Abstract
The extracellular matrix (ECM) is an active and dynamic feature of tissues that not only provides gross structure but also plays key roles in cellular responses. The ever-changing microenvironment responds dynamically to cellular and external signals, and in turn influences cell fate, tissue development, and response to environmental injury or microbial invasion. It is therefore paramount to understand how the ECM components interact with each other, the environment and cells, and how they mediate their effects. Among the ECM components that have recently garnered increased attention, proteoglycans (PGs) deserve special note. Recent evidence strongly suggests that they play a crucial role both in health maintenance and disease development. In particular, proteoglycans dictate whether homeostasis or cell death will result from a given injury, by triggering and modulating activation of the innate immune system, via a conserved array of receptors that recognize exogenous (infectious) or endogenous (tissue damage) molecular patterns. Innate immune activation by proteoglycans has important implications for the understanding of cell-matrix interactions in health and disease. In this review, we will summarize the current state of knowledge of innate immune signaling by proteoglycans, discuss the implications, and explore future directions to define progress in this area of extracellular matrix biology.
Keywords: innate immunity, proteoglycans, Toll-like receptors
INTRODUCTION
The extracellular matrix (ECM) is an active and dynamic feature of tissues that not only provides gross structure but also plays key roles in cellular responses. The ever-changing microenvironment responds dynamically to cellular and external signals, and in turn influences cell fate, tissue development, and response to environmental injury or microbial invasion. It is therefore paramount to understand how the ECM components interact with each other, the environment and cells, and how they mediate their effects.
Among the ECM components that have recently garnered increased attention, proteoglycans (PGs) deserve special note. Recent evidence strongly suggests that they play a crucial role both in health maintenance and disease development (1–3). In particular, proteoglycans dictate whether homeostasis or cell death will result from a given injury, by triggering and modulating activation of the innate immune system (3–5), via a conserved array of receptors that recognize exogenous (infectious) or endogenous (tissue damage) molecular patterns. Innate immune activation by proteoglycans has important implications for the understanding of cell-matrix interactions in health and disease. In this review, we will summarize the current state of knowledge of innate immune signaling by proteoglycans, discuss the implications, and explore future directions to define progress in this area of extracellular matrix biology.
PROTEOGLYCANS
Proteoglycans (PGs) form a diverse family of molecules distinguished by their composition: they consist of a protein core (proteo-) bound to large glycosaminoglycan (GAG) chains (-glycan), whereby the glycan component contributes to up to 90% of the molecular weight (6). This structure distinguishes PGs from glycoproteins (short oligosaccharide chains attached to proteins) and GAGs (polysaccharides without a protein component), and also informs their function as central mediators of matrix signaling, cell fate, and tissue or organ development. PGs can be classified based on their location, predominant GAG, and the structure of protein modules utilized (1). The largest classes of PGs are cell membrane-bound (e.g., betaglycan and glypican), pericellular (e.g., perlecan and agrin), and particularly extracellular (e.g., aggrecan, versican, neurocan, and biglycan). Among the extracellular PGs, the largest family is the small, leucine-rich PGs (SLRPs). To date, there are five described SLRP classes based on their protein core structure, three canonical and two noncanonical (lacking glycosaminoglycan chains, and therefore not further discussed here). As their name indicates, SLRPs are characterized by a relatively small protein core of 36–42 kDa that contains numerous leucine-rich repeats. SLRPs are ubiquitously expressed and play a central role in the regulation of organ development and response to injury (1–5, 7) via binding to other ECM molecules (e.g., collagen), cytokines, growth factors, and receptors (8). More recently, there is accumulating evidence that some PGs, and in particular SLRPs, can interact with the innate immune system via Toll-like receptor activation.
TOLL-LIKE RECEPTORS AND INNATE IMMUNITY
Unlike adaptive immunity, innate immunity is an evolutionarily conserved, first-line response system that does not require prior education for its activation. Specific sensors of the innate immune system recognize and respond to endogenous and exogenous stimuli. These sensors consist of several pattern recognition receptors (PRRs) that recognize specific molecular patterns. The prototypical PPRs are the Toll-like receptors (TLRs). At this time, 11 TLRs have been identified in humans (9) and 12 in mice (10), each recognizing either pathogen-associated (PAMPs) or endogenous or “danger-associated” molecular patterns (DAMPs) (11, 12). PPRs are restricted to specific cellular compartments. Thus, TLR1, 2, 4, 5, 6, and 11 are located on the cell surface, whereas TLR3, 7, 8, 9, and 10 are in endosomes. Cell surface TLRs recognize bacterial components and extracellular DAMPs, whereas endosomal TLRs recognize single- and double-stranded RNA and CpG-DNA or intracellular DAMPs (13, 14). TLR signaling depends on the specific cell type in which it is activated but generally utilizes two principal pathways, by activating either the myeloid differentiation gene 88 (MyD88) (15) or the TIR-domain-containing adaptor-inducing interferon β (TRIF) adaptor proteins (16). TLRs always form homo- or heterodimers to engage their ligands, resulting in coordinate dimerization of the cytosolic TIR domains that are then detected by two adaptor proteins, the TIR domain-containing adapter protein (TIRAP) and the TIRAP-inducing IFN-β (TRIF)-related adaptor molecule (TRAM). TIRAP then recruits MyD88, whereas TRAM recruits TRIF. These divergent signaling pathways result in the activation of different downstream kinases and gene expression profiles. The MyD88-dependent pathway induces the activation of nuclear factor κ B (NF-κB), which induces the expression of inflammatory cytokines, whereas the TRIF-dependent pathway induces the expression of type I IFN (17). However, TLR signaling is now understood to play a role in cell fate determination, organ patterning, and development as well (18). Thus, TLR activation serves many functions, depending on context (e.g., concomitant growth factor exposure) and cell type activated (e.g., mature immune cells vs. embryonic mesenchymal or endodermal cells). Furthermore, although TLR activation has been most widely evaluated for its proinflammatory effects, it can also have potent anti-inflammatory and prosurvival effects, e.g., via induction of IL-10 and IL-6, respectively (19, 20). This has important implications for the activity and role of PGs in TLR signaling. Most TLRs activate MyD88, whereas TRIF signaling is activated by TLR3 (21), and by TLR4 when it is located in the endosome (22).
Intracellular PRRs, such as the nucleotide oligomerization domain (NOD)-like receptors form part of the inflammasome pathway (23) and principally identify pathogens that have accessed the cell and are subverting cellular functions to support replication (24–28). The combination of these PPRs allows for a diverse innate immune response to a wide variety of inflammatory signals.
Innate immune responses are mediated by the activation of a broad group of myeloid and lymphoid cells, including neutrophils, macrophages, and the more recently described innate lymphoid cells (29). These cells actively respond to changes in the local tissue microenvironment, such as the release of PGs, a response that must be carefully finetuned to avoid chronic inflammation and aberrant repair. A part of this response repertoire is the “maturation” of cells into different subtypes: for example, macrophages can be activated by Th1 cytokines or microbial molecular patterns to become “classically activated” and promote inflammation, or by Th2 cytokines (e.g., IL-4 and IL-13) to become “alternatively activated” and promote repair. In reality, a spectrum of responses can be identified and is likely influenced by the relative preponderance of specific triggers and receptor ligands. In aggregate, plasticity in the signaling pathways and cellular interactions dictates a large gamut of responses to extracellular matrix perturbations and is responsible for sometimes contradictory findings in experimental reports, as will be discussed below.
PRINCIPLES OF PG INTERACTION WITH TLRs
Although TLRs recognize specific molecular patterns, the specific interactions of specific TLRs with their ligands are not always clear or even direct. For example, lipopolysaccharide (LPS) is the cognate ligand for TLR4, yet does not bind to TLR4 itself; instead, it is bound to a complex of TLR4 with MD2 (30). In another example from the GAG family, hyaluronan (HA) and heparan sulfate fragments have been widely shown to activate TLR2 and TLR4 (31–35) but no physical interaction has ever been demonstrated. TLR2 and TLR4 have been implicated the most in PG signaling. This is perhaps not surprising, as TLR2 recognizes peptidoglycans and TLR4 recognizes LPS (36, 37), both sugar-containing molecules that are best suited as molecular patterns. In this context, it should be highlighted upfront that any studies on activation of TLR by presumably sterile endogenous ligands must contend with the risk of confounding by ubiquitous bacterial or viral TLR contaminants (38); this was particularly problematic in “older” studies when purified preparations were not as readily available. The reader must always, therefore, exercise critical reading of the utilized methodology, and demand separate lines of evidence for a convincing report. Nevertheless, enough independent, high-quality studies on PG-TLR interactions have emerged to warrant the conclusion that PG-TLR interactions are indeed present.
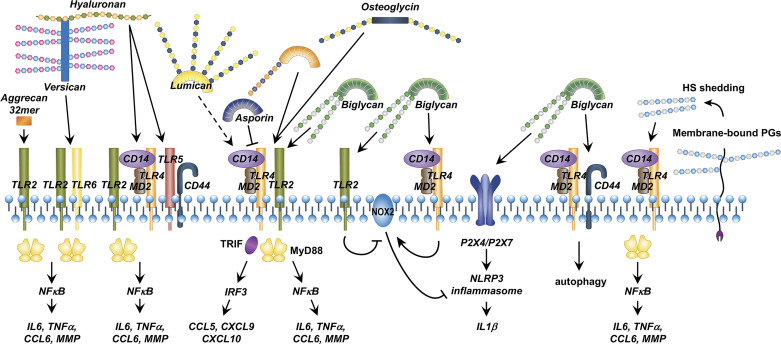
There are at least three potential mechanisms by which PGs interact with PRRs: direct interaction, interaction via their GAG side chains, and interaction via receptor complexes (Fig. 1). First, a wide array of intrinsic DAMPs, including proteins and GAGs, activate TLRs (39) and heterodimeric TLR complexes serve to expand the array of ligands that are directly recognized by TLRs (40, 41) to induce immune activation.
Figure 1.
Summary: Proteoglycan (PG) interactions with the innate immune system. At the cell membrane, PGs can engage Toll-like receptor (TLR) homo- or heterodimer complexes, or heteromeric complexes of TLRs with other receptors such as CD44. Glycosaminoglycan (GAG) chains can also activate TLRs, as can hyaluronan, which is often bound to PGs. Intracellularly, at least one PG (biglycan) can activate the NLRP3 inflammasome. MMP, matrix metalloprotease; TRIF, TIR-domain-containing adaptor-inducing interferon β.
Second, GAGs like heparan sulfate are recognized by innate immune receptors such as TLR4 (31, 42–44). It is therefore likely that PGs activate innate immunity via their GAG side chains, either directly or after shedding of these side chains during inflammation.
Third, TLRs could form complexes with other receptors like CD44 (34) and MARCO (45), which enable them to further expand the spectrum of potential agonists. Although this possibility may apply to PG-stimulated innate immune signaling, it remains to be definitively proven.
Beyond these established or putative interactions, PGs may also regulate TLR signaling indirectly by activating intracellular kinases and influencing inflammatory gene expression. Finally, at least one PG, biglycan, activates an intracellular innate immune component, the NLRP3 inflammasome. It is likely that other PG-innate immune interactions will emerge in the future. We will now explore known PG-innate immune interactions in more detail.
BIGLYCAN
Biglycan is one of the most studied PGs with regard to innate immune signaling. Biglycan was originally isolated from bone tissue but is expressed by many different immune, vascular, and mesenchymal cells, and derives its name from the presence of two GAG chains in the molecule (1, 46, 47). Genetically induced absence of biglycan results in connective tissue and vascular abnormalities, including corneal dystrophy. During inflammation or tissue injury, biglycan is released by proteolysis of the ECM or de novo synthesis by immune cells like macrophages and dendritic cells (48, 49). Biglycan is a strong agonist of TLR2 and TLR4 (49, 50), and leads to diverging or opposing effects depending on TLR engagement and cell type. Signaling of biglycan through TLR2 and TLR4 requires the entire molecule, including the GAG side chains (49) and absence of CD14 abolishes proinflammatory effects of biglycan downstream of TLR2 and TLR4 (51). Since CD14 is a coreceptor with TLR4 and TLR2 for lipopolysaccharide and lipoteichoic acid, respectively, interactions of the side chain with CD14 are crucial for biglycan signaling (52, 53). Conversely, biglycan activation of CD44-mediated TLR4-dependent macrophage autophagy in kidney injury models (54). Thus, by engaging alternate innate immune co-receptors (CD14 or CD44), biglycan can either activate inflammation and tissue damage or dampen inflammation and induce tissue repair, respectively. The factors that dictate coreceptor availability, engagement, and preference are still not well understood.
Soluble biglycan released either by generalized inflammation or local injury can accumulate in specific tissues such as the kidneys, causing recruitment of leukocytes via the chemokines CXCL1, CXCL2, CCL2, and CCL20 (downstream of TLR2/TLR4/MyD88 activation, recruiting neutrophils, macrophages, and Th17 lymphocytes) and CXCL9, CXCL10, and CCL5 (downstream of TLR4/TRIF activation, recruiting macrophages, Th1 and Th17 lymphocytes) (50, 55, 56). It is currently unclear whether circulating biglycan preferentially homes into certain tissues above others.
In macrophages, biglycan also regulates the elaboration of reactive oxygen species by modulating the expression, activity, and stability of NADPH oxidase (NOX)2 via TLR2 and TLR4. Biglycan-mediated TLR2 activation stimulated the expression of heat shock protein 70, which bound to NOX2 and impaired the inhibitory function of NOX2 on IL1β, thus being proinflammatory. However, biglycan also inhibited IL1β via TLR4 activation by promoting NOX2 synthesis through a TRIF-mediated mechanism, and activation through a MyD88-dependent mechanism, thereby being anti-inflammatory (57). Thus, biglycan can either promote or inhibit reactive oxygen species and inflammation depending on the balance of TLR2 or TLR4 expression and activity.
Beyond its direct TLR activating capacity, biglycan can further modulate innate immune responses by interacting with lipid signaling pathways, notably, sphingosine kinase, which regulates the activation of sphingolipids, i.e., highly conserved, ubiquitous lipids that mediate cell responses to inflammation, proliferation, autophagy, and death. The interaction of biglycan with lipid signaling is also evident in mesenchymal (heart valvular) cells, where soluble biglycan exposure induces ICAM-1 and MCP-1 expression via TLR4 while TLR2 and ERK1/2 pathway activation by biglycan lead to an inflammatory response (58) as well as production of phospholipid transfer protein (59). Furthermore, biglycan induced the expression of bone morphogenetic protein-2 (BMP2) and alkaline phosphatase via TLR2 and ERK-1/2 activation, and potentiated oxidized LDL effects on BMP2 and alkaline phosphatase expression (60). Thus, biglycan may be involved in the pathobiology of valvular disease by influencing inflammatory and metabolic responses. Effects of biglycan have been reported in other mesenchymal cells as well. For example, in human chondrocytes, biglycan induces the expression of IL1β, IL-6, matrix metalloprotease (MMP)-13, and IL-17 via TLR4/MyD88/NFκB activation (61).
Biglycan activation of TLR2 and TLR4 in endothelial cells results in increased hypoxia-inducible factor (HIF)-1α mRNA, leading to VEGF expression, migration, proliferation, and vascular tube formation (62). This may suggest a role for biglycan both in tissue recovery after injury and in pathological processes like tumor angiogenesis.
In liver and kidney epithelia, biglycan activation of TLR2 stabilizes HIF-2α, which in turn induces synthesis of erythropoietin. Consequently, mice overexpressing soluble biglycan demonstrate polycythemia and increased iron-binding capacity (63), which can lead to ischemic phenomena in human patients (64), suggesting a potential role of biglycan in polycythemia-induced complications.
Finally, biglycan can also interact with the intracellular inflammasome pathway. Inflammasome activation normally requires priming through TLRs (for expression of pro-IL1β) and subsequent activation of the inflammasome and caspase 1 that cleaves pro-IL1β and pro-IL18 into their mature, active forms. Biglycan is able to fulfill both roles, via interaction with TLR2, TLR4, and the purinergic receptors P2X4 and P2X7 (65).
In conclusion, biglycan is emerging as a central mediator of both inflammatory and repair mechanisms via an intricate signaling loop involving TLRs, coreceptors like CD14 and CD44, and interactions with other factors such as lipids and other ECM molecules. Further research will be needed to shed light to the full mechanistic spectrum of biglycan innate immune signaling as well as potential therapeutic applications (66).
AGGRECAN
Aggrecan, an extracellular PG, derives its name from its tendency to aggregate into large complexes with hyaluronan and link protein (1). Aggrecan, also called a hyalectan because it can bind hyaluronan and lectins in the ECM (67), is the principal load-bearing PG of cartilage and thus its signaling effects have been primarily investigated in articular tissue and cells. Genetic aggrecan deficiency in humans and/or animal models leads to musculoskeletal developmental deficiencies (67, 68) and more recently described cardiovascular defects (69). Aggrecan fragments are released during inflammation and tissue damage by the action of the enzymes A-disintegrin-and-metalloproteinase-with-thrombospondin motif (ADAMTS)4 or ADAMTS5 and matrix metalloproteases (MMPs) (70, 71). A 32-mer aggrecan fragment activates TLR2 in synovial cells, chondrocytes, and macrophages, and induces CCL2, IL-6, and further MMP expression (70, 72), leading to further cartilage destruction, inflammation, and increased pain sensitivity (70, 72). Interestingly, in synovial cells, a synthetic 32-mer aggrecan fragment did not activate TLR2 (71), suggesting that other cofactors may be necessary for TLR activation by aggrecan fragments.
LUMICAN
Lumican is a ubiquitously expressed SLRP that derives its name from its role in regulating corneal transparency (1). Lumican is widely distributed in interstitial connective tissue and its deficiency leads to disorganized collagen organization, cutis laxa, and corneal opacification (73–75). As we described with biglycan and aggrecan, lumican is also elevated in osteoarthritis cartilage and synovial fluid and promotes inflammation via activation of TLR4 (76). Along similar mechanistic lines, lumican potentiated TLR4 activation and exacerbated inflammation and kidney injury in a murine model of LPS-stimulated systemic inflammation (77). Lumican binds LPS as well as CD14 and may therefore be able to shuttle LPS to TLR4 more efficiently (78).
Lumican is also elevated in systemic inflammation, for example, in the serum of patients with sepsis (79). In a mouse model of polymicrobial sepsis (via cecal ligation and puncture), lumican was secreted from activated omental fibroblasts and acted in a paracrine fashion to promote bacterial clearance via TLR4 activation in macrophages. Lumican enriches TLR4 at the cell membrane by attaching to caveolin 1 in lipid rafts and to the TLR4 coreceptor CD14 via two epitopes, which become exposed after detachment of lumican from the collagen matrix (79). Lumican colocalizes with TLR4 and promotes MyD88-mediated signaling both at the cell membrane and in the endosome (79) promoting a type I interferon response, suggesting TRIF-mediated signaling. However, lumican also inhibits TLR9 activation in macrophages and dendritic cells, possibly by sequestering intracellular CpG. Interestingly, biglycan also binds CpG DNA and suppresses TLR9 responses (79) suggesting similar mechanisms of action by soluble, as opposed to ECM-bound, PGs. Interestingly, even though lumican promotes inflammation, it also promotes epithelial homeostasis via innate immune activation (80). The same group reported that lumican promotes bacterial clearance in a murine bacterial keratitis model by promoting macrophage influx via CXCL1 expression (81). Thus, lumican induction of innate immunity appears to be a “double-edged” sword: it can act as a prohomeostatic and antibacterial danger signal in acute infection, but in chronic inflammation it may potentiate tissue destruction.
VERSICAN
Versican, a large ECM hyalectan PG, derives its name from its versatile functions (1). Versican is expressed in many tissues, both transiently (e.g., during development) and stably, and plays a crucial role in tissue morphogenesis and development of diverse organs such as the heart, joints, and the skin (67, 82). Growing evidence suggests that versican is important in the innate immune response to lung infection. Versican is induced by activation of the TRIF signaling pathway (downstream of either TLR3 or TLR4), through IFNα and IFNβ (83). It also plays a significant role in the regulation of the immune response to injury and strongly modulates TLR activation (either positively or negatively, depending on context) (83, 84). As seen with other PGs, there may be divergent effects of the full-length versican molecule and its degradation products, such as versikine (85). This review will only address its direct activity as an innate immune agonist.
Because increased versican expression is found in many human cancers (86), most of the TLR-activating effects of versican have been studied in the context of cancer and appear to be acting in a paracrine fashion on tumor-associated immune cells. Thus, versican activation of TLR2 induces expression of the matrix metalloprotease MT1-MMP in glioma-associated microglia, which promotes cancer cell invasiveness (87). In lung and ovarian cancer, versican acts as a paracrine activator of macrophages via the formation of TLR2/TLR6/CD14 complexes and inducing TNFα secretion. The result of this activation is the promotion of tumor progression and metastatic growth by tumor-associated macrophages (88, 89). In tumor-associated dendritic cells, cancer-derived versican engages and activates TLR2, and leads to a dysfunctional state (less ability to activate alloreactive lymphocytes), thus potentially contributing to immune escape by cancer cells (90). On the other hand, versikine, a versican degradation product containing the N-terminal domain of the V1 versican isoform, generated by proteolysis at the Glu441 position (91), may activate TLR2 and the interferon-IRF8 signaling pathway and thus activate antigen-presenting cells and promote immunosurveillance of cancer cells (85). Thus, versican metabolism may regulate the immune response to cancer through a shift from full-length to degradation products induced by the cancer microenvironment.
DECORIN
Decorin gained its name because it binds to and “decorates” collagen fibrils in the extracellular matrix (1). It is ubiquitously expressed in the mesenchyme and its deficiency leads to aberrant structure in skin, eye, and tendon tissues (92, 93). Decorin is a SLRP that is a ligand for receptor tyrosine kinases, including epidermal growth factor receptor (EGFR) (1), an endogenous inhibitor of TGFβ1, and a tumor suppressor (94, 95). However, recently, soluble decorin was shown to be an endogenous ligand of TLR2 and TLR4, and thus influence the response to infection, inflammation, and cancer progression (96). Decorin was elevated in the plasma of patients with sepsis, mouse models of sepsis, and promoted TLR4-induced inflammation. This effect was due to direct signaling of decorin through TLR2 and TLR4 that upregulated the expression of the tumor suppressor gene programmed-cell-death-4 (PDCD4). In a cancer model, this proinflammatory effect of decorin resulted in reduced tumor size (96).
OSTEOGLYCIN
Osteoglycin, which, along with lumican, is the only keratan sulfate PG in the group of TLR-activating PGs discussed in this review, was originally derived from bone, hence its name (1), but more recent reports implicate it in myocardial integrity and remodeling after injury. A genomic study found a strong correlation of osteoglycin abundance with left ventricular mass in humans and identified osteoglycin as a positive regulator of left ventricular cardiac mass in a rat model (97). Mechanistically, a 72 kDa variant of osteoglycin has been isolated in human and murine viral myocarditis tissue. This variant directly binds to TLR4, potentially via their respective leucine-rich repeats, and activates TLR4/MyD88 signaling (98). This promotes inflammation both in a systemic LPS sepsis model, as well as in a viral myocarditis model (98). Thus, osteoglycin expression triggered by infectious or sterile injury of the myocardial tissue may trigger innate immune pathways that lead to pathological remodeling and ultimately decreased cardiac function.
ASPORIN
Asporin is an atypical SLRP in that it may not have a GAG sidechain. It derives its name from its aspartate-enriched N-terminal region and relative sequence homology to decorin, and it is often found along with decorin in skeletal and cartilage tissue (1). However, contrary to decorin, asporin inhibits TLR2 and TLR4 signaling in a model of periodontal disease (99). Even though there was evidence of direct binding of asporin to TLR2 and TLR4 in this study, it is unclear how the inhibition took place. The authors proposed an inhibition of TLR interaction with ligands as a potential mechanism for this effect (99).
SERGLYCIN AND BIKUNIN
Serglycin and bikunin are not known to have direct interaction with TLRs, but they modulate TLR signaling and thus indirectly impact this pathway.
Serglycin is the only intracellular PG described to date. It is stored in cytoplasmic granules and modulates the activity of inflammatory mediators, chemokines, cytokines, and growth factors. However, serglycin can also bind to the prototypic hyaluronan receptor CD44 (100) and promotes the activation of NF-κB and downstream cytokine expression after LPS (101) or IL1β exposure (102) in human chondrocytes, suggesting that it is, at least indirectly, involved in innate immune signaling. Interestingly, in endothelial cells, serglycin promotes cytokine expression only after IL1β but not LPS exposure (103) suggesting some cell-specificity in serglycin signaling pathways.
Bikunin is a circulating PG that contains two tandem Kunitz-type protease inhibitory domains that give it its name. It is most often found in association with inter α trypsin inhibitor protein in the plasma and extracellular matrix (104–106). Bikunin inhibits LPS-induced increase of cytoplasmic free Ca2+ in immune and structural cells (107) and protects epithelia from injury after IL1β and TNFα exposure (108) through a Ca2+-inhibitory effect. This effect of Ca2+ inhibition was seen in several cell types (107, 109, 110) suggesting that this is likely a class effect on Ca2+ channels. Bikunin also inhibits the activation of neutrophils after LPS exposure, by reducing the activity of ERK1/2 and p38 (111).
INDIRECT PROTEOGLYCAN SIGNALING VIA TLRs: HYALURONAN AND HEPARAN SULFATE
Beyond the direct PG signaling through TLRs, indirect PG signaling modulating innate immunity via effects on the known TLR agonist glycosaminoglycans HA and heparan sulfate have been described. These interactions involve binding and shedding: the hyalectan PGs aggrecan, versican, neurocan, and brevican bind HA via their N-terminal domain (1), whereas heparan sulfate can be shed by membrane-bound PGs (like syndecan) during inflammation and activate TLRs (31, 43, 112–114).
Since there have been several outstanding reviews on hyaluronan-immune interactions recently (115–123), we will not describe these in detail. HA fragments are generated from the native ECM during injury via breakdown from reactive oxygen species (124, 125) and hyaluronidases (126–132) or de novo synthesis (133), and can engage cell surface receptors like CD44, RHAMM, HARE, LYVE1, layilin, and the innate immune receptors TLR2, TLR4, and TLR5 (118, 134–144). HA size determines receptor signaling, perhaps due to differential receptor clustering on the membrane (118, 145). Higher-molecular-weight HA possesses multivalent sites for CD44 binding, whereas oligosaccharides of HA have only one or two binding sites (146–148). High-molecular-weight HA is generally anti-inflammatory and antiangiogenic (120), whereas HA fragments are proinflammatory (149), proangiogenic, promote tumor progression (145, 150), and can either stimulate or inhibit inflammation depending on cell type and disease model (145, 151–155). HA fragments engage a receptor complex of CD44 and TLRs and induce inflammation (34, 156, 157). HA signals through TLR2 and TLR4 (118), and more recently, TLR5 has been found to participate in the TLR4 receptor signaling complex (138). In addition, a 6-mer form of HA and the TLR2-specific agonist Pam3Cys promoted chemotaxis of macrophages, effects that were inhibited in Rhamm−/− but not in Cd44−/− mice, suggesting that oligomeric forms of HA preferentially signal via RHAMM (158). In general, HA fragments induce TLR-mediated inflammation (118, 157, 159), whereas high-molecular-weight HA is inert or anti-inflammatory (156, 160) or prohomeostatic (19, 118).
Interestingly, a recent paper described the involvement of miRNAs in the inflammatory responses to short fragment HA. Treatment of chondrocytes with a 6-mer of HA resulted in upregulation of miR146a that then affected downstream signaling (161). Thus, treatment with a miR146a mimic decreased HA6-stimulated TLR4 and NF-κB expression, whereas an inhibitor of miR146a increased the expression of these proinflammatory molecules (161). In addition, also in chondrocytes, the expression of miR9 was increased by HA6 treatment that subsequently inhibited NF-κB activity (162). These data suggest that HA fragments also induce protective mechanisms to limit inflammatory responses to endogenous danger signals.
Hyalectans (aggrecan, versican, neurocan, and brevican) attach themselves to HA and can form giant ECM complexes. Thus, it is highly likely that hyalectan binding to, or release from HA affects the conformation and availability of HA to engage TLR. Thus, indirectly, hyalectans may promote or inhibit TLR signaling by influencing the ability of HA to engage with the innate immune system.
Heparan sulfate is the dominant GAG in several PGs (1). Notably, shedding of heparan sulfate is seen in endothelial and epithelial cells after injury and can act as a proinflammatory stimulus that impedes homeostasis (113, 163–165). Heparan sulfate is a potent activator of TLR4 (31, 43, 112–114, 163) suggesting that PGs may either indirectly mediate TLR activation by the release of this GAG sidechain, or perhaps activate innate immunity by engagement of heparan sulfate with TLR4 in situ.
PG-INNATE IMMUNE INTERACTIONS IN HUMAN DISEASE AND THE PROMISE OF THERAPEUTIC INTERVENTIONS
Given the importance of cell-matrix interactions in the pathogenesis of inflammation and disease, it is perhaps not surprising that a clear picture that PGs play a central role in human disease and can be potential treatment targets is emerging.
It is perhaps expected that PGs will be released in a wide variety of inflammatory and tissue injury states, and as such can be nonspecific, but sensitive, biomarkers of inflammation and disease activity. Increased circulating biglycan concentrations have been found in chronic obstructive pulmonary disease (COPD), cancer, obesity, metabolic syndrome, autoimmune diseases, and kidney and liver diseases (2, 46, 50, 66). In systemic sclerosis, the expression of versican, decorin, and biglycan was associated with skin fibrosis (166). Serum biglycan was also associated with hepatic fibrosis in chronic infectious hepatitis (167, 168). Circulating biglycan was also elevated in several autoimmune liver diseases such as primary sclerosing cholangitis, primary biliary cholangitis, and autoimmune hepatitis (169). Circulating decorin was elevated in patients with sepsis (96). In sum, these reports underscore an important role for biglycan, and probably other PGs, in mediating matrix-immunity interactions and tissue remodeling in human disease.
Biglycan and other PGs (especially decorin) play prominent roles in kidney disease. Circulating biglycan concentrations correlate with inflammatory chemokine (CXCL9/10) concentrations in human lupus nephritis (55), which mechanistically promotes inflammatory cell influx into the diseased kidney via its TLR activating effects (55). Biglycan, along with decorin, lumican, and fibromodulin, were elevated in all stages of human diabetic nephropathy (170). Furthermore, non-TLR-mediated roles of PGs in renal disease have been discussed in outstanding review articles (2, 171).
Biglycan is increased in degenerative aortic valves in human patients in a stage-dependent manner (172). Beyond signaling induction, in human tissues, decorin and biglycan can attach LDL particles to collagen-rich, elastin-deficient extracellular matrix, thus promoting valvular degeneration (173). This interaction with LDL and oxidized LDL (60) underscores the potential role of PGs in pathogenesis of atherosclerosis and cardiovascular disease, particularly since atherogenic exposures such as cigarette smoke are associated with increased serum biglycan concentrations (174). However, biglycan is not strictly detrimental in human vascular disease: for example, adventitial biglycan in the aorta promotes structural integrity in the mouse (175) and genetic biglycan deficiency is associated with earlier-onset thoracic aortic aneurysms and dissections in patients with Marfan syndrome (176). This may reflect a dichotomy between structurally bound and soluble biglycan in terms of their respective functions.
The yin-yang association of biglycan and decorin is also evident in musculoskeletal disease. For example, high biglycan and low decorin expression were observed in patients with Kashin–Beck disease, a degenerative osteoarthropathy (177). An adverse effect of biglycan in arthritis may be linked to its activation of the TLR4/MyD88/NF-κB pathway (61). On the other hand, tethered biglycan is crucial for structural integrity of the connective tissue, and a missense mutation in the biglycan gene results in syndesmoepimetaphysial dysplasia (178), a condition associated with short stature and osteoarthritis.
PGs also play a role in cancer (7) where biglycan expression is epigenetically induced in tumor endothelia and appears to promote metastatic potential by activation of TLR2, TLR4, and ERK1/2 (179). Biglycan expression is inversely associated with cancer-free survival while, in contrast, decorin expression reduces metastatic potential and improves survival (180). However, the role of biglycan in cancer is not entirely resolved, as there are reports of biglycan expression correlating with improved survival in patients with bladder cancer and diffuse B-cell lymphoma, an effect, which may be partly induced by increased immune surveillance via local TLR activation (7). Interestingly, lumican is associated with poor prognosis in gastric and colon cancer but favorable prognosis in pancreatic adenocarcinoma (180), suggesting perhaps differential effects dependent on local cell signaling. Finally, versican appears to be associated with ovarian cancer and breast cancer and correlates with invasiveness and cancer progression (7).
A number of peptides have been developed that interfere with HA-receptor interactions, thereby inhibiting downstream activation of the innate immune system. The two original studies used different approaches to generate peptides, but both blocked inflammatory responses (181, 182), and further optimization of peptides has recently been reviewed (183, 184). Similarly, 4-methylumbelliferone (4MU), a blocker of HA synthesis has also been used to abrogate innate immune responses (185, 186), and also LPS-mediated inflammation (187), whereas hyaluronidase treatment appears to improve outcomes in viral (influenza) pneumonia (188). Collectively, these reports establish the importance of HA in innate immune signaling for inflammation and potential therapeutic strategies to control the adverse effects of these pathways.
Heparanase, an endoglycosidase that degrades heparan sulfate, has been implicated in the promotion of tumor growth, angiogenesis, and metastasis. A number of strategies, including heparin-like inhibitors of heparinase, neutralizing monoclonal antibodies, and small molecule inhibitors of this enzyme are being tested for their efficacy in various types of cancer (189).
KNOWLEDGE GAPS, OPPORTUNITIES, AND FUTURE DIRECTIONS
The study of PGs in the context of immune activation is exciting, impactful, and promising in terms of potential therapeutic implications and applications. Nevertheless, much remains to be elucidated. There may be unrecognized class effects of PG interactions with the innate immune system that can be uncovered: for example, effects mediated by similarities in the GAG side chains, or by interactions of the SLRP leucine-rich repeat domains with TLRs. It is notable that most proteoglycans that interact with PRRs are chondroitin sulfate proteoglycans. This raises the possibility that chondroitin sulfate or its fragments contribute to TLR signaling, as some emerging data suggest (190, 191). Structural studies will further help to shed light on these potential interactions. The interplay of PGs with other ECM molecules, such as HA, is also of significant interest. The ECM is an extraordinarily dynamic space, and constant turnover is observed in homeostasis and during the response to injury. Thus, it is likely that interactions of different PGs with other ECM molecules, and the combination of PG effects on immune cells, may ultimately dictate the effects in a context and tissue-specific manner. Thus, enrichment of a PG in diseased tissue, the unmasking of previously cryptic epitopes by partial degradation, and the presence or absence of coreceptors may influence the immune effects of PG molecules. In this context, it is important to remind the reader of the observation that circulating and ECM-bound PGs have often very different functions, i.e., proinflammatory versus prohomeostatic. Thus, shedding of PGs during the injury process (as happens for example with syndecan-1 and syndecan-4) may contribute to both the development and resolution of inflammation, depending on context (cofactors, target cells, and engagement of receptors) (192). The mechanisms of action, potential engagement of innate immune receptors, and pro- or anti-inflammatory pathways activated by these shed PGs are obvious current gaps in our knowledge. Ultimately, the development of specific agonists or antagonists may enable clinicians to translate these scientific insights into therapeutics. Given that PGs have profound effects on many human diseases, the potential for novel insights into the mechanisms of disease and the development of effective and targeted therapeutics is tremendous.
GRANTS
S. Garantziotis is supported by grants from the Division of Intramural Research, National Institute of Environmental Health Sciences (NIEHS) (ES102605, ES102465, and ES103342). R. C. Savani holds the William Buchanan Chair in Pediatrics at the University of Texas (UT) Southwestern Medical Center. R.C. Savani has a Sponsored Research Agreement with Mallinckrodt Pharmaceuticals that is independent of this work.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.C.S. conceived and designed research; S.G. prepared figures; S.G. and R.C.S. drafted manuscript; S.G. and R.C.S. edited and revised manuscript; S.G. and R.C.S. approved final version of manuscript.
ACKNOWLEDGMENTS
This article is part of the special collection “Deciphering the Role of Proteoglycans and Glycosaminoglycans in Health and Disease.” Dr. Liliana Schaefer served as Guest Editor of this collection.
REFERENCES
- 1.Iozzo RV, Schaefer L. Proteoglycan form and function: a comprehensive nomenclature of proteoglycans. Matrix Biol 42: 11–55, 2015. doi: 10.1016/j.matbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schaefer L. Small leucine-rich proteoglycans in kidney disease. J Am Soc Nephrol 22: 1200–1207, 2011. doi: 10.1681/ASN.2010050570. [DOI] [PubMed] [Google Scholar]
- 3.Schaefer L, Tredup C, Gubbiotti MA, Iozzo RV. Proteoglycan neofunctions: regulation of inflammation and autophagy in cancer biology. FEBS J 284: 10–26, 2017. doi: 10.1111/febs.13963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frey H, Schroeder N, Manon-Jensen T, Iozzo RV, Schaefer L. Biological interplay between proteoglycans and their innate immune receptors in inflammation. FEBS J 280: 2165–2179, 2013. doi: 10.1111/febs.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaefer L. Complexity of danger: the diverse nature of damage-associated molecular patterns. J Biol Chem 289: 35237–35245, 2014. doi: 10.1074/jbc.R114.619304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alberts BJ, Lewis J, Raff M, Roberts K, Walter P. The extracellular matrix of animals. In: Molecular Biology of the Cell (4th ed.). New York: Garland Science, 2002. [Google Scholar]
- 7.Roedig H, Damiescu R, Zeng-Brouwers J, Kutija I, Trebicka J, Wygrecka M, Schaefer L. Danger matrix molecules orchestrate CD14/CD44 signaling in cancer development. Semin Cancer Biol 62: 31–47, 2020. doi: 10.1016/j.semcancer.2019.07.026. [DOI] [PubMed] [Google Scholar]
- 8.Bryant CE, Gay NJ, Heymans S, Sacre S, Schaefer L, Midwood KS. Advances in Toll-like receptor biology: modes of activation by diverse stimuli. Crit Rev Biochem Mol Biol 50: 359–379, 2015. doi: 10.3109/10409238.2015.1033511. [DOI] [PubMed] [Google Scholar]
- 9.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 140: 805–820, 2010. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol 5: 461, 2014. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brubaker SW, Bonham KS, Zanoni I, Kagan JC. Innate immune pattern recognition: a cell biological perspective. Annu Rev Immunol 33: 257–290, 2015. doi: 10.1146/annurev-immunol-032414-112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L, Aderem A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between Toll-like receptors. Proc Natl Acad Sci USA 97: 13766–13771, 2000. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413: 732–738, 2001. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 14.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature 408: 740–745, 2000[Erratum inNature409: 646, 2001]. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 15.Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway CA Jr.. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell 2: 253–258, 1998. doi: 10.1016/S1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- 16.Hoebe K, Du X, Georgel P, Janssen E, Tabeta K, Kim SO, Goode J, Lin P, Mann N, Mudd S, Crozat K, Sovath S, Han J, Beutler B. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature 424: 743–748, 2003. doi: 10.1038/nature01889. [DOI] [PubMed] [Google Scholar]
- 17.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell 124: 783–801, 2006. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Anthoney N, Foldi I, Hidalgo A. Toll and Toll-like receptor signalling in development. Development 145: dev156018, 2018. doi: 10.1242/dev.156018. [DOI] [PubMed] [Google Scholar]
- 19.Liang J, Zhang Y, Xie T, Liu N, Chen H, Geng Y, Kurkciyan A, Mena JM, Stripp BR, Jiang D, Noble PW. Hyaluronan and TLR4 promote surfactant-protein-C-positive alveolar progenitor cell renewal and prevent severe pulmonary fibrosis in mice. Nat Med 22: 1285–1293, 2016. doi: 10.1038/nm.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanin DE, Prendergast CT, Mountford AP. IL-10 production in macrophages is regulated by a TLR-driven CREB-mediated mechanism that is linked to genes involved in cell metabolism. J Immunol 195: 1218–1232, 2015. doi: 10.4049/jimmunol.1500146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301: 640–643, 2003. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 22.Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-β. Nat Immunol 9: 361–368, 2008. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zelensky AN, Gready JE. The C-type lectin-like domain superfamily. FEBS J 272: 6179–6217, 2005. doi: 10.1111/j.1742-4658.2005.05031.x. [DOI] [PubMed] [Google Scholar]
- 24.Girardin SE, Boneca IG, Carneiro LA, Antignac A, Jehanno M, Viala J, Tedin K, Taha MK, Labigne A, Zahringer U, Coyle AJ, DiStefano PS, Bertin J, Sansonetti PJ, Philpott DJ. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science 300: 1584–1587, 2003. doi: 10.1126/science.1084677. [DOI] [PubMed] [Google Scholar]
- 25.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem 278: 8869–8872, 2003. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 26.Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 21: 677–687, 2015. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol 13: 397–411, 2013. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF 3. Cell 122: 669–682, 2005. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Gasteiger G, D'Osualdo A, Schubert DA, Weber A, Bruscia EM, Hartl D. Cellular innate immunity: an old game with new players. J Innate Immun 9: 111–125, 2017. doi: 10.1159/000453397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazgaeen L, Gurung P. Recent advances in lipopolysaccharide recognition systems. Int J Mol Sci 21: 379, 2020. doi: 10.3390/ijms21020379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson GB, Brunn GJ, Kodaira Y, Platt JL. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J Immunol 168: 5233–5239, 2002. doi: 10.4049/jimmunol.168.10.5233. [DOI] [PubMed] [Google Scholar]
- 32.Powell JD, Horton MR. Threat matrix: low-molecular-weight hyaluronan (HA) as a danger signal. Immunol Res 31: 207–218, 2005. doi: 10.1385/IR:31:3:207. [DOI] [PubMed] [Google Scholar]
- 33.Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, Horton MR. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol 177: 1272–1281, 2006. doi: 10.4049/jimmunol.177.2.1272. [DOI] [PubMed] [Google Scholar]
- 34.Taylor KR, Yamasaki K, Radek KA, Di Nardo A, Goodarzi H, Golenbock D, Beutler B, Gallo RL. Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on Toll-like receptor 4, CD44, and MD-2. J Biol Chem 282: 18265–18275, 2007. doi: 10.1074/jbc.M606352200. [DOI] [PubMed] [Google Scholar]
- 35.Tesar BM, Jiang D, Liang J, Palmer SM, Noble PW, Goldstein DR. The role of hyaluronan degradation products as innate alloimmune agonists. Am J Transplant 6: 2622–2635, 2006. doi: 10.1111/j.1600-6143.2006.01537.x. [DOI] [PubMed] [Google Scholar]
- 36.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol 2: 675–680, 2001. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 37.Cook DN, Hollingsworth JW Jr, Schwartz DA. Toll-like receptors and the genetics of innate immunity. Curr Opin Allergy Clin Immunol 3: 523–529, 2003. doi: 10.1097/00130832-200312000-00016. [DOI] [PubMed] [Google Scholar]
- 38.Lee-Sayer SS, Dong Y, Arif AA, Olsson M, Brown KL, Johnson P. The where, when, how, and why of hyaluronan binding by immune cells. Front Immunol 6: 150, 2015. doi: 10.3389/fimmu.2015.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piccinini AM, Midwood KS. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm 2010: 1–21, 2010. doi: 10.1155/2010/672395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farhat K, Riekenberg S, Heine H, Debarry J, Lang R, Mages J, Buwitt-Beckmann U, Roschmann K, Jung G, Wiesmuller KH, Ulmer AJ. Heterodimerization of TLR2 with TLR1 or TLR6 expands the ligand spectrum but does not lead to differential signaling. J Leukoc Biol 83: 692–701, 2008. doi: 10.1189/jlb.0807586. [DOI] [PubMed] [Google Scholar]
- 41.Jin MS, Lee JO. Structures of the toll-like receptor family and its ligand complexes. Immunity 29: 182–191, 2008. doi: 10.1016/j.immuni.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Akbarshahi H, Axelsson JB, Said K, Malmstrom A, Fischer H, Andersson R. TLR4 dependent heparan sulphate-induced pancreatic inflammatory response is IRF3-mediated. J Transl Med 9: 219, 2011. doi: 10.1186/1479-5876-9-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goodall KJ, Poon IK, Phipps S, Hulett MD. Soluble heparan sulfate fragments generated by heparanase trigger the release of pro-inflammatory cytokines through TLR-4. PloS one 9: e109596, 2014. doi: 10.1371/journal.pone.0109596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olivares-Silva F, Landaeta R, Aranguiz P, Bolivar S, Humeres C, Anfossi R, Vivar R, Boza P, Munoz C, Pardo-Jimenez V, Peiro C, Sanchez-Ferrer CF, Diaz-Araya G. Heparan sulfate potentiates leukocyte adhesion on cardiac fibroblast by enhancing Vcam-1 and Icam-1 expression. Biochim Biophys Acta Mol Basis Dis 1864: 831–842, 2018. doi: 10.1016/j.bbadis.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 45.Bowdish DM, Sakamoto K, Kim MJ, Kroos M, Mukhopadhyay S, Leifer CA, Tryggvason K, Gordon S, Russell DG. MARCO, TLR2, and CD14 are required for macrophage cytokine responses to mycobacterial trehalose dimycolate and Mycobacterium tuberculosis. PLoS Pathog 5: e1000474, 2009. doi: 10.1371/journal.ppat.1000474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Appunni S, Rubens M, Ramamoorthy V, Anand V, Khandelwal M, Sharma A. Biglycan: an emerging small leucine-rich proteoglycan (SLRP) marker and its clinicopathological significance. Mol Cell Biochem 476: 3935–3950, 2021. doi: 10.1007/s11010-021-04216-z. [DOI] [PubMed] [Google Scholar]
- 47.Diehl V, Huber LS, Trebicka J, Wygrecka M, Iozzo RV, Schaefer L. The role of decorin and biglycan signaling in tumorigenesis. Front Oncol 11: 801801, 2021. doi: 10.3389/fonc.2021.801801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hsieh LT, Nastase MV, Zeng-Brouwers J, Iozzo RV, Schaefer L. Soluble biglycan as a biomarker of inflammatory renal diseases. Int J Biochem Cell Biol 54: 223–235, 2014. doi: 10.1016/j.biocel.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schaefer L, Babelova A, Kiss E, Hausser HJ, Baliova M, Krzyzankova M, Marsche G, Young MF, Mihalik D, Gotte M, Malle E, Schaefer RM, Grone HJ. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest 115: 2223–2233, 2005. doi: 10.1172/JCI23755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moreth K, Frey H, Hubo M, Zeng-Brouwers J, Nastase MV, Hsieh LT, Haceni R, Pfeilschifter J, Iozzo RV, Schaefer L. Biglycan-triggered TLR-2- and TLR-4-signaling exacerbates the pathophysiology of ischemic acute kidney injury. Matrix Biol 35: 143–151, 2014. doi: 10.1016/j.matbio.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roedig H, Nastase MV, Frey H, Moreth K, Zeng-Brouwers J, Poluzzi C, Hsieh LT, Brandts C, Fulda S, Wygrecka M, Schaefer L. Biglycan is a new high-affinity ligand for CD14 in macrophages. Matrix Biol 77: 4–22, 2019. doi: 10.1016/j.matbio.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 52.Ranoa DRE, Kelley SL, Tapping RI. Human lipopolysaccharide-binding protein (LBP) and CD14 independently deliver triacylated lipoproteins to Toll-like receptor 1 (TLR1) and TLR2 and enhance formation of the ternary signaling complex. J Biol Chem 288: 9729–9741, 2013. doi: 10.1074/jbc.M113.453266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249: 1431–1433, 1990. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 54.Poluzzi C, Nastase MV, Zeng-Brouwers J, Roedig H, Hsieh LT, Michaelis JB, Buhl EM, Rezende F, Manavski Y, Bleich A, Boor P, Brandes RP, Pfeilschifter J, Stelzer EHK, Munch C, Dikic I, Brandts C, Iozzo RV, Wygrecka M, Schaefer L. Biglycan evokes autophagy in macrophages via a novel CD44/Toll-like receptor 4 signaling axis in ischemia/reperfusion injury. Kidney Int 95: 540–562, 2019. doi: 10.1016/j.kint.2018.10.037. [DOI] [PubMed] [Google Scholar]
- 55.Nastase MV, Zeng-Brouwers J, Beckmann J, Tredup C, Christen U, Radeke HH, Wygrecka M, Schaefer L. Biglycan, a novel trigger of Th1 and Th17 cell recruitment into the kidney. Matrix Biol 68-69: 293–317, 2018. doi: 10.1016/j.matbio.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 56.Zeng-Brouwers J, Beckmann J, Nastase MV, Iozzo RV, Schaefer L. De novo expression of circulating biglycan evokes an innate inflammatory tissue response via MyD88/TRIF pathways. Matrix Biol 35: 132–142, 2014. doi: 10.1016/j.matbio.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hsieh LT, Frey H, Nastase MV, Tredup C, Hoffmann A, Poluzzi C, Zeng-Brouwers J, Manon-Jensen T, Schroder K, Brandes RP, Iozzo RV, Schaefer L. Bimodal role of NADPH oxidases in the regulation of biglycan-triggered IL-1β synthesis. Matrix Biol 49: 61–81, 2016. doi: 10.1016/j.matbio.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song R, Ao L, Zhao KS, Zheng D, Venardos N, Fullerton DA, Meng X. Soluble biglycan induces the production of ICAM-1 and MCP-1 in human aortic valve interstitial cells through TLR2/4 and the ERK1/2 pathway. Inflamm Res 63: 703–710, 2014. doi: 10.1007/s00011-014-0743-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Derbali H, Bosse Y, Cote N, Pibarot P, Audet A, Pepin A, Arsenault B, Couture C, Despres JP, Mathieu P. Increased biglycan in aortic valve stenosis leads to the overexpression of phospholipid transfer protein via Toll-like receptor 2. Am J Pathol 176: 2638–2645, 2010. doi: 10.2353/ajpath.2010.090541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Song R, Zeng Q, Ao L, Yu JA, Cleveland JC, Zhao KS, Fullerton DA, Meng X. Biglycan induces the expression of osteogenic factors in human aortic valve interstitial cells via Toll-like receptor-2. Arterioscler Thromb Vasc Biol 32: 2711–2720, 2012. doi: 10.1161/ATVBAHA.112.300116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Avenoso A, D'Ascola A, Scuruchi M, Mandraffino G, Calatroni A, Saitta A, Campo S, Campo GM. The proteoglycan biglycan mediates inflammatory response by activating TLR-4 in human chondrocytes: inhibition by specific siRNA and high polymerized hyaluronan. Arch Biochem Biophys 640: 75–82, 2018. doi: 10.1016/j.abb.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 62.Hu L, Zang MD, Wang HX, Li JF, Su LP, Yan M, Li C, Yang QM, Liu BY, Zhu ZG. Biglycan stimulates VEGF expression in endothelial cells by activating the TLR signaling pathway. Mol Oncol 10: 1473–1484, 2016. doi: 10.1016/j.molonc.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Frey H, Moreth K, Hsieh LT, Zeng-Brouwers J, Rathkolb B, Fuchs H, Gailus-Durner V, Iozzo RV, de Angelis MH, Schaefer L. A novel biological function of soluble biglycan: induction of erythropoietin production and polycythemia. Glycoconj J 34: 393–404, 2017. doi: 10.1007/s10719-016-9722-y. [DOI] [PubMed] [Google Scholar]
- 64.Hart RG, Kanter MC. Hematologic disorders and ischemic stroke. A selective review. Stroke 21: 1111–1121, 1990. doi: 10.1161/01.str.21.8.1111. [DOI] [PubMed] [Google Scholar]
- 65.Babelova A, Moreth K, Tsalastra-Greul W, Zeng-Brouwers J, Eickelberg O, Young MF, Bruckner P, Pfeilschifter J, Schaefer RM, Grone HJ, Schaefer L. Biglycan, a danger signal that activates the NLRP3 inflammasome via toll-like and P2X receptors. J Biol Chem 284: 24035–24048, 2009. doi: 10.1074/jbc.M109.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roedig H, Nastase MV, Wygrecka M, Schaefer L. Breaking down chronic inflammatory diseases: the role of biglycan in promoting a switch between inflammation and autophagy. FEBS J 286: 2965–2979, 2019. doi: 10.1111/febs.14791. [DOI] [PubMed] [Google Scholar]
- 67.Watanabe H. Aggrecan and versican: two brothers close or apart. Am J Physiol Cell Physiol 322: C967–C976, 2022. doi: 10.1152/ajpcell.00081.2022. [DOI] [PubMed] [Google Scholar]
- 68.Ergun-Longmire B, Wajnrajch MP, Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, Dungan K, Hershman JM, Hofland J, Kalra S, Kaltsas G, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrere B, Levy M, McGee EA, McLachlan R, Morley JE, New M, Purnell J, Sahay R, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP (Editors). Growth and growth disorders. In: Endotext. South Dartmouth, MA: MDText.com, Inc., 2000. [Google Scholar]
- 69.Koch CD, Lee CM, Apte SS. Aggrecan in cardiovascular development and disease. J Histochem Cytochem 68: 777–795, 2020. doi: 10.1369/0022155420952902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miller RE, Ishihara S, Tran PB, Golub SB, Last K, Miller RJ, Fosang AJ, Malfait AM. An aggrecan fragment drives osteoarthritis pain through Toll-like receptor 2. JCI Insight 3: e95704, 2018. doi: 10.1172/jci.insight.95704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sharma N, Drobinski P, Kayed A, Chen Z, Kjelgaard-Petersen CF, Gantzel T, Karsdal MA, Michaelis M, Ladel C, Bay-Jensen AC, Lindemann S, Thudium CS. Inflammation and joint destruction may be linked to the generation of cartilage metabolites of ADAMTS-5 through activation of Toll-like receptors. Osteoarthritis Cartilage 28: 658–668, 2020. doi: 10.1016/j.joca.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 72.Lees S, Golub SB, Last K, Zeng W, Jackson DC, Sutton P, Fosang AJ. Bioactivity in an aggrecan 32-mer fragment is mediated via Toll-like receptor 2. Arthritis Rheumatol 67: 1240–1249, 2015. doi: 10.1002/art.39063. [DOI] [PubMed] [Google Scholar]
- 73.Chakravarti S, Magnuson T, Lass JH, Jepsen KJ, LaMantia C, Carroll H. Lumican regulates collagen fibril assembly: skin fragility and corneal opacity in the absence of lumican. J Cell Biol 141: 1277–1286, 1998. doi: 10.1083/jcb.141.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chakravarti S, Petroll WM, Hassell JR, Jester JV, Lass JH, Paul J, Birk DE. Corneal opacity in lumican-null mice: defects in collagen fibril structure and packing in the posterior stroma. Invest Ophthalmol Vis Sci 41: 3365–3373, 2000. [PMC free article] [PubMed] [Google Scholar]
- 75.Giatagana EM, Berdiaki A, Tsatsakis A, Tzanakakis GN, Nikitovic D. Lumican in carcinogenesis—revisited. Biomolecules 11: 1319, 2021. doi: 10.3390/biom11091319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barreto G, Senturk B, Colombo L, Bruck O, Neidenbach P, Salzmann G, Zenobi-Wong M, Rottmar M. Lumican is upregulated in osteoarthritis and contributes to TLR4-induced pro-inflammatory activation of cartilage degradation and macrophage polarization. Osteoarthritis Cartilage 28: 92–101, 2020. doi: 10.1016/j.joca.2019.10.011. [DOI] [PubMed] [Google Scholar]
- 77.Lu XM, Ma L, Jin YN, Yu YQ. Lumican overexpression exacerbates lipopolysaccharide-induced renal injury in mice. Mol Med Rep 12: 4089–4094, 2015. doi: 10.3892/mmr.2015.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu F, Vij N, Roberts L, Lopez-Briones S, Joyce S, Chakravarti S. A novel role of the lumican core protein in bacterial lipopolysaccharide-induced innate immune response. J Biol Chem 282: 26409–26417, 2007. doi: 10.1074/jbc.M702402200. [DOI] [PubMed] [Google Scholar]
- 79.Maiti G, Frikeche J, Lam CY, Biswas A, Shinde V, Samanovic M, Kagan JC, Mulligan MJ, Chakravarti S. Matrix lumican endocytosed by immune cells controls receptor ligand trafficking to promote TLR4 and restrict TLR9 in sepsis. Proc Natl Acad Sci USA 118: 2021. doi: 10.1073/pnas.2100999118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lohr K, Sardana H, Lee S, Wu F, Huso DL, Hamad AR, Chakravarti S. Extracellular matrix protein lumican regulates inflammation in a mouse model of colitis. Inflamm Bowel Dis 18: 143–151, 2012. doi: 10.1002/ibd.21713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shao H, Scott SG, Nakata C, Hamad AR, Chakravarti S. Extracellular matrix protein lumican promotes clearance and resolution of Pseudomonas aeruginosa keratitis in a mouse model. PloS one 8: e54765, 2013. doi: 10.1371/journal.pone.0054765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Islam S, Versican WH. A dynamic regulator of the extracellular matrix. J Histochem Cytochem 68: 763–775, 2020. doi: 10.1369/0022155420953922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chang MY, Kang I, Gale M Jr, Manicone AM, Kinsella MG, Braun KR, Wigmosta T, Parks WC, Altemeier WA, Wight TN, Frevert CW. Versican is produced by Trif- and type I interferon-dependent signaling in macrophages and contributes to fine control of innate immunity in lungs. Am J Physiol Lung Cell Mol Physiol 313: L1069–L1086, 2017. doi: 10.1152/ajplung.00353.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kang I, Harten IA, Chang MY, Braun KR, Sheih A, Nivison MP, Johnson PY, Workman G, Kaber G, Evanko SP, Chan CK, Merrilees MJ, Ziegler SF, Kinsella MG, Frevert CW, Wight TN. Versican deficiency significantly reduces lung inflammatory response induced by polyinosine-polycytidylic acid stimulation. J Biol Chem 292: 51–63, 2017. doi: 10.1074/jbc.M116.753186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hope C, Foulcer S, Jagodinsky J, Chen SX, Jensen JL, Patel S, Leith C, Maroulakou I, Callander N, Miyamoto S, Hematti P, Apte SS, Asimakopoulos F. Immunoregulatory roles of versican proteolysis in the myeloma microenvironment. Blood 128: 680–685, 2016. doi: 10.1182/blood-2016-03-705780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Isogai Z, Shinomura T, Yamakawa N, Takeuchi J, Tsuji T, Heinegard D, Kimata K. 2B1 antigen characteristically expressed on extracellular matrices of human malignant tumors is a large chondroitin sulfate proteoglycan, PG-M/versican. Cancer Res 56: 3902–3908, 1996. [PubMed] [Google Scholar]
- 87.Hu F, Dzaye O, Hahn A, Yu Y, Scavetta RJ, Dittmar G, Kaczmarek AK, Dunning KR, Ricciardelli C, Rinnenthal JL, Heppner FL, Lehnardt S, Synowitz M, Wolf SA, Kettenmann H. Glioma-derived versican promotes tumor expansion via glioma-associated microglial/macrophages Toll-like receptor 2 signaling. Neuro Oncol 17: 200–210, 2015. doi: 10.1093/neuonc/nou324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, Luo JL, Karin M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature 457: 102–106, 2009. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li D, Wang X, Wu JL, Quan WQ, Ma L, Yang F, Wu KY, Wan HY. Tumor-produced versican V1 enhances hCAP18/LL-37 expression in macrophages through activation of TLR2 and vitamin D3 signaling to promote ovarian cancer progression in vitro. PLoS One 8: e56616, 2013. doi: 10.1371/journal.pone.0056616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tang M, Diao J, Gu H, Khatri I, Zhao J, Cattral MS. Toll-like receptor 2 activation promotes tumor dendritic cell dysfunction by regulating IL-6 and IL-10 receptor signaling. Cell Rep 13: 2851–2864, 2015. doi: 10.1016/j.celrep.2015.11.053. [DOI] [PubMed] [Google Scholar]
- 91.Nandadasa S, Foulcer S, Apte SS. The multiple, complex roles of versican and its proteolytic turnover by ADAMTS proteases during embryogenesis. Matrix Biol 35: 34–41, 2014. doi: 10.1016/j.matbio.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen S, Birk DE. The regulatory roles of small leucine-rich proteoglycans in extracellular matrix assembly. FEBS J 280: 2120–2137, 2013. doi: 10.1111/febs.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reed CC, Iozzo RV. The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconj J 19: 249–255, 2002. doi: 10.1023/A:1025383913444. [DOI] [PubMed] [Google Scholar]
- 94.Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-β by the proteoglycan decorin. Nature 346: 281–284, 1990. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- 95.Yamaguchi Y, Ruoslahti E. Expression of human proteoglycan in Chinese hamster ovary cells inhibits cell proliferation. Nature 336: 244–246, 1988. doi: 10.1038/336244a0. [DOI] [PubMed] [Google Scholar]
- 96.Merline R, Moreth K, Beckmann J, Nastase MV, Zeng-Brouwers J, Tralhao JG, Lemarchand P, Pfeilschifter J, Schaefer RM, Iozzo RV, Schaefer L. Signaling by the matrix proteoglycan decorin controls inflammation and cancer through PDCD4 and MicroRNA-21. Sci Signal 4: ra75, 2011. doi: 10.1126/scisignal.2001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Petretto E, Sarwar R, Grieve I, Lu H, Kumaran MK, Muckett PJ, Mangion J, Schroen B, Benson M, Punjabi PP, Prasad SK, Pennell DJ, Kiesewetter C, Tasheva ES, Corpuz LM, Webb MD, Conrad GW, Kurtz TW, Kren V, Fischer J, Hubner N, Pinto YM, Pravenec M, Aitman TJ, Cook SA. Integrated genomic approaches implicate osteoglycin (Ogn) in the regulation of left ventricular mass. Nat Genet 40: 546–552, 2008. doi: 10.1038/ng.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rienks M, Papageorgiou A, Wouters K, Verhesen W, Leeuwen RV, Carai P, Summer G, Westermann D, Heymans S. A novel 72-kDa leukocyte-derived osteoglycin enhances the activation of toll-like receptor 4 and exacerbates cardiac inflammation during viral myocarditis. Cell Mol Life Sci 74: 1511–1525, 2017. doi: 10.1007/s00018-016-2423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yamaba S, Yamada S, Kajikawa T, Awata T, Sakashita H, Tsushima K, Fujihara C, Yanagita M, Murakami S. PLAP-1/asporin regulates TLR2- and TLR4-induced inflammatory responses. J Dent Res 94: 1706–1714, 2015. doi: 10.1177/0022034515606859. [DOI] [PubMed] [Google Scholar]
- 100.Toyama-Sorimachi N, Sorimachi H, Tobita Y, Kitamura F, Yagita H, Suzuki K, Miyasaka M. A novel ligand for CD44 is serglycin, a hematopoietic cell lineage-specific proteoglycan. Possible involvement in lymphoid cell adherence and activation. J Biol Chem 270: 7437–7444, 1995. doi: 10.1074/jbc.270.13.7437. [DOI] [PubMed] [Google Scholar]
- 101.D'Ascola A, Scuruchi M, Avenoso A, Bruschetta G, Campo S, Mandraffino G, Campo GM. Serglycin is involved in inflammatory response in articular mouse chondrocytes. Biochem Biophys Res Commun 499: 506–512, 2018. doi: 10.1016/j.bbrc.2018.03.178. [DOI] [PubMed] [Google Scholar]
- 102.Scuruchi M, D'Ascola A, Avenoso A, Mandraffino GG, Campo SS, Campo GM. Serglycin as part of IL-1β induced inflammation in human chondrocytes. Arch Biochem Biophys 669: 80–86, 2019. doi: 10.1016/j.abb.2019.05.021. [DOI] [PubMed] [Google Scholar]
- 103.Reine TM, Vuong TT, Jenssen TG, Kolset SO. Serglycin secretion is part of the inflammatory response in activated primary human endothelial cells in vitro. Biochim Biophys Acta 1840: 2498–2505, 2014. doi: 10.1016/j.bbagen.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 104.Salier JP, Rouet P, Raguenez G, Daveau M. The inter-α-inhibitor family: from structure to regulation. Biochem J 315: 1–9, 1996. doi: 10.1042/bj3150001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhuo L, Hascall VC, Kimata K. Inter-α-trypsin inhibitor, a covalent protein-glycosaminoglycan-protein complex. J Biol Chem 279: 38079–38082, 2004. doi: 10.1074/jbc.R300039200. [DOI] [PubMed] [Google Scholar]
- 106.Zhuo L, Kimata K. Structure and function of inter-α-trypsin inhibitor heavy chains. Connect Tissue Res 49: 311–320, 2008. doi: 10.1080/03008200802325458. [DOI] [PubMed] [Google Scholar]
- 107.Kanayama N, Maehara K, Suzuki M, Fujise Y, Terao T. The role of chondroitin sulfate chains of urinary trypsin inhibitor in inhibition of LPS-induced increase of cytosolic free Ca2+ in HL60 cells and HUVEC cells. Biochem Biophys Res Commun 238: 560–564, 1997. doi: 10.1006/bbrc.1997.7344. [DOI] [PubMed] [Google Scholar]
- 108.el Maradny E, Kanayama N, Halim A, Maehara K, Terao T. Urinary trypsin inhibitor has a protective effect on the amnion. Gynecol Obstet Invest 38: 169–172, 1994. doi: 10.1159/000292472. [DOI] [PubMed] [Google Scholar]
- 109.Kanayama N, el Maradny E, Halim A, Liping S, Maehara K, Kajiwara Y, Terao T. Urinary trypsin inhibitor prevents uterine muscle contraction by inhibition of Ca++ influx. Am J Obstet Gynecol 173: 192–199, 1995. doi: 10.1016/0002-9378(95)90189-2. [DOI] [PubMed] [Google Scholar]
- 110.Kanayama N, Maehara K, She L, Belayet HM, Khatun S, Tokunaga N, Terao T. Urinary trypsin inhibitor suppresses vascular smooth muscle contraction by inhibition of Ca2+ influx. Biochim Biophys Acta 1381: 139–146, 1998. doi: 10.1016/s0304-4165(98)00022-1. [DOI] [PubMed] [Google Scholar]
- 111.Kanayama S, Yamada Y, Onogi A, Shigetomi H, Ueda S, Tsuji Y, Haruta S, Kawaguchi R, Yoshida S, Sakata M, Sado T, Kitanaka T, Oi H, Yagyu T, Kobayashi H. Bikunin suppresses expression of pro-inflammatory cytokines induced by lipopolysaccharide in neutrophils. J Endotoxin Res 13: 369–376, 2007. doi: 10.1177/0968051907086464. [DOI] [PubMed] [Google Scholar]
- 112.Christianson HC, Belting M. Heparan sulfate proteoglycan as a cell-surface endocytosis receptor. Matrix Biol 35: 51–55, 2014. doi: 10.1016/j.matbio.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 113.Platt JL, Wrenshall LE, Johnson GB, Cascalho M. Heparan sulfate proteoglycan metabolism and the fate of grafted tissues. Adv Exp Med Biol 865: 123–140, 2015. doi: 10.1007/978-3-319-18603-0_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rangarajan S, Richter JR, Richter RP, Bandari SK, Tripathi K, Vlodavsky I, Sanderson RD. Heparanase-enhanced shedding of syndecan-1 and its role in driving disease pathogenesis and progression. J Histochem Cytochem 68: 823–840, 2020. doi: 10.1369/0022155420937087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Avenoso A, Bruschetta G, D'Ascola A, Scuruchi M, Mandraffino G, Saitta A, Campo S, Campo GM. Hyaluronan fragmentation during inflammatory pathologies: a signal that empowers tissue damage. Mini Rev Med Chem 20: 54–65, 2020. doi: 10.2174/1389557519666190906115619. [DOI] [PubMed] [Google Scholar]
- 116.Avenoso A, Bruschetta G, D'Ascola A, Scuruchi M, Mandraffino G, Gullace R, Saitta A, Campo S, Campo GM. Hyaluronan fragments produced during tissue injury: a signal amplifying the inflammatory response. Arch Biochem Biophys 663: 228–238, 2019. doi: 10.1016/j.abb.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 117.Garantziotis S, Savani RC. Hyaluronan biology: a complex balancing act of structure, function, location and context. Matrix Biol 78–79: 1–10, 2019. doi: 10.1016/j.matbio.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med 11: 1173–1179, 2005. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 119.Jiang D, Liang J, Li Y, Noble PW. The role of Toll-like receptors in non-infectious lung injury. Cell Res 16: 693–701, 2006. doi: 10.1038/sj.cr.7310085. [DOI] [PubMed] [Google Scholar]
- 120.Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human diseases. Physiol Rev 91: 221–264, 2011. doi: 10.1152/physrev.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol 23: 435–461, 2007. doi: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- 122.Liang J, Jiang D, Noble PW. Hyaluronan as a therapeutic target in human diseases. Adv Drug Deliv Rev 97: 186–203, 2016. doi: 10.1016/j.addr.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tighe RM, Garantziotis S. Hyaluronan interactions with innate immunity in lung biology. Matrix Biol 78–79: 84–99, 2019. doi: 10.1016/j.matbio.2018.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Monzon ME, Fregien N, Schmid N, Falcon NS, Campos M, Casalino-Matsuda SM, Forteza RM. Reactive oxygen species and hyaluronidase 2 regulate airway epithelial hyaluronan fragmentation. J Biol Chem 285: 26126–26134, 2010. doi: 10.1074/jbc.M110.135194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Soltes L, Mendichi R, Kogan G, Schiller J, Stankovska M, Arnhold J. Degradative action of reactive oxygen species on hyaluronan. Biomacromolecules 7: 659–668, 2006. doi: 10.1021/bm050867v. [DOI] [PubMed] [Google Scholar]
- 126.Csoka AB, Frost GI, Stern R. The six hyaluronidase-like genes in the human and mouse genomes. Matrix Biol 20: 499–508, 2001. doi: 10.1016/S0945-053X(01)00172-X. [DOI] [PubMed] [Google Scholar]
- 127.De Angelis JE, Lagendijk AK, Chen H, Tromp A, Bower NI, Tunny KA, Brooks AJ, Bakkers J, Francois M, Yap AS, Simons C, Wicking C, Hogan BM, Smith KA. Tmem2 regulates embryonic Vegf signaling by controlling hyaluronic acid turnover. Dev Cell 40: 123–136, 2017. doi: 10.1016/j.devcel.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 128.Stern R, Jedrzejas MJ. Hyaluronidases: their genomics, structures, and mechanisms of action. Chem Rev 106: 818–839, 2006. doi: 10.1021/cr050247k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Totong R, Schell T, Lescroart F, Ryckebusch L, Lin YF, Zygmunt T, Herwig L, Krudewig A, Gershoony D, Belting HG, Affolter M, Torres-Vazquez J, Yelon D. The novel transmembrane protein Tmem2 is essential for coordination of myocardial and endocardial morphogenesis. Development 138: 4199–4205, 2011. doi: 10.1242/dev.064261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yamaguchi Y, Yamamoto H, Tobisawa Y, Irie F. TMEM2: a missing link in hyaluronan catabolism identified? Matrix Biol 78–79: 139–146, 2019. doi: 10.1016/j.matbio.2018.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yamamoto H, Tobisawa Y, Inubushi T, Irie F, Ohyama C, Yamaguchi Y. A mammalian homolog of the zebrafish transmembrane protein 2 (TMEM2) is the long-sought-after cell-surface hyaluronidase. J Biol Chem 292: 7304–7313, 2017. doi: 10.1074/jbc.M116.770149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yoshida H, Nagaoka A, Kusaka-Kikushima A, Tobiishi M, Kawabata K, Sayo T, Sakai S, Sugiyama Y, Enomoto H, Okada Y, Inoue S. KIAA1199, a deafness gene of unknown function, is a new hyaluronan binding protein involved in hyaluronan depolymerization. Proc Natl Acad Sci USA 110: 5612–5617, 2013. doi: 10.1073/pnas.1215432110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Garantziotis S. Modulation of hyaluronan signaling as a therapeutic target in human disease. Pharmacol Ther 232: 107993, 2022. doi: 10.1016/j.pharmthera.2021.107993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol 144: 789–801, 1999. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bono P, Rubin K, Higgins JM, Hynes RO. Layilin, a novel integral membrane protein, is a hyaluronan receptor. Mol Biol Cell 12: 891–900, 2001. doi: 10.1091/mbc.12.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Forteza RM, Casalino-Matsuda SM, Falcon NS, Valencia Gattas M, Monzon ME. Hyaluronan and layilin mediate loss of airway epithelial barrier function induced by cigarette smoke by decreasing E-cadherin. J Biol Chem 287: 42288–42298, 2012. doi: 10.1074/jbc.M112.387795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hardwick C, Hoare K, Owens R, Hohn HP, Hook M, Moore D, Cripps V, Austen L, Nance DM, Turley EA. Molecular cloning of a novel hyaluronan receptor that mediates tumor cell motility. J Cell Biol 117: 1343–1350, 1992[Erratum inJ Cell Biol118: 753, 1992]. doi: 10.1083/jcb.117.6.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hussain S, Johnson CG, Sciurba J, Meng X, Stober VP, Liu C, Cyphert-Daly JM, Bulek K, Qian W, Solis A, Sakamachi Y, Trempus CS, Aloor JJ, Gowdy KM, Foster WM, Hollingsworth JW, Tighe RM, Li X, Fessler MB, Garantziotis S. TLR5 participates in the TLR4 receptor complex and promotes MyD88-dependent signaling in environmental lung injury. Elife 9: e50458, 2020. doi: 10.7554/eLife.50458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mouta Carreira C, Nasser SM, di Tomaso E, Padera TP, Boucher Y, Tomarev SI, Jain RK. LYVE-1 is not restricted to the lymph vessels: expression in normal liver blood sinusoids and down-regulation in human liver cancer and cirrhosis. Cancer Res 61: 8079–8084, 2001. [PubMed] [Google Scholar]
- 140.Prevo R, Banerji S, Ferguson DJ, Clasper S, Jackson DG. Mouse LYVE-1 is an endocytic receptor for hyaluronan in lymphatic endothelium. J Biol Chem 276: 19420–19430, 2001. doi: 10.1074/jbc.M011004200. [DOI] [PubMed] [Google Scholar]
- 141.Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon JC. Oligosaccharides of hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med 195: 99–111, 2002. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Weigel JA, Raymond RC, McGary C, Singh A, Weigel PH. A blocking antibody to the hyaluronan receptor for endocytosis (HARE) inhibits hyaluronan clearance by perfused liver. J Biol Chem 278: 9808–9812, 2003. doi: 10.1074/jbc.M211462200. [DOI] [PubMed] [Google Scholar]
- 143.Weigel JA, Raymond RC, Weigel PH. The hyaluronan receptor for endocytosis (HARE) is not CD44 or CD54 (ICAM-1). Biochem Biophys Res Commun 294: 918–922, 2002. doi: 10.1016/S0006-291X(02)00558-2. [DOI] [PubMed] [Google Scholar]
- 144.Zhou B, Weigel JA, Fauss L, Weigel PH. Identification of the hyaluronan receptor for endocytosis (HARE). J Biol Chem 275: 37733–37741, 2000. doi: 10.1074/jbc.M003030200. [DOI] [PubMed] [Google Scholar]
- 145.Toole BP, Ghatak S, Misra S. Hyaluronan oligosaccharides as a potential anticancer therapeutic. Curr Pharm Biotechnol 9: 249–252, 2008. doi: 10.2174/138920108785161569. [DOI] [PubMed] [Google Scholar]
- 146.Lesley J, Hascall VC, Tammi M, Hyman R. Hyaluronan binding by cell surface CD44. J Biol Chem 275: 26967–26975, 2000. doi: 10.1074/jbc.M002527200. [DOI] [PubMed] [Google Scholar]
- 147.Schmits R, Filmus J, Gerwin N, Senaldi G, Kiefer F, Kundig T, Wakeham A, Shahinian A, Catzavelos C, Rak J, Furlonger C, Zakarian A, Simard JJ, Ohashi PS, Paige CJ, Gutierrez-Ramos JC, Mak TW. CD44 regulates hematopoietic progenitor distribution, granuloma formation, and tumorigenicity. Blood 90: 2217–2233, 1997. [PubMed] [Google Scholar]
- 148.Wolny PM, Banerji S, Gounou C, Brisson AR, Day AJ, Jackson DG, Richter RP. Analysis of CD44-hyaluronan interactions in an artificial membrane system: insights into the distinct binding properties of high and low molecular weight hyaluronan. J Biol Chem 285: 30170–30180, 2010. doi: 10.1074/jbc.M110.137562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Horton MR, McKee CM, Bao C, Liao F, Farber JM, Hodge-DuFour J, Pure E, Oliver BL, Wright TM, Noble PW. Hyaluronan fragments synergize with interferon-gamma to induce the C-X-C chemokines mig and interferon-inducible protein-10 in mouse macrophages. J Biol Chem 273: 35088–35094, 1998. doi: 10.1074/jbc.273.52.35088. [DOI] [PubMed] [Google Scholar]
- 150.Toole BP, Zoltan-Jones A, Misra S, Ghatak S. Hyaluronan: a critical component of epithelial-mesenchymal and epithelial-carcinoma transitions. Cells Tissues Organs 179: 66–72, 2005. doi: 10.1159/000084510. [DOI] [PubMed] [Google Scholar]
- 151.Campo GM, Avenoso A, D'Ascola A, Prestipino V, Scuruchi M, Nastasi G, Calatroni A, Campo S. 4-mer hyaluronan oligosaccharides stimulate inflammation response in synovial fibroblasts in part via TAK-1 and in part via p38-MAPK. Curr Med Chem 20: 1162–1172, 2013. doi: 10.2174/0929867311320090005. [DOI] [PubMed] [Google Scholar]
- 152.Gao F, Yang CX, Mo W, Liu YW, He YQ. Hyaluronan oligosaccharides are potential stimulators to angiogenesis via RHAMM mediated signal pathway in wound healing. CIM 31: 106–116, 2008. doi: 10.25011/cim.v31i3.3467. [DOI] [PubMed] [Google Scholar]
- 153.Ghatak S, Misra S, Toole BP. Hyaluronan constitutively regulates ErbB2 phosphorylation and signaling complex formation in carcinoma cells. J Biol Chem 280: 8875–8883, 2005. doi: 10.1074/jbc.M410882200. [DOI] [PubMed] [Google Scholar]
- 154.Kim MY, Muto J, Gallo RL. Hyaluronic acid oligosaccharides suppress TLR3-dependent cytokine expression in a TLR4-dependent manner. PloS One 8: e72421, 2013. doi: 10.1371/journal.pone.0072421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Misra S, Toole BP, Ghatak S. Hyaluronan constitutively regulates activation of multiple receptor tyrosine kinases in epithelial and carcinoma cells. J Biol Chem 281: 34936–34941, 2006. doi: 10.1074/jbc.C600138200. [DOI] [PubMed] [Google Scholar]
- 156.Garantziotis S, Li Z, Potts EN, Kimata K, Zhuo L, Morgan DL, Savani RC, Noble PW, Foster WM, Schwartz DA, Hollingsworth JW. Hyaluronan mediates ozone-induced airway hyperresponsiveness in mice. J Biol Chem 284: 11309–11317, 2009[Erratum inJ Biol Chem291: 19257–19258, 2016]. doi: 10.1074/jbc.M802400200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 157.Garantziotis S, Li Z, Potts EN, Lindsey JY, Stober VP, Polosukhin VV, Blackwell TS, Schwartz DA, Foster WM, Hollingsworth JW. TLR4 is necessary for hyaluronan-mediated airway hyperresponsiveness after ozone inhalation. Am J Respir Crit Care Med 181: 666–675, 2010. doi: 10.1164/rccm.200903-0381OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 158.Foley JP, Lam D, Jiang H, Liao J, Cheong N, McDevitt TM, Zaman A, Wright JR, Savani RC. Toll-like receptor 2 (TLR2), transforming growth factor-β, hyaluronan (HA), and receptor for HA-mediated motility (RHAMM) are required for surfactant protein A-stimulated macrophage chemotaxis. J Biol Chem 287: 37406–37419, 2012. doi: 10.1074/jbc.M112.360982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Voelcker V, Gebhardt C, Averbeck M, Saalbach A, Wolf V, Weih F, Sleeman J, Anderegg U, Simon J. Hyaluronan fragments induce cytokine and metalloprotease upregulation in human melanoma cells in part by signalling via TLR4. Exp Dermatol 17: 100–107, 2008. doi: 10.1111/j.1600-0625.2007.00638.x. [DOI] [PubMed] [Google Scholar]
- 160.Lazrak A, Creighton J, Yu Z, Komarova S, Doran SF, Aggarwal S, Emala CW Sr, Stober VP, Trempus CS, Garantziotis S, Matalon S. Hyaluronan mediates airway hyperresponsiveness in oxidative lung injury. Am J Physiol Lung Cell Mol Physiol 308: L891–L903, 2015. doi: 10.1152/ajplung.00377.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Avenoso A, D'Ascola A, Scuruchi M, Mandraffino G, Campo S, Campo GM. miR146a up-regulation is involved in small HA oligosaccharides-induced pro-inflammatory response in human chondrocytes. Biochim Biophys Acta Gen Subj 1865: 129731, 2021. doi: 10.1016/j.bbagen.2020.129731. [DOI] [PubMed] [Google Scholar]
- 162.Scuruchi M, D'Ascola A, Avenoso A, Zappone A, Mandraffino G, Campo S, Campo GM. miR9 inhibits 6-mer HA-induced cytokine production and apoptosis in human chondrocytes by reducing NF-kB activation. Arch Biochem Biophys 718: 109139, 2022. doi: 10.1016/j.abb.2022.109139. [DOI] [PubMed] [Google Scholar]
- 163.Kliment CR, Tobolewski JM, Manni ML, Tan RJ, Enghild J, Oury TD. Extracellular superoxide dismutase protects against matrix degradation of heparan sulfate in the lung. Antioxid Redox Signal 10: 261–268, 2008. doi: 10.1089/ars.2007.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.LaRiviere WB, Liao S, McMurtry SA, Oshima K, Han X, Zhang F, Yan S, Haeger SM, Ransom M, Bastarache JA, Linhardt RJ, Schmidt EP, Yang Y. Alveolar heparan sulfate shedding impedes recovery from bleomycin-induced lung injury. Am J Physiol Lung Cell Mol Physiol 318: L1198–L1210, 2020. doi: 10.1152/ajplung.00063.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rehm M, Bruegger D, Christ F, Conzen P, Thiel M, Jacob M, Chappell D, Stoeckelhuber M, Welsch U, Reichart B, Peter K, Becker BF. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation 116: 1896–1906, 2007. doi: 10.1161/CIRCULATIONAHA.106.684852. [DOI] [PubMed] [Google Scholar]
- 166.Hesselstrand R, Westergren-Thorsson G, Scheja A, Wildt M, Akesson A. The association between changes in skin echogenicity and the fibroblast production of biglycan and versican in systemic sclerosis. Clin Exp Rheumatol 20: 301–308, 2002. [PubMed] [Google Scholar]
- 167.Ciftciler R, Ozenirler S, Yucel AA, Cengiz M, Erkan G, Buyukdemirci E, Sonmez C, Esendagli GY. The importance of serum biglycan levels as a fibrosis marker in patients with chronic hepatitis B. J Clin Lab Anal 31: e22109, 2017. doi: 10.1002/jcla.22109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sobhy A, Fakhry MM, H AA, Ashmawy AM, Omar Khalifa H. Significance of biglycan and osteopontin as non-invasive markers of liver fibrosis in patients with chronic hepatitis B virus and chronic hepatitis C virus. J Investig Med 67: 681–685, 2019. doi: 10.1136/jim-2018-000840. [DOI] [PubMed] [Google Scholar]
- 169.Vesterhus M, Nielsen MJ, Hov JR, Saffioti F, Manon-Jensen T, Leeming DJ, Moum B, Boberg KM, Pinzani M, Karlsen TH, Karsdal MA, Thorburn D. Comprehensive assessment of ECM turnover using serum biomarkers establishes PBC as a high-turnover autoimmune liver disease. JHEP Rep 3: 100178, 2021. doi: 10.1016/j.jhepr.2020.100178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Schaefer L, Raslik I, Grone HJ, Schonherr E, Macakova K, Ugorcakova J, Budny S, Schaefer RM, Kresse H. Small proteoglycans in human diabetic nephropathy: discrepancy between glomerular expression and protein accumulation of decorin, biglycan, lumican, and fibromodulin. FASEB J 15: 559–561, 2001. doi: 10.1096/fj.00-0493fje. [DOI] [PubMed] [Google Scholar]
- 171.Zou W, Wan J, Li M, Xing J, Chen Q, Zhang Z, Gong Y. Small leucine rich proteoglycans in host immunity and renal diseases. J Cell Commun Signal 13: 463–471, 2019. doi: 10.1007/s12079-018-0489-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Barth M, Selig JI, Klose S, Schomakers A, Kiene LS, Raschke S, Boeken U, Akhyari P, Fischer JW, Lichtenberg A. Degenerative aortic valve disease and diabetes: Implications for a link between proteoglycans and diabetic disorders in the aortic valve. Diab Vasc Dis Res 16: 254–269, 2019. doi: 10.1177/1479164118817922. [DOI] [PubMed] [Google Scholar]
- 173.Song R, Fullerton DA, Ao L, Zheng D, Zhao KS, Meng X. BMP-2 and TGF-β1 mediate biglycan-induced pro-osteogenic reprogramming in aortic valve interstitial cells. J Mol Med (Berl) 93: 403–412, 2015. doi: 10.1007/s00109-014-1229-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mandraffino G, Imbalzano E, Mamone F, Aragona CO, Lo Gullo A, D'Ascola A, Alibrandi A, Cinquegrani A, Mormina E, Versace A, Basile G, Sardo MA, Cinquegrani M, Carerj S, Saitta A. Biglycan expression in current cigarette smokers: a possible link between active smoking and atherogenesis. Atherosclerosis 237: 471–479, 2014. doi: 10.1016/j.atherosclerosis.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 175.Heegaard AM, Corsi A, Danielsen CC, Nielsen KL, Jorgensen HL, Riminucci M, Young MF, Bianco P. Biglycan deficiency causes spontaneous aortic dissection and rupture in mice. Circulation 115: 2731–2738, 2007. doi: 10.1161/CIRCULATIONAHA.106.653980. [DOI] [PubMed] [Google Scholar]
- 176.Meester JA, Vandeweyer G, Pintelon I, Lammens M, Van Hoorick L, De Belder S, Waitzman K, Young L, Markham LW, Vogt J, Richer J, Beauchesne LM, Unger S, Superti-Furga A, Prsa M, Dhillon R, Reyniers E, Dietz HC, Wuyts W, Mortier G, Verstraeten A, Van Laer L, Loeys BL. Loss-of-function mutations in the X-linked biglycan gene cause a severe syndromic form of thoracic aortic aneurysms and dissections. Genet Med 19: 386–395, 2017. doi: 10.1038/gim.2016.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wang M, Xue S, Fang Q, Zhang M, He Y, Zhang Y, Lammi MJ, Cao J, Chen J. Expression and localization of the small proteoglycans decorin and biglycan in articular cartilage of Kashin-Beck disease and rats induced by T-2 toxin and selenium deficiency. Glycoconj J 36: 451–459, 2019. doi: 10.1007/s10719-019-09889-9. [DOI] [PubMed] [Google Scholar]
- 178.Cho SY, Bae JS, Kim NKD, Forzano F, Girisha KM, Baldo C, Faravelli F, Cho TJ, Kim D, Lee KY, Ikegawa S, Shim JS, Ko AR, Miyake N, Nishimura G, Superti-Furga A, Spranger J, Kim OH, Park WY, Jin DK. BGN mutations in X-linked spondyloepimetaphyseal dysplasia. Am J Hum Genet 98: 1243–1248, 2016. doi: 10.1016/j.ajhg.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Maishi N, Ohba Y, Akiyama K, Ohga N, Hamada J, Nagao-Kitamoto H, Alam MT, Yamamoto K, Kawamoto T, Inoue N, Taketomi A, Shindoh M, Hida Y, Hida K. Tumour endothelial cells in high metastatic tumours promote metastasis via epigenetic dysregulation of biglycan. Sci Rep 6: 28039, 2016. doi: 10.1038/srep28039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Appunni S, Anand V, Khandelwal M, Gupta N, Rubens M, Sharma A. Small Leucine Rich Proteoglycans (decorin, biglycan and lumican) in cancer. Clin Chim Acta 491: 1–7, 2019. doi: 10.1016/j.cca.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 181.Mummert ME, Mohamadzadeh M, Mummert DI, Mizumoto N, Takashima A. Development of a peptide inhibitor of hyaluronan-mediated leukocyte trafficking. J Exp Med 192: 769–779, 2000. doi: 10.1084/jem.192.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Savani RC, Hou G, Liu P, Wang C, Simons E, Grimm PC, Stern R, Greenberg AH, DeLisser HM, Khalil N. A role for hyaluronan in macrophage accumulation and collagen deposition after bleomycin-induced lung injury. Am J Respir Cell Mol Biol 23: 475–484, 2000. doi: 10.1165/ajrcmb.23.4.3944. [DOI] [PubMed] [Google Scholar]
- 183.Esguerra KV, Tolg C, Akentieva N, Price M, Cho CF, Lewis JD, McCarthy JB, Turley EA, Luyt LG. Identification, design and synthesis of tubulin-derived peptides as novel hyaluronan mimetic ligands for the receptor for hyaluronan-mediated motility (RHAMM/HMMR). Integr Biol (Camb) 7: 1547–1560, 2015. doi: 10.1039/c5ib00222b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hauser-Kawaguchi A, Luyt LG, Turley E. Design of peptide mimetics to block pro-inflammatory functions of HA fragments. Matrix Biol 78–79: 346–356, 2019. doi: 10.1016/j.matbio.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 185.Nagy N, Kuipers HF, Frymoyer AR, Ishak HD, Bollyky JB, Wight TN, Bollyky PL. 4-methylumbelliferone treatment and hyaluronan inhibition as a therapeutic strategy in inflammation, autoimmunity, and cancer. Front Immunol 6: 123, 2015. doi: 10.3389/fimmu.2015.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Nagy N, Kuipers HF, Marshall PL, Wang E, Kaber G, Bollyky PL. Hyaluronan in immune dysregulation and autoimmune diseases. Matrix Biol 78-79: 292–313, 2019. doi: 10.1016/j.matbio.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.McKallip RJ, Ban H, Uchakina ON. Treatment with the hyaluronic acid synthesis inhibitor 4-methylumbelliferone suppresses LPS-induced lung inflammation. Inflammation 38: 1250–1259, 2015. doi: 10.1007/s10753-014-0092-y. [DOI] [PubMed] [Google Scholar]
- 188.Bell TJ, Brand OJ, Morgan DJ, Salek-Ardakani S, Jagger C, Fujimori T, Cholewa L, Tilakaratna V, Ostling J, Thomas M, Day AJ, Snelgrove RJ, Hussell T. Defective lung function following influenza virus is due to prolonged, reversible hyaluronan synthesis. Matrix Biol 80: 14–28, 2019. doi: 10.1016/j.matbio.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Vlodavsky I, Singh P, Boyango I, Gutter-Kapon L, Elkin M, Sanderson RD, Ilan N. Heparanase: From basic research to therapeutic applications in cancer and inflammation. Drug Resist Updat 29: 54–75, 2016. doi: 10.1016/j.drup.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Jin M, Iwamoto T, Yamada K, Satsu H, Totsuka M, Shimizu M. Disaccharide derived from chondroitin sulfate A suppressed CpG-induced IL-6 secretion in macrophage-like J774.1 cells. Cytokine 51: 53–59, 2010. doi: 10.1016/j.cyto.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 191.Wu F, Zhou C, Zhou D, Ou S, Liu Z, Huang H. Immune-enhancing activities of chondroitin sulfate in murine macrophage RAW 264.7 cells. Carbohydr Polym 198: 611–619, 2018. doi: 10.1016/j.carbpol.2018.06.071. [DOI] [PubMed] [Google Scholar]
- 192.Gopal S. Syndecans in inflammation at a glance. Front Immunol 11: 227, 2020. doi: 10.3389/fimmu.2020.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]