PURPOSE
An association with a reduction in the risk of all-cause mortality (ACM) and the use of adjuvant as compared with early postradical prostatectomy salvage radiation therapy (sRT) in men with pN1 prostate cancer (PC) has been observed. Yet, whether this finding applies irrespective of the number of positive lymph nodes (LNs) after adjusting for the time-dependent use and duration of androgen deprivation therapy is unknown and is addressed in the current study.
METHODS
Univariable and multivariable Cox regression was used to evaluate whether the ACM risk ratio for time-dependent use of adjuvant versus early sRT per unit increase in positive pelvic LNs was significantly reduced. Adjusted ACM estimates were calculated among men who received adjuvant, early salvage, or no RT stratified by one to three or four or more positive pelvic LNs.
RESULTS
After a median follow-up of 7.02 years, 986 (5.50%) men died, with 223 (22.62%) of PC. Adjuvant compared with early sRT was associated with a significantly lower ACM risk per unit increase in positive pelvic LNs (adjusted hazard ratio: 0.92; 95% CI, 0.85 to 0.99; P = .03). A significant difference in the 7-year adjusted ACM estimates favoring aRT versus early sRT was observed in men with four or more positive LNs (7.74% v 23.36%) in that the 95% CI for the 15.62% difference (5.90 to 25.35) excluded 0.00, but this was not true for men with 1-3 positive LNs (14.27% v 13.89%; 95% CI for the 0.38% difference [–7.02 to 7.79]).
CONCLUSION
Adjuvant compared with early sRT in men with pN1 PC was associated with a decreased ACM risk, and this reduction increased with each additional positive pelvic LN.
INTRODUCTION
Level 1 evidence to guide the optimal timing of postradical prostatectomy (RP) radiation therapy (RT) is lacking in men with pN1 prostate cancer (PC). Moreover, very few men with pN1 PC were included in three randomized trials and accompanying meta-analysis1 that compared the impact of adjuvant with early salvage radiation therapy (sRT) on progression and concluded that early sRT was preferable. Yet, the result from the meta-analysis1 may be inappropriately extrapolated to men with pN1 PC. More recently, a study2 revealed a significant association with a reduction in the risk of all-cause mortality (ACM) and the use of adjuvant (aRT) as compared with early sRT in men with pN1 or pT3/4 and pGleason score 8-10 PC. A previous study3 found a significant association with the use of aRT compared with early sRT and decreased biochemical failure and distant metastasis in men with pN1 PC, but in that study, an adjustment for the time-dependent use and duration of androgen deprivation therapy (ADT) was not performed nor was the ACM end point explored. In addition, in a previous report,4 an association with a reduction in ACM risk when using aRT as compared with early sRT was not observed in men with more than four positive lymph nodes (LNs). However, again, in that study, an adjustment for the time-dependent use and duration of ADT was not made, yet ADT's use, timing, and duration can significantly affect the time to death.
CONTEXT
Key Objective
Could a possible reduction in all-cause mortality risk when using adjuvant versus early salvage radiation therapy (sRT) in men found to have more than four positive pelvic lymph nodes (pN1) after radical prostatectomy for prostate cancer have been missed given that previous studies did not adjust for the time-dependent use and duration of androgen deprivation therapy, which are known to affect the time to death in this patient population?
Knowledge Generated
After adjustment for the time-dependent use and duration of androgen deprivation therapy, adjuvant RT use was associated with a significant reduction in all-cause mortality risk compared with early salvage radiation therapy. Importantly, the magnitude of this reduction increased by 8% with each additional positive pelvic LN.
Relevance
Men with pN1 prostate cancer and 4 or more as compared with 1-3 positive pelvic LNs appear to benefit the most from the use of adjuvant RT.
Given the lack of level 1 evidence to guide the timing of post-RP RT in men with pN1 PC and the worse prognosis with the increasing number of positive LNs and/or positive LN density,5,6 whether the association of a reduction in ACM risk when using adjuvant as compared with early sRT applies irrespective of the number of positive pelvic LNs after adjusting for the time-dependent use and duration of ADT is clinically relevant and is explored in the current study.
METHODS
Patient Population and Treatment
The study cohort comprised 17,913 men with a median (interquartile range [IQR]) age of 64 (59-68) years with pT2-4N1M0 PC consecutively treated between March 7, 1995, and October 5, 2017, with RP and pelvic LN assessment at the University Hospital Hamburg-Eppendorf (Hamburg, Germany) and then followed for possible treatment with aRT or early sRT. The median (IQR) number of LNs removed among all men in the study cohort was 12 (IQR: 7-20), and the median number of positive LNs was 1 (IQR: 1-3). The use of aRT, early sRT, or no RT among the 17,913 men stratified by the number of positive pelvic LNs (0, 1-3, 4 or more) is shown in Figure 1. Adjuvant RT and early sRT to the pelvic LNs (45 Gy) and prostatic bed (median dose: 68.4 Gy) were delivered at a median of 3.42 months (IQR: 2.79-4.14 months) and 21.36 months (IQR: 8.28-43.42 months) after RP, respectively.
FIG 1.
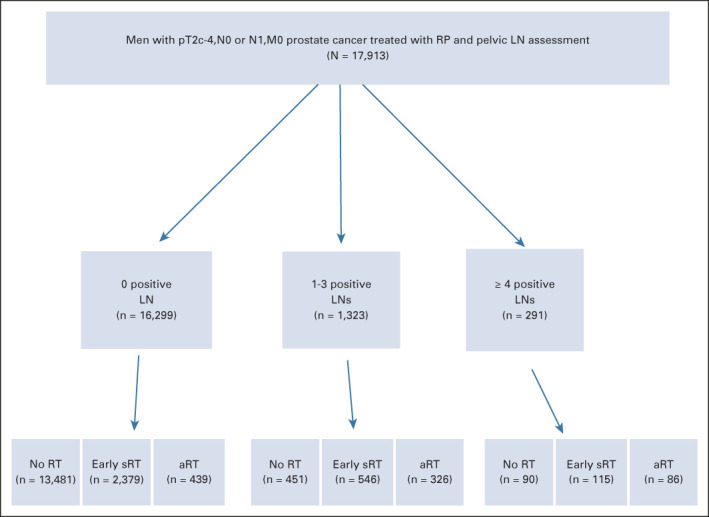
CONSORT diagram illustrating the distribution of adjuvant, early salvage, or no radiation therapy use over the study period among the 17,913 men in the study cohort stratified by the number of positive pelvic LNs. Given time 0 is defined as the date of RP, the numbers for men who received No, aRT or early sRT are time-dependent and correspond to the values at last follow up. aRT, adjuvant RT; LN, lymph node; RP, radical prostatectomy, RT, radiation therapy; sRT, salvage RT.
Prostatectomy and LN specimens underwent review by a pathologist with expertise in genitourinary pathology. In accord with federal and institutional guidelines, men signed an institutional review board–approved, protocol-specific informed consent form permitting prospective collection of deidentified data at baseline and follow-up, which were entered into a secure, password-protected database for outcome analysis. A minority of data were collected retrospectively.
Follow-Up and Determination of the Cause of Death
Follow-up started on the day of RP and concluded on the date of last follow-up or the date of death, whichever came first. The database was last updated on October 2, 2020. For men undergoing early sRT, the median prostate-specific antigen (PSA) level was 0.30 ng/mL (IQR: 0.20-0.59) measured on day 1 of early sRT before treatment. The PSA measurement on day 1 of early sRT preceded by several months the trigger PSA that documented PSA recurrence during which time the following tasks could occur: the referral to radiation oncology, early sRT planning, physics consultation to create the RT plan, and finally scheduling an early sRT start date. Therefore, to provide time for these tasks to occur, the randomized trials of adjuvant versus early sRT1 allowed up to 4 months from observation of the trigger PSA that indicated PSA recurrence to the start of early sRT. Therefore, the median PSA that we report would be expected to be higher than the actual trigger PSA level, which identified recurrence and therefore, may more closely approximate the 0.1 or 0.2 ng/mL level used in the randomized trials1 evaluating the impact of adjuvant versus early salvage RT on progression. During follow-up, patients had a PSA test and rectal examination and were seen every 3 months for 1 year, every 6 months for an additional 4 years, and then annually thereafter. Salvage ADT was delivered after PSA failure, clinical or radiographic evidence of progression after receiving aRT or early sRT. At the time of progression to castrate-resistant M0 or M1 disease, the practice patterns followed the treatment guidelines set forth by the European Association of Urology guideline.7 To assign PC-specific mortality as the cause of death, castrate-resistant metastatic PC on the basis of a rising PSA level in the setting of a testosterone level of < 20 ng/dL before death needed to be confirmed and the treating oncologist or urologist at the time of death needed to assign PC as the primary cause of death and record this on the death certificate.
Statistical Analysis
Comparison of the distribution of clinical factors and postoperative treatment.
For the entire study cohort, comparisons of the distribution of clinical factors and post-RP treatment stratified by no RT and aRT versus early sRT were made using a Mantel-Haenszel χ2 metric8 for categorical covariates; in the case of a small sample size, a Fisher exact test9 was used. For continuous covariates, such as age and year of treatment, medians and their distributions were compared using a Wilcoxon two-sample test.10
Univariable and multivariable hazard ratios for ACM risk.
Univariable and multivariable Cox regression11 was used to evaluate whether the ACM risk ratio for time-dependent use of adjuvant versus early sRT per unit increase in positive pelvic LNs was significantly reduced. Here, the ACM adjusted hazard ratio (AHR) represents the change in the risk of ACM when delivering aRT versus early sRT for each unit increase in the number of positive pelvic LNs. We define early sRT (t) as the time-dependent11 baseline group and report results for the treatment comparisons of adjuvant versus early sRT (t) and no RT versus early sRT (t). Men who received neither aRT nor early sRT (ie, no RT group) never experienced PSA failure during the conduct of the study or were treated with salvage ADT alone at progression. In addition to PC prognostic factors, all models were adjusted for age at RP, the number of positive pelvic LNs, the number of LNs sampled, and the time-dependent use12 of post-RP ADT, which could be in the adjuvant or salvage setting. Time zero was defined as the date of RP.
Treatment propensity score.
The treatment propensity score (PS)13 represents the probability of treatment assignment conditional on observed baseline prognostic covariates. PSs were estimated using multinomial logistic regression, with treatment (aRT or aADT, early sRT or sADT, and no RT) as the outcome and age in years at RP (continuous), year of RP (continuous), a PSA level of < 4 ng/mL versus others, pT3 or higher and pGS 8-10 and margin-positive versus others, and a persistent post-RP PSA versus undetectable as prognostic covariates. To minimize the potential bias when estimating the treatment effect of aRT, early sRT, or no RT on ACM risk in the Cox model,11 we adjust using a treatment PS. Age is used twice in the adjusted Cox model11 because physicians incorporate age in decisions regarding treatment selection and age is also prognostic for ACM risk.
Adjusted estimates of ACM.
For the purpose of illustration, adjusted estimates of ACM (1 minus Kaplan-Meier14 estimates of overall survival) after RP stratified by no RT and the time-dependent treatment groups of aRT or early sRT among men with one to three or four or more positive LNs were calculated using the extended Kaplan-Meier method with time-dependent treatment groups.12 These estimates were adjusted for the treatment PS13 and the fixed covariates of age at RP15 and the time-dependent use12 of post-RP ADT. We calculated estimates and 95% CIs of ACM at the median follow-up of 7 years for the no RT, aRT, and early sRT groups among men with one to three and four or more positive LNs. In addition, the difference in these 7-year point estimates and 95% CI of that difference between men who received aRT versus early sRT and no RT versus early sRT were calculated. A 95% CI for the difference that did not include 0.00 defined a significant difference in 7-year point estimates between the two treatment arms being compared.
A two-sided P value of ≤ .05 was considered statistically significant. R (version 4.0.3; R Foundation for Statistical Computing) was used to calculate Kaplan-Meier estimates with time-dependent treatment covariates. SAS (version 9.4; SAS Institute Inc) was used for all other calculations.
RESULTS
Description and Comparison of the Distribution of Clinical Factors and Postoperative Treatment
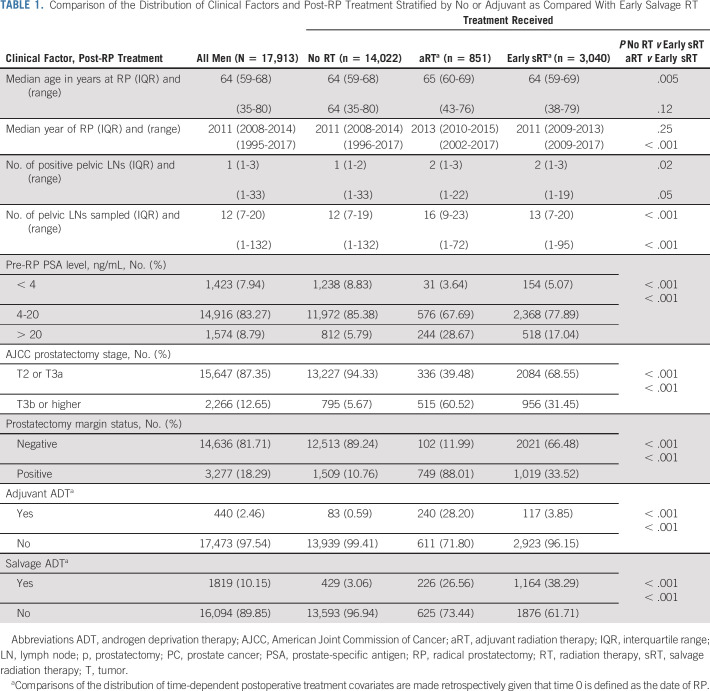
Of the 17,913 men in the study cohort, 851 (4.75%) received aRT (ie, PSA level < 0.1 ng/mL) generally within 6 months of RP and 3,040 (16.97%) underwent early sRT. Of the 3,040 men who received early sRT, 567 had a persistent PSA (18.65%) defined as a PSA that remained detectable at least 6 weeks after RP and were categorized into the early sRT group. Adjuvant ADT and sADT were used in 440 (2.46%) and 1819 (10.15%) men, respectively. Adjuvant ADT was given for a median of 10.32 months (IQR, 5.59-23.20 months). Among the 17,913 men, 1,614 (9.01%) were found to have pN1 PC, with 1,323 (81.97%) having 1-3 and 291 (18.03%) having 4 or more positive pelvic LNs. Of the 1,614 men with pN1 PC, adjuvant RT was given to 412 (25.53%) and 340 (21.07%) received adjuvant ADT.
As shown in Table 1, men who underwent adjuvant compared with early sRT had a significantly higher proportion with a PSA level > 20 ng/mL (28.6% v 17.04%), pT3b or higher (60.52% v 31.45%), margin-positive PC (88.01% v 33.52%), and aADT use (28.20% v 3.85%), whereas sADT use was significantly lower (26.56% v 38.29%) and all P values were < .001.
TABLE 1.
Comparison of the Distribution of Clinical Factors and Post-RP Treatment Stratified by No or Adjuvant as Compared With Early Salvage RT
Univariable and Multivariable Hazard Ratios for ACM Risk
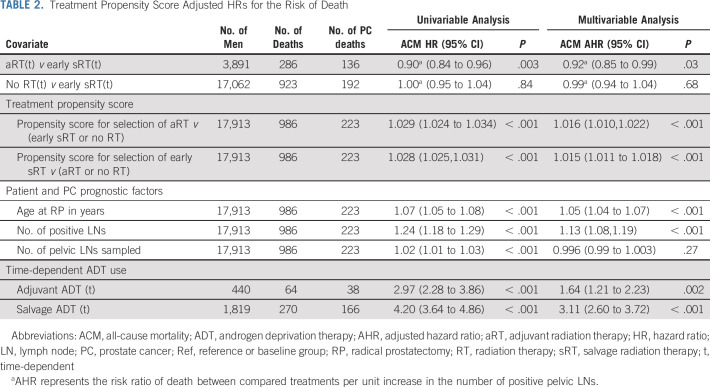
Men included in the study had a minimum follow-up of 2.50 years and a maximum follow-up of 23.20 years and underwent RP. After a median follow-up (IQR) of 7.02 (4.21-9.17) years, 986 (5.50%) men died, with 223 (22.62%) of PC. As shown in Table 2, adjuvant compared with early sRT was associated with a significantly lower ACM risk and this reduction in ACM risk increased by 8% (AHR: 0.92; 95% CI, 0.85 to 0.99; P = .03) with each additional positive pelvic LN, whereas no significant association with ACM risk was observed when comparing no RT with early sRT (AHR: 0.99; 95% CI, 0.94 to 1.04; P = .66). Moreover, both the time-dependent use and duration of aADT (1.64; 95% CI, 1.21 to 2.23; P = .002) and sADT (3.11; 95% CI, 2.60 to 3.72; P < .001) were significantly associated with an increased risk of ACM.
TABLE 2.
Treatment Propensity Score Adjusted HRs for the Risk of Death
Adjusted Estimates of ACM
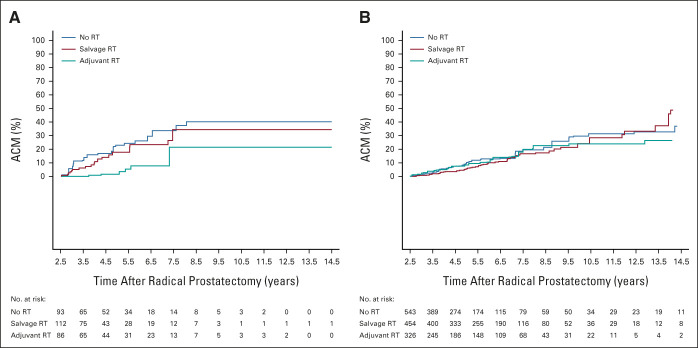
As illustrated in Figure 2A, a significant difference in the 7-year adjusted ACM estimates favoring the use of aRT versus early sRT was observed in men with 4 or more positive LNs (7.74%; 95% CI, 3.57 to 16.35 v 23.36%; 95% CI, 16.66 to 32.20) in that the 95% CI for 15.62% difference (5.90 to 25.35), excluded 0.00, but this was not true for men with one to three positive LNs (14.27%; 95% CI, 9.12 to 21.95 v 13.89%; 95% CI, 10.44 to 18.36; 95% CI for the 0.38% difference [–7.02 to 7.79]), as shown in Figure 2B. No significant difference was found in the 7-year adjusted ACM estimates for men who did not receive RT versus early sRT whether one to three (13.27%; 95% CI, 9.55 to 18.28 v 13.89%; 95% CI, 10.44 to 18.36; 95% CI for the 0.62% difference [–5.21 to 6.45]) or four or more (33.67%; 95% CI, 25.24 to 43.99 v 23.36%; 95% CI, 16.66 to 32.20; 95% CI for the 10.31% difference [–1.85 to 22.47]) pelvic LNs were involved.
FIG 2.
Adjusted estimates of ACM among (A) the 291 men with four or more and (B) the 1,323 men with one to three positive lymph nodes comparing time-dependent adjuvant or no RT with time-dependent early salvage RT. x-axis begins at the minimum follow-up time of 2.5 years. ACM, all-cause mortality; RT, radiation therapy.
DISCUSSION
In this study, we found that adjuvant compared with early sRT in men with pN1 PC was associated with a decreased ACM risk and this reduction increased with each additional positive pelvic LN. Specifically, we observed an 8% reduction in the risk of ACM for every additional positive pelvic LN found at RP. Given that the estimated death rate in men with one to three positive pelvic LNs who underwent early sRT at the median follow-up of 7 years was 14%, observing an 8% reduction/positive LN in the case of up to three positive LNs among men receiving aRT would result in a maximum absolute reduction in ACM of 0.08 × 3 × 14% or approximately 3%, which would require a very large sample size and subsequent number of events to observe. This can explain why the difference at 7 years in the ACM estimates for men undergoing adjuvant compared with early sRT was not significantly different among men with one to three positive LNs (Fig 2B), whereas it was significant for men with 4 or more positive LNs (Fig 2A) where the absolute difference in ACM estimates at 7 years for this comparison would be expected to be much larger than 3% given the number of positive LNs.
The clinical significance of this finding is two-fold. First, it is important to weigh the potential short- and long-term toxicity of pelvic RT16 against the possible but modest absolute reduction in the risk of ACM when considering its use in men with a single or a few positive LNs. Second, although on the basis of a previous report,4 one could conclude that men with four or more positive pelvic LNs would not benefit from aRT, the data in this study provide evidence to support that aRT in the setting of four or more positive LNs has the potential to translate into a reduced risk of ACM. In addition, a probable reason why a previous study4 might not have found a benefit when using aRT as compared with early sRT in the setting of more than 4 positive LNs is the lack of adjustment for the time-dependent use and duration of ADT. Specifically, the use, timing, and duration of both aADT and sADT affected ACM risk in the current study, as noted in Table 2 where the AHRs were 1.64 (P = .002) and 3.11 (P < .001), respectively.
Several points deserve clarification. First, we included the year of RP in the treatment PS13 as a prognostic covariate to help ensure that the probability of treatment assignment to adjuvant versus early sRT conditional on the year of RP was balanced with respect to practice patterns that evolved during the conduct of the study. In addition, the EAU7 guidelines were followed at the time of progression to castrate-resistant M0 or M1 PC irrespective of whether the patient had received prior adjuvant or early sRT. Therefore, it is very unlikely that as standards of care evolved over time that salvage therapies for progression to M0 castrate-resistant and then M1 castrate-resistant disease states differed among men who had previously underwent adjuvant versus early sRT following RP. Third, if positron emission tomographic (PET) imaging was used more frequently in the adjuvant compared with early salvage RT group, this could potentially confound our results by detecting recurrences earlier in the adjuvant as compared with early sRT groups and then offering treatment that could prolong survival. However, reimbursement for PSMA PET scanning in the post-RP PSA failure setting did not begin until recently in Germany. Given how costly PSMA PET scanning is, PSMA PET use during the study period would have been very limited and therefore very unlikely to be a confounding factor in our study. Moreover, in a study17 where pelvic LN histopathology was used to confirm the prostate-specific membrane antigen (PSMA) PET-computed tomography (CT) findings, the sensitivity was 40% and the positive predictive value was 75%. Therefore, 60% of pN1 PC would not be visualized on a preoperative PSMA PET-CT and a PSMA PET-CT, showing that cN1 disease is a false positive in 25% of the cases. Therefore, management of men found to have pelvic LN positive disease on PSMA PET-CT remains RP and pelvic LN assessment or RT and appropriate systemic therapy.
Fourth, the median number of LNs sampled was 12 given the extended LN sampling that was routinely performed. Therefore, the magnitude of potential reduction in ACM when one to three positive LNs are found in a patient whose total LN sampling includes < 12 LNs may be larger than what is reported in the current study. However, in the case of the patient in whom at least 12 LNs are sampled, the magnitude of the possible modest reduction in men with one to three positive LNs should be personalized by considering life expectancy using a validated, metric such as the Adult Comorbidity Evaluation-2718 and weighed against the potential toxicities of pelvic RT.16 Fifth, the divergence of the ACM curves occurs at 3 years after RP as shown in Figure 2A in men with four or more positive LNs, meaning that most men who were healthy enough to undergo RP would likely benefit, and this should be considered when deciding on whether to recommend adjuvant RT. Finally, although a recent report19 of an overall survival benefit was observed when adding 2 years of abiraterone to conventional ADT in men with newly diagnosed clinical N1 PC, how the risk of ACM would be affected with the use of adjuvant RT when 2 years of abiraterone is administered in addition to conventional ADT in the setting of pN1 PC requires additional study given that only 3% of men in that study19 had prior RP or RT and then relapsed.
Given these considerations, these findings provide evidence to support considering the use of adjuvant RT in men with pN1 PC and using a personalized approach on the basis of the number of positive pelvic LNs and other comorbidities.
Derya Tilki
Honoraria: Janssen, Ipsen, Exact Sciences, Apogepha, AstraZeneca, Advanced Accelerator Applications, Roche, Takeda, miR Scientific
Consulting or Advisory Role: miR Scientific, AstraZeneca, Roche
Research Funding: Janssen
Ming-Hui Chen
Employment: Boehringer Ingelheim
Markus Graefen
Honoraria: Astellas Pharma, Bayer, Takeda, Janssen, Medtronic
Consulting or Advisory Role: Medtronic
Travel, Accommodations, Expenses: Astellas Pharma, Bayer, Janssen, Takeda
No other potential conflicts of interest were reported.
Footnotes
See accompanying editorial on page 2179
AUTHOR CONTRIBUTIONS
Collection and assembly of data: Hartwig Huland, Markus Graefen
Data analysis and interpretation: Derya Tilki, Ming-Hui Chen, Jing Wu
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Derya Tilki
Honoraria: Janssen, Ipsen, Exact Sciences, Apogepha, AstraZeneca, Advanced Accelerator Applications, Roche, Takeda, miR Scientific
Consulting or Advisory Role: miR Scientific, AstraZeneca, Roche
Research Funding: Janssen
Ming-Hui Chen
Employment: Boehringer Ingelheim
Markus Graefen
Honoraria: Astellas Pharma, Bayer, Takeda, Janssen, Medtronic
Consulting or Advisory Role: Medtronic
Travel, Accommodations, Expenses: Astellas Pharma, Bayer, Janssen, Takeda
No other potential conflicts of interest were reported.
REFERENCES
- 1.Vale CL, Fisher D, Kneebone A, et al. : Adjuvant or early salvage radiotherapy for the treatment of localised and locally advanced prostate cancer: A prospectively planned systematic review and meta-analysis of aggregate data. Lancet 396:1422-1431, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tilki D, Chen MH, Wu J, et al. : Adjuvant versus early salvage radiation therapy for men at high risk for recurrence following radical prostatectomy for prostate cancer and the risk of death. J Clin Oncol 39:2284-2293, 2021 [DOI] [PubMed] [Google Scholar]
- 3.Tilki D, Preisser F, Tennstedt P, et al. : Adjuvant radiation therapy is associated with better oncological outcome compared with salvage radiation therapy in patients with pN1 prostate cancer treated with radical prostatectomy. BJU Int 119:717-723, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Abdollah F, Karnes RJ, Suardi N, et al. : Impact of adjuvant radiotherapy on survival of patients with node-positive prostate cancer. J Clin Oncol 32:3939-3947, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Daneshmand S, Quek ML, Stein JP, et al. : Prognosis of patients with lymph node positive prostate cancer following radical prostatectomy: Long-term results. J Urol 172:2252-2255, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Froehner M, Koch R, Farahzadi S, et al. : Long-term mortality in patients with positive lymph nodes at the time of radical prostatectomy. Urol Int 103:427-432, 2019 [DOI] [PubMed] [Google Scholar]
- 7.Cornford P, van den Bergh RCN, Briers E, et al. : EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer. Part II-2020 update: Treatment of relapsing and metastatic prostate cancer. Eur Urol 79:263-282, 2021 [DOI] [PubMed] [Google Scholar]
- 8.Agresti A: Categorical Data Analysis (ed 3). Hoboken, NJ, John Wiley & Sons, 2012 [Google Scholar]
- 9.Fisher RA: On the interpretation of Χ2 from contingency tables, and the calculation of P. J R Stat Soc 85:87-94, 1922 [Google Scholar]
- 10.Hollander M, Wolfe D, Chicken E: Nonparametric Statistical Methods (ed 3). Hoboken, NJ, John Wiley & Sons, 2014 [Google Scholar]
- 11.Klein J, Moeschberger M: Survival Analysis: Techniques for Censored and Truncated Data. Norwell, MA, Springer, 2013 [Google Scholar]
- 12.Snapinn S, Jiang Q, Iglewicz B: Illustrating the impact of a time-varying covariate with an extended Kaplan-Meier estimator. Am Stat 59:301-307, 2005 [Google Scholar]
- 13.Newgard CD, Hedges JR, Arthur M, et al. : Advanced statistics: The propensity score—A method for estimating treatment effect in observational research. Acad Emerg Med 11:953-961, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Kaplan EL, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457-481, 1958 [Google Scholar]
- 15.Cupples LA, Gagnon DR, Ramaswamy R, et al. : Age-adjusted survival curves with application in the Framingham Study. Stat Med 14:1731-1744, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Murthy V, Maitre P, Kannan S, et al. : Prostate-only versus whole-pelvic radiation therapy in high-risk and very high-risk prostate cancer (POP-RT): Outcomes from phase III randomized controlled trial. J Clin Oncol 39:1234-1242, 2021 [DOI] [PubMed] [Google Scholar]
- 17.Hope TA, Eiber M, Armstrong WR, et al. : Diagnostic accuracy of 68Ga-PSMA-11 PET for pelvic nodal metastasis detection prior to radical prostatectomy and pelvic lymph node dissection: A multicenter prospective phase 3 imaging trial. JAMA Oncol 7:1635-1642, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piccirillo JF, Tierney RM, Costas I, et al. : Prognostic importance of comorbidity in a hospital-based cancer Registry. JAMA 291:2441-2447, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Attard G, Murphy L, Clarke NW, et al. : Abiraterone acetate and prednisolone with or without enzalutamide for high-risk non-metastatic prostate cancer: A meta-analysis of primary results from two randomised controlled phase 3 trials of the STAMPEDE platform protocol. Lancet 399:447-460, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]