Abstract
Our article Newly diagnosed neuromyelitis optica spectrum disorders following vaccination: Case report and systematic review had instigated a critique that there were more cases of post-COVID-19-vaccination NMOSD. Indeed, after the systematic review was performed in July 2021, many reports have been published, and we have seen two new patients at our center as well. However, Finsterer's question on the subclinical activity of NMOSD prior to vaccination, although an interesting notion, was debatable. NMOSD is a relapsing disease with severe attacks. Investigations in our patients did not reveal robust evidence of prior subclinical attacks so far.
Abbreviations: AQP4, aquaporin-4; COVID-19, Coronavirus disease; EDSS, expanded disability status scale; IVMP, intravenous methylprednisolone; LETM, longitudinally extensive transverse myelitis; MOGAD, myelin oligodendrocyte glycoprotein antibody-associated disease; MRC, Medical Research Council; MS, multiple sclerosis; NMOSD, neuromyelitis optica spectrum disorder; OCT, optical coherence tomography
1. Response to the commentary
Dear Editors,
The authors are pleased that our article Newly diagnosed neuromyelitis optica spectrum disorders following vaccination: Case report and systematic review (Anamnart et al., 2022) had instigated a critique. Finsterer raised concerns that our cases were not the only cases of post-Coronavirus (COVID-19)-vaccination NMOSD and that we should have provided evidence that there had been no NMOSD activity in our patients before COVID-19 vaccination (Finsterer, 2022). We believe that these points need to be clarified.
Indeed, ours were not the only cases of newly diagnosed NMOSD after COVID-19 vaccination since mass vaccination efforts were made across the globe during this pandemic. Both our cases presented in the middle of 2021. We performed a systematic review in July 2021 for NMOSD with onset within 30 days after vaccination (Anamnart et al., 2022; Langer-Gould et al., 2014) and submitted the manuscript in August. At that time, there had been no published reports of post-COVID-19-vaccination NMOSD. Therefore, those published afterward were not included in our systematic review. In fact, after submission, there have been two new cases in our center as well.
2. Case reports
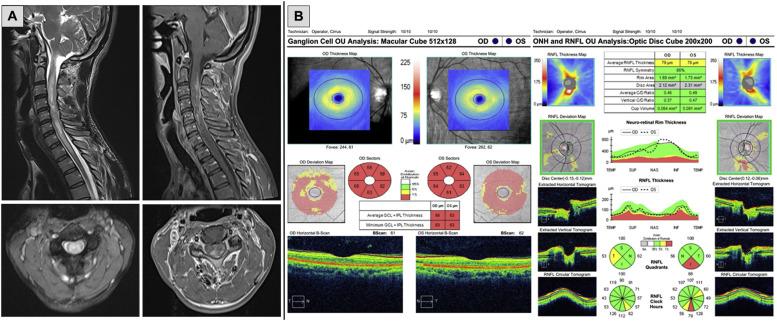
A 50-year-old healthy man received his second dose of the ChAdOx1 nCoV-19 vaccine in November 2021. Four days later, he developed left hemiparesis. The symptoms progressed to quadriparesis, quadruspasticity, painful tonic spasms, and urinary retention over one month. MRI revealed longitudinally extensive transverse myelitis (LETM) from C2 to T1 levels (Fig. 1), and his serum aquaporin-4 (AQP4) antibody was positive. His weakness improved with intravenous methylprednisolone (IVMP). With maintenance rituximab, there had been no relapses within five months of follow-up, and he was fully ambulatory with EDSS 3.5.
Fig. 1.
Spinal cord MRI and optical coherence tomography of the first patient.
The second case was a 70-year-old lady with no prior medical conditions. Ten days after the first dose of the ChAdOx1 nCoV-19 vaccine, she had electric shock-like pain along the left C5-T4 dermatomes which spontaneously resolved over two months. The same pain returned after the second dose of ChAdOx1 nCoV-19, and a spinal MRI showed focal T2 hyperintensity with faint enhancement at the T1 level. However, she was only seen by a neurologist a month later when her symptoms progressed to left arm weakness with MRC grade 4. Repeated spinal MRI showed LETM from C1 to T1 levels, and serum AQP4-IgG was positive. She received IVMP with partial recovery. At the three-month follow-up, her EDSS was 2, and there were no relapses with rituximab.
Fig. 1 shows a T2 hyperintense lesion at C2-T1 levels with cord edema and inhomogeneous gadolinium enhancement. Although this patient only had signs and symptoms of transverse myelitis, his optical coherence tomography shows thinning of the ganglion cell layer bilaterally (Fig. 1). There was no visual disturbance, and detailed history revealed no toxic-metabolic or hereditary clues. Therefore, the etiology of optic neuropathy was undetermined.
3. Subclinical NMOSD activity?
Typically, NMOSD is a severe relapsing demyelinating disease. The diagnosis is made by clinical syndromes, the presence of AQP4 antibody, and the aid of characteristic MRI findings. NMOSD is known to cause severe attack-related weakness with LETM (Lennon et al., 2004). Unlike multiple sclerosis (MS), where there might be disease progression despite not having any relapses, the existence of subclinical NMOSD is questionable (Akaishi et al., 2020). Thinning of the retinal nerve fiber layer in the eyes without overt optic neuritis was observed in NMOSD patients during follow-up (Pisa et al., 2020). However, whether there is subclinical inflammation of the optic nerve remains controversial (Jeong et al., 2016). Patients with the first episode of demyelinating disease are unlikely to have any baseline MRI, optical coherence tomography (OCT), or serum AQP4 antibody before disease onset.
Both our new patients and the two previously reported patients had no prior neurological abnormalities, and none had any baseline investigations. All four presented with transverse myelitis. However, three patients were also sent for OCT, which came back normal in two. The first patient in this case report had thin ganglion cell layers bilaterally without decreased visual acuity or color vision abnormality (Fig. 1). His MRI also showed T2 hyperintensity at both optic nerves without enhancement. These findings suggested subclinical bilateral optic neuropathy, but the etiology was undetermined. The absence of evidence of current optic nerve inflammation could not argue for or against a preceding episode of subclinical optic neuritis. None had any asymptomatic demyelinating brain or spine lesions on MRI.
Apart from NMOSD, myelin oligodendrocytes glycoprotein antibody-associated disease (MOGAD) presenting after vaccination have been reported as well (Francis et al., 2022). However, there was no difference in the frequency of serum SARS-CoV-2 IgG in the normal population and newly diagnosed MOGAD patients (Mariotto et al., 2022). The association between infection/vaccination and CNS inflammatory demyelinating diseases is an interesting area for future studies.
Author contribution
Dr. Tisavipat was involved in drafting and revising the manuscript for content, including medical writing for content, study concept and design, interpretation of data, and acquisition of data. Dr. Anamnart was involved in drafting and revising the manuscript for content, including medical writing for content, study concept and design, interpretation of data, and acquisition of data. Dr. Owattanapanich was involved in the study concept and design and study supervision. Dr. Apiwattanakul was involved in the acquisition of data. Dr. Savangned was involved in the acquisition of data. Dr. Siritho was involved in revising the manuscript for content and analysis, and interpretation of the data. Dr. Prayoonwiwat was involved in revising the manuscript for content and analysis, and interpretation of the data. Dr. Rattanathamsakul was involved in revising the manuscript for content and analysis, and interpretation of the data. Dr. Jitprapaikulsan was involved in drafting and revising the manuscript for content, including medical writing for content, study concept and design, interpretation of data, acquisition of data, and study supervision.
Consent to participate
Patients’ consents were obtained.
Ethics approval
The Siriraj Institutional Review Board approved this study.
Funding sources
No funding was received.
References
- Akaishi T., Takahashi T., Misu T., Abe M., Ishii T., Fujimori J., Aoki M., Fujihara K., Nakashima I. Progressive patterns of neurological disability in multiple sclerosis and neuromyelitis optica spectrum disorders. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-70919-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anamnart C., Tisavipat N., Owattanapanich W., Apiwattanakul M., Savangned P., Prayoonwiwat N., Siritho S., Rattanathamsakul N., Jitprapaikulsan J. Newly diagnosed neuromyelitis optica spectrum disorders following vaccination: case report and systematic review. Mult. Scler. Rel. Disord. 2022;58 doi: 10.1016/j.msard.2021.103414. [DOI] [PubMed] [Google Scholar]
- Finsterer J. Neuromyelitis optica complicating COVID vaccinations. Mult. Scler. Rel. Disord. 2022 doi: 10.1016/j.msard.2022.103809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis A., Palace J., Fugger L. MOG antibody-associated disease after vaccination with ChAdOx1 nCoV-19. Lancet Neurol. 2022;21(3):217–218. doi: 10.1016/S1474-4422(22)00043-6. [DOI] [PubMed] [Google Scholar]
- Jeong I.H., Kim H.J., Kim N.-H., Jeong K.S., Park C.Y. Subclinical primary retinal pathology in neuromyelitis optica spectrum disorder. J. Neurol. 2016;263(7):1343–1348. doi: 10.1007/s00415-016-8138-8. [DOI] [PubMed] [Google Scholar]
- Langer-Gould A., Qian L., Tartof S.Y., Brara S.M., Jacobsen S.J., Beaber B.E., Sy L.S., Chao C., Hechter R., Tseng H.F. Vaccines and the risk of multiple sclerosis and other central nervous system demyelinating diseases. JAMA Neurol. 2014;71(12):1506–1513. doi: 10.1001/jamaneurol.2014.2633. [DOI] [PubMed] [Google Scholar]
- Lennon V.A., Wingerchuk D.M., Kryzer T.J., Pittock S.J., Lucchinetti C.F., Fujihara K., Nakashima I., Weinshenker B.G. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364(9451):2106–2112. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- Mariotto S., Carta S., Dinoto A., Lippi G., Salvagno G.L., Masin L., Alberti D., Marignier R., Ferrari S. Is there a correlation between MOG-associated disorder and SARS-CoV-2 infection? Eur. J. Neurol. 2022 doi: 10.1111/ene.15304. n/a(n/a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisa M., Ratti F., Vabanesi M., Radaelli M., Guerrieri S., Moiola L., Martinelli V., Comi G., Leocani L. Subclinical neurodegeneration in multiple sclerosis and neuromyelitis optica spectrum disorder revealed by optical coherence tomography. Mult. Scler. J. 2020;26(10):1197–1206. doi: 10.1177/1352458519861603. [DOI] [PubMed] [Google Scholar]