PURPOSE
The NCI-COG Pediatric MATCH trial assigns patients age 1-21 years with relapsed or refractory solid tumors, lymphomas, and histiocytic disorders to phase II studies of molecularly targeted therapies on the basis of detection of predefined genetic alterations. Patients with tumors harboring mutations or fusions driving activation of the mitogen-activated protein kinase (MAPK) pathway were treated with the MEK inhibitor selumetinib.
METHODS
Patients received selumetinib twice daily for 28-day cycles until disease progression or intolerable toxicity. The primary end point was objective response rate; secondary end points included progression-free survival and tolerability of selumetinib.
RESULTS
Twenty patients (median age: 14 years) were treated. All were evaluable for response and toxicities. The most frequent diagnoses were high-grade glioma (HGG; n = 7) and rhabdomyosarcoma (n = 7). Twenty-one actionable mutations were detected: hotspot mutations in KRAS (n = 8), NRAS (n = 3), and HRAS (n = 1), inactivating mutations in NF1 (n = 7), and BRAF V600E (n = 2). No objective responses were observed. Three patients had a best response of stable disease including two patients with HGG (NF1 mutation, six cycles; KRAS mutation, 12 cycles). Six-month progression-free survival was 15% (95% CI, 4 to 34). Five patients (25%) experienced a grade 3 or higher adverse event that was possibly or probably attributable to study drug.
CONCLUSION
A national histology-agnostic molecular screening strategy was effective at identifying children and young adults eligible for treatment with selumetinib in the first Pediatric MATCH treatment arm to be completed. MEK inhibitors have demonstrated promising responses in some pediatric tumors (eg, low-grade glioma and plexiform neurofibroma). However, selumetinib in this cohort with treatment-refractory tumors harboring MAPK alterations demonstrated limited efficacy, indicating that pathway mutation status alone is insufficient to predict response to selumetinib monotherapy for pediatric cancers.
INTRODUCTION
Alterations in RAS/RAF/MEK/ERK genes result in the dysregulation of the classical mitogen-activated protein kinase (MAPK) pathway. This pathway has been identified as one of the most frequently dysregulated signaling cascades in the pathogenesis of many human cancers.1,2 Aberrant activation of MAPK signaling is most often because of gain-of-function mutations in RAS or BRAF genes or loss or inactivating mutation of NF1.3
CONTEXT
Key Objective
Outcomes for children and young adults with recurrent or refractory cancers following dose-intensive chemotherapy are poor. The NCI-COG Pediatric MATCH Trial was designed to provide a national framework for histology-agnostic trials of investigational molecularly targeted therapies in biomarker-selected populations. Treatment arm E evaluated the safety and efficacy of the MEK inhibitor selumetinib in patients harboring activating alterations in the mitogen-activated protein kinase (MAPK) signaling pathway genes (ClinicalTrials.gov identifier: NCT03213691).
Knowledge Generated
Twenty patients with diverse tumor types and MAPK pathway gene alterations were treated on this trial. No objective responses were observed, although three patients achieved best response of stable disease.
Relevance
The study design was effective at identifying patients eligible for treatment with selumetinib. In contrast to prior experience with less aggressive pediatric tumor types with few somatic mutations, such as low-grade glioma, selumetinib demonstrated limited efficacy in our cohort, indicating that MAPK pathway gene mutation status alone is insufficient to predict response for pediatric cancers.
Genetic alterations in the MAPK pathway have been identified in a diverse group of pediatric and adult cancers and serve to inhibit proapoptotic signaling and result in unchecked cell proliferation.4-8 Accordingly, there has been great interest in the potential therapeutic use of targeted drugs at multiple points in this pathway, irrespective of histology.9,10 Although only rare mutations have been identified in the genes encoding MEK proteins, MEK1 and MEK2 have been identified as therapeutic targets because of their central role in the MAPK signaling pathway and lack of cross-pathway signaling distal to RAF.11,12
Pediatric and young adult malignancies with recurrent activating MAPK pathway alterations include low-grade glioma (LGG; > 90% of tumors, most frequently BRAF fusions or V600E mutations),13 malignant peripheral nerve sheath tumors (MPNST; NF1 inactivation in > 80%),14 melanoma (> 80%, most frequently with activating BRAF mutations),15 Langerhans cell histiocytosis (> 80%, most frequently BRAF V600E),16,17 rhabdomyosarcoma (RMS; > 40%, most frequently activating NRAS/KRAS/HRAS mutations),18 and high-grade glioma (HGG; 10-20%, most frequently inactivation of NF1).19
Selumetinib is a potent orally bioavailable selective inhibitor of MEK1/MEK2. Evidence of selumetinib activity has been observed in several pediatric cancer types with MAPK pathway alterations. Several recent reports have described selumetinib to be well tolerated and result in prolonged disease stability in children with progressive LGGs harboring alterations in BRAF or NF1.20,21 In April 2020 (after completion of enrollment on the current study), the US Food and Drug Administration (FDA) approved selumetinib for the treatment of inoperable plexiform neurofibromas (PNs) in children age ≥ 2 years on the basis of the results of a phase II trial, which demonstrated a 3-year progression-free survival (PFS) of 84% in patients receiving selumetinib when compared with 15% of patients in a natural history study.22
The National Cancer Institute-Children's Oncology Group Pediatric Molecular Analysis for Therapy Choice (NCI-COG Pediatric MATCH) trial was activated in July 2017 to provide a national framework for histology-agnostic trials of investigational molecularly targeted therapies in biomarker-selected populations.23,24 Centralized tumor sequencing is performed through the Pediatric MATCH screening protocol, which allows enrollment of patients from COG institutions across the United States. Patients with tumors that harbor an actionable genetic alteration for a MATCH treatment arm are eligible for treatment in a phase II trial. Here, we report the results of the first completed NCI-COG Pediatric MATCH treatment subprotocol (arm E): the MEK inhibitor selumetinib for treatment of patients with tumors harboring alterations in the MAPK signaling pathway (ClinicalTrials.gov identifier: NCT03213691).
METHODS
Study Design and Eligibility
Both the NCI-COG Pediatric MATCH screening protocol and all treatment subprotocols were reviewed by the NCI central institutional review board. The study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from parents/guardians, and assent was also obtained from the patient where appropriate. Patients age 1-21 years with treatment-refractory or recurrent solid tumors (including CNS tumors), lymphomas, and histiocytic disorders are eligible for screening on Pediatric MATCH (ClinicalTrials.gov identifier: NCT03155620).
Formalin-fixed paraffin-embedded tumor specimens obtained at a time of refractory/recurrent disease (median of 115 days before arm E enrollment, range: 34-1,343 days) are subjected to DNA- and RNA-based molecular profiling using an investigational targeted Ampliseq panel (Thermo Fisher Scientific, Waltham, MA). Single-nucleotide variants (variant allele fraction > 0.05), insertions and deletions (variant allele fraction > 0.1), amplifications (≥ 7 copies), and selected fusions are analyzed in 143 cancer genes (Data Supplement, online only).25 Blood samples are also sequenced using the same DNA panel to identify germline variants but not used to determine treatment assignments.
If an actionable tumor genetic alteration is detected, on the basis of predetermined levels of preclinical and clinical evidence (Data Supplement), patients are assigned (matched) to a Pediatric MATCH treatment subprotocol. Specific actionable alterations for arm E (selumetinib; ClinicalTrials.gov identifier: NCT03213691) of Pediatric MATCH were defined in ARAF, BRAF, NRAS, KRAS, HRAS, MAP2K1, GNA11, GNAQ, NF1, and BRAF (Data Supplement). Patients with tumors harboring an actionable BRAF V600 mutation were preferentially assigned to a separate subprotocol (arm G) evaluating the BRAF-V600E inhibitor vemurafenib; if such patients had previously been treated with vemurafenib or another BRAF inhibitor, they were excluded from that protocol but eligible for arm E.
Any tumor histology was eligible for enrollment in arm E with the exception of LGG (WHO grade I or II), given that previous clinical trials of selumetinib for children with that tumor type had already demonstrated activity.20,21,26 In addition to the requirement for detection of an actionable mutation by MATCH screening, criteria for treatment included age ≥ 12 months and ≤ 21 years, Karnofsky or Lansky performance score ≥ 50%, radiographically measurable disease, body surface area > 0.5 m2, ability to swallow intact oral capsules, and adequate organ function. Patients were excluded from treatment if they had a history of known significant ophthalmologic conditions, uncontrolled infection, or concomitant use of CYP3A4/CYP2C19-inducing or -inhibiting agents.
Selumetinib (AZD6244 hydrogen sulfate) was given orally at the FDA-approved pediatric dose of 25 mg/m2/dose twice daily every day, with a maximum dose of 75 mg/dose.26-30 Patients received drug continuously with each cycle lasting 28 days. Patients were eligible to receive therapy for up to 2 years, as long as there was no evidence of progressive disease or toxicity that met protocol-defined criteria for discontinuation of protocol therapy.
Adverse Events and Dose Modifications
Adverse events (AEs) were reported according to the NCI Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. All patients who received at least one dose of protocol therapy were considered evaluable for toxicity. Dose-limiting toxicity (DLT) was defined differently for hematologic and nonhematologic toxicity. Treatment could be withheld for DLTs for up to 14 days. If the DLT resolved to baseline or eligibility parameters within 14 days of discontinuing therapy, the patient could resume selumetinib at a reduced dose; if not, the patient was removed from protocol therapy. If a recurrent or second DLT occurred at a reduced dose of selumetinib, the patient was removed from protocol therapy.
Measurement of Response
Any eligible patient who received at least one dose of protocol therapy was evaluable for response. Tumor disease evaluations were obtained every other cycle for three occurrences, and then every three cycles. For documentation of objective response (complete response [CR] or partial response [PR]), confirmatory scans were required to be obtained after the next consecutive cycle. The revised RECIST, version 1.1 was used to determine response and progression, with specific criteria outlined for CNS tumors, non-CNS solid tumors, lymphomas, and histiocytoses.31 Central review was required for any patient who was deemed to have a CR or PR and was used to determine final assessment of response.
Statistical Considerations
The study's primary end point was objective response rate. The primary study cohort used a single-stage design with a minimum of 20 patients and a projected accrual duration of 48 months. The overall response rate was compared against a null benchmark value of 5%. With this design (alpha = 10%), the power was 90% to detect an improvement in response rate from 5%, if the treatment was ineffective, to 25%, if the agent was sufficiently effective to warrant further study.
The study's secondary end points included PFS, defined as time from initiation of protocol therapy until the occurrence of disease progression, disease recurrence, or death from any cause. PFS along with the 95% confidence intervals was estimated using the Kaplan-Meier method. Chi-square tests (or Fisher's exact tests as appropriate) were used to evaluate the association between patient's baseline characteristics and treated/untreated cohorts. All statistical analyses were done in SAS (version 9.4) or R (version 4.0).
RESULTS
Patient Characteristics and Demographics
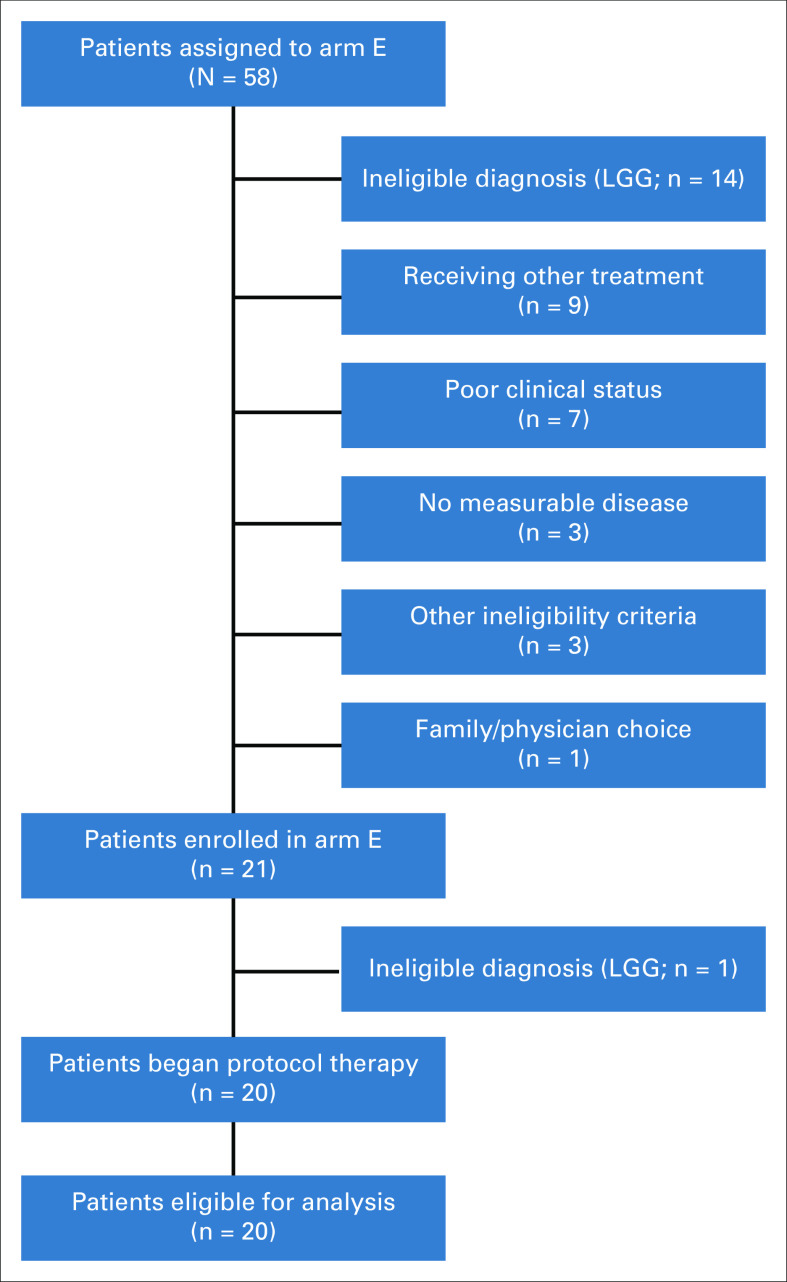
Fifty-eight patients from 42 study sites had actionable tumor alterations and were therefore matched to arm E (selumetinib; Fig 1). Pediatric MATCH screening protocol treatment assignments had been confirmed for 577 patients when the final arm E match was made, resulting in an arm E match rate of 10% (58/577 patients). Of these 58 matched patients, 21 (from 18 sites) enrolled in arm E between December 2017 and August 2019, and 20 initiated protocol therapy; one patient with LGG was erroneously enrolled but identified as ineligible before treatment. The most common reasons provided for subjects who were matched but did not enroll (Fig 1) were ineligible diagnosis of LGG (n = 14), patient receiving other treatment (n = 9), and worsening clinical status (n = 7). All 20 patients treated on arm E were evaluable for response and toxicities. Data as of December 31, 2020, were used in the manuscript.
FIG 1.
Patient flow diagram of NCI-COG Pediatric MATCH arm E. Other reasons for ineligibility were inadequate cardiac function (n = 2) and known ophthalmologic condition (n = 1). LGG, low-grade glioma.
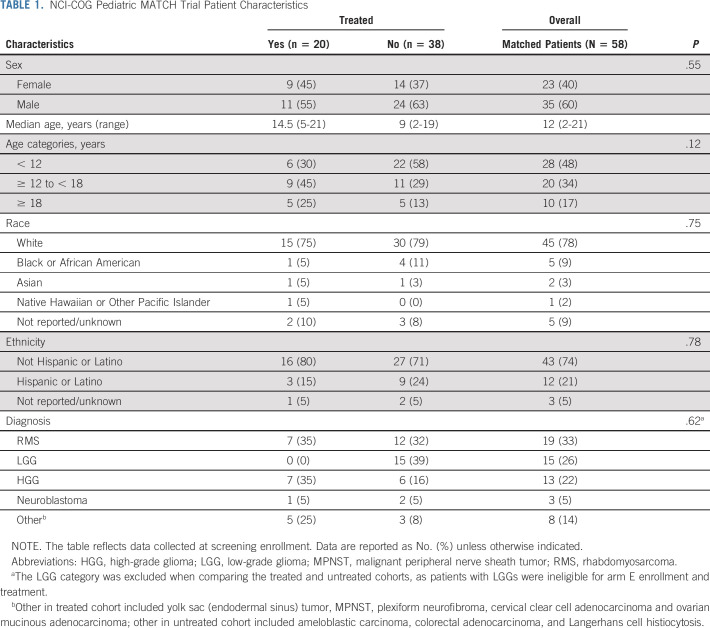
Demographics and characteristics of matched (n = 58) and treated (n = 20) patients are shown in Table 1. Patients receiving therapy ranged in age from 5 to 21 years (median: 14 years), with near-equal numbers of male and female patients. Most treated patients were White (15/20, 75%) and reported non-Hispanic/Latino ethnicity (16/20, 80%). No demographic differences were noted between the treated and untreated patient cohorts (Table 1).
TABLE 1.
NCI-COG Pediatric MATCH Trial Patient Characteristics
The distribution of diagnoses is shown in Table 1 and Figure 2. Among all matched patients, RMS (19/58, 33%), LGG (15/58, 26%), and HGG (13/58, 22%) were most common (Fig 2A). In treated patients, RMS and HGG were also most frequent (each 7/20, 35%; Fig 2B). One patient each with neuroblastoma, yolk sac (endodermal sinus) tumor, MPNST, PN, cervical clear cell adenocarcinoma, and ovarian mucinous adenocarcinoma were also treated.
FIG 2.
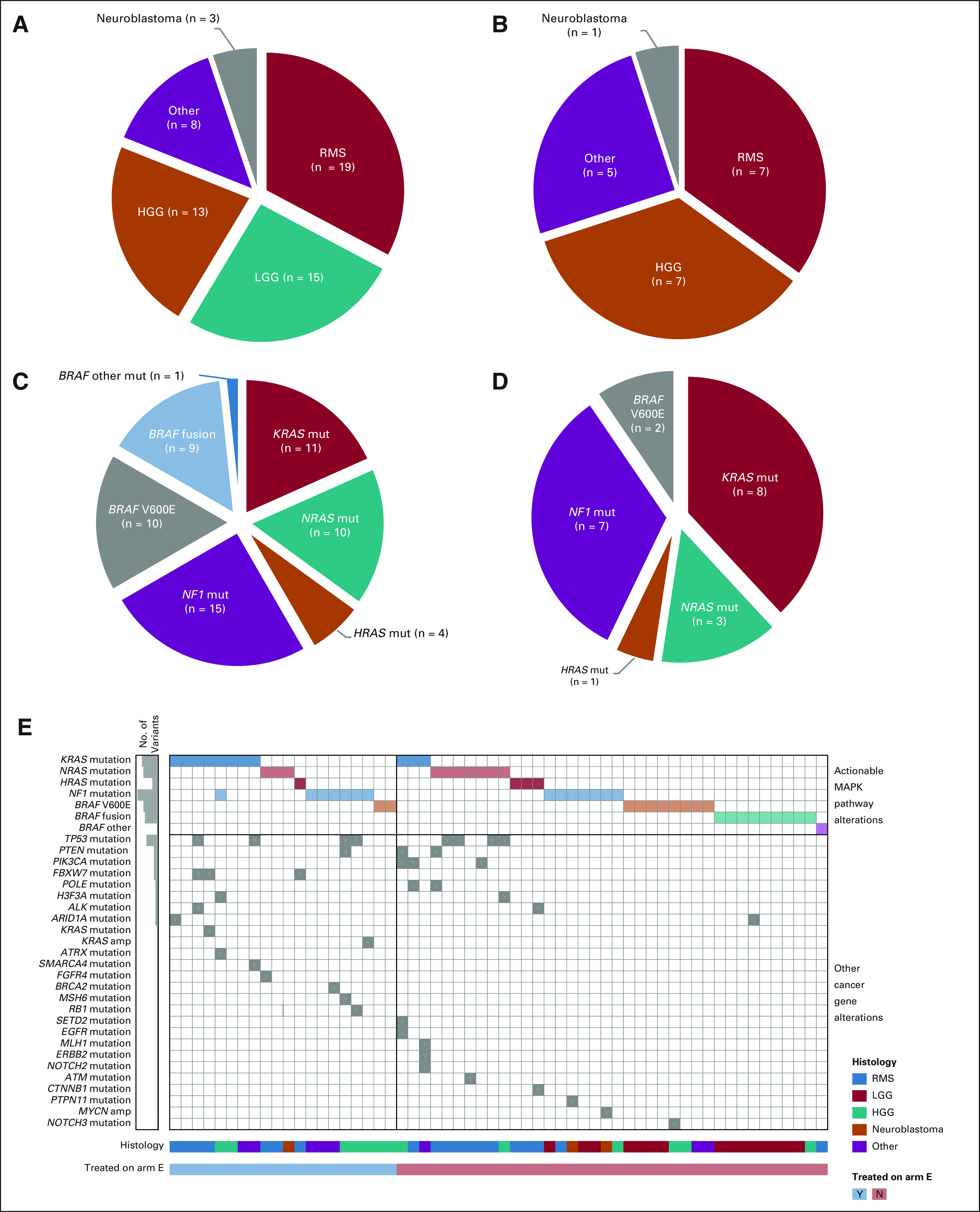
Diagnoses and actionable MAPK pathway mutations of arm E patients. (A) Pie chart of the histologic diagnoses of the 58 matched patients. (B) Pie chart of the histologic diagnoses of the 20 treated patients. (C) Pie chart of the types of actionable MAPK pathway tumor alterations detected in the 58 matched patients (n = 60 mutations). (D) Pie chart of the types of actionable MAPK pathway tumor alterations detected in the 20 treated patients (n = 21 mutations). (E) Tumor variants detected by gene and patient. The genes with variants are indicated for each patient (each column represents a patient). The 20 patients treated in arm E are on the left side of the figure; the remainder of the matched patients are on the right. The histogram on the left gives the number of variants detected for each alteration type. Actionable MAPK pathway alterations are in the top rows of the figure (KRAS mutation to BRAF other); other cancer gene alterations identified by the tumor panel testing are listed in the rows below. HGG, high-grade glioma; LGG, low-grade glioma; MAPK, mitogen-activated protein kinase; Mut, mutation; RMS, rhabdomyosarcoma.
Landscape of MAPK Pathway Alterations
Patients matched to arm E.
The actionable MAPK pathway tumor alterations detected in the 58 matched patients are shown in Figures 2C and 2E and in the Data Supplement. Activating hotspot mutations in RAS genes were most common (n = 25: KRAS × 11, NRAS × 10, HRAS × 4), followed by NF1-inactivating mutations (n = 15), BRAF V600E mutations (n = 10), and other activating BRAF mutations (n = 1). Fusions in the BRAF gene (n = 9) were observed in the matched patient cohort but not in patients treated on study, consistent with the exclusion of LGGs from arm E. Additional nonactionable alterations detected in cancer genes are shown in Figure 2E.
Patients treated in arm E.
The spectrum of actionable alterations identified in the cohort of 20 treated patients (Figs 2D and 2E, and the Data Supplement) was similar to that in the matched cohort, with activating RAS gene mutations (KRAS × 8, NRAS × 3, HRAS × 1) and inactivating mutations in NF1 (n = 7) most common. BRAF V600E mutations were identified in two patients. One HGG had concurrent actionable mutations in KRAS and NF1. Nonactionable tumor alterations (median of one per tumor, range: 0-3), were also identified by the panel (Fig 2E and the Data Supplement) including multiple mutations in TP53 (n = 4) and FBXW7 (n = 3). Two nonactionable KRAS alterations (a KRAS amplification in an HGG with NF1 mutation and a KRAS p.Ala146Pro mutation in an RMS with KRAS p.Gly12Val mutation) were detected. Four of the tumor mutations identified were also detected in patient-matched blood (germline) samples: NF1 × 2 (patient diagnoses: MPNST and PN), MSH6 (HGG), and TP53 (HGG).
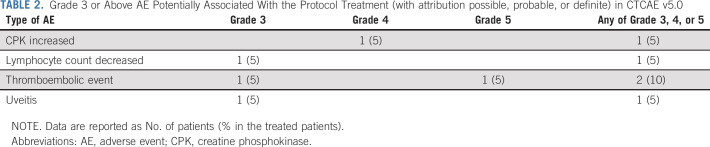
AEs
Of the 20 evaluable patients, 5 (25%) experienced a grade 3 or higher AE with possible or probable attribution to selumetinib (Table 2 and the Data Supplement). Three patients had grade 3 events (uveitis, decreased lymphocyte count, and thromboembolic event), one patient had a grade 4 elevated creatinine phosphokinase, and one patient had a grade 5 toxicity with death occurring because of thromboembolic event (pulmonary embolus). Three patients required dose modifications for AEs and two of these patients ultimately discontinued selumetinib because of DLT (uveitis and elevated creatinine phosphokinase). Of the 10 patients who had radiographs showing open tibial growth plates before the start of therapy, eight remained on therapy long enough to undergo repeat tibial growth plate monitoring, and none of these patients had evidence of growth plate thickening.
TABLE 2.
Grade 3 or Above AE Potentially Associated With the Protocol Treatment (with attribution possible, probable, or definite) in CTCAE v5.0
Evaluation of Activity and Efficacy
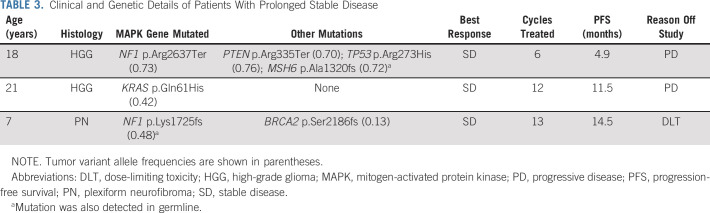
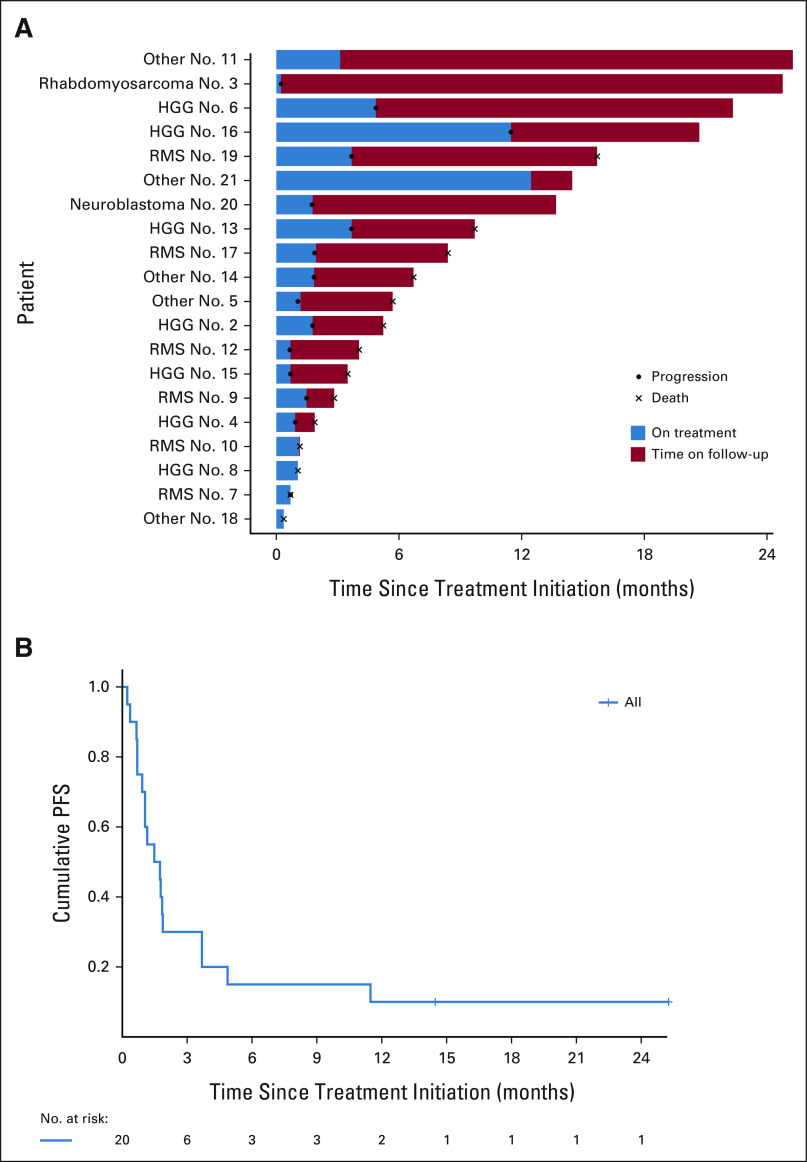
No objective responses (PR or CR) were observed in the 20 treated patients. Median number of cycles completed was 2 (range: 1-13). Three patients had a best overall response of stable disease (SD; Table 3). One patient with HGG (KRAS mutation) had SD for 12 cycles before progressing on therapy. A second patient with HGG (NF1 and PTEN mutations) had SD for six cycles before progressing on therapy. One patient with PN (NF1 and BRCA2 mutations) had SD for 13 cycles until removal from protocol therapy because of dose-limiting toxicity (uveitis). Six-month PFS was 15% (95% CI, 4 to 34; Fig 3).
TABLE 3.
Clinical and Genetic Details of Patients With Prolonged Stable Disease
FIG 3.
(A) Swimmer plot and (B) Kaplan-Meier curve of the 20 treated arm E patients. HGG, high-grade glioma; LGG, low-grade glioma; PFS, progression-free survival; RMS, rhabdomyosarcoma.
DISCUSSION
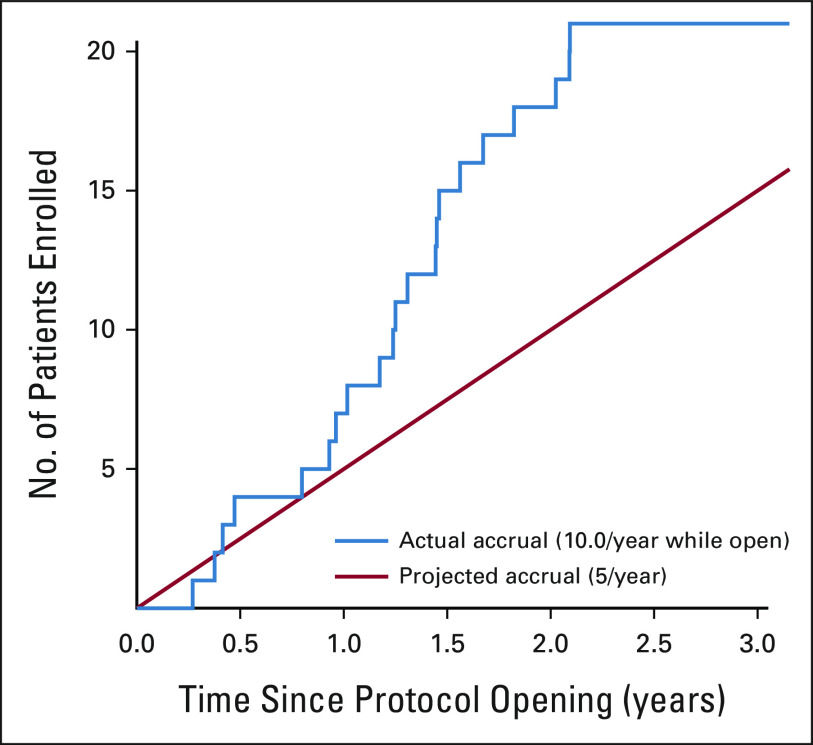
In this study, we report the results of the first completed NCI-COG Pediatric MATCH phase II treatment trial: the MEK inhibitor selumetinib (arm E) for the treatment of patients with tumors harboring alterations in the MAPK signaling pathway. The nationwide histology-agnostic molecular screening strategy used in Pediatric MATCH was effective at identifying children and young adults eligible for treatment with selumetinib, with 58 patients from 42 different study sites matched to arm E in less than two years (Table 1). The 20-patient treatment cohort enrolled in 21 months, well ahead of the projected accrual duration of 48 months (Appendix Figure A1, online only). Adolescent and young adult patients (age 15-21 years) were well represented in the trial, comprising half of the cohort (Data Supplement). As observed in other pediatric and adult trials of selumetinib, the agent is relatively well tolerated. Few dose-limiting toxicities were seen in this trial using the FDA-approved dose of 25 mg/m2/dose twice daily (Table 2 and the Data Supplement). The toxicities seen were consistent with previous trials, including uveitis, elevated creatinine phosphokinase, and thromboembolic events.
A wide variety of diagnoses were represented in the matched and treated arm E patient cohorts, with 12 and eight histologies represented, respectively (Table 1, Figs 2A and 2B, and the Data Supplement). Diverse MAPK pathway tumor alterations typical of pediatric cancers were detected, with mutations and fusions targeting five genes (KRAS, NRAS, HRAS, NF1, and BRAF) in both the matched and treated patient cohorts (Figs 2C-2E). Most patients matched to arm E (47/58, 81%) had one of three tumor types: RMS, HGG, or LGG. RMS and HGG are prototypes of aggressive, genomically complex MAPK-driven tumors of childhood with multiple molecular mechanisms underlying tumor development and progression.18,19 Aberrant MAPK signaling in RMS in study patients most commonly resulted from activating mutations in RAS genes (detected in 17 of 19 [89%] matched and all seven treated RMS patients on study), whereas in HGG, a more varied set of pathway-activating alterations was observed (Fig 2E, and the Data Supplement). By contrast, LGG in children (most commonly WHO grade I pilocytic astrocytoma) generally follows a more indolent course and serves as a model of a single-pathway MAPK-driven malignancy. Since previous trials of selumetinib for children with LGG demonstrated activity,20,21 these patients were excluded from treatment on Pediatric MATCH arm E, and our study cohort was therefore primarily composed of patients with RMS and HGG (14/20 patients, 70%).
The overall clinical activity of selumetinib was disappointing in this cohort of children and young adults with treatment-refractory tumors harboring activating MAPK pathway alterations: no objective responses and a 6-month PFS of 15% were observed (Fig 3). This lack of activity stands in marked contrast to previous reports of selumetinib activity for treatment of other pediatric tumor types: both sporadic and neurofibromatosis type I–associated LGG and PN, for which the agent has received FDA approval. Durable responses have also been reported in close to 90% of adult patients with histiocytic disorders (similarly characterized by single MAPK pathway alterations) treated with the MEK inhibitor cobimetinib32; a phase II trial for pediatric patients is ongoing (ClinicalTrials.gov identifier: NCT04079179). The response rate to selumetinib observed in this study is comparable with the trametinib (MEK inhibitor) arm of the adult NCI-MATCH (EAY131) trial where overall response rate in tumors with BRAF fusions or non-V600 mutations was 3%.33 Importantly, the current study did not rigorously evaluate selumetinib activity in tumors harboring MAPK pathway alterations that were not well represented in treated patients, such as BRAF mutations and fusions (n = 2 and 0, respectively), and these results should not be interpreted to suggest lack of activity of MEK inhibitors in pediatric cancers with these variants.
In combination with prior reported experience with single-agent MEK inhibition in both pediatric and adult malignancies, our data suggest two potential explanations for the lack of activity observed for selumetinib in Pediatric MATCH.20,21,33-39 First, the tumor types for which single-agent activity of MEK inhibitors has been demonstrated in children (sporadic and NF1-associated LGG; PN) are less clinically aggressive and molecularly defined almost exclusively by single gene driver mutations in the MAPK pathway. There is limited evidence of MEK inhibitor monotherapy efficacy in more genetically complex tumors such as RMS or HGG, which comprised 70% of our treated patient cohort. Evidence of this differential genomic complexity was seen in the molecular profiling for our cohort of patients matched to arm E; consistent with larger genomic studies of these tumor types,13,18 only 1/15 (6.7%) LGG tested had a second cancer gene alteration detected, whereas 13/19 (68%) RMS had at least one other cancer gene mutation identified (Fig 2E).13 Second, the Pediatric MATCH data add to the accumulating evidence that targeting RAS-mutated tumors with single MAPK pathway–directed agents is not an effective approach. In our cohort, hotspot mutations in KRAS, NRAS, or HRAS were detected in 12 of 20 (60%) selumetinib-treated patients (Figs 2B and 2E, and the Data Supplement).
Taken together, these data support the potential for MEK inhibition monotherapy to contribute to disease control in selected histologies that are primarily defined by single alterations in the MAPK pathway and an otherwise limited mutation burden, but not in more genomically complex and clinically aggressive tumor types. Activating MAPK pathway mutations are necessary, but not sufficient, for efficacy of MEK inhibitors for pediatric tumors. It is likely that selumetinib and other MEK inhibitors will require combination with additional molecularly targeted, immunotherapeutic, or cytotoxic agents to achieve optimal efficacy in patients with genomically complex tumors. Combination BRAF-V600E/MEK inhibition has been used to overcome toxicity of paradoxical BRAF activation by first-generation BRAF-V600E inhibitors and also to address resistance from acquisition of new mutations in melanoma and other BRAF V600E-mutated tumors.40-43 Addition of immune checkpoint inhibitors to MAPK-pathway targeted inhibitors may contribute to enhanced efficacy, although this may also broaden toxicity profile.44,45 In the example of Philadelphia chromosome–positive acute lymphoblastic leukemia, addition of chemotherapy to tyrosine kinase inhibition was associated with increased durability of responses.46,47 The rapid accrual to this arm of the Pediatric MATCH trial demonstrates the high burden of MAPK alterations in pediatric cancers and the utility of a nationwide molecular screening approach to facilitate further study of molecularly targeted agents targeting this pathway across tumor histologies.
APPENDIX
FIG A1.
Projected and observed accrual to arm E. Arm E was activated in July 2017 with the first patient enrolling in December 2017. The trial was temporarily closed after the 20th patient was enrolled in August 2019 and permanently closed in September 2020.
Carl E. Allen
Consulting or Advisory Role: Genentech/Roche, Sobi
Research Funding: NovImmune
P. Mickey Williams
Research Funding: Illumina (Inst)
Patents, Royalties, Other Intellectual Property: I was a coinventor of the DLBCL cell of origin patent recently filed by the NIH
Brent Coffey
Stock and Other Ownership Interests: Pfizer, AbbVie
Stacey L. Berg
Other Relationship: I am a member of the Children's Oncology Group Developmental Therapeutics Steering Committee. Some clinical trials may be partially industry funded. My institution may receive some funding for these trials, Pediatric Early Phase Clincial Trials Network
Alok Jaju
Stock and Other Ownership Interests: Gilead Sciences
Douglas S. Hawkins
Research Funding: Loxo (Inst), Bristol Myers Squibb (Inst), Merck Sharp & Dohme (Inst), Bayer (Inst), Lilly (Inst), Eisai (Inst), Amgen (Inst), Seattle Genetics (Inst), Incyte (Inst), Jazz Pharmaceuticals (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Katherine A. Janeway
Honoraria: Foundation Medicine, Takeda
Consulting or Advisory Role: Bayer, Ipsen
Travel, Accommodations, Expenses: Bayer
D. Williams Parsons
Patents, Royalties, Other Intellectual Property: Coinventor on current and pending patents related to cancer genes discovered through sequencing of several adult cancer types. Participates in royalty sharing related to those patents
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the 2021 ASCO Annual Virtual Meeting, June 4-8, 2021.
SUPPORT
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
The Children's Oncology Group Data Sharing policy describes the release and use of COG individual subject data for use in research projects in accordance with National Clinical Trials Network (NCTN) Program and NCI Community Oncology Research Program (NCORP) Guidelines. Only data expressly released from the oversight of the relevant COG Data and Safety Monitoring Committee (DSMC) are available to be shared. Data sharing will ordinarily be considered only after the primary study manuscript is accepted for publication. For phase III studies, individual-level deidentified data sets that would be sufficient to reproduce results provided in a publication containing the primary study analysis can be requested from the NCTN/NCORP Data Archive at https://nctn-data-archive.nci.nih.gov/. Data are available to researchers who wish to analyze the data in secondary studies to enhance the public health benefit of the original work and agree to the terms and conditions of use. For non–phase 3 studies, data are available following the primary publication. An individual-level deidentified data set containing the variables analyzed in the primary results paper can be expected to be available upon request. Requests for access to COG protocol research data should be sent to datarequest@childrensoncologygroup.org. Data are available to researchers whose proposed analysis is found by COG to be feasible and of scientific merit and who agree to the terms and conditions of use.
For all requests, no other study documents, including the protocol, will be made available and no end date exists for requests. In addition to above, release of data collected in a clinical trial conducted under a binding collaborative agreement between COG or the NCI Cancer Therapy Evaluation Program (CTEP) and a pharmaceutical/biotechnology company must comply with the data sharing terms of the binding collaborative/contractual agreement and must receive the proper approvals.
AUTHOR CONTRIBUTIONS
Conception and design: Olive S. Eckstein, Carl E. Allen, P. Mickey Williams, David R. Patton, Brent Coffey, Todd A. Alonzo, Stacey L. Berg, Elizabeth Fox, Naoko Takebe, James V. Tricoli, Katherine A. Janeway, Nita L. Seibel, D. Williams Parsons
Administrative support: David R. Patton, Naoko Takebe
Provision of study materials or patients: David R. Patton, Nilsa C. Ramirez, Elizabeth Fox, Naoko Takebe
Collection and assembly of data: Olive S. Eckstein, Carl E. Allen, P. Mickey Williams, David R. Patton, Brent Coffey, Lauren Saguilig, Nilsa C. Ramirez, Joyce Mhlanga, Elizabeth Fox, Margaret M. Mooney, Katherine A. Janeway, Nita L. Seibel, D. Williams Parsons
Data analysis and interpretation: Olive S. Eckstein, Carl E. Allen, P. Mickey Williams, Sinchita Roy-Chowdhuri, David R. Patton, Brent Coffey, Joel M. Reid, Jin Piao, Lauren Saguilig, Todd A. Alonzo, Stacey L. Berg, Alok Jaju, Elizabeth Fox, Douglas S. Hawkins, Naoko Takebe, Katherine A. Janeway, Nita L. Seibel, D. Williams Parsons
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase II Study of Selumetinib in Children and Young Adults With Tumors Harboring Activating Mitogen-Activated Protein Kinase Pathway Genetic Alterations: Arm E of the NCI-COG Pediatric MATCH Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Carl E. Allen
Consulting or Advisory Role: Genentech/Roche, Sobi
Research Funding: NovImmune
P. Mickey Williams
Research Funding: Illumina (Inst)
Patents, Royalties, Other Intellectual Property: I was a coinventor of the DLBCL cell of origin patent recently filed by the NIH
Brent Coffey
Stock and Other Ownership Interests: Pfizer, AbbVie
Stacey L. Berg
Other Relationship: I am a member of the Children's Oncology Group Developmental Therapeutics Steering Committee. Some clinical trials may be partially industry funded. My institution may receive some funding for these trials, Pediatric Early Phase Clincial Trials Network
Alok Jaju
Stock and Other Ownership Interests: Gilead Sciences
Douglas S. Hawkins
Research Funding: Loxo (Inst), Bristol Myers Squibb (Inst), Merck Sharp & Dohme (Inst), Bayer (Inst), Lilly (Inst), Eisai (Inst), Amgen (Inst), Seattle Genetics (Inst), Incyte (Inst), Jazz Pharmaceuticals (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Katherine A. Janeway
Honoraria: Foundation Medicine, Takeda
Consulting or Advisory Role: Bayer, Ipsen
Travel, Accommodations, Expenses: Bayer
D. Williams Parsons
Patents, Royalties, Other Intellectual Property: Coinventor on current and pending patents related to cancer genes discovered through sequencing of several adult cancer types. Participates in royalty sharing related to those patents
No other potential conflicts of interest were reported.
REFERENCES
- 1.Davies H, Bignell GR, Cox C, et al. : Mutations of the BRAF gene in human cancer. Nature 417:949-954, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Pratilas CA, Xing F, Solit DB: Targeting oncogenic BRAF in human cancer. Curr Top Microbiol Immunol 355:83-98, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao Y, Adjei AA: The clinical development of MEK inhibitors. Nat Rev Clin Oncol 11:385-400, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Braicu C, Buse M, Busuioc C, et al. : A comprehensive review on MAPK: A promising therapeutic target in cancer. Cancers (Basel) 11:1618, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burotto M, Chiou VL, Lee JM, et al. : The MAPK pathway across different malignancies: A new perspective. Cancer 120:3446-3456, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakraborty R, Hampton OA, Shen X, et al. : Mutually exclusive recurrent somatic mutations in MAP2K1 and BRAF support a central role for ERK activation in LCH pathogenesis. Blood 124:3007-3015, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eleveld TF, Oldridge DA, Bernard V, et al. : Relapsed neuroblastomas show frequent RAS-MAPK pathway mutations. Nat Genet 47:864-871, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knight T, Irving JA: Ras/Raf/MEK/ERK pathway activation in childhood acute lymphoblastic leukemia and its therapeutic targeting. Front Oncol 4:160, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chappell WH, Steelman LS, Long JM, et al. : Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors: Rationale and importance to inhibiting these pathways in human health. Oncotarget 2:135-164, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santarpia L, Lippman SM, El-Naggar AK: Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin Ther Targets 16:103-119, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marks JL, Gong Y, Chitale D, et al. : Novel MEK1 mutation identified by mutational analysis of epidermal growth factor receptor signaling pathway genes in lung adenocarcinoma. Cancer Res 68:5524-5528, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murugan AK, Dong J, Xie J, et al. : MEK1 mutations, but not ERK2 mutations, occur in melanomas and colon carcinomas, but none in thyroid carcinomas. Cell Cycle 8:2122-2124, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Ryall S, Zapotocky M, Fukuoka K, et al. : Integrated molecular and clinical analysis of 1,000 pediatric low-grade gliomas. Cancer Cell 37:569-583, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemberg KM, Wang J, Pratilas CA: From genes to -omics: The evolving molecular landscape of malignant peripheral nerve sheath tumor. Genes (Basel) 11:691, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilmott JS, Johansson PA, Newell F, et al. : Whole genome sequencing of melanomas in adolescent and young adults reveals distinct mutation landscapes and the potential role of germline variants in disease susceptibility. Int J Cancer 144:1049-1060, 2019 [DOI] [PubMed] [Google Scholar]
- 16.Allen CE, Merad M, McClain KL: Langerhans-cell histiocytosis. N Engl J Med 379:856-868, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diamond EL, Durham BH, Haroche J, et al. : Diverse and targetable kinase alterations drive histiocytic neoplasms. Cancer Discov 6:154-165, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shern JF, Selfe J, Izquierdo E, et al. : Genomic classification and clinical outcome in rhabdomyosarcoma: A report from an international consortium. J Clin Oncol 39:2859-2871, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackay A, Burford A, Carvalho D, et al. : Integrated molecular meta-analysis of 1,000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell 32:520-537, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fangusaro J, Onar-Thomas A, Young PT, et al. : Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: A multicentre, phase 2 trial. Lancet Oncol 20:1011-1022, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fangusaro J, Onar-Thomas A, Poussaint TY, et al. : A phase 2 trial of selumetinib in children with recurrent optic pathway and hypothalamic low-grade glioma without NF1: A pediatric brain tumor consortium study. Neuro Oncol 23:1777-1788, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gross AM, Wolters PL, Dombi E, et al. : Selumetinib in children with inoperable plexiform neurofibromas. N Engl J Med 382:1430-1442, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allen CE, Laetsch TW, Mody R, et al. : Target and agent prioritization for the Children's Oncology Group-National Cancer Institute Pediatric MATCH Trial. J Natl Cancer Inst 109:djw274, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seibel NL, Janeway K, Allen CE, et al. : Pediatric oncology enters an era of precision medicine. Curr Probl Cancer 41:194-200, 2017 [DOI] [PubMed] [Google Scholar]
- 25.Lih CJ, Harrington RD, Sims DJ, et al. : Analytical validation of the next-generation sequencing Assay for a nationwide signal-finding clinical trial: Molecular analysis for therapy choice clinical trial. J Mol Diagn 19:313-327, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banerjee A, Jakacki RI, Onar-Thomas A, et al. : A phase I trial of the MEK inhibitor selumetinib (AZD6244) in pediatric patients with recurrent or refractory low-grade glioma: A Pediatric Brain Tumor Consortium (PBTC) study. Neuro Oncol 19:1135-1144, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adjei AA, Cohen RB, Franklin W, et al. : Phase I pharmacokinetic and pharmacodynamic study of the oral, small-molecule mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) in patients with advanced cancers. J Clin Oncol 26:2139-2146, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boers-Sonderen MJ, Desar IM, Blokx W, et al. : A prolonged complete response in a patient with BRAF-mutated melanoma stage IV treated with the MEK1/2 inhibitor selumetinib (AZD6244). Anticancer Drugs 23:761-764, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Leijen S, Soetekouw PM, Jeffry Evans TR, et al. : A phase I, open-label, randomized crossover study to assess the effect of dosing of the MEK 1/2 inhibitor Selumetinib (AZD6244; ARRY-142866) in the presence and absence of food in patients with advanced solid tumors. Cancer Chemother Pharmacol 68:1619-1628, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leijen S, Middleton MR, Tresca P, et al. : Phase I dose-escalation study of the safety, pharmacokinetics, and pharmacodynamics of the MEK inhibitor RO4987655 (CH4987655) in patients with advanced solid tumors. Clin Cancer Res 18:4794-4805, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Diamond EL, Durham BH, Ulaner GA, et al. : Efficacy of MEK inhibition in patients with histiocytic neoplasms. Nature 567:521-524, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson DB, Zhao F, Noel M, et al. : Trametinib activity in patients with solid tumors and lymphomas harboring BRAF non-V600 mutations or fusions: Results from NCI-MATCH (EAY131). Clin Cancer Res 26:1812-1819, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dombi E, Baldwin A, Marcus LJ, et al. : Activity of selumetinib in neurofibromatosis type 1-related plexiform neurofibromas. N Engl J Med 375:2550-2560, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Infante JR, Fecher LA, Falchook GS, et al. : Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: A phase 1 dose-escalation trial. Lancet Oncol 13:773-781, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Champer M, Miller D, Kuo DY: Response to trametinib in recurrent low-grade serous ovarian cancer with NRAS mutation: A case report. Gynecol Oncol Rep 28:26-28, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ho AL, Grewal RK, Leboeuf R, et al. : Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med 368:623-632, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dummer R, Schadendorf D, Ascierto PA, et al. : Binimetinib versus dacarbazine in patients with advanced NRAS-mutant melanoma (NEMO): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 18:435-445, 2017 [DOI] [PubMed] [Google Scholar]
- 39.Chen AP, Kummar S, Moore N, et al. : Molecular Profiling-Based Assignment of Cancer Therapy (NCI-MPACT): A randomized multicenter phase II trial. JCO Precis Oncol 5:133-144, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robert C, Karaszewska B, Schachter J, et al. : Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 372:30-39, 2015 [DOI] [PubMed] [Google Scholar]
- 41.Flaherty KT, Infante JR, Daud A, et al. : Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 367:1694-1703, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paton EL, Turner JA, Schlaepfer IR: Overcoming resistance to therapies targeting the MAPK pathway in BRAF-mutated tumours. J Oncol 2020:1079827, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kakadia S, Yarlagadda N, Awad R, et al. : Mechanisms of resistance to BRAF and MEK inhibitors and clinical update of US Food and Drug Administration-approved targeted therapy in advanced melanoma. Onco Targets Ther 11:7095-7107, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gutzmer R, Stroyakovskiy D, Gogas H, et al. : Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAF(V600) mutation-positive melanoma (IMspire150): Primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 395:1835-1844, 2020 [DOI] [PubMed] [Google Scholar]
- 45.Ferrucci PF, Di Giacomo AM, Del VM, et al. : KEYNOTE-022 part 3: A randomized, double-blind, phase 2 study of pembrolizumab, dabrafenib, and trametinib in BRAF-mutant melanoma. J Immunother Cancer 8:e001806, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schultz KR, Bowman WP, Aledo A, et al. : Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: A Children's Oncology Group study. J Clin Oncol 27:5175-5181, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wassmann B, Pfeifer H, Goekbuget N, et al. : Alternating versus concurrent schedules of imatinib and chemotherapy as front-line therapy for Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL). Blood 108:1469-1477, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The Children's Oncology Group Data Sharing policy describes the release and use of COG individual subject data for use in research projects in accordance with National Clinical Trials Network (NCTN) Program and NCI Community Oncology Research Program (NCORP) Guidelines. Only data expressly released from the oversight of the relevant COG Data and Safety Monitoring Committee (DSMC) are available to be shared. Data sharing will ordinarily be considered only after the primary study manuscript is accepted for publication. For phase III studies, individual-level deidentified data sets that would be sufficient to reproduce results provided in a publication containing the primary study analysis can be requested from the NCTN/NCORP Data Archive at https://nctn-data-archive.nci.nih.gov/. Data are available to researchers who wish to analyze the data in secondary studies to enhance the public health benefit of the original work and agree to the terms and conditions of use. For non–phase 3 studies, data are available following the primary publication. An individual-level deidentified data set containing the variables analyzed in the primary results paper can be expected to be available upon request. Requests for access to COG protocol research data should be sent to datarequest@childrensoncologygroup.org. Data are available to researchers whose proposed analysis is found by COG to be feasible and of scientific merit and who agree to the terms and conditions of use.
For all requests, no other study documents, including the protocol, will be made available and no end date exists for requests. In addition to above, release of data collected in a clinical trial conducted under a binding collaborative agreement between COG or the NCI Cancer Therapy Evaluation Program (CTEP) and a pharmaceutical/biotechnology company must comply with the data sharing terms of the binding collaborative/contractual agreement and must receive the proper approvals.