Abstract
No hyperthermophilic microorganisms have previously been shown to anaerobically oxidize acetate, the key extracellular intermediate in the anaerobic oxidation of organic matter. Here we report that two hyperthermophiles, Ferroglobus placidus and “Geoglobus ahangari,” grow at 85°C by oxidizing acetate to carbon dioxide, with Fe(III) serving as the electron acceptor. These results demonstrate that acetate could potentially be metabolized within the hot microbial ecosystems in which hyperthermophiles predominate, rather than diffusing to cooler environments prior to degradation as has been previously proposed.
It is well known that hyperthermophilic microorganisms inhabit the hot (ca. 80 to 110°C), anaerobic environments surrounding hydrothermal zones and the deep subsurface, but much remains to be learned about the functioning of hot microbial ecosystems (7, 13, 14). The direct measurement of in situ rates of microbial processes in hot microbial ecosystems has proven to be technically difficult. Therefore, concepts on microbial processes in such environments have largely been inferred from the physiological properties of the hyperthermophilic microorganisms that are available in pure culture.
In anaerobic environments at more moderate temperatures, such as aquatic sediments and the terrestrial subsurface, complex organic matter is anaerobically oxidized to carbon dioxide via the cooperative activity of microbial food chains (10, 13). Fermentative microorganisms partially oxidize sugars and amino acids to carbon dioxide with the concomitant production of hydrogen, but the primary carbon end products of fermentation are short-chain acids, of which acetate is by far the most abundant. Therefore, the effective processing of organic matter in these anaerobic sedimentary environments requires the activity of acetate-degrading anaerobic microorganisms. Depending upon the availability of electron acceptors, acetate may either be oxidized to carbon dioxide, with the reduction of electron acceptors such as nitrate, Fe(III), or sulfate, or be converted to methane and carbon dioxide by methanogens.
Acetate is also the most important organic acid in hot microbial ecosystems because it is a major fermentation product of hyperthermophiles and because it may enter hot microbial ecosystems from hotter, sterile environments in which organic matter is abiotically hydrolyzed to acetate (2, 5, 13). A recent review (13) suggested that acetate is not degraded in hot microbial ecosystems but rather must first diffuse into cooler environments before it can be microbially degraded. This model is based on the fact that although there were some preliminary reports that hyperthermophiles might grow in an anaerobic medium in which acetate was provided as a potential electron donor (6, 16), no data demonstrating anaerobic acetate degradation or growth with acetate as the sole electron donor were provided, and subsequent studies (1) questioned some of these results.
In order to further evaluate the potential for hyperthermophilic microorganisms to anaerobically degrade acetate, we studied acetate metabolism in several hyperthermophilic Archaea, that are available in pure culture. Ferroglobus placidus (DSM 10642), Archaeoglobus profundus (DSM 5631), and Archaeoglobus veneficus (DSM 11195) were obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen. Pyrobaculum aerophilum (DSM 7523) was a gift from Imke Schröder (Department of Microbiology and Molecular Genetics, University of California, Los Angeles).
It was previously stated that P. aerophilum could grow in an acetate-containing medium in which nitrate was provided as an electron acceptor (16). However, the study provided no data on acetate consumption or acetate-dependent nitrate reduction that would indicate whether this organism actually anaerobically oxidized acetate, and there were no quantitative data on cell growth. When we attempted to grow P. aerophilum on acetate with nitrate as the electron acceptor in the growth medium previously specified for this organism (16), it failed to grow, even though it readily grew in the same medium with yeast extract or peptone as the electron donor and nitrate as the electron acceptor. This result is consistent with another report that also suggested that P. aerophilum does not actually grow via acetate oxidation coupled to nitrate reduction (1). In addition to nitrate, P. aerophilum is capable of using Fe(III) as an electron acceptor (8), but P. aerophilum did not grow with acetate as the sole electron donor and Fe(III) as the electron acceptor in media in which it grew with hydrogen as the electron donor and Fe(III) as the electron acceptor. Furthermore, the closely related Pyrobaculum islandicum did not grow with acetate as the electron donor in media (8) that supported growth with hydrogen, yeast extract, or peptone as the electron donor and S0 or Fe(III) as the electron acceptor.
It has also been reported that A. veneficus can grow with acetate as the electron donor and sulfite as the electron acceptor (6). However, no data supporting this conclusion were provided. We were able to grow this organism in the suggested medium (6) with sulfite as the electron acceptor and hydrogen as the electron donor, but it did not grow with acetate as the sole electron donor and sulfite or any other commonly used electron acceptors, including Fe(III). The closely related A. profundus also did not grow with acetate as the electron donor when its preferred electron acceptor, sulfate, or Fe(III) was provided as the electron acceptor, but it did grow in the same medium with hydrogen as the electron donor and sulfate as the electron acceptor. These results are consistent with the model (13) in which hyperthermophiles do not anaerobically oxidize acetate.
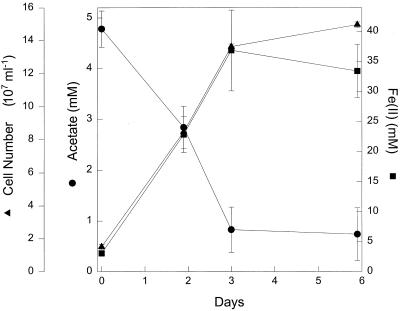
F. placidus is another hyperthermophile closely related to A. veneficus (4). This organism is known for its ability to grow anaerobically with Fe(II) as the electron donor and nitrate as the electron acceptor. F. placidus did not grow with acetate as the electron donor and nitrate as the electron acceptor in a medium (4) that supported growth on Fe(II) and nitrate. F. placidus also did not grow on acetate with thiosulfate as an electron acceptor, even though it was able to grow in that medium if hydrogen was provided as an electron donor, as previously reported (4). These results are not surprising because previous studies had indicated that F. placidus did not grow with organic compounds as sole electron donors (4). However, recent studies have demonstrated that the metabolic versatility of hyperthermophilic microorganisms may be expanded when Fe(III) is provided as an electron acceptor (15). Therefore, the standard growth medium for F. placidus was altered by replacing the nitrate with 100 mmol of poorly crystalline Fe(III) oxide per liter (12) as the electron acceptor, and the concentration of KH2PO4 was increased to 0.5 g/liter, in order to account for phosphate adsorption onto the Fe(III) oxide. F. placidus readily grew at 85°C in the Fe(III) oxide medium (Fig. 1). Growth was concurrent with loss of acetate and accumulation of Fe(II) resulting from Fe(III) reduction. The stoichiometry of Fe(III) reduced to acetate consumed was 7.8 ± 1.0 (mean ± standard deviation; n = 3). This is consistent with the stoichiometry expected for oxidation of acetate to carbon dioxide with Fe(III) serving as the sole electron acceptor according to the following reaction: acetate− + 8Fe(III) + 4H2O → 2HCO3− + 8Fe(II) + 9H+.
FIG. 1.
Growth of F. placidus at 85°C with acetate as an electron donor and poorly crystalline Fe(III) as an electron acceptor. The results are the means of triplicate cultures; error bars indicate the standard deviations.
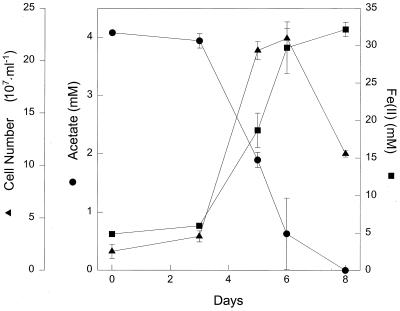
“Geoglobus ahangari” is a hyperthermophilic microorganism isolated from sediments from Guaymas Basin in the Gulf of California (K. Kashefi, J. Tor, C. V. G. Van Praugh, A.-L. Reysenbach, and D. R. Lovley, unpublished data). This organism was enriched and isolated in media with pyruvate as the electron donor and Fe(III) as the electron acceptor. Phylogenetic analysis has indicated that the closest known genera to Geoglobus are Ferroglobus and Archaeoglobus (Kashefi et al., unpublished). Like F. placidus, “G. ahangari” grew at 85°C in a medium with acetate as the sole electron donor and Fe(III) oxide as the sole electron acceptor (Fig. 2). The stoichiometry of Fe(III) reduced to acetate consumed was 7.1 ± 0.7 (mean ± standard deviation; n = 3), which is consistent with acetate oxidation to carbon dioxide with Fe(III) serving as the sole electron acceptor.
FIG. 2.
Growth of “G. ahangari” at 85°C with acetate as an electron donor and poorly crystalline Fe(III) as an electron acceptor. The results are the means of triplicate cultures; error bars indicate the standard deviations.
The results presented here provide the first documented example of anaerobic acetate oxidation at the temperatures found in hot microbial ecosystems. The finding that hyperthermophiles can anaerobically oxidize acetate demonstrates that this key intermediate in the anaerobic degradation of organic carbon does not necessarily have to be exported to cooler environments in order to be oxidized, as has previously been proposed. Fe(III) is considered to be available as an electron acceptor in modern, hot, anaerobic ecosystems such as the deep subsurface and areas around hydrothermal vents (3, 7, 9). Thus, consortia of hyperthermophiles consisting of fermentative microorganisms and acetate-oxidizing Fe(III) reducers may cooperate in the oxidation of fermentable compounds in hyperthermophilic environments in a manner analogous to that of the mesophilic food chains involved in processing organic matter in cooler Fe(III)-containing sedimentary environments (9, 12). F. placidus and “G. ahangari” were able to use only Fe(III) as the electron acceptor for anaerobic acetate oxidation. Efforts to recover anaerobic acetate-oxidizing hyperthermophiles capable of using other electron acceptors are warranted.
Acknowledgments
This research was supported by grant MCB-0085365 from The National Science Foundation.
REFERENCES
- 1.Afshar S, Kim C, Monbouquette H G, Schröder I. Effect of tungstate on nitrate reduction by the hyperthermophilic archaeon Pyrobaculum aerophilum. Appl Environ Microbiol. 1998;64:3004–3008. doi: 10.1128/aem.64.8.3004-3008.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amend J P, Amend A C, Valenza M. Determination of volatile fatty acids in the hot springs of Vulcano, Aeolian Islands, Italy. Org Geochem. 1998;28:699–705. [Google Scholar]
- 3.Gold T. The deep, hot biosphere. Proc Natl Acad Sci USA. 1992;89:6045–6049. doi: 10.1073/pnas.89.13.6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hafenbradl D, Keller M, Dirmeier R, Rachel R, Roßnagel P, Burggraf S, Huber H, Stetter K O. Ferroglobus placidus gen nov., sp. nov., a novel hyperthermophilic archaeum that oxidizes Fe+2 at neutral pH under anoxic conditions. Arch Microbiol. 1996;166:308–314. doi: 10.1007/s002030050388. [DOI] [PubMed] [Google Scholar]
- 5.Helgesen H C, Knox A M, Owens C E, Shock E L. Petroleum, oil field waters, and authigenic mineral assemblages: are they in metastable equilibrium in hydrocarbon reservoirs? Geochim Cosmochim Acta. 1993;57:3295–3339. [Google Scholar]
- 6.Huber H, Jannasch H, Rachel R, Fuchs T, Stetter K O. Archaeoglobus veneficus sp. nov., a novel facultative chemolithotrophic hyperthermophilic sulfite reducer, isolated from abyssal black smokers. Syst Appl Microbiol. 1997;20:374–380. [Google Scholar]
- 7.Karl D M. Ecology of free-living hydrothermal vent microbial communities. In: Karl D M, editor. The microbiology of deep-sea hydrothermal vents. New York, N.Y: CRC Press; 1995. pp. 35–124. [Google Scholar]
- 8.Kashefi K, Lovley D R. Reduction of Fe(III), Mn(IV), and toxic metals at 100°C by Pyrobaculum islandicum. Appl Environ Microbiol. 2000;66:1050–1056. doi: 10.1128/aem.66.3.1050-1056.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lovley D R. Fe(III) and Mn(IV) Reduction. In: Lovley D R, editor. Environmental microbe-metal interactions. Washington, D.C.: ASM Press; 2000. pp. 3–30. [Google Scholar]
- 10.Lovley D R, Chapelle F H. Deep subsurface microbial processes. Rev Geophys. 1995;33:365–381. [Google Scholar]
- 11.Lovley D R, Phillips E J P. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl Environ Microbiol. 1986;51:683–689. doi: 10.1128/aem.51.4.683-689.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lovley D R, Phillips E J P. Requirement for a microbial consortium to completely oxidize glucose in Fe(III)-reducing sediments. Appl Environ Microbiol. 1989;55:3234–3236. doi: 10.1128/aem.55.12.3234-3236.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slobodkin A I, Zavarzina D G, Sokolova T G, Bonch-Osmolovskaya E A. Dissimilatory reduction of inorganic electron acceptors by thermophilic anaerobic prokaryotes. Microbiology. 1999;68:600–622. [Google Scholar]
- 14.Stetter K O. Hyperthermophilic procaryotes. FEMS Microbiol Rev. 1996;18:149–158. [Google Scholar]
- 15.Vargas M, Kashefi K, Blunt-Harris E L, Lovley D R. Microbiological evidence for Fe(III) reduction on early Earth. Nature. 1998;395:65–67. doi: 10.1038/25720. [DOI] [PubMed] [Google Scholar]
- 16.Völkl P, Huber R, Drobner E, Rachel R, Burggraf S, Trincone A, Stetter K O. Pyrobaculum aerophilum sp. nov. a novel nitrate-reducing hyperthermophilic archaeum. Appl Environ Microbiol. 1993;59:2918–2926. doi: 10.1128/aem.59.9.2918-2926.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]