Abstract
Background
Various noninvasive tools based on anthropometric indicators, blood lipids, and liver enzymes, etc. have been developed to screen for nonalcoholic fatty liver disease (NAFLD), with different diagnostic performance and cutoff values among studies. We aimed to validate and compare eight NAFLD-related models developed by simple indicators and to define their cutoff values in Chinese community population.
Methods
A cross-sectional study was conducted in a health examination cohort of 3259 people. NAFLD was diagnosed by ultrasonography. General, anthropometric and biochemical data were collected. Fatty liver index (FLI), fatty liver disease index (FLD), Zhejiang University index (ZJU), lipid accumulation product (LAP), regression formula of controlled attenuation parameter (CAP), waist-to-height ratio (WHtR), triglyceride and glucose index (TyG), and visceral adiposity index (VAI) were calculated. The accuracy and cutoff points to detect NAFLD were evaluated by area under the receiver operator characteristic curve and the maximum Youden index analysis, respectively. A head-to-head comparison between these models and Decision Curve Analysis (DCA) was conducted.
Results
In eight noninvasive diagnostic models of NAFLD, AUCs of FLI and FLD for NAFLD were higher than those of other models in the whole (0.852 and 0.852), male (0.826 and 0.824), and female (0.897 and 0.888) population, respectively. DCA showed that FLI, FLD, and ZJU have higher net benefit to screen for NAFLD compared to other models.
Conclusions
FLI and FLD could be the most accurate and applicable of eight models for the noninvasive diagnosis of NAFLD in both male and female groups.
Keywords: fatty liver index, fatty liver disease index, nonalcoholic fatty liver disease, noninvasive diagnosis, Zhejiang University Index
Introduction
Nonalcoholic fatty liver disease (NAFLD) is becoming increasingly prevalent, affecting more than one-quarter of adults in the world, with prevalence of 20.09% in China [1]. The spectrum of NAFLD covers from simple steatosis or nonalcoholic fatty liver (NAFL) to nonalcoholic steatohepatitis (NASH). It may progress to fibrosis, leading to cirrhosis and hepatocellular carcinoma [2]. NAFLD has imposed a severe burden on the whole world [3–5].
The golden standard for diagnosis of NAFLD is liver biopsy but it may cause severe complications, such as bleeding, pain, and death [6]. Imaging techniques such as ultrasonography, computed tomography, MRI, and magnetic resonance spectroscopy as noninvasive approaches are well studied to detect NAFLD [7]. But the costs associated with imaging techniques limit its widespread use for large-scale screening of asymptomatic individuals like community population. Moreover, the increasing incidence of NAFLD calls for practical and cost-effective screening and monitoring. Predictive models based on clinical and laboratory biomarkers are prevalent in recent years [8]. The practical advantages of biomarkers include their high applicability, their good inter-laboratory reproducibility, and their potential widespread availability.
A variety of noninvasive models based on biomarkers have been developed for NAFLD screening in the past two decades [8]. Current NAFLD-related models including fatty liver index (FLI), fatty liver disease index (FLD), Zhejiang University index (ZJU), lipid accumulation product (LAP), regression formula of controlled attenuation parameter (CAP), waist-to-height ratio (WHtR), triglyceride and glucose index (TyG), and visceral adiposity index (VAI) were well documented [9–16]. However, the performance and cutoff values of these models are varied by sex, region, and race. For example, the cutoff values of FLI were <30 and >60 for total population to rule out and in NAFLD in the primary study [10], and it was <10 for female and <25 for male to rule out NAFLD, ≥20 for female and ≥35 for male to rule in NAFLD in a validation study in Taiwan [17]. Although some of the models have been validated independently, their diagnostic performances are difficult to compare, as they have been designed and validated against different standards (liver biopsy, ultrasonography, or magnetic resonance spectroscopy) [8,18].
This study aimed to validate and compare the current noninvasive models developed by simple biomarkers, so as to find suitable screening methods and determine the cutoff values for NAFLD in the eastern Chinese community population.
Methods
Study population
We conducted a cross-sectional study. Participants were from a community in Nanjing, eastern China who received health check-up services from July to September 2018. All participants have signed informed consents. The study design was approved by the Institutional Ethics Review Committee of Nanjing Medical University and in accordance with the ethical standards in the Declaration of Helsinki.
The exclusion criteria of this study were subjects who: (1) had uncompleted clinical data; (2) had a significant alcoholic consumption (men >140 g or women >70 g per week in the past 12 months); (3) had viral/autoimmune hepatitis, drug-induced liver damage or other liver diseases; (4) have undergone gastrointestinal surgery; and (5) had a history of mental disorders.
Measurement
All participants received physical examination, laboratory examination and upper abdominal ultrasonography. Weight, height, and waist circumstance (WC) were all measured wearing minimal clothing and without socks by the same nurses and machine. BMI was calculated as: BMI = weight (kg)/height (m) 2. Whole blood samples were collected after 10-h overnight fasting and serum samples were separated for immediate analysis. Fasting plasma glucose (FPG), triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and γ-glutamyl transferase (GGT) were measured by a biochemical analyzer (Mindray BS-860, Shenzhen Mindray Bio-Medical Electronics Co., Ltd, Shenzhen, China). The abdominal ultrasonography was performed by a LOGIQ-E9 ultrasound system (General Electric Healthcare, Milwaukee, Wisconsin, USA).
Diagnosis and classification of nonalcoholic fatty liver disease
The diagnosis of NAFLD was based on ultrasonography after excluding alcohol abuse and other liver diseases, which is in accord with the Guidelines for Diagnosis and Treatment of NAFLD issued by Fatty Liver and Alcoholic Liver Disease Study Group of the Chinese Liver Disease Association in 2010 and the updated one in 2018 [19,20]. Two experienced ultrasound experts performed the examination, and NAFLD would be diagnosed by the presence of the following findings: (1) The near-field echo of the liver is diffusely increased and more than the kidney and the spleen. The far-field echo of the liver is attenuated gradually. (2) The intrahepatic duct structure is not clear. (3) The liver is mildly to moderately enlarged, with round and blunt edges. (4) Color Doppler flow imaging shows that the blood flow signal in the liver is reduced or difficult to display, but the intrahepatic blood vessels are normal. (5) The echo of the capsule of right liver lobe and septum is unclear or incomplete. Those with the symptoms of item 1 and one of items 2–4 above would be mild fatty liver; those with the symptoms of item 1 and two of items 2–4 above would be moderate fatty liver; those with the symptoms of item 1, two of items 2–4 above, and item 5 above would be severe fatty liver.
Calculation of predictive models
Eight models were validated and compared in this study. The formulas of models were checked by tracing back to the original literature (Table 1). TG in FLI and TyG; and FPG in TyG are in mg/dL. TG in FLD, LAP, and VAI; FPG in ZJU; and HDL-C in VAI are all in mmol/L. WC and height are in centimeter. BMI is in kg/m2 and AST, ALT, and GGT are in IU/L.
Table 1.
Characteristics of nonalcoholic fatty liver disease-related models
| Model | Full name | Country, year | Formula |
|---|---|---|---|
| FLI [10] | Fatty Liver Index | Italy, 2006 | FLI = e 0.953 × ln TG + 0.139 × BMI + 0.718 × ln (GGT) + 0.053 × WC − 15.745)/(1 + e 0.953 × ln TG + 0.139 × BMI + 0.718 × ln (GGT) + 0.053 × WC − 15.745) × 100 |
| FLD [14] | Fatty liver disease index | China, 2013 | FLD index = BMI + TG + 3 (ALT/AST ratio) + 2HG (presence of HG, HG = 1; absence of HG, HG = 0) |
| ZJU [15] | Zhejiang University index | China, 2015 | ZJU index = BMI + FPG + TG + 3 ALT/AST ratio + 2 (if female) |
| LAP [12] | Lipid accumulation product | Italy, 2010 | LAP (male) = (WC – 65) × TG LAP (female) = (WC – 58) × TG |
| CAP [16] | Regression formula of controlled attenuation parameter | China, 2018 | CAP = 113.163 + 0.252 × ALT + 6.316 × BMI |
| WHtR [9] | Waist-to-Height Ratio | Japan, 1995 | WHtR = WC/height × 100 |
| TyG [11] | Triglyceride and glucose index | Mexico, 2008 | TyG = ln[(TG × FPG)/2] |
| VAI [13] | Visceral adiposity index | Italy, 2012 | VAI (male) = (WC/(39.68 + (1.88 × BMI))) × (TG/1.03) × (1.31/HDL-C) VAI (female) = (WC/(39.58 + (1.89 × BMI))) × (TG/0.81) × (1.52/HDL-C) |
ALT, alanine aminotransferase; AST, aspartic transaminase; BP, blood pressure; DM, diabetes mellitus; FPG, fasting plasma glucose; GGT, gamma-glutamyl transpeptidase; HG, hyperglycemia; HDL-C, high-density lipoprotein cholesterol; TG, triglyceride; TB, total bilirubin; TC, total cholesterol; WC, waist circumference; platelet count.
Statistical analysis
Continuous variables are presented as mean ±SD or median (interquartile range), and categorical variables are presented as numbers and percentages. The Student’s t-test or Mann–Whitney U test for continuous variables, and chi-squared test or Kruskal–Wallis test for categorical variables were used to compare the parameters between NAFLD and non-NAFLD groups. Receiver operating characteristic (ROC) curves of models were developed to predict the presence of NAFLD. Comparisons of the areas under receiver operating characteristic (AUC) curves between every two models were performed, using the method of DeLong et al. [21]. Decision Curve Analysis (DCA) was also conducted to compare these models. Youden index and the discriminant ability at each cutoff value for NAFLD-related models were used to determine the optimal cutoff value to diagnose NAFLD in total, male and female population. Descriptive analyses, correlation analyses, and ROC analyses were performed using SPSS (version 23.0, IBM Corp., Chicago, Illinois, USA). The ROC comparison of NAFLD-related models using method of DeLong et al. was performed by MedCalc (version 11.4.2.0, MedCalc Inc., Mariakerke, Belgium) and DCA was performed by R software (version 3.6.2, https://www.r-project.org/). A two-sided P < 0.05 was considered statistically significant.
Results
Characteristics of the study population
After excluding 391 participants according to our exclusion criteria [significant alcohol consumption (n = 257), positive hepatitis C virus (n = 19), use of medication associated with fatty liver (n = 103) and with mental disorders (n = 12)], a total of 3259 subjects were included in the analysis. As shown in Table 2, overall characteristics of the population were depicted and eight models namely FLI, FLD, ZJU, TyG, CAP, LAP, WHtR, and VAI were also displayed. Seventy-three percent of the study population was male, and mean age of participants was 40.59 ± 9.30 years. NAFLD was present in 1169 of 3259 participants (35.9%). Patients with mild fatty liver accounted for 69.8% (n = 816); moderate accounted for 24.0% (n = 280) and severe accounted for 6.2% (n = 73). NAFLD group had higher levels of NAFLD-related indexes than non-NAFLD group.
Table 2.
Characteristics of the study population and comparison between subjects with and without nonalcoholic fatty liver disease
| Variables | All participants (n = 3259) | Non-NAFLD (n = 2090) | NAFLD (n = 1169) | χ2/t/Z | P |
|---|---|---|---|---|---|
| Male (n, %) | 2387 (73.2) | 1378 (65.9) | 1009 (86.3) | 158.887 | <0.001a |
| Age (years) | 40.59 ± 9.30 | 39.61 ± 9.24 | 42.34 ± 9.15 | −8.1 | <0.001b |
| WC (cm) | 83.01 ± 9.47 | 79.65 ± 8.55 | 89.03 ± 7.93 | −31.48 | <0.001b |
| BMI (kg/m2) | 23.46 ± 2.97 | 22.37 ± 2.61 | 25.42 ± 2.54 | −32.28 | <0.001b |
| SBP (mmHg) | 125.45 ± 15.34 | 122.37 ± 14.31 | 130.97 ± 15.59 | −15.54 | <0.001b |
| DBP (mmHg) | 75.88 ± 10.46 | 73.73 ± 9.66 | 79.73 ± 10.74 | −15.85 | <0.001b |
| TC (mmol/L) | 4.60 ± 0.86 | 4.49 ± 0.81 | 4.79 ± 0.90 | −9.44 | <0.001b |
| TG (mmol/L) | 1.16 (0.84, 1.70) | 0.99 (0.75, 1.33) | 1.66 (1.19, 2.28) | −26.61 | <0.001c |
| TBIL (mmol/L) | 13.86 (10.87, 17.84) | 13.92 (10.97, 18.00) | 13.73 (10.76, 17.66) | −1.16 | 0.248c |
| DBIL (mmol/L) | 4.13 (3.35, 5.15) | 4.22 (3.37, 5.27) | 4.01 (3.29, 5.00) | −3.30 | 0.001c |
| HDL-C (mmol/L) | 1.24 ± 0.32 | 1.33 ± 0.33 | 1.08 ± 0.25 | 24.11 | <0.001b |
| LDL-C (mmol/L) | 2.70 ± 0.74 | 2.64 ± 0.71 | 2.81 ± 0.78 | −6.04 | <0.001b |
| ALT (IU/L) | 19 (14, 29) | 16 (12, 23) | 26 (19, 38) | −23.33 | <0.001c |
| AST (IU/L) | 19 (16, 23) | 18 (15, 21) | 21 (17, 25) | −14.83 | <0.001c |
| GGT (IU/L) | 19 (13, 31) | 16 (12, 24) | 29 (19, 42) | −24.08 | <0.001c |
| Scr (mmol/L) | 66.94 ± 13.29 | 65.69 ± 13.44 | 69.04 ± 12.76 | −6.96 | <0.001b |
| FPG (mmol/L) | 4.75 (4.48, 5.05) | 4.70 (4.45, 4.97) | 4.85 (4.55, 5.21) | −9.16 | <0.001c |
| FLI | 17.30 (6.79, 38.74) | 9.86 (4.58, 20.60) | 41.27 (23.94, 60.09) | −33.39 | <0.001c |
| FLD | 28.34 ± 4.29 | 26.54 ± 3.46 | 31.58 ± 3.68 | −38.97 | <0.001b |
| ZJU | 33.68 ± 4.22 | 31.95 ± 3.36 | 36.79 ± 3.80 | −36.35 | <0.001b |
| TyG | 8.89 ± 2.97 | 7.87 ± 2.48 | 10.70 ± 2.93 | −27.88 | <0.001b |
| CAP | 267.45 ± 20.73 | 259.44 ± 17.70 | 281.77 ± 17.87 | −34.43 | <0.001b |
| LAP | 22.66 (12.88, 39.44) | 16.45 (9.72, 25.53) | 40.59 (27.18, 61.68) | −31.75 | <0.001c |
| WHtR | 48.87 ± 4.90 | 47.10 ± 4.37 | 52.02 ± 4.15 | −31.37 | <0.001b |
| VAI | 1.34 (0.85, 2.14) | 1.05 (0.72, 1.54) | 2.08 (1.37, 3.25) | −19.14 | <0.001c |
Data are mean ± SD or median (interquartile range) for continuous variables; P values present comparisons between NAFLD patients and non-NAFLD subjects.
Chi-squared test.
Independent-samples T test.
Mann–Whitney U test.
ALT, alanine aminotransferase; AST, aspartic transaminase; CAP, controlled attenuation parameter; DBIL, direct bilirubin; DBP, diastolic blood pressure; FLD, fatty liver disease index; FLI, fatty liver index; FPG, fasting plasma glucose; GGT, gamma-glutamyl transpeptidase; HDL-C, high-density lipoprotein cholesterol; LAP, lipid accumulation product; LDL-C, low-density lipoprotein cholesterol; NAFLD, nonalcoholic fatty liver disease; platelet count; SBP, systolic blood pressure; Scr, serum creatinine; TBIL, total bilirubin; TC, total cholesterol; TG, triglyceride; TyG, triglyceride and glucose index; VAI, visceral adiposity index; WC, waist circumference; WHtR, waist-to-height ratio; ZJU, Zhejiang University index.
Validation and comparison of nonalcoholic fatty liver disease-related models
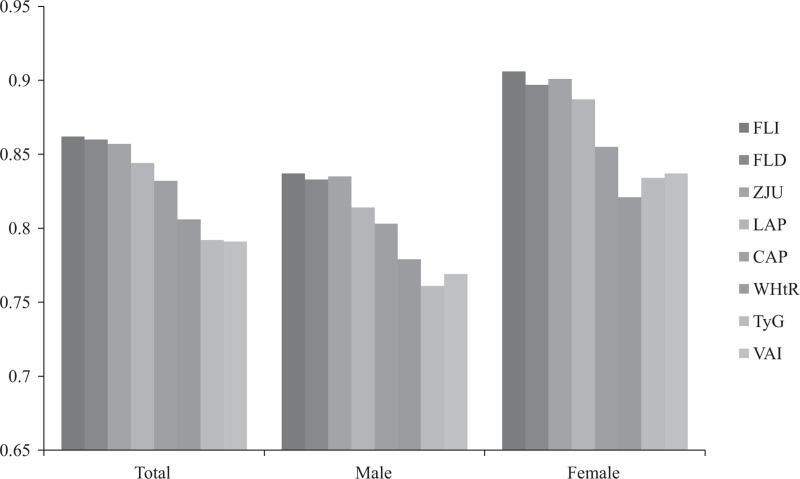
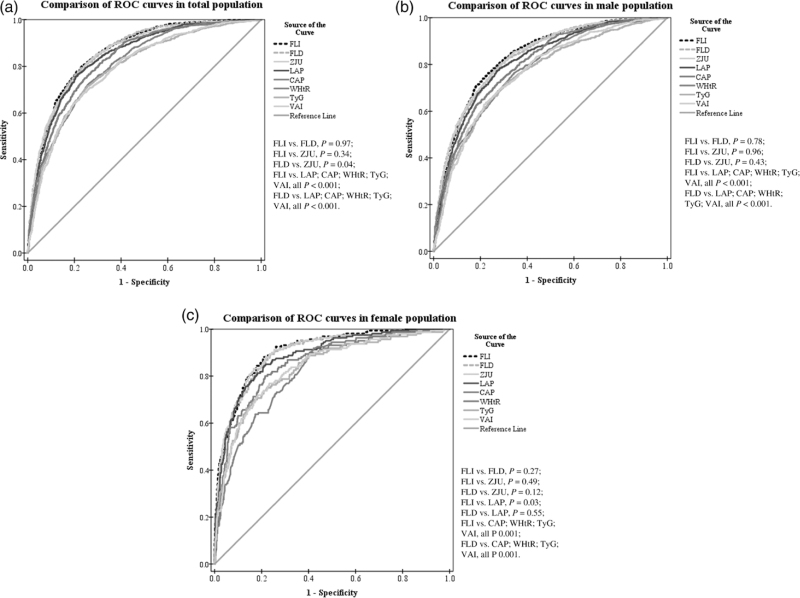
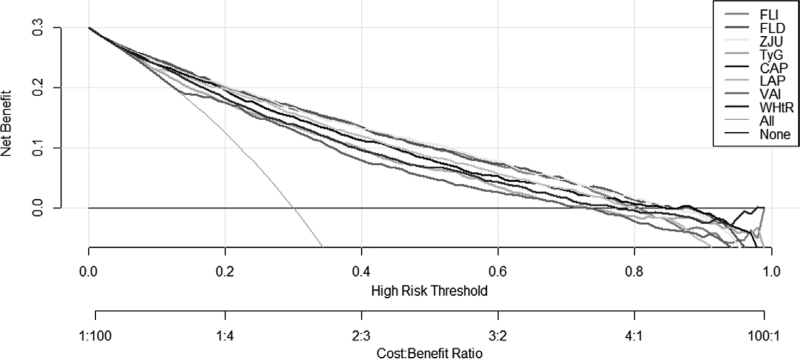
The odds ratios (ORs) of 1-unit increment of NAFLD-related models for mild, moderate, and severe NAFLD were analyzed. We calculated crude OR and adjusted OR (adjusted for age, sex, and BMI). As shown in Table S1, Supplemental Digital Content 1, http://links.lww.com/EJGH/A759, univariate and multivariate analysis showed that all of the eight models were positively associated with NAFLD (all P < 0.001). In Table 3 and Fig. 1, ROC analysis results of NAFLD-related models for predicting NAFLD were presented. All of the models had moderate discrimination in the overall, male and female population (all AUC > 0.75, all P < 0.001). Compared to total and male population, AUCs of all models were higher in female, and FLI (0.897, 0.875–0.917), FLD (0.888, 0.865–0.908), and ZJU (0.892, 0.869–0.911) had a high AUC next to 0.9. The AUCs of FLI (0.852, 0.839–0.864), FLD (0.852, 0.839–0.864) were higher than other models to predict NAFLD in the overall population (Table 3, Fig. 2a). The results of comparison showed that the differences in FLI and FLD compared with ZJU were NS in male and female population, and it was indicated that the AUC of FLI and FLD were higher than LAP, CAP, WHtR, and TyG (all P < 0.001) (Fig. 2b and c). The DCA showed that FLI, FLD, and ZJU have higher net benefit to screen for NAFLD compared to other models (Fig. 3).
Table 3.
Areas under receiver operating characteristics curves of nonalcoholic fatty liver disease-related models for predicting nonalcoholic fatty liver disease
| Models | Total | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|---|
| AUC | SE | 95% CI | AUC | SE | 95% CI | AUC | SE | 95% CI | |
| FLI | 0.852 | 0.01 | 0.839–0.864 | 0.826 | 0.01 | 0.810–0.841 | 0.897 | 0.01 | 0.875–0.917 |
| FLD | 0.852 | 0.01 | 0.839–0.864 | 0.824 | 0.01 | 0.809–0.839 | 0.888 | 0.01 | 0.865–0.908 |
| ZJU | 0.847 | 0.01 | 0.835–0.860 | 0.826 | 0.01 | 0.810–0.841 | 0.892 | 0.01 | 0.869–0.911 |
| LAP | 0.835 | 0.01 | 0.822–0.847 | 0.804 | 0.01 | 0.788–0.820 | 0.879 | 0.01 | 0.856–0.900 |
| CAP | 0.823 | 0.01 | 0.810–0.836 | 0.794 | 0.01 | 0.778–0.810 | 0.847 | 0.02 | 0.821–0.870 |
| WHtR | 0.797 | 0.01 | 0.783–0.811 | 0.768 | 0.01 | 0.751–0.785 | 0.812 | 0.02 | 0.784–0.837 |
| TyG | 0.792 | 0.01 | 0.777–0.806 | 0.761 | 0.01 | 0.743–0.778 | 0.834 | 0.02 | 0.807–0.859 |
| VAI | 0.791 | 0.01 | 0.777–0.805 | 0.769 | 0.01 | 0.751–0.786 | 0.837 | 0.02 | 0.810–0.861 |
Fig. 1.
Areas under receiver operating characteristic curves of nonalcoholic fatty liver disease-related models in total, male and female population.
Fig. 2.
Comparison of Receiver-operating characteristic (ROC) curves of the NAFLD-related models for the detection of NAFLD in the validation. (a) Comparison of ROCs in total population. (b) Comparison of ROCs in male population. (c) Comparison of ROCs in female population. Comparisons of the areas under receiver operating characteristic (AUC) curves between FLI and other indexes were performed by the method of DeLong et al. NAFLD, nonalcoholic fatty liver disease.
Fig. 3.
Decision Curve Analysis for NAFLD Screening. NAFLD, nonalcoholic fatty liver disease.
Cutoff values of models for the detection of nonalcoholic fatty liver disease
The optimal cutoff values were determined by the maximum of Youden index. The sensitivity and specificity of NAFLD-related models at the optimal cutoff values were displayed in Table 4. ZJU had the same optimal cutoff point (>33.7) in all population. FLI >20.6 (sensitivity = 85.3% and specificity = 75.1%) was the optimal cutoff point of NAFLD screening in total population. In male and female population, the cutoff values were different (25.3, sensitivity = 78.4% and specificity = 72.4%; 8.4, sensitivity = 88.1% and specificity = 78.2%). The diagnostic performance of FLI at 30 and 60 was also analyzed. The specificity is much higher than sensitivity at the cutoff of 30 and 60 (in total population, sensitivity = 66.1% and 24.9%, specificity = 84.9% and 96.9%). The optimal cutoff points of FLD were >28.7 (sensitivity = 79.6% and specificity = 75.2%) in whole population, >29.0 (sensitivity = 80.0% and specificity = 70.0%) in male population, >26.1 (sensitivity = 88.2% and specificity = 75.6%) in female population. Besides, the cutoff values of FLI and FLD for 90% sensitivity and 90% specificity among different population were analyzed and shown in Table S2, Supplemental Digital Content 1, http://links.lww.com/EJGH/A759. In the total population, FLI >13.5 as cutoff point had the sensitivity of 90.0% and FLI >37.6 had the specificity of 90.0%. The cutoff values of FLD for 90% sensitivity and 90% specificity in the total population were >27.2 and >30.9.
Table 4.
Cutoff values and diagnostic accuracy of nonalcoholic fatty liver disease-related models in total, male and female population
| Model | Total | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cutoffa | Sensitivity (%) | Specificity (%) | Cutoffa | Sensitivity (%) | Specificity (%) | Cutoffa | Sensitivity (%) | Specificity (%) | |
| FLI | 20.6 | 85.3 | 75.1 | 25.3 | 78.4 | 72.4 | 8.4 | 88.1 | 78.2 |
| 30 | 66.1 | 84.9 | 30 | 71.2 | 78.4 | 30 | 34.1 | 97.9 | |
| 60 | 24.9 | 96.9 | 60 | 28 | 95.3 | 60 | 5.4 | 100 | |
| FLD | 28.7 | 79.6 | 75.2 | 29.0 | 80.0 | 70.0 | 26.1 | 88.2 | 75.6 |
| ZJU | 33.7 | 80.6 | 73.8 | 33.7 | 80.4 | 69.3 | 33.7 | 82.6 | 82.3 |
| LAP | 26.9 | 75.6 | 77.7 | 26.9 | 77.3 | 71.1 | 19.7 | 84.4 | 77.9 |
| CAP | 269.7 | 75 | 74.2 | 272.4 | 72.2 | 71.6 | 259.9 | 79.5 | 77.1 |
| WHtR | 49.7 | 72.5 | 72.5 | 49.4 | 76.6 | 63.6 | 45.5 | 91.9 | 56.3 |
| TyG | 9.1 | 71.9 | 73.1 | 9.1 | 72.5 | 67.7 | 8.9 | 70.2 | 82.1 |
| VAI | 1.6 | 68.3 | 76.1 | 1.7 | 62.9 | 78.3 | 1.6 | 71.2 | 81.8 |
Apart from 30 to 60 cutoff points of FLI, optimal cutoff values of all scores were determined by the maximum of Youden index.
CAP, controlled attenuation parameter; FLD, fatty liver disease index; FLI, fatty liver index; LAP, lipid accumulation product; NAFLD, nonalcoholic fatty liver disease; TyG, triglyceride and glucose index; VAI, visceral adiposity index; WHtR, waist-to-height ratio; ZJU, Zhejiang University index.
Discussion
More than a dozen models have been proposed to detect NAFLD patients, and most of them had a good diagnostic performance in the original study such as the Steato Test [22], FLI [10], FLD index [14], Hepatic steatosis index [23], Framingham steatosis index [24], homeostasis model assessment of insulin resistance (HOMA-IR) [25], and index of NASH (ION) [26]. However, some indicators in these models are costly and not available in most laboratories in the undeveloped countries, such as α2-macroglobulin, apolipoprotein A-I in the Steato Test, and the insulin test in HOMA-IR and ION. In order to determine a simple, accurate, and cost-effective model for NAFLD screening on a large scale, we screened out NAFLD-related models developed by simple indicators. We validated eight NAFLD-related models (FLI, FLD, ZJU, LAP, CAP, WHtR, TyG, and VAI) and compared the performance of these models in eastern Chinese community population. Our study showed that all of these models have a moderate discrimination, and FLI and FLD have better performance than other models in this population.
FLI, consisting of TG, GGT, BMI, and WC, was developed by Bedogni et al. [10] in northern Italy, 2006. It has been validated to a great extent and has been proved to be correlated with insulin resistance, coronary heart disease, and early atherosclerosis [27,28]. Studies have shown that FLI has a moderate discrimination (AUC, 0.83–0.88) in Taiwan, northern and western Chinese mainland [17,29,30]. Our study has testified the feasibility of FLI in eastern Chinese mainland and proved that FLI has good applicability in the community population. FLD based on BMI, TG, ALT to AST ratio and FPG was proposed by Fuyan et al. in eastern China with an AUC of 0.82 [14]. It has been validated by Zhu et al. with an AUC of 0.87 in western China, but it was no better than FLI (AUC, 0.88) [29]. Our study supported that FLD has the same diagnostic performance as FLI. Besides, the DCA also suggested that FLI and FLD have higher net benefit than other models. The population in Zhu et al.’s study was western Chinese while our population was eastern Chinese, which can cause a great difference. There are a variety of differences in culture and lifestyle between western and eastern China, which can cause heterogeneity among different populations. For instance, various dietary patterns can lead to changes in dietary nutrient intake, resulting in different anthropometric data, and biochemical measurements [31,32]. The prevalence of NAFLD in this study is higher than Zhu et al’s. Besides, the lean population proportion in our study is bigger than the previous study.
ZJU also has a great performance in this study but it does not perform as well as FLD in the whole population. ZJU was developed based on BMI, FPG, TG, ALT to AST ratio and sex with an AUC of 0.82 in eastern China, and in the validation cohort using pathological data, the ZJU index had a good accuracy (AUC, 0.896) for the detection of steatosis [15]. Some recent studies have validated ZJU and compared to other models including FLI using large population, with conflicting results [29,33]. The underlying reason may be the difference of NAFLD prevalence and characteristics of population.
LAP, CAP, WHtR, TyG, and VAI in our study have AUCs of over 0.75 in all population. WC as a good surrogate parameter of visceral fat [34,35], is the common component of FLI, LAP, WHtR, and VAI. Visceral adiposity has a significant association with increased free fatty acids, which can be transported to liver and expose the liver to fat accumulation, liver insulin resistance and inflammation [36]. CAP was developed based on 141 histological diagnosed NAFLD patients and has higher accuracy than FibroScan [16]. The sample size in the primary study may be too small to develop a model applying to general population. To our knowledge, CAP has not been external validated. TyG has a strong correlation with the degree of NAFLD, but the AUC of TyG to detect NAFLD is not in parallel with the high-risk correlation. TyG has been used to reflect insulin resistance, which is very important in the development of NAFLD [37]. Apart from insulin resistance, transaminases and anthropometric indicators also play vital roles in the prediction of NAFLD. It may be the reason why the diagnostic performance of TyG is not in line with the relationship to NAFLD, and is not as well as FLI and FLD.
Cutoff value is an important concern when applying models to specific population. We also determined cutoff values of these models in this population. The cutoff values of FLI and FLD differ in sex and are inconsistent with previous studies. The optimal cutoff points of the FLI in the present study were 20.6, 25.3, and 8.4 in the total, male and female population, respectively. Western countries mostly identified FLI <30 as non-NAFLD and >60 as NAFLD without gender difference [10]. Li et al. determined the optimal cutoff point of FLI as 20 in the north Chinese population, which was similar to our findings [30]. But they did not detect the gender difference of the cutoff values. A validation study of FLI in Taiwan also showed lower cutoff values for NAFLD than Western populations [17]. Body size, body composition and fat distribution have difference among different races and ethnic groups due to the environment, nutrition factors and culture [38,39], which leads to the diversity of anthropometric and serological measurements. Women had higher percent of fat mass, extremity fat, and lower lean mass compared to men at the same level of age and BMI [40]. This may explain the lower cutoff values in female subjects. The optimal cutoff values of FLD in different population are more stable (28.7, 29.0, and 26.1 for total, male and female, respectively) than FLI, while the cutoff value for female subjects is also lower than male. So it is essential to apply corresponding cutoff values among different population.
Our results showed that FLI and FLD can be used to screen for NAFLD in eastern Chinese community population. The expenditure of these models in eastern China has not been studied. Jinzhou et al. compared the expenditure of several NAFLD-related models in western China [29]. FLI costs 20 Yuan per capita, which is lower than FLD and ZJU. So FLI may have advantages in expenditure and accessibility compared to other models. The cost-effectiveness of these models should be further studied.
The strength of this study includes large-scale population, comprehensive analysis of the variables and head to head comparison of included models. There are also some limitations in our study. Ultrasonography as a diagnostic method for NAFLD has limited sensitivity [41]. But ultrasound is a preferable method for large-scale screening of asymptomatic individuals like community population. Another limitation is we did not adjust for other factors such as physical activity, diet and smoke, which may have correlation with the risk of metabolic symptoms. Additionally, our study was retrospective, further prospective studies are needed to evaluate the applied values of these models.
Conclusion
In conclusion, we validated and compared eight NAFLD-related models for noninvasive diagnosis of NAFLD in eastern Chinese community population. FLI and FLD are more accurate and applicable in our study. The cutoff values should be used according to sex. The cost-effectiveness of NAFLD-related models should be further evaluated.
Acknowledgements
We thank all doctors and nurses who dedicated to the data collection and kind suggestions.
This work was supported in part by the Six Talent Peak Project in Jiangsu Province (grant number 2019 WSN-049), Natural Science Foundation of Jiangsu Province (grant number BK20181369), Research and Innovation Team Project of School of Nursing, Nanjing Medical University, and the Priority Academic Program Development of Jiangsu Higher Education Institutions (grant number PAPD [2018] 87).
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Liuxin Zhang and Mengting Zhang contributed equally to the writing of this article.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website, www.eurojgh.com.
References
- 1.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018; 15:11–20. [DOI] [PubMed] [Google Scholar]
- 2.Ji YX, Huang Z, Yang X, Wang X, Zhao LP, Wang PX, et al. The deubiquitinating enzyme cylindromatosis mitigates nonalcoholic steatohepatitis. Nat Med 2018; 24:213–223. [DOI] [PubMed] [Google Scholar]
- 3.Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 2016; 64:1577–1586. [DOI] [PubMed] [Google Scholar]
- 4.Perumpail BJ, Khan MA, Yoo ER, Cholankeril G, Kim D, Ahmed A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol 2017; 23:8263–8276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang XJ, She ZG, Li H. Time to step-up the fight against NAFLD. Hepatology 2018; 67:2068–2071. [DOI] [PubMed] [Google Scholar]
- 6.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD; American Association for the Study of Liver Diseases. Liver biopsy. Hepatology 2009; 49:1017–1044. [DOI] [PubMed] [Google Scholar]
- 7.Duarte-Rojo A, Altamirano JT, Feld JJ. Noninvasive markers of fibrosis: key concepts for improving accuracy in daily clinical practice. Ann Hepatol 2012; 11:426–439. [PubMed] [Google Scholar]
- 8.Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic Fatty liver disease. Gastroenterology 2019; 156:1264–1281.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JS, Aoki K, Kawakubo K, Gunji A. A study on indices of body fat distribution for screening for obesity. Sangyo Eiseigaku Zasshi 1995; 37:9–18. [DOI] [PubMed] [Google Scholar]
- 10.Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, Tiribelli C. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol 2006; 6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord 2008; 6:299–304. [DOI] [PubMed] [Google Scholar]
- 12.Bedogni G, Kahn HS, Bellentani S, Tiribelli C. A simple index of lipid overaccumulation is a good marker of liver steatosis. BMC Gastroenterol 2010; 10:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petta S, Amato MC, Di Marco V, Cammà C, Pizzolanti G, Barcellona MR, et al. Visceral adiposity index is associated with significant fibrosis in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2012; 35:238–247. [DOI] [PubMed] [Google Scholar]
- 14.Fuyan S, Jing L, Wenjun C, Zhijun T, Weijing M, Suzhen W, Yongyong X. Fatty liver disease index: a simple screening tool to facilitate diagnosis of nonalcoholic fatty liver disease in the Chinese population. Dig Dis Sci 2013; 58:3326–3334. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Xu C, Xun Y, Lu Z, Shi J, Yu C, Li Y. ZJU index: a novel model for predicting nonalcoholic fatty liver disease in a Chinese population. Sci Rep 2015; 5:16494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng G, He N, Zhou YF, Li XP, Niu C, Liu ML, et al. A simpler diagnostic formula for screening nonalcoholic fatty liver disease. Clin Biochem 2019; 64:18–23. [DOI] [PubMed] [Google Scholar]
- 17.Yang BL, Wu WC, Fang KC, Wang YC, Huo TI, Huang YH, et al. External validation of fatty liver index for identifying ultrasonographic fatty liver in a large-scale cross-sectional study in Taiwan. PLoS One 2015; 10:e0120443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vanni E, Bugianesi E. Editorial: utility and pitfalls of Fatty Liver Index in epidemiologic studies for the diagnosis of NAFLD. Aliment Pharmacol Ther 2015; 41:406–407. [DOI] [PubMed] [Google Scholar]
- 19.Jian-gao F; Chinese Liver Disease Association. Guidelines for management of nonalcoholic fatty liver disease: an updated and revised edition. Zhonghua Gan Zang Bing Za Zhi 2010; 18:163–166. [PubMed] [Google Scholar]
- 20.National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Association CM, Fatty Liver Expert Committee, Chinese Medical Doctor Association. Guidelines of prevention and treatment for nonalcoholic fatty liver disease: a 2018 update. Zhonghua Gan Zang Bing Za Zhi 2018; 26:195–203. [DOI] [PubMed] [Google Scholar]
- 21.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988; 44:837–845. [PubMed] [Google Scholar]
- 22.Poynard T, Ratziu V, Naveau S, Thabut D, Charlotte F, Messous D, et al. The diagnostic value of biomarkers (SteatoTest) for the prediction of liver steatosis. Comp Hepatol 2005; 4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis 2010; 42:503–508. [DOI] [PubMed] [Google Scholar]
- 24.Long MT, Pedley A, Colantonio LD, Massaro JM, Hoffmann U, Muntner P, Fox CS. Development and validation of the Framingham steatosis index to identify persons with hepatic steatosis. Clin Gastroenterol Hepatol 2016; 14:1172–1180.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28:412–419. [DOI] [PubMed] [Google Scholar]
- 26.Otgonsuren M, Estep MJ, Hossain N, Younossi E, Frost S, Henry L, et al. Single non-invasive model to diagnose non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). J Gastroenterol Hepatol 2014; 29:2006–2013. [DOI] [PubMed] [Google Scholar]
- 27.Gastaldelli A, Kozakova M, Højlund K, Flyvbjerg A, Favuzzi A, Mitrakou A, Balkau B; RISC Investigators. Fatty liver is associated with insulin resistance, risk of coronary heart disease, and early atherosclerosis in a large European population. Hepatology 2009; 49:1537–1544. [DOI] [PubMed] [Google Scholar]
- 28.Kim D, Choi SY, Park EH, Lee W, Kang JH, Kim W, et al. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology 2012; 56:605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu J, He M, Zhang Y, Li T, Liu Y, Xu Z, Chen W. Validation of simple indexes for nonalcoholic fatty liver disease in western China: a retrospective cross-sectional study. Endocr J 2018; 65:373–381. [DOI] [PubMed] [Google Scholar]
- 30.Li C, Guo P, Zhang R, Zhang M, Li Y, Huang M, et al.; Nutrition and Health Institute. Both WHR and FLI as better algorithms for both Lean and Overweight/Obese NAFLD in a Chinese population. J Clin Gastroenterol 2019; 53:e253–e260. [DOI] [PubMed] [Google Scholar]
- 31.Huang Q, Jiang H, Zhang B, Wang H, Jia X, Huang F, et al. Threshold-effect association of dietary cholesterol intake with dyslipidemia in Chinese adults: results from the China Health and Nutrition Survey in 2015. Nutrients 2019; 11:2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Dai Y, Tian T, Zhang J, Xie W, Pan D, et al. The effects of dietary pattern on metabolic syndrome in Jiangsu Province of China: based on a nutrition and diet investigation project in Jiangsu Province. Nutrients 2021; 13:4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li L, You W, Ren W. The ZJU index is a powerful index for identifying NAFLD in the general Chinese population. Acta Diabetol 2017; 54:905–911. [DOI] [PubMed] [Google Scholar]
- 34.Pinho CPS, Diniz ADS, Arruda IKG, Leite APDL, Petribu MMV, Rodrigues IG. Waist circumference measurement sites and their association with visceral and subcutaneous fat and cardiometabolic abnormalities. Arch Endocrinol Metab 2018; 62:416–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fang H, Berg E, Cheng X, Shen W. How to best assess abdominal obesity. Curr Opin Clin Nutr Metab Care 2018; 21:360–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tchernof A, Després JP. Pathophysiology of human visceral obesity: an update. Physiol Rev 2013; 93:359–404. [DOI] [PubMed] [Google Scholar]
- 37.Gastaldelli A. Insulin resistance and reduced metabolic flexibility: cause or consequence of NAFLD? Clin Sci (Lond) 2017; 131:2701–2704. [DOI] [PubMed] [Google Scholar]
- 38.Khalil SF, Mohktar MS, Ibrahim F. The theory and fundamentals of bioimpedance analysis in clinical status monitoring and diagnosis of diseases. Sensors (Basel) 2014; 14:10895–10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rush EC, Freitas I, Plank LD. Body size, body composition and fat distribution: comparative analysis of European, Maori, Pacific Island and Asian Indian adults. Br J Nutr 2009; 102:632–641. [DOI] [PubMed] [Google Scholar]
- 40.Schorr M, Dichtel LE, Gerweck AV, Valera RD, Torriani M, Miller KK, Bredella MA. Sex differences in body composition and association with cardiometabolic risk. Biol Sex Differ 2018; 9:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fishbein M, Castro F, Cheruku S, Jain S, Webb B, Gleason T, Stevens WR. Hepatic MRI for fat quantitation: its relationship to fat morphology, diagnosis, and ultrasound. J Clin Gastroenterol 2005; 39:619–625. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.