Abstract
Increased plasma mitochondrial DNA concentrations are associated with poor outcomes in multiple critical illnesses, including COVID-19. However, current methods of cell-free mitochondrial DNA quantification in plasma are time-consuming and lack reproducibility. Here, we used next-generation sequencing to characterize the size and genome location of circulating mitochondrial DNA in critically ill subjects with COVID-19 to develop a facile and optimal method of quantification by droplet digital PCR. Sequencing revealed a large percentage of small mitochondrial DNA fragments in plasma with wide variability in coverage by genome location. We identified probes for the mitochondrial DNA genes, cytochrome B and NADH dehydrogenase 1, in regions of relatively high coverage that target small sequences potentially missed by other methods. Serial assessments of absolute mitochondrial DNA concentrations were then determined in plasma from 20 critically ill subjects with COVID-19 without a DNA isolation step. Mitochondrial DNA concentrations on the day of enrollment were increased significantly in patients with moderate or severe acute respiratory distress syndrome (ARDS) compared with those with no or mild ARDS. Comparisons of mitochondrial DNA concentrations over time between patients with no/mild ARDS who survived, patients with moderate/severe ARDS who survived, and nonsurvivors showed the highest concentrations in patients with more severe disease. Absolute mitochondrial DNA quantification by droplet digital PCR is time-efficient and reproducible; thus, we provide a valuable tool and rationale for future studies evaluating mitochondrial DNA as a real-time biomarker to guide clinical decision-making in critically ill subjects with COVID-19.
Keywords: ARDS, COVID-19, mitochondrial DNA
INTRODUCTION
Acute respiratory distress syndrome (ARDS) is the main cause of death due to COVID-19 (1). Early application of advanced therapies, such as prone positioning (2), has decreased mortality in severe ARDS, however, identifying which patients are developing severe disease is challenging in real time. Novel biomarkers of disease severity have the potential to allow closer surveillance for clinical deterioration and earlier implementation of advanced treatments, as well as suggest relevant mechanisms that may be modifiable.
Circulating, cell-free mitochondrial DNA (mtDNA) has emerged as one such biomarker that may be important clinically and mechanistically. MtDNA is a double-stranded, circular molecule that encodes 13 mRNAs that are essential subunits of the OXPHOS complexes and two rRNAs and 22 tRNAs that are needed to translate these on mitochondrial ribosomes. Interestingly, mtDNA is also a key mediator of the antiviral response (3) that potentiates systemic inflammation via innate immune pathways, such as Toll-like receptor 9 (TLR9) (4) and inflammasomes (5). Increased plasma mtDNA concentrations determined by quantitative PCR (qPCR) have been found to be associated with poor outcomes in multiple critical illnesses, including ARDS (4, 6–9). Recently, plasma mtDNA concentrations assessed at the time of hospital admission among persons with COVID-19 were found to predict the need for mechanical ventilation and risk of mortality (10). However, time-consuming procedures utilized in prior quantification methods, such as DNA isolation and the generation of a standard curve, limit the clinical utility of mtDNA measured in such assessments (11). Furthermore, qPCR methods are highly variable among prior studies, with limited reproducibility (8, 12).
Droplet digital PCR (ddPCR) provides absolute DNA quantification without the need for a standard curve by partitioning individual samples into thousands of droplets for individual reactions. This method is superior to qPCR at low levels of DNA concentrations, making it feasible to obtain results from plasma without a DNA isolation step (11, 13). Still, there are several issues that need to be addressed to optimize mtDNA quantification by ddPCR in the setting of critical illness. First, mtDNA is damaged and fragmented during cellular injury, thus smaller fragments may be outside the limit of several commonly used target sequences (14). Second, certain regions of mtDNA may be more prone to release from cells into circulation, which may explain discrepancies in results based on the mtDNA sequence being targeted (i.e., specific primers used) (8, 15). Third, numerous mtDNA insertions into the nuclear genome exist as mutated pseudogenes, also called nuclear mitochondrial DNA segments (NUMTs) (16). Therefore, target sequences that are homologous to NUMTs may lead to false-positive results.
The objective of this study was to evaluate mtDNA concentration determined by ddPCR as a marker of disease severity in critically ill subjects with COVID-19. To do this, we refined a novel method of next-generation sequencing (NGS) with an mtDNA enrichment step to determine the size and genome location of cell-free mtDNA fragments in plasma. Next, we used these data to identify optimal target sequences for mtDNA quantification by ddPCR from plasma without DNA isolation. Finally, we leveraged this method to determine the association of serial assessments of plasma mtDNA concentration with markers of disease severity in critically ill subjects with COVID-19.
METHODS
Prospective Enrollment and Sample Collection
Blood samples were collected prospectively from adult subjects (≥18 yr old) with COVID-19 who were admitted to the intensive care units (ICUs) of the University of California San Diego (UCSD) Health System and Rady Children’s Hospital from April 9, 2020, to August 7, 2020. All studies were approved by the local Institutional Review Board (IRB, No. 190699), and written informed consent was obtained before blood draws. Blood samples were collected on days 1, 3, 5, 7, 9, and 11 after enrollment. Blood was collected in EDTA-coated collection tubes and processed to generate platelet-poor plasma as described previously (10) before being stored at −80°C. The presence or absence of ARDS and ARDS severity were determined at the timing of each collection per the Berlin definition (5). Chest radiographs were obtained every morning within 4 h of blood draws and read by a board-certified radiologist to determine if bilateral opacifications were present that would meet the Berlin criteria for ARDS. / ratio defined by the partial pressure of oxygen (Po2 in arterial blood divided by the fraction of inspired oxygen delivered () was available daily in all patients who were treated with positive pressure ventilation to determine ARDS severity. Modified Murray lung injury score (17) and the Sequential Organ Failure Assessment (SOFA) score (18) were also recorded at each time point.
Library Preparation and High-Throughput Sequencing
Plasma samples were centrifuged again at 18,000 g for 15 min at 4°C to remove any cellular debris and then DNA was isolated from 200 μL of plasma using the DNA Blood Mini Kit (Qiagen, Germantown, MD) as per the manufacturer’s instructions. Purified DNA was used to generate sequencing libraries using the KAPA Hyper Prep Kit (KAPA Biosystems, Wilmington, MA) with 10–12 cycles of amplification as needed. To ensure small DNA fragments were not lost, Ampure cleanup protocols were modified and a 1:1.4 ratio was used as published previously (14). To enrich mtDNA for sequencing, resulting libraries were hybridized using mtDNA-specific, biotinylated, probes (myBaits, Arbor Biosciences) according to the manufacturer’s instructions. Sequencing was then performed using the Illumina HiSeq 4000 Sequencing System (Illumina, San Diego, CA) with the generation of 100 bp paired-end (PE) reads to a depth of ∼5 million paired reads per sample. Quality control of the raw FastQ files was performed using the software tool, FastQC. Low-quality reads were trimmed by Trimmomatic v0.36 before alignment.
MtDNA Alignment, Size Distribution, and Coverage
PE sequences were trimmed with FastP (19) and aligned to the human genome version hg38 using STAR aligner (20). Reads aligning to mtDNA were retained for further analysis with reads with primary alignments to the nuclear genome filtered out to avoid alignment of NUMTs. Read length was determined as the distance (+1) of the first and last aligned bases of the PE read. Coverage was calculated as a histogram of length 16,569, in which each bin represents an mtDNA nucleotide, and the value represents the number of PE fragments that cover that nucleotide.
Absolute quantification of mtDNA in plasma by ddPCR.
We utilized target sequences from the NADH dehydrogenase 1 (ND1) and cytochrome B (CYB) regions of the mitochondrial genome. The following CYB primers and probes targeted an 85-bp region from 14,848 to 14,932 of the mitochondrial genome:
Forward: 5′-CTCACTCCTTGGCGCCTGCC-3′
Reverse: 5′-GGCGGTTGAGGCGTCTGGTG-3′
Probe: (FAM) 5′CCTCCAAATCACCACAGGACTATTCCTAGCCATGCA-3′- (BHQ-1). The following ND1 primers and probes targeted a 69-bp region from 3,484 to 3,553 of the mitochondrial genome:
Forward: 5′-CCCTAAAACCCGCCACATCT-3′
Reverse: 5′GAGCGATGGTGAGAGCTAAGGT-3′
Probe: 5′ (FAM) CCATCACCCTCTACATCACCGCCC (BHQ-1) 3′.
We prepared a 20 µL ddPCR reaction volume containing 10 µL of 2× ddPCR supermix for probes, 1 μL of 20× primer/probe mix for a final concentration of 900 nM forward/900 nM reverse/250 nM probe, 1 µL of plasma sample, and 8 µL of water. The 20 μL ddPCR reaction mix with 70 μL droplet generating oil was placed on a plastic cartridge and sealed before droplet generation in the droplet generator machine (Bio-Rad Laboratories, Hercules, CA). Droplets from the cartridge were then transferred onto a 96-well ddPCR plate. Plates were incubated at 95°C for 10 min and then cycled at 94°C for 30 s and at 60°C for 60 s for a total of 45 times using the Applied Biosystems Veriti 96-well Thermal Cycler (Applied Biosystems, Waltham, MA). The droplets from each sample were read on a QX200 droplet reader machine (Bio-Rad Laboratories, Hercules, CA) and analyzed individually using QuantSoft version 1.7.4 software. PCR-positive and PCR-negative droplets were counted to provide absolute quantification of target mtDNA sequences. The fraction of positive droplets in a sample was used to determine the concentration of the target sequence in copies/microliter, then multiplied by 20 to account for the dilution factor.
Determining Potential for NUMT Amplification
The minimum Hamming distance was defined as the minimum number of mismatches between ddPCR target sequences and the nuclear genome. To determine the Hamming distance for each target sequence, we determined the nuclear sequence which minimizes the number of mismatches with the mitochondrial fragment (or its reverse complement) by determining the best alignment to the nuclear genome using Blastn in the ungapped mode (21) and recording the number of mismatches.
Statistics
Analyses included descriptive statistics (mean, Inter Quartile Range) on entry and over time. Mean concentrations of plasma mtDNA were compared using analysis of variance for day 1 samples. Linear mixed models with random effects for participants were used to assess differences over time. Data were logarithmically transformed to reduce skewness and normalize data. Analyses were performed using Prism (GraphPad, La Jolla, CA), JMP (JMP Pro 16.0.0), and SAS (Version 9.4).
RESULTS
High-Throughput Sequencing and ddPCR Probe Comparison
Our average mtDNA sequencing yield was 3.5 ± 2.1% with an average of 221,548 ± 133,075 mtDNA reads/sample included in our downstream analyses. Sequencing data were uploaded to a shared server (BioProject) and may be accessed online at: https://www.ncbi.nlm.nih.gov/bioproject/810499. We averaged the total mtDNA reads by genome location that showed wide variability, with the highest sequencing yield from the CYB gene (Fig. 1A). Size distribution analysis showed that 18.1% of mtDNA fragments were less than 100 bp (Fig. 1B). Based on these results, we identified ddPCR probes within CYB and ND1 that would be ideal for plasma mtDNA quantification based on their small target sequences of 69 bp and 85 bp, respectively, and relatively high sequencing yield compared with other mtDNA regions. Next, we compared the copy number obtained by ddPCR for the CYB and ND1 probes, and the minimum Hamming distance of each target sequence to the nuclear genome. There was a larger percentage of total mtDNA reads from the CYB target sequence compared with ND1 (Fig. 1C), yet ND1 yielded a higher copy number by ddPCR in 16 out of the 18 samples sequenced (Fig. 1D). Minimum Hamming distance was used to evaluate the potential that these sequences would also amplify NUMTs. When compared with the nuclear genome, the ND1 and CYB target sequences had minimum Hamming distances of 16 and 5, respectively. A correlation analysis of copy number determined by ddPCR revealed a near perfect correlation between the ND1 versus CYB probes (Fig. 1E), indicating they would likely yield very similar results when compared with clinical outcomes.
Figure 1.
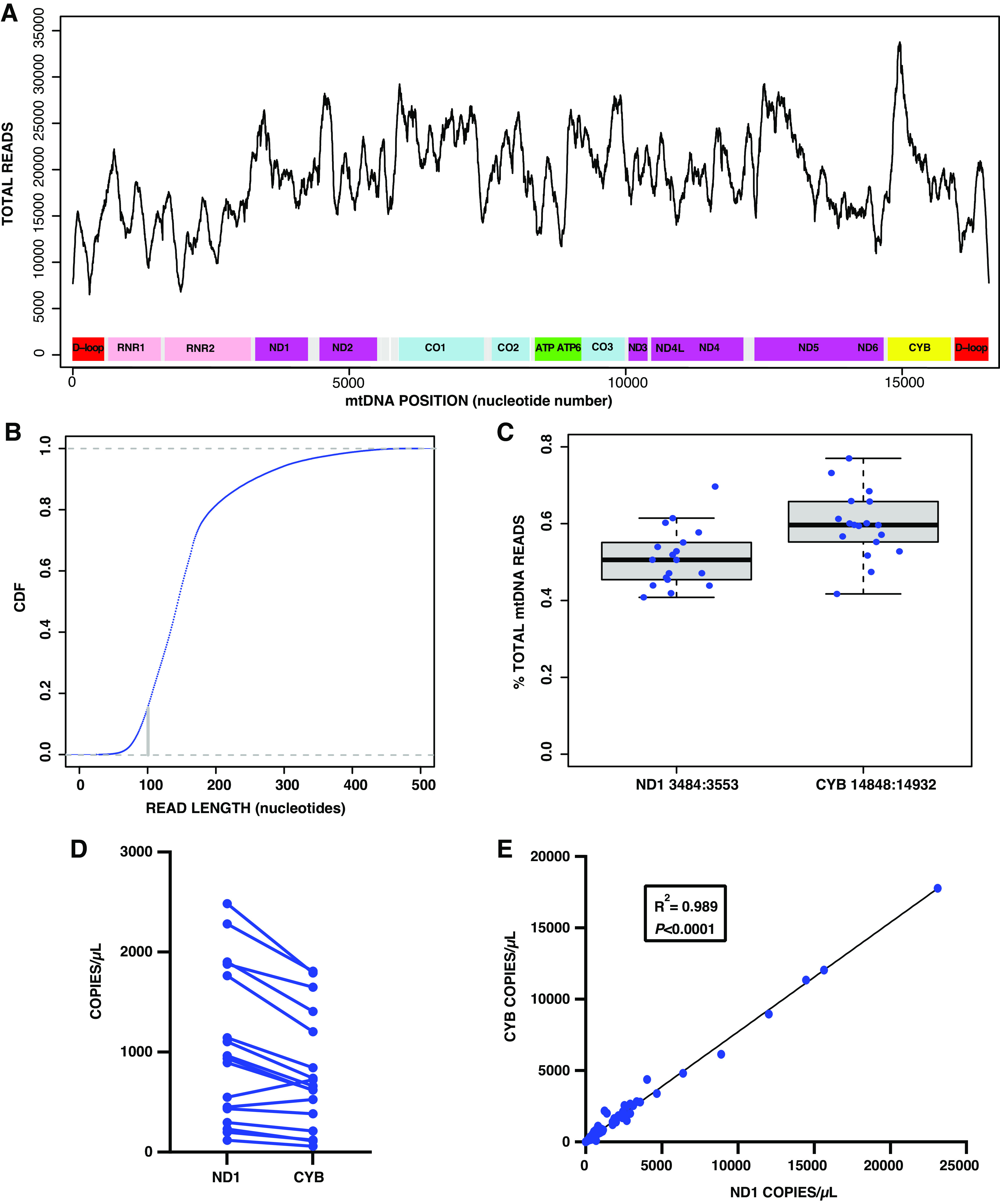
Sequencing and mtDNA target sequence comparisons. A: average reads by mitochondrial genome location. B: size distribution of cell-free mtDNA reads. C: CYB vs. ND1 target sequence reads. D: CYB vs. ND1 copy number in plasma by droplet digital PCR. E: linear regression of CYB vs. ND1 copy number. For A, B, and C: n = 18 subjects; E: n = 111 samples. CYB, cytochrome B; mtDNA, mitochondrial DNA; ND1, NADH dehydrogenase 1.
Plasma mtDNA and disease severity in COVID-19.
Serial measurements of CYB and ND1 were performed in plasma from 20 critically ill patients with COVID-19. Day 1 blood samples were collected within 24 h of ICU admission in 18 of the 20 patients enrolled. One patient was enrolled in an ICU stepdown unit, and another was enrolled as a transfer from an outside hospital on day 6 after initial ICU admission. We broadened our protocol later into enrollment to allow more frequent sampling, thus we have day 2 samples in five patients, and day of discharge samples in nine patients. Samples were also drawn within 48 h of death in two of the nonsurvivors. Demographic data are shown in Table 1. Five patients did not meet ARDS criteria as they were not treated with positive pressure ventilation, two patients developed mild ARDS, 10 developed moderate ARDS, and three had severe ARDS. Seventeen patients survived to discharge, and two patients died after receiving full critical care measures. One patient preferred to be treated with comfort measures only on day 2 and died on day 5. Day 1 concentrations of ND1 and CYB are shown in Fig. 2. CYB (Fig. 2A) and ND1 (Fig. 2B) concentrations were significantly higher in patients who had moderate/severe ARDS on study day 1 compared with those who had no/mild ARDS. One patient from the moderate/severe group was excluded from this comparison due to a missing day 1 sample, but the day 2 sample values were 3.3 copies/µL for both Log (10) CYB and Log (10) ND1. A simple linear regression of day 1CYB and ND1 concentrations versus modified Murray lung injury and SOFA scores showed a positive and significant correlation for both analyses (Fig. 2, C–F).
Table 1.
Patient demographics
| Parameter | Total |
|---|---|
| Patients included | 20 |
| Survival to hospital discharge | 17 |
| Median age, IQR | 62 (47–71) |
| Male sex | 13 |
| White | 17 |
| Asian | 2 |
| African American | 1 |
| Hispanic | 13 |
| Median body mass index, IQR | 32.13 (27.71–35.39) |
| Treated with mechanical ventilation | 15 |
| No ARDS | 5 |
| Mild ARDS | 2 |
| Moderate ARDS | 10 |
| Severe ARDS | 3 |
| Hypertension | 12 |
| Heart failure | 2 |
| Chronic obstructive pulmonary disease | 4 |
| History of ischemic stroke | 1 |
| Connective tissue disease | 1 |
| Diabetes with organ damage | 4 |
| Former smoker | 5 |
| Aspirin | 3 |
| Therapeutic anticoagulation | 2 |
| Ace inhibitor or angiotensin receptor blocker | 9 |
| Metformin | 4 |
| Beta blocker | 4 |
| Statin | 4 |
| Immunosuppression | 1 |
ARDS, acute respiratory distress syndrome; IQR, interquartile range.
Figure 2.
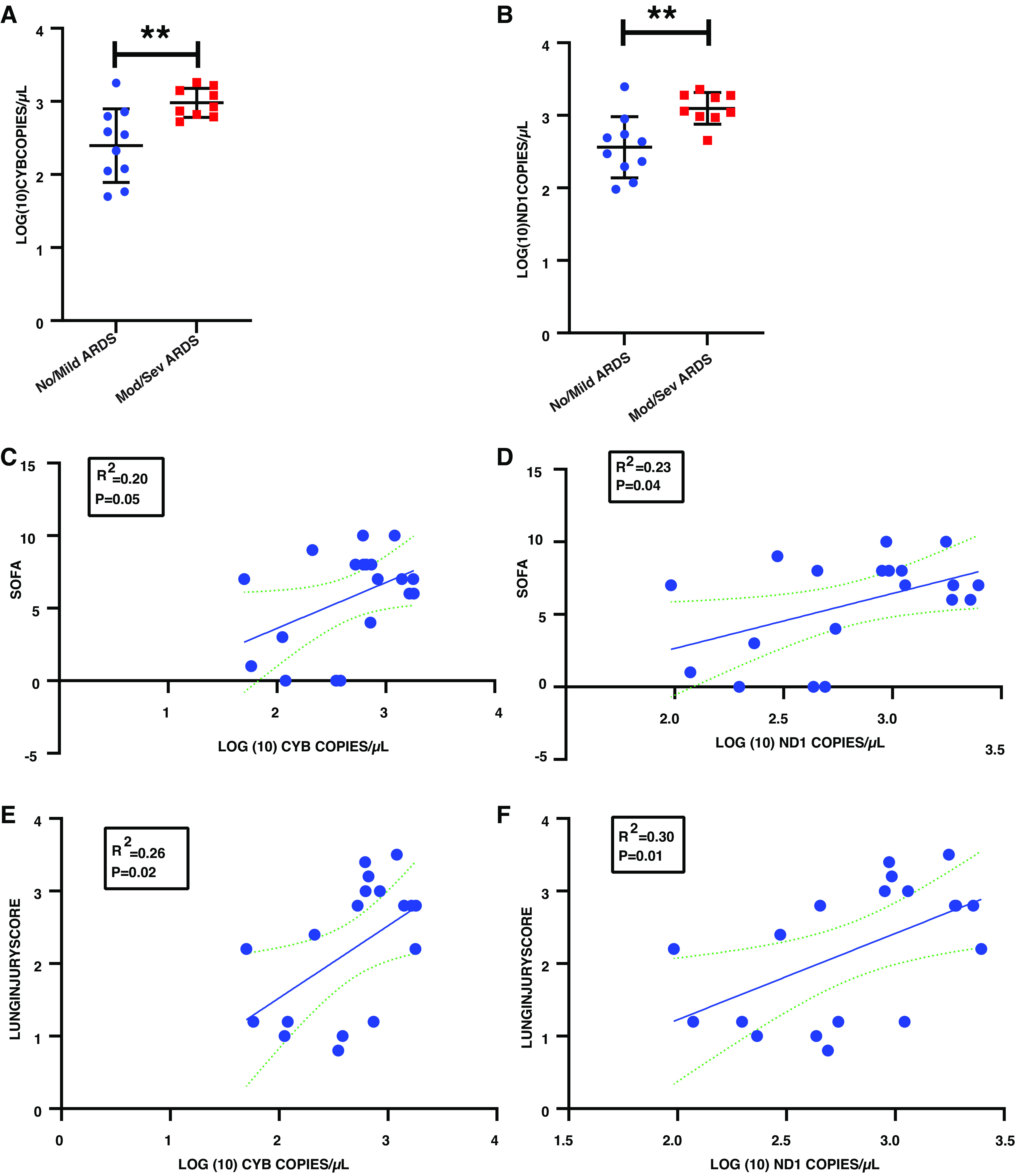
Plasma mtDNA and disease severity. A: CYB copy number in no/mild vs. moderate/severe (mod/sev) ARDS on study day 1. B: ND1 copy number in no/mild vs. mod/sev ARDS on study day 1. C: CYB copy number on study day 1 and sequential organ failure assessment (SOFA) score. D: ND1 copy number on study day 1 and SOFA score. E: CYB copy number on study day 1 and modified Murray lung injury score. F: ND1 copy number on study day 1 and modified Murray lung injury score. **P < 0.01, n = 19 subjects. ARDS, acute respiratory distress syndrome; CYB, cytochrome B; mtDNA, mitochondrial DNA; ND1, NADH dehydrogenase 1.
Serial mtDNA Assessments and ARDS Outcomes
Figure 3 shows the plots of ND1 and CYB concentrations by study day and ARDS and survival outcomes, including patients with no/mild ARDS who survived, those with moderate/severe ARDS who survived, and nonsurvivors. Those with no/mild ARDS had an average of 3.67 assessments compared with 6.36 and 6.00, respectively, for moderate/severe ARDS survivors and nonsurvivors. Unadjusted pairwise comparisons show significantly lower mtDNA concentrations over time in no/mild ARDS compared with the moderate/severe ARDS group (P = 0.0213 for CYB and 0.0068 for ND1) and nonsurvivors (P = 0.0001 for CYB and ND1). The moderate/severe ARDS group also had significantly lower mtDNA concentrations compared with nonsurvivors (P = 0.0012 for CYB and 0.0108 for ND1), and only the nonsurvivor group had increasing levels over time. We note that the patient in the nonsurvivor group who had the lowest CYB and ND1 concentrations elected not to be treated with life support measures, including mechanical ventilation.
Figure 3.
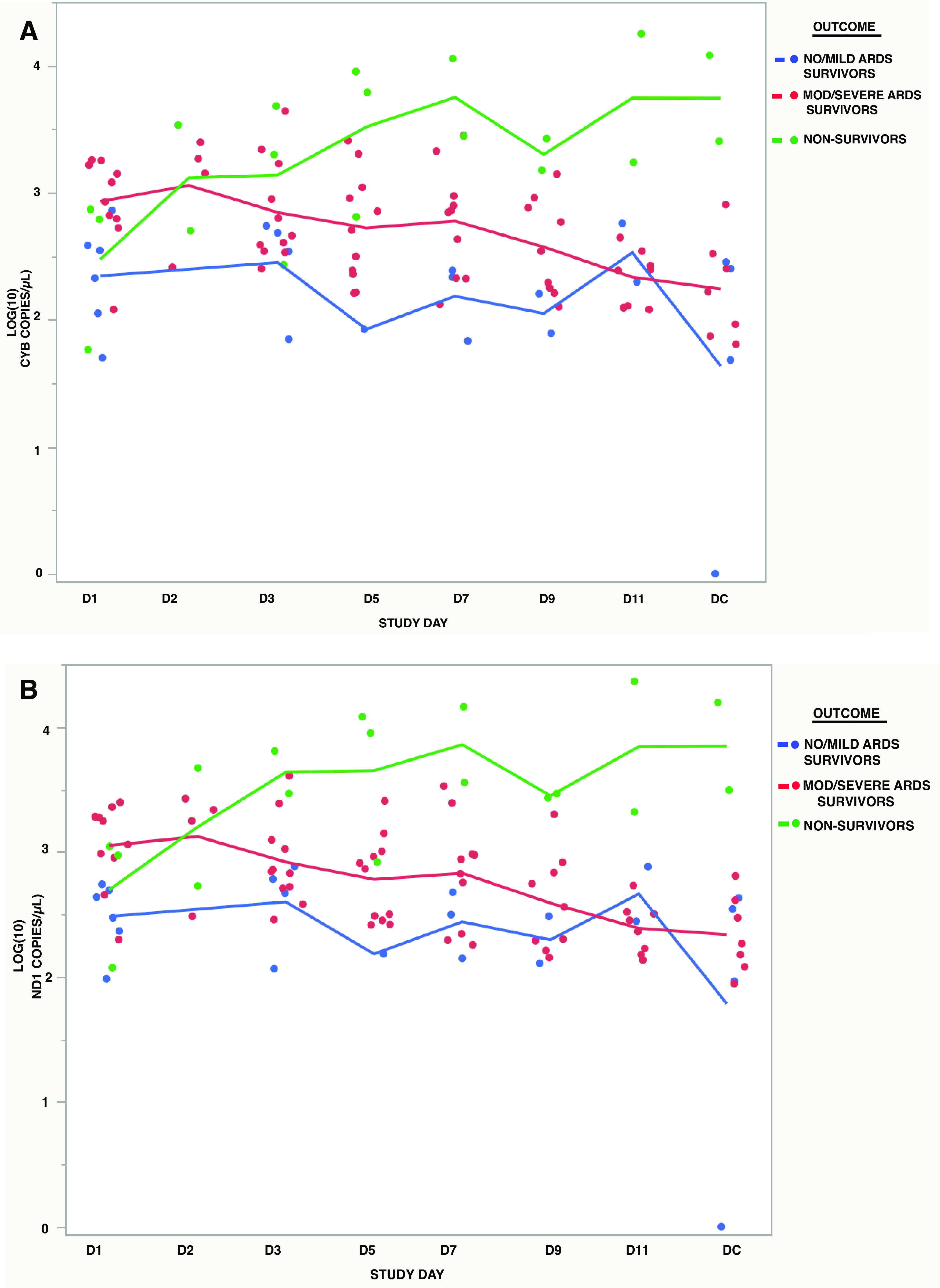
Serial assessments of mtDNA and ARDS outcomes. A: serial CYB concentrations in patients with no/mild ARDS who survived vs. those with mod/severe ARDS who survived vs. nonsurvivors. B: serial ND1 concentrations in patients with no/mild ARDS who survived vs. those with mod/severe ARDS who survived vs. nonsurvivors. n = 6 subjects for no/mild ARDS survivors, n = 11 subjects for mod/severe ARDS survivors, and n = 3 subjects for nonsurvivors. ARDS, acute respiratory distress syndrome; CYB, cytochrome B; mtDNA, mitochondrial DNA; ND1, NADH dehydrogenase 1.
DISCUSSION
In this study of critically ill subjects with COVID-19, we used NGS to determine the optimal size and genome locations for ddPCR target sequences to determine mtDNA concentrations in plasma. Importantly, ddPCR provides absolute quantification without a standard curve, and assessments were performed without DNA isolation, making results more reproducible and rapidly available. Plasma mtDNA concentrations determined by this methodology were directly related to ARDS severity.
Our findings add to recent work by Scozzi et al. (10), who found that plasma mtDNA concentrations determined by qPCR at hospital admission for COVID-19 are an indicator of severe illness and mortality. Interestingly, Andargie et al. (22) quantified mtDNA by ddPCR in DNA isolated from plasma of subjects with COVID-19, and found that mtDNA concentrations determined early in the hospital course did not predict mortality. The major strength of our study is that we quantified mtDNA by ddPCR without a DNA isolation step, and we performed serial assessments which showed that plasma mtDNA concentrations are associated with COVID-19 severity over time. Obviating the need for DNA isolation eliminates a potential source of error, as DNA isolation methods are highly variable and small DNA fragments may be lost (14). Thus, the methods we describe may improve the reproducibility of mtDNA quantification in future studies, in addition to making serial assessments more facile. These methods could also be translated to investigations of the numerous other diseases where mtDNA has been implicated, such as interstitial pulmonary fibrosis (23), sarcoidosis (24), and systemic lupus erythematosus (25).
Our sequencing results showed that target sequences in low coverage regions or those targeting larger fragment sizes may partially explain the wide variability in published concentrations for plasma mtDNA among critically ill cohorts (8, 12). Previous studies have also suggested that probes targeting multiple regions of the genome should be used to more accurately determine mtDNA concentrations (8). However, probes from the ND1 and CYB regions in our study showed a near perfect correlation in copy number obtained, indicating that only one probe may be necessary when evaluating associations with clinical outcomes. Our data also suggest that small target sequences are important as nearly 20% of mtDNA fragments sequenced were <100 bp. We show an ND1 target sequence that is ideal given it has a small target sequence of 69 bp, which may explain the consistently higher copy number obtained compared with CYB at 85 bp. The ND1 target sequence also had a minimum Hamming distance of 16 compared with the nuclear genome, meaning 23% of nucleotides were mismatched in the most similar region, which makes amplification of NUMTs highly unlikely.
Our study does have limitations that are worth addressing. First, we show results for a small cohort of 20 critically ill patients with COVID-19, and larger studies of more diverse populations, including non-COVID-19 cohorts, are warranted. Evaluations for differences in results based on sex or ethnicity may be particularly important given the unique, maternal inheritance of mtDNA, and racial disparities in COVID-19 and ARDS outcomes (26, 27). Second, ddPCR is a relatively new technology that is not likely to be widely available in clinical laboratories. This limits the immediate translational ability. We are also unable to perform a direct comparison between copy numbers obtained with our target sequences and those utilized in prior studies due to differences in methodologies. Third, cell-free mtDNA is known to exist both within and outside of extracellular vesicles (EVs), which may have important biological implications (28). While we provide new insights into the size and genome location of cell-free mtDNA, we are not able to comment on possible differences between the EV and non-EV fractions. Fourth, patients with more severe diseases tended to have more assessments over time compared with those with mild disease, which is a source of bias in the mixed regression model we used to evaluate serial assessments. Still, our study validates ddPCR in plasma without DNA isolation as a method of cell-free mtDNA quantification in the critically ill subjects with COVID-19 and highlights the potential importance of serial measurements.
In conclusion, we present facile and reproducible methods to quantify cell-free mtDNA in plasma by ddPCR. Serial mtDNA assessments correlated with ARDS severity, as mtDNA concentrations were significantly lower over time in patients with milder disease compared with those with more severe disease. These methods and results provide practical relevance for future studies designed to determine whether mtDNA provides a useful biomarker in managing critically ill patients with COVID-19.
DATA AVAILABILITY
The sequencing data that support the findings of this study are openly available in The National Center for Biotechnology Information shared server (BioProject) at https://www.ncbi.nlm.nih.gov/bioproject/810499.
GRANTS
This work was supported by the US Department of Veterans Affairs IK2BX004338-01 (to M.L.H.) and National Institutes of Health (NIH) Grants R01AR069876 (to G.S.S.), F31AG062099 (to A.G.S.), T32CA009370-39 (to A.G.M.), T32HL134632-04 (to M.T.L.) R01HL113614 (to M.N.G.), R01GM127823 (to M.N.G.), K08NS109200 (to N.G.C.), and K24DK110427 (to J.H.I.). Additional support from the UCSD IGM Genomics Center, the Altman Clinical & Translational Research Institute (ACTRI) at UCSD (NIH) UL1TR001442, the Genomics and Sequencing Core of the San Diego Center for AIDS Research (NIH) P30AI036214, the Doris Duke Foundation (to N.G.C.), the California Institute for Regenerative Medicine EDUC2-08388 (to E.H.), the Audrey Geisel Chair in Biomedical Sciences and a grant from the NOMIS Foundation (to G.S.S.), the UAB-UCSD O’Brien Center for Acute Kidney Injury Research (NIH) P30DK079337, and American Thoracic Society Research Foundation Unrestricted Critical Care Grant (to M.L.H.) and Academic Sleep Pulmonary Integrated Research/Clinical Fellowship (to M.T.L).
DISCLOSURES
G. Cutter has the following conflicts of interest to disclose: Data and Safety Monitoring Boards: AI Therapeutics, AMO Pharma, Astra-Zeneca, Avexis Pharmaceuticals, Biolinerx, Brainstorm Cell Therapeutics, Bristol Meyers Squibb/Celgene, CSL Behring, Galmed Pharmaceuticals, Green Valley Pharma, Horizon Pharmaceuticals, Immunic, Mapi Pharmaceuticals LTD, Merck, Mitsubishi Tanabe Pharma Holdings, Opko Biologics, Prothena Biosciences, Novartis, Regeneron, Sanofi-Aventis, Reata Pharmaceuticals, NHLBI (Protocol Review Committee), University of Texas Southwestern, University of Pennsylvania, Visioneering Technologies, Inc. Consulting or Advisory Boards: Alexion, Antisense Therapeutics, Biogen, Clinical Trial Solutions LLC, Genzyme, Genentech, GW Pharmaceuticals, Immunic, Klein-Buendel Incorporated, Merck/Serono, Osmotica Pharmaceuticals, Perception Neurosciences, Protalix Biotherapeutics, Recursion/Cerexis Pharmaceuticals, Regeneron, Roche, SAB Biotherapeutics. G. Cutter is employed by the University of Alabama at Birmingham and is President of Pythagoras, Inc., a private consulting company located in Birmingham, Alabama. J. H. Ix leads an Investigator Initiated Research Grant supported by Baxter International, has served on Advisory Boards for Akebia, AstraZeneca, and Bayer, and serves as a member of a Data and Safety Monitoring Board for Sanifit International. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
M.L.H., G.S.S., K.J., M.N.G., R.G.S., R.S., and J.H.I. conceived and designed research; M.L.H., M.O., M.T.L., N.G.C., M.L.R., A.G.M., A.G.S., P.G.T., S.P., A.J.L., E.H., S.T., C.N., K.J., and R.S. performed experiments; M.L.H., M.O., M.T.L., G.S.S., A.G.M., A.G.S., S.P., K.J., G.C., R.G.S., R.S., and J.H.I. analyzed data; M.L.H., M.T.L., N.G.C., G.S.S., N.S., M.N.G., R.G.S., and J.H.I. interpreted results of experiments; M.L.H., A.J.L., N.S., G.C., and R.S. prepared figures; M.L.H. drafted manuscript; M.L.H., M.O., M.T.L., N.G.C., M.L.R., G.S.S., A.G.M., A.G.S., P.G.T., S.P., A.J.L., N.S., K.J., G.C., R.G.S., R.S., and J.H.I. edited and revised manuscript; M.L.H., M.O., M.T.L., N.G.C., M.L.R., G.S.S., A.G.M., A.G.S., P.G.T., S.P., A.J.L., N.S., E.H., S.T., C.N., K.J., G.C., M.N.G., R.G.S., R.S., and J.H.I. approved final version of manuscript.
REFERENCES
- 1.Tzotzos SJ, Fischer B, Fischer H, Zeitlinger M. Incidence of ARDS and outcomes in hospitalized patients with COVID-19: a global literature survey. Crit Care 24: 516, 2020. doi: 10.1186/s13054-020-03240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guérin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, Mercier E, Badet M, Mercat A, Baudin O, Clavel M, Chatellier D, Jaber S, Rosselli S, Mancebo J, Sirodot M, Hilbert G, Bengler C, Richecoeur J, Gainnier M, Bayle F, Bourdin G, Leray V, Girard R, Baboi L, Ayzac L. PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 368: 2159–2168, 2013. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 3.West AP, Khoury-Hanold W, Staron M, Tal MC, Pineda CM, Lang SM, Bestwick M, Duguay BA, Raimundo N, MacDuff DA, Kaech SM, Smiley JR, Means RE, Iwasaki A, Shadel GS. Mitochondrial DNA stress primes the antiviral innate immune response. Nature 520: 553–557, 2015. doi: 10.1038/nature14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krychtiuk KA, Ruhittel S, Hohensinner PJ, Koller L, Kaun C, Lenz M, Bauer B, Wutzlhofer L, Draxler DF, Maurer G, Huber K, Wojta J, Heinz G, Niessner A, Speidl WS. Mitochondrial DNA and toll-like receptor-9 are associated with mortality in critically ill patients. Crit Care Med 43: 2633–2641, 2015. doi: 10.1097/CCM.0000000000001311. [DOI] [PubMed] [Google Scholar]
- 5.Wu G, Zhu Q, Zeng J, Gu X, Miao Y, Xu W, Lv T, Song Y. Extracellular mitochondrial DNA promote NLRP3 inflammasome activation and induce acute lung injury through TLR9 and NF-κB. J Thorac Dis 11: 4816–4828, 2019. doi: 10.21037/jtd.2019.10.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakahira K, Kyung SY, Rogers AJ, Gazourian L, Youn S, Massaro AF, Quintana C, Osorio JC, Wang Z, Zhao Y, Lawler LA, Christie JD, Meyer NJ, Mc Causland FR, Waikar SS, Waxman AB, Chung RT, Bueno R, Rosas IO, Fredenburgh LE, Baron RM, Christiani DC, Hunninghake GM, Choi AM. Circulating mitochondrial DNA in patients in the ICU as a marker of mortality: derivation and validation. PLoS Med 10: e1001577, 2013. doi: 10.1371/journal.pmed.1001577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faust HE, Reilly JP, Anderson BJ, Ittner CAG, Forker CM, Zhang P, Weaver BA, Holena DN, Lanken PN, Christie JD, Meyer NJ, Mangalmurti NS, Shashaty MGS. Plasma mitochondrial DNA levels are associated with ARDS in trauma and sepsis patients. Chest 157: 67–76, 2020. doi: 10.1016/j.chest.2019.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lubkin DT, Bishawi M, Barbas AS, Brennan TV, Kirk AD. Extracellular mitochondrial DNA and N-formyl peptides in trauma and critical illness: a systematic review. Crit Care Med 46: 2018–2028, 2018. doi: 10.1097/CCM.0000000000003381. [DOI] [PubMed] [Google Scholar]
- 9.Simmons JD, Freno DR, Muscat CA, Obiako B, Lee Y-L. L, Pastukh VM, Brevard SB, Gillespie MN. Mitochondrial DNA damage associated molecular patterns in ventilator-associated pneumonia: prevention and reversal by intratracheal DNase I. J Trauma Acute Care Surg 82: 120–125, 2017. doi: 10.1097/TA.0000000000001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scozzi D, Cano M, Ma L, Zhou D, Zhu JH, O'Halloran JA, Goss CW, Rauseo AM, Liu Z, Sahu SK, Peritore V, Rocco M, Ricci A, Amodeo R, Aimati L, Ibrahim M, Hachem RR, Kreisel D, Mudd PA, Kulkarni HS, Gelman AE. Circulating mitochondrial DNA is an early indicator of severe illness and mortality from COVID-19. JCI Insight 6, 2021. doi: 10.1172/jci.insight.143299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor SC, Laperriere G, Germain H. Droplet Digital PCR versus qPCR for gene expression analysis with low abundant targets: from variable nonsense to publication quality data. Sci Rep 7: 2409, 2017. doi: 10.1038/s41598-017-02217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrington JS, Huh JW, Schenck EJ, Nakahira K, Siempos II, Choi AMK. Circulating mitochondrial DNA as predictor of mortality in critically ill patients: a systematic review of clinical studies. Chest 156: 1120–1136, 2019. doi: 10.1016/j.chest.2019.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye W, Tang X, Liu C, Wen C, Li W, Lyu J. Accurate quantitation of circulating cell-free mitochondrial DNA in plasma by droplet digital PCR. Anal Bioanal Chem 409: 2727–2735, 2017. doi: 10.1007/s00216-017-0217-x. [DOI] [PubMed] [Google Scholar]
- 14.Zhang R, Nakahira K, Guo X, Choi AM, Gu Z. Very short mitochondrial DNA fragments and heteroplasmy in human plasma. Sci Rep 6: 36097, 2016. doi: 10.1038/srep36097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.García N, Chávez E. Mitochondrial DNA fragments released through the permeability transition pore correspond to specific gene size. Life Sci 81: 1160–1166, 2007. doi: 10.1038/srep36097. [DOI] [PubMed] [Google Scholar]
- 16.Malik AN, Shahni R, Rodriguez-de-Ledesma A, Laftah A, Cunningham P. Mitochondrial DNA as a non-invasive biomarker: accurate quantification using real time quantitative PCR without co-amplification of pseudogenes and dilution bias. Biochem Biophys Res Commun 412: 1–7, 2011. doi: 10.1016/j.bbrc.2011.06.067. [DOI] [PubMed] [Google Scholar]
- 17.Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis 138: 720–723, 1988[Erratum inAm Rev Respir Dis139: 1065, 1989]. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- 18.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22: 707–710, 1996. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 19.Chen S, Zhou Y, Chen Y, Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34: i884–i890, 2018. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29: 15–21, 2013. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol 215: 403–410, 1990. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 22.Andargie TE, Tsuji N, Seifuddin F, Jang MK, Yuen PS, Kong H, Tunc I, Singh K, Charya A, Wilkins K, Nathan S, Cox A, Pirooznia M, Star RA, Agbor-Enoh S. Cell-free DNA maps COVID-19 tissue injury and risk of death and can cause tissue injury. JCI Insight 6, 2021. doi: 10.1172/jci.insight.147610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryu C, Sun H, Gulati M, Herazo-Maya JD, Chen Y, Osafo-Addo A, Brandsdorfer C, Winkler J, Blaul C, Faunce J, Pan H, Woolard T, Tzouvelekis A, Antin-Ozerkis DE, Puchalski JT, Slade M, Gonzalez AL, Bogenhagen DF, Kirillov V, Feghali-Bostwick C, Gibson K, Lindell K, Herzog RI, Dela Cruz CS, Mehal W, Kaminski N, Herzog EL, Trujillo G. Extracellular mitochondrial DNA is generated by fibroblasts and predicts death in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 196: 1571–1581, 2017. doi: 10.1164/rccm.201612-2480OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryu C, Brandsdorfer C, Adams T, Hu B, Kelleher DW, Yaggi M, Manning EP, Walia A, Reeves B, Pan H, Winkler J, Minasyan M, Dela Cruz CS, Kaminski N, Gulati M, Herzog EL. Plasma mitochondrial DNA is associated with extrapulmonary sarcoidosis. Eur Respir J 54: 1801762, 2019. doi: 10.1183/13993003.01762-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang H, Li T, Chen S, Gu Y, Ye S. Neutrophil extracellular trap mitochondrial DNA and its autoantibody in systemic lupus erythematosus and a proof-of-concept trial of metformin. Arthritis Rheumatol 67: 3190–3200, 2015. doi: 10.1002/art.39296. [DOI] [PubMed] [Google Scholar]
- 26.Mude W, Oguoma VM, Nyanhanda T, Mwanri L, Njue C. Racial disparities in COVID-19 pandemic cases, hospitalisations, and deaths: A systematic review and meta-analysis. J Glob Health 11: 05015, 2021. doi: 10.7189/jogh.11.05015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erickson SE, Shlipak MG, Martin GS, Wheeler AP, Ancukiewicz M, Matthay MA, Eisner MD; National Institutes of Health National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network. Racial and ethnic disparities in mortality from acute lung injury. Crit Care Med 37: 1–6, 2009. doi: 10.1097/CCM.0b013e31819292ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Todkar K, Chikhi L, Desjardins V, El-Mortada F, Pépin G, Germain M. Selective packaging of mitochondrial proteins into extracellular vesicles prevents the release of mitochondrial DAMPs. Nat Commun 12: 1971, 2021. doi: 10.1038/s41467-021-21984-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The sequencing data that support the findings of this study are openly available in The National Center for Biotechnology Information shared server (BioProject) at https://www.ncbi.nlm.nih.gov/bioproject/810499.


