Abstract
Objective
Recent research has documented psychological distress in advanced breast cancer (ABC) patients, but few studies have examined how death anxiety is affected by the symptom burden. Therefore, this study aims to explore the association among symptom burden, death anxiety and psychological distress (depression and anxiety) in ABC patients.
Methods
This cross-sectional study used the Death and Dying Anxiety Scale (DADDS), 9-item Patient Health Questionnaire (PHQ-9), General Anxiety Disorder-7 (GAD-7) and MD Anderson Symptom Inventory (MDASI) to assess death anxiety, depression, anxiety, and symptom burden, respectively. Bias-corrected bootstrapping methods were used to estimate indirect effects and 95% confidence intervals.
Results
Two hundred ABC patients completed the questionnaires. All of the respondents were females, with a mean age of 50±10 years. Initial correlation analyses revealed significant associations of death anxiety with depression (r=0.57, P<0.001), anxiety (r=0.60, P<0.001) and symptom burden (r=0.43, P<0.001). Moreover, depression (r=0.53, P<0.001) and anxiety (r=0.45, P<0.001) were significantly correlated with symptom burden. An analysis using Hayes’ PROCESS macro revealed the partial effecting role of death anxiety in the relationship between depression and symptom burden, and between anxiety and symptom burden (contributions to the total effect of 0.247 and 0.469, respectively).
Conclusions
This study provides insight into the relationship between death anxiety and symptom burden. The results suggest that interventions addressing death anxiety may be more effective for alleviating the depression and anxiety experienced by ABC patients with a symptom burden.
Keywords: Advanced breast cancer, death anxiety, psychological distress, symptom burden, depression, anxiety
Introduction
In China, breast cancer is the most common cancer and the third leading cause of cancer-related deaths in women (1). The incidence and associated mortality of breast cancer have been continuously increasing over the past several years. The median age at diagnosis of breast cancer in Chinese women is approximately 50 years, which is around 10 years younger than that of European and American patients (2). Nearly one-third of patients with early-stage breast cancer will develop advanced breast cancer (ABC) (3), as such, China has a large ABC patient population.
Some side effects inevitably increase in prevalence in parallel with longer survival of ABC patients (4). The burden of disease and side effects are both more severe in ABC than early-stage breast cancer patients. Although many ABC survivors are psychologically well-adjusted, residual physical and psychological symptoms are nonetheless common. Physical symptoms include pain, fatigue and insomnia, among others (5). Regarding psychological symptoms, approximately 84% of ABCs were found to have clinical anxiety, and 25% had clinical depression (6,7). Psychological conditions, such as depression and anxiety, increase the physical symptom burden (8) of breast cancer patients, and vice versa (9). However, few studies have examined the underlying mechanism in detail.
The most fundamental type of anxiety is death anxiety (10). ABC is a major trauma for many females, and typically causes psychological and social issues, including anxiety and, in particular, death anxiety (i.e., the perceived threat of death in everyday life). The North American Nursing Diagnosis Association International (NANDA-I) defines death anxiety as a “vague uneasy feeling of discomfort or dread generated by perceptions of a real or imagined threat to one’s existence” (11). According to previous studies (12,13), the presence of depression and anxiety predicts death anxiety; moreover, death anxiety is associated with physical suffering. However, few studies have assessed the impact of death anxiety on the relationship between symptom burden and psychological distress. Considering the high prevalence of death anxiety, and its correlations with physical and psychological symptoms, we studied psychological conditions, such as depression and anxiety, in ABC patients, and examined how death anxiety is affected by symptom burden. We hypothesized that death anxiety affects the relationship between symptom burden and psychological distress.
Materials and methods
Participants and procedures
A convenience sample was recruited from April 2019 to December 2019 at Peking University Cancer Hospital. The eligibility criteria were as follows: 1) Adult (aged ≥18 years) female patients; 2) diagnosed with ABC; 3) able to read and speak Chinese fluently; and 4) no cognitive impairment according to medical records and attending oncologist. A brief interview was held to assess patients according to the following exclusion criteria: 1) Major communication difficulties; 2) inability to commit to the investigation (i.e., too ill to participate); or 3) refusal to participate.
All participants in this cross-sectional study were also enrolled in the single-center Managing Cancer and Living Meaningfully (CALM) clinical trial, which examined the efficacy of a brief psychotherapy intervention for Chinese ABC patients (14). The study was approved by the Institutional Review Board of Peking University Cancer Hospital on April 15, 2019 (2019YJZ23).
The participants were fully informed about the purpose and process of the study before signing the consent form. The questionnaires were anonymized based on the assignment of numbers. A researcher collected the questionnaires once they had been completed, and conducted an audit to ensure that there were no missing data.
Measures
Demographic and clinical characteristics
The participants completed a demographic survey concerned with age, marital status, educational level, employment status, religion, whether they had children, residential area (urban or rural), economic status and experiences associated with death that could induce death anxiety (e.g. death-related events) (15,16). Clinical data, including the date of breast cancer diagnosis, metastasis site and type(s) of treatment, were collected via medical record review.
Symptom burden
Symptom burden was assessed with the Chinese version of the MD Anderson Symptom Inventory (MDASI-C) (17). The MDASI (18) was introduced in 2000 to assess symptom burden and interference with daily life in cancer patients. It comprises 19 items, including 13 pertaining to core symptom severity (pain, fatigue, nausea, sleep disturbance, distress, shortness of breath, difficulty remembering things, lack of appetite, drowsiness, dry mouth, sadness, vomiting, and numbness) and 6 assessing symptom interference (general activity, mood, work, relationships with other people, walking, and enjoyment of life). The MDASI-C uses an 11-point response scale (range: 0−10), where higher scores indicate a more severe symptom burden or interference. The internal consistency (reliability) of the MDASI-C is high (range: 0.83−0.90). A score of 5−6 is taken to indicate moderate symptoms, while scores of ≥7 indicate severe symptoms (17).
Death anxiety
The Death and Dying Anxiety Scale (DADDS) (19) was used to measure death anxiety. The DADDS is suitable for assessing patients with advanced or metastatic cancer, and is specifically used to evaluate the death anxiety of cancer patients. The DADDS quantifies regret regarding lost time and opportunities, fear of the death process, and worry about the impact of death on relatives. It comprises 15 items, each rated on a scale ranging from 0 to 5; thus, total scores range from 0 to 75. Higher scores indicate more severe death anxiety. In our study, the Cronbach’s α was 0.927.
Depression
Depression was assessed with the 9-item Patient Health Questionnaire (PHQ-9) (20). The PHQ-9 is a self-report depression screening scale used worldwide to assess advanced cancer patients (21). Depression is rated based on nine Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition (DSM-IV) criteria; the answer options range from 0 (not at all) to 3 (nearly every day), and total scores range between 0 and 27. The Chinese version of the PHQ-9 (22) has good reliability and validity, with a score of 10 being the optimal cutoff for diagnosing depression. In our study, the Cronbach’s α was 0.812.
Anxiety
Anxiety was assessed with the General Anxiety Disorder-7 (GAD-7) (23). The GAD-7 is a 7-item patient self-report scale based on the diagnostic criteria of the DSM-IV. Each item is scored from 0 (not at all) to 3 (nearly every day), and total scores thus range between 0 and 21. The Chinese version of the GAD-7 (24) has good reliability and validity, with a score of 20 being the optimal cutoff for diagnosing anxiety. In our study, the Cronbach’s α was 0.904.
Statistical analysis
Because of the exploratory nature of the study, no formal sample size calculation was performed. To characterize the sample, descriptive statistics were generated (median and interquartile range for continuous variables and absolute numbers and percentages for categorical variables). Furthermore, the Mann-Whitney U test was used to analyze depression, anxiety, death anxiety, and symptom burden according to patient characteristics. And the multivariable analysis on the anxiety and depression was examined by logistic regression analysis. The Pearson correlation coefficient and Spearman’s rho were utilized to examine the relationships of demographic, clinical and psychological characteristics with death anxiety, and depressive and anxiety symptoms. To enhance clinical interpretability, we used cutoff points to distinguish between patients with moderate-to-severe and those with severe symptoms. Next, analyses were performed to determine whether death anxiety affects the relationship between symptom burden and psychological distress. The effecting role of death anxiety between symptom burden and depression/anxiety was evaluated by Hayes’ PROCESS. PROCESS (25) estimates the indirect effect and bias-corrected confidence intervals (CIs) using bootstrapping. Death anxiety as the possible effector was used in two analyses, one with depression as the dependent variable (DV), and one with anxiety as DV. The model 4 of PROCESS was used with 5,000 bootstrapping samples for all analyses. Statistical analysis was carried out using IBM SPSS statistical (Version 20.0; IBM Corp., New York, USA) and AMOS software (Version 20.0; IBM Corp., New York, USA). P<0.05 were taken to indicate statistical significance.
Results
Patient characteristics
A total of 1,653 patients were screened between April 2019 and December 2019. Of the 347 eligible patients approached, 253 provided informed consent; 42 of those patients refused to complete the study questionnaire because of the nature of the topic (i.e., death), and 11 were unable to complete the questionnaire because of poor physical status. Thus, 200 patients were included in the final analysis (response rate =79.1%).
There were no differences in sociodemographic or clinical characteristics between respondents and nonrespondents. The mean age of the 200 participants was 50 years. There were 27 (13.5%) participants with moderate-to-severe death anxiety (DADDS score ≥45), 48 (24.0%) with depression (PHQ-9 score ≥10), and 31 (15.5%) with anxiety (GAD-7 score ≥10). The other characteristics are shown in Table 1 .
Table 1. Patients’ characteristics (N=200).
| Characteristics | n (%) |
| IQR, interquartile range; CNY, Chinese Yuan; DADDS, Death and Dying Anxiety Scale; MDASI, MD Anderson Symptom Inventory; PHQ-9, 9-item Patient Health Questionnaire; GAD-7, General Anxiety Disorder-7. | |
| Age (year) | |
|
50±10 |
| IQR | 43−57 |
| Marital status | |
| Married | 182 (91.0) |
| Not married | 18 (9.0) |
| Education | |
| High school and below | 124 (62.0) |
| College school and above | 76 (38.0) |
| Working state | |
| Employed | 119 (59.5) |
| Unemployed or retired | 81 (40.5) |
| Religion | |
| Yes | 41 (20.5) |
| No | 159 (79.5) |
| Having children | |
| Yes | 188 (94.0) |
| No | 12 (6.0) |
| Residential location | |
| Urban area | 159 (79.5) |
| Rural area | 41 (20.5) |
| Income per month (CNY) | |
| <7,000 | 159 (79.5) |
| ≥7,000 | 41 (20.5) |
| Financial strain | |
| Yes | 144 (72.0) |
| No | 56 (28.0) |
| Separation and death in 5 years | |
| Yes | 80 (40.0) |
| No | 120 (60.0) |
| Life-threatening event in 5 years | |
| Yes | 17 (8.5) |
| No | 183 (91.5) |
| Metastasis | |
| Organs | 167 (83.5) |
| Lymph nodes or nearby tissues | 33 (16.5) |
| Surgery | |
| Yes | 164 (82.0) |
| No | 36 (18.0) |
| Radiotherapy | |
| Yes | 108 (54.0) |
| No | 92 (46.0) |
| Chemotherapy | |
| Yes | 192 (96.0) |
| No | 8 (4.0) |
| Endocrinotherapy | |
| Yes | 133 (66.5) |
| No | 67 (33.5) |
| Comorbidity | |
| Yes | 40 (20.0) |
| No | 160 (80.0) |
| Illness duration in months | |
|
75.7±56.8 |
| IQR | 35−104 |
| Death anxiety (DADDS) | |
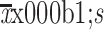
|
20.6±17.5 |
| IQR | 7.0−30.5 |
| None to little (<15) | 94 (47.0) |
| Little to mild (15−29) | 54 (27.0) |
| Mild to moderate (30−44) | 25 (12.5) |
| Moderate to great (45−59) | 21 (10.5) |
| Great to extreme (60−75) | 6 (3.0) |
| Symptom burden (MDASI) | |
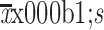
|
1.3±1.8 |
| IQR | 0−2 |
| Depression (PHQ-9) | |
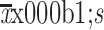
|
4.6±4.9 |
| IQR | 1−6 |
| Anxiety (GAD-7) | |
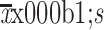
|
6.7±5.1 |
| IQR | 3−9 |
Tested variables according to patient characteristics
The results of the Mann-Whitney U test used to analyze depression, anxiety, death anxiety and symptom burden according to patient characteristics are shown in Table 2 . Patients with religious beliefs (Z=−2.04, P=0.042), and those who had a financial burden (Z=−2.16, P=0.031) or had undergone radiotherapy (Z=−3.37, P<0.001), had higher depression scores.
Table 2. Difference in depression, anxiety, death anxiety and symptom burden between patient characteristics (N=200).
| Characteristics | Depression | Anxiety | Death anxiety | Symptom burden | |||||||
| Median (IQR) | Z | Median (IQR) | Z | Median (IQR) | Z | Median (IQR) | Z | ||||
| CNY, Chinese Yuan; IQR, interquartile range; *, P<0.05; **, P<0.01; ***, P<0.001. | |||||||||||
| Marital status | 1.11 | −0.68 | −0.86 | −0.18 | |||||||
| Married | 6.0 (3.0−9.0) | 3.0 (1.0−6.8) | 16.0 (7.0−31.8) | 1.0 (0−2.0) | |||||||
| Not married | 4.5 (3.0−8.8) | 2.0 (1.0−5.8) | 13.0 (7.5−22.0) | 1.0 (0−1.0) | |||||||
| Education | 1.33 | −2.43* | 1.14 | −0.71 | |||||||
| High school and below | 6.0 (3.0−9.0) | 2.0 (1.0−6.0) | 14.5 (7.0−29.3) | 1.0 (0−2.0) | |||||||
| College school and above | 6.0 (3.8−9.3) | 4.0 (2.0−7.0) | 16.5 (8.8−32.3) | 2.0 (0−2.0) | |||||||
| Working state | −0.95 | −1.96* | −2.33* | −0.33 | |||||||
| Employed | 6.0 (3.0−10.0) | 3.0 (1.0−8.0) | 18.0 (8.5−35.0) | 1.0 (0−2.0) | |||||||
| Unemployed or retired | 6.0 (3.0−8.0) | 2.0 (1.0−6.0) | 12.0 (6.0−21.0) | 2.0 (0−2.0) | |||||||
| Religion | −2.04* | 1.26 | 1.43 | −0.80 | |||||||
| Yes | 8.0 (4.0−11.0) | 4.0 (2.0−7.0) | 17.0 (9.0−38.0) | 2.0 (0−2.0) | |||||||
| No | 6.0 (3.0−9.0) | 3.0 (1.0−6.0) | 15.0 (7.0−29.0) | 1.0 (0−2.0) | |||||||
| Having children | −0.75 | −1.25 | 1.91 | 1.04 | |||||||
| Yes | 6.0 (3.0−9.0) | 3.0 (1.0−6.0) | 15.0 (7.0−30.0) | 1.0 (0−2.0) | |||||||
| No | 7.5 (1.5−16.0) | 4.5 (1.3−12.3) | 20.0 (10.5−51.8) | 1.5 (0−4.0) | |||||||
| Residential location | −0.21 | −0.21 | −0.32 | −0.47 | |||||||
| Urban area | 6.0 (3.0−9.0) | 3.0 (1.0−6.0) | 16.0 (7.0−31.0) | 1.0 (0−2.0) | |||||||
| Rural area | 6.0 (3.0−10.0) | 2.0 (1.0−6.5) | 14.0 (7.0−26.5) | 1.0 (0−2.0) | |||||||
| Income per month (CNY) | −0.65 | −1.99* | 1.59 | −0.05 | |||||||
| <7,000 | 6.0 (3.0−9.0) | 2.0 (1.0−6.0) | 15.0 (7.0−27.0) | 1.0 (0−2.0) | |||||||
| ≥7,000 | 6.0 (3.5−10.0) | 5.0 (2.0−9.5) | 23.0 (8.0−37.0) | 2.0 (0−2.8) | |||||||
| Financial strain | −2.16* | −1.87 | 1.07 | −2.28* | |||||||
| Yes | 6.0 (3.0−10.0) | 3.0 (1.0−7.0) | 16.0 (8.0−32.0) | 2.0 (0−2.0) | |||||||
| No | 5.0 (2.0−7.8) | 2.0 (1.0−5.0) | 14.5 (3.3−29.0) | 1.0 (0−1.0) | |||||||
| Separation and death in 5 years | −0.23 | −0.92 | −0.50 | −1.34 | |||||||
| Yes | 6.0 (3.0−10.0) | 3.0 (1.0−7.0) | 16.0 (7.0−31.5) | 1.0 (0−2.0) | |||||||
| No | 6.0 (3.0−9.0) | 3.0 (1.0−6.0) | 15.0 (7.0−30.8) | 0 (0−2.0) | |||||||
| Life-threatening events in 5 years | 1.18 | −2.09* | −3.15** | −2.02* | |||||||
| Yes | 6.0 (5.0−13.5) | 6.0 (2.0−11.0) | 39.0 (16.5−54.5) | 1.0 (0−5.0) | |||||||
| No | 6.0 (3.0−9.0) | 3.0 (1.0−6.0) | 15.0 (7.0−29.0) | 0.5 (0−2.0) | |||||||
| Metastasis | −0.03 | −1.26 | 1.56 | −0.13 | |||||||
| Organs | 6.0 (3.0−9.0) | 3.0 (1.0−6.0) | 15.0 (7.0−29.0) | 1.0 (0−2.0) | |||||||
| Lymph nodes or nearby tissues | 6.0 (3.0−9.5) | 3.0 (1.0−10.0) | 21.0 (7.5−42.5) | 0 (0−2.0) | |||||||
| Surgery | 1.24 | −2.25* | −0.33 | −0.61 | |||||||
| Yes | 6.0 (3.0−10.0) | 3.0 (1.0−7.0) | 15.5 (7.0−30.0) | 1.0 (0−2.0) | |||||||
| No | 5.5 (1.3−8.0) | 2.0 (0−5.0) | 16.0 (5.5−41.8) | 0 (0−2.0) | |||||||
| Radiotherapy | −3.37*** | −1.98* | 1.22 | −3.05** | |||||||
| Yes | 6.5 (4.0−11.0) | 3.0 (1.0−7.0) | 17.0 (8.0−30.8) | 1.0 (0−2.0) | |||||||
| No | 4.5 (2.0−8.0) | 2.0 (1.0−6.0) | 14.5 (6.3−32.8) | 0 (0−1.0) | |||||||
| Chemotherapy | −0.94 | −0.91 | −0.40 | −0.31 | |||||||
| Yes | 6.0 (3.0−9.0) | 3.0 (1.0−6.8) | 15.0 (7.0−31.0) | 1.0 (0−2.0) | |||||||
| No | 5.5 (1.3−7.8) | 2.0 (0.3−5.0) | 19.0 (2.5−27.5) | 1.0 (0−1.0) | |||||||
| Endocrinotherapy | −0.49 | −0.14 | −0.51 | −0.13 | |||||||
| Yes | 6.0 (3.0−9.0) | 3.0 (1.0−6.0) | 16.0 (7.0−29.5) | 0.5 (0−2.0) | |||||||
| No | 6.0 (3.0−10.0) | 3.0 (1.0−7.0) | 15.0 (8.0−36.0) | 1.0 (0−2.0) | |||||||
| Comorbidity | −0.80 | −2.04* | −2.39* | −0.60 | |||||||
| Yes | 6.0 (2.0−9.0) | 2.0 (0.3−4.0) | 10.5 (5.3−22.5) | 2.0 (0−1.8) | |||||||
| No | 6.0 (3.0−9.0) | 3.0 (1.0−7.0) | 17.0 (8.0−33.5) | 1.0 (0−2.0) | |||||||
Anxiety scores were higher in patients with a college education or above (Z=−2.43, P=0.015), as well as in those who were still working (Z=−1.96, P=0.050), had a relatively high income (Z=−1.99, P=0.047), experienced life-threatening events within the last 5 years (Z=−2.09, P=0.037), had undergone surgery (Z=−2.25, P=0.025) or radiotherapy (Z=−1.98, P=0.048), or had comorbidities (Z=−2.04, P=0.041).
Scores on the death anxiety scale were higher in patients who were still working (Z=−2.33, P=0.020), had experienced life-threatening events within the last 5 years (Z=−3.15, P=0.002), or had comorbidities (Z=−2.39, P=0.017). Patients with a financial burden (Z=−2.28, P=0.023), as well as those who experienced life-threatening events within the last 5 years (Z=−2.02, P=0.044) or had undergone radiotherapy (Z=−3.05, P=0.002), experienced a greater symptom burden. The multivariable analysis shows that depression and anxiety are affected by patients’ employment, religion and financial strain (Supplementary Table S1 ).
Table S1. Multivariable analysis on anxiety and depression.
| Variables | Depression | Anxiety | |||
| OR | 95% CI | OR | 95% CI | ||
| CNY, Chinese Yuan; OR, odds ratio; 95% CI, 95% confidence interval; *, P<0.05. | |||||
| College school and above | 0.834 | 0.37, 1.88 | 1.196 | 0.47, 3.05 | |
| Employed | 1.444 | 0.69, 3.02 | 2.795* | 1.06, 7.40 | |
| Religion | 2.331* | 1.04, 5.23 | 1.088 | 0.40, 3.00 | |
| Income ≥7,000 CNY | 2.268 | 0.84, 6.14 | 2.421 | 0.83, 7.06 | |
| Financial strain | 3.125* | 1.19, 8.19 | 2.856 | 0.94, 8.66 | |
| Life-threatening events in 5 years | 0.897 | 0.26, 3.07 | 1.161 | 0.31, 4.37 | |
| Surgery | 1.695 | 0.56, 5.15 | 1.100 | 0.35, 3.49 | |
| Radiotherapy | 2.118 | 0.99, 4.52 | 1.466 | 0.61, 3.53 | |
| Comorbidity | 0.941 | 0.38, 2.33 | 0.678 | 0.21, 2.24 | |
Symptom burden and interference
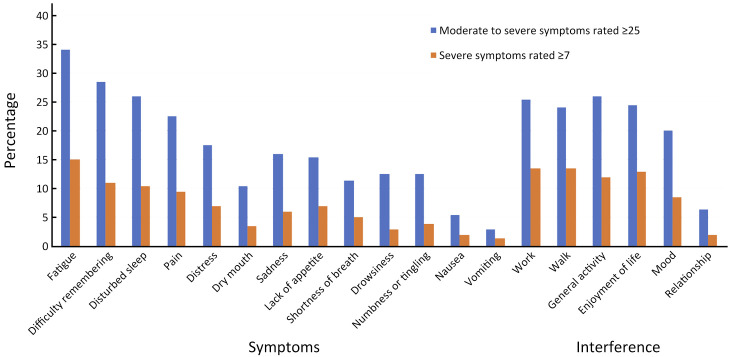
Table 3 (Figure 1 ) presents the median and interquartile range for symptom data, in decreasing order of severity. All symptoms except nausea and vomiting were reported to be moderate to severe by at least 10.0% of the patients. The seven most severe MDASI symptoms, in decreasing order of magnitude, were fatigue, difficulty remembering, disturbed sleep, pain, distress, dry mouth and sadness. More than 26.0% of the patients reported moderate-to-severe fatigue, difficulty remembering or disturbed sleep (score of ≥5 on the 0−10-point scale). Severe fatigue, difficulty remembering and disturbed sleep (score of ≥7) were reported by more than 10.5% of patients. Pain, distress and lack of appetite were reported to be severe by at least 7.0% of the patients.
Table 3. Descriptive statistics for symptoms and interference (N=200).
| Variables | Scores [median (IQR)] | n (%) | |
| Moderate to severe symptoms (rated ≥5) | Severe symptoms (rated ≥7) | ||
| IQR, interquartile range; *, higher scores indicate greater symptom severity (0, symptom not present; 10, symptom as bad as you can imagine); #, higher scores indicate greater symptom interference (0, does not interfere; 10, completely interferes). | |||
| Symptom* | |||
| Fatigue | 3 (1−5) | 68 (34.0) | 30 (15.0) |
| Difficulty remembering | 2 (0−5) | 57 (28.5) | 22 (11.0) |
| Disturbed sleep | 2 (0−5) | 52 (26.0) | 21 (10.5) |
| Pain | 1 (0−4) | 45 (22.5) | 19 (9.5) |
| Distress | 1 (0−3) | 35 (17.5) | 14 (7.0) |
| Dry mouth | 1 (0−3) | 21 (10.5) | 7 (3.5) |
| Sadness | 0 (0−3) | 32 (16.0) | 12 (6.0) |
| Lack of appetite | 0 (0−3) | 31 (15.5) | 14 (7.0) |
| Shortness of breath | 0 (0−2) | 23 (11.5) | 10 (5.0) |
| Drowsiness | 0 (0−3) | 25 (12.5) | 6 (3.0) |
| Numbness or tingling | 0 (0−2) | 25 (12.5) | 8 (4.0) |
| Nausea | 0 (0−1) | 11 (5.5) | 4 (2.0) |
| Vomiting | 0 (0−0) | 6 (3.0) | 3 (1.5) |
| Interference# | |||
| Work | 1 (0−5) | 51 (25.5) | 27 (13.5) |
| Walk | 1 (0−4) | 48 (24.0) | 27 (13.5) |
| General activity | 1 (0−5) | 52 (26.0) | 24 (12.0) |
| Enjoyment of life | 1 (0−4) | 49 (24.5) | 26 (13.0) |
| Mood | 1 (0−3) | 40 (20.0) | 17 (8.5) |
| Relationship | 0 (0−0) | 13 (6.5) | 4 (2.0) |
Figure 1.
Descriptive statistics for symptoms and interference.
Effecting role of death anxiety in relationship between psychological distress and symptom burden
Covariates were identified based on the results of the Mann-Whitney U test of patient characteristics. As Table 4 indicates, after controlling for employment status, religion, financial strain, life-threatening events, radiotherapy and comorbidities, the results of partial correlation analyses showed significant associations of psychological distress and death anxiety with symptom burden. Moreover, death anxiety was significantly correlated with depression (r=0.57, P<0.001), anxiety (r=0.60, P<0.001) and symptom burden (r=0.43, P<0.001).
Table 4. Partial correlations among study variables (N=200).
| Variables | r | |
| Depression | Anxiety | |
| ***, P<0.001. | ||
| Depression | − | |
| Anxiety | 0.70*** | − |
| Death anxiety | 0.57*** | 0.60*** |
| Symptom burden | 0.53*** | 0.45*** |
Depression (r=0.53, P<0.001) and anxiety (r=0.45, P<0.001) were both correlated with symptom burden. An indirect effect of depression on symptom burden (via death anxiety) was demonstrated by analysis using Hayes’ PROCESS macro, thus supporting the hypothesis that death anxiety affects the relationship between symptom burden and psychological distress.
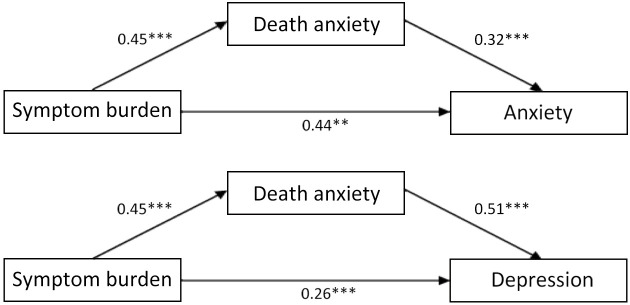
The results for specific parameters are shown in Table 5 and Figure 2 . The bootstrapping method was used; the results of indirect effect show that death anxiety significantly affected the relationship between symptom burden and anxiety (95% CI=0.08−0.23, P<0.001), and depression (95% CI=0.15−0.35, P<0.001), respectively; all the results do not include zero. Therefore, death anxiety can be considered to a moderator between anxiety/depression and symptom burden; and the contribution of the effect to the total effect in these two cases was 0.144/0.582=0.2474 (24.74%) and 0.230/0.490=0.4694 (46.94%), respectively.
Table 5. Effecting results of symptom burden on death anxiety and psychological distress.
| Path | a | b | c’ | ab | 95% CI | P |
| SB, symptom burden; 95% CI, 95% confidence interval; a, pathway between symptom burden and death anxiety; b, pathway between death anxiety and psychological distress; c’, direct effect pathway between symptom burden and psychological distress; ab, indirect effect pathway between symptom burden and psychological distress. | ||||||
| SB → Depression | 0.45 | 0.51 | 0.26 | 0.23 | 0.15−0.35 | <0.001 |
| SB → Anxiety | 0.45 | 0.32 | 0.44 | 0.14 | 0.08−0.23 | <0.001 |
Figure 2.
Models depicting the effect of symptom burden on psychological distress through death anxiety. **, P<0.01; ***, P<0.001.
Discussion
To our knowledge, the current study is the first to examine the associations of physical symptom burden with death anxiety and psychological distress among a sample of Mainland Chinese ABC patients. This study supported the relationships of physical symptoms with depression and anxiety reported in many previous studies. According to our results, a high symptom burden is associated with various physical and psychosocial problems in Chinese breast cancer patients (26). Western studies also showed that most of the physical problems experienced by breast cancer patients were associated with anxiety and, in particular, depression (8). In addition, Grotmol et al. found that breast cancer patients with a relatively poor prognosis and depression reported a significantly higher symptom burden (27).
More than half of the ABC patients in our study experienced at least moderate fatigue, as well as difficulty remembering and disturbed sleep. These results are largely consistent with those of a previous study reporting the most common severe symptoms in advanced cancer patients in China (17); these symptoms are treatment targets in early-stage breast cancer patients (28). Symptom management is an essential component of treatment and palliative care for advanced cancer patients. A previous study showed that systematic symptom monitoring can significantly prolong the survival of cancer patients (by 5.2 months) (29). Assessment of fatigue (30), cognitive functions (31) and sleep problems (32) seems to be adequate. These studies show the importance of a scientific approach to symptom management, which can achieve excellent results.
In the current study, the ABC patients’ physical symptoms were related to psychological problems such as depression and anxiety. Patients with a larger symptom burden also tended to have a higher risk of depression and/or anxiety. This confirms that breast and general cancer patients with symptoms such as pain (33,34), fatigue (35-37) and/or insomnia (38) are more likely to experience depression and/or anxiety.
There are also studies reporting that breast and general cancer patients experiencing more severe psychological distress in turn suffer more with physical symptoms (39-42). Moreover, depression and anxiety are associated with cancer-specific mortality (43). Taken together, the data show that cancer patients’ physical symptoms are highly related to their mental status. Thus, clinical interventions targeting not just physical symptoms, but also psychological ones, would be more effective for breast cancer patients; addressing depression and/or anxiety is essential to aid the diagnosis and treatment of breast cancer patients.
We also found that symptom burden had a significant and positive relationship with death anxiety. The effect of symptom burden on death anxiety has been documented in the literature; for example, pain is a well-recognized risk factor for death anxiety (13,44,45). We speculate that death anxiety and symptom burden may both worsen without effective treatment. However, interventions aiming to alleviate death anxiety have not been widely used in the treatment of cancer patients in China, because of a lack of awareness and inequalities in medical resource allocation (46). It is important to recognize the need for both symptom and death anxiety management. Interventions targeting both physical and psychological symptoms to alleviate death anxiety may lead to better outcomes. One study showed that the Dying Well Education Program decreased the fear of death in Korean women with breast cancer (47). A well-designed death education program can help individuals prepare for their death, enjoy the present moment, and live a meaningful life.
The findings of this study need to be considered in the context of certain limitations. First, this cross-sectional study had a relatively small sample size and thus is not representative of the entire ABC patient population. Second, the lack of a control group and long-term follow-up data limit our ability to draw conclusions regarding the impact of death anxiety. Ideally, early-stage patients should have been used as a control group. A previous study (48) showed that the death anxiety of ABC patients is significantly higher than that of early-stage patients, suggesting that death anxiety could impact the relationship between psychological distress and physical burden in different disease stages. Furthermore, patients who are economically advantaged may have been overrepresented in this study, so the findings need to be replicated in less affluent, i.e., rural, areas. Finally, patient-reported outcomes (PROs) are considered a potential source of bias; although PROs highlight the emotional experience of individual patients, the application of psychiatric diagnostic criteria by a practicing psychiatrist is also important.
This study revealed a moderating role of death anxiety in the association between the somatization of psychological symptoms and symptom burden among ABC patients, which could inform future prevention and treatment measures aiming to alleviate physical symptoms. Death anxiety is a sensitive topic; such that most patients, relatives and clinical workers prefer to avoid discussing it, and even related topics. However, addressing death anxiety may be important for reducing general anxiety and depression in patients with a symptom burden. ABC patients, oncologists and psycho-oncologists in China should thus devote more attention to death anxiety. Death anxiety should be considered in the treatment plan, alongside depression, anxiety and physical symptoms.
Conclusions
This study showed that death anxiety affected the relationships of depression and anxiety with physical symptoms in ABC patients. Thus, special attention should be paid to the measurement and treatment of death anxiety. Although the current study exposes a partial relationship between death anxiety, symptom burden and psychological distress among cancer patients, the real-world clinical situation could be much more complicated among cancer patients’ psychological distress and symptom burden. For example death anxiety among cancer patients could be caused by more than one single variable (symptom burden); symptom burden and death anxiety could impact on each other rather than one-way influences. Therefore, more qualitative and quantality clinical data are needed in the future to help us understand more detailed mechanisms among these variables, and how they impacted each other. A case-control study is needed to further explore the impact of death anxiety on the relationship between psychological distress and physical symptom burden in various cancer stages.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Acknowledgements
We would like to express our thanks to Dr. Yan Wei (Peking University Cancer Hospital) for project assistance during the whole survey. The research was funded by the Beijing Municipal Health and Scientific and Technological Achievements and Appropriate Technology Promotion Projects in China (No. 2018-TG-48).
References
- 1.Sun D, Cao M, Li H, et al Cancer burden and trends in China: A review and comparison with Japan and South Korea. Chin J Cancer Res. 2020;32:129–39. doi: 10.21147/j.issn.1000-9604.2020.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song QK, Li J, Huang R, et al Age of diagnosis of breast cancer in China: Almost 10 years earlier than in the United States and the European Union. Asian Pac J Cancer Pre. 2014;15:10021–5. doi: 10.7314/apjcp.2014.15.22.10021. [DOI] [PubMed] [Google Scholar]
- 3.Berman AT, Thukral AD, Hwang WT, et al Incidence and patterns of distant metastases for patients with early-stage breast cancer after breast conservation treatment. Clin Breast Cancer. 2013;13:88–94. doi: 10.1016/j.clbc.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Caswell-Jin JL, Plevritis SK, Tian L, et al Change in survival in metastatic breast cancer with treatment advances: Meta-analysis and systematic review. JNCI Cancer Spectr. 2018;2:pky062. doi: 10.1093/jncics/pky062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rinnerthaler G, Gampenrieder SP, Greil R ASCO 2018 highlights: metastatic breast cancer. Memo. 2018;11:276–9. doi: 10.1007/s12254-018-0450-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valderrama Rios MC, Sánchez Pedraza R Anxiety and depression disorders in relation to the quality of life of breast cancer patients with locally advanced or disseminated stage. Rev Colomb Psiquiatr (Engl Ed) 2018;47:211–20. doi: 10.1016/j.rcp.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Yang M, Ma F, Lan B, et al Validity of distress thermometer for screening of anxiety and depression in family caregivers of Chinese breast cancer patients receiving postoperative chemotherapy. Chin J Cancer Res. 2020;32:476–84. doi: 10.21147/j.issn.1000-9604.2020.04.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He Y, Gao C, Pang Y, et al Psychosomatic symptoms affect radiotherapy setup errors in early breast cancer patients. Chin J Cancer Res. 2021;33:323–30. doi: 10.21147/j.issn.1000-9604.2021.03.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park EM, Gelber S, Rosenberg SM, et al Anxiety and depression in young women with metastatic breast cancer: A cross-sectional study. Psychosomatics. 2018;59:251–8. doi: 10.1016/j.psym.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fritsche I, Jonas E, Kayser DN, et al Existential threat and compliance with pro-environmental norms. J Environmental Psychology. 2010;30:67–79. doi: 10.1016/j.jenvp.2009.08.007. [DOI] [Google Scholar]
- 11.Lodhi MK, Cheema UI, Stifter J, et al Death anxiety in hospitalized end-of-life patients as captured from a structured electronic health record: differences by patient and nurse characteristics. Res Gerontol Nurs. 2014;7:224–34. doi: 10.3928/19404921-20140818-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menzies RE, Sharpe L, Dar-Nimrod I The relationship between death anxiety and severity of mental illnesses. Br J Clin Psychol. 2019;58:452–67. doi: 10.1111/bjc.12229. [DOI] [PubMed] [Google Scholar]
- 13.Neel C, Lo C, Rydall A, et al Determinants of death anxiety in patients with advanced cancer. BMJ Support Palliat Care. 2015;5:373–80. doi: 10.1136/bmjspcare-2012-000420. [DOI] [PubMed] [Google Scholar]
- 14.Rodin G, Lo C, Rydall A, et al Managing Cancer and Living Meaningfully (CALM): A randomized controlled trial of a psychological intervention for patients with advanced cancer. J Clin Oncol. 2018;36:2422–32. doi: 10.1200/JCO.2017.77.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang H, Zhang J, Lu Y, et al A Chinese version of a Likert-type death anxiety scale for colorectal cancer patients. Int J Nursing Sciences. 2016;3:337–41. doi: 10.1016/j.ijnss.2016.11.002. [DOI] [Google Scholar]
- 16.Tomer A, Eliason G Toward a comprehensive model of death anxiety. Death Stud. 1996;20:343–65. doi: 10.1080/07481189608252787. [DOI] [PubMed] [Google Scholar]
- 17.Wang XS, Wang Y, Guo H, et al Chinese version of the M. D. Anderson Symptom Inventory:Validation and application of symptom measurement in cancer patients. Cancer. 2004;101:1890–901. doi: 10.1002/cncr.20448. [DOI] [PubMed] [Google Scholar]
- 18.Cleeland CS, Mendoza TR, Wang XS, et al Assessing symptom distress in cancer patients: The M. D. Anderson Symptom Inventory. Cancer. 2000;89:1634–46. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 19.Lo C, Hales S, Zimmermann C, et al. Measuring death-related anxiety in advanced cancer: preliminary psychometrics of the Death and Dying Distress Scale. J Pediatr Hematol Oncol 2011;33 Suppl 2:S140-5.
- 20.Kroenke K, Spitzer RL, Williams JB The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ell K, Xie B, Quon B, et al Randomized controlled trial of collaborative care management of depression among low-income patients with cancer. J Clin Oncol. 2008;26:4488–96. doi: 10.1200/JCO.2008.16.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen S, Fang Y, Chiu H, et al Validation of the nine-item Patient Health Questionnaire to screen for major depression in a Chinese primary care population. Asia Pac Psychiatry. 2013;5:61–8. doi: 10.1111/appy.12063. [DOI] [PubMed] [Google Scholar]
- 23.Spitzer RL, Kroenke K, Williams JB, et al A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–7. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 24.He X, Li C, Qian J, et al Reliability and validity of a generalized anxiety scale in general hospital outpatients. Shanghai Jing Shen Yi Xue. 2010;22:200–3. doi: 10.3969/j.issn.1002-0829.2010.04.002. [DOI] [Google Scholar]
- 25.Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. New York: The Guilford Press, 2013.
- 26.Leonhart R, Tang L, Pang Y, et al Physical and psychological correlates of high somatic symptom severity in Chinese breast cancer patients. Psychooncology. 2017;26:656–63. doi: 10.1002/pon.4203. [DOI] [PubMed] [Google Scholar]
- 27.Grotmol KS, Lie HC, Loge JH, et al Patients with advanced cancer and depression report a significantly higher symptom burden than non-depressed patients. Palliat Support Care. 2019;17:143–9. doi: 10.1017/S1478951517001183. [DOI] [PubMed] [Google Scholar]
- 28.Imanian M, Imanian M, Karimyar M Sleep quality and fatigue among breast cancer patients undergoing chemotherapy. Int J Hematol Oncol Stem Cell Res. 2019;13:196–200. [PMC free article] [PubMed] [Google Scholar]
- 29.Basch E, Deal AM, Dueck AC, et al Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318:197–8. doi: 10.1001/jama.2017.7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Vulpen JK, Peeters PHM, Velthuis MJ, et al Effects of physical exercise during adjuvant breast cancer treatment on physical and psychosocial dimensions of cancer-related fatigue: A meta-analysis. Maturitas. 2016;85:104–11. doi: 10.1016/j.maturitas.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Reuter-Lorenz PA, Cimprich B Cognitive function and breast cancer: promise and potential insights from functional brain imaging. Breast Cancer Res Treat. 2013;137:33–43. doi: 10.1007/s10549-012-2266-3. [DOI] [PubMed] [Google Scholar]
- 32.Matthews EE, Janssen DW, Djalilova DM, et al Effects of exercise on sleep in women with breast cancer: A systematic review. Sleep Med Clin. 2018;13:395–417. doi: 10.1016/j.jsmc.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 33.Wang HL, Kroenke K, Wu J, et al Predictors of cancer-related pain improvement over time. Psychosom Med. 2012;74:642–7. doi: 10.1097/PSY.0b013e3182590904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Syrjala KL, Jensen MP, Mendoza ME, et al Psychological and behavioral approaches to cancer pain management. J Clin Oncol. 2014;32:1703–11. doi: 10.1200/JCO.2013.54.4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jim HS, Sutton SK, Jacobsen PB, et al Risk factors for depression and fatigue among survivors of hematopoietic cell transplantation. Cancer. 2016;122:1290–7. doi: 10.1002/cncr.29877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hopwood P, Stephens RJ Depression in patients with lung cancer: prevalence and risk factors derived from quality-of-life data. J Clin Oncol. 2000;18:893–903. doi: 10.1200/JCO.2000.18.4.893. [DOI] [PubMed] [Google Scholar]
- 37.Loge JH, Abrahamsen AF, Ekeberg, et al Fatigue and psychiatric morbidity among Hodgkin’s disease survivors. J Pain Symptom Manage. 2000;19:91–9. doi: 10.1016/s0885-3924(99)00148-7. [DOI] [PubMed] [Google Scholar]
- 38.Ashraf S, Jafri MAS, Alsharedi MF. Insomnia in cancer patients. J Clin Oncol 2021:39(Suppl 15): e18651.
- 39.Breen SJ, Baravelli CM, Schofield PE, et al Is symptom burden a predictor of anxiety and depression in patients with cancer about to commence chemotherapy. Med J Aust. 2009;190:S99–104. doi: 10.5694/j.1326-5377.2009.tb02480.x. [DOI] [PubMed] [Google Scholar]
- 40.Akechi T, Nakano T, Akizuki N, et al Somatic symptoms for diagnosing major depression in cancer patients. Psychosomatics. 2003;44:244–8. doi: 10.1176/appi.psy.44.3.244. [DOI] [PubMed] [Google Scholar]
- 41.Fitzgerald P, Lo C, Li M, et al The relationship between depression and physical symptom burden in advanced cancer. BMJ Support Palliat Care. 2015;5:381–8. doi: 10.1136/bmjspcare-2012-000380. [DOI] [PubMed] [Google Scholar]
- 42.McFarland DC, Shaffer KM, Tiersten A, et al Physical symptom burden and its association with distress, anxiety, and depression in breast cancer. Psychosomatics. 2018;59:464–71. doi: 10.1016/j.psym.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang YH, Li JQ, Shi JF, et al Depression and anxiety in relation to cancer incidence and mortality: a systematic review and meta-analysis of cohort studies. Mol Psychiatry. 2020;25:1487–99. doi: 10.1038/s41380-019-0595-x. [DOI] [PubMed] [Google Scholar]
- 44.Gonen G, Kaymak SU, Cankurtaran ES, et al The factors contributing to death anxiety in cancer patients. J Psychosoc Oncol. 2012;30:347–58. doi: 10.1080/07347332.2012.664260. [DOI] [PubMed] [Google Scholar]
- 45.Bergqvist J, Strang P The will to live − breast cancer patients perceptions’ of palliative chemotherapy. Acta Oncol. 2017;56:1168–74. doi: 10.1080/0284186X.2017.1327719. [DOI] [PubMed] [Google Scholar]
- 46.Tang L, Groot I, Bultz BD Psychosocial oncology in China − challenges and opportunities. Chin-German J Clin Oncol. 2009;8:123–8. doi: 10.1007/s10330-010-0605-6. [DOI] [Google Scholar]
- 47.Kim BR, Cho OH, Yoo YS The effects of dying well education program on Korean women with breast cancer. Appl Nurs Res. 2015;30:61–6. doi: 10.1016/j.apnr.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 48.Eggen AC, Reyners AKL, Shen G, et al Death anxiety in patients with metastatic non-small cell lung cancer with and without brain metastases. J Pain Symptom Manage. 2020;60:422–9.e1. doi: 10.1016/j.jpainsymman.2020.02.023. [DOI] [PubMed] [Google Scholar]