Abstract
Background:
Vitamin D plays an essential role in the regulation of bone metabolism. The current meta-analysis aimed to assess the effectiveness of vitamin D fortification on special bone biomarkers.
Methods:
Five main databases (PubMed/Medline, ISI Web of Knowledge, Science Direct, Scopus, Cochrane Library as well as Science Direct, and Scopus) were considered for this systematic review, until Jan 2020. All randomized controlled trials were included to evaluate the probable relationship between consumption of vitamin D fortification products and bone biomarkers profile in this review.
Results:
Among serum bone biomarkers (osteocalcin and telopeptides of type-1 collagen) investigated, only the level of telopeptides of type-1 collagen significantly decreased after fortification of vitamin D in the intervention group. A significant increase in vitamin D was seen in those older than 18 yr old, while the increase in younger children was not statistically significant between intervention and control groups.
Conclusion:
Vitamin D fortification was not associated with a significant improvement in bone mass density (BMD), while it resulted in decreased PTH levels. Vitamin D fortified foods have some benefits on bone health due to increase in the level of vitamin D and IGF-1; and decreasing PTH and CTx levels.
Keywords: Vitamin D, Bone density, Osteocalcin, Parathyroid hormone, Insulin-like growth factor I
Introduction
Vitamin D deficiency has become a major health challenge among many countries in the 21st century and is associated with an increased risk of several serious chronic diseases. The health benefits of this fat-soluble vitamin have been reported to include the prevention of chronic diseases, including those of the musculoskeletal system and bone metabolism (1–3).
The main source of vitamin D synthesis in man is dermal synthesis following exposure to ultraviolet B waves of sunlight (4). Various environmental and cultural factors including season, latitude, use of sunscreens, aging, skin pigmentation and even job can affect the number of absorbed sunlight rays by the skin and cutaneous synthesis of this vitamin (5). Although dietary sources such as liver, oily fish and egg yolk are rich in vitamin D, they are not routinely consumed in many countries and cultures. Only a small portion of the daily-required vitamin D can be obtained by a non-fortified diet (6).
The main biological function of vitamin D is regulating calcium absorption and also maintaining bone health. This role is mediated by increasing the intestinal absorption of calcium and phosphorus, thus promotion of bone mineralization. Osteoporosis, characterized by loss of bone density and bone tissue degradation, is one of the main complications caused by vitamin D deficiency. Osteoporosis increases the risk of spontaneous fractures, especially in the elderly population (4). Two specific markers have been recommended as references by the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) and the International Osteoporosis Foundation (IOF). Serum C-terminal telopeptide of type-I collagen (CTx) and serum procollagen type-I N propeptide (PINP) are bone markers for bone resorption and bone turnover, respectively (7).
Because of the high prevalence of vitamin D insufficiency and deficiency (8), and the wide spectrum of morbidities that can occur due to deficiency of this vitamin, food fortification has been considered in many countries in recent years, and some clinical trials have been carried out aiming at investigating the clinical effects of vitamin D fortification (9). The current review focuses on vitamin D fortified products and the subsequent health outcomes on the musculoskeletal system, using meta-analysis of randomized controlled studies. The most recent systematic review covering a similar topic was published by Tangestani et al. (4). There are two main differences between their paper and the current review. One is the timescale covered by our review, and the other relates to the types of studies, with the requirement to have a control group and to include studies in which vitamin D was used alone (or plus calcium), without other nutrients.
The aim of the current meta-analysis was to assess the effectiveness of vitamin D fortification on serum bone biomarkers.
Methods
Strategy of literature search
This systematic review was conducted based on the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (10). The primary outcome of interest was change in bone measurements and the primary intervention was consuming vitamin D fortified food. To find the related articles, five main scientific databases (PubMed/Medline, ISI web of knowledge, Cochrane Library, Science Direct, and Scopus) were searched and then the search was completed by finding more articles in Google scholar. The search was done without any time limits until Jan 2020 using the combination of keywords as follows: “vit D” or “vitamin D” combined with “fortified” or “fortification” or “fortified food” or “fortifi*”. Search strategy was adapted for each database. To avoid missing any article, we searched broadly and did not use bone-related terms in the selected keywords.
Selection criteria and Data extraction
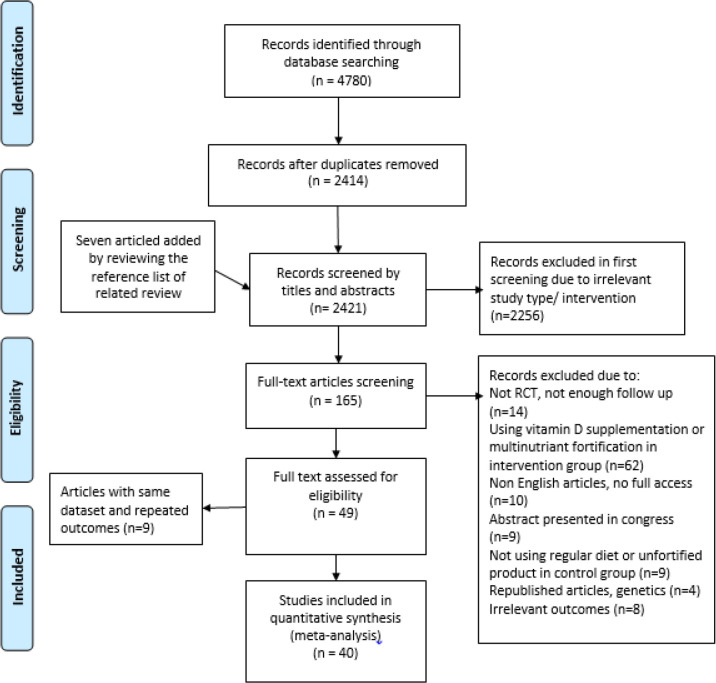
All English studies that evaluated the impact of vitamin D fortified food on bone measurements were enrolled. The inclusion criteria were: 1) randomized controlled trials, 2) subjects in intervention group consumed vitamin D (or calcium-vitamin D) fortified food and in control group received unfortified food (or a regular diet) for at least one month, 3) containing quantitative data about the mean change of bone measurements in each group (or providing the mean and standard deviation of bone measures at baseline and in different time points of the study). The exclusion criteria were as followed: i) book chapters, editorials, review articles and abstracts presented in congresses (without any full texts), ii) studies with multi-nutrient fortified product in the intervention group, iii) studies with the use of fortified product in the control group. Duplicate studies were removed by title and abstract screening (Fig. 1).
Fig. 1:
Flow diagram of the study selection
Two reviewers (M.E and R.S) extracted the important information of each article. The extracted data is shown in supplementary table.
Quality assessment and Synthesis of data
The Jadad scale was used for quality assessment of the articles (11). In this scale, there are questions about randomization, drop-out and blinding. If an article scored 3 or more, it is considered as good quality (12).
In order to calculate the effect size, the mean difference and standard deviation (13) of difference were extracted.
If the data were only presented as a graph, we used the GetData Graph Digitizer 2.24 to extract the needed data (14–16). Moreover, to determine the influence of variables such as duration of intervention and age groups, subgroup analysis was conducted.
Publication bias
To assess the publication bias, the funnel plot asymmetry and Egger‘s regression test were used. Trim and fill analysis was conducted in the case of probable evidence of publication bias. We used Comprehensive Meta-Analysis (CMA) V2 software to conduct the meta-analysis (17).
Results
Summary of searches and study selection process
We identified 4780 citations of which 2414 articles remained after removing duplicates. Likewise, by reviewing the reference list of related publications, seven articles were added to the list. Overall, 2421 citations were screened by title and abstracts, of which 165 records remained after excluding irrelevant study type/intervention. After full-text assessment, 116 records were excluded. We also removed nine records with the same dataset or repeated outcomes. Finally, 40 studies were included in the meta-analysis (Fig. 1).
According to the JADAD score, 13 studies obtained a complete Jadad score (complete 5 scores). The quality was inadequate (<3) in 8 studies, while others had a score of three or more (supplementary table).
Characteristics of the included studies
The characteristic summary of included studies is shown in supplementary table. These items were published between 1995 and 2019, and were from fourteen different countries. Participants in seventeen studies were only females (5, 18–33) and in two studies were only males (34, 35). Eight studies addressed children and adolescents (36–43). The mean age of subjects varied from 2 yr (42) to 84 years (18). The duration of intervention varied from one month (44) to thirty months (23–25). Interventions varied by calcium addition, dosages, and food staples including seventeen reports that used vitamin D alone (5, 13, 18, 22, 29–33, 38, 39, 41, 42, 44–49) and the rest of the studies used calcium plus vitamin D to fortify food staples. The dosage of vitamin D fortification varied from 40 IU/d to 28000 IU/d. Dairy products were the dominant fortified foods used in most studies, while others used fortified orange juice, bread, biscuit and snack bars.
Pooled estimate of the effect of vitamin d fortified food intake
Bone biomarkers
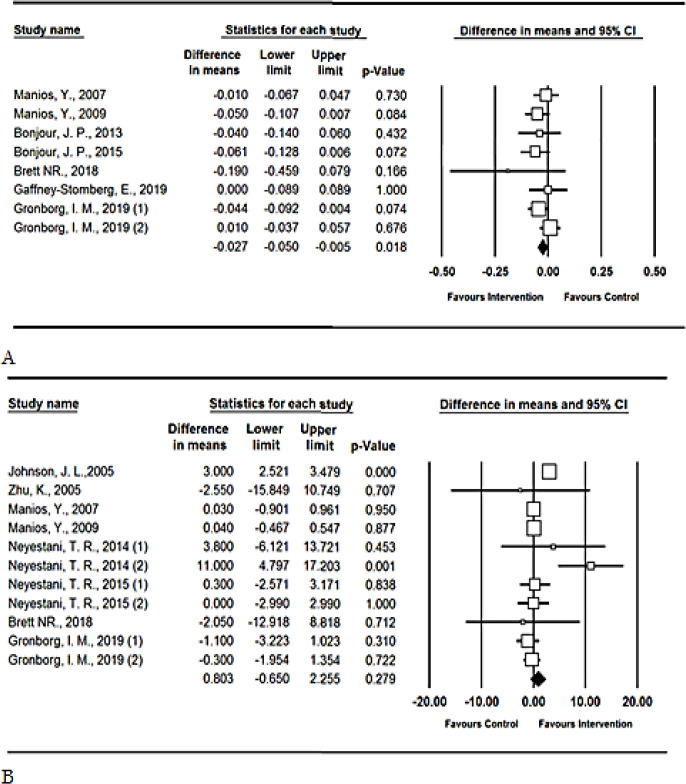
In the pooled analysis of eight reports, serum telopeptides of type-1 collagen (C-terminal: CTx) were significantly decreased in the intervention groups (difference in means=−0.027, P-value=0.018, CI 95%: −0.05 to −0.005). Only one study included 2–8-yr-old children (42). By conducting sensitivity analysis, by omitting this study the corresponding point estimate was not significantly altered, suggesting stability of the results. In contrast, serum osteocalcin did not alter significantly (difference in means=0.803, P-value=0.279, CI 95%: −0.65 to 2.255) (Fig. 2). Subgroup analysis revealed that the results were similar in adolescents and older population. The duration of intervention was more than six months in only two studies (20, 37), which did not indicate different results in comparison with studies of less than six months of interventions (Table 1).
Fig. 2:
Forest plot for the assessing the relationship between using vitamin D fortified food and a) telopeptides of type-1 collagen (C-terminal: CTx); b) serum osteocalcin.
Table 1:
Pooled estimation of vitamin D fortified food intake on Bone Mass Densitometry, hormones and bone biomarkers
| Variable | Difference in means | Lower limit | Upper limit | P-value | |||
|---|---|---|---|---|---|---|---|
| BMD | |||||||
| Total | 0.008 | −0.014 | 0.03 | 0.467 | |||
| Femoral neck | −0.008 | −0.034 | −0.017 | 0.516 | |||
| Lumbar | 0.006 | −0.008 | 0.02 | 0.384 | |||
| Spine | 0.081 | 0.047 | 0.116 | <0.001 | |||
| Pelvis | 0.005 | −0.001 | 0.012 | 0.12 | |||
| Arms | 0.024 | −0.023 | 0.072 | 0.315 | |||
| Legs | −0.003 | −0.015 | 0.009 | 0.662 | |||
| Hormones | |||||||
| Vitamin D | Subgroup * | 16.518 | 11.618 | 21.418 | <0.001 | ||
| Duration of intervention | ≤ 6 months | 18.074 | 12.23 | 23.918 | <0.001 | ||
| > 6 months | 10.938 | 4.009 | 17.867 | 0.002 | |||
| Age groups | < 18 year-olds | 8.686 | −0.497 | 17.869 | 0.064 | ||
| ≥ 18 year-olds | 19.453 | 12.859 | 26.048 | <0.001 | |||
| PTH | Subgroup * | −5.148 | −7.341 | −2.955 | <0.001 | ||
| Duration of intervention | ≤ 6 months | −5.158 | −7.777 | −2.539 | <0.001 | ||
| > 6 months | −4.854 | −8.749 | −0.96 | 0.015 | |||
| Age groups | < 18 year-olds | −8.262 | −13.497 | −3.02 | 0.002 | ||
| ≥ 18 year-olds | −4.181 | −6.503 | −1.859 | <0.001 | |||
| IGF1 | 42.789 | 14.607 | 70.971 | 0.003 | |||
| Bone biomarkers | |||||||
| Ctx | −0.027 | −0.05 | −0.005 | 0.018 | |||
| OC | Subgroup * | 0.803 | −0.65 | 2.255 | 0.279 | ||
| Age groups | < 18 year-olds | 3.886 | −3.165 | 10.936 | 0.28 | ||
| ≥ 18 year-olds | 0.381 | −1.099 | 1.861 | 0.614 |
Subgroup analysis conducted if there were at least three articles in each subgroup
Hormones
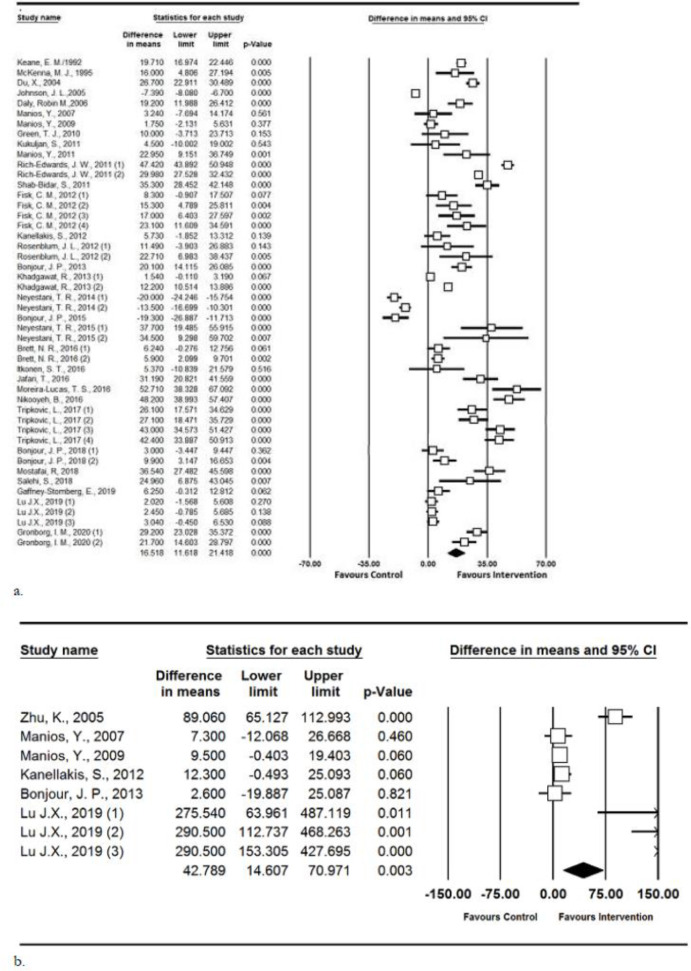
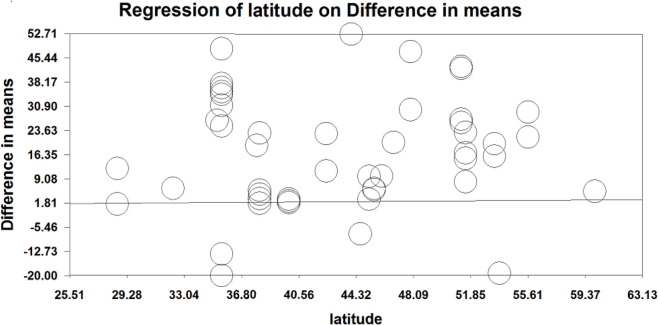
Pooled estimation for serum vitamin D levels showed a significant increase in serum vitamin D in the intervention vs. control groups (difference in means=16.518 nmol/L, P-value<0.001, CI 95%: 11.618 to 21.418). Subgroup analysis according to duration of intervention indicated that there are no significant differences in the serum vitamin D levels if the duration of intervention was less or more than 6 months. Furthermore, the significant increase in vitamin D was seen in those older than 18 yr old (difference in means=19.453, P-value<0.001, CI 95%: 12.859 to 26.048), while the increase in those younger than 18 was not statistically significant between intervention and control groups (difference in means=8.686, P-value=0.064, CI 95%: −0.497 to 17.869). Meta-regression did not show statistically significant associations between mean difference of vitamin D level and latitude of where the study was undertaken (P=0.37) (Figs. 3,4).
Fig. 3:
Forest plot for the assessing the relationship between using vitamin D fortified food and a) Serum 25(OH)D; b) IGF1
Fig. 4:
Meta-regression between the effect of latitude on serum vitamin D
The analysis of serum PTH in 25 reports showed a significant effect of the intervention (difference in means=−5.148, P-value<0.001, CI 95%: −7.341 to −2.955); serum PTH level decreased significantly after the intervention. Subgroup analysis, according to age categorization, indicated that the point estimates were similar in both older and younger than 18-year-old participants (−4.181 vs. −8.262 respectively). More data on subgroup analysis is shown in Table 1.
Pooled results showed a significant difference for serum IGF-1 in favor of the intervention groups (difference in means=42.789, P-value=0.003, CI 95%: 14.607 to 70.971) (Fig. 3). The results of the studies conducted by Zhu et al. and Lu J.X. are different from the other studies. These two studies were in children (< 15-year-old) (37, 43), while the other studies were in participants older than 50-year-old. Sensitivity analysis indicated that by omitting these studies the pooled estimate was still in favor of intervention (Difference in means=9.38, P-value=0.008, CI 95%: 2.48 to 16.29).
Bone Mass Density (BMD)
Pooled estimation of fortified food intake (vitamin D intervention groups versus placebo groups) on all evaluated BMD such as total, femoral neck, and lumbar BMD are shown in Table 1. The analysis indicated that vitamin D fortification was not associated with a significant increase in BMD in each specific anatomical site except spine significant improved in the intervention group (difference in means=0.081, P-value<0.001, CI 95%: 0.047 to 0.116).
Publication bias
Publication bias was assessed by Egger‘s test. We did not find any evidence of publication bias for most of the outcomes of interest such as OC (P=0.9), CTx (P=0.22) and BMD (P=0.1). However, the P-value of the Egger test was <0.05 for serum PTH and 25(OH) D. We used trim and fill method to adjust the asymmetry of the funnel plot. For serum PTH, the imputed estimate (difference in means: −3.88; 95% CI −6.05 to −1.7) was the same as previous cumulative effect (difference in means: −5.14) which indicates that the results were robust.
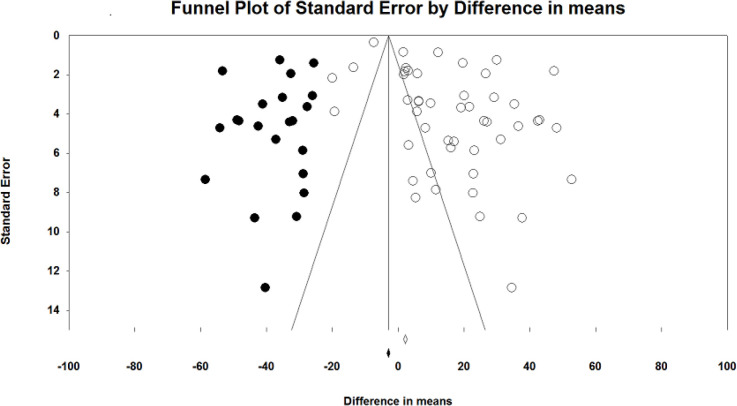
For serum 25 (OH) vitamin D, adding imputed studies to the funnel plot, made it symmetrical. This indicates that if some negative unpublished studies exist, the final point estimate did not change substantially. A Funnel plot of the 25 (OH) vitamin D is shown in Fig. 5.
Fig. 5:
Trim and Fill analysis of the funnel plot of 25(OH) vitamin D to adjust for asymmetries. Solid points indicate imputed studies
Discussion
This meta-analysis indicated an overall beneficial effect of vitamin D fortified foods in increasing serum IGF1 and decreasing serum PTH and CTx, while its effect on osteocalcin and bone mass density was not remarkable.
It is of interest to find the association between vitamin D fortification and health outcomes in the musculoskeletal system and bone biomarkers. There was increasing evidence that vitamin D plus calcium supplementation reduces the risk of bone loss and fracture (50). While, the optimal vitamin D status and whether vitamin D fortification should be considered alone or in combination with calcium, remains controversial (51).
In this current meta-analysis, no statistically significant effect on BMD was found after vitamin D fortification, apart from in the spine. The results may be influenced by the small sample size because the spine BMD was only reported in two studies (19, 23). Vitamin D supplementation was associated with an increase in BMD in the femoral neck (52). These results may be due to the aspects of the study population or the vitamin D dosage used across trials. A higher dose of vitamin D may be required to achieve the potential benefits, which is inconsistent with US Preventive Services Task Force guidelines (53).
The effects of vitamin D fortification on serum osteocalcin and telopeptides of type-1 collagen were also investigated in this review. The level of telopeptides of type-1 collagen was significantly decreased in the intervention group in comparison to the control group, but the level of osteocalcin was decreased more (but not significantly) in the control group in comparison to the intervention group.
Reduced levels of serum osteocalcin and increased level of serum telopeptides of type-1 collagen are associated with vitamin D deficiency (54). Osteocalcin is a protein synthesized by osteoblasts and is a specific and sensitive serum marker of bone turnover and formation (55). Alteration in serum osteocalcin and serum markers of collagen turnover in response to supplementation occur within two weeks (56). Vitamin D supplementation might increase the expression of osteocalcin (57), however, vitamin D supplementation did not alter serum osteocalcin which is inconsistent with our results on vitamin D fortification (57–59). Moreover, the pretreatment level of vitamin D should be evaluated before assessing the effect of vitamin D fortification on bone turnover markers.
In addition, current meta-analysis revealed that there is a significant reduction in serum PTH level after consumption of vitamin D fortified products. PTH is an important hormone in bone remodeling associated with vitamin D levels. Although studies revealed that, there is a significant relationship between PTH levels and BMD but this association is still controversial (60–62).
Insulin-like growth factors (IGFs) are important factors in bone remodeling and bone cell proliferation. Studies have demonstrated a positive association between serum IGF and vitamin D level and BMD which is inconsistent with the results of this meta-analysis, in which we found a significant increase in this marker after using vitamin D fortified foods (63, 64).
As expected, the serum level of vitamin D increased significantly in those who consumed vitamin D fortified foods. This increase was smaller in adolescents and children (younger than 18-year-old). This could be due to the higher need for vitamin D related to the rapid growth and higher metabolic rate in this age group. Therefore, to reach a higher serum level of vitamin D in children and teens, perhaps higher doses of fortification or more servings should be consumed.
The strengths of this meta-analysis include the broad inclusion of randomized controlled trials and there were no time limitations for including studies. Moreover, we did not include studies with interventions other than fortifying vitamin D, or vitamin D plus calcium, to explore the specific net effect of this vitamin in fortified products.
There are some limitations of this study. First, we only included English language manuscripts. Second, different experimental groups were investigated with respect to the age and gender-related inclusions across trials. About the population characteristics, it should be noted that some articles assessed the vitamin D fortification on diabetics or post-menopausal women, while others evaluate its effect on healthy adults or children. Moreover, due to the heterogeneity in latitudes and season of study interventions, the net effects of vitamin D fortification in this report may have difference.
Finally, calcium, magnesium and phosphorous are all necessary nutrients for bone and should be considered in study design. Moreover, publication bias is an inherent limitation of any meta-analysis. Therefore, the result of the current meta-analysis may be affected by this bias as well.
Conclusion
More clinical data about the relationship between consumption of vitamin D fortified products and BMD as well as bone markers are needed. Based on the results of the current meta-analysis, consumption of vitamin D fortified food significantly increases vitamin D levels, regardless of the duration of intervention and latitude. It could also significantly increase IGF-1 and reduce PTH and CTx, while its effect on osteocalcin is not remarkable.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
This work was supported by Research Council of Mashhad University of Medical Sciences, Mash-had, Iran. We also thank Clinical Research Development Unit of Ghaem Hospital for participation in data analysis.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Charoenngam N, Shirvani A, Holick MF. (2019). Vitamin D for skeletal and non-skeletal health: What we should know. J Clin Orthop Trauma, 10:1082–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emadzadeh M, Rashidmayvan M, Sahebi R, et al. (2020). The effect of vitamin D fortified products on anthropometric indices: A systematic review and meta-analysis. Complement Ther Clin Pract, 41:101242. [DOI] [PubMed] [Google Scholar]
- 3.Emadzadeh M, Sahebi R, Khedmatgozar H, et al. (2020). A systematic review and meta-analysis of the effect of Vitamin D-fortified food on glycemic indices. Biofactors, 46 (4):502–513. [DOI] [PubMed] [Google Scholar]
- 4.Tangestani H, Djafarian K, Emamat H, et al. (2020). Efficacy of vitamin D fortified foods on bone mineral density and serum bone biomarkers: A systematic review and meta-analysis of interventional studies. Crit Rev Food Sci Nutr, 60 (7):1094–1103. [DOI] [PubMed] [Google Scholar]
- 5.Jafari T, Faghihimani E, Feizi A, Iraj B, et al. (2016). Effects of vitamin D-fortified low fat yogurt on glycemic status, anthropometric indexes, inflammation, and bone turnover in diabetic postmenopausal women: A randomised controlled clinical trial. Clin Nutr, 35:67–76. [DOI] [PubMed] [Google Scholar]
- 6.Moulas AN, Vaiou M. (2018). Vitamin D fortification of foods and prospective health outcomes. J Biotechnol, 285:91–101. [DOI] [PubMed] [Google Scholar]
- 7.Vasikaran S, Eastell R, Bruyere O, et al. (2011). Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int, 22:391–420. [DOI] [PubMed] [Google Scholar]
- 8.Heshmat R, Mohammad K, Majdzadeh S, et al. (2008). Vitamin D deficiency in Iran: A multi-center study among different urban areas. Iran J Public Health, 37 (Supple 2):72–78. [Google Scholar]
- 9.Pilz S, März W, Cashman KD, et al. (2018). Rationale and plan for vitamin D food fortification: a review and guidance paper. Front Endocrinol (Lausanne), 9:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman DG. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med, 151:264–269. [DOI] [PubMed] [Google Scholar]
- 11.Jadad AR, Moore RA, Carroll D, et al. (1996). Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials, 17:1–12. [DOI] [PubMed] [Google Scholar]
- 12.Lundh A, Gøtzsche PC. (2008). Recommendations by Cochrane Review Groups for assessment of the risk of bias in studies. BMC Med Res Methodol, 8:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mostafai R, Mohammadi R, Nachvak SM, et al. (2018). Fortified yogurt with vitamin D as a cost-effective food to prevent diabetes: A randomized double-blind clinical trial. J Funct Foods, 42:137–145. [Google Scholar]
- 14.Mazidi M, Gao H-K, Rezaie P, Ferns GA. (2016). The effect of ginger supplementation on serum C-reactive protein, lipid profile and glycaemia: a systematic review and meta-analysis. Food Nutr Res, 60:32613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazidi M, Rezaie P, Karimi E, Kengne AP. (2017). The effects of bile acid sequestrants on lipid profile and blood glucose concentrations: a systematic review and meta-analysis of randomized controlled trials. Int J Cardiol, 227:850–857. [DOI] [PubMed] [Google Scholar]
- 16.Zein H, Tran VL-H, Abdelmotaleb Ghazy A, et al. (2015). How to extract data from graphs using plot digitizer or Getdata graph digitizer. Technical Report. [Google Scholar]
- 17.Borenstein M, Hedges L, Higgins J, Rothstein H. (2005). “Comprehensive meta analysis Version 2”Biostat. https://www.meta-analysis.com/downloads/Meta-Analysis-Manual.pdf
- 18.Keane EM, Rochfort A, Cox J, et al. (1992). Vitamin-D-fortified liquid milk–a highly effective method of vitamin D administration for house-bound and institutionalised elderly. Gerontology, 38:280–284. [DOI] [PubMed] [Google Scholar]
- 19.Moschonis G, Manios Y. (2006). Skeletal site-dependent response of bone mineral density and quantitative ultrasound parameters following a 12-month dietary intervention using dairy products fortified with calcium and vitamin D: the Postmenopausal Health Study. Br J Nutr, 96:1140–1148. [DOI] [PubMed] [Google Scholar]
- 20.Manios Y, Moschonis G, Trovas G, Lyritis GP. (2007). Changes in biochemical indexes of bone metabolism and bone mineral density after a 12-mo dietary intervention program: the Postmenopausal Health Study. Am J Clin Nutr, 86:781–789. [DOI] [PubMed] [Google Scholar]
- 21.Manios Y, Moschonis G, Panagiotakos DB, et al. (2009). Changes in biochemical indices of bone metabolism in post-menopausal women following a dietary intervention with fortified dairy products. J Hum Nutr Diet, 22:156–65. [DOI] [PubMed] [Google Scholar]
- 22.Green TJ, Skeaff CM, Rockell JE. (2010). Milk fortified with the current adequate intake for vitamin D (5μg) increases serum 25-hydroxyvitamin D compared to control milk but is not sufficient to prevent a seasonal decline in young women. Asia Pac J Clin Nutr, 19:195–199. [PubMed] [Google Scholar]
- 23.Moschonis G, Katsaroli I, Lyritis GP, Manios Y. (2010). The effects of a 30-month dietary intervention on bone mineral density: the Postmenopausal Health Study. Br J Nutr, 104:100–107. [DOI] [PubMed] [Google Scholar]
- 24.Manios Y, Moschonis G, Lyritis G. (2011). Seasonal variations of vitamin D status in Greek postmenopausal women receiving enriched dairy products for 30 months: the Postmenopausal Health Study. Eur J Clin Nutr, 65:412–4. [DOI] [PubMed] [Google Scholar]
- 25.Tenta R, Moschonis G, Koutsilieris M, Manios Y. (2011). Calcium and vitamin D supplementation through fortified dairy products counterbalances seasonal variations of bone metabolism indices: the Postmenopausal Health Study. Eur J Nutr, 50:341–349. [DOI] [PubMed] [Google Scholar]
- 26.Kanellakis S, Moschonis G, Tenta R, et al. (2012). Changes in parameters of bone metabolism in postmenopausal women following a 12-month intervention period using dairy products enriched with calcium, vitamin D, and phylloquinone (vitamin K 1) or menaquinone-7 (vitamin K 2): the Postmenopausal Health Study II. Calcif Tissue Int, 90:251–262. [DOI] [PubMed] [Google Scholar]
- 27.Bonjour J-P, Benoit V, Payen F, Kraenzlin M. (2013). Consumption of yogurts fortified in vitamin D and calcium reduces serum parathyroid hormone and markers of bone resorption: a double-blind randomized controlled trial in institutionalized elderly women. J Clin Endocrinol Metab, 98:2915–2921. [DOI] [PubMed] [Google Scholar]
- 28.Bonjour J-P, Benoit V, Atkin S, Walrand S. (2015). Fortification of yogurts with vitamin D and calcium enhances the inhibition of serum parathyroid hormone and bone resorption markers: A double blind randomized controlled trial in women over 60 living in a community dwelling home. J Nutr Health Aging, 19:563–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Itkonen ST, Skaffari E, Saaristo P, et al. (2016). Effects of vitamin D 2-fortified bread v. supplementation with vitamin D 2 or D 3 on serum 25-hydroxyvitamin D metabolites: an 8-week randomised-controlled trial in young adult Finnish women. Br J Nutr, 115:1232–1239. [DOI] [PubMed] [Google Scholar]
- 30.Tripkovic L, Wilson LR, Hart K, et al. (2017). Daily supplementation with 15 μg vitamin D2 compared with vitamin D3 to increase wintertime 25-hydroxyvitamin D status in healthy South Asian and white European women: a 12-wk randomized, placebo-controlled food-fortification trial. Am J Clin Nutr, 106:481–490. [DOI] [PubMed] [Google Scholar]
- 31.Bonjour J-P, Dontot-Payen F, Rouy E, et al. (2018). Evolution of Serum 25OHD in Response to Vitamin D3–Fortified Yogurts Consumed by Healthy Menopausal Women: A 6-Month Randomized Controlled Trial Assessing the Interactions between Doses, Baseline Vitamin D Status, and Seasonality. J Am Coll Nutr, 37:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grønborg IM, Tetens I, Andersen EW, et al. (2019). Effect of vitamin D fortified foods on bone markers and muscle strength in women of Pakistani and Danish origin living in Denmark: a randomised controlled trial. Nutr J, 18:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grønborg IM, Tetens I, Christensen T, et al. (2020). Vitamin D-fortified foods improve wintertime vitamin D status in women of Danish and Pakistani origin living in Denmark: a randomized controlled trial. Eur J Nutr, 59:741–753. [DOI] [PubMed] [Google Scholar]
- 34.Daly RM, Bass S, Nowson C. (2006). Long-term effects of calcium–vitamin-D3-fortified milk on bone geometry and strength in older men. Bone, 39:946–953. [DOI] [PubMed] [Google Scholar]
- 35.Kukuljan S, Nowson CA, Sanders KM, et al. (2011). Independent and combined effects of calcium-vitamin D3 and exercise on bone structure and strength in older men: an 18-month factorial design randomized controlled trial. J Clin Endocrinol Metab, 96:955–963. [DOI] [PubMed] [Google Scholar]
- 36.Xueqin D, Zhu K, Trube A, et al. (2004). School-milk intervention trial enhances growth and bone mineral accretion in Chinese girls aged 10–12 years in Beijing. Br J Nutr, 92:159–168. [DOI] [PubMed] [Google Scholar]
- 37.Zhu K, Du X, Cowell CT, et al. (2005). Effects of school milk intervention on cortical bone accretion and indicators relevant to bone metabolism in Chinese girls aged 10–12 y in Beijing. Am J Clin Nutr, 81:1168–1175. [DOI] [PubMed] [Google Scholar]
- 38.Rich-Edwards JW, Ganmaa D, Kleinman K, et al. (2011). Randomized trial of fortified milk and supplements to raise 25-hydroxyvitamin D concentrations in schoolchildren in Mongolia. Am J Clin Nutr, 94:578–584. [DOI] [PubMed] [Google Scholar]
- 39.Khadgawat R, Marwaha R, Garg M, et al. (2013). Impact of vitamin D fortified milk supplementation on vitamin D status of healthy school children aged 10–14 years. Osteoporos Int, 24:2335–2343. [DOI] [PubMed] [Google Scholar]
- 40.Neyestani T, Hajifaraji M, Omidvar N, et al. (2014). Calcium-vitamin D-fortified milk is as effective on circulating bone biomarkers as fortified juice and supplement but has less acceptance: a randomised controlled school- based trial. J Hum Nutr Diet, 27:606–616. [DOI] [PubMed] [Google Scholar]
- 41.Brett NR, Lavery P, Agellon S, et al. (2016). Dietary vitamin D dose-response in healthy children 2 to 8 y of age: a 12-wk randomized controlled trial using fortified foods. Am J Clin Nutr, 103:144–152. [DOI] [PubMed] [Google Scholar]
- 42.Brett NR, Parks CA, Lavery P, et al. (2018). Vitamin D status and functional health outcomes in children aged 2–8 y: a 6-mo vitamin D randomized controlled trial. Am J Clin Nutr, 107:355–364. [DOI] [PubMed] [Google Scholar]
- 43.Lu J, Pan H, Hu X, et al. (2019). Effects of milk powder intervention on bone mineral density and indicators related to bone metabolism in Chinese adolescents. Osteoporos Int, 30:2231–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fisk CM, Theobald HE, Sanders TA. (2012). Fortified malted milk drinks containing low-dose ergocalciferol and cholecalciferol do not differ in their capacity to raise serum 25-hydroxyvitamin D concentrations in healthy men and women not exposed to UV-B. J Nutr, 142:1286–1290. [DOI] [PubMed] [Google Scholar]
- 45.Johnson J, Mistry V, Vukovich M, et al. (2005). Bioavailability of vitamin D from fortified process cheese and effects on vitamin D status in the elderly. J Dairy Sci, 88:2295–2301. [DOI] [PubMed] [Google Scholar]
- 46.Neyestani TR, Nikooyeh B, Kalayi A, et al. (2015). A vitamin D-calcium-fortified yogurt drink decreased serum PTH but did not affect osteocalcin in subjects with type 2 diabetes. Int J Vitam Nutr Res, 85:61–69. [DOI] [PubMed] [Google Scholar]
- 47.Moreira-Lucas TS, Duncan AM, Rabasa-Lhoret R, et al. (2016). Effect of Vitamin D Fortified Cheese on Oral Glucose Tolerance in Individuals Exhibiting Marginal Vitamin D Status and an Increased Risk for Developing Type 2 Diabetes: A Double-Blind, Randomized Placebo-Controlled Clinical Trial. Faseb J, 30:917.1–917.1. [DOI] [PubMed] [Google Scholar]
- 48.Nikooyeh B, Neyestani TR, Zahedirad M, et al. (2016). Vitamin D-fortified bread is as effective as supplement in improving vitamin D status: a randomized clinical trial. J Clin Endocrinol Metab, 101:2511–2519. [DOI] [PubMed] [Google Scholar]
- 49.Salehi S, Sadeghi F, Akhlaghi M, et al. (2018). Vitamin D3-fortified milk did not affect glycemic control, lipid profile, and anthropometric measures in patients with type 2 diabetes, a triple-blind randomized clinical trial. Eur J Clin Nutr, 72:1083–1092. [DOI] [PubMed] [Google Scholar]
- 50.Boonen S, Lips P, Bouillon R, et al. (2007). Need for additional calcium to reduce the risk of hip fracture with vitamin D supplementation: evidence from a comparative metaanalysis of randomized controlled trials. J Clin Endocrinol Metab, 92:1415–1423. [DOI] [PubMed] [Google Scholar]
- 51.Papadimitropoulos E, Shea B, Wells GA, et al. (1997). Vitamin D with or without calcium for treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev, 4: CD000519. [Google Scholar]
- 52.Lips P. (2001). Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev, 22:477–501. [DOI] [PubMed] [Google Scholar]
- 53.Kuehn BM. (2012). USPSTF: Taking vitamin D and calcium doesn’t prevent fractures in older women. JAMA, 308:225–226. [DOI] [PubMed] [Google Scholar]
- 54.Baroncelli GI, Bertelloni S, Ceccarelli C, et al. (2000). Bone turnover in children with vitamin D deficiency rickets before and during treatment. Acta Paediatr, 89:513–8. [DOI] [PubMed] [Google Scholar]
- 55.Seibel M. (2000). Molecular markers of bone turnover: biochemical, technical and analytical aspects. Osteoporos Int, 11 Suppl 6:S18–29. [DOI] [PubMed] [Google Scholar]
- 56.Calvo MS, Eyre DR, Gundberg CM. (1996). Molecular basis and clinical application of biological markers of bone turnover. Endocr Rev, 17:333–368. [DOI] [PubMed] [Google Scholar]
- 57.Sukumar D, Shapses S, Schneider S. (2015). Vitamin D supplementation during short-term caloric restriction in healthy overweight/obese older women: effect on glycemic indices and serum osteocalcin levels. Mol Cell Endocrinol, 410:73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Je SH, Joo N-S, Choi B-h, et al. (2011). Vitamin K supplement along with vitamin D and calcium reduced serum concentration of undercarboxylated osteocalcin while increasing bone mineral density in Korean postmenopausal women over sixty-years-old. J Korean Med Sci, 26:1093–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Von Hurst P, Stonehouse W, Kruger M, Coad J. (2010). Vitamin D supplementation suppresses age-induced bone turnover in older women who are vitamin D deficient. J Steroid Biochem Mol Biol, 121:293–296. [DOI] [PubMed] [Google Scholar]
- 60.Martinez M, Del Campo M, Sanchez-Cabezudo M, et al. (1994). Relations between calcidiol serum levels and bone mineral density in postmenopausal women with low bone density. Calcif Tissue Int, 55:253–256. [DOI] [PubMed] [Google Scholar]
- 61.Khaw K-T, Sneyd M-J, Compston J. (1992). Bone density parathyroid hormone and 25-hydroxyvitamin D concentrations in middle aged women. BMJ, 305:273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chapuy M, Schott A, Garnero P, et al. (1996). Healthy elderly French women living at home have secondary hyperparathyroidism and high bone turnover in winter. EPIDOS Study Group. J Clin Endocrinol Metab, 81:1129–1133. [DOI] [PubMed] [Google Scholar]
- 63.Jehle PM, Schulten K, Schulz W, et al. (2003). Serum levels of insulin-like growth factor (IGF)-I and IGF binding protein (IGFBP)-1 to -6 and their relationship to bone metabolism in osteoporosis patients. Eur J Intern Med, 14:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O’Connor KG, Tobin JD, Harman SM, et al. (1998). Serum levels of insulin-like growth factor-I are related to age and not to body composition in healthy women and men. J Gerontol A Biol Sci Med Sci, 53:M176–82. [DOI] [PubMed] [Google Scholar]