Abstract
A higher incidence of female infertility has been reported with an unexpectedly early appearance in recent years. The female infertility treatment and application of assisted reproductive technology have recently gained immense interest from scientists. Many studies have discussed the beneficial effects of acupuncture on female infertility. With advancements in science and medical technology, acupuncture-related research has increased in investigating its effectiveness in treating female infertility. This review focuses on a compilation of research in recent years on acupuncture for female infertility treatment and the exploration of the underlying mechanism. For this purpose, literature was searched using various search engines like PubMed, Web of Science, and Google Scholar. The search was refined by only focusing on recent studies on acupuncture effectiveness and mechanism in female infertility and evaluating pregnancy outcomes.
1. Introduction
The reproduction cycle continued to work, but the primary focus was on arranging life commodities for the family in ancient times due to limited resources. The advancement in science and technology and the modernization of civilization led to an increase in the pace of people's lives. Their roles gradually diversified, which translated into an increase in the burden of responsibilities. Similarly, fertility seems no longer a “must” thing to ponder upon with the increasing enrichment of human spiritual culture. Although stupendous progress in human civilization is inevitable and cannot be segregated from spiritual beliefs, fertility problems demand attention.
An estimated 8 to 12% of couples of childbearing ages suffer infertility globally [1], and the incidence rate of female infertility is reported at 15% [2], making it the third primary disease after cancer and cardiovascular diseases. In addition, female infertility affects the women's psychological well-being and results in disturbed intrafamily harmony. With an increasing incidence rate of female infertility each year, it has become one of the urgent problems to be solved and has aroused the widespread concern of researchers. Infertility is a condition characterized by the failure to establish a clinical pregnancy after 12 months of regular unprotected sex [3]. Many factors, behaviors, and pathologies can hinder the pregnancy process and lead to infertility, except for artificial contraception [4].
Furthermore, increased age has also been regarded as one of the important contributing factors to female fertility [5]. The rate of female infertility incidence among younger women has increased in recent years [6]. Due to unhealthy lifestyles and environmental factors, the incidence of reproductive and endocrine diseases in female infertility is gradually growing, such as premature ovarian insufficiency (POI), polycystic ovary syndrome (PCOS), chronic endometritis (CE), and endometrial polyps [7]. They all hinder the occurrence of pregnancy from different nodes and lead to adverse pregnancy outcomes.
The recent increase in female infertility cases has also promoted the development and application of assisted reproductive technology, such as artificial insemination (AI), in vitro fertilization-embryo transfer (IVF-ET), and intracytoplasmic sperm injection (ICSI). However, the current success rate of AI and ET technology is reported to be 15% [8, 9] and 30 to 40% [10], respectively. Therefore, further improving the success rate of assisted reproductive technology is also the hotspot and focus of current research. The combined application of complementary and alternative medicine (CAM) and assisted reproductive technology may become a solution to improve the clinical pregnancy rate.
Acupuncture is an alternative auxiliary therapy that has existed for thousands of years and is widely used in clinical practice. There is much research evidence to support the therapeutic effect of acupuncture [11]. Traditional acupuncture is comprised of inserting a “needle” into specific parts (i.e., topically disinfected acupoints), followed by manipulation to enhance the sense of acupuncture after the needle enters the skin and reaches the site of action [12]. Later, with the progress of science and technology, to make the effect of acupuncture more obvious and lasting, a series of similar treatment methods have been derived, such as electroacupuncture (EA), acupoint catgut embedding (ACE), and transcutaneous electrical acupoint stimulation (TEAS) [13]. The working principle of EA, ACE, and TEAS is the same as that of traditional acupuncture, with only difference in the action parts and operating devices.
Being a novel subject, the development of assisted reproductive technology has gained much interest in recent years. The clinical efficacy of acupuncture in female infertility has undoubtedly aroused the interest of many reproductive doctors, and the research on acupuncture in female infertility has gradually increased, which provides a lot of evidence basis for the application of acupuncture in female infertility. This review summarizes the current application of acupuncture in treating female infertility. It further explores acupuncture's potential mechanism and application potential as an auxiliary and alternative therapy option for treating female infertility. This paper is also envisaged to promote research on acupuncture further and optimize the clinical treatment plan.
2. Causes of Female Infertility
Understanding female infertility requires prior knowledge and understanding of the pregnancy cycle. The pregnancy cycle starts with sperms entering the vagina and traveling upwards through the cervix into the uterus. The sperms then travel to fallopian tubes, meeting the ova and causing its fertilization. The fertilized egg follows the movement of cilia in the lining of the fallopian tube toward the uterine cavity. Once in the uterine cavity, it is implanted in the endometrium; that is, the embryo is injected to complete the pregnancy [14]. Thus, successful conception requires sperms auspiciously reaching the cervix, sperms-ova meet-up in the fallopian tubes, unhindered fertilization, successful traveling of the zygote into the uterine cavity, and successful embryo implantation. Female infertility can be caused due to the following reasons.
2.1. Abnormal Cervical Mucus
Abnormal cervical mucus will affect the penetration of sperm [15], making it much more difficult for them to enter the cervical opening. Under physiological conditions, cervical mucus can prevent pathogenic microorganisms from entering the uterus and infecting the endometrium [16], thus making it naturally viscous. During ovulation, to facilitate sperm entering the uterus, mucus permeability is selectively regulated under the biochemical interaction of a series of proteins and molecules [17, 18], primarily the expression levels of the trefoil factor family (TFF) peptide [15], resulting in reduced viscosity of the cervical mucus [19, 20]. This process is highly dependent on the regulation of sex hormones [21], so cervical mucus at different periods of the menstrual cycle has different mucin concentrations, which is the main reason why cervical mucus becomes thinner during ovulation [22]. The TFF is a polypeptide containing the P domain, which maintains the mucosal barrier. There are three types of human TFF, that is, TFF1, TFF2, and TFF3. TFF3 is secreted by gelatin forming mucin (GFM) cells that produce mucin [23]. It has been shown that TFF expression changes with the menstrual cycle, where the expression of TFF1 and TFF2 decreases during the ovulation period, while the expression of TFF3 significantly increases [24].
Cervical inflammation caused by pathogenic microorganism infection can also cause abnormal cervical mucus. Moreover, due to the infiltration of inflammatory secretions, patients often show sweeping changes in the amount, color, and quality of secretions [25]. At the same time, pathogens of reproductive tract infection like Chlamydia trachomatis and Neisseria gonorrhoeae [26, 27] are usually transmitted through sexual intercourse, which can directly lead to infertility by affecting sperm motility and impeding sperm entry.
2.2. Oocyte Quality
Oocyte quality is an independent factor in successful pregnancy [28, 29]. Each follicle comprises an oocyte with encapsulated granulosa cells and follicular membrane cells [30]. The average development and maturation of oocytes depend on the “nutrients” provided by granulosa cells and theca cells, such as steroid hormones, growth factors, and cytokines [31]. Studies have shown that mitochondria in granulosa cells are the core of steroid hormones required for follicular development [32]. Degraded granulosa cells are associated with mitochondrial swelling, the leading cause of oocyte apoptosis and follicular atresia [33]. Abnormal follicular development is not conducive to fertilization, fertilized egg implantation, and embryonic development [34]. Relative to it, the diseases leading to infertility mainly include POI and, more seriously, premature ovarian failure (POF) [35].
Increasing age will inevitably lead to ovaries aging [36], causing a gradual decrease in the number of follicular cells as well as the follicular pool in females, resulting in a decline in levels of inhibin B (INH-B) and anti-Müllerian hormone (AMH) secreted by granulosa cells in follicles [37], which are indicative of the declining function of ovaries, resulting in a reduction in the responsiveness of follicles to reproductive hormones [5]. “Fewer available follicles” and “decreased ovarian secretion function” are irreversible factors that contribute to female infertility [38].
In addition, bad living habits can also affect the quality of follicles by disrupting the average metabolic level [39], which is also the main reason for the gradual younger onset of POI/POF. For example, Zhang et al. [40] reported that women with BMI >29 kg/m2 showed lower fertility due to adverse effects of inferior follicular development on oocyte quality. Similarly, spicy food, improper sleep, and mental stress cause chronic inflammation in the body [41, 42], which result in reactive oxygen species (ROS) production and accumulation in granulosa cells to induce oxidative stress [43], which will further damage the function of organelles such as granulosa mitochondria and endoplasmic reticulum [44, 45], thus impairing the fertilization ability and normal development of oocytes [46], thus leading to infertility and adverse pregnancy outcomes. Similarly, the inflammatory-induced macrophage infiltration and significant secretion of proinflammatory factors increase the incidence of ovarian fibrosis [47–49], which compromises the ova's normal development and destroys the ovary's secretory function.
2.3. Ovulatory Dysfunction
Ovulatory dysfunction is the leading cause of infertility. The hypothalamic-pituitary-ovary (HPO) axis is essential in controlling female reproduction and the key to timely ovulation [50, 51]. World Health Organization (WHO) divides ovulatory dysfunction into three categories [52]: type I is gonadotropin-induced hypogonadism, mainly including hypothalamus and pituitary dysfunction, accounting for about 10% of ovulatory dysfunction; type II is HPO axis dysfunction, often due to some endocrine diseases, such as polycystic ovary syndrome (PCOS) and obesity; and type III is ovarian insufficiency. Ovulatory dysfunction mainly includes irregular ovulation and nonovulation, and such patients often presented with menstrual thinning or amenorrhea [53]. Hence, female infertility caused by ovulatory dysfunction often suggests a disordered reproductive hormone balance in patients.
PCOS is the most common ovulatory dysfunctional disease in women, accounting for approximately 80% of anovulatory infertility [54]. Research statistics show that PCOS affects 7 to 10% of women of childbearing age [55]. In recent years, it has been reported to be an endocrine disease caused by genetic, endocrine, and environmental factors [56]. Furthermore, abnormal metabolism results in persistently “high luteinizing hormone (LH)” status in patients, resulting in ovulatory dysfunction. Lifestyle adjustment is the first-line treatment for PCOS [53]. Therefore, a healthy lifestyle is essential for adjusting endocrine metabolism. In addition, Chavarro et al. [57] showed that following a nutritional diet plan benefits female fertility. Therefore, most cases of female infertility caused by ovulatory dysfunction can be prevented by changing diet and lifestyle.
Hyperthyroid hormones, caused by hyperprolactinemia (HPRL) and hypothyroidism, can interfere with the pulsed secretion of gonadotropin-releasing hormone, hinder the normal feedback regulation of HPO axis, and lead to ovulatory dysfunction. High thyroid-stimulating hormone (TSH) status induced by hyperprolactinemia and hypothyroidism can interfere with the pulsed secretion of GnRH and hinder the normal feedback regulation of the HPO axis, leading to ovulatory dysfunction [53, 58]. Furthermore, hypothalamic-pituitary failure (HPF) directly results in GnRH and/or gonadotropin (Gn) deficiency like idiopathic gonadotropic hypogonadism (IGH), panhypopituitarism, and Langerhans cell histiocytosis [50, 59].
Psychological stress-induced ovulation disorder infertility has received extensive attention in recent years. A series of neuroendocrine signal transduction produced by psychological stress will eventually be manifested as the continuous activation of the hypothalamus-pituitary-adrenal gland (HPA) axis [60], translating into the reduced release of central GnRH [61], disrupting the menstrual cycle, and inhibiting ovulation. Chronic stress directly acts on the hypothalamus to produce more corticotropin-releasing hormone (CRH) [62], while activation of the HPA axis results in more cortisol. Bolt's study has shown that cortisol and estradiol have the same coactivator to “fine-tune” transcription [63], and there is a significant overlap between glucocorticoids and estrogen-controlled signaling molecular networks [64], which indicates that elevated glucocorticoids will antagonize estrogen effects and disrupt regular ovulation. In a randomized controlled trial by Michopoulos [65], ovulation resumed in six of eight women treated with cognitive-behavioral therapy (CBT).
2.4. Tubal Obstruction
The anatomical-based tubal obstruction will directly hinder the sperm and egg combination, causing fertilization to fail. According to statistics, about 30% of the world's infertile women have tubal lesions [66]. Moreover, other common pathogenic contributing factors are Neisseria gonorrhoeae, Chlamydia trachomatis, endometriosis, and surgery [26, 67], leading to tubal infertility. Cilia in the fallopian tube become active during ovulation to transport the fertilized egg. However, pathological conditions such as tubal inflammation and hydrosalpinx result in cilia destruction, thereby reducing the ciliary movement [68], thus preventing the ova and sperm combination for successful fertilization and increasing the risk of infertility or ectopic pregnancy.
2.5. Endometrial Damage
Embryo implantation is the result of the interaction between blastocyst and endometrium. The key to its success lies in embryo quality and endometrium conditions. This process cannot be avoided even with assisted reproductive technology, so a good endometrium is necessary for a successful pregnancy. Endometrial receptivity (ER) is commonly used to evaluate the ability of an embryo's endometrial receptivity [69] and is closely related to the time of embryo implantation [70]. In most women, the endometrial implantation window is approximately five to seven days after ovulation [71]. Still, various pathological factors will shorten or shift the implantation window, which is not conducive to embryo implantation.
CE is a significant factor that damages the normal function and is often neglected due to the lack of apparent clinical manifestations [72]. Various “stimuli” promote changes in the number of white blood cells, cytokine production, and growth factors in the endometrial microenvironment [73], such as plasma cell infiltration and tissue edema [74], thus exacerbating their adverse effects on ER. In addition, the endometrium is regularly subjected to sex hormones under the regulation of the HPO axis, resulting in periodic growth changes. This growth pattern of the endometrium is of pivotal importance for a successful pregnancy, which CE compromises. Studies [73, 75] showed that antiapoptotic expressions, such as estrogen receptor, progesterone receptor, Ki-67 cell proliferation marker, B-cell lymphoma-2 (BCL-2), and BCL-2-associated X (BAX), were increased in endometrial epithelial cells and stroma of CE patients, leading to endometrial polyps and endometriosis [76]. Thus, it can be concluded that the immune disorder in the endometrial microenvironment is an essential factor leading to endometrial lesions. Endometrial polyps, endometriosis, adenomyosis, fibroids, and other space-occupying lesions can directly damage the endometrium and hinder embryo implantation. Surgical resection is the preferred treatment [77], but as an intrauterine operation, surgery has the possibility of compromising fertility, such as intrauterine adhesions [78].
In addition, CE has also been found to change the pattern of uterine contractions during ovulation and the luteal phase [79]. Under physiological conditions, in the proliferative phase, the uterus tends to contract from the fundus to the cervix, which is beneficial for menstrual discharging; in the ovulation and luteal phase, the retrograde contraction from the cervix to the fundus of the uterus is the primary process, which is conducive to the migration of sperm to the fallopian tube. However, CE reduces the probability of retrograde contractions during ovulation and the luteal phase by 3.3 folds [80], which will reduce the likelihood of successful pregnancy. It is also associated with additional discomfort symptoms, such as dysmenorrhea and pelvic pain [72, 81].
3. Acupuncture
Acupuncture is regarded as a multidimensional procedure that can prove being helpful in treating many diseases with minimal invasiveness using needles. It has been practiced for thousands of years, yet scientists started investigating its effects after the 18th century [82]. With the increasing application of acupuncture in clinical practice, researchers also developed a strong aptitude for researching acupuncture to treat various ailments. After decades of clinical practice and development, acupuncture was reported to possess significant clinical effects [11]. During early times, acupuncture was only realized through doctors' manipulations [83], which had high requirements for doctors' acupuncture skills. With the development of science and technology and the continuous integration of modern technology and medical technology, various acupuncture-derived therapies were introduced to strengthen the effect of acupuncture and facilitate clinical treatment, like EA and ACE.
3.1. Traditional Acupuncture
Traditional acupuncture involves inserting needles into meridians and acupoints [12]. To enhance the sense of needling, doctors often use auxiliary manipulation after needling the skin, such as repeatedly lifting and inserting and rotating the needling back and forth [83]. Primary scientific studies have shown [84] that manual stimulation of acupuncture needles can activate muscle afferent nerves on the one hand and exert its effect by locally generating electrical signals on the other. Manually operated acupuncture needles effectively regulate peripheral and central neural pathway activity [85]. When acupuncture is inserted into specific parts, the conduction through meridians will promote the coordination and balance of blood and energy throughout the body, producing a series of physio-psychological reactions [86, 87]. However, the manual operation of traditional acupuncture has high requirements on the physical strength of the doctor. Therefore, after inserting the needle into the corresponding acupoint, the doctor often uses electricity to translate the acupuncture into a more obvious therapeutic effect.
3.2. EA
EA comprises stimulating the hand-inserted needles with microcurrent waves (including continuous waves and intermittent waves), which can enhance the effect of acupuncture [88]. Furthermore, neural signals generated by EA stimulation were found to cause the hypothalamus to release endogenous opioids [89], such as β-endorphins, which are thought to play an essential role in regulating the effects of stress on reproductive function [90]. Previous studies have shown that [91, 92] acupuncture can change the level of plasma β-endorphin, thereby affecting the secretion of GnRH and Gn and then regulating the HPO axis to control female reproduction. Moreover, Stener [92, 93] reported that repeated EA therapy could reduce the high pulsatility index (PI) value in women's uterine arteries to an average level, which produced beneficial effects in increasing ER. Similarly, it can also improve the phenomenon of anovulation in PCOS patients.
Kim and Bae [94] showed that EA could produce a balance that enhanced the NK cell activity and promoted Th1/Th2 cell reaction. Moreover, Li et al. [95] demonstrated that acupuncture could inhibit free radicals (FR) metabolic imbalance and thus help achieve an antioxidant stress effect, which shows that acupuncture can regulate immunity and antioxidation, which can help regulate the immune balance in the endometrial microenvironment, treat CE, and increase ER.
3.3. ACE
ACE therapy is to embed absorbable sutures into acupoints, which will continuously stimulate the embedded part, to achieve the purpose of disease treatment, health care, and body strengthening [96]. The effect of catgut embedding is like that of acupuncture, but it is more lasting than acupuncture, and its stimulation can be maintained for two weeks or even longer [97]. A randomized controlled trial (RCT) conducted by Qin et al. [98] showed that ACE as a complementary therapy was beneficial for improving the rate of regular ovulation and clinical pregnancy.
4. Acupuncture and Infertility
4.1. Clinical Research
Female infertility results from an abnormal reproductive endocrine function [99]. Many diseases lead to female infertility, hindering the process of pregnancy from various nodes. Because acupuncture has the function of adjusting the neuroendocrine system [100], its application in the field of reproduction has attracted extensive attention. Acupuncture can act on multiple parts of the female reproductive system to improve its function through the action of neuroendocrine signaling molecules, to improve female fertility. Currently, acupuncture is mainly applied as a clinical adjuvant treatment of infertility conditions, such as IVF-ET and PCOS, as shown in Table 1.
Table 1.
Characteristics of clinical studies.
| First author | Year | Research type | Type of infertility | Intervention | Course of treatment | Evaluating indicator | ||
|---|---|---|---|---|---|---|---|---|
| T | C | P < 0.05 | P > 0.05 | |||||
| Andersen D | 2010 | RCT | IVF-ET | Acupuncture | N | 1 time | —— | CPR, PPR, LBR (−) |
| Moy I | 2010 | RCT | IVF-ET | Acupuncture | N | 1 time | —— | CPR (−) |
| Rashidi BH | 2013 | RCT | IVF-ET | Acupuncture | N | 4 times | NHQE⬆ | NRO, CPR, PPR, BPR (−) |
| Villahermosa DI | 2013 | RCT | IVF-ET | Acupuncture | N | 4 times | CPR⬆ | —— |
| Chen Q | 2015 | RCT | IVF-ET | Acupuncture | N | 12 weeks | (E2, P)⬆, EAI⬇ | CPR (−) |
| Jiang D | 2015 | RCT | PCOS | Acupuncture + WM | WM | 12 weeks | (CPR, ET/EM, CM)⬆, AR⬇ | —— |
| Wang X | 2016 | RCT | IVF-ET | Acupuncture | N | 30 times | (CPR, EM, Menstruation)⬆ | —— |
| Zhou L | 2016 | RCT | IVF-ET | Acupuncture | N | 15 ± 2 times | (E2, NRO, NHQE, CPR)⬆ | —— |
| Qu F | 2017 | RCT | IVF-ET | TEAS | N | 2 times | (CPR, LBR, NPY)⬆ | NRO, FR, NHQE, TGF-α, G-CSF (−) |
| Wu XK | 2017 | RCT | PCOS | Acupuncture + WM | WM | 16 weeks | —— | LBR (−) |
| Xu J | 2018 | RCT | PCOS | Acupuncture + WM | WM | 8 weeks | (CPR, COR)⬆, (T, LH)⬇ | AR, ET (−) |
| Yu L | 2018 | RCT | PCOS | Acupuncture + WM | WM | 24 times | (COR, ET/EM, E2, P)⬆ | CPR (−) |
| Altutunji AZ | 2019 | RCT | IVF-ET | Acupuncture | N | 2 weeks | (CPR, PPR, E2, P)⬆, OHSS incidence⬇ | AMH (−) |
| Kusuma AC | 2019 | RCT | IVF-ET | EA | False EA | 6 times | (NRO, FR, BCL-2)⬆, BAX⬇ | GDF9, BMP15 (−) |
| Shuai Z | 2019 | RCT | IVF-ET | TEAS | N | 16 ± 2 times | (CPR, LBR)⬆ | —— |
| Wu HC | 2019 | RCT | IVF-ET | EA | False EA | 12 weeks | (NHQE, CPR, PI3K/Akt mRNA)⬆ | NRO (−) |
| Dehghani AS | 2020 | RCT | IVF-ET | Acupuncture | N | 2 times | (CPR, PPR)⬆, BPR⬇ | —— |
| Guven PG | 2020 | RCT | IVF-ET | Acupuncture | N | 3 times | (CPR, PPR, LBR)⬆, STAI-1 score⬇ | —— |
| Xiang S | 2021 | RCT | IVF-ET | EA | N | 24 times | (NHQE, LBR, IRS-1/PI3K/GLUT4 mRNA)⬆ | NRO, CPR (−) |
| Zhai ZJ | 2021 | RCT | IVF-ET | TEAS (20 mA/30 mA/40 mA) | TEAS (5 mA) | 10–13 times | (NRO, ET)⬆ | NHQE, CPR (−) |
Note. T: Test group; C: Control group; RCT: Randomized controlled trial; IVF-ET: In vitro fertilization - embryo transfer; PCOS: Polycystic ovary syndrome; EA: Electroacupuncture; TEAS: Transcutaneous electrical acupoint stimulation; N: None; WM: Western medicine; CPR: Clinical pregnancy rate; PPR: Persistent pregnancy rate; LBR: live birth rate; BPR: Biochemical pregnancy rate; COR: Cycle ovulation rate; AR: Abortion rate; FR: Fertilization rate; NRO: Number of retrieved oocytes; NHQE: Number of high-quality embryos; ET: Endometrial thickness; EM: Endometrial morphology; EAI: Endometrial arterial impedance; CM: Cervical mucus; E2: Estradiol; P: Progesterone; STAI-1: State-trait anxiety inventory-1; OHSS: Ovarian hyperstimulation syndrome; NPY: Neuropeptide Y; TGF-α: Transforming growth factor-α; G-CSF: Granulocyte colony stimulating factor; BCL-2: B-cell lymphoma-2; BAX: Bcl2-Associated X; PI3K: Phosphatidylinositol-3-kinase; AKT: Protein kinase B; IRS-1: Insulin receptor substrate-1; GLUT4: Glucose transporter 4; GDF9: Growth differentiation factor 9; BMP15: Bone morphogenetic protein 15; ⬆: Improve; ⬇: Reduce; (−): Unchanged.
Six RCTs [101–106] of IVF-ET patients treated with acupuncture showed a significantly increased clinical pregnancy rate than those without acupuncture. However, four other studies [107–110] reported the opposite results. Following a thorough analysis, we found that the RCTs that showed increased pregnancy rates were mainly from the last two to three years, while the RCTs that showed adverse effects were more than a decade old. Thus, it can be hypothesized that this could be due to the continuous development of acupuncture technology over time, which also improved the clinical efficacy of acupuncture.
Rashidi et al. [109] showed that acupuncture could improve the rate of high-quality embryos in IVF patients. Chen and Hau [110] showed that acupuncture could increase the estradiol (E2) and progesterone (P) levels in IVF patients and promote the endometrial artery blood flow; all of these are conducive to embryo transfer. Similarly, after follow-up, three studies showed significant differences in the rate of persistent pregnancy between the two groups [104–106]. Guven et al. [106] showed that IVF-ET patients treated with acupuncture had a higher live birth rate and a lower state-trait anxiety inventory-1 (STAI-1) score. Wang et al. [102] demonstrated that acupuncture could improve the endometrial morphology and menstruation in IVF-ET patients, and Zhou et al. [103] showed that acupuncture significantly improved blood E2 levels, the number of ova obtained, and the number of high-quality embryos in IVF patients.
Furthermore, Altutunji et al. [104] showed that acupuncture could increase blood E2 and P levels and reduce the incidence of ovarian hyperstimulation syndrome (OHSS). Therefore, it can be found that acupuncture can improve the clinical pregnancy rate of IVF-ET patients by ameliorating the level of sex hormones and promoting the endometrial condition. In addition, acupuncture also reduced the occurrence of ovarian hyperstimulation syndrome (OHSS) and alleviated patients' anxiety to a certain extent.
Three other RCTs [111–113] reported the effect of EA on IVF-ET infertility patients. Kusuma et al. [111] showed that EA could improve the number of ova obtained by IVF. Further testing found that BAX expression decreased, and BCL-2 expression increased in patients with EA treatment, which confirmed that EA could help patients get more follicles by inhibiting granulosa cell apoptosis. However, Wu et al. [112] and Xiang et al. [113] showed opposite results regarding the number of obtained ova but concluded that EA could significantly improve the rate of high-quality embryos in IVF patients. However, their research results on clinical pregnancy rates were different. We speculate that this may be related to the course of EA treatment. Studies with longer EA treatment cycles have shown better efficacy in clinical pregnancy rates than short-term EA treatment [112]. The study of Xiang et al. [113] showed that EA could significantly improve the live birth rate of IVF patients, although there was no significant difference in clinical pregnancy rate. Therefore, we can find that EA can enhance ova quality to a certain extent, but further facilitating the pregnancy outcome may require a long period of repeated EA stimulation.
Three other RCTs [114–116] reported the effect of TEAS on patients with IVF-ET infertility. Qu et al. [114] and Shuai et al. [116] reported that TEAS significantly increased the clinical pregnancy rate and live birth rate in IVF patients compared with those without TEAS intervention. Similarly, Zhai et al. [115] showed that TEAS (20/30/40 mA) showed no significant difference in clinical pregnancy rate compared to TEAS (5 mA), depicting that though TEAS is beneficial for pregnancy outcomes, in IVF-ET patients, the optimal stimulation intensity of TEAS is yet to be explored.
Four RCTs [117–120] investigated the effect of acupuncture on infertility patients with PCOS. Jiang et al. [117] and Xu et al. [119] reported that the group with acupuncture intervention had a significant increase in clinical pregnancy rate compared to the group that received only western medicine treatment regimen. However, Jiang and Xu's findings contradicted the study conducted by Yu et al. [120]. Moreover, it was reported that acupuncture can improve the cycle ovulation rate, E2 and P levels, and endometrial morphology is also envisaged to benefit patients with PCOS. Xu et al. [119] also reported that acupuncture could improve the rate of regular ovulation and reduce serum T and LH levels in infertility patients with PCOS. Nevertheless, Wu et al. [118] reported that acupuncture could not improve the live birth rate. Therefore, it is hypothesized that acupuncture could help PCOS patients ovulate by improving sex hormone levels before pregnancy, thus increasing the pregnancy rates. However, the therapeutic effects of acupuncture before pregnancy may not last until delivery.
4.2. Animal Studies
The detailed information regarding acupuncture in animals is shown in Table 2. Stener-Victorin reported that repeated EA stimulation significantly promoted the release of β-endorphins in the hypothalamus of polycystic ovary (PCO) rats and reduced nerve growth factor (NGF), corticotropin-releasing factor (CRF), and endothelin-1 (ET-1) in the ovaries of PCO rats [95, 121–123]. The β-endorphins can inhibit GnRH secreted by the hypothalamus and LH released by the pituitary [124]. Some scholars argue that endogenous opioid drugs are closely related to the surge of LH before ovulation [125, 126]. Therefore, EA helps improve the “high-LH” state and restore normal ovulation function for PCO rats. The increase in nerve growth factor (NGF) and endothelin-1 (ET-1) was found to be associated with ovarian sympathetic excitation [127, 128], which has been reported in many inflammatory and autoimmune states [129]. The NGF is one of the target neurotrophic factors involved in developing and maintaining ovarian innervation [130]; ET-1 is a vital peptide that regulates blood flow in the ovary and is an effective and durable vasoconstrictor [122]. CRF is related to stress [131]. The repeated EA treatment was also found to improve the frequency and activation of T and natural killer (NK) cells, but the difference was insignificant.
Table 2.
Characteristics of animal studies.
| First author | Year | Experimental object | Modeling drugs | Intervention | Course of treatment (times) | Evaluating indicator | |
|---|---|---|---|---|---|---|---|
| P < 0.05 | P > 0.05 | ||||||
| Stener-victorin E | 2000 | PCO rats | EV | EA | 12 | NGF⬇ | Ovarian morphology (-) |
| Stener-victorin E | 2001 | PCO rats | EV | EA | 12 | CRF⬇ | —— |
| Stener-victorin E | 2003 | PCO rats | EV | EA | 12 | NGF⬇, ET-1⬇ | —— |
| Stener-victorin E | 2004 | PCO rats | EV | EA | 12 | β-Endorphins⬆ | NK cell, CD4 + /CD8 + T cell (-) |
| Zhang WY | 2009 | PCO rats | DHEA | Acupuncture | 5 | (NEI, Ovarian morphology, ET, E2)⬆, T⬇ | TSH, LH, P (-) |
| Huang J | 2020 | PCO rats | LE | EA | 14 | (AMH, PI3K, AKT)⬆, (T, LH, LC3 II/I)⬇ | Ovarian morphology (-) |
| Gao W | 2013 | ETF rats | Mifepristone | Acupuncture | 10 | (NEI, CCL2, CXCL8, uNK cell subsets)⬆ | —— |
| Zhu S | 2016 | COH mice | Gn | EA | 7 | (NEI, IGF-1)⬆ | —— |
| Wang S | 2019 | POF rats | CTX | Acupuncture | 21 | (E2, PI3K, Akt, BCL-2)⬆, (BAX, FSH)⬇ | Ovarian morphology (-) |
| Zhong H | 2019 | POF rats | CTX | EA | 7 | (NF, LS, E2, AMH)⬆, (PI3K, AKT, mTOR, S6K, 4E-BP1, FSH, LH)⬇ | —— |
Note. PCO: Polycystic ovary; ETF: Embryo transfer failure; COH: Controlled ovarian hyperstimulation; POF: Premature ovarian failure; EV: Estradiol valerate; DHEA: Dehydroepiandrosterone; LE: Letrozole; Gn: Gonadotropin; CTX: Cyclophosphamide; EA: Electroacupuncture; NGF: Nerve growth factor; CRF: Corticotropin releasing factor; ET-1: Endothelin-1; FSH: Follicle stimulating hormone; E2: Estradiol; T: Testosterone; ET: Endometrial thickness; NEI: Number of embryo implantation; LH: Luteinizing hormone; AMH: Anti-Mullerian hormone; LC3: Microtubule associated protein 1 light chain 3; PI3K: Phosphatidylinositol-3-kinase; AKT: Protein kinase B; CCL2: CC chemokine subfamily L2; CXCL8: CXC chemokine subfamily L8; IGF-1: Insulin growth factor; BCL-2: B-cell lymphoma-2; BAX: Bcl2-Associated X; NK cell: Natural killer cell; mTOR: mammalian target of rapamycin; S6K: S6 kinase; 4E-BP1: 4E-Binding Protein1; NF: Number of follicles; LS: Litter size; ⬆: Improve; ⬇: Reduce; (-): Unchanged.
Similarly, 12 cycles of EA treatment did not significantly improve ovarian morphology. However, it substantially improved the ovarian neuroendocrine function in PCO rats. The authors speculated that more extended periods of EA might translate into significant effectiveness. Zhang [132] demonstrated that acupuncture could significantly downregulate serum testosterone (T) and upregulate E2 expression in PCO rats, improve ovarian function, promote endometrial development, and promote embryo implantation.
Huang et al. [133] reported that EA could reduce serum T, LH, and AMH contents, reduce the ratio of ovarian LC3-II/I, and upregulate phosphatidylinositol-3-kinase (PI3K) and protein kinase B (PKB, also known as AKT) in PCO rats. The LC3 is a biomarker of autophagy. Cytoplasmic LC3 (LC3-I) will hydrolyze a small peptide and transform it into autophagosome (LC3-II), representing a direct relationship between a higher LC3-II/I ratio and a higher the level of autophagy.
A reduction in ovarian reserve function directly affects female reproduction ability. An increase in FSH and a decrease in E2 levels are typical of decreased ovarian reserve function [134]. Follicular development and maturation result from the oocytes' coregulation, mutual influence, and surrounding granule cells. It has been found that granulosa cells provide nutrients and growth signals for oocytes, and their apoptosis is the primary reason for follicular atresia [135]. A hormone is an important signal molecule in granulosa cell proliferation and apoptosis. In ovarian dysfunction, the sensitivity of follicles to hormones reduces and declines the ovaries' secretary function for estrogen and progesterone [136], both of which are not conducive to follicular development. FSH is an essential hormone for follicular development, which can bind to the FSH receptor on the granular cell membrane to activate upstream protein kinase A (PKA) and Grb2-associated Binder2 (GAB2), and downstream target factors and PI3K/AKT pathway are then activated to slow down the apoptosis of granulosa cells [137, 138]. Zhao et al. [139] showed that repeated EA stimulation significantly increased blood E2 concentration and restored HPO axis function in ovariectomy (OVX) rats. Further analysis showed that EA increased aromatase activity, mRNA, and protein expression in OVX rats, suggesting that FSH can also induce aromatase expression, stimulate ovarian E2 secretion, and promote granulosa cell maturation [136].
Wang et al. [140] showed that manual acupuncture could regulate hormone levels in POF rats (increase E2 and decrease FSH), upregulate PI3K/AKT signaling pathway, and promote follicular development, where higher expressions of PI3K, AKT, BCL-2 gene, and proteins were observed. In contrast, the expression of BAX decreased in POF rats after manual acupuncture, which could help reduce apoptosis of granulosa cells and reduce follicular atresia. In terms of morphology, though, acupuncture did not significantly reduce the apoptosis of granulosa cells in POF rats, suggesting that acupuncture was more about improving ovarian function. However, Zhang et al. [141] reported that EA reduced the phosphorylation of PI3K, AKT, mammalian target of rapamycin (mTOR), S6 kinase (S6K), and 4E-binding protein 1 (4E-BP1) and downregulated the ovarian PI3K/AKT/mTOR activation pathway in POF mouse. In terms of hormone levels, EA can upregulate serum AMH and E2 in POF mice and downregulate serum FSH and LH levels, which agrees with the reports of other researchers; hence, acupuncture is speculated to be beneficial to the level of sex hormones in POF rats.
Female POF mice treated with EA mated with male mice, and the outcome was compared with mice not subjected to EA treatment [141]. The results indicated that the EA group's litter size was significantly increased, indicating that acupuncture can improve the fertility of POF mice. Gao et al. [142] used mifepristone in pregnant rats to establish an “embryo implantation failure model.” After manual acupuncture treatment, compared with the nonacupuncture group, the number of implanted embryos, mRNA, and protein levels of CCL2 (CC chemokine subfamily L2) and CXCL8 (CXC chemokine subfamily L8) in the endometrium and uterine NK (uNK) cell subpopulations were significantly higher. The CCL2, CXCL8, and uNK cells are closely related to placental development [143]. The CCL2 can promote Th2 polarization and maintain a Th2 dominant environment, thus ensuring embryo implantation by regulating immunity [144]; CXCL8, on the other hand, stimulates the invasion of trophoblast cells outside villus in early pregnancy into the decidua by increasing MMP-2 secretion [145]. However, uNK cells in rats only appear during pregnancy. They often promote angiogenesis by expressing various cytokines, such as vascular endothelial growth factor (VEGF), placental growth factor (PGF), and angiopoietin 2 (ANG2), which is conducive to trophoblast invasion and placenta formation [146, 147]. This experiment suggested that acupuncture helps regulate the endometrial microenvironment through cytokine and chemokine pathways to aid embryo implantation during pregnancy. Zhu et al. [148] also reported that repeated EA stimulation could increase the secretion of insulin growth fact-1 (IGF-1) in the endometrium, thus improving the embryo implantation rate in mice. Fu et al. [149] reported that acupuncture could reduce the higher level of E2 in superovulation rats, and further studies found that the expression of estrogen receptor -β increased in rats' pituitary. These results suggest that acupuncture can prevent and reduce the incidence of OHSS by reducing the excessive increase in serum E2, which reflects the bidirectional adjustment ability of acupuncture and high safety.
5. Mechanism of Acupuncture Treatment of Female Infertility
As previously described, acupuncture has a positive treatment effect on female infertility. However, the causes of infertility vary, and understanding the role of acupuncture will help apply it to the appropriate type of infertility to achieve maximum efficacy. Based on the existing studies, it was found that acupuncture is beneficial in regulating female reproductive hormones and various cellular and immune signaling molecules, which are considered helpful in improving multiple aspects of the female reproductive system, such as follicular development, regular ovulation, and embryo implantation. Therefore, acupuncture has great application value in PCOS, POI, and IVF-ET. Furthermore, as a complementary and alternative therapy, acupuncture can improve female fertility and the success rate of assisted reproductive technology. As shown in Figure 1, the therapeutic effect of acupuncture on female infertility is mainly aimed at the following three aspects: reproductive endocrine, follicular development, and embryo implantation. The various mechanistic implications of acupuncture therapy in female infertility are discussed below.
Figure 1.
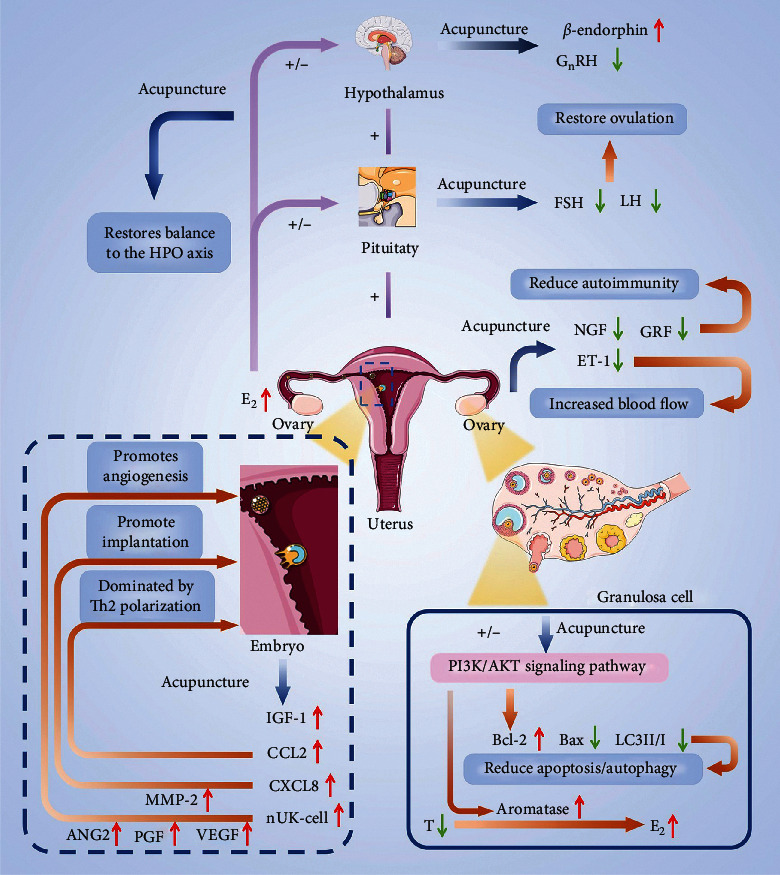
Mechanism of acupuncture in the treatment of infertility. The main function of acupuncture is to regulate reproductive related neuroendocrine signal molecules, in order to balance female reproductive endocrine, improve ovarian function, and promote embryo implantation. The symbol “⬆” represents increased expression and activity of the molecule, and the symbol “⬇” represents a decrease in the expression of this molecule. The symbol “+” represents positive feedback regulation, and the symbol “−” represents negative feedback regulation. Abbreviations are listed at the end of the article.
5.1. Reproductive Endocrine Pathway
The reproductive hormones are at their basic level during the follicular phase, and withdrawal of estrogen and progesterone induces menstruation. Low levels of E2 and P will alternatively promote the secretion of GnRH, encouraging the secretion of Gn (FSH and LH), which is essential for follicles development and results in the secretion of E2 and P. E2 and P are the material basis of endometrial proliferation and transformation and facilitate it in accepting embryos. This serial effect ensures the synchronization of follicular development and endometrial development required for pregnancy. The basic endocrine level of infertile women is “high Gn,” which inhibits follicular growth since higher levels of Gn inhibit the secretion of E2 and P, resulting in no longer regular ovulation, leading to infertility. Acupuncture can restore normal reproductive hormone levels. While reducing GnRH, FSH, LH, and increasing E2 and P, it is more important to restore the regularity of hormone interaction, make the reproductive system usually operate, and then promote pregnancy.
5.2. Improvement in Ovarian Function
There are two main ways to improve ovarian function: reducing granulosa cell apoptosis in follicles and improving the ovarian environment. During follicular development, granulosa cells are responsible for providing nutrients required maturation of the ovum. Follicular atresia and apoptosis are closely related to granulosa cell apoptosis. Acupuncture can increase the expression of BCL-2 and reduce the expression of BAX to reduce the apoptosis of granulosa cells, hence serving as an escort for follicular development. On the other hand, the internal ovarian environment is a prerequisite for follicular development. Acupuncture can reduce the expression of NGF, CRF, and ET-1 to reduce inflammation in the ovary and promote the blood supply in the ovary.
5.3. Improvement in Embryo Implantation
The interaction of an embryo with the endometrium microenvironment to form a biological connection refers to embryo implantation. Acupuncture can promote embryo implantation by three mechanisms, that is, modulating autoimmunity, where acupuncture resists the maternal immune response to embryos (foreign objects) by increasing the expression of CCL2. Similarly, by increasing the expression of CXCL8 and MMP-2, trophoblast cells can invade decidual tissue to establish a physical connection with the endometrium. The second mechanism refers to feeding, where acupuncture increases the expression of VEGF, ANG2, and PGF to promote angiogenesis, to establish the pathway of material exchange between mother and embryo. Finally, the other mechanism embarks on embryonic development, where acupuncture helps the development of embryos in the endometrium by increasing the expression of IGF-1. The classification and summary of relevant mechanisms are shown in Figure 2.
Figure 2.
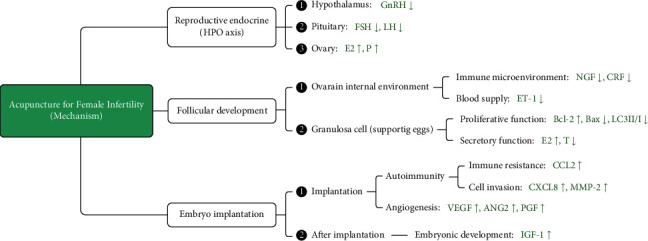
Classification of the mechanisms of acupuncture in treating infertility. The symbol “⬆” represents increased expression and activity of the molecule, and the symbol “⬇” represents a decrease in the expression of this molecule.
6. Summary and Outlook
The etiology of female infertility is complex and can be multifactorial, that is, pathological, physiological, psychological, and social. This review discusses the incidence of female infertility during the whole pregnancy cycle for the first time, which is envisaged to help understand the causes of female infertility and help target the clinical diagnosis and treatment. Female reproduction needs the reproductive system to provide a reasonable “material basis” on the one hand, and the neuroendocrine system to release accurate “signal” regulation on the other hand. Thus, successful pregnancy embodies the perfect cooperation between the reproductive and neuroendocrine systems. This ideal state is reflected in the interaction between signal molecules to achieve “dynamic balance.” A good “material base” is necessary for follicular development and endometrial growth.
Initially, the HPO axis is in a positive activation state: the hypothalamus releases GnRH, which prompts the pituitary gland to release FSH and LH, and the ovary begins to produce E2. Simultaneously, follicles start to develop, and intima begins to proliferate. Until the follicle maturation, LH and E2 levels significantly increase, followed by a negative activation state elicited by the HPO axis, resulting in LH and E2 levels dropping precipitously, thus inducing ovulation. Following this, the level of P rises, and the endometrium begins to prepare for embryo implantation. Hormones, as nutrients, participate in the whole process of follicular and intimal development. At the same time, its interaction precisely controls the entire process. The orderly progress of this process is the “balance state” of the female reproductive endocrine. An abnormality in any of these processes results in missing the pregnancy opportunity or might translate into unacceptable conditions for pregnancy, leading to infertility. For example, high LH will inhibit the production of E2 and P by a feedback mechanism, hindering follicular maturation, resulting in failure of endometrium transformation from proliferative to secretory phase. The key to successful pregnancy lies in the regular appearance of specific signal molecules and the “steady state” of the body's internal environment.
Based on existing studies, it was observed that the mechanism of acupuncture in the treatment of infertility is mainly reflected in four ways, that is, by adjusting HPO axis balance, where acupuncture reduces the “high GnRH” and “high LH” status in PCO rats by increasing the expression of β-endorphins, which is beneficial to follicular development. The second mechanism involves improving the ovary's internal environment by increasing ovarian blood flow and reducing the ovary's immune-inflammatory response by decreasing the expression of NGF, CRF, and ET-1. The third mechanism involves increasing the expression of BCL-2 and decreasing the expression of BAX and LC3-II to LC3-I ratio to reduce granulosa cell apoptosis and promote T's transformation to E2 by activating aromatase. The final mechanism involves helping the embryo implantation by upregulating the expression of IGF-1, CCL2, and CXCL8 signaling molecules to promote placental growth and ensure embryo implantation. All these mechanisms are conducive to restoring women's normal reproductive hormone levels, improving the function of the female reproductive system, and promoting the occurrence of pregnancy. The event of infertility is often the result of an “imbalance” in the human internal environment, such as “high GnRH” and “high LH.” Therefore, acupuncture can comprehensively regulate various signal molecules in the human body and improve reproductive function from multiple angles. Based on the above studies, it is believed that acupuncture as a complementary and alternative therapy in assisted reproduction could be meaningful.
Acupuncture has also been widely used in clinics, where in addition to its curative effect, its simple operation and fewer adverse reactions are additional advantages. Different from the endogenous reaction caused by drugs, as a surgical operation, acupuncture treatment does not involve the use of any chemicals; hence, it is considered relatively safe. At present, the current research results are not entirely consistent. We speculate that it is mainly due to clinical heterogeneity, including different acupuncture schemes and doctors' levels. Although acupuncture is easy to operate, its efficacy is determined by the angle, depth, single duration, optimal acupuncture time, treatment course of acupuncture, different acupuncture manipulations at different acupoints, and differences in individual responsiveness of patients. Differences in these control variables lead to heterogeneity between clinical studies and are the main reason for the inconsistent research results, yet the effectiveness of acupuncture cannot be ignored. Based on the existing clinical reports, we can find that the positive effect of acupuncture was reflected in all aspects conducive to pregnancy, as shown in Table 3. We can divide these evaluation indicators into two categories: prepregnancy indicators (detection indicators), which are the conditions to increase the probability of pregnancy, while the other is the observation index after pregnancy (i.e., to observe the pregnancy outcome intuitively). The reports on negative results only focused on the observation indicators after pregnancy, that is, pregnancy outcome. However, the positive effects of acupuncture on the physical state of infertile women were never denied. We know that exploring the differences in pregnancy outcomes is more challenging than detecting indicators with more stringent requirements in research design and observation duration. The premise of pregnancy is to have a good physical state. It mainly refers to mature follicles, high-quality embryos, and good endometrium, necessary conditions for a successful pregnancy. Therefore, in the process of observation and research, the effectiveness of acupuncture may first be reflected in the above aspects, followed by pregnancy outcomes. Thus, acupuncture is believed to be equally effective in resolving infertility issues through comprehensive analysis. It also necessitates studying the effectiveness of acupuncture in treating females' infertility by comparing results from different treatment cycles among other patient groups. It is believed that acupuncture effectiveness can be significantly reflected in longer treatment cycles, also reflected in the current research results, which are envisaged to help a more comprehensive understanding of acupuncture, improving the overall physiological function of infertile women.
Table 3.
Classification of clinical evaluation indexes of acupuncture in the treatment of infertility.
| Evaluation category | Specific items | |||
|---|---|---|---|---|
| Evaluation index before pregnancy | ||||
|
| ||||
| Cervix | CM | ⬆ | —— | |
| Endometrium | EAI, ET, EM | ⬆ | —— | |
| Ovary | NRO, COR | ⬆ | —— | |
| Embryo | FR, NHQE | ⬆ | —— | |
| Menstruation | Menstrual regularity | ⬆ | —— | |
| Ovarian function markers | NPY, AMH, BCL-2, IRS-1, (PI3K, Akt, GLUT4) mRNA | ⬆ | BAX | ⬇ |
| Hormone level | E2, P | ⬆ | T, LH | ⬇ |
| Security | —— | OHSS incidence | ⬇ | |
|
| ||||
| Evaluation index after pregnancy | ||||
| Pregnancy outcome | CPR, PPR, LBR | ⬆ | BPR, AR | ⬇ |
Note. CM: Cervical mucus; EAI: Endometrial arterial impedance; ET: Endometrial thickness; EM: Endometrial morphology; NRO: Number of retrieved oocytes; COR: Cycle ovulation rate; FR: Fertilization rate; NHQE: Number of high-quality embryos; NPY: Neuropeptide Y; AMH: Anti-Mullerian hormone; BCL-2: B-cell lymphoma-2; IRS-1: Insulin receptor substrate-1; PI3K: Phosphatidylinositol-3-kinase; AKT: Protein kinase B; GLUT4: Glucose transporter 4; BAX: Bcl2-Associated X; E2: Estradiol; P: Progesterone; T: Testosterone; LH: Luteinizing hormone; OHSS: Ovarian hyperstimulation syndrome; CPR: Clinical pregnancy rate; PPR: Persistent pregnancy rate; LBR: live birth rate; BPR: Biochemical pregnancy rate; AR: Abortion rate; ⬆: Improve; ⬇: Reduce.
Therefore, optimizing the acupuncture treatment plan in an all-around way will improve the clinical curative effect and help us explore acupuncture deeply. Similarly, it is suggested that more rigorous and more targeted studies in the future may provide strong evidence and help determine the application suitability of acupuncture in the field of reproduction, making it worthy of further investigation.
7. Conclusion
Acupuncture and related therapy can restore the average balance and regularity of sex hormones in infertile women by decreasing serum FSH and LH levels and increasing E2 and P levels, which are beneficial for follicle development and maturation, regular ovulation, and the normal physiological function of the inner membrane, which is conducive to conception. As a complementary and alternative therapy, acupuncture can play a positive role in PCOS-infertility, POI- infertility, and IVF-ET, with clinical application value.
Acknowledgments
The authors would like to thank the mapping help provided by Shanghai Mumuchuang Culture Communication Co., Ltd. At the same time, the authors would like to thank FreeScience (https://www.home-for-researchers.com) for the help with the English language.
Abbreviations
- POI:
Premature ovarian insufficiency
- PCOS:
Polycystic ovary syndrome
- CE:
Chronic endometritis
- AI:
Artificial insemination
- IVF:
In vitro fertilization
- IVF-ET:
In vitro fertilization-embryo transfer
- ICSI:
Intracytoplasmic sperm injection
- CAM:
Complementary and alternative medicine
- EA:
Electroacupuncture
- ACE:
Acupoint catgut embedding
- TEAS:
Transcutaneous electrical acupoint stimulation
- TFF:
Trefoil factor family
- GFM:
Gelatin forming mucin
- POF:
Premature ovarian failure
- INH-B:
Inhibin B
- AMH:
Anti-Mullerian hormone
- ROS:
Reactive oxygen species
- HPO:
Hypothalamic-pituitary-ovary
- LH:
Luteinizing hormone
- HPRL:
Hyperprolactinemia
- TSH:
Thyroid stimulating hormone
- GnRH:
Gonadotropin-releasing hormone
- HPF:
Hypothalamic-pituitary failure
- Gn:
Gonadotropin
- IGH:
Idiopathic gonadotropic hypogonadism
- HPA:
Hypothalamus-pituitary-adrenal
- CRH:
Corticotropin releasing hormone
- CBT:
Cognitive behavioral therapy
- ER:
Endometrial receptivity
- BCL-2:
B-cell lymphoma-2
- BAX:
BCL-2-associated X
- FR:
Free radicals
- RCT:
Randomized controlled trial
- E2:
Estradiol
- P:
Progesterone
- STAI-1:
State-trait anxiety inventory-1
- OHSS:
Ovarian hyperstimulation syndrome
- PCO:
Polycystic ovary
- NGF:
Nerve growth factor
- CRF:
Corticotropin releasing factor
- ET-1:
Endothelin-1
- LC:
Light chain
- T:
Testosterone
- PI3K:
Phosphatidylinositol-3-kinase
- PKB:
Protein kinase B
- PKA:
Protein kinase A
- GAB2:
Grb2-associated Binder2
- OVX:
Ovariectomy
- Mtor:
Mammalian target of rapamycin
- S6K:
S6 kinase
- 4E-BP1:
4E-Binding Protein1
- CCL2:
CC chemokine subfamily L2
- CXCL8:
CXC chemokine subfamily L8
- MMP-2:
Matrix metalloproteinase-2
- VEGF:
Vascular endothelial growth factor
- PGF:
Placental growth factor
- ANG2:
Angiopoietin 2
- IGF-1:
Insulin growth fact-1
- PI:
Pulsatility index.
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Ombelet W., Cooke I., Dyer S., Serour G., Devroey P. Infertility and the provision of infertility medical services in developing countries. Human Reproduction Update . 2008;14(6):605–621. doi: 10.1093/humupd/dmn042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Datta J., Palmer M. J., Tanton C., et al. Prevalence of infertility and help seeking among 15000 women and men. Human Reproduction . 2016;31(9):2108–2118. doi: 10.1093/humrep/dew123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zegers-Hochschild F., Adamson G. D., Dyer S., et al. The international glossary on infertility and fertility care, 2017. Fertility and Sterility . 2017;108(3):393–406. doi: 10.1016/j.fertnstert.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Vander Borght M., Wyns C. Fertility and infertility: definition and epidemiology. Clinical Biochemistry . 2018;62:2–10. doi: 10.1016/j.clinbiochem.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Hart R. J. Physiological aspects of female fertility: role of the environment, modern lifestyle, and genetics. Physiological Reviews . 2016;96(3):873–909. doi: 10.1152/physrev.00023.2015. [DOI] [PubMed] [Google Scholar]
- 6.Pisarska M. D. Fertility status and overall health. Seminars in Reproductive Medicine . 2017;35(03):203–204. doi: 10.1055/s-0037-1603728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Z. J., Liu J. Y., Huang H. F., et al. Guideline on diagnosis of infertility. Zhonghua Fu Chan Ke Za Zhi . 2019;54(8):505–511. doi: 10.3760/cma.j.issn.0529-567x.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Cohlen B., Bijkerk A., Van der Poel S., Ombelet W. IUI: review and systematic assessment of the evidence that supports global recommendations. Human Reproduction Update . 2018;24(3):300–319. doi: 10.1093/humupd/dmx041. [DOI] [PubMed] [Google Scholar]
- 9.Mikołajczyk M., Goździewicz T., Skrzypczak J. The efficiency of artificial insemination by husband sperm in infertile couples according to the revised WHO 2010 criteria. Ginekologia Polska . 2013;84(10):846–850. doi: 10.17772/gp/1650. [DOI] [PubMed] [Google Scholar]
- 10.European IVF-monitoring Consortium (EIM), European Society of Human Reproduction and Embryology (ESHRE), Calhaz-Jorge C., et al. Assisted reproductive technology in Europe, 2013: results generated from European registers by ESHRE. Human Reproduction . 2017;32(10):1957–1973. doi: 10.1093/humrep/dex264. [DOI] [PubMed] [Google Scholar]
- 11.Chon T. Y., Lee M. C. Acupuncture. Mayo Clinic Proceedings . 2013;88(10):1141–1146. doi: 10.1016/j.mayocp.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Cheong Y. C., Dix S., Hung Y. N. E., Ledger W. L., Farquhar C. Acupuncture and assisted reproductive technology. The Cochrane database of systematic reviews . 2013;7 doi: 10.1002/14651858.CD006920.pub3.CD006920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He J. R., Yu S. G., Tang Y., Illes P. Purinergic signaling as a basis of acupuncture-induced analgesia. Purinergic Signalling . 2020;16(3):297–304. doi: 10.1007/s11302-020-09708-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin J. Treatments for infertility. JAMA . 2015;313(3):p. 320. doi: 10.1001/jama.2014.7051. [DOI] [PubMed] [Google Scholar]
- 15.Lacroix G., Gouyer V., Gottrand F., Desseyn J. L. The cervicovaginal mucus barrier. International Journal of Molecular Sciences . 2020;21(21):p. 8266. doi: 10.3390/ijms21218266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yarbrough V. L., Winkle S., Herbst-Kralovetz M. M. Antimicrobial peptides in the female reproductive tract: a critical component of the mucosal immune barrier with physiological and clinical implications. Human Reproduction Update . 2015;21(3):353–377. doi: 10.1093/humupd/dmu065. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y. Y., Lai S. K., Ensign L. M., Zhong W., Cone R., Hanes J. The microstructure and bulk rheology of human cervicovaginal mucus are remarkably resistant to changes in pH. Biomacromolecules . 2013;14(12):4429–4435. doi: 10.1021/bm401356q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Witten J., Samad T., Ribbeck K. Selective permeability of mucus barriers. Current Opinion in Biotechnology . 2018;52:124–133. doi: 10.1016/j.copbio.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brunelli R., Papi M., Arcovito G., et al. Globular structure of human ovulatory cervical mucus. The FASEB Journal . 2007;21(14):3872–3876. doi: 10.1096/fj.07-8189com. [DOI] [PubMed] [Google Scholar]
- 20.Katz D. F., Slade D. A., Nakajima S. T. Analysis of pre-ovulatory changes in cervical mucus hydration and sperm penetrability. Advances in Contraception: The Official Journal of the Society for the Advancement of Contraception . 1997;13(2-3):143–151. doi: 10.1023/a:1006543719401. [DOI] [PubMed] [Google Scholar]
- 21.Andersch-Björkman Y., Thomsson K. A., Holmén Larsson J. M., Ekerhovd E., Hansson G. C. Large scale identification of proteins, mucins, and their O-glycosylation in the endocervical mucus during the menstrual cycle. Molecular & Cellular Proteomics . 2007;6(4):708–716. doi: 10.1074/mcp.m600439-mcp200. [DOI] [PubMed] [Google Scholar]
- 22.Grande G., Milardi D., Vincenzoni F., et al. Proteomic characterization of the qualitative and quantitative differences in cervical mucus composition during the menstrual cycle. Molecular BioSystems . 2015;11(6):1717–1725. doi: 10.1039/c5mb00071h. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann W. Trefoil factor family (TFF) peptides and their diverse molecular functions in mucus barrier protection and more: changing the paradigm. International Journal of Molecular Sciences . 2020;21(12):p. 4535. doi: 10.3390/ijms21124535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samson M. H., Chaiyarit P., Nortvig H., Vestergaard E. M., Ernst E., Nexo E. Trefoil factor family peptides in human saliva and cyclical cervical mucus. Method evaluation and results on healthy individuals. Clinical Chemistry and Laboratory Medicine . 2011;49(5):861–868. doi: 10.1515/cclm.2011.123. [DOI] [PubMed] [Google Scholar]
- 25.Ortiz-de la Tabla V., Gutiérrez F. Cervicitis: etiology, diagnosis and treatment. Enfermedades Infecciosas y Microbiología Clínica . 2019;37(10):661–667. doi: 10.1016/j.eimce.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 26.Karinen L., Pouta A., Hartikainen A. L., et al. Association between chlamydia trachomatis antibodies and subfertility in the northern Finland birth cohort 1966 (NFBC 1966), at the age of 31 years. Epidemiology and Infection . 2004;132(5):977–984. doi: 10.1017/s0950268804002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell C., Prabhu M. Pelvic inflammatory disease: current concepts in pathogenesis, diagnosis and treatment. Infectious Disease Clinics of North America . 2013;27(4):793–809. doi: 10.1016/j.idc.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uyar A., Torrealday S., Seli E. Cumulus and granulosa cell markers of oocyte and embryo quality. Fertility and Sterility . 2013;99(4):979–997. doi: 10.1016/j.fertnstert.2013.01.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoshino Y. Updating the markers for oocyte quality evaluation: intracellular temperature as a new index. Reproductive Medicine and Biology . 2018;17(4):434–441. doi: 10.1002/rmb2.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsuda F., Inoue N., Manabe N., Ohkura S. Follicular growth and atresia in mammalian ovaries: regulation by survival and death of granulosa cells. Journal of Reproduction and Development . 2012;58(1):44–50. doi: 10.1262/jrd.2011-012. [DOI] [PubMed] [Google Scholar]
- 31.Tanghe S., Van Soom A., Nauwynck H., Coryn M., de Kruif A. Minireview: functions of the cumulus oophorus during oocyte maturation, ovulation, and fertilization. Molecular Reproduction and Development . 2002;61(3):414–424. doi: 10.1002/mrd.10102. [DOI] [PubMed] [Google Scholar]
- 32.Sreerangaraja Urs D. B., Wu W. H., Komrskova K., et al. Mitochondrial function in modulating human granulosa cell steroidogenesis and female fertility. International Journal of Molecular Sciences . 2020;21(10):p. 3592. doi: 10.3390/ijms21103592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von Mengden L., Klamt F., Smitz J. Redox biology of human cumulus cells: basic concepts, impact on oocyte quality, and potential clinical use. Antioxidants and Redox Signaling . 2020;32(8):522–535. doi: 10.1089/ars.2019.7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lazzarino G., Pallisco R., Bilotta G., et al. Altered follicular fluid metabolic pattern correlates with female infertility and outcome measures of in vitro fertilization. International Journal of Molecular Sciences . 2021;22(16):p. 8735. doi: 10.3390/ijms22168735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thurston L., Abbara A., Dhillo W. S. Investigation and management of subfertility. Journal of Clinical Pathology . 2019;72(9):579–587. doi: 10.1136/jclinpath-2018-205579. [DOI] [PubMed] [Google Scholar]
- 36.Fortune J. E., Rivera G. M., Yang M. Y. Follicular development: the role of the follicular microenvironment in selection of the dominant follicle. Animal Reproduction Science . 2004;82-83:109–126. doi: 10.1016/j.anireprosci.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 37.Hansen K. R., Knowlton N. S., Thyer A. C., Charleston J. S., Soules M. R., Klein N. A. A new model of reproductive aging: the decline in ovarian non-growing follicle number from birth to menopause. Human Reproduction . 2008;23(3):699–708. doi: 10.1093/humrep/dem408. [DOI] [PubMed] [Google Scholar]
- 38.Steiner A. Z., Pritchard D., Stanczyk F. Z., et al. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. JAMA . 2017;318(14):p. 1367. doi: 10.1001/jama.2017.14588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu F., Liu W. N., Zhao Q. X., Han M. M. Study on environmental and psychological risk factors for female infertility. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi . 2013;31(12):922–933. in Chinese. [PubMed] [Google Scholar]
- 40.Zhang R., Linpeng S., Li Z., et al. Deficiency in GnRH receptor trafficking due to a novel homozygous mutation causes idiopathic hypogonadotropic hypogonadism in three prepubertal siblings. Gene . 2018;669:42–46. doi: 10.1016/j.gene.2018.05.050. [DOI] [PubMed] [Google Scholar]
- 41.Zhong J., Shi G. Editorial: regulation of inflammation in chronic disease. Frontiers in Immunology . 2019;10:p. 737. doi: 10.3389/fimmu.2019.00737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Umamaheswaran S., Dasari S. K., Yang P., Lutgendorf S. K., Sood A. K. Stress, inflammation, and eicosanoids: an emerging perspective. Cancer and Metastasis Reviews . 2018;37(2-3):203–211. doi: 10.1007/s10555-018-9741-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Biswas S. K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxidative Medicine and Cellular Longevity . 2016;2016 doi: 10.1155/2016/5698931.CD5698931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chong W. C., Shastri M. D., Eri R. Endoplasmic reticulum stress and oxidative stress: a vicious nexus implicated in bowel disease pathophysiology. International Journal of Molecular Sciences . 2017;18(4):p. 771. doi: 10.3390/ijms18040771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nordgren M., Fransen M. Peroxisomal metabolism and oxidative stress. Biochimie . 2014;98:56–62. doi: 10.1016/j.biochi.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 46.Kawwass J. F., Kulkarni A. D., Hipp H. S., Crawford S., Kissin D. M., Jamieson D. J. Extremities of body mass index and their association with pregnancy outcomes in women undergoing in vitro fertilization in the United States. Fertility and Sterility . 2016;106(7):1742–1750. doi: 10.1016/j.fertnstert.2016.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skaznik-Wikiel M. E., Swindle D. C., Allshouse A. A., Polotsky A. J., McManaman J. L. High-fat diet causes subfertility and compromised ovarian function independent of obesity in mice. Obstetrical and Gynecological Survey . 2016;71(9):532–533. doi: 10.1097/ogx.0000000000000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xie F., Anderson C. L., Timme K. R., Kurz S. G., Fernando S. C., Wood J. R. Obesity-dependent increases in oocyte mRNAs are associated with increases in proinflammatory signaling and gut microbial abundance of Lachnospiraceae in female mice. Endocrinology . 2016;157(4):1630–1643. doi: 10.1210/en.2015-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Araújo J. F., Podratz P. L., Sena G. C., et al. The obesogen tributyltin induces abnormal ovarian adipogenesis in adult female rats. Toxicology Letters . 2018;295:99–114. doi: 10.1016/j.toxlet.2018.06.1068. [DOI] [PubMed] [Google Scholar]
- 50.Mikhael S., Punjala-Patel A., Gavrilova-Jordan L. Hypothalamic-pituitary-ovarian axis disorders impacting female fertility. Biomedicines . 2019;7(1):p. 5. doi: 10.3390/biomedicines7010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maggi R., Cariboni A. M., Marelli M. M., et al. GnRH and GnRH receptors in the pathophysiology of the human female reproductive system. Human Reproduction Update . 2016;22(3):358–381. doi: 10.1093/humupd/dmv059. [DOI] [PubMed] [Google Scholar]
- 52.National Collaborating Centre for Women’s and Children’s Health (UK) Fertility: Assessment and Treatment for People with Fertility Problems . London, UK: Royal College of Obstetricians & Gynaecologists; 2013. [PubMed] [Google Scholar]
- 53.American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 194: polycystic ovary syndrome. Obstetrics & Gynecology . 2018;131(6):e157–e171. doi: 10.1097/AOG.0000000000002656. [DOI] [PubMed] [Google Scholar]
- 54.Balen A. H., Morley L. C., Misso M., et al. The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Human Reproduction Update . 2016;22(6):687–708. doi: 10.1093/humupd/dmw025. [DOI] [PubMed] [Google Scholar]
- 55.Snider A. P., Wood J. R. Obesity induces ovarian inflammation and reduces oocyte quality. Reproduction . 2019;158(3):R79–R90. doi: 10.1530/rep-18-0583. [DOI] [PubMed] [Google Scholar]
- 56.Morgante G., Massaro M. G., Di Sabatino A., Cappelli V., De Leo V. Therapeutic approach for metabolic disorders and infertility in women with PCOS. Gynecological Endocrinology . 2018;34(1):4–9. doi: 10.1080/09513590.2017.1370644. [DOI] [PubMed] [Google Scholar]
- 57.Chavarro J. E., Rich-Edwards J. W., Rosner B. A., Willett W. C. Diet and lifestyle in the prevention of ovulatory disorder infertility. Obstetrics & Gynecology . 2007;110(5):1050–1058. doi: 10.1097/01.aog.0000287293.25465.e1. [DOI] [PubMed] [Google Scholar]
- 58.Medenica S., Nedeljkovic O., Radojevic N., Stojkovic M., Trbojevic B., Pajovic B. Thyroid dysfunction and thyroid autoimmunity in euthyroid women in achieving fertility. European Review for Medical and Pharmacological Sciences . 2015;19(6):977–987. [PubMed] [Google Scholar]
- 59.Montefusco L., Harari S., Elia D., et al. Endocrine and metabolic assessment in adults with Langerhans cell histiocytosis. European Journal of Internal Medicine . 2018;51:61–67. doi: 10.1016/j.ejim.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 60.Berga S. L. Stress and reprodution: a tale of false dichotomy? Endocrinology . 2008;149(3):867–868. doi: 10.1210/en.2008-0004. [DOI] [PubMed] [Google Scholar]
- 61.Crowley W. F., Filicori M., Spratt D. I., Santoro N. F. The physiology of gonadotropin-releasing hormone (GnRH) secretion in men and women. Recent Progress in Hormone Research . 1985;41:473–526. doi: 10.1016/b978-0-12-571141-8.50015-9. [DOI] [PubMed] [Google Scholar]
- 62.Hawkins S. M., Matzuk M. M. The menstrual cycle: basic biology. Annals of the New York Academy of Sciences . 2008;1135(1):10–18. doi: 10.1196/annals.1429.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bolt M. J., Stossi F., Newberg J. Y., Orjalo A., Johansson H. E., Mancini M. A. Coactivators enable glucocorticoid receptor recruitment to fine-tune estrogen receptor transcriptional responses. Nucleic Acids Research . 2013;41(7):4036–4048. doi: 10.1093/nar/gkt100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Whirledge S., Xu X., Cidlowski J. A. Global gene expression analysis in human uterine epithelial cells defines new targets of glucocorticoid and estradiol antagonism. Biology of Reproduction . 2013;89(3):p. 66. doi: 10.1095/biolreprod.113.111054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Michopoulos V., Mancini F., Loucks T. L., Berga S. L. Neuroendocrine recovery initiated by cognitive behavioral therapy in women with functional hypothalamic amenorrhea: a randomized, controlled trial. Fertility and Sterility . 2013;99(7):2084–2091.e1. doi: 10.1016/j.fertnstert.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schlegel P. N., Fauser B. C., Carrel D. T., Racowsky C. Biennial Review of Infertility . New York, NY, USA: Springer; 2013. [Google Scholar]
- 67.Torre A., Pouly J. L., Wainer B. Anatomic evaluation of the female of the infertile couple. Journal de gynecologie, obstetrique et biologie de la reproduction . 2010;39:S34–S44. doi: 10.1016/s0368-2315(10)70029-6. [DOI] [PubMed] [Google Scholar]
- 68.Lyons R. A., Saridogan E., Djahanbakhch O. The reproductive significance of human Fallopian tube cilia. Human Reproduction Update . 2006;12(4):363–372. doi: 10.1093/humupd/dml012. [DOI] [PubMed] [Google Scholar]
- 69.Lessey B. A., Young S. L. What exactly is endometrial receptivity? Fertility and Sterility . 2019;111(4):611–617. doi: 10.1016/j.fertnstert.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 70.Dvořan M., Vodička J., Dostál J., et al. Implantation and diagnostics of endometrial receptivity. Ceská Gynekologie . 2018;83(4):291–298. [PubMed] [Google Scholar]
- 71.Tan J., Kan A., Hitkari J., et al. The role of the endometrial receptivity array (ERA) in patients who have failed euploid embryo transfers. Journal of Assisted Reproduction and Genetics . 2018;35(4):683–692. doi: 10.1007/s10815-017-1112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Greenwood S. M., Moran J. J. Chronic endometritis: morphologic and clinical observations. Obstetrics & Gynecology . 1981;58(2):176–184. [PubMed] [Google Scholar]
- 73.Kitaya K., Matsubayashi H., Yamaguchi K., et al. Chronic endometritis: potential cause of infertility and obstetric and neonatal complications. American Journal of Reproductive Immunology . 2016;75(1):13–22. doi: 10.1111/aji.12438. [DOI] [PubMed] [Google Scholar]
- 74.Michels T. C. Chronic endometritis. American Family Physician . 1995;52(1):217–222. [PubMed] [Google Scholar]
- 75.Wu D., Kimura F., Zheng L., et al. Chronic endometritis modifies decidualization in human endometrial stromal cells. Reproductive Biology and Endocrinology . 2017;15(1):p. 16. doi: 10.1186/s12958-017-0233-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Munro M. G. Uterine polyps, adenomyosis, leiomyomas, and endometrial receptivity. Fertility and Sterility . 2019;111(4):629–640. doi: 10.1016/j.fertnstert.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 77.American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Gynecology. Infertility workup for the women’s health specialist: ACOG committee opinion, number 781. Obstetrics & Gynecology . 2019;133(6):e377–e384. doi: 10.1097/AOG.0000000000003271. [DOI] [PubMed] [Google Scholar]
- 78.Sanders B. Uterine factors and infertility. Journal of Reproductive Medicine . 2006;51(3):169–176. [PubMed] [Google Scholar]
- 79.Pinto V., Matteo M., Tinelli R., Mitola P. C., De Ziegler D., Cicinelli E. Altered uterine contractility in women with chronic endometritis. Fertility and Sterility . 2015;103(4):1049–1052. doi: 10.1016/j.fertnstert.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 80.Coughlan C., Ledger W., Wang Q., et al. Recurrent implantation failure: definition and management. Reproductive BioMedicine Online . 2014;28(1):14–38. doi: 10.1016/j.rbmo.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 81.Puente E., Alonso L., Laganà A. S., Ghezzi F., Casarin J., Carugno J. Chronic endometritis: old problem, novel insights and future challenges. International journal of fertility & sterility . 2020;13(4):250–256. doi: 10.22074/ijfs.2020.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhuang Y., Xing J. J., Li J., Zeng B. Y., Liang F. History of acupuncture research. International Review of Neurobiology . 2013;111:1–23. doi: 10.1016/b978-0-12-411545-3.00001-8. [DOI] [PubMed] [Google Scholar]
- 83.Hu N. J., Lin C., Li J., et al. Remarks on the relationship between deqi and effect of acupuncture. Zhongguo Zhen Jiu . 2014;34(4):413–416. [PubMed] [Google Scholar]
- 84.Kaufman M. P., Waldrop T. G., Rybicki K. J., Ordway G. A., Mitchell J. H. Effects of static and rhythmic twitch contractions on the discharge of group III and IV muscle afferents. Cardiovascular Research . 1984;18(11):663–668. doi: 10.1093/cvr/18.11.663. [DOI] [PubMed] [Google Scholar]
- 85.Zhou W., Benharash P. Effects and mechanisms of acupuncture based on the principle of meridians. Journal of acupuncture and meridian studies . 2014;7(4):190–193. doi: 10.1016/j.jams.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 86.Lee H., Lee J. Y., Kim Y. J., et al. Acupuncture for symptom management of rheumatoid arthritis: a pilot study. Clinical Rheumatology . 2008;27(5):641–645. doi: 10.1007/s10067-007-0819-3. [DOI] [PubMed] [Google Scholar]
- 87.Tan E. K., Millington G. W. M., Levell N. J. Acupuncture in dermatology: an historical perspective. International Journal of Dermatology . 2009;48(6):648–652. doi: 10.1111/j.1365-4632.2009.03899.x. [DOI] [PubMed] [Google Scholar]
- 88.Zhao J. J., Rong P. J., Shi L., Ben H., Zhu B. Somato stimulation and acupuncture therapy. Chinese Journal of Integrative Medicine . 2016;22(5):394–400. doi: 10.1007/s11655-015-2088-3. [DOI] [PubMed] [Google Scholar]
- 89.Andersson S., Lundeberg T. Acupuncture — from empiricism to science: functional background to acupuncture effects in pain and disease Pain and disease. Medical Hypotheses . 1995;45(3):271–281. doi: 10.1016/0306-9877(95)90117-5. [DOI] [PubMed] [Google Scholar]
- 90.Rivier C., Rivest S. Effect of stress on the activity of the hypothalamic-pituitary-gonadal Axis: peripheral and central Mechanisms1. Biology of Reproduction . 1991;45(4):523–532. doi: 10.1095/biolreprod45.4.523. [DOI] [PubMed] [Google Scholar]
- 91.Chen B. Y., Yu J. Relationship between blood radioimmunoreactive beta-endorphin and hand skin temperature during the electro-acupuncture induction of ovulation. Acupuncture & Electro-Therapeutics Research . 1991;16(1):1–5. doi: 10.3727/036012991816358053. [DOI] [PubMed] [Google Scholar]
- 92.Victorin E. S., Lundeberg T., Waldenström U., et al. Effects of electro-acupuncture on nerve growth factor in rats with experimentally induced polycystic ovaries. Biology of Reproduction . 2000;63:1507–1513. doi: 10.1095/biolreprod63.5.1497. [DOI] [PubMed] [Google Scholar]
- 93.Stener-Victorin E., Waldenström U., Andersson S. A., Wikland M. Reduction of blood flow impedance in the uterine arteries of infertile women with electro-acupuncture. Human Reproduction . 1996;11(6):1314–1317. doi: 10.1093/oxfordjournals.humrep.a019378. [DOI] [PubMed] [Google Scholar]
- 94.Kim S. K., Bae H. Acupuncture and immune modulation. Autonomic Neuroscience . 2010;157(1-2):38–41. doi: 10.1016/j.autneu.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 95.Li Z. R., Shen M. H., Peng Y. J. Progress in researches on the effect of acupuncture in antagonizing oxygen stress. Chinese Journal of Integrative Medicine . 2005;11(2):156–160. doi: 10.1007/bf02836477. [DOI] [PubMed] [Google Scholar]
- 96.Huo J., Zhao J., Yuan Y., Wang J. Research status of the effect mechanism on catgut-point embedding therapy. Zhongguo Zhen Jiu . 2017;37(11):1251–1254. doi: 10.13703/j.0255-2930.2017.11.031. [DOI] [PubMed] [Google Scholar]
- 97.Wu J., Fu Q., Yang S., Wang H., Li Y. Efficacy and safety of acupoint catgut embedding for diarrhea-predominant irritable bowel syndrome and constipation-predominant irritable bowel syndrome: a systematic review and meta-analysis. Evidence-based Complementary and Alternative Medicine: eCAM . 2020;2020 doi: 10.1155/2020/5812320.5812320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Qin W., Zhao K., Yang H. Effect of acupoint catgut embedding therapy combined with Chinese medicine for nourishing the kidneys and promoting blood circulation and improving blood glucose and lipid levels as well as the pregnancy rate in obese PCOS patients with infertility. Experimental and Therapeutic Medicine . 2016;12(5):2909–2914. doi: 10.3892/etm.2016.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Inhorn M. C., Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Human Reproduction Update . 2015;21(4):411–426. doi: 10.1093/humupd/dmv016. [DOI] [PubMed] [Google Scholar]
- 100.Wang J. X., Yang Y., Song Y., Ma L. X. Positive effect of acupuncture and cupping in infertility treatment. Medical Acupuncture . 2018;30(2):96–99. doi: 10.1089/acu.2017.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.di Villahermosa D. I. M., dos Santos L. G., Nogueira M. B., Vilarino F. L., Barbosa C. P. Influence of acupuncture on the outcomes of in vitro fertilisation when embryo implantation has failed: a prospective randomised controlled clinical trial. Acupuncture in Medicine . 2013;31(2):157–161. doi: 10.1136/acupmed-2012-010269. [DOI] [PubMed] [Google Scholar]
- 102.Wang X., Jing F., Wang C., et al. The influence for success rate of warm acupuncture for tube baby of infertility patients with kidney yang deficiency:a randomized controlled trial. Zhongguo Zhen Jiu . 2016;36(9):906–910. doi: 10.13703/j.0255-2930.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 103.Zhou L., Xia Y., Ma X., et al. Effects of menstrual cycle-based acupuncture therapy on IVF-ET in patients with decline in ovarian reserve. Zhongguo Zhen Jiu . 2016;36(1):25–28. [PubMed] [Google Scholar]
- 104.Altutunji A. Z., Liu L., Cai J., Wang Z., Gao Y. The effect of acupuncture on anti-mullerian hormone and assisted reproduction outcome in Polycystic Ovary Syndrome patients undergoing in vitro fertilization. JPMA. The Journal of the Pakistan Medical Association . 2019;69(Suppl 3)(8):S4–S8. [PubMed] [Google Scholar]
- 105.Dehghani A. S., Homayouni K., Kanannejad Z., Kanannejad Z. The effect of acupuncture on the day of embryo transfer on the in vitro fertilization outcomes: an RCT. International journal of reproductive biomedicine . 2020;18(3):209–214. doi: 10.18502/ijrm.v18i3.6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Guven P. G., Cayir Y., Borekci B. Effectiveness of acupuncture on pregnancy success rates for women undergoing in vitro fertilization: a randomized controlled trial. Taiwanese Journal of Obstetrics & Gynecology . 2020;59(2):282–286. doi: 10.1016/j.tjog.2020.01.018. [DOI] [PubMed] [Google Scholar]
- 107.Andersen D., Løssl K., Nyboe Andersen A., et al. Acupuncture on the day of embryo transfer: a randomized controlled trial of 635 patients. Reproductive BioMedicine Online . 2010;21(3):366–372. doi: 10.1016/j.rbmo.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 108.Moy I., Milad M. P., Barnes R., Confino E., Kazer R. R., Zhang X. Randomized controlled trial: effects of acupuncture on pregnancy rates in women undergoing in vitro fertilization. Fertility and Sterility . 2011;95(2):583–587. doi: 10.1016/j.fertnstert.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 109.Rashidi B. H., Tehrani E. S., Hamedani N. A., Pirzadeh L. Effects of acupuncture on the outcome of in vitro fertilisation and intracytoplasmic sperm injection in women with polycystic ovarian syndrome. Acupuncture in Medicine . 2013;31(2):151–156. doi: 10.1136/acupmed-2012-010198. [DOI] [PubMed] [Google Scholar]
- 110.Chen Q., Hau C. Impacts on pregnancy outcome treated with acupuncture and moxibustion in IVF-ET patients. Zhongguo Zhen Jiu . 2015;35(4):313–317. [PubMed] [Google Scholar]
- 111.Kusuma A. C., Oktari N., Mihardja H., et al. Electroacupuncture enhances number of mature oocytes and fertility rates for in vitro fertilization. Medical Acupuncture . 2019;31(5):289–297. doi: 10.1089/acu.2019.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wu H. C., Zhang J. W., Sun Z. G., Xiang S., Qiao Y., Lian F. Effects of electroacupuncture on expression of PI3K/Akt/Foxo3a in granulosa cells from women with shen (kidney) deficiency syndrome undergoing in vitro fertilization-embryo transfer. Chinese Journal of Integrative Medicine . 2019;25(4):252–258. doi: 10.1007/s11655-019-2948-3. [DOI] [PubMed] [Google Scholar]
- 113.Xiang S., Xia M. F., Song J. Y., Liu D., Lian F. Effect of electro-acupuncture on expression of IRS-1/PI3K/GLUT4 pathway in ovarian granulosa cells of infertile patients with polycystic ovary syndrome-insulin resistance of phlegm-dampness syndrome. Chinese Journal of Integrative Medicine . 2021;27(5):330–335. doi: 10.1007/s11655-020-3219-z. [DOI] [PubMed] [Google Scholar]
- 114.Qu F., Wang F. F., Wu Y., et al. Transcutaneous electrical acupoint stimulation improves the outcomes of in vitro fertilization: a prospective, randomized and controlled study. Explore . 2017;13(5):306–312. doi: 10.1016/j.explore.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 115.Zhai Z. J., Liu J. E., Lei L. L., Wang S. Effects of transcutaneous electrical acupoint stimulation on ovarian responses and pregnancy outcomes in patients undergoing IVF-et: a randomized controlled trial. Chinese Journal of Integrative Medicine . 2021;28(5):434–439. doi: 10.1007/s11655-021-3457-8. [DOI] [PubMed] [Google Scholar]
- 116.Shuai Z., Li X., Tang X., Lian F., Sun Z. Transcutaneous electrical acupuncture point stimulation improves pregnancy outcomes in patients with recurrent implantation failure undergoing in vitro fertilisation and embryo transfer: a prospective, randomised trial. Acupuncture in Medicine . 2019;37(1):33–39. doi: 10.1136/acupmed-2017-011483. [DOI] [PubMed] [Google Scholar]
- 117.Jiang D., Zhang Y., Wu X., Wu S. Infertility in polycystic ovary syndrome treated with acupuncture and clomiphene: a randomized controlled trial. Zhongguo Zhen Jiu . 2015;35(2):114–118. [PubMed] [Google Scholar]
- 118.Wu X. K., Stener-Victorin E., Kuang H. Y., et al. Effect of acupuncture and clomiphene in Chinese women with polycystic ovary syndrome: a randomized clinical trial. JAMA . 2017;317(24):p. 2502. doi: 10.1001/jama.2017.7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xu J., Hong X., Zeng J., Wang X., Chen J. Efficacy of acupuncture as adjunctive treatment on infertility patients with polycystic ovary syndrome. Zhongguo Zhen Jiu . 2018;38(4):p. 358. doi: 10.1186/s13063-021-05426-y. [DOI] [PubMed] [Google Scholar]
- 120.Yu L., Cao L., Xie J., Shi Y. Therapeutic effects on ovulation and reproduction promotion with acupuncture and clomiphene in polycystic ovary syndrome. Zhongguo Zhen Jiu . 2018;38(3):263–268. doi: 10.13703/j.0255-2930.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 121.Victorin E. S., Lundeberg T., Waldenström U., Bileviciute-Ljungar I., Janson P. O. Effects of electro-acupuncture on corticotropin releasing-factor (CRF) in rats with experimentally induced polycystic ovaries. Neuropeptides . 2001;35:227–231. doi: 10.1054/npep.2002.0878. [DOI] [PubMed] [Google Scholar]
- 122.Victorin E. S., Lundeberg T., Cajander S., et al. Steroid-induced polycystic ovaries in rats: effect of electro-acupuncture on concentration of endothelin-1, nerve growth factor and NGF mRNA expression in the ovaries, adrenal glands and the central nervous system. Reproductive Biology and Endocrinology . 2003;1:p. 33. doi: 10.1186/1477-7827-1-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stener-Victorin E., Lindholm C. Immunity and beta-endorphin concentrations in hypothalamus and plasma in rats with steroid-induced polycystic ovaries: effect of low-frequency electroacupuncture. Biology of Reproduction . 2004;70(2):329–333. doi: 10.1095/biolreprod.103.022368. [DOI] [PubMed] [Google Scholar]
- 124.Wildt L., Sir-Petermann T., Leyendecker G., Waibel-Treber S., Rabenbauer B. Opiate antagonist treatment of ovarian failure. Human Reproduction . 1993;8(suppl 2):2168–2174. doi: 10.1093/humrep/8.suppl_2.168. [DOI] [PubMed] [Google Scholar]
- 125.Smith M. J., Jennes L. Neural signals that regulate GnRH neurones directly during the oestrous cycle. Reproduction . 2001;122(1):1–10. doi: 10.1530/rep.0.1220001. [DOI] [PubMed] [Google Scholar]
- 126.Jenkins P. J., Grossman A. The control of the gonadotrophin releasing hormone pulse generator in relation to opioid and nutritional cues. Human Reproduction . 1993;8(suppl 2):154–161. doi: 10.1093/humrep/8.suppl_2.154. [DOI] [PubMed] [Google Scholar]
- 127.Lara H. E., Dorfman M., Venegas M., et al. Changes in sympathetic nerve activity of the mammalian ovary during a normal estrous cycle and in polycystic ovary syndrome: studies on norepinephrine release. Microscopy Research and Technique . 2002;59(6):495–502. doi: 10.1002/jemt.10229. [DOI] [PubMed] [Google Scholar]
- 128.Lara H. E., Dissen G. A., Leyton V., et al. An increased intraovarian synthesis of nerve growth factor and its low affinity receptor is a principal component of steroid-induced polycystic ovary in the Rat. Endocrinology . 2000;141(3):1059–1072. doi: 10.1210/endo.141.3.7395. [DOI] [PubMed] [Google Scholar]
- 129.Levi-Montalcini R., Skaper S. D., Dal Toso R., Petrelli L., Leon A. Nerve growth factor: from neurotrophin to neurokine. Trends in Neurosciences . 1996;19(11):514–520. doi: 10.1016/s0166-2236(96)10058-8. [DOI] [PubMed] [Google Scholar]
- 130.Dissen G. A., Hill D. F., Costa M. E., Les Dees C. W., Lara H. E., Ojeda S. R. A role for trkA nerve growth factor receptors in mammalian ovulation. Endocrinology . 1996;137(1):198–209. doi: 10.1210/endo.137.1.8536613. [DOI] [PubMed] [Google Scholar]
- 131.Bala R., Singh V., Rajender S., Singh K. Environment, lifestyle, and female infertility. Reproductive Sciences . 2021;28(3):617–638. doi: 10.1007/s43032-020-00279-3. [DOI] [PubMed] [Google Scholar]
- 132.Zhang W. Y., Huang G. Y., Liu J. Influences of acupuncture on infertility of rats with polycystic ovarian syndrome. Zhongguo Zhong Xi Yi Jie He Za Zhi . 2009;29(11):997–1000. [PubMed] [Google Scholar]
- 133.Huang J., Tang C. L., Liao D. M. Effect of electroacupuncture on levels of serum sex hormones and expression of ovarian auto-phagy related factors in rats with polycystic ovary syndrome. Zhen Ci Yan Jiu . 2020;45(8):640–644. doi: 10.13702/j.1000-0607.190744. [DOI] [PubMed] [Google Scholar]
- 134.Bandyopadhyay S., Chakrabarti J., Banerjee S., et al. Galactose toxicity in the rat as a model for premature ovarian failure: an experimental approach readdressed. Human Reproduction . 2003;18(10):2031–2038. doi: 10.1093/humrep/deg414. [DOI] [PubMed] [Google Scholar]
- 135.Matsuda-Minehata F., Inoue N., Goto Y., Manabe N. The regulation of ovarian granulosa cell death by pro- and anti-apoptotic molecules. Journal of Reproduction and Development . 2006;52(6):695–705. doi: 10.1262/jrd.18069. [DOI] [PubMed] [Google Scholar]
- 136.Li X., Lang L., Ji Y., Wang B., Zhao C. Study on process of the signaling pathway of follicle stimulating hormone to promote the ovarian granulosa cell proliferation and differentiation. Journal of Beijing Union University . 2012;26:46–50. [Google Scholar]
- 137.Tsai-Turton M., Luong B. T., Tan Y., Luderer U. Cyclophosphamide-induced apoptosis in COV434 human granulosa cells involves oxidative stress and glutathione depletion. Toxicological Sciences . 2007;98(1):216–230. doi: 10.1093/toxsci/kfm087. [DOI] [PubMed] [Google Scholar]
- 138.Hunzicker-Dunn M. E., Lopez-Biladeau B., Law N. C., Fiedler S. E., Carr D. W., Maizels E. T. PKA and GAB2 play central roles in the FSH signaling pathway to PI3K and AKT in ovarian granulosa cells. Proceedings of the National Academy of Sciences of the United States of America . 2012;109(44):E2979–E2988. doi: 10.1073/pnas.1205661109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhao H., Tian Z. Z., Chen B. Y. Electroacupuncture stimulates hypothalamic aromatization. Brain Research . 2005;1037(1-2):164–170. doi: 10.1016/j.brainres.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 140.Wang S., Lin S., Zhu M., et al. Acupuncture reduces apoptosis of granulosa cells in rats with premature ovarian failure via restoring the PI3K/akt signaling pathway. International Journal of Molecular Sciences . 2019;20(24):p. 6311. doi: 10.3390/ijms20246311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang H., Qin F., Liu A., et al. Electro-acupuncture attenuates the mice premature ovarian failure via mediating PI3K/AKT/mTOR pathway. Life Sciences . 2019;217:169–175. doi: 10.1016/j.lfs.2018.11.059. [DOI] [PubMed] [Google Scholar]
- 142.Gao W., Tang X., Chen Z., et al. Effects of acupuncture on CCL2 and CXCL8 expression and the subset of uNK cells in rats with embryo implantation failure. Evidence-based Complementary and Alternative Medicine . 2013;2013:p. 12. doi: 10.1155/2013/678390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hirota Y., Osuga Y., Hasegawa A., et al. Interleukin (IL)-1β stimulates migration and survival of first-trimester villous cytotrophoblast cells through endometrial epithelial cell-derived IL-8. Endocrinology . 2009;150(1):350–356. doi: 10.1210/en.2008-0264. [DOI] [PubMed] [Google Scholar]
- 144.Chaouat G., Ledée-Bataille N., Dubanchet S., Zourbas S., Sandra O., Martal J. Th1/Th2 paradigm in pregnancy: paradigm lost? Cytokines in pregnancy/early abortion: reexamining the Th1/Th2 paradigm. International Archives of Allergy and Immunology . 2004;134(2):93–119. doi: 10.1159/000074300. [DOI] [PubMed] [Google Scholar]
- 145.De Oliveira L., Lash G. E., Murray-Dunning C., et al. Role of interleukin 8 in uterine natural killer cell regulation of extravillous trophoblast cell invasion. Placenta . 2010;31(7):595–601. doi: 10.1016/j.placenta.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 146.Li X. F., Jones D. S. C., Zhang E., et al. Angiogenic growth factor messenger ribonucleic acids in uterine natural killer cells. Journal of Clinical Endocrinology and Metabolism . 2001;86(4):1823–1834. doi: 10.1210/jc.86.4.1823. [DOI] [PubMed] [Google Scholar]
- 147.Dosiou C., Giudice L. C. Natural killer cells in pregnancy and recurrent pregnancy loss: endocrine and immunologic perspectives. Endocrine Reviews . 2005;26(1):44–62. doi: 10.1210/er.2003-0021. [DOI] [PubMed] [Google Scholar]
- 148.Zhu S., Liu J., Li C., et al. Impacts on thepregnancy outcome in the mice of controlled ovarian hyperstimulation treated with acupuncture at different time points. Zhongguo Zhen Jiu . 2016;36(11):1181–1185. doi: 10.13703/j.0255-2930.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 149.Fu H., Sun J., Tan Y., et al. Effects of acupuncture on the levels of serum estradiol and pituitary estrogen receptor beta in a rat model of induced super ovulation. Life Sciences . 2018;197:109–113. doi: 10.1016/j.lfs.2018.02.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.


