Keywords: adaptations, blood flow restriction, electromyographic, occlusion, resistance training
Abstract
The purpose of this study was to examine the acute effects of low-load blood flow restriction (LLBFR) and low-load non-BFR (LL) on neuromuscular function after a bout of standardized fatiguing leg extension muscle actions. Fourteen men (mean age ± SD = 23 ± 4 yr) volunteered to participate in this investigation and randomly performed LLBFR and LL on separate days. Resistance exercise consisted of 75 isotonic unilateral leg extension muscle actions performed at 30% of one-repetition maximum. Before (pretest) and after (posttest) performance of each bout of exercise, strength and neural assessments were determined. There were no pretest to posttest differences between LLBFR and LL for maximal voluntary isometric contraction (MVIC) torque or V wave/M wave responses (muscle compound action potentials assessed during a superimposed MVIC muscle action), which exhibited decreases (collapsed across condition) of 41.2% and 26.2%, respectively. There were pretest to posttest decreases in peak twitch torque (36.0%) and surface electromyography amplitude (sEMG) (29.5%) for LLBFR but not LL and larger decreases in voluntary activation for LLBFR (11.3%) than for LL (4.5%). These findings suggested that LLBFR elicited greater fatigue-induced decreases in several indexes of neuromuscular function relative to LL. Despite this, both LLBFR and LL resulted in similar decrements in performance as assessed by maximal strength.
NEW & NOTEWORTHY The application of blood flow restriction induces greater acute neuromuscular fatigue relative to nonrestricted conditions. Resistance exercise with blood flow restriction elicited a greater reduction in twitch responses. These neuromuscular differences might explain the more favorable adaptations achieved with blood flow restriction that are likely a function of metabolic stress and subsequent changes in efferent neural drive.
INTRODUCTION
The utility of low-load blood flow restriction (LLBFR) resistance training has been demonstrated in asymptomatic and symptomatic populations, and it may have additional applications among diabetic and coronary artery disease patients (1–4). It is generally reported that chronic high-load (HL), low-load (LL), and LLBFR resistance exercise elicit comparable increases in muscle mass, whereas subsequent increases in muscle strength exhibit less consistency (5, 6). Specifically, LLBFR typically elicits greater training-induced strength increases than LL but similar or smaller strength increases than HL. It has been hypothesized that the differences in strength-related outcomes between LLBFR and HL may reflect training specificity whereby HL training more closely resembles maximal strength testing than LLBFR (7). This does not, however, explain the training-induced differences in muscle strength between LLBFR and LL which both utilize low training loads and have evaluated training-induced strength outcomes with maximal strength assessments. Therefore, it is possible that relative to LL the greater training-induced increases in muscle strength associated with LLBFR are facilitated by neural adaptations.
The underlying mechanisms mediating neural adaptations, however, remain largely hypothetical and not easily identifiable. Thus, it is possible that exercise load is not a primary mechanism facilitating neural adaptions that has, acutely, resulted in similar decreases in voluntary muscle activation [excitation] following acute resistance exercise utilizing low/moderate and heavy exercise loads (8). Regardless, previous literature (9–11) has demonstrated a superiority of HL versus LL or LLBFR training on neural adaptions, although there is discrepancy in this regard (12, 13). Furthermore, a recent review (14) indicated that LLBFR elicited a greater overall effect on muscle excitation than LL and was similar to HL. Additionally, we (15) and others (11, 12, 16, 17) have observed significant (but not always meaningful) differences between LLBFR and LL in several indexes of neural function (e.g., muscle excitation, efficiency of electrical activity, twitch properties) after chronic interventions. For example, as a result of LLBFR resistance exercise, there were greater decreases in the ratio of twitch to maximal voluntary isometric contraction torque [twitch:maximal voluntary isometric contraction (MVIC)], earlier recruitment of high-threshold motor units, and greater increases in muscle excitability relative to LL (non-BFR conditions) (12, 16, 17). On the contrary, there were no differences in muscle excitation, action potential conduction velocity, motor unit recruitment, or firing rate after acute and chronic LLBFR and LL resistance training (15, 18–20). Therefore, there are clear inconsistencies within the known, available literature regarding neural function after LLBFR and LL resistance training, which warrants additional research.
Some of the challenges with identifying differences in neural adaptations between LLBFR and LL resistance training may be due to underpowered analyses and inconsistent/insufficient normalization procedures when examining neuromuscular function. For example, we (15, 19) and others (11, 12, 16–18, 20) have utilized different normalization procedures, applications, and conditioning of the surface electromyographic (sEMG) signal that have inherent limitations (e.g., stationarity, filtering, electrode placement, amplitude cancellation, cross talk, between- or within-subject comparisons) and may reduce statistical power to delineate potentially small but meaningful differences between LLBFR and LL resistance exercise on neural adaptations (21–23). To overcome some of these limitations and challenges associated with signal processing, sEMG can be normalized to maximal compound muscle action potential (M wave), providing a more robust examination of central efferent drive apart from peripheral factors, assuming that proper sEMG placement and signal processing are performed under identical testing conditions (22, 24). Of note, it has also been suggested (14) that to examine potential differences between LLBFR and LL resistance exercise on neural adaptations, the exercise protocols should implement a standard set and repetition scheme as opposed to performing repetitions to failure.
There is a general lack of research that has examined the acute effects of standardized LLBFR and LL with a multifaceted, robust neuromuscular approach. Of the available information in this regard, there exists large heterogeneity among the implementation of LLBFR, including varying BFR occlusion pressures, neural assessments, and exercise protocols (25). Thus, a comprehensive examination of acute neuromuscular responses associated with LLBFR and LL would provide valuable insight into the potential mechanisms mediating muscular adaptations, both acutely and chronically. Therefore, the purpose of this study was to examine the acute effects of LLBFR and LL on neuromuscular function after a bout of standardized fatiguing leg extension muscle actions. Based on previous LLBFR and LL studies (12, 16, 17), it was hypothesized that LLBFR would be associated with greater fatigue-induced decreases in maximum strength and larger increases in muscle excitation.
METHODS
Participants
Fourteen men (n = 14; mean age ± SD = 23 ± 4 yr; body mass = 79.3 ± 13.5 kg; height = 176.2 ± 12.4 cm) volunteered to participate in this investigation and randomly performed LLBFR and LL on separate days. The participants had no known cardiovascular, pulmonary, metabolic, muscular, and/or coronary heart disease or regularly used prescription medication. All participants were recreationally active (Tier 1) at the time of testing, but no participants had been actively participating in resistance training for at least the past 6 mo (26). Individuals were excluded if they were currently taking any supplements or participating in caloric restriction dietary practices. This study was approved by the University Institutional Review Board for Human Subjects, and all subjects completed a health history questionnaire and gave written informed consent before testing.
Experimental Design
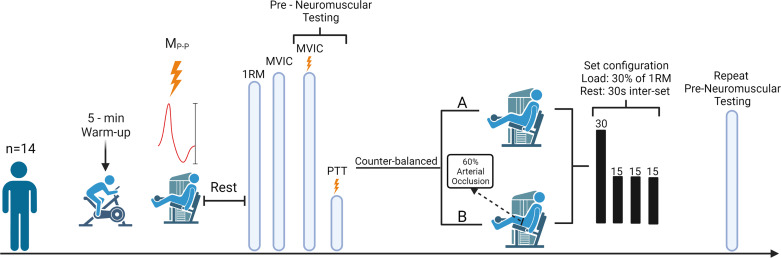
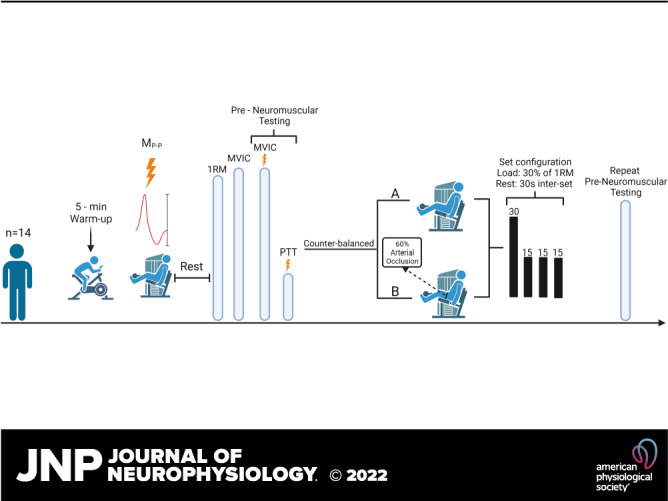
A randomized, counterbalanced, repeated-measures, within-group crossover design was used for this study (Fig. 1). Fourteen men performed 75 isotonic, unilateral submaximal [30% of 1 repetition maximum (1RM)] leg extension muscle actions with BFR and without BFR (non-BFR) that were randomly allocated and performed on separate days. Blood flow restriction was achieved with a 10-cm-wide cuff and a rapid cuff inflator (Hokanson Rapid Cuff Inflator; Hokanson Inc., Belleview, WA) that was applied at 60% of arterial occlusion pressure. During each visit, maximal voluntary isometric contraction (MVIC) torque and neuromuscular fatigue were assessed.
Figure 1.
Overview of experimental procedures performed on testing visits 1 and 2 of the low-load blood flow restriction (LLBFR) and low-load non-blood flow restriction (LL) resistance exercise protocols. Specifically, after a 5-min self-paced warmup, subjects were fitted to the isotonic device and completed a submaximal isotonic warmup before determination of the optimal stimulation location, intensity, and quantification of maximum peak-to-peak amplitude of the potentiated singlet (MP-P). After a rest period, subjects performed 1-repetition maximum (1RM) and maximal voluntary isometric contraction (MVIC) testing before performing a series of superimposed MVIC muscle actions with doublet stimuli to quantify muscle compound action potentials during (M wave) and after (V wave) each stimulus. Each superimposed MVIC muscle action was followed by a potentiated doublet stimulus to quantify peak twitch torque (PTT). After preneuromuscular testing, subjects completed LLBFR or LL [randomly allocated, counterbalanced (B or A, respectively)] that consisted of 75 isotonic, unilateral, submaximal (30% of 1RM) leg extension muscle actions. Immediately after each LLBFR and LL exercise protocol, subjects performed postneuromuscular testing. The schematic was created with BioRender.com.
Procedures
The first laboratory visit consisted of an orientation session to familiarize the participants with the testing protocols and measure subject characteristics. During the orientation, subjects performed submaximal and maximal isotonic and isometric leg extension muscle actions on a plate-loaded, seated leg extension device (Power Lift & Connor Athletic Products, Iowa). The subjects were also introduced to the stimulation procedures.
Maximal strength assessments.
Before each exercise protocol and maximal strength testing, the participants performed a 5-min warm-up at a self-selected pace on a stationary cycle ergometer (Corival; Lode B.V., Groningen, The Netherlands). After a brief rest period, the subjects were fitted to the isotonic leg extension device (Power Lift, part no. 81000 A; Iowa) and the seat was adjusted so the lateral epicondyle of the femur aligned with the axis of rotation of the leg extension device. The participants then performed 5–10 submaximal (40–80% of perceived maximum effort) isotonic leg extension muscle actions that were achieved by addition of weight to the device between repetitions based on subjective feedback.
1RM.
After the warm-up and optimal stimulation location and intensity determination, the participants rested for 5 min and then performed 1RM testing (Fig. 1). Participants’ 1RM strength was determined with the unilateral leg extension device that was custom fitted with a calibrated pancake load cell (model 41; Honeywell Inc., North Carolina). All 1RM testing was performed according to the guidelines by the National Strength and Conditioning Association (27), which include a light warm-up of 10 repetitions followed by two or three sets of 5 repetitions with progressively heavier loads until participants can no longer complete a leg extension muscle action through a full 90° range of motion. The heaviest load lifted throughout the entire range of motion was defined as the participant’s 1RM.
MVIC.
Participants were allotted a brief rest period after 1RM testing and then performed two 3-s maximal isometric muscle actions of the leg extensors at a knee joint angle of 90° (180° corresponds to full extension at the knee). Knee joint angle was achieved with a handheld goniometer (Smith & Nephew Rolyan Inc., Menomonee Falls, WI), and the lever arm was isolated in place on a subject-by-subject basis by adjusting the starting point via a belt and clamp system on the isotonic device. To ensure that there was no movement of the lever arm during each isometric muscle action (voluntary and/or evoked), the device was supramaximally loaded. The highest isometric torque produced during the two MVIC attempts was used to create force thresholds for subsequent MVIC trials. Specifically, target MVIC force was observed in real time on the computer monitor to ensure that maximum force was obtained during each of the subsequent MVICs and superimposed evoked potentials.
Exercise protocol.
The exercise protocol consisted of 75 unilateral submaximal (30% of 1RM) isotonic leg extension muscle actions performed across four sets (1 × 30, 3 × 15), and the sets were separated by 30 s of rest. All isotonic muscle actions were performed through a full 90° range of motion at a controlled pace (1 s concentric, 1 s eccentric), which was paced by a metronome and monitored by the research team.
Blood flow restriction.
Blood flow restriction was applied with a 10-cm-wide cuff and a rapid cuff inflator (Hokanson Rapid Cuff Inflator; Hokanson Inc., Belleview, WA). The pressure was initially applied at 30 mmHg and intermittently inflated and deflated until target pressure was achieved. Optimal pressure was calculated at 60% of the lowest amount of pressure necessary to completely occlude blood as indicated by the ultrasound (28). Across the 14 participants, total arterial occlusion pressure was 165 ± 20 mmHg. The cuff was inflated immediately before performance of the 75 submaximal repetitions and was deflated immediately after completion of the 75 submaximal repetitions. The total duration of BFR was ∼5 min.
Stimulation procedures.
Singlet (50 µs) and doublet (2 singlets interspersed by 10 ms) rectangular pulsed stimuli were performed to quantify indexes of neuromuscular fatigue. All stimuli were delivered at 400 V, and only amperage was modulated. For all stimulation procedures, participants performed (voluntarily and/or potentiated) unilateral isometric leg extension muscle actions while the leg was at a 90° angle. Optimal stimulation location was determined by the simultaneous inspection of the evoked muscle action potentials of the three superficial muscles of the quadriceps and the subsequent torque response that was elicited from each stimulus. These exploratory singlet stimuli were delivered at a low amperage (25–50 mA) with a handle-held cathode (Compex Motor Point Pen; Compex, Mississauga, ON, Canada) and a disposal anode (Digitimer Ltd, Welwyn Garden City, UK) fixed over the greater trochanter during all stimuli. The cathode was initially applied over the femoral nerve beginning in the lateral corner of the femoral triangle. The optimal location for stimulation was determined on each visit and marked with indelible, permanent black ink, ensuring that the location was not different between visits for any of the subjects. After the determination of optimal stimulation location, the amperage was progressively increased by 20–50 mA until a plateau in the muscle compound action potentials was observed for all three superficial muscles of the quadriceps and a plateau in the corresponding torque response occurred and was captured via the pancake load cell. The amperage was then multiplied by 120%, and this resultant amperage (supramaximal stimulus) value was used for all further singlet and doublet stimuli applications. Optimal location and intensity were determined after the submaximal isotonic warm-up, before 1RM testing on each testing visit.
Measurements
Torque assessments.
During each MVIC muscle action and twitch response, the raw torque signals were determined with a custom-fitted pancake load cell (model 41; Honeywell Inc., North Carolina) and were sampled at 10,000 Hz. The raw torque signals were filtered (high pass 15 Hz) and analyzed off-line (LabVIEW v. 12.0; National Instruments, Austin, TX). Force was then calculated from a linear regression equation (R2 = 0.99) that was derived from voltages and corresponding external loads that were hung from the lever arm (0–100 kg in 5-kg increments), gravity corrected. This force (N) was then multiplied by lever arm length on a subject-by-subject basis to determine torque (Nm).
Stimulation-derived assessments.
The maximum peak-to-peak amplitude (MP-P) was determined from the vastus lateralis by performing three separate, potentiated, supramaximal singlet stimuli separated by 60 s of rest. The MP-P was quantified as the maximum peak-to-peak amplitude of the unrectified EMG signal elicited among the three supramaximal stimuli (18, 24) and was performed before the LLBFR and LL exercise (Fig. 1).
M and V waves were determined during and after the superimposed doublet stimulus, respectively, which was performed during the separate 3- to 6-s MVIC muscle actions. The doublet stimulus was delivered when the subject reached a plateau in their previously determined MVIC torque that was transposed and displayed in real time on a computer monitor. If the subject failed to reach their previously determined MVIC torque or did not reach a visible plateau, the attempt was repeated after a brief rest period. In general, each participant performed two to four MVIC trials with the superimposed doublet stimuli before and immediately after each of the LLBFR and LL protocols (Fig. 1). M and V waves were quantified as the maximum peak-to-peak amplitude of the muscle compound action potential of the unrectified EMG signal assessed during and after (8–20 ms) the superimposed doublet stimuli, respectively (18).
Voluntary activation (VA) was determined as the percentage of the superimposed doublet twitch torque relative to the potentiated doublet twitch torque [(1 − superimposed doublet twitch torque/potentiated doublet twitch torque) × 100] (29, 30). The potentiated doublet twitch was generated, at rest, 3–5 s after the cessation of each MVIC effort. The twitch force associated with the highest 10-ms average torque generated of the potentiated doublet twitches was considered peak twitch torque (PTT) (10). Trials were repeated if subjects did not fully relax between the cessation of their MVIC effort and the subsequent potentiated doublet twitch.
Electromyography.
During the LLBFR and LL visits, pregelled surface electrodes (Ag/AgCl, AccuSensor; Lynn Medical, Wixom, MI) were placed in a bipolar arrangement (50 mm center to center) on the vastus lateralis of the exercising leg. The electrodes were placed at 66% of the distance from the anterior superior iliac spine to the lateral border of the patella (31), and the longitudinal axis of the bipolar electrodes was placed parallel to the angle of pennation (20°) of the muscle fibers (32). The reference electrode was placed over the anterior superior iliac spine, and before each electrode placement the skin was shaved, carefully abraded, and cleaned with alcohol.
The raw sEMG signals were digitized at 2,000 Hz with a 32-bit analog-to-digital converter (model MP150; Biopac Systems, Inc.) and stored in a personal computer (ATIV Book 9 Intel Core i7; Samsung Inc., Dallas, TX) for subsequent analyses. The EMG signals were amplified (gain: ×1,000) with differential amplifiers (EMG 100; Biopac Systems, Inc., Santa Barbara, CA) with a common mode rejection ratio of 110 dB min and an impedance of 2 MΩ. The sEMG signals were digitally band-pass filtered (4th-order Butterworth, zero-phase shift) at 10–500 Hz. The sEMG amplitude (µV root-mean-square, µVrms) values were calculated for a time period that corresponded to 500 ms (1,000 data points) immediately before the superimposed doublet stimuli during the MVIC muscle actions. MVIC torque was also derived from this same 500-ms time period and was determined as the highest 10-ms torque value that was calculated from a running average. All sEMG and torque signal processing was performed off-line with custom written software (LabVIEW v. 13.0; National Instruments, Austin, TX).
Data Analysis
Normalization.
The absolute sEMG amplitude values during the MVIC muscle actions were normalized to the previously determined MP-P values that were collected on each testing visit. Thus, all sEMG amplitude values were expressed as a percentage of the MP-P, which has been purported to be a more robust representation of muscle excitation apart from peripheral factors (21, 22).
Reliability.
Test-retest reliability for MVIC, PTT, sEMG, MP-P, M wave, V wave, and VA was assessed from testing visits 1 and 2 before the LLBFR and LL exercise protocols. Repeated-measures ANOVAs were used to assess systematic error, and model 2,1 (26) was used to calculate intraclass correlation coefficients (ICCs) and standard errors of measurement (SEM). The 95% confidence intervals for the means of the dependent variables were calculated with the studentized t distribution.
Statistical Analyses
Separate 2 [Condition (LLBFR, LL)] × 2 [Time (Pretest, Posttest)] repeated-measures ANOVAs were used to examine MVIC, PTT:MVIC, sEMG, VA, and V wave/M wave. For all ANOVA-based analyses, significant interactions or main effects (in the absence of an interaction) were examined with follow-up Bonferroni-corrected dependent-samples t tests. Greenhouse–Geisser corrections were applied when sphericity was not met according to Mauchly’s test of sphericity, and partial eta squared effect sizes () were calculated for each ANOVA. Additionally, unplanned post hoc Pearson correlation analyses were performed to investigate the relationships among posttest muscle strength and indexes of central and peripheral fatigue for the LLBFR and LL conditions. All statistical analyses were performed with IBM SPSS v. 27 (Armonk, NY), and an α of P ≤ 0.05 was considered statistically significant for all comparisons.
RESULTS
Reliability
Table 1 includes the test-retest reliability values from visit 1 and 2 measurements for MVIC, PTT, sEMG, MP-P, M wave, V wave, and VA. There were no mean differences for visit 1 versus visit 2 (P > 0.05) for any of the variables. The ICC values for all measured variables ranged from 0.647 to 0.944 and the SEM values ranged from 0.7% to 17.3% of the grand mean.
Table 1.
Test-retest reliability assessed from between visits 1 and 2 for all participants
| Variables | Visit 1 | Visit 2 | P Value | ICC | ICC95% | SEM | Grand Mean |
|---|---|---|---|---|---|---|---|
| MVIC, Nm | 186.74 ± 55.81 | 199.41 ± 52.32 | 0.244 | 0.848 | 0.546–0.951 | 17.3 | 193.1 |
| PTT, Nm | 85.29 ± 52.60 | 94.86 ± 63.41 | 0.265 | 0.923 | 0.770–0.975 | 13.25 | 90.04 |
| sEMG, µV | 814.1 ± 317.6 | 847.3 ± 297.6 | 0.531 | 0.895 | 0.677–0.966 | 81.6 | 830.7 |
| MP-P, µV | 7,662.8 ± 2378.2 | 7,450.2 ± 2500.0 | 0.709 | 0.787 | 0.321–0.932 | 920.3 | 7,556.5 |
| M wave, µV | 7,739.5 ± 2158.8 | 8,000.3 ± 1752.3 | 0.530 | 0.832 | 0.480–0.946 | 659.5 | 7,869.9 |
| V wave, µV | 3,196.8 ± 1495.9 | 2,734.4 ± 1410.6 | 0.141 | 0.818 | 0.456–0.941 | 513.3 | 2,965.6 |
| VA, % | 96.8 ± 1.8 | 97.6 ± 1.0 | 0.076 | 0.647 | 0.012–0.882 | 0.72 | 97.2 |
Values are means ± SE for n = 14 participants. ICC, intraclass correlation coefficient; MP-P, maximum peak-to-peak amplitude; MVIC, maximal voluntary isometric contraction; M wave, maximum compound muscle action potential; PTT, peak twitch torque; SEM, standard error of measurement; sEMG, normalized surface electromyography amplitude; VA, voluntary activation; V wave, maximum compound muscle action potential immediately following the superimposed doublet twitch.
Strength Assessments
MVIC.
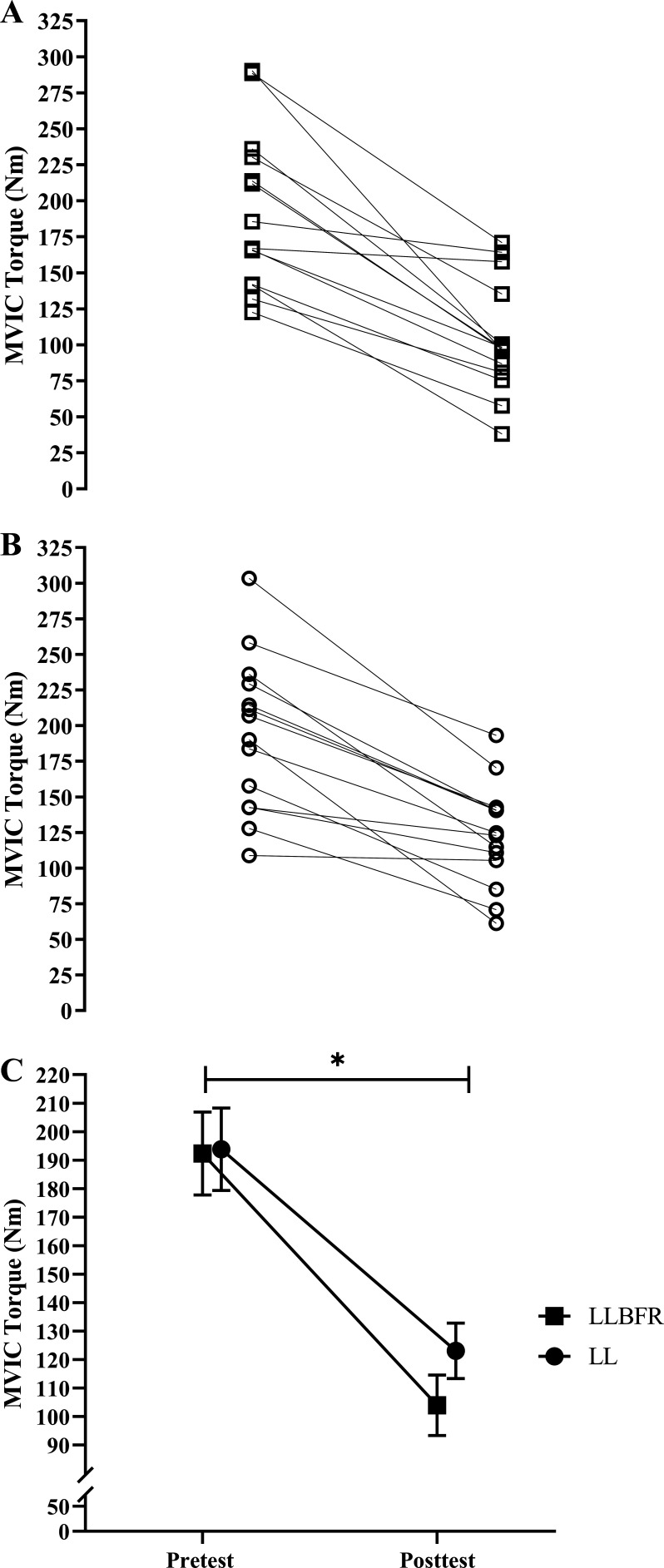
There was no significant (P = 0.112, = 0.183) two-way interaction for MVIC torque. There was, however, a significant main effect for Time (P < 0.001, = 0.818) but not Condition (P = 0.369, = 0.062). Specifically, collapsed across Condition, MVIC torque decreased from pretest (193.1 ± 53.5 Nm) to posttest (113.6 ± 38.4 Nm) (Fig. 2).
Figure 2.
Individual (A and B) and mean ± SE (C) absolute changes in maximal voluntary isometric contraction (MVIC) torque. MVIC torque was determined as the highest 10-ms average torque generated during the 500 ms before each superimposed doublet twitch stimulus, which was assessed immediately before (pretest) and after (posttest) performance of low-load blood flow restriction (LLBFR; A, □) and low-load non-blood flow restriction (LL; B, ○) leg extension muscle actions. There was no significant interaction, but there was a main effect for Time. *Significant (P < 0.05) main effect for Time, collapsed across Condition (pretest > posttest). Note that in C the effects of LLBFR and LL have been displayed separately across Time, although there was no interaction.
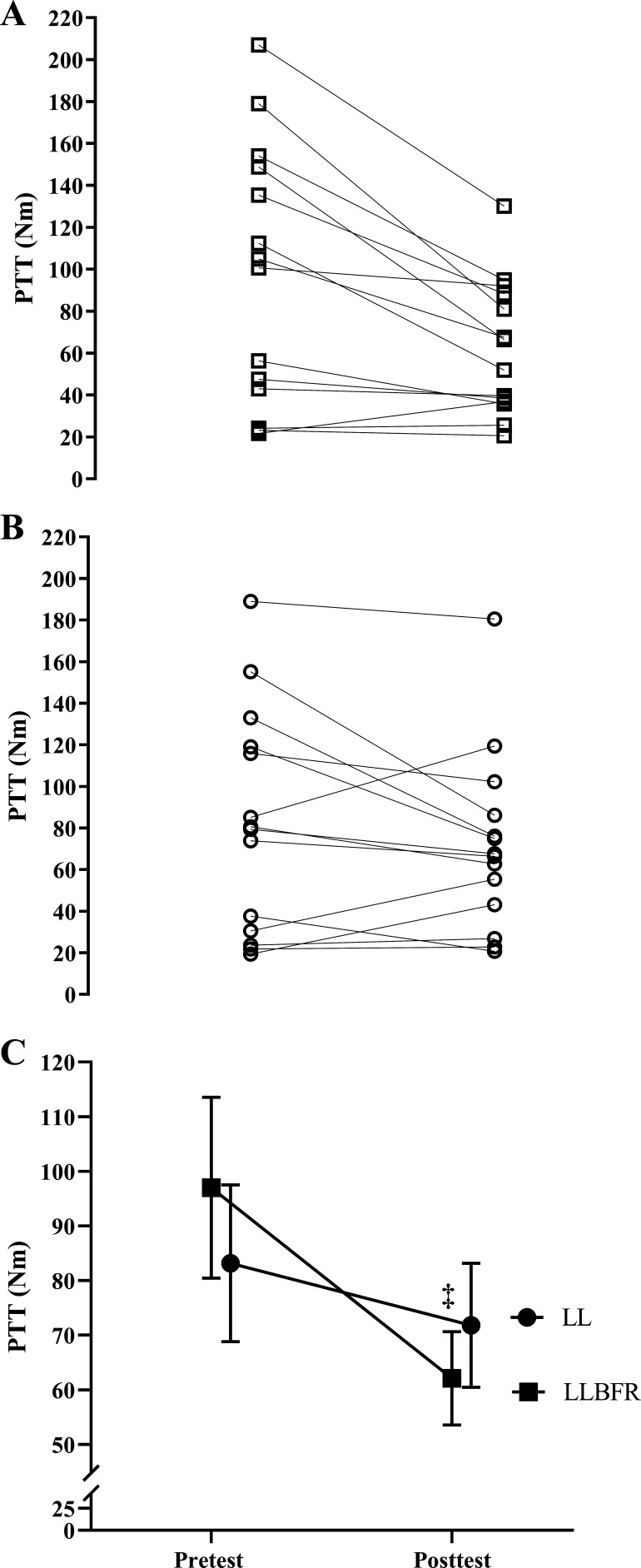
PTT.
There was a significant (P = 0.017, = 0.364) two-way interaction for PTT and significant (P = 0.003, d = 0.968) follow-up simple main effects for LLBFR but no significant (P = 0.098–0.177, d = 0.042–0.477) simple main effects for LL, pretest, or posttest. Specifically, PTT decreased from pretest to posttest for LLBFR (97.0 ± 62.0 Nm to 62.1 ± 31.9 Nm) but not LL (83.2 ± 53.7 Nm to 71.8 ± 42.5 Nm), and PTT was not different at pretest or posttest between LLBFR and LL (Fig. 3).
Figure 3.
Individual (A and B) and mean ± SE (C) absolute changes in peak twitch torque (PTT). PTT was determined as the highest 10-ms average torque generated during the potentiated doublet twitch stimulus that was assessed immediately before (pretest) and after (posttest) performance of low-load blood flow restriction (LLBFR; A, □) and low-load non-blood flow restriction (LL; B, ○) leg extension muscle actions. There was a significant interaction for PTT that was decomposed into follow-up dependent-samples t tests. ‡Significant (P < 0.05) simple main effect for Time (pretest > posttest). There were no other significant effects for PTT.
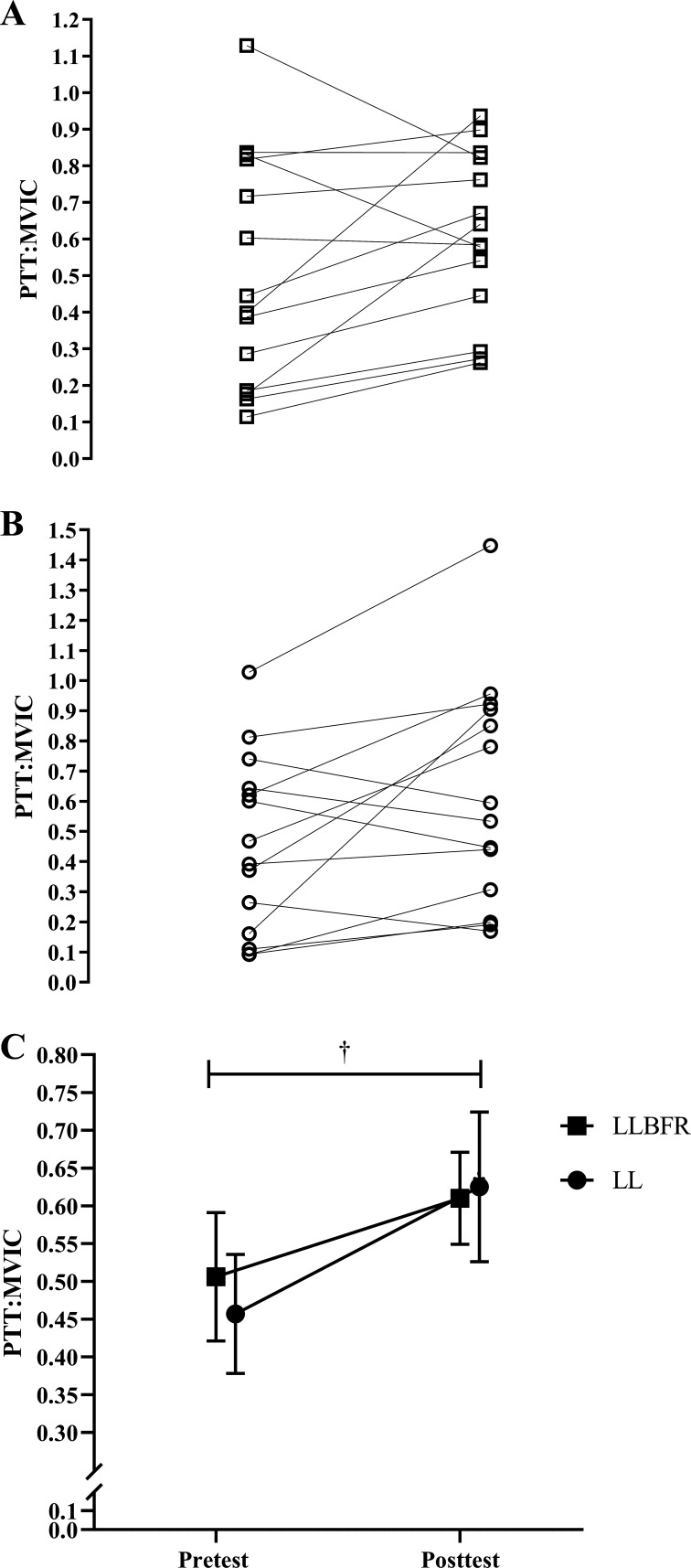
PTT:MVIC.
There was no significant (P = 0.284, = 0.088) two-way interaction for PTT:MVIC. There was, however, a significant main effect for Time (P = 0.040, = 0.286) but not Condition (P = 0.787, = 0.006). Specifically, collapsed across Condition, PTT:MVIC increased from pretest (0.482 ± 0.288) to posttest (0.617 ± 0.293) (Fig. 4).
Figure 4.
Individual (A and B) and mean ± SE (C) absolute changes in peak twitch torque-to-maximal voluntary isometric contraction ratio (PTT:MVIC). MVIC torque was determined as the highest 10-ms average torque generated during the 500 ms before each superimposed doublet twitch stimulus, and PTT was determined as the highest 10-ms average torque generated during the potentiated doublet twitch stimulus. PTT:MVIC was assessed immediately before (pretest) and after (posttest) performance of low-load blood flow restriction (LLBFR; A, □) and low-load non-blood flow restriction (LL; B, ○) leg extension muscle actions. There was no significant interaction, but there was a main effect for Time. †Significant (P < 0.05) main effect for Time, collapsed across Condition (pretest < posttest). Note that in C the effects of LLBFR and LL have been displayed separately across Time, although there was no interaction.
Neuromuscular Fatigue Indexes
sEMG amplitude.
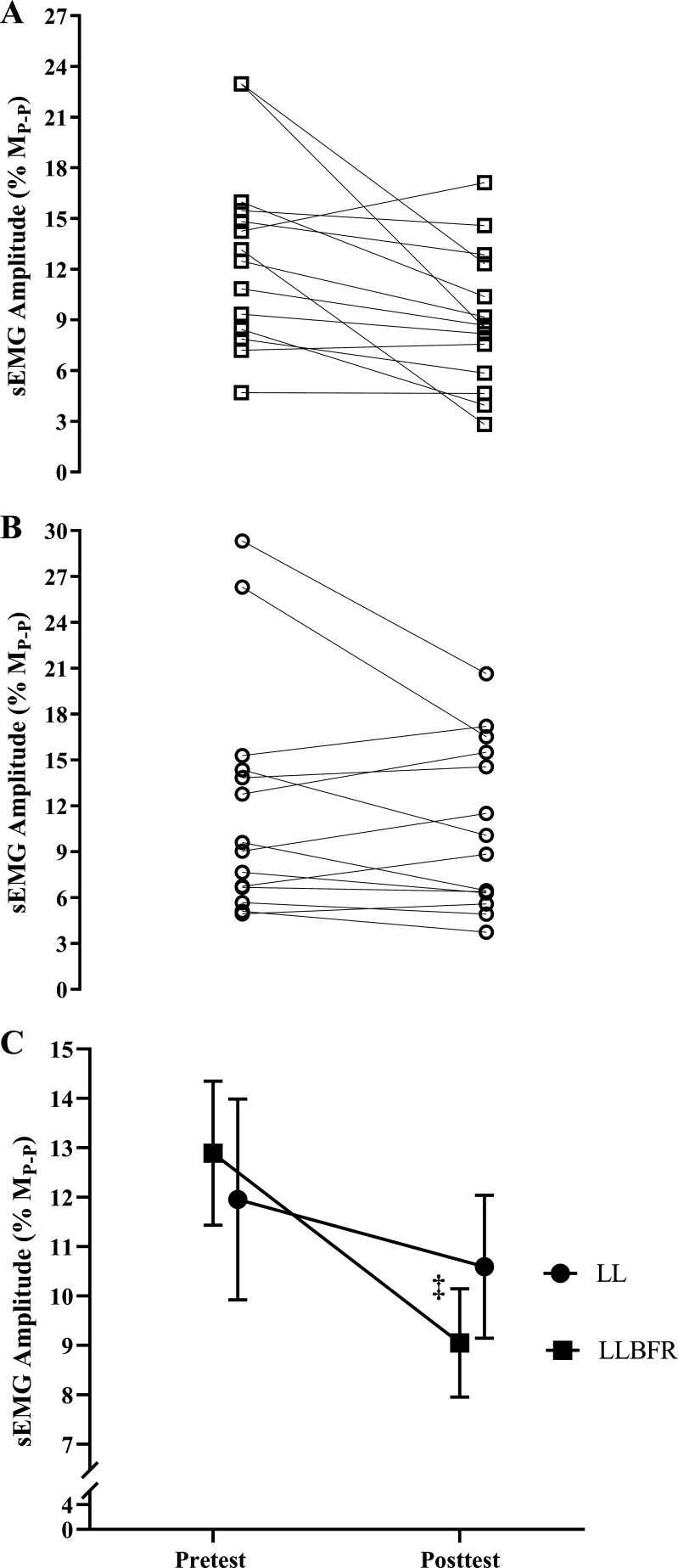
There was a significant (P = 0.048, = 0.268) two-way interaction for normalized sEMG amplitude and significant (P = 0.011, d = 0.790) follow-up simple main effects for LLBFR but no significant (P = 0.216–0.531, d = 0.172–0.348) simple main effects for LL, pretest, or posttest. Specifically, normalized sEMG amplitude decreased from pretest to posttest for the LLBFR condition (0.129 ± 0.055 to 0.091 ± 0.041) but did not change from pretest to posttest for LL (0.120 ± 0.076 to 0.106 ± 0.054), and sEMG was not different at pretest or posttest between LLBFR and LL (Fig. 5).
Figure 5.
Individual (A and B) and mean ± SE (C) normalized [to maximum peak-to-peak amplitude of the potentiated singlet (MP-P)] changes in surface electromyographic (sEMG) amplitude (% of MP-P). sEMG was assessed during the 500 ms before each superimposed doublet twitch stimulus that were assessed immediately before (pretest) and after (posttest) performance of low-load blood flow restriction (LLBFR; A, □) and low-load non-blood flow restriction (LL; B, ○) leg extension muscle actions. MP-P was determined before the exercise protocols and as the maximum peak-to-peak amplitude of the unrectified EMG signal elicited among the supramaximal stimuli. There was a significant interaction for sEMG amplitude that was decomposed into follow-up dependent-samples t tests. ‡Significant (P < 0.05) simple main effect for Time (pretest > posttest). There were no other significant effects for sEMG amplitude.
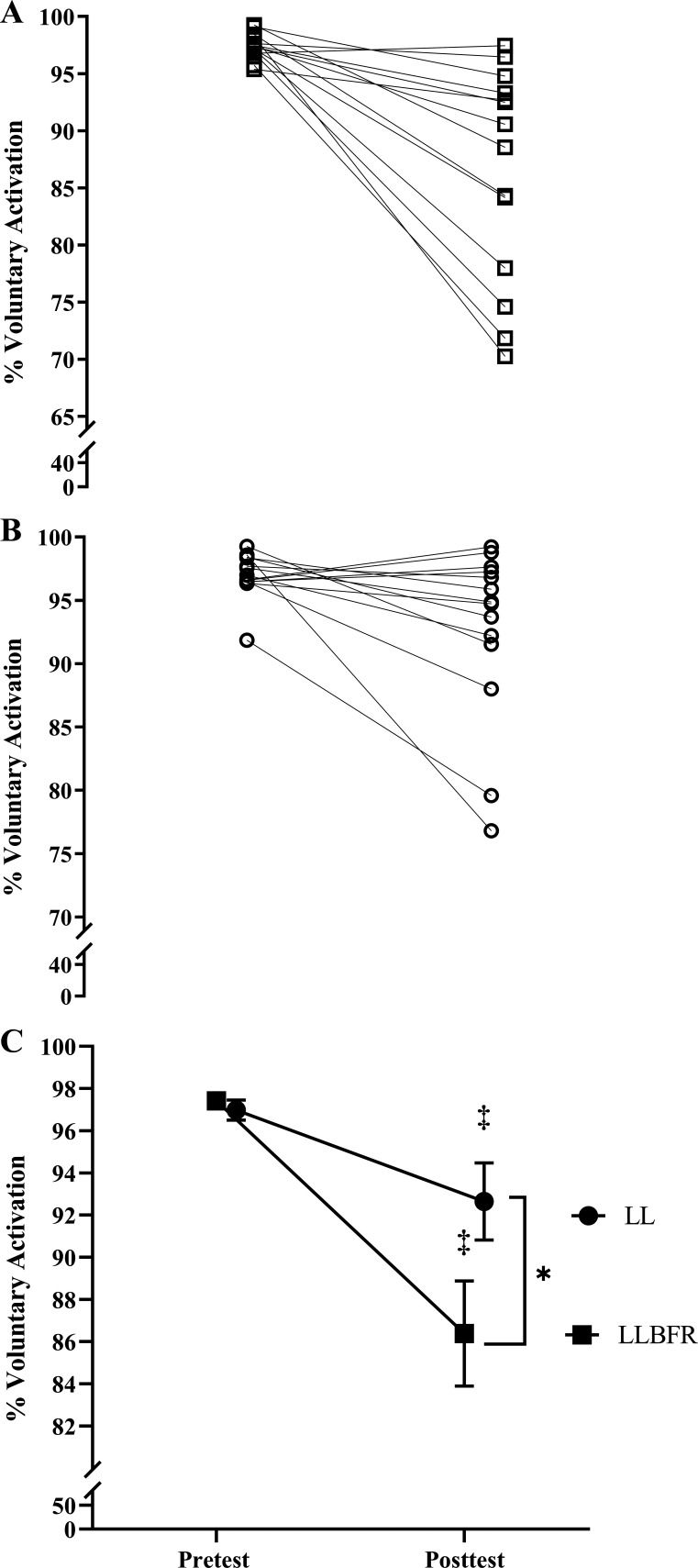
VA.
There was a significant (P = 0.004, = 0.489) two-way interaction for VA and significant follow-up simple main effects for LLBFR (P = 0.001, d = 1.195), LL (P = 0.027, d = 0.655), and posttest (P = 0.005, d = 0.906) but no significant simple main effect for pretest (P = 0.308, d = 0.283). Specifically, VA decreased from pretest to posttest for LLBFR (97.4 ± 1.1% to 86.4 ± 9.3%) and LL (97.0 ± 1.8% to 92.6 ± 6.8%), and VA was lower at posttest for LLBFR than LL (Fig. 6).
Figure 6.
Individual (A and B) and mean ± SE (C) % of voluntary activation that was assessed immediately before (pretest) and after (posttest) performance of low-load blood flow restriction (LLBFR; A, □) and low-load non-blood flow restriction (LL; B, ○) leg extension muscle actions. Voluntary activation was determined as % of the superimposed doublet twitch torque relative to the potentiated doublet twitch torque. There was a significant interaction for voluntary activation that was decomposed into follow-up dependent-samples t tests. *Significant (P < 0.05) simple main effect for Condition (LLBFR < LL); ‡significant (P < 0.05) simple main effect for Time (pretest > posttest).
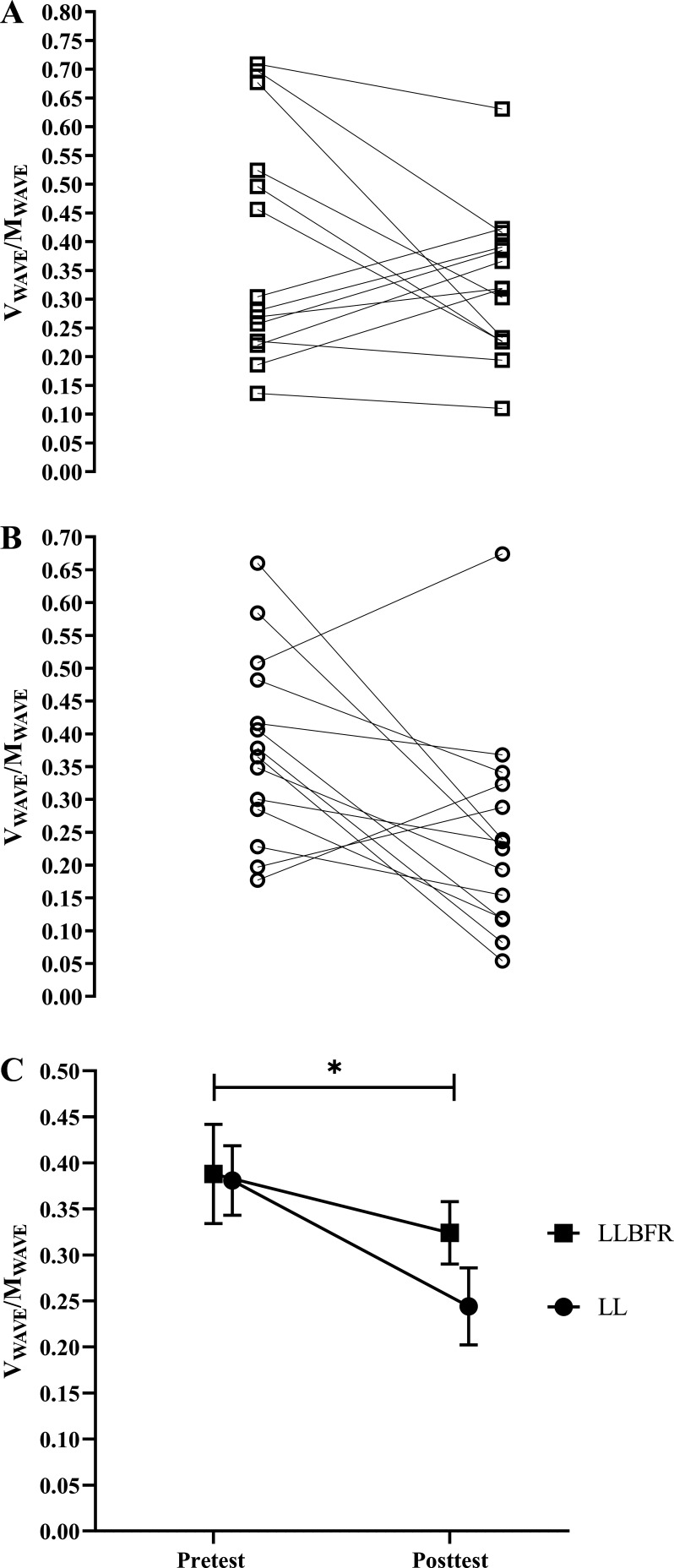
V wave/M wave.
There was no significant (P = 0.167, = 0.142) two-way interaction for V wave/M wave. There was, however, a significant main effect for Time (P = 0.040, = 0.286) but not Condition (P = 0.313, = 0.078). Specifically, collapsed across Condition, V wave/M wave decreased from pretest (0.385 ± 0.171) to posttest (0.284 ± 0.147) (Fig. 7).
Figure 7.
Individual (A and B) and mean ± SE (C) absolute changes in the ratio of maximum peak-to-peak amplitude of the muscle compound action potential during (M wave) and after (V wave) the superimposed doublet stimulus that was assessed before (pretest) and after (posttest) performance of low-load blood flow restriction (LLBFR; A, □) and low-load non-blood flow restriction (LL; B, ○) leg extension muscle actions. There was no significant interaction, but there was a main effect for Time. *Significant (P < 0.05) main effect for Time, collapsed across Condition (pretest > posttest). Note that in C the effects of LLBFR and LL have been displayed separately across Time, although there was no interaction.
Pearson Correlation Analyses
For LLBFR, posttest MVIC torque was positively correlated (r = 0.722, P = 0.002) with posttest PTT and negatively correlated with posttest V wave/M wave (r = −0.478, P = 0.042). There were no other significant correlations (r = −0.090–0.240, P = 0.204–0.379) for LLBFR. For LL, posttest MVIC torque was positively correlated (r = 0.491, P = 0.037) with posttest sEMG amplitude, but there were no other significant correlations (r = −0.002–0.263, P = 0.182–0.497).
DISCUSSION
The application of LLBFR resulted in greater acute fatigue-induced decreases in sEMG and VA relative to LL but similar decreases in V wave/M wave. These fatigue-induced decreases in neural drive contributed, in part, to the reductions in PTT for LLBFR but not LL and similar MVIC torque (decreases) and PTT:MVIC (increases) responses between conditions. Collectively, our findings suggested that LLBFR induced greater acute neuromuscular fatigue that was both central and peripheral in origin.
The similar fatigue-induced decreases in MVIC torque between LLBFR and LL in the present study were not consistent with previous investigations. For example, these previous investigations (17, 33), which also implemented a standardized 75-repetition protocol (1 × 30, 3 × 15), reported greater fatigue-induced decreases in MVIC torque following LLBFR (20.5–39%) than LL (0–17%) unilateral forearm flexion or leg extension muscle actions performed at 20% of 1RM. On the contrary, the use of a repetitions to failure design elicited similar decreases (37%) in MVIC torque between LLBFR and LL following unilateral leg extension muscle actions performed at 20% of 1RM (34). Performing sets to failure opposed to implementing a standardized 75-repetition protocol, however, may eliminate potential differences among acute fatigue responses between LLBFR and LL (14, 34). Thus, unlike the present study, previous investigations (17, 33) utilizing a standardized number of repetitions have generally reported greater fatigue-induced decreases in MVIC torque following LLBFR and LL resistance exercise. The dissociation between our findings and others (17, 33) may be a function of training load, which was lower in these previous investigations (20% of 1RM compared with 30% of 1RM used in the present study). Although training loads of 15–30% of 1RM have been effective at promoting muscle adaptation when combined with BFR (35), the magnitude of MVIC torque reduction was dependent upon the BFR occlusion pressure when combined with training loads < 30% of 1RM (25). Specifically, when training loads of 15–20% of 1RM were implemented, MVIC torque decreased to a greater extent when combined with BFR applied at 80–90% relative to 40–50% of total arterial occlusion pressure (25). There were, however, no differences in the decreases in MVIC torque across arterial occlusion pressures when a training load of 30% of 1RM was utilized (25). Therefore, it is possible that the differences in MVIC torque responses in these previous investigations (20, 35, 36) were due, in part, to a lower training load and the implementation of various BFR occlusion pressures [60% of total arterial occlusion pressure (individualized based on blood flow); 98, 121, and 147 mmHg (absolute pressures); and 1.3–1.5 times systolic blood pressure (individualized based on blood pressure)]. Unlike these previous investigations (20, 35, 36), in the present study we utilized a training load of 30% of 1RM and a BFR pressure of 60% of total arterial occlusion pressure (individualized based on blood flow) that did not result in differences in the MVIC torque responses between conditions.
In the present study, there were decreases in PTT for LLBFR but not LL (Fig. 3) and similar increases in PTT:MVIC (Fig. 4) for both conditions. The PTT responses provide insight into muscle contraction dynamics independent of descending neural drive and may be sensitive to localized muscle fatigue (e.g., metabolite buildup and excitation-contraction coupling failure) (16, 36, 37, 46). For example, Cook et al. (34) demonstrated that there was a 37% decrease in MVIC torque and a similar 40% decrease in postactivated potentiated PTT but no changes in neural drive after three sets to failure of LLBFR, LL, and HL leg extension muscle actions. In conjunction with these findings (34), in the present study there was a 41.2 ± 17.8% decrease in MVIC torque, collapsed across conditions, but a 36.0 ± 32.8% decrease in PTT for LLBFR and a nonsignificant 13.7 ± 49.1% decrease for LL. Thus, PTT is sensitive to and provides insight regarding the development of peripheral fatigue (36). MVIC torque, however, has been described as a global measure of fatigue and is thus affected by changes in both peripheral and central fatigue as a function of excitation-contraction dynamics and descending efferent neural drive, respectively (37). Therefore, the PTT-to-MVIC ratio is unique from MVIC torque and provides a more robust examination of central fatigue while accounting for the peripheral contribution (i.e., PTT) (38). Although PTT decreased for LLBFR and not LL, there were no differences in PTT assessed at pretest or posttest between conditions. Similarly, there were no differences in PTT:MVIC that increased similarly for LLBFR and LL. Together, these findings suggest that both conditions elicited central fatigue (as assessed by PTT:MVIC), while LLBFR may have induced greater peripheral fatigue (as assessed by PTT).
The observed decreases in MVIC torque and PTT (only for LLBFR; Fig. 3) and increases in PTT:MVIC for LLBFR and LL were also associated with several other indexes of central fatigue. For example, the decreases in sEMG amplitude (only for LLBFR; Fig. 5), VA (Fig. 6), and V wave/M wave (Fig. 7) suggested that both conditions induced an overall reduction in efferent neural drive. For example, sEMG represents a global measure of muscle excitation and is thought to be, at least partially, reflective of descending efferent neural drive (39, 40). In theory, during maximal muscle actions all motor units are excited and fire at their optimal frequencies. Contrary to this assumption, sEMG amplitude decreased, which coincided with decreases in VA and V wave/M wave. Specifically, VA is inversely related to and provides insight into the magnitude of central fatigue discriminate of peripheral factors (41). Assessments of VA have also been applied to track training-induced neural adaptations as a result of resistance exercise, but the validity of VA to delineate neural adaptations remains questionable (42). More recently, the assessment of central fatigue as derived from VA has been critically examined, exhibits poor validity, and may be influenced profoundly by peripheral factors (43). Perhaps of greater utility than sEMG amplitude and VA, V wave-to-M wave ratio provides an indirect assessment of efferent neural drive, and it has also been applied to examine the chronic effects of resistance training that may influence motor unit recruitment, firing rate, motor neuron excitability, and/or synaptic transmission (18, 23, 44, 45). The V wave constitutes a reflex response that is the net result of potentiated twitch-induced antidromic action potentials and descending efferent neural drive, with the latter increasing with increases in cortical excitation (44). Thus, after a period of resistance training, increases in V wave/M wave might be indicative of training-induced increases in cortical excitability. Similarly, after an acute bout of fatiguing exercise, decreases in V wave/M wave may demarcate a reduction in neural drive (i.e., central fatigue). Therefore, in the present study, the decreases in MVIC torque and increases in PTT:MVIC could be explained, in part, by the reduction in efferent neural drive as evidenced by the decreases in V wave/M wave. It is tempting to speculate that the decreases in sEMG amplitude that occurred for LLBFR as well as the larger decreases in VA relative to LL reflected a greater reduction in efferent neural drive. Contrarily, V wave/M wave responses decreased to a greater extent (although not significantly) after LL (36.0%) than after LLBFR (16.5%). Additionally, post hoc Pearson correlation analyses indicated that posttest MVIC torque was positively correlated (r = 0.722, P = 0.002) with PTT but negatively correlated with V wave/M wave (r = −0.478, P = 0.042) for LLBFR. For LL, posttest MVIC torque was positively correlated with sEMG amplitude (r = 0.491, P = 0.037) alone. These findings, alternatively, would suggest the opposite, by which LLBFR preserved efferent neural drive (or compensated for peripheral fatigue development) relative to LL whereby MVIC torque decreased with voluntary muscle excitation.
The underlying mechanisms facilitating the observed reduction in efferent neural drive after LLBFR and LL are likely multifaceted. Previously, it has been proposed that central fatigue may develop from inhibitory responses mediated by exercise-induced buildup of metabolites, increases in perceived effort, and/or pain associated with exercise (47–49). A global, hypothetical construct exists, termed the “sensory tolerance limit,” describes the centrally mediated integration of these physiological responses occurring at the local muscular level (48). For example, group III and IV afferent nerve endings monitor mechanical tension and metabolic accumulation within the muscle (50). During very intense exercise bouts or those performed with ischemia (as is the case with BFR) a more selective subgroup of group III and IV afferent nerve endings (metabo-nociceptors) suppress neural drive, perhaps through the inhibition of descending efferent neural drive and/or a reduction in corticospinal excitability. These group III and IV afferent nerve endings also respond to noxious stimuli including perturbations in discomfort and/or pain. For example, increasing the sensation of pain during exercise (induced via ischemia or hypertonic solution) reduced MVIC torque, time to failure, and VA relative to nonischemic or control/isotonic solution conditions (47, 49). Thus, it has been purported that the stimulation of group III and IV afferent nerve endings ultimately results in central fatigue, although initiated secondary to the development of peripheral fatigue and/or pain (47–49).
Therefore, it is possible that the reduction in efferent neural drive following LLBFR and LL was related to increased activation of group III/IV afferent nerve endings due to metabolite buildup and/or increased perception of pain. The larger decreases in PTT, sEMG, and VA for LLBFR indirectly suggested that the application of BFR exacerbated this response. For example, the magnitude of metabolite buildup is larger for LLBFR than for LL and may contribute to the greater training-induced muscular adaptations associated with LLBFR than LL (51, 52). LLBFR has also been associated with greater discomfort and ratings of perceived exertion relative to LL (53), but the BFR occlusion pressure was not individualized (200 mmHg pressure) and may have resulted in occlusion pressures that were higher than necessary to promote muscle adaptations (28, 54, 55). Contrarily, LLBFR appears to elicit comparable perceptual responses across exercise modalities, muscle groups, and populations and may attenuate delayed-onset muscle soreness (56–60). Collectively, the present findings suggest that LLBFR may induce a greater buildup of metabolites but does not adversely affect exercise performance or efferent neural drive relative to LL. Thus, LLBFR may provide a unique exercise stimulus that elicits peripheral perturbations and preserves efferent neural drive.
Limitations
The present study examined the acute neuromuscular responses to LLBFR and LL using sEMG, M wave, V wave, and VA as indexes of muscle fatigue. These indexes were used to make inferences regarding the magnitude of efferent neural drive (sEMG, V wave/M wave, and VA), but none of these assessments directly identifies the location or intensity of descending efferent neural drive. Specifically, sEMG records and quantifies the global summation of muscle action potentials, but it cannot discriminate motor unit recruitment and firing rate characteristics (22, 40). The acquisition of sEMG can be affected by a number of methodological factors and may underestimate muscle excitation because of overlapping positive and negative phases of the muscle action potentials (amplitude cancellation) that is more prevalent at higher contraction intensities (e.g., MVIC muscle actions) (40, 61). Additionally, the V wave is thought to provide a measure of efferent neural drive (supraspinal) but may be influenced by other, unrelated factors including increased synaptic transmission or reduced inhibition (23, 44). Despite these inherent limitations, attempts were made to reduce extrinsic interference adversely affecting our neural parameters and subsequent interpretations. Specifically, consistent with recommendations for sEMG recording, placement, and interpretation, the electrodes were placed in identical locations between visits and only within-subject comparisons were made (21, 22, 62). Additionally, all sEMG and V wave assessments were normalized to the M wave, which may provide more robust analyses (21, 22). Because of the difficulty associated with assessing some of these parameters (MP-P, VA), several attempts or repetitions were performed to allow for a critical examination of these parameters. As a result, our interpretations of efferent neural drive and central fatigue were made after a collective assessment of these parameters independently that separately resulted in similar outcomes. These findings that exhibited unique differences between LLBFR and LL may not translate to differences among chronic neural adaptations and may not be evident during longer-duration studies. Furthermore, in the present study the neuromuscular and performance measures were evaluated during isolated muscle actions (evoked and/or voluntary isometric muscle actions) that do not reflect the nature of the fatiguing exercise (isotonic). Although it is difficult to examine these neural parameters during dynamic muscle actions, future investigations may consider utilizing isokinetic pretest and posttest strength measures that may exhibit increased applicability and may limit the variability associated with the application of these stimulation procedures during dynamic muscle actions. Together, this may provide greater insight regarding the magnitude of central and peripheral fatigue as it relates to LLBFR and LL resistance exercise.
Summary
The application of LLBFR elicited larger changes in PTT, sEMG amplitude, and VA relative to LL but similar changes in MVIC torque, PTT:MVIC, and V wave/M wave. The concomitant decreases in VA and V wave/M wave for both conditions and decreases in sEMG amplitude for LLBFR suggested that the decreases in MVIC torque and increases in PTT:MVIC were due, in part, to a reduction in efferent neural drive. The similar MVIC torque responses between conditions may have been a function of training load that has been dissociated from MVIC-specific torque responses. The decreases in PTT associated with LLBFR may reflect a more robust buildup of metabolites. The reductions in efferent neural drive associated with LLBFR and LL could be attributed to the activation of group III/IV afferent nerve endings resulting from the fatigue-induced buildup of metabolites. Future studies remain warranted to determine the chronic effects of LLBFR and LL resistance exercise on neural adaptations as well as in clinical and special populations such as midlife adults and women.
GRANTS
E.C.H. is supported by a research grant from the NASA Planetary Science Division, under the Solar System Exploration Research Virtual Institute Cooperative Agreement (NNH16ZDA001N) program titled “Radiation Effects on Volatiles and Exploration of Asteroids and Lunar Surfaces (REVEALS)” related to crew safety and human space exploration.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
E.C.H. conceived and designed research; E.C.H., P.M.R., C.E.P., D.H.G.R., and A.M.W. performed experiments; E.C.H. analyzed data; E.C.H. and J.L.K. interpreted results of experiments; E.C.H. prepared figures; E.C.H. drafted manuscript; E.C.H., P.M.R., C.E.P., D.H.G.R., A.M.W., and J.L.K. edited and revised manuscript; E.C.H., P.M.R., C.E.P., D.H.G.R., A.M.W., and J.L.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the participants for participation throughout the duration of the study.
Figure 1 and Graphical Abstract created with BioRender and published with permission.
REFERENCES
- 1.Shimizu R, Hotta K, Yamamoto S, Matsumoto T, Kamiya K, Kato M, Hamazaki N, Kamekawa D, Akiyama A, Kamada Y, Tanaka S, Masuda T. Low-intensity resistance training with blood flow restriction improves vascular endothelial function and peripheral blood circulation in healthy elderly people. Eur J Appl Physiol 116: 749–757, 2016. doi: 10.1007/s00421-016-3328-8. [DOI] [PubMed] [Google Scholar]
- 2.Kambič T, Novaković M, Tomažin K, Strojnik V, Jug B. Blood flow restriction resistance exercise improves muscle strength and hemodynamics, but not vascular function in coronary artery disease patients: a pilot randomized controlled trial. Front Physiol 10: 656, 2019. doi: 10.3389/fphys.2019.00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angelopoulos P, Mylonas K, Tsigkas G, Tsepis E, Billis E, Fousekis K. Blood flow restriction training in cardiovascular disease patients. In: Contemporary Advances in Sports Science, edited by Taiar R. London, UK: IntechOpen, 2021, p. 21–34. [Google Scholar]
- 4.Saatmann N, Zaharia OP, Loenneke JP, Roden M, Pesta DH. Effects of blood flow restriction exercise and possible applications in type 2 diabetes. Trends Endocrinol Metab 32: 106–117, 2021. doi: 10.1016/j.tem.2020.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Lixandrão ME, Ugrinowitsch C, Berton R, Vechin FC, Conceição MS, Damas F, Libardi CA, Roschel H. Magnitude of muscle strength and mass adaptations between high-load resistance training versus low-load resistance training associated with blood-flow restriction: a systematic review and meta-analysis. Sports Med 48: 361–378, 2018. doi: 10.1007/s40279-017-0795-y. [DOI] [PubMed] [Google Scholar]
- 6.Centner C, Wiegel P, Gollhofer A, König D. Effects of blood flow restriction training on muscular strength and hypertrophy in older individuals: a systematic review and meta-analysis. Sports Med 49: 95–108, 2019. [Erratum in Sports Med 49: 109–111, 2019]. doi: 10.1007/s40279-018-0994-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buckner SL, Jessee MB, Mattocks KT, Mouser JG, Counts BR, Dankel SJ, Loenneke JP. Determining strength: a case for multiple methods of measurement. Sports Med 47: 193–195, 2017. doi: 10.1007/s40279-016-0580-3. [DOI] [PubMed] [Google Scholar]
- 8.Robbins DW, Goodale TL, Docherty D, Behm DG, Tran QT. The effects of load and training pattern on acute neuromuscular responses in the upper body. J Strength Cond Res 24: 2996–3007, 2010. doi: 10.1519/JSC.0b013e3181f67474. [DOI] [PubMed] [Google Scholar]
- 9.Jenkins ND, Housh TJ, Buckner SL, Bergstrom HC, Cochrane KC, Hill EC, Smith CM, Schmidt RJ, Johnson GO, Cramer JT. Neuromuscular adaptations after 2 and 4 weeks of 80% versus 30% 1 repetition maximum resistance training to failure. J Strength Cond Res 30: 2174–2185, 2016. doi: 10.1519/JSC.0000000000001308. [DOI] [PubMed] [Google Scholar]
- 10.Jenkins ND, Miramonti AA, Hill EC, Smith CM, Cochrane-Snyman KC, Housh TJ, Cramer JT. Greater neural adaptations following high- vs. low-load resistance training. Front Physiol 8: 331, 2017. doi: 10.3389/fphys.2017.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sousa J, Neto GR, Santos HH, Araújo JP, Silva HG, Cirilo-Sousa MS. Effects of strength training with blood flow restriction on torque, muscle activation and local muscular endurance in healthy subjects. Biol Sport 34: 83–90, 2017. doi: 10.5114/biolsport.2017.63738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manimmanakorn A, Manimmanakorn N, Taylor R, Draper N, Billaut F, Shearman JP, Hamlin MJ. Effects of resistance training combined with vascular occlusion or hypoxia on neuromuscular function in athletes. Eur J Appl Physiol 113: 1767–1774, 2013. doi: 10.1007/s00421-013-2605-z. [DOI] [PubMed] [Google Scholar]
- 13.Cook SB, Scott BR, Hayes KL, Murphy BG. Neuromuscular adaptations to low-load blood flow restricted resistance training. J Sports Sci Med 17: 66–73, 2018. [PMC free article] [PubMed] [Google Scholar]
- 14.Centner C, Lauber B. A systematic review and meta-analysis on neural adaptations following blood flow restriction training: what we know and what we don’t know. Front Physiol 11: 887, 2020. doi: 10.3389/fphys.2020.00887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill EC, Housh TJ, Keller JL, Smith CM, Schmidt RJ, Johnson GO. Early phase adaptations in muscle strength and hypertrophy as a result of low-intensity blood flow restriction resistance training. Eur J Appl Physiol 118: 1831–1843, 2018. doi: 10.1007/s00421-018-3918-8. [DOI] [PubMed] [Google Scholar]
- 16.Moore DR, Burgomaster KA, Schofield LM, Gibala MJ, Sale DG, Phillips SM. Neuromuscular adaptations in human muscle following low intensity resistance training with vascular occlusion. Eur J Appl Physiol 92: 399–406, 2004. doi: 10.1007/s00421-004-1072-y. [DOI] [PubMed] [Google Scholar]
- 17.Fatela P, Mendonca GV, Veloso AP, Avela J, Mil-Homens P. Blood flow restriction alters motor unit behavior during resistance exercise. Int J Sports Med 40: 555–562, 2019. doi: 10.1055/a-0888-8816. [DOI] [PubMed] [Google Scholar]
- 18.Colomer-Poveda D, Romero-Arenas S, Vera-Ibáñez A, Viñuela-García M, Márquez G. Effects of 4 weeks of low-load unilateral resistance training, with and without blood flow restriction, on strength, thickness, V wave, and H reflex of the soleus muscle in men. Eur J Appl Physiol 117: 1339–1347, 2017. doi: 10.1007/s00421-017-3622-0. [DOI] [PubMed] [Google Scholar]
- 19.Hill EC, Housh TJ, Keller JL, Smith CM, Anders JV, Schmidt RJ, Johnson GO, Cramer JT. Low-load blood flow restriction elicits greater concentric strength than non-blood flow restriction resistance training but similar isometric strength and muscle size. Eur J Appl Physiol 120: 425–441, 2020. doi: 10.1007/s00421-019-04287-3. [DOI] [PubMed] [Google Scholar]
- 20.de Castro FMP, Alves GF, Oliveira LP, Tourinho Filho H, Puggina EF. Strength training with intermittent blood flow restriction improved strength without changes in neural aspects on quadriceps muscle. Sci Sports 34: e175–e185, 2019. doi: 10.1016/j.scispo.2018.10.012. [DOI] [Google Scholar]
- 21.Arabadzhiev TI, Dimitrov VG, Dimitrov GV. The increase in surface EMG could be a misleading measure of neural adaptation during the early gains in strength. Eur J Appl Physiol 114: 1645–1655, 2014. doi: 10.1007/s00421-014-2893-y. [DOI] [PubMed] [Google Scholar]
- 22.Vigotsky AD, Halperin I, Lehman GJ, Trajano GS, Vieira TM. Interpreting signal amplitudes in surface electromyography studies in sport and rehabilitation sciences. Front Physiol 8: 985, 2017. doi: 10.3389/fpls.2017.00985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siddique U, Rahman S, Frazer AK, Pearce AJ, Howatson G, Kidgell DJ. Determining the sites of neural adaptations to resistance training: a systematic review and meta-analysis. Sports Med 50: 1107–1128, 2020. doi: 10.1007/s40279-020-01258-z. [DOI] [PubMed] [Google Scholar]
- 24.Trezise J, Collier N, Blazevich AJ. Anatomical and neuromuscular variables strongly predict maximum knee extension torque in healthy men. Eur J Appl Physiol 116: 1159–1177, 2016. doi: 10.1007/s00421-016-3352-8. [DOI] [PubMed] [Google Scholar]
- 25.de Queiros VS, de França IM, Trybulski R, Vieira JG, Dos Santos IK, Neto GR, Wilk M, de Matos DG, Vieira WHB, Novaes JD, Makar P, Cabral BG, Dantas PM. Myoelectric activity and fatigue in low-load resistance exercise with different pressure of blood flow restriction: a systematic review and meta-analysis. Front Physiol 12: 786752, 2021. doi: 10.3389/fphys.2021.786752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKay AK, Stellingwerff T, Smith ES, Martin DT, Mujika I, Goosey-Tolfrey VL, Sheppard J, Burke LM. Defining training and performance caliber: a participant classification framework. Int J Sports Physiol Perform 17: 317–331, 2022. doi: 10.1123/ijspp.2021-0451. [DOI] [PubMed] [Google Scholar]
- 27.Haff G, Triplett NT; National Strength & Conditioning Association (U.S.) (Editors). Essentials of Strength Training and Conditioning (4th ed.). Champaign, IL: Human Kinetics, 2016. [Google Scholar]
- 28.Reis JF, Fatela P, Mendonca GV, Vaz JR, Valamatos MJ, Infante J, Mil-Homens P, Alves FB. Tissue oxygenation in response to different relative levels of blood-flow restricted exercise. Front Physiol 10: 407, 2019. doi: 10.3389/fphys.2019.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allen GM, Gandevia SC, McKenzie DK. Reliability of measurements of muscle strength and voluntary activation using twitch interpolation. Muscle Nerve 18: 593–600, 1995. doi: 10.1002/mus.880180605. [DOI] [PubMed] [Google Scholar]
- 30.Behm DG, St-Pierre DM, Perez D. Muscle inactivation: assessment of interpolated twitch technique. J Appl Physiol (1985) 81: 2267–2273, 1996. doi: 10.1152/jappl.1996.81.5.2267. [DOI] [PubMed] [Google Scholar]
- 31.Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol 10: 361–374, 2000. doi: 10.1016/S1050-6411(00)00027-4. [DOI] [PubMed] [Google Scholar]
- 32.Fukunaga T, Ichinose Y, Ito M, Kawakami Y, Fukashiro S. Determination of fascicle length and pennation in a contracting human muscle in vivo. J Appl Physiol (1985) 82: 354–358, 1997. doi: 10.1152/jappl.1997.82.1.354. [DOI] [PubMed] [Google Scholar]
- 33.Yasuda T, Brechue WF, Fujita T, Sato Y, Abe T. Muscle activation during low-intensity muscle contractions with varying levels of external limb compression. J Sports Sci Med 7: 467–474, 2008. [PMC free article] [PubMed] [Google Scholar]
- 34.Cook SB, Murphy BG, Labarbera KE. Neuromuscular function after a bout of low-load blood flow-restricted exercise. Med Sci Sports Exerc 45: 67–74, 2013. doi: 10.1249/MSS.0b013e31826c6fa8. [DOI] [PubMed] [Google Scholar]
- 35.Loenneke JP, Wilson JM, Marín PJ, Zourdos MC, Bemben MG. Low intensity blood flow restriction training: a meta-analysis. Eur J Appl Physiol 112: 1849–1859, 2012. doi: 10.1007/s00421-011-2167-x. [DOI] [PubMed] [Google Scholar]
- 36.Desmedt JE, Hainaut K. Kinetics of myofilament activation in potentiated contraction: staircase phenomenon in human skeletal muscle. Nature 217: 529–532, 1968. doi: 10.1038/217529a0. [DOI] [PubMed] [Google Scholar]
- 37.Pensini M, Martin A, Maffiuletti NA. Central versus peripheral adaptations following eccentric resistance training. Int J Sports Med 23: 567–574, 2002. doi: 10.1055/s-2002-35558. [DOI] [PubMed] [Google Scholar]
- 38.Duchateau J, Hainaut K. Training effects of sub-maximal electrostimulation in a human muscle. Med Sci Sports Exerc 20: 99–104, 1988. doi: 10.1249/00005768-198802000-00015. [DOI] [PubMed] [Google Scholar]
- 39.Farina D, Fosci M, Merletti R. Motor unit recruitment strategies investigated by surface EMG variables. J Appl Physiol (1985) 92: 235–247, 2002. doi: 10.1152/jappl.2002.92.1.235. [DOI] [PubMed] [Google Scholar]
- 40.Farina D, Merletti R, Enoka RM. The extraction of neural strategies from the surface EMG: an update. J Appl Physiol (1985) 117: 1215–1230, 2014. doi: 10.1152/japplphysiol.00162.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gandevia SC, Allen GM, Butler JE, Taylor JL. Supraspinal factors in human muscle fatigue: evidence for suboptimal output from the motor cortex. J Physiol 490: 529–536, 1996. doi: 10.1113/jphysiol.1996.sp021164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Folland JP, Williams AG. The adaptations to strength training: morphological and neurological contributions to increased strength. Sports Med 37: 145–168, 2007. doi: 10.2165/00007256-200737020-00004. [DOI] [PubMed] [Google Scholar]
- 43.Dotan R, Woods S, Contessa P. On the reliability and validity of central fatigue determination. Eur J Appl Physiol 121: 2393–2411, 2021. doi: 10.1007/s00421-021-04700-w. [DOI] [PubMed] [Google Scholar]
- 44.Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P. Neural adaptation to resistance training: changes in evoked V-wave and H-reflex responses. J Appl Physiol (1985) 92: 2309–2318, 2002. doi: 10.1152/japplphysiol.01185.2001. [DOI] [PubMed] [Google Scholar]
- 45.Mendonca GV, Vila-Chã C, Teodósio C, Goncalves AD, Freitas SR, Mil-Homens P, Pezarat-Correia P. Contralateral training effects of low-intensity blood-flow restricted and high-intensity unilateral resistance training. Eur J Appl Physiol 121: 2305–2321, 2021. doi: 10.1007/s00421-021-04708-2. [DOI] [PubMed] [Google Scholar]
- 46.Miyamoto N, Kanehisa H, Fukunaga T, Kawakami Y. Effect of postactivation potentiation on the maximal voluntary isokinetic concentric torque in humans. J Strength Cond Res 25: 186–192, 2011. doi: 10.1519/JSC.0b013e3181b62c1d. [DOI] [PubMed] [Google Scholar]
- 47.Kennedy DS, McNeil CJ, Gandevia SC, Taylor JL. Fatigue-related firing of distal muscle nociceptors reduces voluntary activation of proximal muscles of the same limb. J Appl Physiol 116: 385–394, 2014. doi: 10.1152/japplphysiol.01166.2013. [DOI] [PubMed] [Google Scholar]
- 48.Hureau TJ, Romer LM, Amann M. The ‘sensory tolerance limit’: a hypothetical construct determining exercise performance? Eur J Sport Sci 18: 13–24, 2018. doi: 10.1080/17461391.2016.1252428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Norbury R, Smith SA, Burnley M, Judge M, Mauger AR. The effect of elevated muscle pain on neuromuscular fatigue during exercise. Eur J Appl Physiol 122: 113–126, 2022. doi: 10.1007/s00421-021-04814-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amann M, Wan HY, Thurston TS, Georgescu VP, Weavil JC. On the influence of group III/IV muscle afferent feedback on endurance exercise performance. Exerc Sport Sci Rev 48: 209–216, 2020. doi: 10.1249/JES.0000000000000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suga T, Okita K, Morita N, Yokota T, Hirabayashi K, Horiuchi M, Takada S, Omokawa M, Kinugawa S, Tsutsui H. Dose effect on intramuscular metabolic stress during low-intensity resistance exercise with blood flow restriction. J Appl Physiol (1985) 108: 1563–1567, 2010. doi: 10.1152/japplphysiol.00504.2009. [DOI] [PubMed] [Google Scholar]
- 52.Loenneke JP, Fahs CA, Wilson JM, Bemben MG. Blood flow restriction: the metabolite/volume threshold theory. Med Hypotheses 77: 748–752, 2011. doi: 10.1016/j.mehy.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 53.Suga T, Dora K, Mok E, Sugimoto T, Tomoo K, Takada S, Hashimoto T, Isaka T. Exercise adherence-related perceptual responses to low-load blood flow restriction resistance exercise in young adults: a pilot study. Physiol Rep 9: e15122, 2021. doi: 10.14814/phy2.15122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Counts BR, Dankel SJ, Barnett BE, Kim D, Mouser JG, Allen KM, Thiebaud RS, Abe T, Bemben MG, Loenneke JP. Influence of relative blood flow restriction pressure on muscle activation and muscle adaptation. Muscle Nerve 53: 438–445, 2016. doi: 10.1002/mus.24756. [DOI] [PubMed] [Google Scholar]
- 55.Loenneke JP, Kim D, Fahs CA, Thiebaud RS, Abe T, Larson RD, Bemben DA, Bemben MG. The influence of exercise load with and without different levels of blood flow restriction on acute changes in muscle thickness and lactate. Clin Physiol Funct Imaging 37: 734–740, 2017. doi: 10.1111/cpf.12367. [DOI] [PubMed] [Google Scholar]
- 56.Brandner CR, Warmington SA. Delayed onset muscle soreness and perceived exertion after blood flow restriction exercise. J Strength Cond Res 31: 3101–3108, 2017. doi: 10.1519/JSC.0000000000001779. [DOI] [PubMed] [Google Scholar]
- 57.Lixandrão ME, Roschel H, Ugrinowitsch C, Miquelini M, Alvarez IF, Libardi CA. Blood-flow restriction resistance exercise promotes lower pain and ratings of perceived exertion compared with either high- or low-intensity resistance exercise performed to muscular failure. J Sport Rehabil 28: 706–710, 2019. doi: 10.1123/jsr.2018-0030. [DOI] [PubMed] [Google Scholar]
- 58.Hill EC, Housh TJ, Smith CM, Keller JL, Schmidt RJ, Johnson GO. Eccentric and concentric blood flow restriction resistance training on indices of delayed onset muscle soreness in untrained women. Eur J Appl Physiol 119: 2363–2373, 2019. doi: 10.1007/s00421-019-04220-8. [DOI] [PubMed] [Google Scholar]
- 59.Freitas ED, Miller RM, Heishman AD, Aniceto RR, Larson R, Pereira HM, Bemben D, Bemben MG. The perceptual responses of individuals with multiple sclerosis to blood flow restriction versus traditional resistance exercise. Physiol Behav 229: 113219, 2021. doi: 10.1016/j.physbeh.2020.113219. [DOI] [PubMed] [Google Scholar]
- 60.Silva JC, Domingos-Gomes JR, Freitas ED, Neto GR, Aniceto RR, Bemben MG, Lima-Dos-Santos A, Cirilo-Sousa MS. Physiological and perceptual responses to aerobic exercise with and without blood flow restriction. J Strength Cond Res 35: 2479–2485, 2021. doi: 10.1519/JSC.0000000000003178. [DOI] [PubMed] [Google Scholar]
- 61.Keenan KG, Farina D, Merletti R, Enoka RM. Amplitude cancellation reduces the size of motor unit potentials averaged from the surface EMG. J Appl Physiol 100: 1928–1937, 2006. doi: 10.1152/japplphysiol.01282.2005. [DOI] [PubMed] [Google Scholar]
- 62.Barbero M, Merletti R, Rainoldi A. Atlas of Muscle Innervation Zones: Understanding Surface Electromyography and Its Applications. Milan: Springer, 2012. [Google Scholar]