Lung protective ventilation, usually referred to as ventilation with low tidal volumes (VT) and low inspiratory pressures (Pinsp), has repeatedly been shown to reduce mortality in patients with acute lung injury [1]. Conservative oxygen supplementation, a strategy that prevents arterial hyperoxemia through restricted use of oxygen [2], could be seen as another way to protect the lungs as use of low fractions of inspired oxygen (FiO2) reduces the direct toxic effects of oxygen on pulmonary tissue. Ventilation with low driving pressure (ΔP) and less mechanical power (MP) may also improve outcomes [3, 4
Targeting low VT and low pressures is a rather simple task, as it often involves nothing more than setting VT that suits the ideal bodyweight. Conservative oxygen supplementation may also be seen as not so difficult, notwithstanding that use of low FiO2 increases the risk of arterial hypoxia. Targeting low ΔP can be more of a challenge. ΔP is simple to monitor as it requires a simple calculation at the bedside, and a reduction in ΔP can be straightforwardly achieved by limiting VT [3], that is when VT are not already low. Use of high positive end-expiratory pressure (PEEP) may decrease ΔP if it increases the size of the functional lung. However, high PEEP may not always recruit collapsed lung units, and can instead cause pulmonary overdistention, thereby increasing ΔP. Targeting less MP is by far the most complex and difficult intervention. MP is not so easy to monitor as it requires a complex formula that uses VT, Pinsp, ΔP, inspiratory flow, and also respiratory rate (RR). And with that, it is uncertain which of these elements to ‘prioritize’. Most difficult herein, surely, is that changing the one setting may require an adjustment in the other, and these actually may have opposite effects on MP—for example, limiting VT to lower MP may only be possible by increasing RR, but the latter actually will increase MP. Last but not least, the everchanging pulmonary condition makes this all even more problematic, requiring almost near-constant adjustments to keep all settings within safe limits.
With the increasing complexity of lung protective ventilation, the question can be asked who should be involved in this intervention that has a huge potential to improve patient outcomes—clearly, this cannot be done by doctors or respiratory therapists, as these healthcare workers are too little present at the bedside. And it is also not possible to have this work done by nurses who have many other things to take care of. Next, we are currently facing an unsustainable situation in medical staffing. Already in 2000 it was forecasted that demand, i.e., numbers of critically ill patients, would continue to grow, while supply, i.e., intensivists and pulmonologists, would remain near constant, yielding deficits of specialists in intensive care units (ICUs) in the United States [5]. There have been no signs that this projection was wrong, and similar prognoses can be made regarding ICU nurses and other healthcare providers within our specialty, now also in the United Kingdom [6]. The recent pandemic taught us that hospital systems, including ICUs, can easily become disrupted, perhaps most of all because of the already scarce available ICU nurses. And this is probably most often the case in countries where there are too few health care workers. Recent news regarding alarming departures of nurses from ICUs enhances the feeling of urgency.
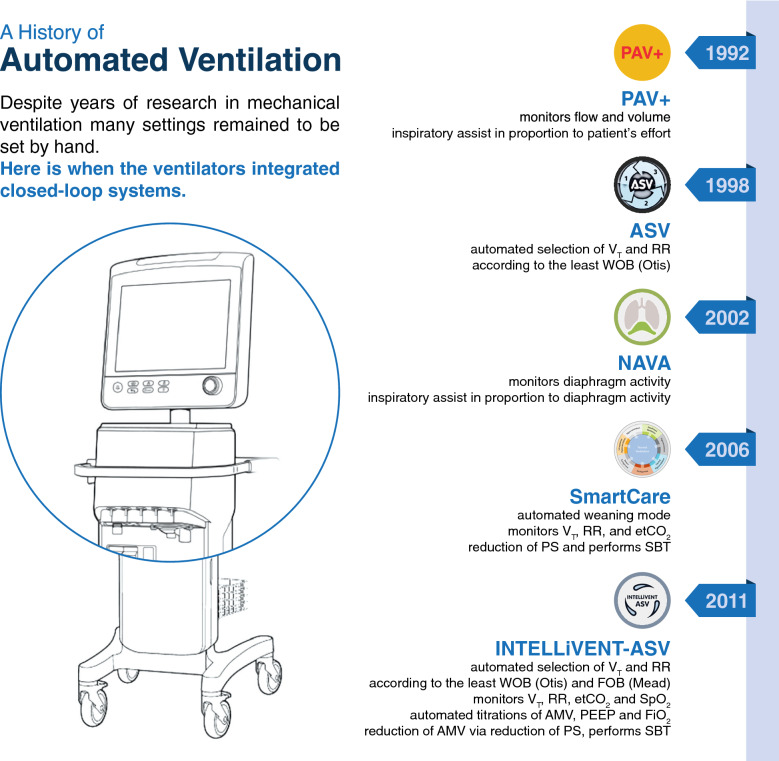
Although it is already considered normal in our daily lives for complex or routine tasks to be taken over by robots, we see this only sporadically happening within the walls of hospitals, including in ICUs. However, the question is not if, but when the complex task of lung protective ventilation will be automated [7]. Actually, so-called ‘closed-loop’ ventilation modes have already entered the critical care arena, and are increasingly used. Examples of automated ventilation modes are presented in Fig. 1. These modes are all based on closed-loop principles, wherein proportional assist ventilation (PAV) + and Neurally Adjusted Ventilatory Assist (NAVA) deliver proportional assist and measure patient efforts, and SmartCare, Adaptive Support Ventilation (ASV) and INTELLiVENT-ASV integrate algorithms to target ventilation and oxygenation goals in accordance to changes in lung mechanics (for further details, see Fig. 1).
Fig. 1.
A history of automated ventilation. Overview of currently available closed–loop ventilation modes, with brief explanations of how they work. The here described examples of automated ventilation modes are Proportional Assist Ventilation with load–adjustable gain factors (PAV +), available on Puritan Bennett ventilators (Puritan Bennett, Minneapolis, USA), SmartCare, available on Dräger ventilators (Dräger, Lübeck, Germany), Neurally Adjusted Ventilatory Assist ventilation (NAVA), available on Maquet ventilators (Getinge, Goteborg, Sweden), and Adaptive Support Ventilation (ASV) and its successor INTELLiVENT–ASV, available on Hamilton ventilators (Hamilton Medical AG, Bonaduz, Switzerland). Abbreviations: VT: tidal volume, RR: respiratory rate, etCO2: end-tidal carbon dioxide, PS: pressure support, SBT: spontaneous breathing trial, WOB: Work of Breathing, FOB: Force of Breathing, SpO2: pulse oximetry, FiO2: fraction of inspired oxygen
Evidence for benefit of using automated ventilation modes is steadily growing. Benefit are improved safety and effectiveness, and by that a better efficacy. INTELLiVENT-ASV has not only found to be safe, but also effective with regard to titration of VT and Pinsp, and indirectly ΔP and MP [8]. Compared to conventional ventilation, INTELLiVENT-ASV provides ventilation with fewer episodes of hypoxemia, and with lower ΔP and less MP [9–11]. PAV + has been found to decrease ΔP, by decreasing VT when the functional lung size becomes smaller, and by increasing VT only when the functional lung size increases [12]. SmartCare and PAV + have been found to decrease duration of weaning [13, 14], and to shorten duration of ventilation and stay in ICU [14], and NAVA may increase survival [14].
But benefits of automated ventilation should not only include safety, effectiveness and efficacy. Automation should also reduce workloads. We are uncertain how to measure this adequately. While use of INTELLiVENT-ASV is associated with a reduction in the number of interactions between caregivers and ventilators [15], this may not necessarily mean it reduces the workload. In addition, it may take time to implement automated ventilation, as it requires a change in the role of caregivers. Especially at first use it could be more time-consuming to ‘supervise an autopilot’ than ‘being the pilot’. Also, if alarm settings are set wrong, i.e., too tight, automated ventilation may actually increase the number of alarms, and thereby workloads. Last but not least, it takes time ‘trusting’ the new.
In conclusion, automated ventilation has a great potential to improve lung protective ventilation, and with that the outcome of critically ill patients. In the context of the growing shortages in ICU staffing, research should not only focus on safety, effectiveness and efficiency, but certainly also on workloads associated with (implementation of) automated ventilation.
Declarations
Conflicts of interest
LAB-K visited Hamilton Medical in 2021 to take part in an advisory board meeting and to give lectures. The expenses for lodging were covered, she had her travel expenses reimbursed and received an advisory- and speaker’s fee of € 1500. MJS attended a workshop organized by Hamilton in 2018. The expenses for lodging were covered for the invited experts, and participants from abroad had their travel expenses reimbursed. Additionally, speakers received a speaker’s fee of CHF 800. He is the Team Leader of Medical Affairs at Hamilton Medical AG, Switzerland, since 2022. ASN received personal speaker fees from Dräger.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.MacIntyre N, Rackley C, Khusid F. Fifty years of mechanical ventilation-1970s to 2020. Crit Care Med. 2021;49(4):558–574. doi: 10.1097/CCM.0000000000004894. [DOI] [PubMed] [Google Scholar]
- 2.Damiani E, Adrario E, Girardis M, Romano R, Pelaia P, Singer M, et al. Arterial hyperoxia and mortality in critically ill patients: a systematic review and meta-analysis. Crit Care. 2014;18(6):711. doi: 10.1186/s13054-014-0711-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372(8):747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 4.Urner M, Jüni P, Hansen B, Wettstein MS, Ferguson ND, Fan E. Time-varying intensity of mechanical ventilation and mortality in patients with acute respiratory failure: a registry-based, prospective cohort study. Lancet Respir Med. 2020;8(9):905–913. doi: 10.1016/S2213-2600(20)30325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angus DC, Kelley MA, Schmitz RJ, White A, Popovich J., Jr Caring for the critically ill patient. Current and projected workforce requirements for care of the critically ill and patients with pulmonary disease: can we meet the requirements of an aging population? JAMA. 2000;284(21):2762–2770. doi: 10.1001/jama.284.21.2762. [DOI] [PubMed] [Google Scholar]
- 6.British Medical Association . Medical staffing in England: a defining moment for doctors and patients. London: BMA House; 2021. [Google Scholar]
- 7.Mamdani M, Slutsky AS. Artificial intelligence in intensive care medicine. Intensive Care Med. 2021;47(2):147–149. doi: 10.1007/s00134-020-06203-2. [DOI] [PubMed] [Google Scholar]
- 8.Botta M, Wenstedt EFE, Tsonas AM, Buiteman-Kruizinga LA, van Meenen DMP, Korsten HHM, et al. Effectiveness, safety and efficacy of INTELLiVENT-adaptive support ventilation, a closed-loop ventilation mode for use in ICU patients—a systematic review. Expert Rev Respir Med. 2021;15(11):1403–1413. doi: 10.1080/17476348.2021.1933450. [DOI] [PubMed] [Google Scholar]
- 9.De Bie AJR, Neto AS, van Meenen DM, Bouwman AR, Roos AN, Lameijer JR, et al. Fully automated postoperative ventilation in cardiac surgery patients: a randomised clinical trial. Br J Anaesth. 2020;125(5):739–749. doi: 10.1016/j.bja.2020.06.037. [DOI] [PubMed] [Google Scholar]
- 10.Buiteman-Kruizinga LA, Mkadmi HE, Schultz MJ, Tangkau PL, van der Heiden PLJ. Comparison of mechanical power during adaptive support ventilation versus nonautomated pressure-controlled ventilation-a pilot study. Crit Care Explor. 2021;3(2):e0335. doi: 10.1097/CCE.0000000000000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buiteman-Kruizinga LA, Mkadmi HE, Serpa Neto A, Kruizinga MD, Botta M, Schultz MJ, et al. Effect of INTELLiVENT-ASV versus conventional ventilation on ventilation intensity in patients with COVID-19 ARDS-An observational study. J Clin Med. 2021;10(22):5409. doi: 10.3390/jcm10225409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Georgopoulos D, Xirouchaki N, Tzanakis N, Younes M. Data on respiratory variables in critically ill patients with acute respiratory failure placed on proportional assist ventilation with load adjustable gain factors (PAV+) Data Brief. 2016;8:484–493. doi: 10.1016/j.dib.2016.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burns KE, Lellouche F, Nisenbaum R, Lessard MR, Friedrich JO. Automated weaning and SBT systems versus non-automated weaning strategies for weaning time in invasively ventilated critically ill adults. Cochrane Database Syst Rev. 2014;2014(9):Cd008638. doi: 10.1002/14651858.CD008638.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kampolis CF, Mermiri M, Mavrovounis G, Koutsoukou A, Loukeri AA, Pantazopoulos I. Comparison of advanced closed-loop ventilation modes with pressure support ventilation for weaning from mechanical ventilation in adults: a systematic review and meta-analysis. J Crit Care. 2021;68:1–9. doi: 10.1016/j.jcrc.2021.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Bialais E, Wittebole X, Vignaux L, Roeseler J, Wysocki M, Meyer J, et al. Closed-loop ventilation mode (IntelliVent®-ASV) in intensive care unit: a randomized trial. Minerva Anestesiol. 2016;82(6):657–668. [PubMed] [Google Scholar]