1. Overview
Gastric cancer originates from the glandular epithelia of the stomach. According to the latest statistics of China in 2020, the incidence and mortality of gastric cancer all rank 3rd among all malignancies. There were approximately 1.2 million newly diagnosed cases of gastric cancer worldwide, 40% of which came from China. Only 20% of gastric cancers are diagnosed in its early stage, most of which are in advanced stage, and the overall 5-year survival rate is less than 50%. In recent years, benefiting from the popularity of gastroscopy, the proportion of early gastric cancer (EGC) has gradually increased.
The overall strategy for gastric cancer treatment is surgery-based comprehensive therapy. This clinical guideline is formulated to further standardize the treatment of gastric cancer in China, enhance the gastric cancer diagnosis and treatment capacity of medical institutions, improve the prognosis of gastric cancer patients, and ensure medical quality and medical safety. Gastric cancer in this guideline refers to gastric adenocarcinoma (hereinafter gastric cancer for short), including esophagogastric junction (EGJ) cancer.
2. Diagnosis
Diagnosis and differential diagnosis of gastric cancer should be made according to the clinical manifestations, endoscopy, histopathology and imaging examination.
2.1. Symptoms
Patients with gastric cancer in its early stage generally have no specific symptoms, and symptoms similar to gastritis or ulcer can appear with the progress of illness, including the following symptoms: 1) epigastric satiety and discomfort, worsen after meal; 2) anorexia, belching, acid reflux, nausea, vomiting, melena, etc. In addition to the above symptoms, patients with advanced gastric cancer often appear 1) weight loss, anemia and fatigue; 2) gastric pain, if the pain continues to aggravate and radiates to the lumbar back, it probably suggests potential invasion of the pancreas and celiac plexus. Once the tumor is perforated, symptoms of gastric perforation, such as severe abdominal pain, may occur; 3) nausea and vomiting, often caused by an obstruction or gastric dysfunction owing to the tumor. Patients with cardia cancer can appear progressively aggravated dysphagia and reflux, and patients with gastric antrum cancer resulting in pylorus obstruction can vomit the retained food; 4) hemorrhage and melena, hemorrhage of the digestive tract could be caused by tumor invasion of blood vessels. Minor hemorrhage can only be diagnosed by the positive result of defecate occult blood, while massive hemorrhage can be manifested as hematemesis and melena; and 5) other symptoms such as diarrhea (due to lack of stomach acid or faster gastric emptying) and symptoms of metastases. In addition to these, advanced patients may present with severe emaciation, anemia, edema, fever, jaundice and cachexia.
2.2. Signs
Patients with gastric cancer, especially early-stage cancer, often show no obvious signs, and advanced gastric cancer can appear with the following signs: 1) deep tenderness in the upper abdomen, sometimes accompanied by mild muscular resistance, which is often the only physical sign available; 2) upper abdominal mass, advanced gastric cancer located in the pyloric antrum or gastric body, sometimes with palpable upper abdominal mass; Krukenberg tumor should be considered in female patients with a palpable mass in the lower abdomen; 3) gastrointestinal obstruction: pyloric obstruction can show stomach type and succussion splash, lumen stenosis caused by small intestine or mesenteric metastasis can lead to partial or complete intestinal obstruction; 4) ascites sign, peritoneal metastasis can result in hemorrhagic ascites; 5) supraclavicular lymph node enlargement; 6) anterior rectal fossa mass; 7) umbilical mass, etc. Among them, lymph node enlargement in supraclavicular fossa, ascites sign, pelvic mass in the lower abdomen, umbilical mass, planting nodule in the anterior rectal fossa, and intestinal obstruction were all important signs indicating advanced gastric cancer. Therefore, these signs not only have important diagnostic value but also provide sufficient clinical basis for the formulation of diagnosis and treatment strategies.
2.3. Imaging
2.3.1. X-ray gas-barium double-contrast imaging
It is superior to conventional computed tomography (CT) or magnetic resonance imaging (MRI) in terms of localized diagnosis, which is of guiding significance for surgeons to choose the appropriate operation and gastrectomy range.
2.3.2. Ultrasonography (US)
It can be used as a routine imaging examination in patients with gastric cancer due to its simple operation, flexible visualization, non-invasion and non-radiation. After filling the gastric cavity, conventional ultrasound can show the gastric wall hierarchy of the lesion site and evaluate the depth of invasion, which contributes to T staging of gastric cancer. The blood supply in the lesion can be detected by color doppler flow imaging. Double-contrast ultrasound can observe the microcirculation perfusion of the lesion and surrounding tissues based on the morphological characteristics of the lesion. Besides, ultrasound can contribute to identifying whether the important organs or lymph nodes of the abdomen and pelvic cavity, neck and supraclavicular lymph nodes are invaded; Ultrasound-guided biopsy of liver and lymph nodes is helpful for tumor diagnosis and staging.
2.3.3. CT
CT examination should be the first choice for clinical staging. Multi-slice spiral CT is widely used in China, and thoracic, abdominal and pelvic scanning is particularly recommended. CT enhancement scan should be made except for contraindications of contrast enhancement agent, and continuous scanning with 1 mm thickness is routinely used, and 3D image reconstruction using multiplanar is recommended, which contributes to identifying tumor sites and the relationship between tumor and adjacent organs (liver, pancreas, diaphragm, colon, etc.) or vessels and differentiating the tumor and regional lymph nodes, and increases the staging confidence and accuracy. To better display lesions, an oral negative contrast agent (generally 500−800 mL water before scan) is recommended. The supine position is generally adopted, and special position (such as prone position or lateral position) will be used for inspection purposes and patient compliance if the tumor is located in the lower part of the stomach or antrum. Multiphase enhancement scanning is recommended. The sensitivity to diagnose advanced gastric cancer by CT is about 65%−90%, and that of EGC is about 50%: T staging accuracy is 70%−90%, N staging is 40%−70%. Therefore, CT is not recommended as the preferred method for the initial diagnosis of gastric cancer, but it is recommended as the preferred method in the staging of gastric cancer.
2.3.4. MRI
MRI is recommended if patients are allergic to CT contrast agent or diagnosed with metastasis by other imaging examinations. MRI is helpful in determining peritoneal metastasis. Enhanced MRI is the first choice or important supplementary examination for liver metastasis of gastric cancer, especially injection of liver-specific contrast agent is more helpful to diagnose and determine the number and location of metastatic lesions. The accuracy of abdominal MRI is consistent with enhanced CT in terms of determining distant metastasis of gastric cancer. Accuracy of N staging of gastric cancer and sensitivity of diagnosis of lymph node invasion are superior to CT. MRI multi-b value diffusion weighted imaging (DWI) is of value for N/T staging of gastric cancer. Soft tissues can be easily identified by MRI. With the improvement of MR scanning technology, it is recommended to use MRI according to the medical ability of the hospital when the advanced carcinoma of the gastroesophageal junction cannot be diagnosed by CT or endoscopic ultrasound (EUS) cannot be completed due to the tumor.
2.3.5. Positron emission tomography (PET)-CT
PET-CT can assist in gastric cancer staging, but it is not recommended routinely. If patients are suspected of distant metastasis by CT, PET-CT can be used to evaluate the patients’ general condition. In addition, studies have shown that PET-CT has certain values in evaluating the efficacy of radiotherapy, chemotherapy, or targeted therapy, but it is not recommended routinely. There is a negative correlation between metabolism of the tumor and normal tissues in some histological types of gastric cancer, such as mucinous adenocarcinoma, signet-ring cell carcinoma and poorly differentiated adenocarcinoma, which are usually with low 18F-FDG uptake. Therefore, these patients should be carefully applied.
2.3.6. Emission computed tomography (ECT)
Bone scintigraphy is the most widely used, experienced, cost-effective and highly sensitive method for detecting bone metastases from gastric cancer, but it has certain false-negative rates in the lesions of spine and bone marrow, which can be combined with MRI to improve the diagnosis level. Bone scintigraphy can be performed in patients with highly suspected bone metastases.
2.3.7. Tumor biomarkers
Tumor biomarkers are widely used in clinical diagnosis, and the combined application of tumor markers contributes to the dynamic observation of tumor occurrence and development, clinical efficacy, and prognosis evaluation, thereby improving the detection rate and differential diagnosis accuracy. Carbohydrate antigen (CA)72-4, carcinoembryonic antigen (CEA) and CA199 are routinely recommended, which can be combined with alpha fetoprotein (AFP) and CA125 in some patients. CA125 has certain diagnostic and prognostic values for peritoneal metastasis and AFP for gastric cancer with special pathological types. The sensitivity and specificity of CA242, tumor-specific growth factor (TSGF), pepsinogen (PG) I, and PG II remain to be recognized. At present, an automatic chemiluminescence immune analyzer is commonly used in tumor biomarkers detection.
2.3.8. Endoscopy
(1) Screening
1) Screening subjects
The incidence of gastric cancer is relatively low (33/100,000). Endoscopic examination for gastric cancer screening needs to consume a large number of human and material resources, and the acceptability is low for patients. Therefore, it is possible and effective to screen for high-risk groups of gastric cancer. It is recommended that in China gastric cancer patients over 40 years old or with a family history of gastric cancer should be screened. Anyone who meets the following clause 1) and one of the clauses 2)−6) should be classified as a high-risk group of gastric cancer, which is recommended for screening: 1) over 40 years old, regardless of gender; 2) population in areas with a high incidence of gastric cancer; 3) Helicobacter pylori infection; 4) previously suffered from chronic atrophic gastritis, gastric ulcer, gastric polyp, residual stomach after surgery, hypertrophic gastritis, pernicious anemia and other pre-gastric cancer diseases; 5) first-degree relatives of patients with gastric cancer; and 6) with other high-risk factors for gastric cancer (high salt, pickled diet, smoking, heavy drinking, etc.).
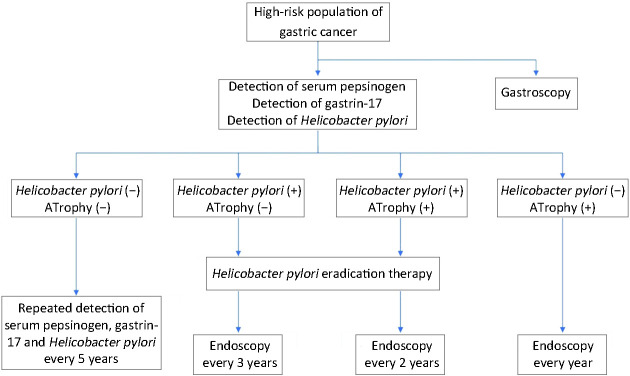
2) Screening methods ( Figure 1 )
Figure 1.
Method of gastric cancer screening.
Serum PG: The screening standard of a high-risk group of gastric cancer is defined as PG I concentration ≤70 μg/L or PG I/PG II ≤3.0. The risk of gastric cancer was stratified according to the results of the serum PG test and Helicobacter pylori antibody test, which determined further examination strategy.
Gastrin 17 (G-17): Serum G-17 concentration can help us to diagnose atrophic gastritis in gastric antrum (decreased G-17 level) or confined to the gastric body (increased G-17 level).
Upper gastrointestinal barium meal: X-ray barium meal examination may find gastric lesions, without a high sensitivity and specificity, which has been replaced by endoscopic examination. It is not recommended for gastric cancer screening.
Endoscopic screening: endoscopic and endoscopic biopsy are currently the gold standard for the diagnosis of gastric cancer. Painless gastroscopy has developed rapidly in recent years and has been applied to the endoscopic screening of high-risk gastric cancer groups, greatly improving the compliance of patients to accept endoscopy.
(2) Endoscopy
1) White light endoscopy: White light endoscopy is the basis of endoscopy. For lesion or suspected lesion area, white light endoscopic observation should be performed first to record the natural state of the lesion area, and then other endoscopic examination techniques should be performed.
2) Chromoendoscopy: Chromoendoscopy is based on the white light endoscopy, spraying the pigment dye onto the surface of the mucosa to be observed so that the lesion is more obvious than the normal mucosa. Physical staining (indigo carmine, methylene blue), refers to the physical covering relationship between the dye and the lesion. Since the microstructure of the lesion surface is different from that of the surrounding normal mucosa, different reflections of light are generated after the dye coating, thus highlighting the boundary between the lesion area and the surrounding normal tissues. Chemical staining (acetic acid, adrenalin) refers to the chemical reaction between the dye and the lesion area; it changes the color of the lesion area and highlights the lesion boundary.
3) Digital chromoendoscopy: Digital chromoendoscopy can observe the superficial microvascular morphology of mucous membranes through special light. The common digital chromoendoscopy includes narrow band imaging (NBI), Fuji intelligent color enhancement (FICE), and i-Scan.
4) Magnifying endoscopy: Magnifying endoscopy can amplify gastric mucosa and observe small changes in the surface of the gastric mucosa gland and microscopic changes of mucosal microvascular network. It can be used to identify benign and malignant lesions of gastric mucosa and determine the boundaries and extent of malignant lesions.
5) Endoscopic ultrasonography: Endoscopic ultrasonography is an endoscopic technique that combines ultrasound and endoscopic techniques. It is used to assess the extent of gastric cancer invasion and lymph node status.
6) Other endoscopic techniques: Confocal caser endomicroscopy (CLE), which can show up to 1,000 times of magnification, achieving the purpose of optical biopsy. Fluorescence endoscopy: A fluorescence endoscopy imaging system can detect and identify precancerous lesions and some hidden malignant lesions that are difficult to detect with common endoscopy. However, the above methods have high requirements for equipment and are still rarely used in clinical practice.
(3) Operational specifications for gastroscopy
Gastroscopy is a necessary means of diagnosis of gastric cancer, which can determine the location of the tumor and obtain tissue samples for pathological examination. Adequate preparation must be made before the endoscopic examination, and defoaming agents and mucous removers are recommended. After transoral endoscopic insertion, the endoscopic observation was conducted from the upper end of the esophagus to the cavity under direct vision, and the esophagus, cardia, gastric body, gastric antrum, pylorus, duodenal bulb and descending part of the duodenum were observed successively. When the endoscope is retracted, it is sequentially withdrawn from the duodenum, gastric antrum, stomach horn, stomach, stomach fundus and esophagus. Observe the whole upper digestive tract, especially the large curvature, small curvature, anterior wall,and posterior wall of the gastric wall, as well as the color, smoothness, mucus, peristalsis and the shape of the inner cavity. If the lesion is found, the specific location and scope of the lesion should be determined and recorded in detail on the record sheet. During the inspection, if there are mucus and bubbles, use water or rinse with a defoaming agent or defoaming agent in time, and then continue to observe. Ensure the number and quality of endoscopic images: to ensure the complete observation of the entire gastric cavity, additional images should be retained if lesions are found. Also, to ensure the clarity of each image, at least 40 images are recommended by Chinese experts. If necessary, digital chromoendoscopy, magnifying endoscopy, or other endoscopic techniques can be selected as appropriate.
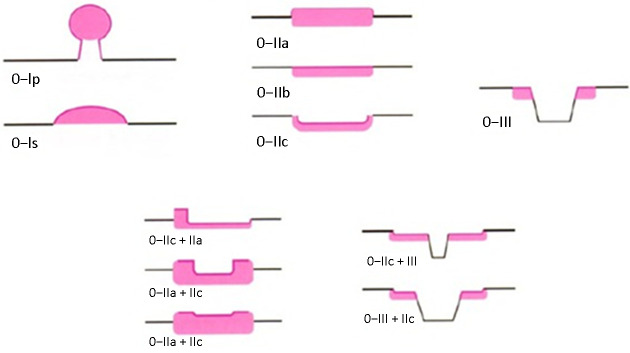
(4) Endoscopic classification of EGC ( Figure 2 )
Figure 2.
Endoscopic classification of gastric cancer.
1) Endoscopic classification of EGC was updated according to the Paris classification standard 2002 and the Paris classification standard 2005. Superficial gastric cancer (Type 0) is divided into uplift lesions (0−I), flat lesions (0−II) and depressed tubulovillous adenoma lesions (0−III). Type 0−I is divided into pedicled type (0−Ip) and non-pedicled type (0−Is). According to three types of lesions: slightly uplift, the flat and slight sag, type 0−II can be divided into three subtypes: 0−IIa, 0−IIb and 0−IIc.
2) Identification point of type 0−I and type 0−IIa is whether the height of the bulge reaches 2.5 mm (the thickness of the biopsy forceps closed), the identification point of type 0−III and type 0−IIc is whether the depth of the depression reaches 1.2 mm (the biopsy forceps open the thickness of a single forceps). At the same time, lesions with slight bulge and slight depression were classified into 0−IIc+IIa and 0−IIa+IIc according to the ratio of bulge/sag. The lesions combined with depressions and slight depressions were classified into 0−III+IIc and 0−IIc+III according to the ratio of depression/slight depression.
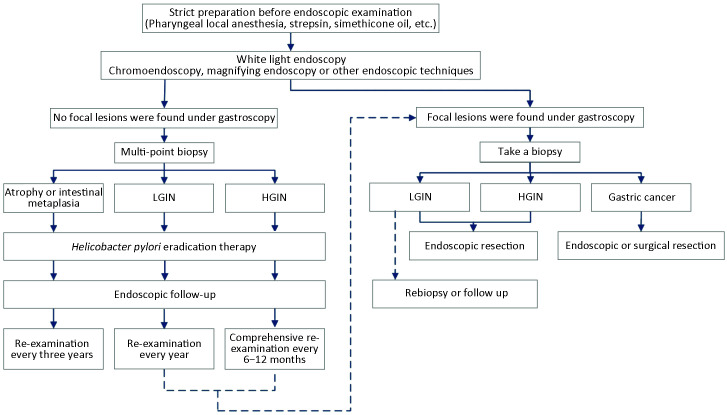
3) Procedures of EGC screening and follow-up (Figure 3 ).
Figure 3.
Procedures of gastric cancer screening and follow-up. LGIN, low-grade intraepithelial neoplasia; HGIN, high-grade intraepithelial neoplasia.
(5) Pathological biopsy
1) If no suspicious lesion is found after special endoscopic techniques such as endoscopic observation and staining, the biopsy is not required.
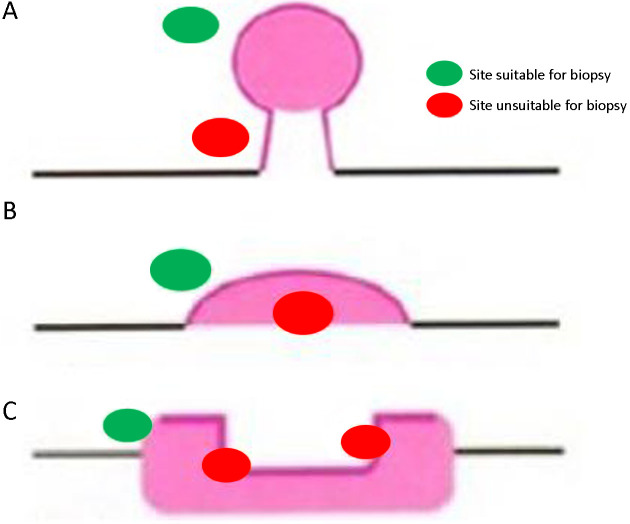
2) Biopsy site: to improve the positive rate of biopsy, biopsy site should be selected for different types of lesions (Figure 4 ).
Figure 4.
Biopsy site selected for different types of lesions. (A) Pedicled lesions: biopsy should be performed on head of the lesion, not the pedicle; (B) Lumpy lesions: biopsy should be performed at the top of the lesion, not at the base of the lesion; (C) Ulcerative lesions: biopsy should be performed on the inside of the ulcer, not on the bottom or outside of the ulcer.
3) When the lesion is suspected to be an early-stage neoplastic lesion, 1−2 pieces of biopsy should be taken when the lesion diameter is less than 2 cm, and 1 piece of biopsy can be added for every 1 cm of lesion diameter. When lesions tend to be advanced cancer, the necrotic area should be avoided and 6−8 pieces of biopsy are collected.
4) Handling of endoscopically biopsied specimens
A) Preparation of biopsied specimens: After the biopsy specimens are obtained, the specimens should be flattened immediately so that the basal layer of the mucosa is attached to the filter paper.
B) Fixation of biopsied specimens: Place the specimen in an adequate (greater than 10 times the volume of the specimen) 10% neutral buffer of formalin. The fixation time before embedding must be more than 6 h and less than 48 h.
C) Paraffin-embedded: Remove the filter paper and embed the tissue in a vertical orientation. When embedding, the hot tweezers can not directly touch the specimen. Do not take the tissue with tweezers until the wax surface is cool, in case of burns on the tissue.
D) Hematoxylin and eosin (HE) staining and mounting: After trimming the paraffin block, slice the fixed materials into 6−8 tissue pieces serially, and place them on the same slide. Conventional HE staining and mounting are then performed.
2.3.9. EUS
EUS is considered as the most accurate method of gastrointestinal tumor local staging, which is equivalent or superior to CT in T staging for gastric cancer (especially early cancer) and N staging. It is commonly used to distinguish the mucosa and submucosa lesions, dynamically observe the relationship between the tumor and adjacent organs, and guide biopsies of lymph nodes. Thus, it improves accuracy of local T and N staging. However, as EUS is an operator-dependent examination, it is recommended at the high-level hospital or center. EUS is necessary for patients with a schedule of endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD) and other endoscopic therapies. EUS can find lymph nodes with a diameter of more than 5 mm. The main criteria for judging lymph node metastasis, allowing for the type, boundary, and size of lymph node echo, are as follows: circular and quasi-circular hypoechoic structures, echo similar to or lower than that of tumor tissues, a clear boundary, a uniform internal echo, and the diameter >1 cm. In contrast, non-specific inflammatory enlarged lymph nodes often present oval or triangular hyperechoic changes with blurred borders and uniform internal echoes.
Standardized operation process and comprehensive and exhaustive scanning are the basis of accurate staging. EUS with the intention of gastric cancer staging should examine carefully at least from pyloric retraction to EGJ. To accurately assess the first station lymph node, retraction from the duodenal bulb is recommended. During the retraction process, perform the staging evaluations, and retain the images of typical tumors and important anatomical markers (i.e., landmark images). Staging accuracy can be improved, and images can be backtracked if dynamic multimedia data can be retained. In the process of scanning, attention should be paid to the filling of the gastric cavity, the selection of appropriate probe frequency, and the proper placement of the probe. A suitable focal length makes images evident. The compression of lesions should be avoided for fear of the wrong staging.
2.4. Diagnostic criteria and contents of gastric cancer
2.4.1. Qualitative diagnosis
Gastroscopic examination, endoscopic biopsy and pathological examination are performed to determine whether the lesion is cancer. During this preoperative diagnostic process, the properties and characteristics of lesions are closely related to the nature and biobehavioral characteristics of gastric cancer, such as the differentiation degree, the special molecular expression. In addition to the histological type, Laurén classification and human epidermal growth factor receptor 2 (HER2) expression status also need to be examined and clarified.
2.4.2. Staging diagnosis
The primary purpose of staging diagnosis of gastric cancer is to fully understand the severity and characteristics of the disease before formulating a treatment plan, and to provide sufficient evidence for selecting a reasonable treatment mode. The severity of gastric cancer can be mainly reflected in local infiltration depth, lymph node metastasis and the presence or absence of distant metastasis. Appropriate auxiliary diagnostic modalities should be selected in clinical work to obtain accurate staging information.
2.4.3. Clinical manifestations
Clinical manifestations can not be used as the main basis for the diagnosis of gastric cancer. However, the existence of comorbidities and complications that may affect the overall treatments should be considered when formulating treatment strategies.
2.5. Differential diagnosis
2.5.1. Benign gastric ulcer
Patients with benign gastric ulcers have a longer course of the disease, compared with those with gastric cancer. They have a history of recurrent pain of a typical ulcer, without loss of appetite. Antacids are useful in these cases. Most of them have no apparent signs unless complicated by severe complications such as hemorrhage and pyloric obstruction. There will be no recent noticeable weight loss, anemia, abdominal mass, or left supraclavicular lymph node enlargement in these patients. More important differential diagnostic modalities are barium X-ray examination and gastroscopy examination. A benign ulcer in barium X-ray examination is usually a circular or elliptical niche with a diameter of less than 2.5 cm and neat edge, through which peristaltic waves can pass. Under gastroscopy, the base of the mucosa of a benign ulcer is flat, covered with white or yellow-white moss, and surrounded by edema and hyperemia. And mucosal folds are concentrated toward the ulcer. Thus, a cancerous ulcer is very different from this, and the detailed characteristics of a cancerous ulcer are shown in the part of the diagnosis of gastric cancer.
2.5.2. Gastric lymphoma
Gastric lymphoma accounts for 2%−7% of gastric malignancies. More than 95% of primary gastric malignant lymphomas are non-Hodgkin’s lymphomas, which often infiltrate the gastric wall extensively and form a large shallow ulcer. The main clinical manifestations of gastric lymphoma are upper abdominal discomfort, gastrointestinal bleeding and abdominal mass.
2.5.3. Gastrointestinal stromal tumor
Mesenchymal-derived tumor, which accounts for 3% of gastric tumors, demonstrates expansive growth and may infiltrate into submucosal or subserosal areas to form spherical or lobulated masses. Patients with small tumors have slight symptoms. They may suffer upper abdominal discomfort or gastrointestinal symptoms similar to ulcer disease. When the tumor is large, it can be palpable as an abdominal mass, often with upper gastrointestinal bleeding.
2.5.4. Neuroendocrine neoplasm (NEN)
NEN is a group of heterogeneous neoplasms originating from peptidogenic neurons and neuroendocrine cells, all of which have malignant potential. These tumors are characterized by the ability to store and secrete different peptides and neuroamines. Although gastrointestinal or pancreatic NEN is rare, accounting for less than 2% of gastrointestinal malignancies, it is currently the second most common gastrointestinal malignancy after colorectal cancer in the United States. The gold standard of its diagnosis is still based on histology biopsy pathology. However, the conventional HE staining has not been enough to provide full diagnostic information for NENs. Synaptophysin (Syn) and chromogranin A (CgA) staining is a mandatory item for the diagnosis of NEN in current immunohistochemical staining methods. Moreover, NEN should be graded according to the mitotic image and Ki-67 percentage.
2.5.5. Benign gastric tumor
Benign gastric tumor accounts for about 2% of all gastric tumors. It can be divided into epithelial cell tumor and mesenchymal tissue tumor according to the tissue source. The former is usually gastric adenoma, while the latter is more common in leiomyoma, lipoma and schwannoma. Generally, tumors are small in size and develop slowly, which occur mostly in the gastric antrum and gastric body. There are few obvious clinical manifestations in patients with benign tumors. Lesions in the barium X-ray examination mostly present circular or elliptical filling defect, rather than niche. It shows a submucosal mass under gastroscopy.
3. Pathology and staging of gastric cancer
3.1. Terms and definitions
3.1.1. Gastric carcinoma
Gastric carcinoma is a malignant tumor originating from the gastric mucosa epithelial cells.
3.1.2. Intraepithelial neoplasia/dysplasia
Intraepithelial neoplasia/dysplasia is a kind of precancerous lesion of gastric cancer. The terms intraepithelial neoplasia and dysplasia are commonly used. There are three diagnoses involving gastric intraepithelial neoplasia or dysplasia, as follows:
(1) No intraepithelial neoplasia (dysplasia): Benign lesions such as gastric mucosal inflammation, metaplasia and reactive hyperplasia.
(2) Indeterminate intraepithelial neoplasia (dysplasia): Not a final diagnostic term, but a pragmatic description used when it is difficult to determine the nature of the morphological changes in gastric mucosa and cells. It is often used for small biopsy specimens, especially for small biopsy specimens with prominent inflammatory, where it is challenging to distinguish reactive lesions from proliferative lesions in the proliferative zone of the mucous neck and the metaplasia zone of the intestinal metaplasia. For such cases, the diagnosis can be confirmed by deep resection and re-handling.
(3) Intraepithelial neoplasia (dysplasia): It is a gastric mucosal epithelial hyperplasia characterized by varying degrees of cellular and structural atypia, which is neoplastic hyperplasia in nature but has no evidence of clear invasive growth. The lesion involves the entire length of the fovea, including the superficial epithelium, which is an essential basis for diagnosis. Gastric intraepithelial neoplasia (dysplasia) can be divided into two types: adenoma type (intestinal type) and small concave or pyloric type (stomach type), according to the tissue structure and cytological characteristics. In a macroscopic examination, gastric mucosal intraepithelial neoplasia (dysplasia) may present as polypoid, flat or slightly concave. Gastric mucosal intraepithelial neoplasia (dysplasia) can be divided into low- and high-grade based on the degree of lesions.
1) Low-grade intraepithelial neoplasia: It is a slight change in mucosal structure. The cells of the glandular epithelium presented mild to moderate atypia, and the nuclei became longer but still polar, located in the basal part of the glandular epithelium. Nuclear fission is visible. The term low-grade adenomas may also be used for polypoid lesions.
2) High-grade intraepithelial neoplasia: The structure of mucosal gland of the lesion is overtly heteromorphic. The cells change from column to cuboid, with enlarging nucleus, increasing ratio of nucleus to plasma and apparent nucleoli. There is an increase in mitotic activity, where pathological mitosis can be observed. The singularly important manifestations are nuclear extension to the side of glandular cavity and loss of cell polarity. For polypoid lesions, high-grade adenomas can also be used.
3.1.3. Early gastric carcinoma
Early gastric carcinoma is defined as invasive gastric cancer that invades no more deeply than the submucosa, irrespective of lymph node metastasis.
3.1.4. Advanced gastric carcinoma
Advanced gastric carcinoma is defined as invasive gastric cancer that invades the muscular layer or deeper, regardless of lymph node metastasis.
3.1.5. Adenocarcinoma of EGJ
Adenocarcinoma of EGJ is defined as an adenocarcinoma that spans the esophagogastric junction. Anatomically, the EGJ refers to the place where the tubular esophagus becomes the cystic stomach, that is, the end of the esophagus and the beginning of the stomach. EGJ corresponds to the horizontal level of peritoneal reflex or the angle of His, and the distal edge of the esophageal sphincter. It is important to note that the squamocolumnar junction (SCJ) does not always coincide with the EGJ histologically.
3.2. Specimen type and fixation
3.2.1. Specimen type
Common types of specimens in daily work include biopsy specimens, EMR/ESD and curative resected specimens (proximal gastrectomy specimens, distal gastrectomy specimens, and total gastrectomy specimens).
3.2.2. Specimen fixation
(1) Specimens should be fixed timely and adequately. Use 10% neutral buffer formalin fixative solution (containing 4% formaldehyde), and fix samples immediately (within half an hour as far as possible after surgical resection). The fixative solution should be more than ten times the volume of specimens, and specimens should be fixed for 6−72 h at average room temperature.
(2) Endoscopically biopsied specimens: After the specimen is obtained, the endoscopic physician or assistant should immediately remove the tissue from the biopsy forceps with a small and thin needle, and flatten it with a small needle on the finger. Next, take a small piece of filter paper, place the flattened mucous membrane on the filter paper, and immediately place them into the fixing solution.
(3) EMR/ESD specimens: The specimen should be spread out with the mucosal side up and pinned at the edges on a corkboard (or foam board) with stainless steel pins by endoscopy physicians. Excessive stretching or wrinkling of the specimen should also be avoided as it can destroy the tissue. The oral and anal margins are marked. Upon completion of the above steps, immediately immerse the specimens into the fixing solution entirely.
(4) Resected specimens: The stomach is, in principle, opened along the greater curvature, unless the tumor is located on the greater curvature. After placing gauzes on a corkboard (or foam board), the resected stomach is fixed on the board with the mucosal side up, pinned at the edges with stainless steel pins, and fixed in a fixing solution as soon as possible (within 30 min after isolating) with the mucosal side downwards.
3.3. Guideline for handling and describing specimens
When collecting and handling specimens, basic information including name, department, bed number, hospital number, specimen type, quantity, etc. should be checked.
3.3.1. Handling of biopsy specimens
(1) Description and record: Describe the size and number of tissues taken for inspection.
(2) Collection and handling: All the mucosa collected specimens taken for inspection should be handled, which should be wrapped in filter paper to avoid loss. When handling, add with eosin, which is helpful for the technician to identify when embedding and slicing. Those with substantial differences in size should be placed separately into different dehydration boxes to prevent small pieces of biopsy tissue from missing or overcutting. Care must be taken to embed the flattened mucosa vertically (i.e., the mucosa is perpendicular to the bottom of the embedding box). The number of tissue pieces embedded in a paraffin block should not exceed three sections that are embedded vertically and parallel to each other. The white edge of the paraffin block without tissue should be removed with a knife as far as possible. It is recommended that each glass slide contain 6−8 serial tissue sections for sequential observation.
3.3.2. Handling of endoscopically resected specimens (EMR/ESD)
(1) Inspection and record: The size of the specimen (maximum diameter × maximum diameter × thickness) should be measured and recorded. As regards the specimen of EGJ, the length and width of the esophagus and the stomach should be measured respectively. Record the color and the features of the mucosal surface, such as whether there are grossly discernible macroscopic lesions, whether the contour of the lesion is regular, whether there is a visible bulge or depression, and whether there is erosion, ulcer, etc. And then measure and describe the size of the lesion (maximum diameter × minimum diameter × thickness), macroscopic type (Appendix 1 ) and the length between the lesion and each margin (at least record the length between the lesion and the closest margin of the mucosal side). For complex specimens, communication between clinicians and pathologists or schematic diagrams of specimen extension and reconstruction provided by the surgeon is recommended.
(2) Collection and handling: All the endoscopically resected specimens taken for inspection should be collected and handled. Handle the specimens vertically perpendicular to the closest margin. The base and the lateral mucosal margins should be inked with ink or carbon ink (different colors can be applied to identify the oral and anal sides if possible), which helps map the margins and assess the margins. The specimen of the EGJ should be handled along the orientation of the oral-anal side to better show the relationship between the tumor and the esophagus and stomach. The specimens should be serially sectioned at 2−3 mm intervals in parallel entirely. If a sample is too large, the sample can be modified and recut; namely, the section is divided into several pieces and labeled a or b, etc. Embed the specimens vertically in the same direction, and record the sequence/location of these embedded tissue blocks. (When embedding the first and last sections, if they contain lesions under the microscope, reverse by 180° and then restart to embed so that the margin around the mucosa can be seen in the final section.) Record the corresponding sites of the tissue blocks (it is recommended to attach photos or schematic diagrams and label them). Concerning the multiple section specimens, it is recommended to be labeled and handled separately. Other procedures of handling multiple section specimens are the same as that of a single resection specimen if not considering the side section margin.
3.3.3. Handling of resected specimens
(1) Inspection and record: First, follow the characteristics of pylorus and cardia to locate the specimen. Then, measure the length of greater curvature and lesser curvature and the volume of gastric omentum. When observing the mucosal surface, describe the location, the size, the number, the macroscopic types (Appendix 1 ) and appearance of the tumor, and measure the depth and the extent of tumor invasion, and the length of the proximal, distal and circumferential resection margins. When measuring the size of the lesion, for the sample following neoadjuvant treatment, the size of the tumor bed ought to be measured; as for the EMR specimen, the size of the ulcer/mucosal defect/scar and the presence or absence of residual tumor should be described. It is also necessary to assess whether the mucosa of the stomach wall other than the tumor lesion has other changes such as congestion, hemorrhage, ulcer, perforation, etc.; whether the serosa is hyperemia, hemorrhage, exudation, perforation, tumor infiltration, etc.; and whether there are thickening and presence of the stomach wall elasticity around the tumor. If spleen, duodenum, etc. are also excised and sent for inspection, describe them in sequence. It is recommended to report the relationship between the proximal gastric cancer with the EGJ, namely whether there is the involvement of the EGJ. (The relationship descripiton between the tumor and EGJ as follow: the tumor is wholly located in the esophagus, without involving the EGJ; the tumor epicenter is located in the distal esophagus, with involvement of the EGJ; the tumor center is located in the EGJ area; the tumor center is located in the proximal stomach, involving the EGJ). For the specimen with the EGJ involved, the distance between the tumor center and the EGJ is recorded (in cm) (as Siewert Classification, Appendix 2 ). The relationship between distal gastric cancer and duodenum is also recommended reporting.
(2) Collection and handling: A piece of tissue can be sectioned through the tumor center along the line from the oral margin to the anal margin and embedded in blocks (including the tumor, the adjacent mucosa of the tumor, and the margins at both proximal and distal resection). Then record the corresponding orientations and locations of the tissue blocks (Had better attach photos or schematic diagrams to mark.) It is recommended to take the resection margins at both ends longitudinally, or horizontally if the tumor is far from the resection margins at both ends. The closed edge of the closure that is sent separately should be removed after the closure is removed. The deepest part of the tumor invasion and the suspected circumferential circumference of the affected area should be noticed. For radical surgery specimens with early onset of cancer or neoadjuvant treatment, it is recommended to take all suspected lesions and tumor beds. The resection margins of the tissues on the cut stapler should be sent for inspection separately and handled entirely after the nail of the stapler is removed. The area of deepest invasion and the suspected circumferential involvement should be handled carefully. Superficial tumors of early-stage cancer or those following neoadjuvant therapy should have all components that contain all suspected lesions and tumor beds. The area of the surrounding mucosa with erosion, roughness, hyperemia, hemorrhage, ulceration, perforation, etc., the nodules inside the surrounding esophageal/gastric wall and the EGJ should be inspected and handled separately. If other adjacent organs are sent for inspection, those tissues should be inspected and handled. The lymph nodes should be handled in the order the surgeon grouped them. If the surgeon does not remove and group lymph nodes from the specimen, the lymph nodes should be grouped and recorded according to the drainage area of each lymph node. The number and size of lymph nodes, the presence or absence of fusion, and the presence or absence of adhesion to surrounding tissues need to be described. If there is adhesion, the connective tissue around the lymph nodes should be contained. All detected lymph nodes should be handled. Although it is not a prerequisite, the examination of 16, preferably 30 or more regional lymph nodes is recommended for pathological evaluation in radical specimens without new adjuvant therapy. It is recommended that the size of the handled tissue should not be larger than 2.0 cm × 1.5 cm × 0.3 cm.
3.4. Classification, grade and staging of pathological diagnosis
3.4.1. Histological type
Both World Health Organization (WHO) (tumor of the digestive system) and Laurén classification (intestinal type, diffuse type, mixed type, uncertain type) are recommanded (Appendix 3 ).
3.4.2. Histological grade
Tubular adenocarcinomas should be classified as well/moderately/poorly differentiated (or high- and low-grade) according to the degree of differentiation.
3.4.3. Gastric cancer staging
The staging from the American Joint Cancer Committee/Union for International Cancer Control (AJCC/UICC) is recommended.
3.4.4. Pathological evaluation of radical resection specimens after neoadjuvant therapy
The primary features of pathological changes after neoadjuvant therapy include tumor cell degeneration, regression, a large area of necrosis, fibrous tissue hyperplasia, interstitial inflammatory cell infiltration, calcium salt deposition, etc. There may be large cell-free mucous lakes that cannot be considered tumor remnants. Large acellular mucin lakes that are likely to be seen should not be regarded as residual tumors. It is recommended to evaluate the response of treatment with the standards of the College of American Pathologists (CAP)/the National Comprehensive Cancer Network (NCCN) guidelines (Appendix 4 ).
3.5. Contents and standards of pathology report on gastric cancer
The pathology report on gastric cancer should include all items related to the treatment and prognosis of the patient, such as specimen type, tumor location, macroscopic type, size and number, histological type, subtype and grade, depth of tumor invasion, capillary (lymphatic/venous) and nerve invasion, peripheral mucosa, lymph node, circumferential and resection margin, etc. It is recommended that pTNM staging be noted in the ultimate report.
(1) Macroscopic description: Specimen type, tumor location, macroscopic types, size (tumor size should be measured in three dimensions) and number should be recorded.
(2) Main tumor lesion areas: Histological type and grade, Laurén classification (intestinal type, diffuse type, mixed type or uncertain), depth of invasion (including mucosa lamina propria, muscularis mucosa, submucosa, superficial muscularis, deep muscularis, subserosa, serosa and surrounding tissues or organs) should be recorded. When the submucosal invasion is present, the actual depth of submucosal invasion should be measured in the endoscopically resected specimens, and distinction between SM1 (submucosal invasion depth <500 µm) and SM2 (submucosal invasion depth >500 µm) is suggested. For the radical resection specimens with submucosal invasion, it is recommended to identify between SM1 (upper 1/3 of the submucosa), SM2 (middle 1/3 of the submucosa), and SM3 (lower 1/3 of the submucosa), the section margin, and the capillary and nerve invasion. Gastric ulcer lesions or ulcer scars, which can affect EMR/ESD surgery and prognosis, are an essential part of the pathology report. [Note: Lateral and vertical margin should be reported in the endoscopically resected specimens, while the oral, anal and circumferential margin should be included in the radical resection specimens. Moreover, if the pathology changes, such as Invasive carcinoma or intraepithelial neoplasia/dysplasia, are present in the section margin area, the changes and the length of those from the resection margin also should be reported and described. And if a capillary invasion is suspected, immunohistochemistry CD31/CD34 and D2−40 is recommended to determine whether there is a capillary invasion, especially for endoscopically resected specimens. Elastin Van Gieson staining can be used to determine the presence or absence of venous invasion].
(3) Pericarcinous tissues: Record intraepithelial neoplasia/dysplasia and degree, the presence or absence of gastritis and gastritis type.
(4) Lymph node metastasis: For surgical resection specimens, the total number of lymph nodes and the number of involved lymph nodes at each nodal station are recorded. The number of lymph extracapsular invasions is also recommended for recording, which is defined as the infiltration of cancer cells beyond the capsule of the metastatic lymph node.
(5) Response to treatment (Case of neoadjuvant therapy).
(6) Report on comorbidity and complication.
(7) All cases pathologically diagnosed as gastric or EGJ adenocarcinoma should undergo the immunohistochemical assessment of HER2 and mismatch repair (MMR) proteins (MLH1, PMS2, MSH2 and MSH6) and the test of MSI. PDL1 test is recommended to be carried out in qualified units.
(8) Remark column of the report should include important relevant past medical history (e.g., related oncology history and neoadjuvant therapy history).
(9) pTNM staging.
3.6. Several precautions of pathology report in endoscopic resection
(1) Depth of tumor invasion: Depth is determined and recorded only when the vertical margin is negative for cancer invasion. The invasion depth of the submucosa is one of the most important indicators to determine whether the lesion is completely resected, as where there is the deeper the submucosal invasion, there is the higher the risk of lymph node metastasis. Submucosa (SM) can be subclassified as SM1 or T1b1 (tumor invasion is within 500 μm of the muscularis mucosae) or SM2 or T1b2 (tumor invasion is 500 μm or more deep into the muscularis mucosae). The method of measuring the depth of submucosal invasion depends on the degree of destruction of the muscularis mucosae in the tumor tissue. When there are residual muscularis mucosae left, the actual measured length should be recorded from the lower border of the muscularis mucosae to the front of tumor invasion. If the muscularis mucosae are obscure, the length should be measured on the virtual line based on the adjacent normal layer to the front of tumor invasion.
(2) Resection margin: Electrocautery change of the tissue is the marker of the resection margin of the ESD specimen. Negative resection margin means that no tumor cells are found at each horizontal or vertical electrocautery margin of the resected specimen. When the resection margin is negative but close to the margin, the nearest length from the lesion to the margin should be recorded. If the horizontal resection margin is positive, the number of positive resection margin blocks should be recorded. When the vertical margin is involved, the invasion layer, such as lamina propria or submucosa, should be described. Immunohistochemical staining can be helpful to determine whether there is a residual tumor in the margin if necessary, as the change of resection margin following electrocautery affects the observation and assessment of the morphology of tissues, cells and nuclei.
(3) Capillary invasion: The presence or absence of vascular/lymphatic invasion in ESD specimens is an important factor to judge whether surgical treatment is needed. The deeper the tumor invades, the more attention should be paid to the capillary invasion. Specific staining or immunohistochemical staining (e.g., CD34/CD34, D2−40) for tissues with submucosa invasion are often able to reveal capillary invasion that might be easily overlooked when HE staining was performed.
(4) Ulcers and other mucosal lesions: Gastric ulcer lesions or ulcer scars, which can affect EMR/ESD surgery and prognosis, are an important part of the pathology report. Other nonneoplastic changes in the surrounding mucosa, (e.g., inflammation, atrophy and metaplasia), and the severity of these changes should also be recorded.
(5) An additional surgical treatment is recommended when the following conditions are met: Histologically of low differentiated pT1 type, positive capillary infiltration [ly(+)/v(−)], positive horizontal margin (HM1) or positive vertical margin (VM1). The other conditions are determined as curative resection. Still, a regular follow-up is necessary.
(6) Histologic features of poor prognosis include: Poor differentiation, vascular/lymphatic infiltration and positive resection margin.
(7) Definition of positive cutting edge: Positive resection margin is defined as residual cancer cells are visible at the electric scalpel/ultrasonic scalpel resection margin, or the length from the resection margin to the tumor is less than 1 mm.
4. Treatment
4.1. Treatment principles
The fundamental principle is that comprehensive treatments should be adopted in a mode of multidisciplinary team (MDT) (including gastrointestinal surgeons, gastroenterologists, medical oncologists, endoscopists, radiation oncologists, radiologists, interventional radiologists, rehabilitation doctors, nutritionists, molecular biologists, bioinformaticians, etc.). With this multidisciplinary approach, reasonable treatments, (e.g., surgery, chemotherapy, radiotherapy, target therapy and immunotherapy) are applied and performed in a planned way, according to the pathologic type and the clinical staging of tumors, the functional state of patients’ organs and the general condition of patients. The treatment aims are to achieve a curative treatment or a maximum control for tumors, prolong survival and improve quality of life.
(1) EGC without lymph node metastasis. EGC without lymph node metastasis can be a candidate for surgery or endoscopic therapy based on the depth of tumor invasion, which doesnot need adjuvant radiotherapy or chemotherapy after operation.
(2) Local advanced gastric cancers and EGC with lymph node metastasis. For local advanced gastric cancers, and EGC with lymph node metastasis, comprehensive treatments based on the surgery is recommended. Radical surgery directly or radical surgery followed by neoadjuvant chemotherapy may be considered based on the depth of tumor invasion and the extent of lymph node metastasis. A postoperative adjuvant therapy should be taken into account if a curative gastric surgery is achieved for a local advanced gastric cancer. The adjuvant therapy regimen (adjuvant chemotherapy, and adjuvant radiotherapy when necessary) depends on postoperative pathological stage .
(3) Patients with metastatic/recurrent disease. For patients with metastatic/recurrent disease, comprehensive treatment based on drug therapy is recommended. Other therapeutics such as palliative surgery, radiotherapy, radiofrequency ablation, intraperitoneal perfusion and arterial embolization may be considered and provided if necessary. At the same time, the best supportive care, including pain relief, stent implantation and nutritional support, should be given actively.
4.2. Endoscopic treatment for EGC
Treatments for EGC include endoscopic resection and surgery. Compared with traditional surgery, endoscopic resection has the advantages of less trauma, fewer complications, faster recovery and lower cost, and the prognosis is equivalent, with the 5-year survival rate exceeding 90%. Therefore, many international guidelines and this consensus recommend endoscopic resection as the preferred treatment for EGC. EGC endoscopic resection, including EMR and ESD.
4.2.1. Definitions and terms of endoscopic therapy
(1) En blocresection: The lesion is completely removed by endoscopic resection, and a single whole specimen is obtained.
(2) Positive horizontal/vertical margins: Fixed materials should be vertically sectioned serially at 2-mm intervals. If there is tumor cell infiltration found at the lateral resection margin of the specimen, it is defined as positive horizontal margin involvement, and if there is tumor cell infiltration found at the basal resection margin, it is defined as positive vertical margin involvement.
(3) Complete resection/R0 resection: Complete resection specimen is negative in both horizontal and vertical margins.
(4) Curative resection: Curative resection is to achieve complete resection without the risk of lymph node metastasis.
(5) Non-curative resection: Resection is determined as non-curative resection when there is one of the following conditions: incomplete resection (including non-en bloc resection and/or positive margin); risk factors of lymph node metastasis (such as the depth of submucosal invasion more than 500 μm, capillary invasion, poor differentiation, etc.)
(6) Local recurrence: Local recurrence refers to cancer that has recurred (come back) at the original resection site or the area within 1 cm around the original resection site more than 6 months after resection.
(7) Residual: Residual is defined as a tumor lesion found pathologically at the original resection site or the area within 1 cm around the original resection site, within 6 months after resection.
(8) Synchronous recurrence: Synchronous recurrence refers to the discovery of new lesions within 12 months after endoscopic treatment of gastric cancer, that is, secondary lesions that have been present but were missed during the original endoscopic treatment are endoscopically found within 12 months after surgery.
(9) Metachronous recurrence: Metachronous recurrence refers to the new lesions that are found more than 12 months after resection. Most of the lesions occurred in the vicinity of the primary lesion in the stomach, of which the pathological type is the same.
4.2.2. Preoperative evaluation of endoscopic resection
EMR or ESD is indicated based on the following contents.
(1) Histological type: Histopathological type is usually determined by the histopathological examination of the specimen. Although it has been reported that histopathological types can be predicted by endoscopy to some extent, there is still insufficient evidence.
(2) Size: Final size data ought to be obtained from the measurement after resection and pathological examination, instead of measuring by conventional endoscopic examination, because measuring the size of lesions by conventional endoscopic examination is easy to make mistakes and difficult to accurately measure preoperatively.
(3) Ulcerative findings: Pay attention to the presence of ulcers in the lesions. If ulcers present, check whether it is an active ulcer or an ulcer scar. Ulcer histopathology is defined as a mucosal defect of at least a depth of UL-II (deeper than muscularis mucosa). Active ulcers generally show with white exudate covered on the surface in preoperative gastroscopy, excluding superficial erosion. In addition, mucosal folds or wrinkles can be observed converging toward a center during the healing or scarring phase of the ulcer.
(4) At present, the depth of invasion of EGC is assessed by conventional endoscopy, and the magnifying endoscopy is also recommended to assist in the evaluation. Endoscopic ultrasonography (EUS) can be used to evaluate the depth of invasion of gastric cancers because of relatively good sensitivity and specificity for the T staging when the above method is difficult to assess the depth of infiltration.
4.2.3. Methods of endoscopic treatment
(1) EMR: EMR refers to the method of resecting the mucosal lesion en bloc or piecemeal by lifting the lesion with submucosal injection and removing it with a high-frequency steel snare, which is used for the diagnosis and treatment of superficial lesions of gastrointestinal tracts. However, there are not enough prospective studies currently on treating EGC with the EMR. Thus, we do not recommend using EMR on the treatment of EGC.
(2) ESD: ESD is currently recommended as the standard endoscopic treatment for EGC.
1) Definition: ESD is a new technology developing from EMR and a method by which mucosa and submucosa of the lesion are en bloc resected following endoscopically dissecting the layer between mucosa and muscularis propria, after selecting the proper electric scalpel (such as IT knife, Dua knife, Hook knife, etc.) according to different locations, sizes and infiltration depth of the lesion.
2) Steps: The operation mainly includes 5 steps: A) Marking around the lesion; B) Injecting saline into the submucosa to elevate the lesion from the muscularis propria; C) Circumferentially incising the surrounding mucosa using a high-frequency electric knife; D) Subsequent dissecting the connective tissue of the submucosa beneath the lesion to completely separate the mucosa from the muscularis propria, and then an en bloc resection being performed at once; and E) Wound management, including wound vascular management and margin inspection.
(3) Other methods: Other endoscopic treatments include laser therapy, argon knife and microwave therapy. However, these methods can only remove tumors, but neither obtain complete pathological specimens nor ensure that the tumor is curatively resected. Therefore, they are often used for precancerous lesions of gastric cancer and require close follow-up after treatment. And we donot recommend these methods as the preferred treatment for EGC.
4.2.4. Indications for endoscopic treatment of EGC
Currently, the absolute indications of endoscopic therapy for EGC are as follows: Macroscopically intramucosal (cT1a) differentiated carcinomas and there must be no finding of ulceration (scar), i.e., UL(−). When one of the depths of invasion, lesion diameter, degree of differentiation or UL (+) with ulceration exceeds the above criteria, the risk of lymph node metastasis is extremely low. Endoscopic treatment can also be considered. For patients receiving the initial ESD or EMR, subsequent locally recurrent intramucosal lesions may be dealt with under expanded indications (Table 1 ).
Table 1. Absolute indication and expanded indication for endoscopic treatment of early gastric cancer.
| Depth of invasion | Differentiated | Undifferentiated | ||
|
| ||||
| cT1a (M) | ||||
| UL (−) | ≤2 cm | >2 cm | ≤2 cm | >2 cm |
| * | ||||
| UL (+) | ≤3 cm | >3 cm | ||
| * | ||||
| cT1b (SM) | ||||
4.2.5. Contraindications for endoscopic treatment of EGC
At present, contraindications for endoscopic resection that are generally recognized in China are the following: 1) EGCs with definite lymph node metastasis; 2) Propria muscularis infiltration; and 3) Patients with coagulation dysfunction. Besides, the relative contraindications for ESD also include a non-lifting sign, which means that no local bulge can be formed after subcutaneous injection of saline in the base of the lesion, indicating that there is adhesion between submucosa and muscularis at the base of the lesion. If an ESD treatment is attempted at this time, the risk of perforation increases. However, ESD can be safely carried out even when the non-lifting sign is present if the operator is an endoscopist with a proficient ESD operation skills.
4.2.6. Perioperative management
(1) Preoperative preparation: In addition to the preoperative diagnosis, preoperative preparation should include an assessment of each patient’s general condition, exclude the contraindication for anesthesia and endoscopic treatment, and sign the preoperative informed consent after obtaining it from the patient and family.
(2) Postoperative management: Fasting on the first day after operation; with close observation of vital signs, fluid or soft food may be taken if no abnormalities on the second day postoperatively. It is still controversial whether to review endoscopy 1 week after the operation.
(3) Postoperative medication: Ulcer treatment: the ulcers after the endoscopic resection of EGC can be treated with a proton pump inhibitor (PPI) or H2 receptor antagonist (H2RA). Use of antibacterial drugs: the prophylactic use of antibiotics may be considered for patients with a potential large resection range, possible long operation time, and high risk of digestive tract perforation in preoperative evaluation.
4.2.7. Postoperative complications and their management
The common complications after ESD include bleeding, perforation, stricture, abdominal pain, infection, etc.
(1) Management of bleeding: Direct electrocoagulation is recommended for intraoperative bleeding. Hemostatic clamp or electrohemostatic forceps can be used for delayed bleeding.
(2) Management of perforation: Most perforation cases can be repaired by endoscopic clip closure with a metal clamp. If the perforation is large, endoscopic treatment is often difficult to perform, and emergency surgery is required.
(3) Management of stricture: The incidence of gastric stricture or deformation is low, which is mainly seen when the resection area of the cardia, pylorus or gastric antrum is large. Endoscopic columnar balloon dilation is an effective treatment for stricture.
4.2.8. Prognostic evaluation and follow-up
Ought to distinguish between two easily-confused concepts of the endoscopic curative resection and R0 resection. R0 resection means negative resection margin, but the negative resection margin after endoscopic resection does not mean curative resection. The eCura system is recommended as a unified prognostic evaluation criterion in this guideline (Table 2 ). And the follow-up recommendation is shown in Table 3 .
Table 2. eCura evaluation system.
| Staging | Ulceration/depth | Differentiated | Undifferentiated | |||
|
| ||||||
| pT1a (M) | UL (−) | ≤2 cm | >2 cm | ≤2cm | >2 cm | |
| UL (+) | ≤3 cm | >3 cm | ||||
| pT1b (SM) | SM1 | ≤3 cm | >3 cm | |||
| SM2 | ||||||
Table 3. Follow-up methods according to different eCura evaluation levels.
| eCura evaluation levels | Follow-up methods |
| eCura A | Endoscopic follow-up is performed every 6−12 months |
| eCura B | Endoscopy plus abdominal ultrasound or CT follow-up is performed every 6−12 months |
| eCura C1 | Complementary therapy (surgical or non-surgical) or close follow-up is recommended |
| eCura C2 | Surgical treatment or fully informed follow-up is recommended |
eCura C1: When all resection conditions of eCura A or B, except for en bloc resection or an HM0 case with local en bloc resection, are met in a differentiated carcinoma case, the cases are regarded as eCura C1. Local treatments, such as one additional ESD and endoscopic ablation, can be adopted, or close follow-up may also be taken into account considering the burn effect of ESD.
eCura C2: Pathology of the eCura C2 cases indicates a high risk of lymph node metastasis. According to the specific situation of cases, another ESD is a possible choice with the patient’s adequately informed consent, although there is a high risk of lymph node metastasis.
Note: There are still debates about whether to perform additional surgery and the operation timing of additional surgery after eCura C resection, which mainly focuses on the following:
(1) More than 80% of patients with eCura C will not have a local recurrence or lymph node metastasis.
(2) The role and influence of risk factors, such as vascular invasion, nerval invasion, lymphatic invasion and horizontal/vertical resection margin, in the evaluation of recurrence, need to be further refined.
(3) There has been no significant difference in prognoses of eCura C after ESD between patients who underwent additional surgery immediately and those who underwent surgery after local recurrence.
In summary, more clinical evidence is needed to be worked out in further detail to support whether patients with eCura C should receive immediate additional surgery.
4.3. Surgery
4.3.1. Principles of surgery
Surgery is the primary treatment for patients with gastric cancer and the only method to cure gastric cancer at present. Gastric cancer surgery is divided into curative surgery and non-curative surgery. Curative surgery involves complete resection of the primary tumor lesion with a thorough dissection of regional lymph nodes, including standard surgery, modified surgery and expanded surgery. Non-curative surgery mainly includes palliative surgery and reductive surgery.
(1) Curative surgery
1) Standard surgery is performed with curative intent, involving resection of at least two-thirds of the stomach with a D2 lymph node dissection.
2) Modified surgery is mainly for the early-stage tumors, involving subtotal or total gastrectomy with a D1 or D1+ lymph node dissection.
3) Extended surgery involves gastrectomy with combined resection of adjacent involved organs and extended lymphadenectomy exceeding D2.
(2) Non-curative surgery
1) Palliative surgery is mainly for gastric cancer patients with serious symptoms such as bleeding or obstruction, including palliative gastrectomy, gastrojejunostomy, gastric bypass, jejunal nutrition tube placement, etc.
2) Reductive surgery is mainly for patients with non-curative factors such as unresectable liver metastasis or peritoneal metastasis, in the absence of urgent symptoms such as bleeding or obstruction, which is not recommended currently.
4.3.2. Treatment process
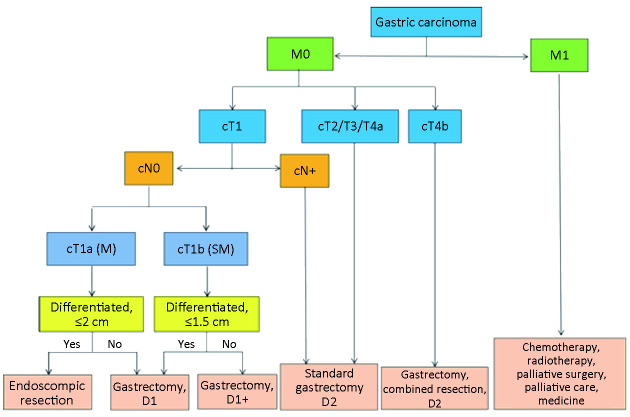
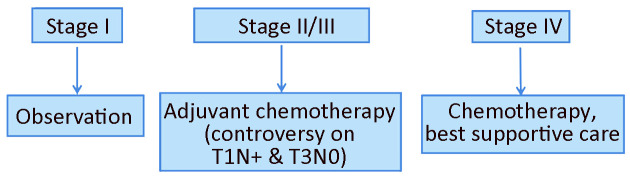
Algorithm of surgery-based standard treatments and algorithm of postoperative treatments is shown in Figure 5 ,6 , respectively, based on the cTNM stage.
Figure 5.
Algorithm of surgery-based standard treatments.
Figure 6.
Algorithm of postoperative treatments.
4.3.3. Criteria of resection margin
(1) For T1 tumors, a resection margin of 2 cm should be ensured. When the tumor border is unclear, preoperative endoscopy will be helpful to mark the resection line.
(2) The proximal margin of at least 3 cm is recommended for T2, or deeper tumors with Borrmann types I and II and at least 5 cm for those with Borrmann types III and IV.
(3) When the above criteria cannot be met, it is advisable to examine the proximal resection margin by the frozen section.
(4) For tumors invading the esophagus, a 3−5 cm margin or frozen section examination of the resection line is required to ensure an R0 resection.
4.3.4. Selection of gastrectomy
The gastric resection extent varies with the tumor location. Distal or total gastrectomy can be considered for tumors that are located in the lower part of the stomach, while total gastrectomy may be performed for carcinoma located in the corpus. For adenocarcinoma located on the proximal side of EGJ, proximal gastrectomy or total gastrectomy should be taken into consideration.
The extent of gastric resection can also be determined by the clinical stage before surgery as follows:
(1) The standard surgical procedure for patients with cT2−T4a or cN(+) tumors is either total or distal gastrectomy.
(2) For cT1N0M0 tumors, besides the above types of gastric resection, proximal gastrectomy, pylorus-preserving gastrectomy (PPG) and Segmental gastrectomy can be considered according to tumor location.
(3) For tumors where the primary or metastatic lesion directly invades adjacent organs, gastrectomy with combined resection of the involved organs may be performed with curative intent. If tumors are located along the greater curvature and harbor metastasis to no. 4sb lymph nodes and total gastrectomy with splenectomy should be taken into account. In other cases, prophylactic splenectomy is not recommended unless the tumor directly invades the spleen.
4.3.5. Lymph node dissection
The extent of systematic lymphadenectomy is defined as follows according to the type of gastrectomy conducted, as what is recommended by the current evidence-based medical evidence and domestic and foreign guidelines (Table 4 ).
Table 4. Extent of lymph node dissection.
| Surgery | D0 | D1 | D1+ | D2 |
| *, Esophagus is invaded. | ||||
| Total gastrectomy | <D1 | No. 1−7 | D1+ No. 8a, 9, 11p
No. 110* |
D1+ No. 8a, 9, 11p, 11d, 12a
No. 19, 20, 110, 111* |
| Distal gastrectomy | <D1 | No. 1, 3, 4sb, 4d, 5, 6, 7 | D1+ No. 8a, 9 | D1+ No. 8a, 9, 11p, 12a |
| Proximal gastrectomy | <D1 | No. 1, 2, 3a, 4sa, 4sb, 7 | D1+ No. 8a, 9, 11p No. 110* | |
| Pylorus-preserving gastrectomy | No. 1, 3, 4sb, 4d, 6, 7 | D1+: D1+ No. 8a, 9 | ||
D1 lymphadenectomy: D1 lymph node dissection involves the resection of the greater and lesser omentum, right and left paracardial lymph node, lymph node along the greater and lesser curvature, suprapyloric and infrapyloric lymph node adjacent to the right gastric artery, as well as lymph node along the left gastric artery. A D1 lymphadenectomy is indicated for cT1aN0 and cT1bN0 tumors with differentiated type and <1.5 cm in diameter. A D1+ lymphadenectomy is indicated for cT1N0 tumors except for the above.
D2 lymphadenectomy: Besides the D1 lymph nodes, the lymph nodes along the common hepatic artery, the celiac artery and the proximal/distal splenic artery, as well as those along the hepatic artery in the hepatoduodenal ligament are additionally resected in a D2 lymphadenectomy, which is indicated for potentially curable T2−T4 tumors as well as cN(+) tumors. (Perigastric lymph node stations are detailed in Appendix 5 ,6 ). The examination of 16 or more regional lymph nodes analgesia for N status and prognosis determination.
When the extent of lymphadenectomy performed does not fully comply with the D level criteria, the actual excision situation can be truthfully recorded as the following examples: D1 (+ No. 8a), D2 (−No. 10), and so on.
Extended lymphadenectomy: Extended lymphadenectomy should be considered in the following situation. (A) D2+ No. 10 lymphadenectomy is recommended for advanced tumors invading the greater curvature of the upper stomach. (B) Dissection of D2+ No. 14v can be performed when harbor metastasis to the No. 6 nodes is suspected in the lower stomach. (C) Complete clearance of D2+ No. 13 should be considered for a potentially curative gastrectomy for tumors invading the duodenum.
The role of resection of LN at the splenic hilum (No. 10) has long been an issue of controversy. There is a variable rate of No. 10 nodes metastasis from different pieces of literature. Therefore, splenic hilar lymph node dissection is not required in patients with stage T1 and T2, while lymphadenectomy of splenic hilar lymph node dissection is only considered for the primary T3−4 tumors with 6 cm or larger in diameter that is located in the greater curvature and upper-middle stomach.
4.3.6. EGJ cancer
There has been no consensus on the approach of gastrectomy and the extent of lymphadenectomy for the EGJ cancer currently so far. Based on currently available evidence, the following recommendations may be made:
(1) EGJ cancer has been defined as cancer with its center located within 2 cm of the EGJ and its diameter ≤4 cm, for which proximal gastrectomy (+ lower esophagectomy) or total gastrectomy (+ lower esophagectomy) can be performed. The extent of lymphadenectomy for cT1 tumors is recommended to include No. 1, 2, 3, 7, 9, 19 and 20, while No. 1, 2, 3, 7, 8a, 9, 11p, 11d, 19, 20 nodes should be cleared for cT2−4 tumors. And dissection of lower mediastinal nodes should be added for the tumor with its center above the EGJ.
(2) A transhiatal abdominal approach is recommended for distal esophageal invasion less than 3 cm. A transthoracic approach may be an option where a greater length than 3 cm of the distal esophagus is involved if the surgery is potentially curative.
4.3.7. Laparoscopic surgery
Indications: gastric cancer with invasion depth within T2, or laparoscopic staging. At present, more and more clinical research results have confirmed the safety and long-term efficacy of laparoscopy for advanced gastric cancer. However, each center should choose its indications carefully according to the experience of its team, and conducts randomized controlled studies to explore the application of laparoscopic technology in gastric cancer surgery.
4.3.8. Reconstruction after gastrectomy
With different types of gastrectomy, there are different methods of digestive tract reconstruction. Various staplers can be considered for reconstruction as needed to increase the safety of anastomotic and reduce the incidence of complications. Based on current evidence-based medical data, the following reconstruction methods are recommended.
(1) Reconstruction after total gastrectomy: Roux-en-Y esophagojejunostomy and Jejunal interposition.
(2) Reconstruction after distal gastrectomy: Billroth I gastroduodenostomy, Billroth II gastrojejunostomy, Roux-en-Y gastrojejunostomy and Jejunal interposition.
(3) Reconstruction after pylorus-preserving gastrectomy: Gastrogastrostomy.
(4) Reconstruction after proximal gastrectomy: Esophagogastrostomy and Jejunal interposition.
4.3.9. Others about surgery
(1) Splenectomy: Splenectomy may be considered for potentially curable primary T2−T4 tumors located in the greater curvature of the upper stomach and directly invading the spleen. Otherwise, splenectomy for the purpose of lymph node dissection is not recommended.
(2) For T1/T2 tumors, the omentum more than 3 cm away from the gastroepiploic arcade is taken into consideration to be retained.
(3) For tumors where the primary or metastatic lesion directly invades adjacent organs, gastrectomy with combined resection of the involved organs may be employed to achieve an R0 resection.
4.3.10. Administration of perioperative medication
(1) Administration of antibiotics
Prophylactic administration: The incision of gastric cancer surgery is a type II incision, so prophylactic antibiotics are recommended. When there is a possibility of being contaminated by gram-negative bacillus, streptococcus, oropharynx anaerobic bacteria (such as streptococcus digestion), the first- and second-generation cephalosporins or cephalomycin should be considered. For the patients with an allergy to β-lactamase, clindamycin + aminoglycosides, or aminoglycosides + metronidazole can be recommended. The antibiotics should be administered through intravenous infusion within 0.5−1 h before skin and mucosa are incised or at the beginning of anesthesia. The operation should not be started until all the infusion is transfused so that the antibiotic concentration in the local tissue is sufficient to kill the bacteria during the operation. And the effective duration of antibiotics should cover the entire surgical procedure. So an extra antibiotic should be added intraoperatively if the following conditions occur: surgery lasts more than 3 h or more than twice the half-life of the drugs, or blood loss exceeds 1,500 mL in an adult. The duration of prophylactic antibiotics for type II incision surgery is 24 h and can be extended to 48 h if necessary. Excessive prolonged drug administration for more than 48 h is not able to further improve the prophylactic effect. On the contrary, the risk of drug-resistant infection will increase if the use of prophylactic antibiotics exceeds 48 h.
Therapeutic administration: The antibiotic treatment plan is formulated based on the pathogen, the infection site, the severity of infections; the physiological and pathological conditions of patients; and the pharmacodynamics and pharmacokinetic characteristics of antibiotics. And the schedule should involve the type and the dosage of the antibiotics, the frequency, the route and the course of the antibiotic administration, as well as the combination and the compatibility of the antibiotics. The withdrawal of antibiotics can only be considered 72−96 h after the temperature is normal and the symptoms are relieved.
(2) Nutritional support treatment
Patient-generated subjective global assessment (PG-SGA), in combination with nutritional risk screening 2002 (NRS-2002) is recommended to screen and assess the nutritional risk.
All patients with NRS-2002 ≥3 or PG-SGA scoring 2−8 should receive nutritional support before surgery, and those who are scheduled for a selective surgery with NRS-2002 ≥3 and PG-SGA score ≥9 should undergo preoperative nutritional support for 10−14 d. Patients with major laparotomy surgery intention, regardless of their nutritional condition, are recommended to use immunonutrition for 5−7 d before surgery and continuing to d 7 after surgery or until the patients can orally ingest more than 60% of the nutrition requirement. Immunoenhanced enteral nutrition should contain ω-3PUFA, arginine, and nucleotide. The effects of any one or two of the above three types of nutrients added separately need to be further studied. Oral enteral nutrition support is preferred for nutritional support if possible.
Patients with moderate malnutrition who are scheduled for major surgery and those with severe malnutrition are advised to receive nutritional treatment for 1−2 weeks before surgery, which is worthwhile even if the surgery is postponed. Postoperative nutritional treatment should be given to patients who are still unable to meet their nutritional requirements through a normal diet for more than 7 d after the operation, and to those who cannot meet 60% of their dietary requirements for more than 1 week postoperatively.
Postoperative enteral nutrition is recommended for patients. Patients are encouraged to resume oral feeding as soon as possible. It is recommended that those who can eat orally ingest nutritional support through the mouth. For patients who could not ingest oral nutrition at an early stage, tube feeding may be applied. In this situation, a nasal jejunal tube for enteral nutrition is a good option for patients with gastric cancer.
Timing of supplementary parenteral nutrition (SPN): For the patients with low nutritional risk of NRS-2002 ≤3 or NUTRIC score ≤5, only when EN fails to meet 60% of the patient’s target energy and protein requirements for more than 7 d, may the SPN support therapy be considered. On the contrary, the SPN is recommended to be employed early for patients with high nutritional risk of NRS-2002 ≥5 or NUTRIC score ≥6, when they are unable to obtain the 60% target energy and protein requirements within 48−72 h through enteral nutrition. And when the enteral nutrient supply reaches 60% of the target requirement, stop SPN.
(3) Pain management
Preoperative administration of opioids or non-selective non-steroidal anti-inflammatory drugs (NSAIDs) is not recommended because there is no benefit to patients.
Pain after surgery is a normal body’s response to surgical stimulation (tissue damage). Effective postoperative analgesia therapy can alleviate the pain of patients and also help their recovery. Multi-modal analgesia regimens are recommended, and NSAIDs are recommended as basic postoperative analgesics in the multi-mode by guidelines of the United States and many European countries. Multi-mode analgesia also includes oral acetaminophen, local infiltration injection of ropivacaine around the incision, and combined epidural analgesia in the middle thoracic segment. The application of opioid analgesics should be avoided or reduced as far as possible, due to the adverse reactions of opioids, including affecting gastrointestinal function recovery, respiratory depression, dizziness, nausea, vomiting, etc.
(4) Management of postoperative nausea and vomiting
Incidence of postoperative nausea and vomiting (PONV) in all hospitalized patients is 20%−30%, mainly occurring within 24−48 h after surgery, with a few lasting for 3−5 d. Risk factors associated with PONV include female, postoperative use of opioid analgesics, non-smoking, and a history of PONV or motion sickness.
Prevention of PONV: First, determine the risk of PONV in patients. Patients without PONV risk factors donot need prophylactic medication. For patients with low or moderate risk, one or two types of prophylactic drugs in Table 5 can be applied. For those with high risk, two or three kinds of drugs can be used.
Table 5. Dosage and time of commonly used drugs to prevent PONV.
| Drugs | Administration time | Adult dose | Children dose |
| PONV, postoperative nausea and vomiting; IV, intravenous injection; ODT, oral disintegrating tablet; PO, per os; IM, intramuscular injection. | |||
| Ondansetron | Before the end of the operation | 4 mg IV | 0.05−0.10 mg/kg IV (Maximum dose 4 mg) |
| 8 mg ODT | |||
| Dolasetron | Before the end of the operation | 12.5 mg IV | 0.35 mg/kg IV (Maximum dose 12.5 mg) |
| Granisetron | Before the end of the operation | 0.35−3.00 mg IV | 0.04 mg/kg IV (Maximum dose 6 mg) |
| Tropisetron | Before the end of the operation | 2 mg IV | 0.1 mg/kg IV (Maximum dose 2 mg) |
| Palonosetron | Before induction of anesthesia | 0.075 mg IV | |
| Aprepitant | Before induction of anesthesia | 40 mg PO | |
| Dexamethasone | After surgery | 4−5 mg IV | 0.15 mg/kg IV (Maximum dose 5 mg) |
| Droperidol | Before the end of the operation | 0.625−1.250 mg IV | 0.010−0.015 mg/kg IV (Maximum dose 1.25 mg) |
| Haloperidol | Before the end of the operation
or after induction of anesthesia |
0.5−2.0 mg IM or IV | |
| Diphenhydramine | During induction of anesthesia | 1 mg/kg IV | 0.5 mg/kg IV (Maximum dose 25 mg) |
| Scopolamine | The night before surgery or 2−4 h
before surgery |
Patch | |
The effects of combined administration of prophylactic agents for PONV with different mechanisms are superior to a single drug. 5-HT3 receptor inhibitors, dexamethasone and haloperidol/haloperidol are drugs with excellent efficacy and mild side-effect for preventing PONV. The gold standard to evaluate the clinical effect of prophylactic and therapeutic drugs for PONV is to achieve no nausea or vomiting for 24 h.
Treatment of PONV: when patients leave anesthesia to recover from continuous nausea and vomiting, the bedside examination should first exclude drug stimulation or mechanical factors, and then antiemetic treatment should be conducted.
If a patient first presents with PONV without prophylactic administration, a small dose of 5-HT3 receptor inhibitor should be initiated, usually 1/4 of the prophylactic dose. Dexamethasone 2−4 mg, haloperidol 0.625 mg, or promethazine 6.25−12.5 mg may also be used. If PONV occurs in PACU, propofol 20 mg intravenous injection can be considered.
If prophylactic medication has been used, the treatment should be switched to other types of drugs. If PONV still occurs in patients after the prevention of triple therapy, it cannot be reused within 6 h and should be replaced with other drugs. Repeat administration of 5-HT3 receptor inhibitors with haloperidol or haloperidol at the same dose if it occurs at 6 h. Repeated use of dexamethasone is not recommended.
(5) Perioperative fluid management
Perioperative fluid balance can improve the prognosis of patients undergoing gastrectomy, which should not only avoid tissue perfusion insufficiency and organ function damage caused by low blood volume but also pay attention to tissue edema and increased cardiac load caused by excessive volume load. Intraoperative target-based treatment strategies can maintain appropriate circulating volume and tissue oxygen supply in patients.
(6) Prevention of stress ulcer
Stress ulcer refers to the acute gastrointestinal mucosal erosion and ulcer lesions that occur under the stress state of various severe trauma, critical illness, or severe psychological disease. In severe cases, gastrointestinal bleeding or even perforation may occur, which may aggravate and worsen the original disease and increase the mortality rate. For patients with severe illness, PPI is better than H2RA, and the standard dose of PPI is recommended for intravenous drip, once every 12 h, for at least 3 consecutive days. When the patient’s condition is stable and can tolerate enteral nutrition or has eaten, clinical symptoms begin to improve or are transferred to the common room. The patient can change to oral medication or gradually stop the drug. For non-severe patients, PPI and H2RA have the same efficacy. Due to the low incidence of severe clinical bleeding, studies have shown that the use of drugs in these patients has no obvious effect on preventing bleeding. Therefore, it is not consistent to recommend the prevention of postoperative stress ulcers in non-severe patients.
(7) Perioperative airway management
Perioperative airway management can effectively reduce complications, shorten hospital stay, reduce readmission rate and risk of death, improve the prognosis of patients, and reduce medical expenses. Common treatments for perioperative airway management include antimicrobial agents, glucocorticoids, bronchodilators (β2 agonists and anticholinergics), and mucolysants. For patients with postoperative respiratory tract infection, antimicrobial agents can be used for treatment according to the guiding principles of clinical application of antimicrobial agents (2015 edition). Glucocorticoids and bronchial diastolic agents were used in combination, inhaled by atomization, 2−3 times a day, for a course of treatment of 7−14 d. Ambroxol hydrochloride, commonly used as a perioperative mucolytic agent, can reduce the decrease of pulmonary surface-active substances caused by mechanical injury during surgery, and reduce the incidence of pulmonary complications such as atelectasis. For patients with poor respiratory function or chronic basic pulmonary diseases such as chronic obstructive pulmonary disease (COPD), the preoperative prophylactic application is recommended until postoperative. It should be noted that ambroxol hydrochloride is an intravenous preparation and is not recommended for aerosol inhalation.
(8) Others
For perioperative medication management and adjustment of patients with underlying diseases, please refer to the topic of perioperative medication management on the UpToDate website. For patients with complex conditions, it is suggested to consult with relevant departments.
4.4. Chemotherapy
Chemotherapy is divided into palliative chemotherapy, adjuvant chemotherapy, neoadjuvant chemotherapy and translational therapy. During chemotherapy, clinical indications should be strictly mastered, contraindication should be excluded, and it should be implemented under the guidance of oncologists. Chemotherapy should give full consideration to patients’ disease stage, age, physical condition, treatment risk, quality of life and patients’ wishes, to avoid excessive or insufficient treatment. Timely evaluation of the efficacy of chemotherapy, close monitoring, prevention of adverse reactions, and appropriate adjustment of drugs and/or dosage. Efficacy was evaluated according to RECIST efficacy evaluation criteria (Appendix 7 ). Adverse reactions were evaluated according to the NCI-CTC standard.
4.4.1. Palliative chemotherapy
The purpose of palliative chemotherapy is to relieve clinical symptoms, improve quality of life and prolong survival. It is suitable for patients with good systemic condition, basically normal function of main organs who cannot be resected, postoperative recurrence and metastasis or palliative resection. It is prohibited for severe organ dysfunction, uncontrolled co-morbidity, and expected survival of fewer than 3 months. Commonly used systemic chemotherapy drugs include 5-fluorouracil (5-FU), capecitabine, tegafur, cisplatin, oxaliplatin, paclitaxel, docetaxel, albumin paclitaxel, irinotecan, epirubicin, etc. It also includes targeted therapies such as trastuzumab and apatinib. The chemotherapy regimen consists of a combination of 2 or 3 drugs, and the combination of 2 drugs includes 5-FU/leucovorin + cisplatin (5-FU/LV+FP), capecitabine + cisplatin (XP), tegafur + cisplatin (SP), 5-FU+oxaliplatin (FOLFOX), capecitabine + oxaliplatin (XELOX), tegafur + oxaliplatin (SOX), capecitabine + paclitaxel, capecitabine + docetaxel, 5-FU/leucovorin + irinotecan (FOLFIRI) and so on. The combination of three drugs is suitable for patients with advanced gastric cancer in good physical condition. Common regimens include: epirubicin + cisplatin + 5-fluorouracil (ECF) and its derivatives (EOX, ECX, EOF), docetaxel + cisplatin + 5-fluorouracil (DCF) and its improved programs (FLOT, DOX, DOS), etc. As a second-line treatment, nab-paclitaxel has the same efficacy as ordinary paclitaxel, and rarely occurs allergic reactions. It is also an optional chemotherapeutic drug at present. Consider single-agent chemotherapy with oral fluorouracils or taxanes for patients with poor performance status and advanced age. For patients with advanced gastric cancer with positive HER2 expression [+++ by immunohistochemical staining, or ++ by immunohistochemical staining and positive (fluorescence in situ hybridization) FISH test], trastuzumab, a molecular targeted therapy drug, can be combined with chemotherapy. Apatinib alone may be considered for patients with advanced gastric cancer who have failed 2 previous chemotherapy regimens and are in good physical condition.
Precautions for palliative chemotherapy are as follows:
(1) Gastric cancer is a heterogeneous malignant tumor. It is difficult to treat and patients should be actively encouraged to participate in clinical research.
(2) For patients with recurrent metastatic gastric cancer, the 3-drug regimen is suitable for those with a large tumor burden and good physical condition. Single-agent chemotherapy is indicated for patients with advanced age, poor physical condition, or mild organ dysfunction.
(3) For patients with disease control after systematic chemotherapy, regular review is still required. According to retrospective and observational studies, sequential single-drug maintenance therapy after standard chemotherapy can improve the quality of life and reduce adverse reactions compared with standard chemotherapy, which can generally be carried out after 4−6 cycles of standard chemotherapy.
(4) Peritoneal metastasis is a special mode of metastasis in patients with advanced gastric cancer, often affecting patients’ food intake and quality of life due to cancerous ascites and cancerous intestinal obstruction. According to abdominal distension, ascites drainage and intraperitoneal perfusion chemotherapy can be performed. After the general condition is improved, combined systemic chemotherapy can be selected.
4.4.2. Adjuvant chemotherapy
Adjuvant chemotherapy is suitable for patients with stages II and III after D2 radical surgery. Adjuvant chemotherapy is not recommended in stage Ia. There is no evidence-based medical evidence for postoperative adjuvant chemotherapy for stage Ib gastric cancer. However, lymph node-positive patients (pT1N1M0) may consider adjuvant chemotherapy. For patients with pT2N0M0, if age <40 years old, histology is poorly differentiated, with nerve bundles, blood vessels or lymphatic vessels infiltration should receive adjuvant chemotherapy, mostly a single-drug regimen, which may reduce recurrence. Combined chemotherapy should be completed within 6 months, and single-agent chemotherapy should not exceed 1 year. The combined regimen of fluorouracil and platinum is recommended for adjuvant chemotherapy. For patients with a poor physical condition, advanced age and intolerance to the combined regimen, oral fluorouracil single-drug chemotherapy is considered.
Precautions for adjuvant chemotherapy are as follows:
(1) Adjuvant chemotherapy begins when the patient’s physical condition returns to normal after surgery, generally starting 4 weeks after surgery. It should be noted that the patient’s postoperative diet needs to be restored before the start of chemotherapy, and perioperative complications need to be alleviated.
(2) Other two-drug combination regimens of fluorouracils combined with platinum may also be considered for adjuvant chemotherapy applications. The latest study suggests that the use of docetaxel in combination with tegafur capsules in patients with stage III gastric cancer has improved outcomes compared with single-agent tegafur capsules. Docetaxel combined with tegafur may be another option for adjuvant chemotherapy.
(3) Observational studies suggest that patients in stage II who receive single-drug chemotherapy are similar to those who receive combination chemotherapy, but patients in stage III benefit more from combination therapy. When choosing a single-agent or combination chemotherapy regimen, it is also necessary to consider the patient’s physical condition, age, underlying disease and pathological type.
(4) During adjuvant chemotherapy, the dose should be adjusted in a standardized and reasonable way, and the nutrition and physical condition of patients should be closely observed, to maintain body weight and immune function. When combined chemotherapy cannot be tolerated, the dose can be reduced or adjusted to a single drug, and the treatment cycle can be ensured as far as possible when maintaining the overall condition.
4.4.3. Neoadjuvant chemotherapy
For locally advanced gastric cancer without distant metastasis (T3/4, N+), neoadjuvant chemotherapy is recommended, which should be combined with platinum and fluorouracil, or combined with paclitaxel to form a three-drug combination chemotherapy regimen based on the two-drug regimen, and should not be applied with a single drug. The duration of neoadjuvant chemotherapy should not exceed 3 months, so the efficacy should be evaluated in time, and the adverse reactions should be judged to avoid increasing surgical complications. Postoperative adjuvant therapy should be based on preoperative staging and the efficacy of neoadjuvant chemotherapy. If effective, the original regimen should be continued, or the therapeutic regimen should be adjusted according to the patient’s tolerance; if ineffective, the regimen should be changed, or targeted drugs such as apatinib could be added.
Precautions for neoadjuvant chemotherapy are as follows:
(1) Whether the three-drug regimen is suitable for all neoadjuvant chemotherapy populations, especially for eastern populations, is still controversial. Small sample prospective randomized controlled studies did not show that the three-drug regimen was more effective than the two-drug regimen, and the survival benefit was more obvious. Several prospective clinical studies of two-drug regimens have been conducted in China, which initially showed good efficacy and perioperative safety. It is suggested to communicate fully with patients and their families based on multidisciplinary cooperation based on clinical practice.
(2) For patients who achieve pathological complete remission (pCR), considering a treatment-effective patient, combined with preoperative staging, in principle, it is recommended to continue the preoperative chemotherapy regimen.
(3) For patients with poor efficacy of neoadjuvant chemotherapy, the MDT team should comprehensively evaluate the value and risk of surgery, the timing, and significance of radiotherapy, and the selection of postoperative drug therapy, etc., and communicate with patients and their families in detail.
4.4.4. Conversion therapy
For patients with locally advanced gastric cancer who are initially unresectable but have no distant metastasis, chemotherapy or concurrent chemoradiotherapy can be considered to make the tumor shrink and transform into resectable. The chemotherapy regiments of conversion therapy refer to neoadjuvant chemotherapy regiments and concurrent chemoradiotherapy regiments refer to the chapter on radiotherapy.
Precautions for conversion therapy are as follows:
(1) Patients who are unresectable in oncology are the population discussed in this section, including severe invasion of the primary tumor, regional lymph node metastasis, fixation and fusion into a group, unable to separate from the surrounding normal tissues or surrounded by large vessels, etc. For patients who cannot be resected due to poor physical condition or basic diseases, conversion therapy is not applicable, palliative chemotherapy and radiotherapy can be considered.
(2) Tumor resectability assessment should be based on the decision of the tumor surgery department. Through imaging, endoscopy, and other means. PET-CT and/or laparoscopic exploration could be performed if necessary to accurately perform clinical staging and develop an overall treatment strategy.
(3) Different from neoadjuvant chemotherapy, the evidence-based medical evidence of conversion therapy is mostly derived from the treatment experience of advanced gastric cancer, and R0 resection can only be achieved after tumor regression. Therefore, conversion therapy emphasizes the effect of tumor shrinkage, so the 3-drug chemotherapy scheme can be actively considered when patients can tolerate it.
(4) Preliminary studies suggest that concurrent chemoradiotherapy may achieve greater tumor regression than radiotherapy or chemotherapy alone, but at present, its applicable population and intervention timing need to be further explored, so it is recommended to conduct concurrent chemoradiotherapy in clinical studies. In clinical practice, evaluation by MDT is recommended to determine the best treatment model.
(5) Gastric cancer with no other non-curative factors at the initial diagnosis and only a single distant metastasis, which is technically resectable, is a special one, for example, only accompanied by liver metastasis, ovarian metastasis, lymph node metastasis in the 16th group, positive cytological examination of abdominal cavity exfoliation or localized peritoneal metastasis. In cohort studies, R0 resection has been performed in some patients after tumor shrinkage with conversion therapy, but it is currently only recommended for active consideration in clinical studies. In clinical practice, the patient’s age, underlying disease, physical condition, compliance, social support, metastatic site, pathological type, efficacy and adverse effects of conversion therapy, and other options other than surgery must be comprehensively evaluated by a MDT and the benefits and risks of surgery should be carefully judged.
(6) Possibility of reresection should be evaluated for local recurrence after radical gastrectomy. In the case of a single distant metastasis after radical surgery, in addition to the above-mentioned fifth point, it is still necessary to consider the comprehensive determination of the first operation stage, adjuvant treatment plan, disease-free survival (DFS) time, recurrence risk factors and other factors.
(7) After conversion therapy, it is recommended that a MDT re-evaluate the feasibility and possibility of radical surgery, and communicate fully with patients and their families about the risks and benefits of treatment. The other perioperative efficacy evaluation and safety management are equivalent to neoadjuvant chemotherapy.
4.5. Radiotherapy
Radiotherapy is one of the most important treatments for malignant tumors. According to the follow-up data of clinical studies and autopsy data, the risk of local-regional recurrence and distant metastasis of gastric cancer after surgery is very high. Radiotherapy can reduce the risk of local recurrence by irradiating the primary tumor location and lymphatic drainage area. Under the guidance of multidisciplinary diagnosis and treatment, combining radiotherapy with surgery, chemotherapy, molecular targeted therapy and other treatment methods, a reasonable treatment plan can be formulated to benefit patients. Currently, both the American NCCN guideline and the European Society for Medical Oncology (ESMO) guideline recommend the treatment mode of chemoradiotherapy before or after surgery for locally advanced gastric cancer under specific circumstances. With the development and wide promotion of D2 surgery, the indications and scope of radiotherapy have become hot topics. For preoperative radiotherapy for locally advanced gastric cancer, especially for GEJ cancer, multiple studies have shown that preoperative concurrent chemoradiotherapy can significantly reduce the tumor burden and improve tumor therapeutic effect.
4.5.1. Indications of radiotherapy
(1) Good physical condition, Karnofsky performance status (KPS)≥70 or Eastern Cooperative Oncology Group (ECOG) 0−2
(2) Preoperative radiotherapy
For surgically resectable or potentially resectable locally advanced gastric cancer, preoperative concurrent chemoradiotherapy can achieve a high R0 resection rate and significantly downgrade the tumor stage, thereby improving long-term prognosis. For unresectable locally advanced gastric cancer, preoperative concurrent chemoradiotherapy can significantly shrink the tumor, transform some tumors into resectable lesions, increase the R0 resection rate and improve the prognosis. On the premise of good patient tolerance, preoperative concurrent chemoradiotherapy combined with chemotherapy can be tried.
(3) Postoperative radiotherapy
1) Postoperative radiotherapy is recommended for patients with positive surgical margins; 2) For patients with R0 resection and lymph node dissection <D2 range, if postoperative pathological T3−4 and/or lymph node metastasis, postoperative concurrent chemoradiotherapy is recommended; 3) For patients with R0 resection and D2 lymph node dissection, postoperative concurrent chemoradiotherapy may be considered for patients with postoperative pathologically confirmed lymph node metastasis.
(4) Cancer patients who refused surgical treatment or could not tolerate surgical treatment due to underlying diseases
(5) Palliative radiotherapy for advanced gastric cancer
For gastric cancer patients with distant metastasis, irradiation of primary or metastatic foci according to the situation can achieve the purpose of relieving obstruction, compression, bleeding or pain, and improve the quality of life. Only the primary lesion and the metastatic lesion causing symptoms are irradiated, and the dose is determined according to the lesion size, location and patient tolerance.
4.5.2. Radiotherapy technology
Intensity modulated radiation therapy (IMRT) techniques include volumetric intensity modulated arc therapy (VMAT) and tomotherapy (TOMO). IMRT technology has better conformal and uniform dose distribution than 3D conformal radiotherapy (3D-CRT). Combined with the simultaneous integrated boost (SIB) within the target or the target area, it can improve the radiation dose of gastric tumors without increasing the exposure dose of normal tissues.
(1) Target area of radiotherapy
For unsurgically resected lesions, conventionally fractionated radiotherapy included primary tumor and metastatic lymph nodes, as well as prophylactic irradiation of lymph nodes in high-risk areas (Table 6 ). The range of radiotherapy for postoperative treatment includes selective irradiation of the tumor bed and anastomotic stoma, as well as prophylactic irradiation of lymph nodes in high-risk areas. If the cutting margin distance is <3 cm, the corresponding anastomotic site should be included; for patients with T4b, especially with lesions of the posterior gastric wall, postoperative radiotherapy is recommended, including the tumor bed ( Table 7 ).
Table 6. Selective irradiation of high-risk lymphatic drainage area.
| Primary site | Areas of lymphatic drainage requiring irradiation |
| *, If group 7−12 lymph node metastasis or N stage is stage 2 or 3, then include group 16b1 lymph nodes; #, If metastasis occurs in group 6 lymph nodes, treatment must include group 14 lymph nodes. | |
| Proximal 1/3 of stomach | 7, 8, 9, 11p, 16a2, 16b1* |
| Middle 1/3 of stomach | 7, 8, 9, 11p, 12a, 13, 14#, 16a2, 16b1* |
| Distal 1/3 of stomach | 7, 8, 9, 11p, 12a, 13, 14#, 16a2, 16b1* |
Table 7. Selective irradiation range of target area after the operation.
| Stage | Stomas | Tumor bed
and organ affected area |
Lymphatic
drainage area |
| T4bNany | Cutting margin
≤3 cm must include |
Yes | Yes |
| T1−4aN+ | No | Yes | |
| T4aN0 | No | Yes | |
| T3N0 | No | Yes |
Cases of palliative care may only irradiate the primary lesion and metastatic lesions that cause symptoms.
(2) Dose of radiotherapy
3D-CRT and IMRT were defined by volume dose, while conventional radiotherapy was defined by isocentric dose. The total amount of conventional radiotherapy in concurrent chemoradiotherapy was 45−50 Gy, and the single dose was 1.8−2.0 Gy. The dose of radical radiotherapy is recommended to increase synchronously or sequentially by 56−60 Gy.
1) Dose of postoperative radiotherapy: recommended clinical target volume (CTV) DT 45−50.4 Gy, each time 1.8 Gy, a total of 25−28 times. For those with tumor and/or residual, local contraction field irradiation after large field irradiation with additional dose DT 5−10 Gy.
2) Dose of preoperative radiotherapy: DT 41.4−45.0 Gy was recommended, each time 1.8 Gy, a total of 23−25 times.
3) Dose of radical radiotherapy: DT 54−60 Gy was recommended, each time 2 Gy, a total of 27−30 times.
4) Radiotherapy dose of patients with metastasis and brain metastasis: 30 Gy/10f or 40 Gy/20f or stereotactic radiosurgery.
(3) Radiotherapy techniques
Different radiotherapy techniques are selected according to the radiotherapy equipment in the hospital, such as conventional radiotherapy, 3D-CRT, IMRT, image-guided radiotherapy, etc. It is suggested to use advanced techniques such as 3D-CRT or IMRT to better protect the surrounding normal tissues such as the liver, spinal cord, kidney and intestines, reduce the toxic and side effects of normal tissues and improve the radiotherapy tolerance.
1) Simulation localization: CT simulation localization is recommended. If there is no CT simulation location, a routine simulation location must be performed. The position was supine and fixed. Avoid overeating 3 h before localization. An oral contrast agent or intravenous angiography is helpful for CT localization and target delineation.
2) Multi-field irradiation with 3 or more fields is recommended.
3) If IMRT is used, verification of the plan must be carried out.
4) Local dose can be increased by intraoperative irradiation or external irradiation.
5) Radioactive particle implantation therapy is not recommended for routine use.
(4) Synchronous chemotherapy
Tegafur or capecitabine was preferred as single agents in synchronous chemotherapy regimens. Clinical studies of combined intravenous chemotherapy can be carried out in hospitals where conditions permit. Recommended dosages for tegafur and the maximum dose in different normal tissues are shown in Tables 8 ,9 , respectively. Recommended dose of capecitabine: 800 mg/m2 bid was given orally on the day of radiotherapy.
Table 8. Recommended dosages for tegafur.
| Body surface area (m2) | Dose (calculated by tegafur) (mg/time) |
| <1.25 | 40 |
| 1.25−<1.5 | 50 |
| ≥1.5 | 60 |
Table 9. Maximum dose in different normal tissues.
| Organ | Maximum dose |
| Lung | V20<25% |
| Heart | V30<30% |
| Spinal cord | Dmax≤45 Gy |
| Kidney | V20<25% |
| Small intestine | V45<195 mL |
| Liver | V30<30%
Dmean<25 Gy |
4.6. Targeted therapy
4.6.1. Trastuzumab
(1) Indications
For patients with advanced gastric or GEJ adenocarcinoma who have overexpression of epidermal growth factor receptor 2 (EGFR2/HER2) (+++ by immunohistochemical staining, or ++ by immunohistochemical staining and positive FISH test). Trastuzumab, a molecular targeted therapy drug, is recommended to be combined with chemotherapy. The adaptive population is the patients who have not received the first-line treatment for the metastatic disease, or the patients who have not received the second line or above treatment against HER2.
(2) Contraindications
Patients with a previous history of congestive heart failure, high-risk uncontrolled arrhythmia, angina require medication, valvular disease with clinical significance, transmural myocardial infarction on electrocardiogram, and poorly controlled hypertension.
(3) Pre-treatment evaluation and in-treatment monitoring
Adverse reactions of trastuzumab mainly include myocardial toxicity, infusion reaction, hematologic toxicity and pulmonary toxicity. Therefore, the patient’s medical history, physical condition, baseline tumor status, HER2 status and cardiac function should be comprehensively evaluated before application. The reaction of the infusion should be closely monitored during the first infusion, and the left ventricular ejection fraction (LVEF) should be closely monitored throughout the treatment. Trastuzumab should be discontinued when the absolute reduction of LVEF relative to pre-treatment is more than 16%, or LVEF is lower than the normal range of local medical institutions and the absolute reduction is more than 10% relative to pre-treatment.
(4) Matters need attention
1) According to the results of the ToGA study, trastuzumab is recommended to be added to 5-FU/capecitabine combined with cisplatin for HER2-positive gastric cancer. In addition, several phase II clinical studies have evaluated the efficacy and safety of trastuzumab in combination with other chemotherapy regimens such as paclitaxel, capecitabine plus oxaliplatin, tegafur plus oxaliplatin, tegafur plus cisplatin, etc.
2) In patients with HER2-positive advanced gastric cancer after first-line chemotherapy progress, if trastuzumab has been applied in the first line, the high-level evidence-based basis for the cross-line application is still lacking, and biopsy is recommended if conditions permit. Although preliminary results of multi-center prospective observational studies in China suggest that the continuation of trastuzumab combined with chemotherapy can prolong median progression-free survival (mPFS), it is still not recommended for clinical practice.
3) Other drugs targeting HER2 include anti-HER2 monoclonal antibody pertuzumab, small-molecule tyrosine kinase inhibitor lapatinib, drug-coupled anti-HER2 monoclonal antibody TDM-1, etc. Currently, none of these drugs has obtained positive results in clinical trials, and none of them is recommended for clinical application.
4.6.2. Apatinib
(1) Indications
Apatinib mesylate is a self-developed new drug in China and is a highly selective vascular endothelial growth factor receptor (VEGFR)-2 inhibitor. It is suitable for third-line and above treatment in patients with advanced gastric adenocarcinoma or GEJ adenocarcinoma, and patients are generally in good condition when receiving apatinib treatment.
(2) Contraindications
The contraindication of apatinib is the same as that of palliative chemotherapy, but special attention should be paid to whether patients have a bleeding tendency, basic diseases of the cardiovascular and cerebrovascular system, and renal function.s
(3) Pre-treatment evaluation and in-treatment monitoring
Adverse reactions to apatinib include elevated blood pressure, proteinuria, hand-foot syndrome, hemorrhage, cardiotoxicity and liver toxicity. The bleeding risk, electrocardiogram, and cardiac and liver function should be closely monitored during treatment.
(4) Matters need attention
1) Apatinib alone or in combination with other drugs is not currently recommended for first-line and second-line treatment, except in clinical studies.
2) Prospective studies have found that patients with early onset of hypertension, proteinuria or hand-foot syndrome have increased disease-control rates, recurrence-free survival, and overall survival. Therefore, it is very important to pay active attention to adverse reactions. When trying to use apatinib again, the treatment process should be precisely managed, and the dose should be adjusted appropriately.
3) Attention should be paid to patient education. For patients with physical condition score ECOG≥2, fourth-line chemotherapy, unresectable primary gastric lesions, poor bone marrow functional reserve, and old and weak or thin female patients, to ensure the safety of patients and improve compliance, oral administration can be started from a low dose such as 500 mg qd.
4.7. Immunotherapy
With the wide application of immune checkpoint inhibitors, first-line chemotherapy combined with programmed cell death protein 1 (PD-1) monoclonal antibody (Checkmate 649) and third-line single-agent PD-1 monoclonal antibody therapy for advanced gastric cancer (Attraction 2) have obtained positive results in randomized phase III clinical studies. In addition, several studies related to immune checkpoint inhibitors have also been carried out in the field of second-line treatment and perioperative treatment. Patients are currently advised to actively participate in clinical research.
4.8. Interventional therapy for gastric cancer
Interventional therapy for gastric cancer mainly includes minimally invasive interventional therapy for gastric cancer, liver metastasis, gastric cancer-related bleeding and gastric outlet obstruction.
(1) Interventional therapy for gastric cancer: Transcatheter arterial embolization (TAE), transcatheter arterial chemoembolization (TACE), or transcatheter arterial infusion (TAI) can be applied for palliative or adjuvant treatment of progressive and non-curative gastric cancer, but the efficacy is not yet definite, which needs to be further confirmed by large-scale and prospective studies.
(2) Interventional therapy for liver metastasis of gastric cancer: Interventional therapy can be used as a local minimally invasive treatment for liver metastasis of gastric cancer in addition to surgical resection. It mainly includes radiofrequency ablation, TAE, TACE and TAI.
(3) Interventional treatment for gastric cancer-related bleeding: Interventional treatment such as TAE has unique advantages in the treatment of gastric cancer-related bleeding (including primary rupture hemorrhage, metastasis hemorrhage and postoperative hemorrhage). Interventional therapy can determine the bleeding location by selective or super-selective arteriography and choose appropriate embolization materials to block the bleeding, which can quickly and efficiently complete hemostasis and relieve the symptoms related to bleeding.
(4) Interventional treatment of gastric outlet obstruction: Patients with advanced gastric cancer may present symptoms related to gastric outlet obstruction. Stent implantation guided by X-ray can be used to alleviate symptoms related to obstruction and improve patients’ quality of life.
4.9. Traditional Chinese Medicine (TCM) treatment
TCM treatment can help improve postoperative complications, reduce adverse reactions to radiotherapy and chemotherapy, and improve patients’ quality of life, which can be used as an important auxiliary means for the treatment of gastric cancer. For patients with advanced age, poor physical condition and severe diseases that cannot tolerate western medicine treatment, TCM treatment can be used as an auxiliary treatment.
In addition to the dialectical treatment of TCM and the use of Chinese herbs, Chinese patent medicines focus on Yiqifuzheng, Qingrejiedu, Huoxuehuayu and Ruanjiansanjie can also be used.
For early detection of precancerous lesions (such as chronic atrophic gastritis, gastric adenoma polyps, residual gastritis, gastric ulcer, etc.), Chinese medicine treatment can be selected, and adjustment of diet structure and lifestyle may delay the development of tumors.
4.10. Support treatment
Gastric cancer support/palliative care aims to alleviate symptoms, relieve pain, improve quality of life, manage treatment-related adverse reactions, and improve compliance with anti-tumor therapy. All gastric cancer patients should be screened, evaluated and treated with supportive/palliative care throughout their lives. This includes not only common physical symptoms such as bleeding, obstruction, pain and nausea/vomiting but also psychological problems such as sleep disorders, anxiety and depression. Meanwhile, relevant rehabilitation guidance and follow-up should be strengthened for cancer survivors.
4.10.1. Basic principles of support/palliative care for patients with gastric cancer
Medical institutions should integrate gastric cancer support/palliative care into the whole process of cancer treatment, and all gastric cancer patients should take support/palliative care early in their treatment and screen for support/palliative care needs at an appropriate time or according to clinical indications. Support/palliative care specialists and MDT, which also includes oncologists, support/palliative care physicians, nurses, dietitians, social workers, pharmacists, mental health professionals, etc., should provide real-time treatment to patients and their families.
4.10.2. Management of support/palliative care for patients with gastric cancer
(1) Bleeding
Bleeding in patients with gastric cancer includes acute and chronic bleeding. Acute bleeding is a common symptom in patients with gastric cancer, which may be caused by the tumor itself or treatment.
1) Vital signs and circulatory status should be monitored for acute bleeding, and fluid resuscitation (blood volume supplement, vasoactive drugs, etc.) should be carried out as soon as possible, and acid inhibition and other hemostatic measures should be given. Patients with acute severe bleeding (hematemesis or black stools) should be evaluated immediately by endoscopic examination.
2) Although endoscopic treatment may initially be effective, the probability of re-bleeding is very high.
3) Commonly available treatment options include injection therapy, mechanical therapy (such as endoscopic clip), ablation therapy (such as argon plasma coagulation), or a combination of these methods.
4) Angiographic embolization may be useful in cases where endoscopic therapy is ineffective.
5) External radiation therapy can effectively control acute and chronic gastrointestinal bleeding in multiple small vessels.
6) Chronic bleeding caused by gastric cancer can be treated by proton pump inhibitors, hemostatic drugs and external radiotherapy. Patients with anemia may be given Erythropoiesis-Stimulating Agents (ESAs), iron agents, folic acid, vitamin B12, and other drugs according to their conditions.
(2) Obstruction
For patients with malignant gastric obstruction, the primary objective of support/palliative care is to reduce nausea/vomiting and resume oral feeding if possible.
1) Endoscopic treatment: the placement of an intestinal stent can relieve outlet obstruction, and the placement of the esophageal stent can relieve EGJ/gastric cardia obstruction.
2) Surgical treatment: gastrojejunostomy can be selected, and gastrectomy can be performed for some selective patients.
3) Some patients may choose external radiation therapy or chemotherapy.
4) When the obstruction is irreversible, gastrostomy can be performed to alleviate the symptoms of obstruction in patients who are not suitable or ineffective for endoscopic dilatation. If the tumor location permits, gastrointestinal decompression can be performed by percutaneous, endoscopic, surgical or interventional radiological means. For patients with obstruction in the middle or distal part of the stomach and inability to eat, a jejunal nutrition tube can be placed if the tumor location permits.
5) If ascites is present, the ascites should be drained before the gastrostomy tube is placed to reduce the risk of infection-related complications.
(3) Cancer pain
1) The chief complaint of patients is the gold standard of pain assessment, and the intensity of pain must be evaluated before analgesia treatment. The assessment includes the cause, characteristics, nature, aggravation or mitigation factors of pain, the impact of pain on patients’ daily life, the efficacy and side effects of analgesic treatment, etc. During the assessment, it is also necessary to determine whether patients have pain caused by tumor emergencies to immediately carry out the corresponding treatment.
2) The WHO three-step analgesia principle is still the most basic principle to be followed in clinical analgesia treatment. Opioids are the cornerstone of cancer pain treatment. If necessary, glucocorticoids, anticonvulsants and other auxiliary drugs should be added, and the adverse reactions of analgesics should be concerned.
3) More than 80% of cancer pain can be alleviated by drug therapy, only a few patients need non-drug analgesic means, including surgery, radiotherapy for pain relief, minimally invasive interventional therapy, etc., so the analgesic effect should be evaluated dynamically and inter-disciplinary cooperation should be actively carried out.
(4) Nausea/vomiting
1) The choice of chemotherapy-induced nausea/vomiting should be based on the vomiting risk of the treatment regimen, previous antiemetic experience, and the patient’s factors, and an adequate dynamic assessment should be conducted for rational management.
2) If nausea/vomiting is suspected to be associated with gastrointestinal obstruction, an endoscopic or fluoroscopy evaluation should be performed to determine the presence of obstruction.
3) Considering other potential factors to vomit: such as vestibular dysfunction, brain metastases, electrolyte imbalance, auxiliary drug therapy (including opioids), the tumor, stomach muscle paralysis induced by chemotherapy or other reasons (such as diabetes), malignant ascites, psychological physiology (including anxiety, prospective nausea/vomiting).
4) Lifestyle management may help reduce nausea/vomiting, such as having more meals a day but less food at each, choosing healthy foods, controlling portion sizes, and avoiding foods that are too cold or too hot. Dietary consultation may also be useful.
(5) Nutritional support
First of all, the nutritional status of each tumor patient should be assessed correctly, and patients with nutritional treatment indications should be screened out for timely treatment. To evaluate the curative effect of nutritional therapy objectively, it is necessary to reevaluate the nutritional status in the process of treatment to adjust the treatment plan in time.
1) Nutritional risk screening should be performed once a patient with a malignant tumor is diagnosed.
2) Currently, the most widely used nutritional risk screening tools for malignant tumors are the NRS-2002 and PG-SGA.
3) Those with an NRS score <3 should be screened once a week during their stay in the hospital, despite no nutritional risk. Patients with NRS scores ≥3 have nutritional risk and need to formulate an individualized nutrition plan and give nutritional intervention according to their clinical conditions.
4) No intervention was required for the PG-SGA score of 0−1 point, and routine follow-up and evaluation are maintained during treatment. Patients with a PG-SGA score of 2−3 need to be educated by a dietitian, nurse practitioner, or physician and may be given medication intervention based on the patient’s existing symptoms and laboratory examination results. Patients with a PG-SGA score of 4−8 require nutritional intervention by a dietitian and may be combined with a physician and nurse practitioner, depending on the severity of symptoms. Patients with a PG-SGA score of 9 require immediate symptom control and/or concurrent nutritional intervention.
5) Medical history, physical examination, and partial laboratory examination are helpful to understand the causes and severity of malnutrition in patients with malignant tumors and to make a comprehensive nutritional assessment.
6) Nutritional risk screening and comprehensive nutritional assessment should be conducted simultaneously with the imaging efficacy evaluation of anti-tumor therapy to comprehensively evaluate the benefits of anti-tumor therapy.
(6) Psychological distress
1) Psychological pain is an unpleasant experience determined by multiple psychological (cognitive, behavioral, emotional), social, spiritual, and/or physical factors that may affect a patient’s ability to cope with tumors, physical symptoms, and treatment. Psychological distress includes things like depression, anxiety, panic, social isolation, and existential crises.
2) Psychological pain should be identified, monitored, and processed at all stages of the disease and in all circumstances.
3) Psychological distress should be assessed and managed according to clinical practice guidelines. An interdisciplinary MDT group should be established to manage and treat the psychological distress of patients and their families.
(7) Anorexia/ cachexy
1) Assess the cause and severity of weight loss, treat reversible anorexia (oral infection, psychological causes, pain, constipation, nausea/vomiting, etc.) early, and assess the effects of medications that affect eating.
2) Develop appropriate exercise programs for patients and provide active nutritional support (enteral or parenteral nutrition).
(8) Other symptoms
1) Constipation: when constipation occurs, it is necessary to evaluate the cause and severity of constipation, expel obstruction, and stool obstruction in time, and treat constipation caused by other reasons. After excluding other reasons, laxatives, gastrointestinal motility drugs, enemas and other treatments can be given. Actively give preventive treatment, such as drinking more water, exercising properly or taking preventive drugs.
2) Sleep/wake disorders: Assess the type and severity of sleep/wake disorders, the patient’s fear and anxiety about death/disease, and treatment-related factors. Provide sleep hygiene education as well as cognitive-behavioral therapy. For refractory sleep/wake disorders, the medication should be given under the guidance of a professional.
4.10.3. Guidelines for healthy behavior for gastric cancer survivors
(1) Maintain a healthy weight for life. Especially in the postoperative period of gastric cancer, the body weight should be monitored regularly, and having more meals a day but less food at each should be encouraged. If necessary, patients should be transeferred to a nutritionist or nutrition department for individual counseling. Pay attention to and actively assess the management of medical and/or psychosocial factors that contribute to weight loss.
(2) Pay attention to the healthy diet from the source of the plant, and adjust the diet according to the sequelae of the treatment needed (e.g., dumping syndrome, intestinal dysfunction).
(3) Take a healthy lifestyle and participate in physical activity. Objective: Try to perform moderate-intensity activities for at least 30 min daily.
(4) Limit alcohol consumption.
(5) Suggest giving up smoking.
4.11. Follow-up
The main purpose of follow-up/monitoring is to detect metastatic recurrence that is acceptable for potential radical treatment, detect tumor recurrence or secondary primary gastric cancer earlier, and timely intervene to improve the overall survival and quality of life of patients. There is no high-level evidence of evidence-based medicine to support which follow-up/monitoring strategies are optimal. Follow-up should be based on the principle of patient individualization and tumor staging. If the patient’s physical condition is not allowed to receive anti-cancer treatment at the time of recurrence, routine tumor follow-up/monitoring of the patient is not recommended.
The main purpose of gastroscopy follow-up after gastric cancer is to find the recurrence of a new tumor or primary tumor under gastroscopy. The anastomosis can be observed under a gastroscope, and a gastric biopsy can be taken to determine the recurrence of the tumor. Gastroscopic examination strategy: It is recommended to perform gastroscopy within 1 year after surgery. If there is any evidence of high-grade atypical hyperplasia or recurrence of gastric cancer during each gastroscopy, it should be reviewed within 1 year. Gastroscopy is recommended once a year. Vitamin B12 and folic acid should be supplemented in patients with megaloblastic anemia after total gastrectomy.
PET-CT and MRI are only recommended for cases where the conventional imaging examination is negative, but the clinician suspects recurrence, such as persistent CEA elevation, abdominal CT examination, or ultrasound negative. PET-CT examinations are not currently recommended as routine follow-up/monitoring tools. The specific methods and frequency of follow-up are shown in Table 10 .
Table 10. Principles of postoperative follow-up for gastric cancer.
| Target | Strategy |
| CEA, carcinoembryonic antigen; CA, carbohydrate antigen; CT, computed tomography. | |
| Follow-up after radical surgery for early gastric cancer | Frequency: Every 6 months for the first 3 years, and then annually for up to 5 years |
| Follow-up items are recommended every time unless particularly stated.
A) Clinical history B) Physical examination C) Blood test including tumor markers (CEA and CA19-9) D) Performance status (PS) E) Body weight F) Ultrasound or chest/abdomen CT (when CEA is abnormal) annually | |
| Follow-up after radical surgery or non-resectable palliative treatment for advanced gastric cancer | Frequency: Every 3 months for the first 2 years, then every 6 months for up to 5 years |
| Follow-up items are recommended every time unless particularly stated.
A) Clinical history B) Physical examination C) Blood test including tumor markers (CEA and CA19-9) D) Performance status (PS) E) Body weight F) Ultrasound or chest/abdomen CT (when CEA is abnormal) every 6 months | |
| New or worsening symptoms | Any time |
Working group members
Group leader: Jiafu Ji
Group members: Rengben Wang, Susheng Shi, Yunpeng Liu, Hongjun Liu, Peng Li, Lin Sheng, Aiwen Wu, Zhiwei Zhou, Aiping Zhou, Xuedong Fang, Dongbing Zhao, Daoyu Hu, Quan Xu, Zhen Zhang, Jun Liang, Han Liang
Translation group members
Jiafu Ji Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Peking University Cancer Hospital & Institute
Zhaode Bu Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Peking University Cancer Hospital & Institute
Jiahui Chen The Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital), Institute of Basic Medicine and Cancer (IBMC), Chinese Academy of Sciences
Appendix 1 Gross classification of gastric cancer
Table A1. Gross classification of early gastric cancer (Paris classification).
| Type | Description |
| Protruded type (0−I) | Type 0−I is subcategorized to type 0−Ip for pedunculated growth and type 0−Is for sessile growth. |
| Superficial type (0−II) | Type 0−II is subcategorized into type 0−IIa for slightly elevated growth, type 0−IIb for flat growth, and type 0−IIc for slightly depressed growth. According to the elevation/depression proportion, the lesions with both superficial elevation and depression are classified into types IIc + IIa for the elevated area in a depressed lesion and types IIa + IIc for a depressed area in an elevated lesion. |
| Excavated type (0−III) | Lesions with both excavation and superficial excavation are classified into type III + IIc for a large excavated lesion in a depressed zone and type IIc + III for a small excavated zone in a depressed lesion, according to the proportion of excavation and superficial excavation. |
Table A2. Gross classification of advanced gastric cancer (Borrmann classification).
| Type | Description |
| I | Polypoid |
| II | Fungating, ulcerated with sharply raised margins |
| III | Ulcerated with poorly defined infiltrative margins |
| III | Diffusely infiltrating |
Appendix 2 Siewert classification of esophagogastric junction (EGJ) tumor
Siewert classification: Siewert and other scholars proposed the typing plan based on the anatomical characteristics of the EGJ, also known as Munich typing. They suggested that distal esophageal adenocarcinoma and cardiac adenocarcinoma should be the same disease, that is adenocarcinoma of the EGJ. Adenocarcinoma of EGJ refers to adenocarcinoma with the tumor center located at the upper and lower 5 cm of the EGJ (Anatomically, EGJ refers to the site where the tubular esophagus becomes the cystic stomach, i.e. the end of the esophagus and the origin of the stomach, which corresponds to the level of Hirschner’s angle or peritoneal reflex or the lower edge of the esophageal sphincter and does not necessarily coincide with the histological boundary of the squamous column.). Tumors are classified into three categories:
Type I: Equivalent to distal esophageal adenocarcinoma. Tumor epicenter located 1−5 cm above esophagogastric junction.
Type II: Equivalent to gastric cardia adenocarcinoma. Tumor epicenter located 1 cm above to 2 cm below EGJ.
Type III: Equivalent to subcardiac adenocarcinoma. Tumor epicenter located 2−5 cm below EGJ.
Appendix 3
Table A3. Histological classification and grade of gastric cancer*.
| Histological classification | ICD-O code |
| *, World Health Organization (WHO) histological classification of gastric tumors (based on the 4th edition of “WHO classification of tumors of the digestive system” in 2010). | |
| Carcinoma | |
| Adenocarcinoma | 8140/3 |
| Papillary adenocarcinoma | 8260/3 |
| Tubular adenocarcinoma | 8211/3 |
| Mucinous adenocarcinoma | 8480/3 |
| Signet-ring cell carcinoma and other poorly cohesive carcinomas | 8490/3 |
| Mixed carcinoma | 8255/3 |
| Adenosquamous carcinoma | 8560/3 |
| Carcinoma with lymphoid stroma (medullary carcinoma) | 8512/3 |
| Hepatoid adenocarcinoma | |
| Squamous cell carcinoma | 8576/3 |
| Undifferentiated carcinoma | 8070/3 |
| 8020/3 | |
| Neuroendocrine neoplasm | |
| Neuroendocrine tumor (NET) | |
| NET G1 | 8240/3 |
| NET G2 | 8249/3 |
| Neuroendocrine carcinoma (NEC) | 8246/3 |
| Small cell carcinoma | 8041/3 |
| Large cell neuroendocrine carcinoma | 8013/3 |
| Mixed adenoneuroendocrine carcinoma | 8244/3 |
| EC cells, 5-HT-secreting NET | 8241/3 |
| Gastrin-secreting NET (gastrinoma) | 8153/3 |
Appendix 4
Table A4. Response evaluation of preoperative adjuvant therapy (TRG).
| TRG | Description |
| TRG, tumor regression grading. Tumor regression grading can only be assessed in primary tumors; Cancer cells refer to surviving cancer cells, excluding degenerative and necrotic cells; Presence of tumor-free mucus lake after neoadjuvant therapy should not be considered as residual cancer. | |
| 0 (Complete response) | No cancer cells, including lymph nodes |
| 1 (Moderately response) | Single cells or small groups of residual cancer cells |
| 2 (Minimal response) | Residual cancer is outgrown by fibrosis |
| 3 (Poor response) | Extensive residual cancer cells; minimal or no treatment effect |
Appendix 5
Table A5. Classification of lymph nodal stations in gastric cancer.
| No. | Lymph node group |
| No. 1 | Right paracardial lymph nodes |
| No. 2 | Left paracardial lymph nodes |
| No. 3 | Lymph nodes along the lesser curvature |
| No. 4sa | Lymph nodes along the short gastric vessels |
| No. 4sb | Lymph nodes along the left gastroepiploic vessels |
| No. 4d | Lymph nodes along the right gastroepiploic vessels |
| No. 5 | Suprapyloric lymph nodes |
| No. 6 | Infrapyloric lymph nodes |
| No. 7 | Lymph nodes along the left gastric artery |
| No. 8a | Lymph nodes along the common hepatic artery (Anterosuperior group) |
| No. 8p | Lymph nodes along the common hepatic artery (Posterior group) |
| No. 9 | Lymph nodes around the celiac artery |
| No. 10 | Lymph nodes at the splenic hilum |
| No. 11p | Lymph nodes along the proximal splenic artery |
| No. 11d | Lymph nodes along the distal splenic artery |
| No. 12a | Lymph nodes in the hepatoduodenal ligament (along the hepatic artery) |
| No. 12b | Lymph nodes in the hepatoduodenal ligament (along the bile duct) |
| No. 12p | Lymph nodes in the hepatoduodenal ligament (behind the portal vein) |
| No. 13 | Lymph nodes on the posterior surface of the pancreatic head |
| No. 14v | Lymph nodes along the superior mesenteric vein |
| No. 14a | Lymph nodes along the superior mesenteric artery |
| No. 15 | Lymph nodes along the top colic vessels |
| No. 16a1 | Lymph nodes in the aortic hiatus |
| No. 16a2 | Lymph nodes around the abdominal aorta (from the upper margin of the celiac trunk to the lower margin of the left renal vein) |
| No. 16b1 | Lymph nodes around the abdominal aorta (from the lower margin of the left renal vein to the upper margin of the inferior mesenteric artery) |
| No. 16b2 | Lymph nodes around the abdominal aorta (from the upper margin of the inferior mesenteric artery to the aortic bifurcation) |
| No. 17 | Lymph nodes on the anterior surface of the pancreatic head |
| No. 18 | Lymph nodes along the inferior margin of the pancreas |
| No. 19 | Infradiaphragmatic lymph nodes |
| No. 20 | Lymph nodes in the esophageal hiatus of the diaphragm |
| No. 110 | Paraesophageal lymph nodes in the lower thorax |
| No. 111 | Supradiaphragmatic lymph nodes |
| No. 112 | Posterior mediastinal lymph nodes |
Appendix 6
Table A6. Lymph node groups by tumor location.
| Lymph node station | Location
|
|||||
| LMU
MUL MLU UML |
LD
L |
LM
M ML |
MU
UM |
U | E+ | |
| No. 1 | 1 | 2 | 1 | 1 | 1 | |
| No. 2 | 1 | M | 3 | 1 | 1 | |
| No. 3 | 1 | 1 | 1 | 1 | 1 | |
| No. 4sa | 1 | M | 3 | 1 | 1 | |
| No. 4sb | 1 | 3 | 1 | 1 | 1 | |
| No. 4d | 1 | 1 | 1 | 1 | 2 | |
| No. 5 | 1 | 1 | 1 | 1 | 3 | |
| No. 6 | 1 | 1 | 1 | 1 | 3 | |
| No. 7 | 2 | 2 | 2 | 2 | 2 | |
| No. 8a | 2 | 2 | 2 | 2 | 2 | |
| No. 8b | 3 | 3 | 3 | 3 | 3 | |
| No. 9 | 2 | 2 | 2 | 2 | 2 | |
| No. 10 | 2 | M | 3 | 2 | 2 | |
| No. 11p | 2 | 2 | 2 | 2 | 2 | |
| No. 11d | 2 | M | 3 | 2 | 2 | |
| No. 12a | 2 | 2 | 2 | 2 | 3 | |
| No. 12b | 3 | 3 | 3 | 3 | 3 | |
| No. 12p | 3 | 3 | 3 | 3 | 3 | |
| No. 13 | 3 | 3 | 3 | M | M | |
| No. 14v | 2 | 2 | 3 | 3 | M | |
| No. 14a | M | M | M | M | M | |
| No. 15 | M | M | M | M | M | |
| No. 16a1 | M | M | M | M | M | |
| No. 16a2 | 3 | 3 | 3 | 3 | 3 | |
| No. 16b1 | 3 | 3 | 3 | 3 | 3 | |
| No. 16b2 | M | M | M | M | M | |
| No. 17 | M | M | M | M | M | |
| No. 18 | M | M | M | M | M | |
| No. 19 | 3 | M | M | 3 | 3 | 2 |
| No. 20 | 3 | M | M | 3 | 3 | 1 |
| No. 110 | M | M | M | M | M | 3 |
| No. 111 | M | M | M | M | M | 3 |
| No. 112 | M | M | M | M | M | 3 |
Appendix 7 Response evaluation criteria of radiation and chemotherapy in gastric cancer
7.1. WHO response evaluation criteria in solid tumors
Complete remission (CR): tumor disappears for more than 1 month.
Partial response (PR): the product of maximum tumor diameter and maximum vertical diameter decreases up to 50%, and none of the other lesions increase for more than 1 month.
Stable disease (SD): the product of two diameters reduces less than 50% or increases less than 25% for more than 1 month.
Progressive disease (PD): the product of two diameters increases more than 25%.
7.2. RECIST 1.1 response evaluation criteria
(1) Evaluation of target lesions
1) CR: All the target lesions disappear. Any pathological lymph nodes (whether target or non-target) must have a reduction in short axis to <10 mm.
2) PR: At least a 30% decrease in the sum of diameters of target lesions, taking as reference the baseline sum diameters.
3) PD: At least a 20% increase in the sum of diameters of target lesions, taking as reference the smallest sum on study (this includes the baseline sum if that is the smallest on the study). In addition to the relative increase of 20%, the sum must also demonstrate an absolute increase of at least 5 mm. (the appearance of one or more new lesions is also considered progression)
4) SD: Neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD, taking as reference the smallest sum diameters while on the study.
(2) Evaluation of non-target lesions
1) CR: Disappearance of all non-target lesions and normalization of tumor marker level.
2) Incomplete response/SD: Persistence of one or more non-target lesion(s) or/and maintenance of tumor marker level above the normal limits.
3) PD: Appearance of one or more new lesions and/or unequivocal progression of existing non-target lesions.
(3) Evaluation of best overall response
The best overall response is the best response recorded from the start of the treatment until disease progression/recurrence. In general, the patient’s best response assignment will depend on the achievement of both measurement and confirmation criteria.