Abstract
Thyroid dysfunction (TD) induced by programmed death-1 (PD-1) or programmed cell death-ligand 1 (PD-L1) immune checkpoint inhibitors (ICIs) has been widely reported. However, the effects of ICI-induced TD on the survival of patients with esophageal squamous cell carcinoma (ESCC) have not been described. Herein, a retrospective study was conducted, which 82 patients with advanced metastatic or recurrent ESCC treated with camrelizumab were enrolled. Twenty patients (24.4%) experienced TD during camrelizumab treatment with or without chemotherapy. The median onset time of TD was 1.7 months. The incidence of TD was 35.6% in patients who previously received thoracic radiotherapy versus 10.8% in patients who did not (P =0.009). Patients with TD had significantly longer median progression-free survival (5.5 months vs 3.5 months, P =0.035) and overall survival (26.7 months vs 11.5 months, P <0.001). TD is frequently observed in ESCC patients treated with camrelizumab and especially in patients who received radiotherapy previously. ESCC patients with TD during ICIs treatment often have better prognosis.
1. Introduction
Esophageal cancer is one of the malignant tumors with terrible prognosis. The substantial majority of esophageal cancer patients are in East Asia, and most patients suffered from squamous cell carcinoma [1]. Patients with metastatic or recurrent esophageal squamous cell carcinoma (ESCC) have very poor prognosis due to insensitivity to chemotherapy [2]. In the past decade, immunotherapy has become the most promising progress in solid tumor treatment. Typically, in the field of lung cancer [3] and melanoma [4], programmed death-1 (PD-1) and programmed cell death-ligand 1 (PD-L1) inhibitors greatly prolongs the survival time of such patients. As a revolutionary treatment, immunotherapy has also been slowly used to treat ESCC [5]. The discrepancy in pathological types of esophageal cancer between Eastern countries and Western countries lead to huge differences in certain genes mutation frequencies [6]. This also indirectly leads to the possibility that immunotherapy may have different clinical profiles in patients with ESCC [6].
Cytotoxic T lymphocyte antigen 4 (CTLA-4) or PD-1 and its ligand PD-L1 are the two most important targets of immune checkpoint inhibitors (ICIs). By inhibiting them, ICIs could activate T cells and thereby play a key role in killing tumor cells. The representative drugs include nivolumab, pembrolizumab, durvalumab, and ipilimumab, which have been widely used in numerous cancer treatment. In recent years, it has been found that compared with traditional chemotherapy, ICIs, like nivolumab [7], pembrolizumab [8], and camrelizumab [9], could greatly prolong the overall survival (OS) of patients with advanced ESCC. Moreover, patients treated with ICIs suffered from less treatment-related side effects and had better quality of life. Camrelizumab is a highly selective humanized PD-1 monoclonal antibody developed in China. It showed durable tumor response and improvement in OS with comparably less toxicity in metastatic ESCC [9]. Combined use of camrelizumab on the basis of chemotherapy can achieve higher tumor remission and longer OS in untreated advanced ESCC [10]. Therefore, camrelizumab has become a new treatment option and standard for advanced ESCC patients in China.
While the therapeutic efficacy is improved, patients treated with ICIs still experience the immunotherapy-related adverse events (irAEs). irAEs are fundamentally different from those of traditional anti-tumor therapy [11]. Prompt identification and treatment of irAEs could help patients obtain the maximum benefit from this promising therapy. Interestingly, the arise and severity of irAEs are closed related to therapeutic effect and prognosis. Generally, patients with irAEs had superior tumor response and longer OS [12–14]. However, some reports demonstrated that irAEs are not related to prognosis in cancer patients who received ICIs [15].
The thyroid is one of the most frequent target organs affected by PD-1 or PD-L1 inhibitors. Hyperthyroidism and hypothyroidism are the two most common thyroid dysfunctions (TDs) in patients treated with ICIs. Generally, TD often manifests as an early onset of transient thyrotoxicosis, followed by hypothyroidism [16]. Several studies reported that 10-20% of lung cancer patients would have TD during ICIs treatment. Cancer-specific survival was often better in patients with TD than that without TD [17, 18]. However, there are few reports on the incidence of TD in ESCC patients and it is also very urgent to know the relationship between TD and the effect of ICIs. Therefore, this retrospective study was designed to investigate the occurence and risk factors of TD induced by camrelizumab and their relationship to outcome in ESCC patients.
2. Methods
2.1. Patients
A retrospective study involving patients with advanced metastatic or recurrent ESCC was conducted. ESCC patients treated with camrelizumab between January 2019 and August 2021 at the Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer hospital) were enrolled in this study. The inclusion criteria were as follows: advanced metastatic or recurrent ESCC confirmed by histology or cytology; age between 18 years and 75 years; received at least two cycles of camrelizumab with or without chemotherapy; no previous thyroid disease, including thyroiditis, hyperthyroidism, hypothyroidism, Graves' disease, and thyroid cancer; regular blood thyroid function test during immunotherapy. The exclusion criteria were as follows: second primary cancer in addition to esophageal cancer in the past or currently; abnormal thyroid function within one week before camrelizumab treatment; previously underwent thyroidectomy; previously prescribed with either levothyroxine or methimazole. Patients' clinicopathological characteristics, such as age, gender, performance status (PS) score, smoking status, tumor location, TNM staging, and previous treatment, were collected from medical records. This study was approved by the institutional review board of The Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer hospital).
2.2. Camrelizumab treatment
All patients received camrelizumab three-week cycle with or without chemotherapy. It was given at a fixed dose of 200 mg/cycle intravenously over approximately 30 minutes. If it was used in combination with chemotherapy, the chemotherapy agents should be used after camrelizumab injection. Camrelizumab would be used for at least two years only stopped in case of disease progression, intolerable adverse events, and patients' decision. Once immune-related adverse events occurred, camrelizumab could be interrupted for up to six weeks.
2.3. Thyroid function tests and classification of TD
Thyroid function tests were carried out in our hospital's clinical laboratory using electrochemiluminescent bridging immunoassay. Blood samples were drawn and tested for free thyroxin (fT4), free triiodothyronine (fT3) and thyroid-stimulating hormone (TSH). Thyroid function tests were performed seven days within the initiation of camrelizumab, as well as every cycle of immunotherapy.
The reference ranges of fT4 and TSH in our hospital were 0.81–1.89 ng/dL and 0.38–4.34 μIU/ml, respectively. Two physicians independently reviewed the thyroid function results. On the basis of fT4 and TSH values, TD was divided into two groups: hypothyroidism and hyperthyroidism. When fT4 was within the normal range and TSH was low (0.38 < μIU/ml) or high (>4.34 μIU/ml), TD was scored as “subclinical hyperthyroidism” or “subclinical hypothyroidism,”, respectively. When TSH was low (0.38 < μIU/ml) and fT4 was high (>1.89 ng/dL), TD was scored as “overt hyperthyroidism,” or vice versa (overt hypothyroidism). TThe Common Terminology Criteria for Adverse Events version 5.0 (CTCAE v5.0.) was utilized to grade TD toxicities. The time of appearance of TD was defined as the time interval from the first use of camrelizumab to the first time TD was diagnosed.
2.4. Tumor response evaluation and outcomes
Every patient underwent computed tomography scans of the chest and upper abdomen, and supraclavicular B-ultrasound every six weeks. Response Evaluation Criteria in Solid Tumors (RECIST) was utilized to assess tumor response. The primary endpoints in this study were progression-free survival (PFS) and OS. The time from the first injection of camrelizumab to cancer progression or death was calculated as PFS. OS was defined as the interval between the first injection of camrelizumab and death from any cause or censored at the date of last follow-up.
2.5. Statistical analysis
The categorical variables were evaluated by Chi-square test. Fisher's exact test was carried out when Chi-square test assumptions were not valid. Survival curves were estimated by the univariate Kaplan–Meier method. The median survival time with corresponding 95% confidence intervals (CIs) were presented. To investigate the influence of risk factors on survival, hazard rations (HRs) and 95% CIs were calculated using univariate Cox proportional hazards models. Cox proportional hazards models were fitted to adjust the effect of TD for potentially prognostic covariates, such as patients' gender, age, performance score (PS), treatment lines, tumor stage, and liver metastasis. All statistical analyses were carried out with SPSS 26.0 for Windows. Statistical significance was considered at P <0.05.
3. Results
3.1. Patients
A total of 82 patients with advanced metastatic or recurrent ESCC treated with camrelizumab were enrolled in this study. The vast majority of patients were men (76/82, 92.7%). The ratio of men to women was 12.7 : 1. The median age of the entire group of patients was 63 years. Twenty-three patients received camrelizumab monotherapy, whereas 59 patients received immunotherapy combined with chemotherapy. Most patients (62/82, 75.6%) had a history of smoking. There were 11, 42, and 29 cases of esophageal cancer in the neck and upper chest, middle chest, and lower chest, respectively. First-line treatment with camrelizumab was administered to 25 patients for a median of six cycles, whereas the remaining 57 received second-line or further treatment for a median of four cycles. The detailed characteristics of patients are shown in Table 1.
Table 1.
Correlations between the occurrence of TD and clinicopathological characteristics.
| Variables | Number (%) | TD | P | |
|---|---|---|---|---|
| Absent | Present | |||
| Gender | ||||
| Female | 6 (7.3) | 4 (66.7) | 2 (33.3) | 0.596 |
| Male | 76 (92.3) | 56 (76.3) | 18 (23.7) | |
| Age (years) | ||||
| ≤ 65 | 53 (64.6) | 38 (71.7) | 15 (28.3) | 0.265 |
| > 65 | 29 (35.4) | 24 (82.8) | 5 (17.2) | |
| Performance status | ||||
| 0 | 15 (18.3) | 8 (53.3) | 7 (46.7) | 0.032 |
| 1 | 51 (62.2) | 39 (76.5) | 12 (23.5) | |
| 2 | 16 (19.5) | 15 (93.8) | 1 (6.3) | |
| Smoking status | ||||
| Never | 20 (24.4) | 13 (65.0) | 7 (35.0) | 0.204 |
| Current and former | 62 (75.6) | 49 (79.0) | 13 (21.0) | |
| Alcohol status | ||||
| Never | 19 (23.2) | 13 (68.4) | 6 (31.6) | 0.405 |
| Current and former | 63 (76.8) | 49 (77.8) | 14 (22.2) | |
| Combined with chemotherapy | ||||
| No | 23 (28.0) | 16 (69.6) | 7 (30.4) | 0.426 |
| Yes | 59 (72.0) | 46 (78.0) | 13 (22.0) | |
| Treatment lines | ||||
| First | 25 (30.5) | 20 (80.0) | 5 (20.0) | 0.540 |
| Second or more | 57 (69.5) | 42 (73.7) | 15 (26.3) | |
| Tumor location | ||||
| Cervical or upper thoracic | 11 (13.4) | 9 (81.8) | 2 (18.2) | 0.819 |
| Middle thoracic | 42 (51.2) | 32 (76.2) | 10 (23.8) | |
| Lower thoracic | 29 (35.4) | 21 (72.4) | 8 (27.6) | |
| Previous surgery | ||||
| No | 45 (54.9) | 36 (80.0) | 9 (20.0) | 0.307 |
| Yes | 37 (45.1) | 26 (70.3) | 11 (29.7) | |
| Previous radiotherapy | ||||
| No | 37 (45.1) | 33 (89.2) | 4 (10.8) | 0.009 |
| Yes | 45 (54.9) | 29 (64.4) | 16 (35.6) | |
| Supraclavicular radiotherapy | ||||
| No | 62 (75.6) | 48 (77.4) | 14 (22.6) | 0.502 |
| Yes | 20 (24.4) | 14 (70.0) | 6 (30.0) | |
| Stage | ||||
| III | 8 (9.8) | 5 (62.5) | 3 (37.5) | 0.363 |
| IV | 74 (90.2) | 57 (77.0) | 17 (23.0) | |
| Liver metastasis | ||||
| No | 60 (73.2) | 42 (70.0) | 18 (30.0) | 0.051 |
| Yes | 22 (26.8) | 20 (90.9) | 2 (9.1) | |
3.2. Association between clinicopathological variables and TD
Twenty patients (24.4%) experienced TD during camrelizumab treatment with or without chemotherapy. The most common TD subtype was subclinical hypothyroidism (11 patients, 55.0%), followed by overt hypothyroidism (5 patients, 25.0%), subclinical hyperthyroidism (2 patients, 10.0%), and overt hyperthyroidism (2 patients, 10.0%). According to CTCAE v5.0, only one patient was graded as 3, and this patient continued to receive immunotherapy after levothyroxine replacement. The vast majority of patients were graded as 1 or 2. The median onset time of TD was 1.7 months (range: 0.3–13.7 months). The median onset time of hypothyroidism was shorter than that of hyperthyroidism (1.7 months vs 2.4 months, P <0.05).
We performed Chi-square test to investigate the association between clinicopathological variables and TD (Shown in Table 1). Patients without liver metastasis were prone to have TD, but the difference was not statistically significant (P =0.051). The incidence of TD was 35.6% in patients who previously received thoracic radiotherapy versus 10.8% in patients who did not (P =0.009). Additionally, patients' PS score was also related to TD (P =0.032). Patients with PS = 0 tended to develop TD during immunotherapy. There is no relationship between the site of radiation therapy and radiation therapy-induced hypothyroidism, calculated by chi-square test (P = 0.502). Moreover, TD was not associated with gender, age, smoking, alcohol, treatment lines, and tumor stage (P > 0.05).
3.3. Clinical response and survival analysis
Thirteen patients (15.9%) were confirmed to have PR, 63 patients SD, and 6 patients PD. PR was positively correlated with the incidence rates of TD (P = 0.002).
The median follow-up time was 15.3 months (range: 3–50 months). Fifty-seven patients discontinued camrelizumab due to cancer progression. Nine patients experienced intolerable irAEs. The most common serious side effects that caused the cessation of camrelizumab were esophageal perforation (3 patients, 3.7%) and pneumonia (3 patients, 3.7%). No patient discontinued immunotherapy due to TD. The median PFS and OS were 3.8 and 18.2 months, respectively. Sixty-three patients (76.8%) progressed during immunotherapy, and 39 patients (47.6%) died. Patients with TD had significantly longer median PFS (5.5 months vs 3.5 months, P =0.035, Figure 1) and OS (26.7 months vs 11.5 months, P <0.001, Figure 2) compared with patients without TD. Multivariate Cox regression analysis for PFS (Figure 3) showed that TD and treatment lines were significantly associated with PFS (TD: HR = 0.47, 95% CI = 0.24–0.94, P = 0.034; treatment lines: HR = 2.44, 95% CI = 1.31–4.56, P = 0.005). Moreover, TD independently predicted OS in univariate and multivariate analyses. Patients with TD had a 0.08-fold decreased risk of death compared with those without TD (P <0.05, shown in Figure 4).
Figure 1.
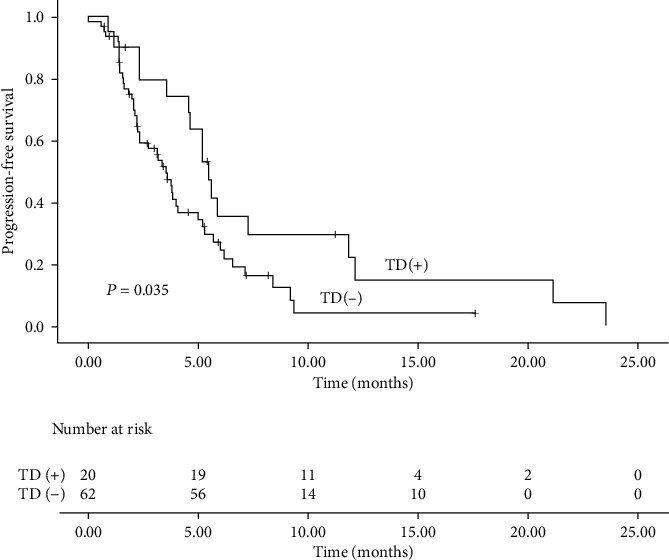
Progression-free survival in ESCC patients with and without TD.
Figure 2.
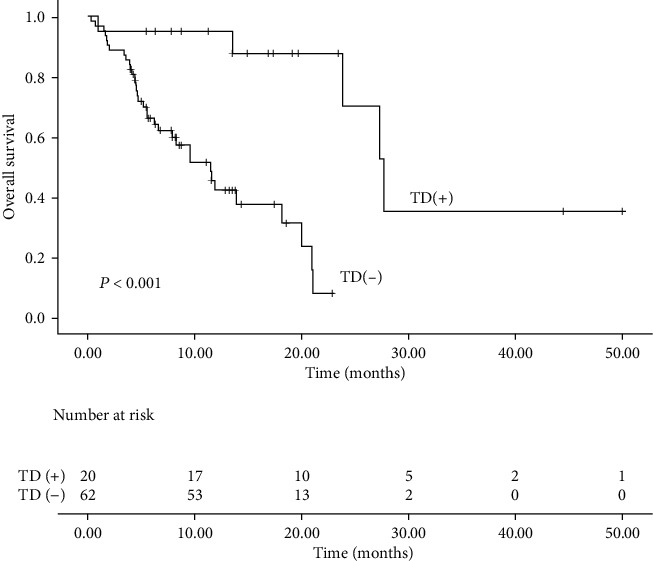
Overall survival in ESCC patients with and without TD.
Figure 3.
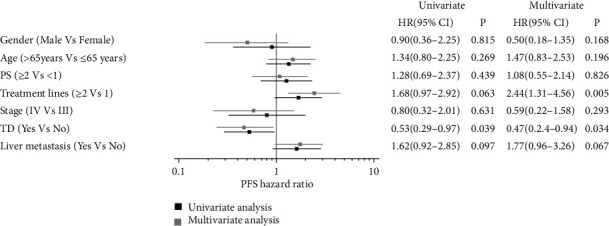
Prognostic factors for PFS by univariate and multivariate analysis in ESCC patients received camrelizumab.
Figure 4.
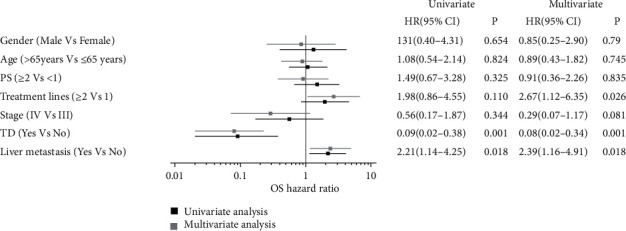
Prognostic factors for OS by univariate and multivariate analysis in ESCC patients received camrelizumab.
4. Discussion
In the present study, we demonstrated that previous radiotherapy and patients' PS score represent predictive biomarkers for TD during camrelizumab treatment. The most common TD subtype was subclinical hypothyroidism. The occurrence of TD is a promising predictor in recurrent or metastatic ESCC patients receiving camrelizumab. TD may be related to improved PFS and OS.
The occurrence and incidence of TD varied among different immunotherapy agents. CTLA-4 inhibitors had a lower rate of TD than PD-1 or PD-L1 inhibitors [19, 20]. TD occurs in less than 5% of patients received ipilimumab. However, up to 10% of patients experienced TD when they were treated with PD-1 inhibitors. The incidence of TD was increased to about 15% in patients receiving the combination regimen [21]. Additionally, the incidence of TD is different for different ICIs, even if they are all PD-1 inhibitors [19]. In the present study, 24.4% of patients developed TD. This value is slightly different from previous reports in the literature [19]. The reason for this difference is unknown. One possible explanation is that the odds of TD is different among different PD-1 inhibitors. In the KEYNOTE-181 study [8], 10.5% of patients who received pembrolizumab monotherapy experienced grade 1–2 TD. The rate of TD increased to 15% when patients were treated with chemoimmunotherapy [22]. Camrelizumab is widely used in different lines of treatment for advanced ESCC in China. It is often associated with high rate of irAEs, especially reactive capillary hemangiomas and TD. The incidence of TD is about 19% when camrelizumab monotherapy is used [9]. When patients were treated with combination regimens, one fourth of them developed TD [23]. Another hypothesis is that more than half of patients in this study received supraclavicular radiotherapy before immunotherapy. Radiotherapy, which promotes the release of neoantigens, was associated with elevated risk of TD [24]. The increased odds of patients experiencing grade 2 or higher TD was confirmed to be related to previous supraclavicular radiotherapy for thyroid cancer [25].
Several studies demonstrated that the occurrence of TD is a promising pronostic factor in cancer patients receiving ICIs, including lung cancer [17, 18] and melanoma [26]. However, no research has investigated the association of TD and prognosis of ESCC. In our study, ESCC patients who received camrelizumab with or without chemotherapy experienced TD had better tumor response and longer OS. Moreover, multivariate analysis revealed that TD is an independent prognostic factor. Patients who experienced TD had 0.47-fold lower risk of disease progression and 0.08 times lower risk of death than those who did not. The underlying molecular mechanism behind this relationship remains unknown. The PD-1/PD-L1 signaling pathway is inhibited by camrelizumab, thereby corresponding T cells are activating. ESCC and thyroid may express many of the similar antigens. Therefore, thyroid is attacked while activating T cells attack ESCC. Angell et al. performed fine needle biopsy on the thyroid tissue of TD patients after nivolumab treatment. It was found that normal thyroid tissue was infiltrated with a large amount of cell debris and CD163 positive lymphocytes [27]. Another possible mechanism is that pre-existing chronic thyroiditis was unleashed by ICI treatment [28]. Patients with high baseline TSH concentration and positive anti-thyroid antibody (ATAb) before ICIs treatment were prone to have TD [29].
The shortcomings of our study are also obvious.. First, the nature of this study is retrospective and the sample size is relatively small. Second, PD-L1 expression was evaluated in a few patients. These factors decrease the reliability of the results. However, the patients enrolled in this study received the same ICI, not a collection of several ICIs, which can avoid bias and offset the heterogeneity. Moreover, we did not investigate the concentration of serum ATAbs before and during camrelizumab treatment. Further investigation should focus on the role of ATAbs in the prognosis of ESCC patients.
In conclusion, our study demonstrated that TD may be a new independent prognostic biomarker for PFS and OS in ESCC patients. However, further prospective studies are warranted to gain full insights into the role of TD as a promising marker during immunotherapy.
Acknowledgments
This study was supported by grants from the Beijing Bethune Charitable Foundation (No flzh202114).
Data Availability
The data of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Chen W., Zheng R., Baade P. D., et al. Cancer statistics in China, 2015. CA: a Cancer Journal for Clinicians . 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Lorenzen S., Schuster T., Porschen R., et al. Cetuximab plus cisplatin-5-fluorouracil versus cisplatin-5-fluorouracil alone in first-line metastatic squamous cell carcinoma of the esophagus: a randomized phase II study of the Arbeitsgemeinschaft Internistische Onkologie. Annals of Oncology . 2009;20(10):1667–1673. doi: 10.1093/annonc/mdp069. [DOI] [PubMed] [Google Scholar]
- 3.Memmott R. M., Wolfe A. R., Carbone D. P., Williams T. M. Predictors of response, progression-free survival, and overall survival in patients with lung cancer treated with immune checkpoint inhibitors. Journal of Thoracic Oncology . 2021;16(7):1086–1098. doi: 10.1016/j.jtho.2021.03.017. [DOI] [PubMed] [Google Scholar]
- 4.Feld E., Mitchell T. C. Immunotherapy in melanoma. Immunotherapy . 2018;10(11):987–998. doi: 10.2217/imt-2017-0143. [DOI] [PubMed] [Google Scholar]
- 5.DaSilva L. L., Aguiar P. N., Jr., de Lima L. G. Immunotherapy for advanced esophageal squamous cell carcinoma-renewed enthusiasm and a lingering challenge. JAMA Oncology . 2021;7(11):1613–1614. doi: 10.1001/jamaoncol.2021.4410. [DOI] [PubMed] [Google Scholar]
- 6.Deng J., Chen H., Zhou D., et al. Comparative genomic analysis of esophageal squamous cell carcinoma between Asian and Caucasian patient populations. Nature Communications . 2017;8(1):p. 1533. doi: 10.1038/s41467-017-01730-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kato K., Cho B. C., Takahashi M., et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. The Lancet Oncology . 2019;20(11):1506–1517. doi: 10.1016/S1470-2045(19)30626-6. [DOI] [PubMed] [Google Scholar]
- 8.Kojima T., Shah M. A., Muro K., et al. Randomized phase III KEYNOTE-181 study of Pembrolizumab versus chemotherapy in advanced esophageal cancer. Journal of Clinical Oncology . 2020;38(35):p. 4138-48. doi: 10.1200/JCO.20.01888. [DOI] [PubMed] [Google Scholar]
- 9.Huang J., Xu J., Chen Y., et al. Camrelizumab versus investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. The Lancet Oncology . 2020;21(6):832–842. doi: 10.1016/S1470-2045(20)30110-8. [DOI] [PubMed] [Google Scholar]
- 10.Luo H., Lu J., Bai Y., et al. Effect of Camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. JAMA . 2021;326(10):916–925. doi: 10.1001/jama.2021.12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brahmer J. R., Lacchetti C., Schneider B. J., et al. Management of Immune-Related Adverse Events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. Journal of Clinical Oncology . 2018;36(17):1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teraoka S., Fujimoto D., Morimoto T., et al. Early immune-related adverse events and association with outcome in advanced non-small cell lung cancer patients treated with Nivolumab: a prospective cohort study. Journal of Thoracic Oncology . 2017;12(12):1798–1805. doi: 10.1016/j.jtho.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 13.Haratani K., Hayashi H., Chiba Y., et al. Association of Immune-Related Adverse Events with Nivolumab Efficacy in non-small-cell lung cancer. JAMA Oncology . 2018;4(3):374–378. doi: 10.1001/jamaoncol.2017.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibney G. T., Kudchadkar R. R., DeConti R. C., et al. Safety, correlative markers, and clinical results of adjuvant nivolumab in combination with vaccine in resected high-risk metastatic melanoma. Clinical Cancer Research . 2015;21(4):712–720. doi: 10.1158/1078-0432.CCR-14-2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamauchi I., Yasoda A., Matsumoto S., et al. Incidence, features, and prognosis of immune-related adverse events involving the thyroid gland induced by nivolumab. PLoS One . 2019;14(5, article e0216954) doi: 10.1371/journal.pone.0216954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iyer P. C., Cabanillas M. E., Waguespack S. G., et al. Immune-related thyroiditis with immune checkpoint inhibitors. Thyroid . 2018;28(10):1243–1251. doi: 10.1089/thy.2018.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Y., Xia R., Xiao H., et al. Thyroid function abnormality induced by PD-1 inhibitors have a positive impact on survival in patients with non-small cell lung cancer. International Immunopharmacology . 2021;91, article 107296 doi: 10.1016/j.intimp.2020.107296. [DOI] [PubMed] [Google Scholar]
- 18.Thuillier P., Joly C., Alavi Z., et al. Thyroid dysfunction induced by immune checkpoint inhibitors is associated with a better progression-free survival and overall survival in non-small cell lung cancer: an original cohort study. Cancer Immunology, Immunotherapy . 2021;70(7):2023–2033. doi: 10.1007/s00262-020-02802-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barroso-Sousa R., Barry W. T., Garrido-Castro A. C., et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncology . 2018;4(2):173–182. doi: 10.1001/jamaoncol.2017.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morganstein D. L., Lai Z., Spain L., et al. Thyroid abnormalities following the use of cytotoxic T-lymphocyte antigen-4 and programmed death receptor protein-1 inhibitors in the treatment of melanoma. Clinical Endocrinology . 2017;86(4):614–620. doi: 10.1111/cen.13297. [DOI] [PubMed] [Google Scholar]
- 21.Postow M. A., Chesney J., Pavlick A. C., et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. The New England Journal of Medicine . 2015;372(21):2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun J. M., Shen L., Shah M. A., et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo- controlled, phase 3 study. Lancet . 2021;398(10302):751–759. doi: 10.1016/S0140-6736(21)01234-4. [DOI] [PubMed] [Google Scholar]
- 23.Zhang B., Qi L., Wang X., et al. Phase II clinical trial using camrelizumab combined with apatinib and chemotherapy as the first-line treatment of advanced esophageal squamous cell carcinoma. Cancer Communications . 2020;40(12):711–720. doi: 10.1002/cac2.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Randhawa A. S., Yadav H. P., Banipal R. P. S., Goyal G., Garg P., Marcus S. Functional and biochemical changes in the thyroid gland following exposure to therapeutic doses of external beam radiotherapy in the head-and-neck cancer patients. Journal of Cancer Research and Therapeutics . 2021;17(4):1025–1030. doi: 10.4103/jcrt.JCRT_148_19. [DOI] [PubMed] [Google Scholar]
- 25.Sukari A., Nagasaka M., Alhasan R., et al. Cancer site and adverse events induced by immune checkpoint inhibitors: a retrospective analysis of real-life experience at a single institution. Anticancer Research . 2019;39(2):781–790. doi: 10.21873/anticanres.13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frelau A., Jali E., Campillo-Gimenez B., et al. Prognostic impact of thyroid dysfunctions on progression-free survival in patients with metastatic melanoma treated with anti-PD-1 antibodies. Melanoma Research . 2021;31(3):208–217. doi: 10.1097/CMR.0000000000000739. [DOI] [PubMed] [Google Scholar]
- 27.Angell T. E., Min L., Wieczorek T. J., Hodi F. S. Unique Cytologic features of thyroiditis caused by immune checkpoint inhibitor therapy for malignant melanoma. Genes Dis . 2018;5(1):46–48. doi: 10.1016/j.gendis.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kari S., Flynn J. C., Zulfiqar M., Snower D. P., Elliott B. E., Kong Y. C. Enhanced autoimmunity associated with induction of tumor immunity in thyroiditis-susceptible mice. Thyroid . 2013;23(12):1590–1599. doi: 10.1089/thy.2013.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brilli L., Danielli R., Campanile M., et al. Baseline serum TSH levels predict the absence of thyroid dysfunction in cancer patients treated with immunotherapy. Journal of Endocrinological Investigation . 2021;44(8):1719–1726. doi: 10.1007/s40618-020-01480-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of this study are available from the corresponding author upon reasonable request.


