Abstract
Background:
Colorectal cancer screening by annual fecal immunochemical test (FIT) with follow-up on abnormal results is a cost-effective strategy to reduce colorectal cancer incidence and mortality. Unfortunately, many patients with abnormal results do not complete a follow-up colonoscopy. We tested whether navigation targeted to patients who are unlikely to complete the procedure may improve adherence and long-term outcomes.
Methods:
Study participants were patients at a large, integrated health system (Kaiser Permanente Northwest) who were ages 50 to 75 and were due for a follow-up colonoscopy after a recent abnormal FIT result. Probability of adherence to follow-up was estimated at baseline using a predictive risk model. Patients whose probability was 70% or lower were randomized to receive patient navigation or usual care, with randomization stratified by probability category (<50%, 50% < 60%, 60% < 65%, 65% ≤ 70%). We compared colonoscopy completion within 6 months between the navigation and usual care groups using Cox proportional hazards regression.
Results:
Participants (n = 415; 200 assigned to patient navigation, 215 to usual care) had a mean age of 62 years, 54% were female, and 87% were non-Hispanic white. By 6 months, 76% of the patient navigation group had completed a colonoscopy, compared with 65% of the usual care group (HR = 1.35; 95% confidence interval, 1.07–1.72; log-rank P value = 0.027).
Conclusions:
In this randomized trial, patient navigation led to improvements in follow-up colonoscopy adherence.
Impact:
More research is needed to assess the value of precision-directed navigation programs.
Introduction
Colorectal cancer claimed the lives of an estimated 50,000 adults in the United States in 2019 (1). Improving screening through fecal immunochemical testing (FIT) or other modalities could reduce colorectal cancer mortality by more than 50% (2), but only if patients with abnormal fecal test results receive follow-up colonoscopies (3, 4). A recent meta-analysis showed an overall follow-up colonoscopy completion rate of 72%, with five recent United States-based studies reporting rates ranging from 56% to 86% (5).
Delays in follow-up colonoscopy can lead to increases in colorectal cancer incidence and mortality. Analyses of Kaiser Permanente Northern California data show that delays of 10 months or longer were associated with a 48% increased risk of colorectal cancer and a two-fold increased risk for advanced-stage disease, compared with colonoscopy within 30 days of an abnormal FIT (6). Lee and colleagues reported a 31% higher risk of colorectal cancer and a two-fold higher risk of advanced stage disease among adults who delayed colonoscopy by 6 months or more than among those who obtained a colonoscopy within 1 to 3 months (7). Modeling by Meester and colleagues predicted a 4% higher colorectal cancer incidence and 16% higher mortality among adults who completed a colonoscopy within 12 months versus within 2 weeks following an abnormal FIT (8).
Research suggests that patient navigation may increase completion of colorectal cancer screening and follow-up. Patient navigators help patients communicate with their health care provider, set up appointments, and access community resources. In previous reports, patient navigation boosted rates of colorectal cancer screening and follow-up by 8 to 31 percentage points (9–14). The New Hampshire Colorectal Cancer Screening Program (NHCRCSP; ref. 10) delivered phone-based navigation to uninsured and underinsured individuals ages 50 to 64 with household incomes at or below 250% of the federal poverty level. The program also provided free colonoscopy to adults eligible for a screening colonoscopy. Findings showed a 27 percentage point boost in colonoscopy uptake over a comparable control group (96% vs. 69%, P < 0.001; ref. 10).
Notably, the successful NHCRCSP program reported that a high proportion (69%) of nonnavigated patients obtained a colonoscopy—that is, they did not need navigation. This finding is important because patient navigation programs typically require extensive resources. If health systems could determine which patients were unlikely to complete a colonoscopy on their own, they could focus on the individuals most in need.
As part of a Centers for Disease Control-funded replication study of the NHCRCSP program (SIP-16-001), we tested the effectiveness of targeted patient navigation for follow-up colonoscopy at Kaiser Permanente Northwest (KPNW). We used a predictive risk model to calculate individuals' probability of obtaining a colonoscopy on their own. We included patients whose probability was less than 70%, and we report the effectiveness of patient navigation, overall and by subgroups defined by age, race, sex, and the estimated probability of a patient obtaining a colonoscopy on their own.
Materials and Methods
Parent study: replication of the New Hampshire colorectal cancer screening program
Here, we present the results of an individual randomized trial of the NHCRCSP program delivered to patients whose probability of obtaining a colonoscopy was less than 70%. This was a subanalysis of a larger study testing the replication of the NHCRCSP program (described elsewhere; refs. 10, 15). The NHCRCSP program is a telephone-based program that focuses on six different topics. Patients receive calls covering four topic areas prior to their colonoscopy, and the final two after they complete it. Calls are delivered by a registered nurse navigator; topics are listed in Supplementary Table S1. All study procedures were reviewed and approved by the University of Washington Institutional Review Board (protocol no.: 00000779), which waived informed consent, because the study involved minimal risks to patients. This subanalysis includes parent-study data (n = 280 participants) from the KPNW site plus an additional 215 KPNW participants recruited for the present analysis. Recruitment took place between May 2018 and January 2019; and follow-up occurred through June 2019. The trial was not registered with clinicaltrials.gov.
Study setting
KPNW is an integrated health care delivery system that serves over 600,000 patients in Oregon and Southwest Washington. Colonoscopy services are delivered at 20 different facilities. In 2019, KPNW's colorectal cancer screening rate was 83% among eligible adults ages 50 to 75. In 2019, a total of 67,619 patients obtained FIT testing and 2,986 patients had an abnormal FIT. KPNW operates a mailed FIT outreach program that mails FIT kits and sends automated reminders to eligible patients. KPNW uses OC-Auto-FIT (Polymedco, Cortlandt Manor, NY), a quantitative FIT with results processed by an automated analyzer. Abnormal FIT results are determined using the manufacturer-recommended hemoglobin concentration cutoff of 20 μg of hemoglobin/g of stool.
Predicted probability of colonoscopy adherence
In this analysis, we applied our previously developed risk prediction model to KPNW patients due for colonoscopy following an abnormal FIT test (16). The model, developed and validated in collaboration with the KPNW gastroenterology department, identifies patients with abnormal FIT results who have a low likelihood of obtaining a follow-up colonoscopy on their own, and thus may benefit from patient navigation.
The variables in the prediction model include demographics (age, sex, race, language, and geographic region); clinical data (smoking status, history of comorbid conditions, body mass index, number of prescriptions, prior flu vaccination, hemorrhoids, and substance abuse); and care utilization data (number of encounters and missed appointments in the past year, and prior colorectal cancer screening; Supplementary Table S2). The current model was developed using Cox proportional hazards regression, and internal validation was performed using bootstrapping with 500 replicates. The model's bootstrap corrected C-statistic was 0.65.
Study procedures
Research staff partnered with KPNW clinic staff to implement the NHCRCSP patient navigation program or usual care for patients who had an abnormal FIT test result and an estimated probability of colonoscopy completion of <70% based on the updated prediction model.
Patient selection and randomization
An automated process identified patients ages 50 to 75 who received an abnormal FIT test result in the past 2 months, a referral to colonoscopy, and no recent colonoscopy (in the past 3 years) or hospitalization (in the prior month). The project analyst calculated individual colonoscopy adherence probabilities using the model described above, and randomized individuals within probability strata (<50%, 50% < 60%, 60% < 65%, 65% ≤ 70%) to receive patient navigation or usual care. The analyst randomized patients on a weekly basis using the SURVEYSELECT procedure in SAS Institute Inc. Patients with a colonoscopy probability over 70% were excluded. Patients randomized to the intervention were entered into a REDCap database, which tracked navigation activities.
Prior to randomization, abnormal FIT results were managed by a centralized licensed nurse who reviewed the patient's chart to determine whether the patient met the criteria for standard referral to colonoscopy. Patients meeting the criteria were referred for a colonoscopy; those for whom it was unclear whether they met the criteria were referred to a physician who performed a more detailed chart review. On the basis of the findings, the physician either placed a colonoscopy referral (for patients meeting clinical criteria), referred the patients to a pre-procedure visit with the gastroenterologist, or sent a letter explaining why the patient did not need a colonoscopy (for patients not meeting clinical criteria). Patients referred to colonoscopy were eligible for randomization. Following randomization, a small number of patients were referred to a pre-procedure visit, based on clinical information obtained during scheduling or during navigation, and thus excluded from the study.
Usual care condition
After referral to colonoscopy, a medical assistant contacted the patient to schedule an appointment. Standard GI department reminders (up to three phone calls, an email message, and a letter) were sent before the appointment, and communications regarding bowel prep were made via phone and through messages in the health plan's website patient portal.
Patient navigation intervention
Patients assigned navigation received navigation services from a registered nurse. The nurse mailed an introductory letter and delivered live calls addressing the six topic areas, scheduled at particular times relative to the colonoscopy procedure. She made up to three attempts to reach a patient for each call. The navigator made calls from 8:00 a.m. to 5:00 p.m. Monday to Thursday.
Outcomes
The primary outcome was time to colonoscopy following an abnormal FIT result. Colonoscopy completion and date of completion were extracted from KPNW's electronic medical record (Epic), the navigator database, and through chart abstractions (the chart abstractor was blinded to randomization assignment). Time-to-colonoscopy was calculated from the date of the abnormal FIT lab test result. Participants were followed until the time of colonoscopy, death, end of membership, or for 180 days, whichever came first. A secondary outcome was colonoscopy appointment cancellations or missed appointments, categorized into five groups: did not miss or cancel the appointment; missed appointment (no-show); cancelled less than 24 hours before appointment; cancelled 24 to 48 hours before appointment; or cancelled 48 hours or more before appointment. Missed or cancelled appointments data were obtained from the navigator database and chart abstraction. Finally, we assessed the proportion of intervention participants who received at least one outreach phone call, and the proportion who received each of the six topic area calls.
Statistical analysis
Analyses were conducted using intent-to-treat principles: all participants were analyzed as part of the group to which they were randomized. We examined the characteristics of our study participants at baseline overall and by study condition and calculated standardized differences in distributions; subsequent hypothesis-testing models were adjusted for variables with standardized differences ≥0.10 (17). We used a Kaplan–Meier estimator to calculate the cumulative incidence of colonoscopy in each condition at 6 months. We used the log-rank test to test for differences in time to colonoscopy. We used Cox proportional hazards regression to estimate the HR and 95% confidence intervals of colonoscopy completion between the two study conditions, using the Efron method for handling tied survival times (18); models were adjusted for age, race, insurance type, prior colonoscopy screening, body mass index, and prior flu vaccination, which had standardized differences ≥0.10. The proportional hazards assumption was verified by visual inspection (log–log plot) and by a global test on the Schoenfeld residuals (19–21). We estimated incidence of colonoscopy by study condition by dividing the number of colonoscopies by the accumulated person-time; confidence intervals of these rates were calculated using the quadratic approximation of the Poisson log likelihood (22).
For the secondary outcome of cancelled or missed appointments, we estimated the proportion of each study condition in each of the five timing groups defined above. Due to small sample sizes, confidence intervals were estimated using exact methods. We used chi-square tests to compare the distribution of responses by study condition.
Finally, to examine our model performance and completion of colonoscopy absent of intervention, we examined Kaplan–Meier plots of colonoscopy completion by probability band in the usual care group. The baseline predicted probability of colonoscopy adherence was calculated using SAS 9.4. All other analyses were conducted using Stata/IC 15.1. We defined statistical significance as P value <0.05 and all P values reported are two-sided.
Heterogeneity of treatment effects
In post hoc secondary analyses, we examined hazard ratios and 95% CIs of colonoscopy receipt for participants in the intervention group, divided into subgroups defined by sex, age (50–54, 55–59, 60–64, 65–69, and 70–75), race (non-white and white), and baseline predicted probability of colonoscopy adherence, per our predictive model. We tested for heterogeneity of treatment effects by examining the interaction between treatment group and subgroup of interest.
Power calculations
We did not perform post hoc power calculations for this study. Power calculations for the parent study showed that a sample size of 280 (140 per condition) yielded a 16 percentage point improvement (69% vs. 85%), using power = 0.90 with a Type I error rate of 5%. This study included these 280 patients plus an additional 215 patients accrued during the extended enrollment interval, for a total of 495 randomized patients (415 in the final analytic sample).
Results
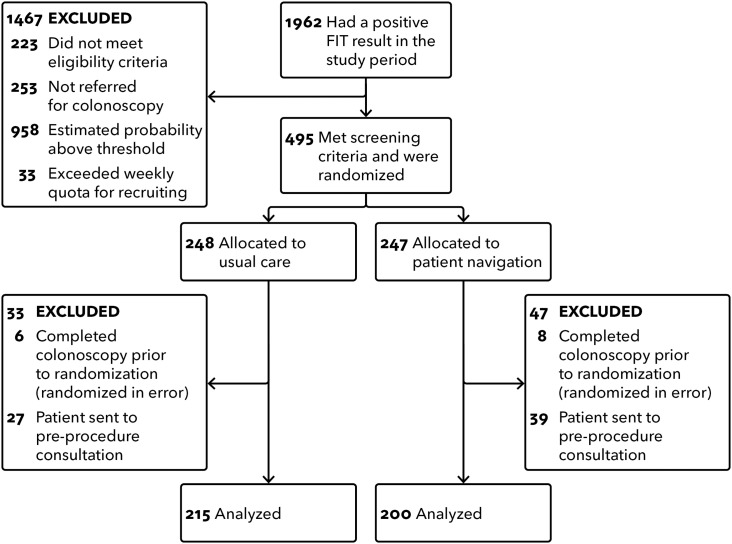
A total of 495 persons met eligibility criteria and were randomized; 415 were included in the analysis (Fig. 1). The 80 excluded patients had completed a recent prior colonoscopy (were randomized in error: six intervention; eight usual care) or were referred to a pre-procedure visit with the gastroenterologist (postrandomization) and were thought to need no further navigation (27 intervention; 39 usual care). Participants' mean age was 62 years, 54% were female, 87% were non-Hispanic white, and 92% spoke English (Table 1). Patients allocated to intervention and usual care were similar across all demographic and clinical variables, except insurance status and prior FIT screening. Among persons who did not complete a colonoscopy, mean follow-up time was 167 days.
Figure 1.
Flow chart of participant selection. The flow chart shows the process used to select study participants for the randomized study of patient navigation and usual care.
Table 1.
Participant characteristics by randomization group.
| Usual care | Patient navigation | Standardized difference | |
|---|---|---|---|
| (n = 215) n (%) | (n = 200) n (%) | ||
| Age group | 0.269 | ||
| 50–54 | 41 (19.1%) | 32 (16.0%) | |
| 55–59 | 27 (12.6%) | 38 (19.0%) | |
| 60–64 | 54 (25.1%) | 63 (31.5%) | |
| 65–69 | 48 (22.3%) | 35 (17.5%) | |
| 70–75 | 45 (20.9%) | 32 (16.0%) | |
| Female | 117 (54.4%) | 107 (53.5%) | 0.018 |
| White | 182 (84.7%) | 177 (88.5%) | 0.113 |
| English speaking | 198 (92.1%) | 182 (91.0%) | 0.039 |
| Geographic region | 0.076 | ||
| Portland, OR | 153 (71.2%) | 144 (72.0%) | |
| Salem, OR | 14 (6.5%) | 16 (8.0%) | |
| Vancouver, WA | 48 (22.3%) | 40 (20.0%) | |
| Insurance type | 0.251 | ||
| Commercial | 103 (47.9%) | 109 (54.5%) | |
| Medicaid | 15 (7.0%) | 23 (11.5%) | |
| Medicare | 97 (45.1%) | 68 (34.0%) | |
| Current tobacco use | 49 (22.8%) | 47 (23.5%) | −0.017 |
| Body mass index | 0.177 | ||
| <25 | 47 (22.6%) | 41 (20.7%) | |
| ≥25–<30 | 58 (27.9%) | 47 (23.7%) | |
| ≥30–<35 | 36 (17.3%) | 43 (21.7%) | |
| ≥35–<40 | 28 (13.5%) | 34 (17.2%) | |
| ≥40 | 39 (18.8%) | 33 (16.7%) | |
| Prior diabetes diagnosis | 81 (37.7%) | 69 (34.5%) | 0.066 |
| Prior colorectal screening | 0.179 | ||
| None | 77 (35.8%) | 67 (33.5%) | |
| Prior colonoscopy | 43 (20.0%) | 29 (14.5%) | |
| Prior FIT only | 95 (44.2%) | 104 (52.0%) | |
| Prior flu vaccination | 123 (57.2%) | 96 (48.0%) | 0.185 |
| Baseline predicted probability of completing a colonoscopy in 6 months | — | ||
| <50% | 27 (12.6%) | 23 (11.5%) | |
| 50%–≤60% | 66 (30.7%) | 56 (28.0%) | |
| 60%–≤65% | 59 (27.4%) | 55 (27.5%) | |
| 65%–≤70% | 63 (29.3%) | 66 (33.0%) | |
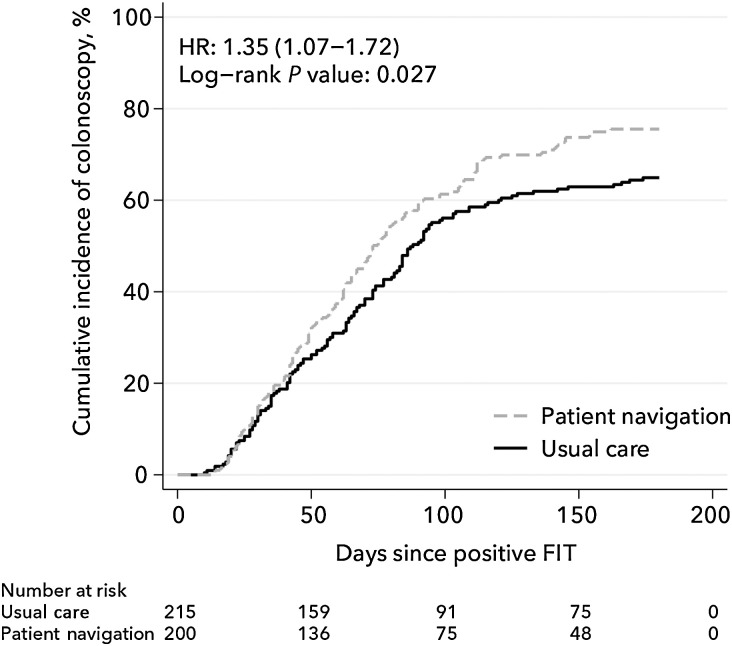
At 180 days, the estimated cumulative incidence of colonoscopy was 75.5% in the navigation group and 64.9% in the usual care group (HR = 1.35; 95% CI, 1.06–1.72; Figure 2). Among those who received colonoscopies, the median time to colonoscopy was 73 days in the navigation group and 88 days in the usual care group.
Figure 2.
Cumulative incidence of colonoscopy estimated using the Kaplan–Meier estimator for the two study conditions: patient navigation and usual care. Cox proportional hazards regression model–derived HR and 95% CI are adjusted for age, race, insurance type, prior colonoscopy screening, body mass index, and prior flu vaccination. The log-rank test P value is unadjusted.
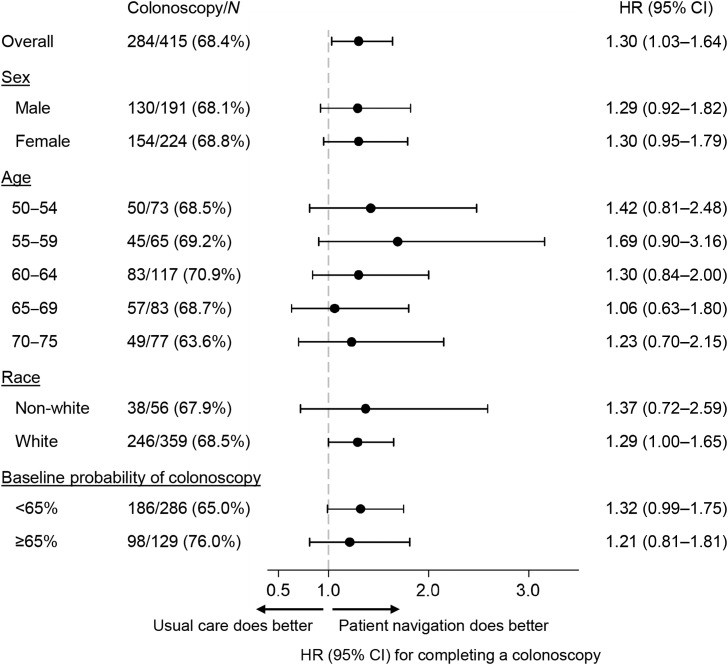
We did not see evidence of heterogeneity in the effect of patient navigation on colonoscopy receipt across subgroups, with similar HRs in subgroups defined by age, sex, race, and baseline estimated probability (Fig. 3). Kaplan–Meier plots and HRs by probability band are shown in Supplementary Fig. S1. There was some indication that navigation performed better in patients assigned to usual care who had lower colonoscopy incidence (Supplementary Table S3). When we examined colonoscopy completion in the usual care group alone, we did not observe a separation in the cumulative incidence of colonoscopy by estimated probability until 40 to 50 days after the positive FIT (Supplementary Fig. S2).
Figure 3.
HRs and 95% CIs are from unadjusted Cox proportional hazards regression models comparing the two study conditions (patient navigation and usual care), fit separately by each subgroup listed.
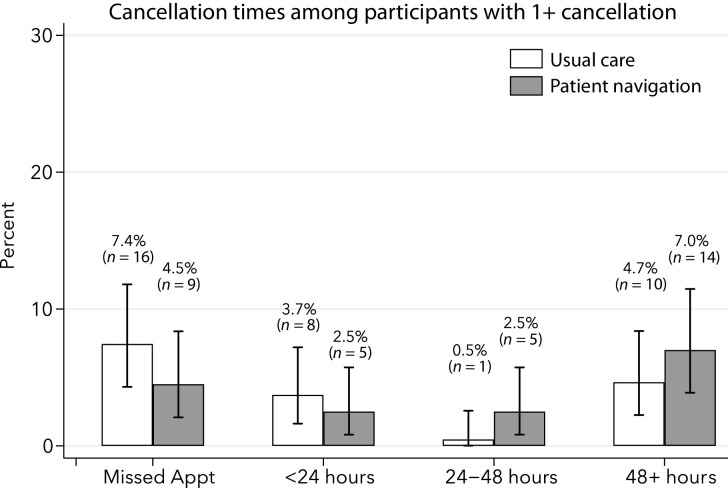
In total, 68 participants (16.4%) had at least one cancellation or missed colonoscopy appointment. We observed differences in the distribution of cancellation times between intervention and usual care groups, with participants in patient navigation having a lower proportion of missed appointments (4.5% vs. 7.4%, Figure 4) and appointments canceled within 24 hours (2.5% vs. 3.7%). Participants in the navigation group were more likely to cancel 24 to 48 or more hours before their appointment (9.5% vs. 5.2%); however, the differences were not significant (chi-square P value = 0.20).
Figure 4.
The proportion of missed or cancelled appointments calculated among participants by study condition: patient navigation and usual care. CIs for the estimated proportions were estimated using exact formulas.
Of the 200 participants allocated to patient navigation and included in the analysis, 100% received at least one phone contact from the navigator and 157 (79%), 147 (74%), 137 (69%), 108 (54%), and 48 (24%) received topic areas 2, 3, 4, 5, and 6, respectively. Call topics 5 and 6 were delivered after the colonoscopy.
Discussion
Among individuals with an abnormal FIT result identified through predictive modeling as having lower than 70% likelihood of obtaining a follow-up colonoscopy, those assigned to patient navigation were significantly more likely to obtain a colonoscopy within 6 months than those assigned to usual care (76% vs. 65%). Moreover, persons receiving navigation had fewer missed and late-cancelled appointments than those receiving usual care. Our findings represent the first efforts to test a probability-based approach to selecting patients for navigation who are due for follow-up colonoscopy.
Risk-prediction models hold promise for identifying patients less likely to complete a colonoscopy. Although we know of no published model that has assessed the probability of colonoscopy adherence following an abnormal FIT result, two models have identified patients unlikely to obtain initial cancer screening tests (23, 24). Blumenthal and colleagues reported on a prediction model developed for screening colonoscopy adherence that achieved an area under the curve estimate of 70.2% (25). To our knowledge, this model has not been evaluated for use in a delivery system. Percac-Lima applied an algorithm to identify primary care patients likely to be nonadherent to cancer screening (24). Study findings showed improvements in cancer screening among patients assigned to navigation, compared with usual care (24). In a separate study, Percac-Lima reported reductions in no-show rates (10.2% vs. 17.5%, P < 0.001) in a patient navigation program that delivered two phone reminders to patients deemed high risk for no-show (4425 patients of 40,075 scheduled; ref. 25). Our study targeted navigation to 495 of 1,962 patients with an abnormal FIT result during the enrollment period, suggesting similar efficiencies.
Although our study was not powered to detect a statistically significant difference across probability groups, our data suggest that patient navigation was more effective among patients who had a lower probability of obtaining colonoscopy on their own. Further research is needed to identify patients who may benefit from additional supports, to tailor the timing and intensity of navigation, and to address barriers for patients who do not complete a colonoscopy even with navigator support. Other research is needed to support the implementation of predictive models (26).
Data from the original NHCRCSP program show that compared with non-navigated patients, navigated patients had lower proportions of appointments that were missed (0% vs. 15.6%, P < 0.001) or canceled within 24 hours of the scheduled time (0.8% vs. 16%, P < 0.001; ref. 10). Our observed usual care proportions of missed and late-cancelled appointments were substantially lower than in the NHCRCSP program, and our navigated patients displayed more modest reductions in missed appointments (7.4% vs. 4.5%; 2.9% difference) and late-canceled appointments (3.7% vs. 2.5%; 1.4% difference). In cost-effectiveness analyses of the NHCRCSP program, each avoided missed or late-canceled appointment led to a $737 cost savings (27), suggesting that even modest improvements in these metrics can lead to substantial savings.
Our evaluation also had several strengths. Our study used an individual randomized design, and we applied a modified intention-to-treat principle [where 80 participants who had a recent prior colonoscopy (n = 14) or referral to a pre-procedure colonoscopy visit (n = 66) were excluded]. Our program was delivered by clinic staff and was much larger than any previous evaluation of follow-up colonoscopy navigation (28–30).
Our study has several limitations. Our study tested patient navigation against usual care in an integrated health system, where baseline follow-up colonoscopy rates were relatively high; greater improvements could likely be achieved in settings with lower follow-up colonoscopy rates. This setting differed substantially from the setting where the program was developed. Our navigator worked daytime hours and was limited to making up to three calls to reach patients. Although the number of call attempts has been rarely reported in similar studies (15, 29), this may have reduced the program's effectiveness. Nevertheless, a high proportion of participants were reached (79% of adults participated in at least two phone calls), and a majority of navigated patients received five of the six topic areas. We did not assess colonoscopy completion among patients whose predicted probability was >70%, although these data may have been useful in further evaluating our model. Moreover, our relatively small sample size limited our power and ability to perform subgroup analysis, and our sample was 87% white, limiting our generalizability to diverse populations. Future research might apply this approach to a non-integrated health system setting serving diverse patients.
Conclusion
Patient navigation is widely endorsed to address low rates of follow-up colonoscopy after an abnormal FIT. Yet, such programs may not be cost-effective and sustainable if not targeted to patients in greatest need. Our findings suggest that directing navigation resources to patients who need them most may improve follow-up colonoscopy rates and reduce missed and late-canceled colonoscopy appointments.
Authors' Disclosures
G.D. Coronado reports personal fees from Exact Sciences and Guardant Health outside the submitted work. E.S. Johnson reports grants from Centers for Disease Control and Prevention during the conduct of the study as well as other salary from Northwest Permanente Medicine outside the submitted work. P.A. Hannon reports grants from Centers for Disease Control and Prevention during the conduct of the study. No disclosures were reported by the other authors.
Acknowledgments
P.A. Hannon, A. Cole, and G.D. Coronado were awarded a cooperative agreement from the Centers for Disease Control and Prevention (Cooperative agreement #U48DP005013) to carry out this research. G.D. Coronado also was awarded a grant from the NCI of the NIH under award number R01CA218923. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Note: Supplementary data for this article are available at Cancer Epidemiology, Biomarkers & Prevention Online (http://cebp.aacrjournals.org/).
Authors' Contributions
G.D. Coronado: Conceptualization, supervision, funding acquisition, investigation, writing–original draft, writing–review and editing. A.M. Rawlings: Formal analysis, writing–original draft, writing–review and editing. A.F. Petrik: Project administration, writing–review and editing. M. Slaughter: Formal analysis, writing–review and editing. E.S. Johnson: Investigation, methodology, writing–review and editing. P.A. Hannon: Conceptualization, funding acquisition, investigation, writing–review and editing. A. Cole: Conceptualization, funding acquisition, investigation, writing–review and editing. T. Vu: Project administration, writing–review and editing. R.R. Mummadi: Supervision, writing–review and editing.
References
- 1. American Cancer Society. Colorectal Cancer Facts & Figures 2017–2019. Atlanta, GA: American Cancer Society; 2017. [Google Scholar]
- 2. Zauber AG. The impact of screening on colorectal cancer mortality and incidence: has it really made a difference? Dig Dis Sci 2015;60:681–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Niikura R, Hirata Y, Suzuki N, Yamada A, Hayakawa Y, Suzuki H, et al. Colonoscopy reduces colorectal cancer mortality: A multicenter, long-term, colonoscopy-based cohort study. PLoS One 2017;12:e0185294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pan J, Xin L, Ma YF, Hu LH, Li ZS. Colonoscopy reduces colorectal cancer incidence and mortality in patients with non-malignant findings: a meta-analysis. Am J Gastroenterol 2016;111:355–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gingold-Belfer R, Leibovitzh H, Boltin D, Issa N, Tsadok Perets T, Dickman R, et al. The compliance rate for the second diagnostic evaluation after a positive fecal occult blood test: A systematic review and meta-analysis. United European Gastroenterol J 2019;7:424–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Corley DA, Jensen CD, Quinn VP, Doubeni CA, Zauber AG, Lee JK, et al. Association between time to colonoscopy after a positive fecal test result and risk of colorectal cancer and cancer stage at diagnosis. JAMA 2017;317:1631–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee YC, Fann JC, Chiang TH, Chuang SL, Chen SL, Chiu HM, et al. Time to colonoscopy and risk of colorectal cancer in patients with positive results from fecal immunochemical tests. Clin Gastroenterol Hepatol 2019;17:1332–40.e3. [DOI] [PubMed] [Google Scholar]
- 8. Meester RG, Zauber AG, Doubeni CA, Jensen CD, Quinn VP, Helfand M, et al. Consequences of increasing time to colonoscopy examination after positive result from fecal colorectal cancer screening test. Clin Gastroenterol Hepatol 2016;14:1445–51.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. DeGroff A, Schroy PC 3rd, Morrissey KG, Slotman B, Rohan EA, Bethel J, et al. Patient navigation for colonoscopy completion: results of an RCT. Am J Prev Med 2017;53:363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rice K, Gressard L, DeGroff A, Gersten J, Robie J, Leadbetter S, et al. Increasing colonoscopy screening in disparate populations: results from an evaluation of patient navigation in the new hampshire colorectal cancer screening program. Cancer 2017;123:3356–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Horne HN, Phelan-Emrick DF, Pollack CE, Markakis D, Wenzel J, Ahmed S, et al. Effect of patient navigation on colorectal cancer screening in a community-based randomized controlled trial of urban African American adults. Cancer Causes Control 2015;26:239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ritvo PG, Myers RE, Paszat LF, Tinmouth JM, McColeman J, Mitchell B, et al. Personal navigation increases colorectal cancer screening uptake. Cancer Epidemiology, Biomarkers & Prevention 2015;24:506–11. [DOI] [PubMed] [Google Scholar]
- 13. Myers RE, Sifri R, Daskalakis C, DiCarlo M, Geethakumari PR, Cocroft J, et al. Increasing colon cancer screening in primary care among African Americans. J Natl Cancer Inst 2014;106:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Honeycutt S, Green R, Ballard D, Hermstad A, Brueder A, Haardorfer R, et al. Evaluation of a patient navigation program to promote colorectal cancer screening in rural Georgia, USA. Cancer 2013;119:3059–66. [DOI] [PubMed] [Google Scholar]
- 15. DeGroff A, Gressard L, Glover-Kudon R, Rice K, Tharpe FS, Escoffery C, et al. Assessing the implementation of a patient navigation intervention for colonoscopy screening. BMC Health Serv Res 2019;19:803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Petrik AF, Keast E, Johnson ES, Smith DH, Coronado GD. Development of a multivariable prediction model to identify patients unlikely to complete a colonoscopy following an abnormal FIT test in community clinics. BMC Health Serv Res 2020Nov 10;20:1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nguyen TL, Collins GS, Spence J, Daurès JP, Devereaux PJ, Landais P, et al. Double-adjustment in propensity score matching analysis: choosing a threshold for considering residual imbalance. BMC Med Res Methodol 2017;17:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hertz-Picciotto I, Rockhill B. Validity and efficiency of approximation methods for tied survival times in Cox regression. Biometrics 1997;53:1151–6. [PubMed] [Google Scholar]
- 19. Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994;81:515–26. [Google Scholar]
- 20. Hess K. Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Stat Med 1995;14:1707–23. [DOI] [PubMed] [Google Scholar]
- 21. Rogers W. Ex post tests and diagnostics for a proportional hazards model. 19:23–27. Reprinted in Stata Technical Bulletin Reprints. College Station, TX: Stata Press; 1994. [Google Scholar]
- 22. Clayton D, Hills M. Statistical models in epidemiology. Oxford: Oxford University Press; 1993. [Google Scholar]
- 23. Percac-Lima S, Ashburner JM, Zai AH, Chang Y, Oo SA, Guimaraes E, et al. Patient navigation for comprehensive cancer screening in high-risk patients using a population-based health information technology system: a randomized clinical trial. JAMA Intern. Med. 2016;176:930–7. [DOI] [PubMed] [Google Scholar]
- 24. Blumenthal DM, Singal G, Mangla SS, Macklin EA, Chung DC. Predicting non-adherence with outpatient colonoscopy using a novel electronic tool that measures prior non-adherence. J Gen Intern Med 2015;30:724–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Percac-Lima S, Cronin PR, Ryan DP, Chabner BA, Daly EA, Kimball AB. Patient navigation based on predictive modeling decreases no-show rates in cancer care. Cancer 2015;121:1662–70. [DOI] [PubMed] [Google Scholar]
- 26. Chanfreau-Coffinier C, Peredo J, Russell MM, Yano EM, Hamilton AB, Lerner B, et al. A logic model for precision medicine implementation informed by stakeholder views and implementation science. Genet Med 2019;21:1139–54. [DOI] [PubMed] [Google Scholar]
- 27. Rice K, Sharma K, Li C, Butterly L, Gersten J, DeGroff A. Cost-effectiveness of a patient navigation intervention to increase colonoscopy screening among low-income adults in New Hampshire. Cancer 2019;125:601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Selby K, Baumgartner C, Levin TR, Doubeni CA, Zauber AG, Schottinger J, et al. Interventions to improve follow-up of positive results on fecal blood tests: a systematic review. Ann Intern Med 2017;167:565–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Green BB, Anderson ML, Wang C-Y, Vernon SW, Chubak J, Meenan RT, et al. Results of nurse navigator follow-up after positive colorectal cancer screening test: a randomized trial. J Am Board Fam Med 2014;27:789–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Raich PC, Whitley EM, Thorland W, Valverde P, Fairclough D. Patient navigation improves cancer diagnostic resolution: an individually randomized clinical trial in an underserved population. Cancer Epidemiol Biomarkers Prev 2012;21:1629–38. [DOI] [PMC free article] [PubMed] [Google Scholar]