-190613. [DOI] [PubMed] [Google Scholar]
- 185.Lee WS, Kim J. Application of animal models in diabetic cardiomyopathy. Diabetes Metab J 45: 129–145, 2021. doi: 10.4093/dmj.2020.0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Riehle C, Bauersachs J. Of mice and men: models and mechanisms of diabetic cardiomyopathy. Basic Res Cardiol 114: 2, 2018. doi: 10.1007/s00395-018-0711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rojo D, McCarthy C, Raingo J, Rubinstein M. Mouse models for V103I and I251L gain of function variants of the human MC4R display decreased adiposity but are not protected against a hypercaloric diet. Mol Metab 42: 101077, 2020. doi: 10.1016/j.molmet.2020.101077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bolzan AD, Bianchi MS. Genotoxicity of streptozotocin. Mutat Res 512: 121–134, 2002. doi: 10.1016/S1383-5742(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 189.Wu KK, Huan Y. Streptozotocin-induced diabetic models in mice and rats. Curr Protoc Pharmacol 5: 5.47–5.47.20, 2008. doi: 10.1002/0471141755.ph0547s40. [DOI] [PubMed] [Google Scholar]
- 190.Furman BL. Streptozotocin-induced diabetic models in mice and rats. Curr Protoc 1: e78, 2021. doi: 10.1002/cpz1.78. [DOI] [PubMed] [Google Scholar]
- 191.Like AA, Rossini AA. Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science 193: 415–417, 1976. doi: 10.1126/science.180605. [DOI] [PubMed] [Google Scholar]
- 192.Cheta D. Animal models of type I (insulin-dependent) diabetes mellitus. J Pediatr Endocrinol Metab 11: 11–19, 1998. doi: 10.1515/jpem.1998.11.1.11. [DOI] [PubMed] [Google Scholar]
- 193.Gurley SB, Clare SE, Snow KP, Hu A, Meyer TW, Coffman TM. Impact of genetic background on nephropathy in diabetic mice. Am J Physiol Renal Physiol 290: F214–F222, 2006. doi: 10.1152/ajprenal.00204.2005. [DOI] [PubMed] [Google Scholar]
- 194.Hahn M, van Krieken PP, Nord C, Alanentalo T, Morini F, Xiong Y, Eriksson M, Mayer J, Kostromina E, Ruas JL, Sharpe J, Pereira T, Berggren PO, Ilegems E, Ahlgren U. Topologically selective islet vulnerability and self-sustained downregulation of markers for β-cell maturity in streptozotocin-induced diabetes. Commun Biol 3: 541, 2020. doi: 10.1038/s42003-020-01243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kume E, Fujimura H, Matsuki N, Ito M, Aruga C, Toriumi W, Kitamura K, Doi K. Hepatic changes in the acute phase of streptozotocin (SZ)-induced diabetes in mice. Exp Toxicol Pathol 55: 467–480, 2004. doi: 10.1078/0940-2993-00351. [DOI] [PubMed] [Google Scholar]
- 196.Shao D, Tian R. Glucose transporters in cardiac metabolism and hypertrophy. Compr Physiol 6: 331–351, 2015. doi: 10.1002/cphy.c150016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Salem KA, Kosanovic M, Qureshi A, Ljubisavljevic M, Howarth FC. The direct effects of streptozotocin and alloxan on contractile function in rat heart. Pharmacol Res 59: 235–241, 2009. doi: 10.1016/j.phrs.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 198.Candela S, Hernandez RE, Gagliardino JJ. Circadian variation of the streptozotocin-diabetogenic effect in mice. Experientia 35: 1256–1257, 1979. doi: 10.1007/BF01963323. [DOI] [PubMed] [Google Scholar]
- 199.Kolb H. Mouse models of insulin dependent diabetes: low-dose streptozocin-induced diabetes and nonobese diabetic (NOD) mice. Diabetes Metab Rev 3: 751–778, 1987. doi: 10.1002/dmr.5610030308. [DOI] [PubMed] [Google Scholar]
- 200.Kromann H, Christy M, Lernmark A, Nedergaard M, Nerup J. The low dose streptozotocin murine model of type 1 (insulin-dependent) diabetes mellitus: studies in vivo and in vitro of the modulating effect of sex hormones. Diabetologia 22: 194–198, 1982. doi: 10.1007/BF00283752. [DOI] [PubMed] [Google Scholar]
- 201.Leiter EH. Multiple low-dose streptozotocin-induced hyperglycemia and insulitis in C57BL mice: influence of inbred background, sex, and thymus. Proc Natl Acad Sci USA 79: 630–634, 1982. doi: 10.1073/pnas.79.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Seager MJ, Singal PK, Orchard R, Pierce GN, Dhalla NS. Cardiac cell damage: a primary myocardial disease in streptozotocin-induced chronic diabetes. Br J Exp Pathol 65: 613–623, 1984. [PMC free article] [PubMed] [Google Scholar]
- 203.Zhu XX, Zhou XP, Zhong XL, Zhong CS, Yu YF. Streptozotocin induced cardiomyopathy in diabetic rats. Chin Med J (Engl) 106: 463–466, 1993. [PubMed] [Google Scholar]
- 204.Pulinilkunnil T, Kienesberger PC, Nagendran J, Waller TJ, Young ME, Kershaw EE, Korbutt G, Haemmerle G, Zechner R, Dyck JR. Myocardial adipose triglyceride lipase overexpression protects diabetic mice from the development of lipotoxic cardiomyopathy. Diabetes 62: 1464–1477, 2013. doi: 10.2337/db12-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sakamoto J, Barr RL, Kavanagh KM, Lopaschuk GD. Contribution of malonyl-CoA decarboxylase to the high fatty acid oxidation rates seen in the diabetic heart. Am J Physiol Heart Circ Physiol 278: H1196–H1204, 2000. doi: 10.1152/ajpheart.2000.278.4.H1196. [DOI] [PubMed] [Google Scholar]
- 206.Wall SR, Lopaschuk GD. Glucose oxidation rates in fatty acid-perfused isolated working hearts from diabetic rats. Biochim Biophys Acta 1006: 97–103, 1989. doi: 10.1016/0005-2760(89)90328-7. [DOI] [PubMed] [Google Scholar]
- 207.Mishra PK. Why the diabetic heart is energy inefficient: a ketogenesis and ketolysis perspective. Am J Physiol Heart Circ Physiol 321: H751–H755, 2021. doi: 10.1152/ajpheart.00260.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chandramouli C, Reichelt ME, Curl CL, Varma U, Bienvenu LA, Koutsifeli P, Raaijmakers AJA, De Blasio MJ, Qin CX, Jenkins AJ, Ritchie RH, Mellor KM, Delbridge LMD. Diastolic dysfunction is more apparent in STZ-induced diabetic female mice, despite less pronounced hyperglycemia. Sci Rep 8: 2346, 2018. doi: 10.1038/s41598-018-20703-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ammar HI, Shamseldeen AM, Shoukry HS, Ashour H, Kamar SS, Rashed LA, Fadel M, Srivastava A, Dhingra S. Metformin impairs homing ability and efficacy of mesenchymal stem cells for cardiac repair in streptozotocin-induced diabetic cardiomyopathy in rats. Am J Physiol Heart Circ Physiol 320: H1290–H1302, 2021. doi: 10.1152/ajpheart.00317.2020. [DOI] [PubMed] [Google Scholar]
- 210.Linthout S, Seeland U, Riad A, Eckhardt O, Hohl M, Dhayat N, Richter U, Fischer JW, Böhm M, Pauschinger M, Schultheiss H-P, Tschöpe C. Reduced MMP-2 activity contributes to cardiac fibrosis in experimental diabetic cardiomyopathy. Basic Res Cardiol 103: 319–327, 2008. doi: 10.1007/s00395-008-0715-2. [DOI] [PubMed] [Google Scholar]
- 211.Marchini GS, Cestari IN, Salemi VMC, Irigoyen MC, Arnold A, Kakoi A, Rocon C, Aiello VD, Cestari IA. Early changes in myocyte contractility and cardiac function in streptozotocin-induced type 1 diabetes in rats. PLoS One 15: e0237305, 2020. doi: 10.1371/journal.pone.0237305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Mukai N, Nakayama Y, Abdali SA, Yoshioka J. Cardiomyocyte-specific Txnip C247S mutation improves left ventricular functional reserve in streptozotocin-induced diabetic mice. Am J Physiol Heart Circ Physiol 321: H259–H274, 2021. doi: 10.1152/ajpheart.00174.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yoshioka M, Kayo T, Ikeda T, Koizuni A. A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes 46: 887–894, 1997. doi: 10.2337/diabetes.46.5.887. [DOI] [PubMed] [Google Scholar]
- 214.Bugger H, Chen D, Riehle C, Soto J, Theobald HA, Hu XX, Ganesan B, Weimer BC, Abel ED. Tissue-specific remodeling of the mitochondrial proteome in type 1 diabetic Akita mice. Diabetes 58: 1986–1997, 2009. doi: 10.2337/db09-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Basu R, Oudit GY, Wang X, Zhang L, Ussher JR, Lopaschuk GD, Kassiri Z. Type 1 diabetic cardiomyopathy in the Akita (Ins2WT/C96Y) mouse model is characterized by lipotoxicity and diastolic dysfunction with preserved systolic function. Am J Physiol Heart Circ Physiol 297: H2096–H2108, 2009. doi: 10.1152/ajpheart.00452.2009. [DOI] [PubMed] [Google Scholar]
- 216.Kambis TN, Shahshahan HR, Kar S, Yadav SK, Mishra PK. Transgenic expression of miR-133a in the diabetic Akita heart prevents cardiac remodeling and cardiomyopathy. Front Cardiovasc Med 6: 45, 2019. doi: 10.3389/fcvm.2019.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Park M, Nishimura T, Baeza-Garza CD, Caldwell ST, Pun PBL, Prag HA, Young T, Sauchanka O, Logan A, Forkink M, Gruszczyk AV, Prime TA, Arndt S, Naudi A, Pamplona R, Coughlan MT, Tate M, Ritchie RH, Caicci F, Kaludercic N, Di Lisa F, Smith RAJ, Hartley RC, Murphy MP, Krieg T. Confirmation of the cardioprotective effect of MitoGamide in the diabetic heart. Cardiovasc Drugs Ther 34: 823–834, 2020. doi: 10.1007/s10557-020-07086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Vadvalkar SS, Matsuzaki S, Eyster CA, Giorgione JR, Bockus LB, Kinter CS, Kinter M, Humphries KM. Decreased mitochondrial pyruvate transport activity in the diabetic heart: role of mitochondrial pyruvate carrier 2 (MPC2) acetylation. J Biol Chem 292: 4423–4433, 2017. doi: 10.1074/jbc.M116.753509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kidokoro K, Cherney DZI, Bozovic A, Nagasu H, Satoh M, Kanda E, Sasaki T, Kashihara N. Evaluation of glomerular hemodynamic function by empagliflozin in diabetic mice using in vivo imaging. Circulation 140: 303–315, 2019. doi: 10.1161/CIRCULATIONAHA.118.037418. [DOI] [PubMed] [Google Scholar]
- 220.Fujita Y, Atageldiyeva KK, Takeda Y, Yanagimachi T, Makino Y, Haneda M. A low-carbohydrate diet improves glucose metabolism in lean insulinopenic Akita mice along with sodium-glucose cotransporter 2 inhibitor. Front Endocrinol (Lausanne) 11: 601594, 2020. doi: 10.3389/fendo.2020.601594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Jun JY, Ma Z, Segar L. Spontaneously diabetic Ins2(+/Akita):apoE-deficient mice exhibit exaggerated hypercholesterolemia and atherosclerosis. Am J Physiol Endocrinol Physiol 301: E145–E154, 2011. doi: 10.1152/ajpendo.00034.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kikawa K, Sakano D, Shiraki N, Tsuyama T, Kume K, Endo F, Kume S. Beneficial effect of insulin treatment on islet transplantation outcomes in Akita mice. PLoS One 9: e95451, 2014. doi: 10.1371/journal.pone.0095451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Miyata KN, Zhao S, Wu CH, Lo CS, Ghosh A, Chenier I, Filep JG, Ingelfinger JR, Zhang SL, Chan JSD. Comparison of the effects of insulin and SGLT2 inhibitor on the Renal Renin-Angiotensin system in type 1 diabetes mice. Diabetes Res Clin Pract 162: 108107, 2020. doi: 10.1016/j.diabres.2020.108107. [DOI] [PubMed] [Google Scholar]
- 224.Salem ES, Grobe N, Elased KM. Insulin treatment attenuates renal ADAM17 and ACE2 shedding in diabetic Akita mice. Am J Physiol Renal Physiol 306: F629–F639, 2014. doi: 10.1152/ajprenal.00516.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Schoeller EL, Albanna G, Frolova AI, Moley KH. Insulin rescues impaired spermatogenesis via the hypothalamic-pituitary-gonadal axis in Akita diabetic mice and restores male fertility. Diabetes 61: 1869–1878, 2012. doi: 10.2337/db11-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hong EG, Jung DY, Ko HJ, Zhang Z, Ma Z, Jun JY, Kim JH, Sumner AD, Vary TC, Gardner TW, Bronson SK, Kim JK. Nonobese, insulin-deficient Ins2Akita mice develop type 2 diabetes phenotypes including insulin resistance and cardiac remodeling. Am J Physiol Endocrinol Physiol 293: E1687–E1696, 2007. doi: 10.1152/ajpendo.00256.2007. [DOI] [PubMed] [Google Scholar]
- 227.Gurley SB, Mach CL, Stegbauer J, Yang J, Snow KP, Hu A, Meyer TW, Coffman TM. Influence of genetic background on albuminuria and kidney injury in Ins2(+/C96Y) (Akita) mice. Am J Physiol Renal Physiol 298: F788–F795, 2010. doi: 10.1152/ajprenal.90515.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kesherwani V, Shahshahan HR, Mishra PK. Cardiac transcriptome profiling of diabetic Akita mice using microarray and next generation sequencing. PLoS One 12: e0182828, 2017. doi: 10.1371/journal.pone.0182828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Tate M, Higgins GC, De Blasio MJ, Lindblom R, Prakoso D, Deo M, Kiriazis H, Park M, Baeza-Garza CD, Caldwell ST, Hartley RC, Krieg T, Murphy MP, Coughlan MT, Ritchie RH. The mitochondria-targeted methylglyoxal sequestering compound, MitoGamide, is cardioprotective in the diabetic heart. Cardiovasc Drugs Ther 33: 669–674, 2019. [Erratum in Cardiovasc Drugs Ther 34: 223, 2020]. doi: 10.1007/s10557-019-06914-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.LaRocca TJ, Fabris F, Chen J, Benhayon D, Zhang S, McCollum L, Schecter AD, Cheung JY, Sobie EA, Hajjar RJ, Lebeche D. Na+/Ca2+ exchanger-1 protects against systolic failure in the Akitains2 model of diabetic cardiomyopathy via a CXCR4/NF-κB pathway. Am J Physiol Heart Circ Physiol 303: H353–H367, 2012. doi: 10.1152/ajpheart.01198.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Hemmeryckx B, Hoylaerts MF, Gallacher DJ, Rong Lu H, Himmelreich U, D’hooge J, Swinnen M, Lijnen HR. Does rosiglitazone affect adiposity and cardiac function in genetic diabetic mice? Eur J Pharmacol 700: 23–31, 2013. doi: 10.1016/j.ejphar.2012.11.062. [DOI] [PubMed] [Google Scholar]
- 232.Zhou Y, Xiao H, Wu J, Zha L, Zhou M, Li Q, Wang M, Shi S, Li Y, Lyu L, Wang Q, Tu X, Lu Q. Type I diabetic Akita mouse model is characterized by abnormal cardiac deformation during early stages of diabetic cardiomyopathy with speckle-tracking based strain imaging. Cell Physiol Biochem 45: 1541–1550, 2018. doi: 10.1159/000487690. [DOI] [PubMed] [Google Scholar]
- 233.Breslow JL. Mouse models of atherosclerosis. Science 272: 685–688, 1996. doi: 10.1126/science.272.5262.685. [DOI] [PubMed] [Google Scholar]
- 234.Whitman SC. A practical approach to using mice in atherosclerosis research. Clin Biochem Rev 25: 81–93, 2004. [PMC free article] [PubMed] [Google Scholar]
- 235.Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest 92: 883–893, 1993. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Piedrahita JA, Zhang SH, Hagaman JR, Oliver PM, Maeda N. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc Natl Acad Sci USA 89: 4471–4475, 1992. doi: 10.1073/pnas.89.10.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Plump AS, Smith JD, Hayek T, Aalto-Setala K, Walsh A, Verstuyft JG, Rubin EM, Breslow JL. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell 71: 343–353, 1992. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 238.von Scheidt M, Zhao Y, Kurt Z, Pan C, Zeng L, Yang X, Schunkert H, Lusis AJ. Applications and limitations of mouse models for understanding human atherosclerosis. Cell Metab 25: 248–261, 2017. doi: 10.1016/j.cmet.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Neuhofer A, Wernly B, Leitner L, Sarabi A, Sommer NG, Staffler G, Zeyda M, Stulnig TM. An accelerated mouse model for atherosclerosis and adipose tissue inflammation. Cardiovasc Diabetol 13: 23, 2014. doi: 10.1186/1475-2840-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Schreyer SA, Vick C, Lystig TC, Mystkowski P, LeBoeuf RC. LDL receptor but not apolipoprotein E deficiency increases diet-induced obesity and diabetes in mice. Am J Physiol Endocrinol Physiol 282: E207–E214, 2002. doi: 10.1152/ajpendo.2002.282.1.E207. [DOI] [PubMed] [Google Scholar]
- 241.Engelbertsen D, To F, Duner P, Kotova O, Soderberg I, Alm R, Gomez MF, Nilsson J, Bengtsson E. Increased inflammation in atherosclerotic lesions of diabetic Akita-LDLr(−)/(−) mice compared to nondiabetic LDLr(−)/(−) mice. Exp Diabetes Res 2012: 176162, 2012. doi: 10.1155/2012/176162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Barrett TJ, Distel E, Murphy AJ, Hu J, Garshick MS, Ogando Y, Liu J, Vaisar T, Heinecke JW, Berger JS, Goldberg IJ, Fisher EA. Apolipoprotein (AI) promotes atherosclerosis regression in diabetic mice by suppressing myelopoiesis and plaque inflammation. Circulation 140: 1170–1184, 2019. doi: 10.1161/CIRCULATIONAHA.119.039476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Renard CB, Kramer F, Johansson F, Lamharzi N, Tannock LR, von Herrath MG, Chait A, Bornfeldt KE. Diabetes and diabetes-associated lipid abnormalities have distinct effects on initiation and progression of atherosclerotic lesions. J Clin Invest 114: 659–668, 2004. doi: 10.1172/JCI200417867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Tse J, Martin-McNaulty B, Halks-Miller M, Kauser K, DelVecchio V, Vergona R, Sullivan ME, Rubanyi GM. Accelerated atherosclerosis and premature calcified cartilaginous metaplasia in the aorta of diabetic male Apo E knockout mice can be prevented by chronic treatment with 17 β-estradiol. Atherosclerosis 144: 303–313, 1999. doi: 10.1016/s0021-9150(98)00325-6. [DOI] [PubMed] [Google Scholar]
- 245.Lamharzi N, Renard CB, Kramer F, Pennathur S, Heinecke JW, Chait A, Bornfeldt KE. Hyperlipidemia in concert with hyperglycemia stimulates the proliferation of macrophages in atherosclerotic lesions: potential role of glucose-oxidized LDL. Diabetes 53: 3217–3225, 2004. doi: 10.2337/diabetes.53.12.3217. [DOI] [PubMed] [Google Scholar]
- 246.Westrick RJ, Winn ME, Eitzman DT. Murine models of vascular thrombosis (Eitzman series). Arterioscler Thromb Vasc Biol 27: 2079–2093, 2007. doi: 10.1161/ATVBAHA.107.142810. [DOI] [PubMed] [Google Scholar]
- 247.Braun A, Trigatti BL, Post MJ, Sato K, Simons M, Edelberg JM, Rosenberg RD, Schrenzel M, Krieger M. Loss of SR-BI expression leads to the early onset of occlusive atherosclerotic coronary artery disease, spontaneous myocardial infarctions, severe cardiac dysfunction, and premature death in apolipoprotein E-deficient mice. Circ Res 90: 270–276, 2002. doi: 10.1161/hh0302.104462. [DOI] [PubMed] [Google Scholar]
- 248.Braun A, Rigotti A, Trigatti BL. Myocardial infarction following atherosclerosis in murine models. Curr Drug Targets 9: 217–223, 2008. doi: 10.2174/138945008783755566. [DOI] [PubMed] [Google Scholar]
- 249.Vasquez EC, Peotta VA, Gava AL, Pereira TM, Meyrelles SS. Cardiac and vascular phenotypes in the apolipoprotein E-deficient mouse. J Biomed Sci 19: 22, 2012. doi: 10.1186/1423-0127-19-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Ahn SJ, Le Master E, Lee JC, Phillips SA, Levitan I, Fancher IS. Differential effects of obesity on visceral versus subcutaneous adipose arteries: role of shear-activated Kir2.1 and alterations to the glycocalyx. Am J Physiol Heart Circ Physiol 322: H156–H166, 2022. doi: 10.1152/ajpheart.00399.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Boardman NT, Pedersen TM, Rossvoll L, Hafstad AD, Aasum E. Diet-induced obese mouse hearts tolerate an acute high-fatty acid exposure that also increases ischemic tolerance. Am J Physiol Heart Circ Physiol 319: H682–H693, 2020. doi: 10.1152/ajpheart.00284.2020. [DOI] [PubMed] [Google Scholar]
- 252.Chen X, Bollinger E, Cunio T, Damilano F, Stansfield JC, Pinkus CA, Kreuser S, Hirenallur-Shanthappa D, Roth Flach RJ. An assessment of thermoneutral housing conditions on murine cardiometabolic function. Am J Physiol Heart Circ Physiol 322: H234–H245, 2022. doi: 10.1152/ajpheart.00461.2021. [DOI] [PubMed] [Google Scholar]
- 253.do Carmo JM, Omoto ACM, Dai X, Moak SP, Mega GS, Li X, Wang Z, Mouton AJ, Hall JE, da Silva AA. Sex differences in the impact of parental obesity on offspring cardiac SIRT3 expression, mitochondrial efficiency, and diastolic function early in life. Am J Physiol Heart Circ Physiol 321: H485–H495, 2021. doi: 10.1152/ajpheart.00176.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Froogh G, Kandhi S, Duvvi R, Le Y, Weng Z, Alruwaili N, Ashe JO, Sun D, Huang A. The contribution of chymase-dependent formation of ANG II to cardiac dysfunction in metabolic syndrome of young rats: roles of fructose and EETs. Am J Physiol Heart Circ Physiol 318: H985–H993, 2020. doi: 10.1152/ajpheart.00633.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Grotle AK, Huo Y, Harrison ML, Ybarbo KM, Stone AJ. GsMTx-4 normalizes the exercise pressor reflex evoked by intermittent muscle contraction in early stage type 1 diabetic rats. Am J Physiol Heart Circ Physiol 320: H1738–H1748, 2021. doi: 10.1152/ajpheart.00794.2020. [DOI] [PubMed] [Google Scholar]
- 256.Komatsu Y, Aoyama K, Yoneda M, Ashikawa S, Nakano S, Kawai Y, Cui X, Furukawa N, Ikeda K, Nagata K. The prebiotic fiber inulin ameliorates cardiac, adipose tissue, and hepatic pathology, but exacerbates hypertriglyceridemia in rats with metabolic syndrome. Am J Physiol Heart Circ Physiol 320: H281–H295, 2021. doi: 10.1152/ajpheart.00657.2020. [DOI] [PubMed] [Google Scholar]
- 257.Leo MD, Peixoto-Nieves D, Yin W, Raghavan S, Muralidharan P, Mata-Daboin A, Jaggar JH. TMEM16A channel upregulation in arterial smooth muscle cells produces vasoconstriction during diabetes. Am J Physiol Heart Circ Physiol 320: H1089–H1101, 2021. doi: 10.1152/ajpheart.00690.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Limberg JK, Smith JA, Soares RN, Harper JL, Houghton KN, Jacob DW, Mozer MT, Grunewald ZI, Johnson BD, Curry TB, Baynard T, Manrique-Acevedo C, Padilla J. Sympathetically mediated increases in cardiac output, not restraint of peripheral vasodilation, contribute to blood pressure maintenance during hyperinsulinemia. Am J Physiol Heart Circ Physiol 319: H162–H170, 2020. doi: 10.1152/ajpheart.00250.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Malhi NK, Allen CL, Stewart E, Horton KL, Riu F, Batson J, Amoaku W, Morris JC, Arkill KP, Bates DO. Serine-arginine-rich protein kinase-1 inhibition for the treatment of diabetic retinopathy. Am J Physiol Heart Circ Physiol 322: H1014–H1027, 2022. doi: 10.1152/ajpheart.00001.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.McCallinhart PE, Cho Y, Sun Z, Ghadiali S, Meininger GA, Trask AJ. Reduced stiffness and augmented traction force in type 2 diabetic coronary microvascular smooth muscle. Am J Physiol Heart Circ Physiol 318: H1410–H1419, 2020. doi: 10.1152/ajpheart.00542.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Ramirez-Perez FI, Cabral-Amador FJ, Whaley-Connell AT, Aroor AR, Morales-Quinones M, Woodford ML, Ghiarone T, Ferreira-Santos L, Jurrissen TJ, Manrique-Acevedo CM, Jia G, DeMarco VG, Padilla J, Martinez-Lemus LA, Lastra G. Cystamine reduces vascular stiffness in Western diet-fed female mice. Am J Physiol Heart Circ Physiol 322: H167–H180, 2022. doi: 10.1152/ajpheart.00431.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Ramirez-Perez FI, Woodford ML, Morales-Quinones M, Grunewald ZI, Cabral-Amador FJ, Yoshida T, Brenner DA, Manrique-Acevedo C, Martinez-Lemus LA, Chandrasekar B, Padilla J. Mutation of the 5'-untranslated region stem-loop mRNA structure reduces type I collagen deposition and arterial stiffness in male obese mice. Am J Physiol Heart Circ Physiol 321: H435–H445, 2021. doi: 10.1152/ajpheart.00076.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Reitz CJ, Alibhai FJ, de Lima-Seolin BG, Nemec-Bakk A, Khaper N, Martino TA. Circadian mutant mice with obesity and metabolic syndrome are resilient to cardiovascular disease. Am J Physiol Heart Circ Physiol 319: H1097–H1111, 2020. doi: 10.1152/ajpheart.00462.2020. [DOI] [PubMed] [Google Scholar]
- 264.Reverte V, Rodriguez F, Oltra L, Moreno JM, Llinas MT, Shea CM, Schwartzkopf CD, Buys ES, Masferrer JL, Salazar FJ. SGLT2 inhibition potentiates the cardiovascular, renal, and metabolic effects of sGC stimulation in hypertensive rats with prolonged exposure to high-fat diet. Am J Physiol Heart Circ Physiol 322: H523–H536, 2022. doi: 10.1152/ajpheart.00386.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Saxton SN, Whitley AS, Potter RJ, Withers SB, Grencis R, Heagerty AM. Interleukin-33 rescues perivascular adipose tissue anticontractile function in obesity. Am J Physiol Heart Circ Physiol 319: H1387–H1397, 2020. doi: 10.1152/ajpheart.00491.2020. [DOI] [PubMed] [Google Scholar]
- 266.Sun Z, Li L, Zhang L, Yan J, Shao C, Bao Z, Liu J, Li Y, Zhou M, Hou L, Jing L, Pang Q, Geng Y, Mao X, Gu W, Wang Z. Macrophage galectin-3 enhances intimal translocation of vascular calcification in diabetes mellitus. Am J Physiol Heart Circ Physiol 318: H1068–H1079, 2020. doi: 10.1152/ajpheart.00690.2019. [DOI] [PubMed] [Google Scholar]
- 267.Sun Z, Zhang L, Li L, Shao C, Liu J, Zhou M, Wang Z. Galectin-3 mediates cardiac remodeling caused by impaired glucose and lipid metabolism through inhibiting two pathways of activating Akt. Am J Physiol Heart Circ Physiol 320: H364–H380, 2021. doi: 10.1152/ajpheart.00523.2020. [DOI] [PubMed] [Google Scholar]
- 268.Watts SW, Darios ES, Contreras GA, Garver H, Fink GD. Male and female high-fat diet-fed Dahl SS rats are largely protected from vascular dysfunctions: PVAT contributions reveal sex differences. Am J Physiol Heart Circ Physiol 321: H15–H28, 2021. doi: 10.1152/ajpheart.00131.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Zheng X, Deacon C, King AJ, Machin DR. Microcirculatory and glycocalyx properties are lowered by high-salt diet but augmented by Western diet in genetically heterogeneous mice. Am J Physiol Heart Circ Physiol 322: H328–H335, 2022. doi: 10.1152/ajpheart.00656.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Bell RM, Mocanu MM, Yellon DM. Retrograde heart perfusion: the Langendorff technique of isolated heart perfusion. J Mol Cell Cardiol 50: 940–950, 2011. doi: 10.1016/j.yjmcc.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 271.Houser SR, Margulies KB, Murphy AM, Spinale FG, Francis GS, Prabhu SD, Rockman HA, Kass DA, Molkentin JD, Sussman MA, Koch WJ, Koch W; American Heart Association Council on Basic Cardiovascular Sciences, Council on Clinical Cardiology, and Council on Functional Genomics and Translational Biology. Animal models of heart failure: a scientific statement from the American Heart Association. Circ Res 111: 131–150, 2012. [Erratum in Circ Res 111: e54, 2012]. doi: 10.1161/RES.0b013e3182582523. [DOI] [PubMed] [Google Scholar]
- 272.Lindsey ML, Brunt KR, Kirk JA, Kleinbongard P, Calvert JW, de Castro Bras LE, DeLeon-Pennell KY, Del Re DP, Frangogiannis NG, Frantz S, Gumina RJ, Halade G, Jones SP, Ritchie RH, Spinale FG, Thorp EB, Ripplinger CM, Kassiri Z. Guidelines for in vivo mouse models of myocardial infarction. Am J Physiol Heart Circ Physiol 321: H1056–H1073, 2021. doi: 10.1152/ajpheart.00459.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Neely JR, Rovetto MJ, Whitmer JT, Morgan HE. Effects of ischemia on function and metabolism of the isolated working rat heart. Am J Physiol 225: 651–658, 1973. doi: 10.1152/ajplegacy.1973.225.3.651. [DOI] [PubMed] [Google Scholar]
- 274.Kuroki MT, Fink GD, Osborn JW. Comparison of arterial pressure and plasma ANG II responses to three methods of subcutaneous ANG II administration. Am J Physiol Heart Circ Physiol 307: H670–H679, 2014. doi: 10.1152/ajpheart.00922.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Lindsey ML, Bolli R, Canty JM Jr, Du XJ, Frangogiannis NG, Frantz S, Gourdie RG, Holmes JW, Jones SP, Kloner RA, Lefer DJ, Liao R, Murphy E, Ping P, Przyklenk K, Recchia FA, Schwartz Longacre L, Ripplinger CM, Van Eyk JE, Heusch G. Guidelines for experimental models of myocardial ischemia and infarction. Am J Physiol Heart Circ Physiol 314: H812–H838, 2018. doi: 10.1152/ajpheart.00335.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Tarnavski O, McMullen JR, Schinke M, Nie Q, Kong S, Izumo S. Mouse cardiac surgery: comprehensive techniques for the generation of mouse models of human diseases and their application for genomic studies. Physiol Genomics 16: 349–360, 2004. doi: 10.1152/physiolgenomics.00041.2003. [DOI] [PubMed] [Google Scholar]
- 277.Harmancey R, Lam TN, Lubrano GM, Guthrie PH, Vela D, Taegtmeyer H. Insulin resistance improves metabolic and contractile efficiency in stressed rat heart. FASEB J 26: 3118–3126, 2012. doi: 10.1096/fj.12-208991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Povlsen JA, Lofgren B, Dalgas C, Birkler RI, Johannsen M, Stottrup NB, Botker HE. Protection against myocardial ischemia-reperfusion injury at onset of type 2 diabetes in Zucker diabetic fatty rats is associated with altered glucose oxidation. PLoS One 8: e64093, 2013. doi: 10.1371/journal.pone.0064093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Cortassa S, Caceres V, Tocchetti CG, Bernier M, de Cabo R, Paolocci N, Sollott SJ, Aon MA. Metabolic remodelling of glucose, fatty acid and redox pathways in the heart of type 2 diabetic mice. J Physiol 598: 1393–1415, 2020. doi: 10.1113/JP276824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Ge ZD, Li Y, Qiao S, Bai X, Warltier DC, Kersten JR, Bosnjak ZJ, Liang M. Failure of isoflurane cardiac preconditioning in obese type 2 diabetic mice involves aberrant regulation of microRNA-21, endothelial nitric-oxide synthase, and mitochondrial complex I. Anesthesiology 128: 117–129, 2018. doi: 10.1097/ALN.0000000000001926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Tsutsumi YM, Patel HH, Lai NC, Takahashi T, Head BP, Roth DM. Isoflurane produces sustained cardiac protection after ischemia-reperfusion injury in mice. Anesthesiology 104: 495–502, 2006. doi: 10.1097/00000542-200603000-00017. [DOI] [PubMed] [Google Scholar]
- 282.Brainard RE, Watson LJ, Demartino AM, Brittian KR, Readnower RD, Boakye AA, Zhang D, Hoetker JD, Bhatnagar A, Baba SP, Jones SP. High fat feeding in mice is insufficient to induce cardiac dysfunction and does not exacerbate heart failure. PLoS One 8: e83174, 2013. [Erratum in PLoS One 9: e113944, 2014]. doi: 10.1371/journal.pone.0083174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Miki T, Itoh T, Sunaga D, Miura T. Effects of diabetes on myocardial infarct size and cardioprotection by preconditioning and postconditioning. Cardiovasc Diabetol 11: 67, 2012. doi: 10.1186/1475-2840-11-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Heaberlin JR, Ma Y, Zhang J, Ahuja SS, Lindsey ML, Halade GV. Obese and diabetic KKAy mice show increased mortality but improved cardiac function following myocardial infarction. Cardiovasc Pathol 22: 481–487, 2013. doi: 10.1016/j.carpath.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Greer JJ, Ware DP, Lefer DJ. Myocardial infarction and heart failure in the db/db diabetic mouse. Am J Physiol Heart Circ Physiol 290: H146–H153, 2006. doi: 10.1152/ajpheart.00583.2005. [DOI] [PubMed] [Google Scholar]
- 286.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev 90: 207–258, 2010. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 287.Mulvihill EE, Varin EM, Ussher JR, Campbell JE, Bang KW, Abdullah T, Baggio LL, Drucker DJ. Inhibition of dipeptidyl peptidase-4 impairs ventricular function and promotes cardiac fibrosis in high fat-fed diabetic mice. Diabetes 65: 742–754, 2016. doi: 10.2337/db15-1224. [DOI] [PubMed] [Google Scholar]
- 288.Rawshani A, Rawshani A, Franzen S, Eliasson B, Svensson AM, Miftaraj M, McGuire DK, Sattar N, Rosengren A, Gudbjornsdottir S. Range of risk factor levels: control, mortality, and cardiovascular outcomes in type 1 diabetes mellitus. Circulation 135: 1522–1531, 2017. doi: 10.1161/CIRCULATIONAHA.116.025961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Shimizu I, Minamino T, Toko H, Okada S, Ikeda H, Yasuda N, Tateno K, Moriya J, Yokoyama M, Nojima A, Koh GY, Akazawa H, Shiojima I, Kahn CR, Abel ED, Komuro I. Excessive cardiac insulin signaling exacerbates systolic dysfunction induced by pressure overload in rodents. J Clin Invest 120: 1506–1514, 2010. doi: 10.1172/JCI40096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Palliyaguru DL, Shiroma EJ, Nam JK, Duregon E, Vieira Ligo Teixeira C, Price NL, Bernier M, Camandola S, Vaughan KL, Colman RJ, Deighan A, Korstanje R, Peters LL, Dickinson SL, Ejima K, Simonsick EM, Launer LJ, Chia CW, Egan J, Allison DB, Churchill GA, Anderson RM, Ferrucci L, Mattison JA, de Cabo R. Fasting blood glucose as a predictor of mortality: Lost in translation. Cell Metab 33: 2189–2200.e3, 2021. doi: 10.1016/j.cmet.2021.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Shang J, Previs SF, Conarello S, Chng K, Zhu Y, Souza SC, Staup M, Chen Y, Xie D, Zycband E, Schlessinger K, Johnson VP, Arreaza G, Liu F, Levitan D, Wang L, van Heek M, Erion M, Wang Y, Kelley DE. Phenotyping of adipose, liver, and skeletal muscle insulin resistance and response to pioglitazone in spontaneously obese rhesus monkeys. Am J Physiol Endocrinol Physiol 312: E235–E243, 2017. doi: 10.1152/ajpendo.00398.2016. [DOI] [PubMed] [Google Scholar]
- 292.Zijlmans DGM, Maaskant A, Sterck EHM, Langermans JAM. Retrospective evaluation of a minor dietary change in non-diabetic group-housed long-tailed macaques (Macaca fascicularis). Animals (Basel) 11: 2749, 2021. doi: 10.3390/ani11092749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Goedeke L, Peng L, Montalvo-Romeral V, Butrico GM, Dufour S, Zhang XM, Perry RJ, Cline GW, Kievit P, Chng K, Petersen KF, Shulman GI. Controlled-release mitochondrial protonophore (CRMP) reverses dyslipidemia and hepatic steatosis in dysmetabolic nonhuman primates. Sci Transl Med 11: eaay0284, 2019. doi: 10.1126/scitranslmed.aay0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Ludwig B, Wolf E, Schonmann U, Ludwig S. Large animal models of diabetes. In: Animal Models of Diabetes: Methods and Protocols, edited by King AJ. New York: Springer Nature, 2020, p. 115–134. [DOI] [PubMed] [Google Scholar]
- 295.Norhammar A, Schenck-Gustafsson K. Type 2 diabetes and cardiovascular disease in women. Diabetologia 56: 1–9, 2013. doi: 10.1007/s00125-012-2694-y. [DOI] [PubMed] [Google Scholar]
- 296.Giles DA, Moreno-Fernandez ME, Stankiewicz TE, Graspeuntner S, Cappelletti M, Wu D, Mukherjee R, Chan CC, Lawson MJ, Klarquist J, Sunderhauf A, Softic S, Kahn CR, Stemmer K, Iwakura Y, Aronow BJ, Karns R, Steinbrecher KA, Karp CL, Sheridan R, Shanmukhappa SK, Reynaud D, Haslam DB, Sina C, Rupp J, Hogan SP, Divanovic S. Thermoneutral housing exacerbates nonalcoholic fatty liver disease in mice and allows for sex-independent disease modeling. Nat Med 23: 829–838, 2017. doi: 10.1038/nm.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]