Abstract
Objectives
Qualitative real-time polymerase chain reaction tests are not designed to provide quantitative or semiquantitative results because cycle threshold (Ct) values are not normalized to standardized controls of known concentration. The aim of this study was to characterize SARS-CoV-2 viral loads based on Ct values, using the QIAstat-Dx® Respiratory SARS-CoV-2 Panel.
Methods
Different lineages of SARS-CoV-2 clinical samples and the World Health Organization international standard were used to assess the linearity of the QIAstat-Dx Respiratory SARS-CoV-2 Panel. Limit of detection for the different lineages was characterized.
Results
Comparable efficiencies and linearity for all samples resulted in R2 ≥0.99, covering a dynamic range of 1,000,000-100 copies/mL for the SARS-CoV-2 assay, showing linear correlation between Ct values and viral load down to 300 copies/mL.
Conclusion
The SARS-CoV-2 Ct values provided by the QIAstat-Dx® Respiratory SARS-CoV-2 Panel could be used as a surrogate for viral load given the linear correlation between Ct values and viral concentration down to limit of detection. This panel allows to obtain reproducible Ct values for SARS-CoV-2 ribonucleic acid downstream of the sample collection, reducing the sample-to-Ct workflow variability. Ct values can help provide a reliable assessment and comparison of viral loads in patients when tested with the QIAstat-Dx Respiratory SARS-CoV-2 Panel.
Keywords: SARS-CoV-2, COVID-19, Cycle threshold value, Viral load, QIAstat-Dx, Disease severity
Introduction
Soon after the outbreak of COVID-19 (World Health Organization 2020), caused by SARS-CoV-2, the first diagnostic methods were developed to respond to the rapid transmission of the virus. The first published real-time reverse transcription-polymerase chain reaction (RT-PCR) assay to detect SARS-CoV-2 ribonucleic acid (RNA) in human samples was developed by Drosten and colleagues and rapidly became endorsed by the World Health Organization (WHO) as the reference method to diagnose COVID-19 (Corman et al., 2020). This assay targets three different regions of the SARS-CoV-2 genome (N gene, E gene, and the RNA-dependent RNA polymerase or RdRP region inside the Orf1ab gene) as a way to minimize potential sensitivity loss due to the unknown genetic variability of this novel virus during the pandemic (Peñarrubia et al., 2020). Several other public entities also made their assays available to detect the RNA from SARS-CoV2 (US Food and Drug Administration 2020). Meanwhile, several companies started getting regulatory approval of commercial in vitro diagnostic (IVD) tests on the basis of RT-PCR, developed to detect the presence of SARS-CoV-2 RNA in human nasopharyngeal fluids samples, such as nasopharyngeal swab, oropharyngeal swab, or nasal aspirates. Common commercial IVD tests are applied to detect and discriminate SARS-CoV-2 presence in samples from patients with and without symptoms of a respiratory illness.
The QIAstat-Dx® Respiratory SARS-CoV-2 panel was one of the first sample-to-result syndromic testing solutions to get CE-IVD marking (catalog no. 691214) and the first syndromic solution get emergency use authorization approval by the Food and Drug Association (catalog no. 691223) in early 2020. The QIAstat-Dx® Respiratory SARS-CoV-2 Panel is a fully automated RT-PCR multiplex assay that detects and discriminates SARS-CoV-2 and 21 other respiratory pathogens (Visseaux et al., 2020). Results are reported as detected/not detected, and corresponding RT-PCR cycle threshold (Ct) value of every detected pathogen is included in the report.
A highly debated topic around the detection of the SARS-CoV-2 RNA is the establishment of an interpretation criterion around the use of the Ct value as a proxy of viral load and how this correlates with contagiousness, measured using viral culture or with patient outcomes, such as progression of the disease or severity (Rao et al., 2020). The ultimate goal of these studies was to determine whether Ct value interpretation could be used to take more accurate patient-management decisions, especially around cohorting, treatment and treatment efficiency, potential resistance emergences, and release of patients. Several organizations like American Association for Clinical Chemistry (AACC), the Infectious Diseases Society of America, and the Association of Public Health Laboratories, Public Health England (PHE), among others have issued guideline documents on interpretation of Ct values. In general, some of these guidelines discourage (AACC) (AACC.org, 2021), advise caution (Infectious Diseases Society of America) (Buchan, 2021), or guide on the interpretation of the Ct values (PHE) (Public Health England 2020). A common conclusion from all these documents is that although Ct values are not directly comparable between different assays, standardized clinical testing workflows using RT-PCR assays are key to interpreting Ct values within those stablished testing layouts (Platten et al., 2021). In addition, PHE guideline suggests that even under these standardized testing workflows, interpretation of Ct values for staging infectious course, prognosis, infectivity, or as an indicator of recovery should be done with context about the clinical history.
In this study, we aimed to study the linearity and limit of detection of the SARS-CoV-2 assay on the QIAstat-Dx® Respiratory SARS-CoV-2 Panel, which can help support Ct value interpretation when using the QIAstat-Dx® Respiratory SARS-CoV-2 Panel to diagnose patients.
Methods and Materials
SARS-CoV-2 Quantification Method
Primer design
A real-time RT-PCR assay was designed targeting the nucleoprotein (N) single-copy coding gene to quantify SARS-CoV-2. Primers were designed to target 82-bp size fragment of the N gene using Primer3 tool implemented in Geneious software v10.2.6 (https://www.geneious.com). The targeted region is placed in positions 28,832-28,913 of the 29,903-bp size reference SARS-CoV-2 genome (sequence ID NC_045512 for GenBank and EPI_ISL_402124 for GISAID). Labeled probe was designed to bind between primers with an annealing temperature (Ta) above the primer of the same direction to optimize PCR performance (see Table 1 ). BLASTN analysis (https://blast.ncbi.nlm.nih.gov/Blast.cgi) analysis was run to discount possible homologies that could produce nonspecific amplifications in the PCR. All three oligonucleotides were ordered to Integrated DNA Technologies (IDT, Coralville, IA), with FAM labeled and double-quenched (ZEN/IBFQ) probe according to supplier recommendations to improve optical performance.
Table 1.
Primers and probe sequences used for SARS-CoV-2 quantification.
| Oligonucleotide | Sequence (5’ à 3’) | Length (bp) | Ta (°C) | Amplicon size (positions in reference genome) |
|---|---|---|---|---|
| Forward primer | TCATCACGTAGTCGCAACAG | 20 | 62.9 | 82 bp (28,832-28,913) |
| Reverse primer | CATTGCCAGCCATTCTAGCA | 20 | 63.9 | |
| Labeled Probea | CAAGAAATTCAACTCCAGGCAGCAGTA | 27 | 67.7 |
.- /56-FAM/CAAGAAATT/ZEN/CAACTCCAGGCAGCAGTA/3IABkFQ/ double-quenched probe.
bp: base pair.
Sample collection and DNA extraction
A customized 300-bp size commercial dsDNA fragment (IDT, Coralville, IA) corresponding to positions 28,723-29,022 of the reference SARS-CoV-2 genome was used for initial RT-PCR validation and test primers performance (see Table 2 ). This gBlock sequence was resuspended in Tris-EDTA buffer 1x (SIGMA, catalog number 93283-100ML) and concentration was calculated by Nanodrop 2000C (ThermoFisher Scientific, Waltham, MA) measure using molecular weight of the sequence.
Table 2.
Samples used for quantification and linearity studies.
| Study | Sample code | Sourcea | Strain name | Strain sequence ID (database) | sample stock (copies/mL)b |
|---|---|---|---|---|---|
| Quantification | dsDNA_ Ngene (gBlock) | IDT | n/a | n/a | 3.78E+13 |
| Clinical sample - 243 | HC | n/a | n/a | 1.4E+07 | |
| Linearity study | Clinical sample - 12 (ancestral lineage) | VdH | c | c | 4.96E+08 |
| Clinical sample - UK 7 (Alpha lineage) | VdH | hCoV-19/Spain/CT-HUVH-88240/2021 | EPI_ISL_954175 (GISAID) |
9.71E+07 | |
| WHO international standard | NIBSC | England/02/2020 | MW059036 (GenBank) | 1.42E+07 |
IDT: Integrated DNA Technologies (Coralville, IA, USA). HC: Hospital Clinic (Barcelona, Spain). VdH: Vall d'Hebron Hospital (Barcelona, Spain). NIBSC: National Institute for Biological Standards and Control (Blanche Lane, Ridge, Herts, UK), material code: 20/146.
Concentration of gBlock was calculated using Nanodrop and Molecular Weight of the sequence. Concentrations of clinical samples and World Health Organization International Standard were obtained during the Standard RT-qPCR curve optimization (sample 243) or quantification step.
Clinical sample 12 corresponds to a clinical sample collected at the beginning of the pandemic, corresponding to the ancestral lineage.
In addition, one representative clinical sample collected from a patient from Hospital Clinic (Barcelona, Spain) was used during the validation of the standard curve. Nucleic acid (NA) extraction for sample quantification was performed using automatic purification procedure with the QIAcube System (QIAGEN N.V., Hilden, Germany) and a customized protocol using the reagents of QIAamp Cador Pathogen Mini Kit (QIAGEN, catalog number 54106).
Standard RT-qPCR curve amplification
A SARS-CoV-2 RT-PCR assay was developed using a quantification standard (QS) to quantify the samples used in the study. Standard curve for SARS-CoV-2 quantification was performed in two steps, according to official RT-PCR guidelines (Hougs et al., 2019; McEnroe, 2020) and reference publications (Broeders et al., 2014; Bustin et al., 2009; Forootan et al., 2017). The first step included the standard curve construction using nine consecutive 10-fold dilutions of quantified dsDNA gBlock stock in 10 replicates by dilution point, starting from 1E+10 copies/mL. Ct values for all replicates were used to establish the linear range, including those dilution points with linear behavior. Linearity (R2), slope (S), and efficiency (E) parameters were calculated, with accepted values of E = 90-110%; S = (-3.1)-(-3.6), and R2 ≥ 0.98. Limit of quantification (LoQ) was considered as the last detected dilution inside the linear range with a coefficient of variation (CV) lower than 25% (calculated as SD/average × 100).
Second step included the validation of the SARS-CoV-2 RT-PCR linear range, and the evaluation of precision, repeatability, and reproducibility of the RT-PCR assay. First, standard curve was validated running seven of the nine initial dilution points in triplicate. For precision evaluation, standard curve was confirmed up to three times in different days and by different operators. Duplicated NA extractions from a clinical sample (ID:243, Hospital Clinic; see Table 2) with unknown titer was selected and tested in four RT-PCR replicates at three 10-fold dilution levels. Appropriate internal controls for each RT-PCR run were also added.
In all cases, RT-PCR amplifications were conducted using 10 replicates in a final volume of 15 μL, containing 600 nM of each primer and 300 nM of labeled probe resuspended in Tris-EDTA buffer 1x (SIGMA, catalog number 93283-100ML) and 1.5 mM trehalose solution (SIGMA, catalog number T9531-25G), and 1.5 μL customized Master Mix including 1.2 mM dNTPs, 4 mM MgCl2, as well as EnzscriptTM reverse transcriptase and antibody mediated Phoenix Hot Start Polymerase. Thermal cycles consisted of an initial retrotranscriptase/denaturing step of 5 minutes at 50°C and 30 s at 95°C, followed by 40 two-step cycles of denaturing at 94°C for 4 seconds, and annealing-extension step at 60°C for 21 seconds. Negative controls were included in all RT-PCR runs to confirm that there was no cross-contamination. RT-PCR reactions were run using QuantStudio™ 5 Real-Time PCR System (ThermoFisher Scientific) and analyzed in QuantStudio™ 5 Design and Analysis Software v1.5.1., with a Threshold of 25,000.
Quantification
A total of three representative samples were quantified for the linearity study: two clinical samples collected from Vall d'Hebron Hospital (Barcelona, Spain) corresponding to different SARS-CoV-2 lineages, together to a third sample corresponding to the first WHO International Standard for SARS-CoV-2 RNA for Nucleic acid Amplification Technique (NAT)-based assays (Blanche Lane, Ridge, Herts, UK) (Table 2). Total NA material extraction protocol and RT-PCR conditions were performed of all three samples following the same protocol previously described for the standard curve construction. In all cases, sample dilutions were amplified in a concentration inside the dynamic range of the standard curve. Final concentration (copies/mL) value was calculated using the linear regression extracted from the standard curve.
Linearity testing and data analysis
All three quantified samples were prepared in ½ logarithm serial dilutions including concentrations values from 2 to 6 magnitude order. Ten replicates by dilution point were run using QIAstat-Dx® Respiratory SARS-CoV-2 Panel cartridges and a QIAstat-Dx® analyzer system (QIAGEN, Hilden, Germany). Linear range, linearity, slope, and efficiency was characterized using the same parameters than the standard curve construction.
Finally, sensitivity levels for all three samples was evaluated by calculating the Limit of detection (LoD). A total of 20 replicates were tested by selected dilution point, and LoD was considered at the last dilution point with ≥95% of positive detection rate (≥19/20 replicates). As a final step, LoD was confirmed running High Negative samples defined as the subsequent semi-log dilution with a detection ratio lower than 95%.
Results
SARS-CoV-2 quantification method
The SARS-CoV-2 RT-PCR QS assay resulted in a final dynamic range of 1.00E+06 to 1.00E+01 copies/mL presented a linearity of R2=0.998 with a slope of -3.3 and an efficiency of 100.4%. This linear range presented a LoQ of 1.00E+02 copies/mL.
Precision of the assay was evaluated running a total of 3 RT-PCR 96-well plates by different days. For Repeatability, two 96-well plates (Plate 1 and 2) were run under the same testing conditions on different days, and the resulted average CV% between plates is CV=6.5%. For Reproducibility, two 96-well plates (Plate 1 and 3) were run in different instruments and by different operators on nonconsecutive days, with an average CV% between plates of CV=2.2%.
Quantification of clinical samples
All SARS-CoV-2 clinical and analytical samples were purified, and genomic material was extracted. Due to limited volume availability, clinical samples were diluted in Universal Transport Media prior to purification (see Table 3 ).
Table 3.
Ct values obtained in the SARS-CoV-2 qPCR assay and quantification results for every sample tested.
| Sample name | Dilution tested | CT obtained in RT-quantitative PCR |
Copies/µL | Final concentration (copies/mL) | ||||
|---|---|---|---|---|---|---|---|---|
| Replicates | Mean | SD | Replicates | Mean | SD | |||
| Clinical sample 1 | 1/1000 | 24.0 | 23.7 | 0.3 | 407 | 496 | 92.7 | 5.0E+08 |
| 23.8 | 456 | |||||||
| 23.4 | 623 | |||||||
| Clinical sample 2 | 1/100 | 23.5 | 23.4 | 0.1 | 923 | 971 | 58.4 | 9.7E+07 |
| 23.4 | 937 | |||||||
| 23.3 | 1,053 | |||||||
| WHO international standard | 1/100 | 27.5 | 27.2 | 0.2 | 114 | 142 | 20.5 | 1.4E+07 |
| 27.0 | 163 | |||||||
| 27.1 | 1489 | |||||||
Ct: cycle threshold; PCR: polymerase chain reaction; WHO: World Health Organization.
Triplicates of QS were included in a RT-PCR to set the standard curve along with triplicates of the sample to be quantified. Calculation of the standard curve and the quantification of the samples resulted in a PCR Efficiency of 100.2%, slope of -3.3 and R2 of 0.999. Final concentration (copies/mL) of the samples were obtained from the mean of the triplicates and applying the dilution factor of the purified sample (see Table 3).
Linearity testing
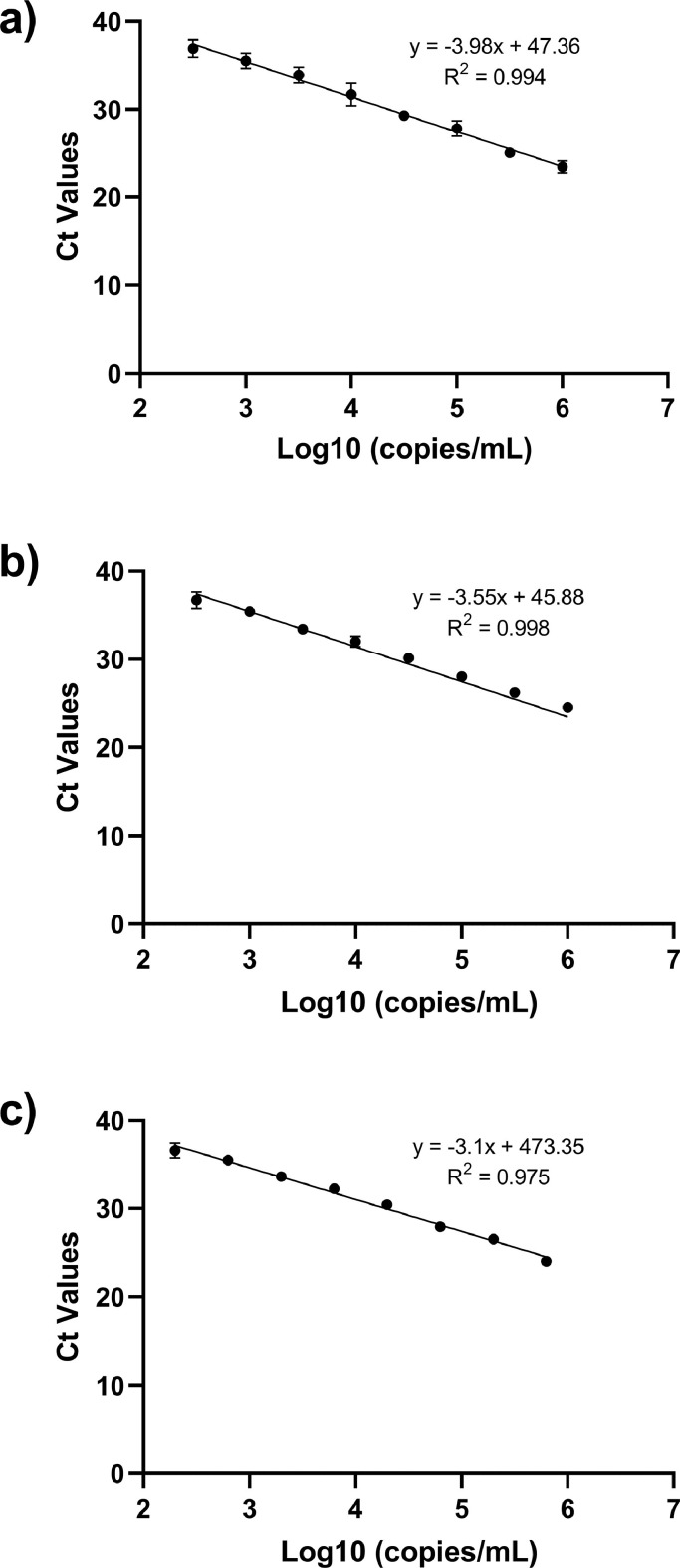
From each sample, a total of 10 replicates of every nine semi-log dilutions were run with the QIAstat-Dx® Respiratory SARS-CoV-2 Panel (see Table 4 ). For linearity evaluation, a corresponding standard curve was performed for every tested sample. In all cases, dilutions inside the dynamic range were included, and the corresponding linear correlation was calculated (see Figure 1 ).
Table 4.
Ct values obtained for the linearity test in every tested sample.
| Sample | Dilution | Copies/mL | Log10 concentration | Hit rate | Ct values for SARS-CoV-2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Individual replicates | Mean | SD | |||||||||
| Clinical sample 1 | Dil1 | 1.00E+06 | 6.0 | 10/10 | 25.2 | 23.5 | 23.5 | 23.2 | 23.4 | 23.4 | 0.7 |
| 23.0 | 23.4 | 23.2 | 22.4 | 23.1 | |||||||
| Dil2 | 3.16E+05 | 5.5 | 10/10 | 25.5 | 25.1 | 25.7 | 25.6 | 25.0 | 25.0 | 0.5 | |
| 24.4 | 24.0 | 24.8 | 25.1 | 25.0 | |||||||
| Dil3 | 1.00E+05 | 5.0 | 10/10 | 27.6 | 27.0 | 28.1 | 27.7 | 26.8 | 27.8 | 0.9 | |
| 29.9 | 26.9 | 28.3 | 27.9 | 28.2 | |||||||
| Dil4 | 3.16E+04 | 4.5 | 10/10 | 30.1 | 28.9 | 29.5 | 29.6 | 29.3 | 29.3 | 0.4 | |
| 29.8 | 29.0 | 28.9 | 28.9 | 28.9 | |||||||
| Dil5 | 1.00E+04 | 4.0 | 10/10 | 30.4 | 32.6 | 33.0 | 32.5 | 32.1 | 31.7 | 1.3 | |
| 29.1 | 30.8 | 31.1 | 33.0 | 32.4 | |||||||
| Dil6 | 3.16E+03 | 3.5 | 10/10 | 32.3 | 34.2 | 33.5 | 34.9 | 33.7 | 33.9 | 0.9 | |
| 34.0 | 32.9 | 34.3 | 35.2 | 34.1 | |||||||
| Dil7 | 1.00E+03 | 3.0 | 10/10 | 35.0 | 35.2 | 35.3 | 36.0 | 37.3 | 35.5 | 0.9 | |
| 35.8 | 34.3 | 34.4 | 35.6 | 35.9 | |||||||
| Dil8 | 3.16E+02 | 2.5 | 8/10 | 37.4 | Neg | 36.6 | 36.4 | 35.2 | 36.9 | 1.0 | |
| 37.4 | 38.3 | Neg | 35.9 | 37.7 | |||||||
| Dil9 | 1.00E+02 | 2.0 | 4/10 | Neg | 32.7 | Neg | Neg | 37.9 | 34.9 | 3.0 | |
| Neg | 31.9 | Neg | Neg | 37.1 | |||||||
| Clinical sample 2 | Dil1 | 1.00E+06 | 6.0 | 10/10 | 24.3 | 24.8 | 24.7 | 24.1 | 24.2 | 24.5 | 0.3 |
| 24.5 | 24.5 | 24.8 | 24.9 | 24.1 | |||||||
| Dil2 | 3.16E+05 | 5.5 | 10/10 | 26.2 | 26.1 | 25.8 | 26.1 | 26.1 | 26.2 | 0.4 | |
| 26.7 | 26.9 | 25.8 | 25.8 | 26.7 | |||||||
| Dil3 | 1.00E+05 | 5.0 | 10/10 | 27.6 | 28.2 | 27.7 | 27.9 | 27.5 | 28.0 | 0.4 | |
| 28.5 | 28.7 | 27.5 | 28.4 | 28.0 | |||||||
| Dil4 | 3.16E+04 | 4.5 | 10/10 | 30.3 | 30.6 | 29.9 | 29.8 | 29.7 | 30.1 | 0.4 | |
| 29.7 | 30.0 | 30.1 | 30.3 | 31.0 | |||||||
| Dil5 | 1.00E+04 | 4.0 | 10/10 | 30.9 | 32.3 | 32.8 | 31.5 | 32.4 | 32.0 | 0.6 | |
| 31.3 | 32.3 | 31.5 | 32.5 | 32.2 | |||||||
| Dil6 | 3.16E+03 | 3.5 | 10/10 | 33.0 | 34.6 | 33.0 | 34.2 | 33.3 | 33.4 | 0.6 | |
| 33.9 | 33.1 | 33.1 | 33.2 | 32.8 | |||||||
| Dil7 | 1.00E+03 | 3.0 | 10/10 | 34.6 | 35.8 | 35.4 | 35.5 | 34.3 | 35.4 | 0.6 | |
| 35.1 | 36.0 | 35.2 | 36.1 | 35.7 | |||||||
| Dil8 | 3.16E+02 | 2.5 | 10/10 | 36.0 | 36.0 | 37.5 | 37.5 | 35.8 | 36.7 | 0.9 | |
| 37.6 | 36.1 | 37.4 | 35.2 | 37.7 | |||||||
| Dil9 | 1.00E+02 | 2.0 | 6/10 | Neg | Neg | Neg | 36.5 | 37.6 | 36.8 | 0.8 | |
| 37.6 | Neg | 36.0 | 37.3 | 35.8 | |||||||
| WHO Standard | Dil1 | 1.90E+06 | 6.3 | 10/10 | 22.5 | 22.6 | 22.4 | 22.2 | 22.2 | 22.4 | 0.3 |
| 22.5 | 21.8 | 22.1 | 22.7 | 22.8 | |||||||
| Dil2 | 6.00E+05 | 5.8 | 10/10 | 24.3 | 24.3 | 24.3 | 24.2 | 24.1 | 24.0 | 0.3 | |
| 24.1 | 23.8 | 24.0 | 23.8 | 23.2 | |||||||
| Dil3 | 1.90E+05 | 5.3 | 10/10 | 26.3 | 26.1 | 27.2 | 26.3 | 26.7 | 26.5 | 0.5 | |
| 27.0 | 25.6 | 26.3 | 26.6 | 26.4 | |||||||
| Dil4 | 6.00E+04 | 4.8 | 10/10 | 28.0 | 27.3 | 27.5 | 27.4 | 27.9 | 27.9 | 0.6 | |
| 29.1 | 27.7 | 27.7 | 28.9 | 27.9 | |||||||
| Dil5 | 1.90E+04 | 4.3 | 10/10 | 30.0 | 31.1 | 31.3 | 30.5 | 30.9 | 30.4 | 0.5 | |
| 30.2 | 30.3 | 29.9 | 30.4 | 29.8 | |||||||
| Dil6 | 6.00E+03 | 3.8 | 10/10 | 31.7 | 32.3 | 31.9 | 32.6 | 31.3 | 32.2 | 0.5 | |
| 32.8 | 32.8 | 32.0 | 32.4 | 32.4 | |||||||
| Dil7 | 1.90E+03 | 3.3 | 10/10 | 33.6 | 33.9 | 33.7 | 34.5 | 33.0 | 33.6 | 0.6 | |
| 34.3 | 32.9 | 33.4 | 32.9 | 33.8 | |||||||
| Dil8 | 6.00E+02 | 2.8 | 10/10 | 35.3 | 35.9 | 34.9 | 35.0 | 35.0 | 35.5 | 0.8 | |
| 36.3 | 34.8 | 35.6 | 34.8 | 37.4 | |||||||
| Dil9 | 1.90E+02 | 2.3 | 9/10 | 35.9 | 37.7 | Neg | 37.4 | 36.2 | 36.6 | 0.9 | |
| 35.7 | 37.6 | 35.8 | 37.6 | 35.5 | |||||||
Neg: Negative result; WHO: World Health Organization.
Figure 1.
Linearity evaluation using QIAstat-Dx® Respiratory SARS-CoV-2 cartridges. a) Clinical sample 1 representing ancestral lineage, b) Clinical sample 2 representing alpha lineage, c) WHO international standard. Ct: cycle threshold; WHO: World Health Organization.
Finally, LoD was evaluated for the three samples, establishing the concentration at which ≥95% of positive detection ratio (See Table 5 ). In order to confirm LoD, an additional dilution was tested with positive detection below LoD (Table 5).
Table 5.
Ct values obtained for the LoD test in every tested sample.
| Sample | Dilution | Copies/mL | Log10 concentration | Hit rate | Ct values for SARS-CoV-2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Individual replicates | Mean | SD | |||||||||
| Clinical sample 1 | LoD | 1.00E+03 | 3.0 | 20/20 | 34.9 | 30.8 | 35.2 | 37.6 | 35.0 | 35.4 | 1.9 |
| 37.5 | 35.6 | 34.6 | 35.2 | 34.9 | |||||||
| 35.7 | 35.6 | 35.7 | 37.6 | 36.7 | |||||||
| 35.7 | 32.2 | 34.6 | 31.7 | 37.0 | |||||||
| High Negative | 3.20E+02 | 2.5 | 16/20 | Neg | 37.6 | Neg | 37.6 | 37.6 | 37.3 | 0.6 | |
| 37.7 | 36.8 | 37.5 | 36.1 | 37.1 | |||||||
| Neg | 37.8 | 37.4 | 36.5 | 37.8 | |||||||
| 37.6 | 37.9 | 35.9 | 37.3 | Neg | |||||||
| Clinical sample 2 | LoD | 3.20E+02 | 2.5 | 20/20 | 35.7 | 36.7 | 35.8 | 36.9 | 37.0 | 36.8 | 0.8 |
| 37.9 | 37.0 | 35.2 | 35.9 | 37.5 | |||||||
| 37.3 | 35.6 | 37.5 | 37.9 | 36.5 | |||||||
| 35.9 | 37.3 | 36.7 | 36.1 | 35.9 | |||||||
| High Negative | 1.00E+02 | 2.0 | 8/19 | 37.6 | Neg | Neg | 36.7 | Neg | 37.1 | 0.7 | |
| 37.6 | Neg | Fail | Neg | 37.6 | |||||||
| 37.5 | 36.2 | Neg | Neg | Neg | |||||||
| 37.7 | 35.9 | Neg | Neg | Neg | |||||||
| WHO Standard | LoD | 6.00E+02 (3.16 Log10 IU/mL) | 2.8 | 20/20 | 34.8 | 35.0 | 35.3 | 37.5 | 36.7 | 35.6 | 0.9 |
| 35.5 | 37.1 | 34.2 | 35.0 | 35.1 | |||||||
| 36.0 | 35.8 | 36.2 | 35.4 | 34.9 | |||||||
| 36.7 | 35.7 | 35.5 | 35.0 | 34.5 | |||||||
| High Negative | 1.90E+02 | 2.3 | 17/19 | 34.4 | 37.1 | Neg | 36.8 | 35.9 | 36.2 | 1.1 | |
| 36.9 | 37.7 | Neg | 37.4 | 37.4 | |||||||
| 36.0 | 37.8 | Fail | 37.2 | 36.7 | |||||||
| 37.2 | 37.7 | 37.7 | 37.7 | 36.4 | |||||||
FAIL: QIAstat-Dx® cartridge run failed and excluded for the hit ratio calculation; LoD: limit of detection; Neg: Negative amplification; WHO: World Health Organization.
As a result, the lower detection level of the SARS-CoV-2 assay on the QIAstat-Dx® Respiratory SARS-CoV-2 panel is in the range of 100-1000 copies/mL, very close to the quantification limit of the assay used to determine concentration in copies/mL. LoD was determined to be between 316 cp/mL (UK-7 sample, Alpha lineage) and 1000cp/mL (sample 12, ancestral lineage).
DISCUSSION
In this study we show that the SARS-CoV-2 RT-PCR assay on the QIAstat-Dx® Respiratory SARS-CoV-2 Panel has a wide linear range spanning from 1,000,000 cp/mL to 100 cp/mL. These linearity results were obtained using both the international SARS-CoV-2 standard material and quantified clinical samples. Additionally, here we reassessed the LoD for SARS-CoV-2 using same three samples representing diverse SARS-CoV-2 lineages, showing how the sensitivity of the assay remains unaffected by genetic variability.
Most criticism from relevant associations about using the RT-PCR Ct value, as proxy of viral load and tool to support clinical decisions, comes from the variability when comparing different systems (AACC.org, 2021; Public Health England 2020; Buchan, 2021)). These guidelines describe in detail the factors that influence the variability of Ct values that go from the pre-examination factors (sampling, specimen type, transport, and storage), examination factors (NA extraction efficiency, RT-PCR characteristics, PCR linearity, reproducibility), and postexamination factors (interpretation).
In this regard, results presented in this study support the reproducibility and linearity of Ct values for clinically relevant SARS-CoV-2 concentrations (covering different variants) obtained with the QIAstat-Dx® Respiratory SARS-CoV-2 Panel. Sample-to-result solutions like the QIAstat-Dx® SARS-CoV-2 panel, with an automated reproducible workflow that includes NA extraction and RT-PCR conditions within a cartridge, remove the mentioned examination and the postexamination factors potentially introducing variability on the Ct value obtention. This solution could facilitate the interpretation of the Ct value to assess the infection phase in a patient when doing a time-course testing in a single patient or in interpatient comparison study to correlate with symptoms or outcomes.
The heterogeneity on COVID-19 severity is well documented (Gudbjartsson et al., 2020; Oran and Topol, 2020; Wang et al., 2020). Patients can have asymptomatic infections and never develop symptoms or clinical abnormalities (Oran and Topol, 2020) or can have symptomatic infections ranging from mild to severe (Gudbjartsson et al., 2020; Wang et al., 2020). In a previous narrative systematic review, authors showed the potential link between SARS-CoV-2 Ct value and clinical outcomes, such as us mortality or disease progression (Rao et al., 2020). As a general agreement during the SARS-CoV-2 pandemic, a qualitative assessment of positive or negative is enough to diagnose a patient. However, the additional information provided by a Ct value on a standardize workflow in combination of the clinical history could help in making clinical and patient-management decisions.
Although analytical characteristics detailed in this study have been established after global laboratory standards as mentioned previously, several limitations must be considered. First, sample dilutions were prepared using simulated negative matrix, and an impact on sensitivity can be generated using negative clinical matrix. However, a matrix equivalency study was performed during the development of the QIAstat-Dx® Respiratory SARS-CoV-2 Panel, with comparable results (data not shown). Second, potential limitation is attributed to the selection of tested dilutions inside the dynamic range. Upper range was limited at 106 copies/mL for this study, and high concentrated samples placed out of the linear range could fall outside the established linearity. An extended study covering highly concentrated samples would be needed to increase the linear range. Finally, although not a limitation of the study, pre-examination factors, such as NPS sampling variability, are also important to consider even when using highly automated platforms such as QIAstat-Dx.
Despite those study limitations, results presented in this study clearly show the excellent analytical characteristics of the QIAstat-Dx® Respiratory SARS-CoV-2 Panel, that in combination with the ease of use of a highly automatized system, creates a solid base to establish a standard laboratory workflow. In combination of the clinical history of patients, the QIAstat-Dx® Respiratory SARS-CoV-2 Panel can be a key in making a correct and useful Ct value interpretation to help clinical decision making.
Acknowledgments
Acknowledgments
The authors thank the Molecular Assay Design and Development team for their support in their corresponding roles.
Conflict of interest
QIAGEN provided support in the form of salaries for all authors (MJF, LP, SJ, RP, CCT, MVV, SNR, GP, DM and JP).
Funding source
This work was solely funded by QIAGEN.
Ethical approval Statement
No ethical approval was required to conduct this work.
Author contributions
Conceptualization: Martí Juanola-Falgarona, Davide Manissero, Josep Pareja, and Luis Peñarrubia. Data curation: Martí Juanola-Falgarona and Luis Peñarrubia. Funding Acquisition: Josep Pareja, Sonia N. Rao, and Davide Manissero. Investigation: Sara Jiménez-Guzmán, Roberto Porco, Marta Varo-Velázquez, and Clàudia Congost-Teixidor. Methodology: Martí Juanola-Falgarona, Luis Peñarrubia, and Josep Pareja. Project administration: Gemma Pueyo and Davide Manissero. Resources: Sara Jiménez-Guzmán, Roberto Porco, Marta Varo-Velázquez, and Clàudia Congost-Teixidor. Supervision: Martí Juanola-Falgarona, Josep Pareja, and Gemma Pueyo. Writing – original draft: Martí Juanola-Falgarona, Josep Pareja, and Luis Peñarrubia. Writing review and editing: Davide Manissero, Sonia N. Rao, Josep Pareja, Gemma Pueyo, and Luis Peñarrubia.
References
- American Association for Clinical Chemistry (AACC.org), AACC recommendation for reporting SARS-CoV-2 cycle threshold (CT) values. https://www.aacc.org/science-and-research/covid-19-resources/statements-on-covid-19-testing/aacc-recommendation-for-reporting-sars-cov-2-cycle-threshold-ct-values, 2021 (accessed 25November 2021).
- Broeders S, Huber I, Grohmann L, Berben G, Taverniers I, Mazzara M, et al. Guidelines for validation of qualitative real-time PCR methods. Trends Food Sci Technol. 2014;37:115–126. [Google Scholar]
- Association for Molecular Pathology. Buchan BW. 2021. Important issues to consider before interpreting and applying CT values in clinical practice - association for molecular pathology.https://www.amp.org/about/news-room/amp-blog-content/important-issues-to-consider-before-interpreting-and-applying-ct-values-in-clinical-practice/ March 12, 2021. [Google Scholar]
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration, Centers for Disease Control and Prevention (U.S.) 2019 Novel coronavirus (2019-nCoV) real-time RT-PCR diagnostic panel. https://www.fda.gov/media/134919/download, 2020 (accessed 26 November 2021).
- Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DKW, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forootan A, Sjöback R, Björkman J, Sjögreen B, Linz L, Kubista M. Methods to determine limit of detection and limit of quantification in quantitative real-time PCR (qPCR) Biomol Detect Quantif. 2017;12:1–6. doi: 10.1016/j.bdq.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Public Health England, Cycle threshold (Ct) in SARS-CoV-2 RT-PCR. https://www.gov.uk/government/publications/cycle-threshold-ct-in-sars-cov-2-rt-pcr, 2020 (accessed 25 November 2021).
- Gudbjartsson DF, Helgason A, Jonsson H, Magnusson OT, Melsted P, Norddahl GL, et al. Spread of SARS-CoV-2 in the Icelandic population. N Engl J Med. 2020;382:2302–2315. doi: 10.1056/NEJMoa2006100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hougs L, Gatto F, Goerlich O, Grohmann L, Lieske K, Mazzara M, et al. Verification of analytical methods for GMO testing when implementing interlaboratory validated methods. Testing and analysis of GMO-containing foods and feed. 2019:245–266. [Google Scholar]
- McEnroe RJ. 2nd ed. Clinical and Laboratory Standards Institute; 2020. Evaluation of Linearity of Quantitative Measurement Procedures. [Google Scholar]
- Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection: A narrative review. Ann Intern Med. 2020;173:362–367. doi: 10.7326/M20-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñarrubia L, Ruiz M, Porco R, Rao SN, Juanola-Falgarona M, Manissero D, et al. Multiple assays in a real-time RT-PCR SARS-CoV-2 panel can mitigate the risk of loss of sensitivity by new genomic variants during the COVID-19 outbreak. Int J Infect Dis. 2020;97:225–229. doi: 10.1016/j.ijid.2020.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platten M, Hoffmann D, Grosser R, Wisplinghoff F, Wisplinghoff H, Wiesmüller G, et al. SARS-CoV-2, CT-values, and infectivity—conclusions to be drawn from side observations. Viruses. 2021;13 doi: 10.3390/v13081459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SN, Manissero D, Steele VR, Pareja J. A narrative systematic review of the clinical utility of cycle threshold values in the context of COVID-19. Infect Dis Ther. 2020;9:573–586. doi: 10.1007/s40121-020-00324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visseaux B, le Hingrat Q, Collin G, Bouzid D, Lebourgeois S, le Pluart D, et al. Evaluation of the qiastat-dx respiratory sars-cov-2 panel, the first rapid multiplex PCR commercial assay for sars-cov-2 detection. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.00630-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liu Y, Liu L, Wang X, Luo N, Li L. Clinical outcomes in 55 patients with severe acute respiratory syndrome coronavirus 2 who were asymptomatic at hospital admission in Shenzhen. China. J Infect Dis. 2020;221:1770–1774. doi: 10.1093/infdis/jiaa119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, Disease outbreak News: pneumonia of unknown Cause – China. https://www.who.int/emergencies/disease-outbreak-news/item/2020-DON229, 2020 (accessed 13 December 2021).