1. Overview
Cervical cancer is one of the most common gynecological malignancies worldwide. The incidence of cervical cancer ranks second for female malignancies in China. There were more than 569,000 new cases of cervical cancer and 311,000 die of the disease worldwide in 2018. More than 85% of the cases occurred in developing countries. In 2015, there were about 111,000 new cases and 34,000 die of the disease in China. The distribution of cervical cancer deaths in China is generally higher in rural areas than in urban areas. The number of deaths from cervical cancer in the central and western regions of our country is about twice that of the eastern region. The median age of onset of cervical cancer patients in China is 51 years old, but it is mainly more likely to occur in two age groups, with 40−50 years old as the most, 60−70 years old as the second, and it is rare before the age of 20 years. However, the age of onset is becoming younger in recent years. Therefore, it is very necessary to standardize the diagnosis and treatment of cervical cancer nationwide. The occurrence of cervical cancer could be effectively controlled by early examination, diagnosis and treatment of precancerous lesions. Experience in Western countries shows that the incidence of cervical cancer has decreased by 70%−90% in closely screened populations. WHO started the global strategy “Accelerate the elimination of cervical cancer” on November 17, 2020.
This guideline applies to cervical squamous cell carcinoma, adenocarcinoma and adenosquamous carcinoma, which accounts for more than 90% of cervical cancers. Several unique pathological types have low incidences, and no consensus has been reached regarding its diagnosis and treatment in China and abroad. Hence, this guideline is not appropriate for cervical cancers with rare pathological types. This guideline references internationally recognized guidelines for the diagnosis and treatment of cervical cancer and amends it with our past guidelines. In current clinical practice, more attention is paid for comprehensive and individualized treatment. Current treatment strategies are influenced by a combination of hospital equipment, technical and patient’s condition. With regards to patients with complex cervical cancer, clinicians should follow these guidelines in a rational manner. For patients that fall out of these guidelines, clinical trials should be recommended.
2. Etiology
At present, it has been established that persistent infection of high-risk human papilloma virus (HPV) is a necessary factor for the occurrence of cervical cancer and precancerous lesions, that is, HPV infection is the most critical link in the process of cervical cancer. Over the lifetime of a female, the probability of infection with high-risk HPV is more than 70%, but less than 10% of females develop cervical cancer or cervical intraepithelial neoplasia (CIN). The main cause is that 80% of females have a transient HPV infection. In addition to the role of persistent high-risk HPV infection, the participation and action of other endogenous and exogenous factors are required to cause the occurrence of cervical cancer. Therefore, the risk factors of cervical cancer are divided into two categories: one is biological factors, that is, high-risk HPV persistent infection; the second is exogenous behavioral risk factors.
2.1. HPV infection
More than 200 subtypes of HPV have been identified, and about 54 subtypes can infect the mucous membranes of the genital tract. According to the different risks of HPV causing cervical cancer, they are divided into high-risk and low-risk types. High-risk HPV (e.g., HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68) are associated with the development of cervical cancer, particularly HPV 16 and 18. Low-risk HPV (e.g., 36, 11, 42, 43 and 44) cause genital and perianal genital warts. Presently, the human papillomavirus vaccine has been on the market in China, and is administered based on appropriate age to prevent cervical precancerous lesions and cervical cancer.
2.2. Behavioral risk factors
Because HPV is primarily sexually transmitted, some risk factors may increase HPV infection such as young age at the beginning of sexual activity, multiple sexual partners, poor sexual hygiene and a history of sexually transmitted, thereby may increase the risk of cervical cancer; menstrual and maternal factors: early marriage, early childbearing, multiple pregnancy and poor hygiene during puerperium and menstruation; smoking; oral contraceptive; autoimmune diseases or long-term immunosuppression (e.g., patients with kidney transplantation need long-term oral immunosuppressive drugs); poor nutritional status, nutritional disorders: such as deficiency of β carotene, folic acid, vitamin A and vitamin C, imbalance of trace elements, etc.
3. Clinical manifestations
3.1. Symptoms
Precancerous lesions and early cervical cancer are asymptomatic, as the disease progresses, there will be contact vaginal bleeding, abnormal vaginal discharge such as bloody vaginal discharge, increased vaginal discharge, irregular vaginal bleeding or postmenopausal vaginal bleeding. Late-stage patients may have severe vaginal hemorrhage and watery vaginal discharge. In addition, there may be symptoms caused by tumor invasion of other organs, such as hematuria that can occur when violating the bladder, bloody stool that can appear when violating the rectum, fistula that can occur when tumor invading bladder or rectum. Parametrial compression of the ureter leads to hydronephrosis and low back pain, and lung metastases may lead to cough, hemoptysis and other related symptoms. Tumor-related infection may present with fever. There may also be renal failure and cachexia.
3.2. Signs
Early-stage cervical cancer (stages IA1 and IA2) may not have any specific signs. Invasive cervical cancer (stage IB1 or above) can be found through gynecological examination, which can be divided into cauliflower type, nodule type, ulcer type and cervical canal type, and the cervical canal type is sometimes manifested as a smooth cervical surface, and only the cervical canal is significantly thickened and the texture becomes rigid. Mass can be found when tumor involving vagina or vaginal vault. Patients with parametrial infiltration can be found parametrial thickening during gynecological examination. For example, in stage IIIB, the carcinoma extends to the pelvic wall. Patients with advanced stage may have enlarged lymph nodes in the groin or supraclavicular region.
4. Diagnostic examination
4.1. Cervical/vaginal cytology examination and HPV test
This is the initial screening method used for early diagnosis of cervical cancer and precancerous lesions. Biopsies are obtained from the transitional zone of the cervical epithelium. At present, cervical thinprep cytologic test (TCT) is the method of choice. The combination of TCT and HPV test helps to improve accuracy. Patients with positive HPV 16 or 18 are advised to refer directly to colposcopy for histological biopsy.
4.2. Cervical colposcopy
Colposcopy plays an important role in finding precancerous lesions, early-stage cervical cancer and determining the site of lesions, which can improve the positive rate of biopsy. In the medical unit that does not have colposcope, using a 3% or 5% acetic acid or Lugol solution to stain cervix, biopsy sites could be observed by the naked eye. Biopsy samples from the cervix should be acquired from “acetowhite changed” or iodine unstained areas and send for pathological examination. Attention should be paid to the importance of cervical curettage during colposcopy, especially when squamous intraepithelial lesions were found extending from the transformation zone to the cervical canal, cytological screening suggested atypical adenosine cells, and no scale-column transformation zone was observed under colposcopy. Only a professional colposcopist can decide to omit cervical curettage, otherwise cervical curettage should be performed in all patients undergoing colposcopic biopsy.
4.3. Gynecological examination
Gynecological examination is the most important means of clinical staging. Clinical staging needs to be decided by two gynecologists with professional title or above. Once the staging is determined, the staging cannot be changed after treatment.
4.3.1. Assessment
Clinical examination and assessment is through direct vulva examination, and examination of the vagina and cervix using a vaginal speculum. Attention should be paid to the location, scope, shape, and volume of cervical tumors and its relationship with surrounding tissues, and as well as the extent of involvement.
4.3.2. Palpation
The texture, infiltration and tumor association to surrounding organs must be determined by palpation. For some submucosal and intraductal infiltration, palpation is more accurate than visual diagnosis. Rectovaginal examination is used to determine whether there is parametrial infiltration, proximity extent between the tumor and pelvic wall, uterosacral ligament, uterine rectal fossa, and surrounding organs.
4.4. Pathological diagnosis
Pathological examination of cervical biopsies after colposcopy or visual observation is the gold standard for final diagnosis. For difficult or rare pathological types (e.g. adenocarcinoma or small cell carcinoma et al), immunohistochemical examination should be performed to identify tumor or determine differential diagnosis. If the diagnosis cannot be confirmed by multiple-site biopsy, further deep tissue biopsy can be used. For patients whose cervical surface biopsy is negative, the vaginal cytological smear is positive, or the imaging examination cannot rule out the cervical duct cancer, the cervical conization should be carried out for pathological examination. Due to the small size of cervical biopsy tissue, the depth and scope of invasion of cervical lesions cannot be completely determined. Therefore, the diagnosis of stage IA1 and stage IA2 early invasive cervical cancer must be confirmed by postoperative pathology of cervical conization.
4.5. Imaging examination
Due to the superficial anatomical site, most cervical cancers can be diagnosed by gynecological examination and cytopathological examination. The value of imaging examination for cervical cancer is mainly to evaluate the extent of local invasion, lymph node metastasis and distant organ metastasis. Furthermore it guides clinical decision-making and assess treatment efficacy. The following are imaging methods used for cervical cancer.
4.5.1. Abdominal and pelvic ultrasound
They are mainly used to detect local cervical lesions, pelvic and retroperitoneal lymph node metastasis, supervicial lymph node metastasis and other abdominal and pelvic organ metastasis. At present, the evaluation of cervical local lesions and systemic metastasis mainly relies on magnetic resonance imaging (MRI) and computed tomography (CT) examination.
4.5.2. Pelvic MRI
MRI is a multi-sequencing and multi-parameter imaging modality with excellent soft tissue resolution, which has no radiation and is the best imaging method for cervical cancer detection. Its advantages include: 1) detecting lesions and determining the size and location, excluding endogenous lesions especially for patients with CIN3; 2) defining the extent of lesion invasion, providing an important basis for staging before treatment, revealing the depth of cervical stromal invasion, determining whether the lesion is confined to the cervix, or invading the parauterine or pelvic wall, presenting the extent of vaginal lesions. But sometimes it is difficult to distinguish between intruding into the vaginal cavity and directly invading the vaginal wall; 3) detecting the invasion of the bladder and rectal wall, but the diagnosis needs to be confirmed by endoscopic examination; and 4) detecting lymph node metastasis in the pelvic cavity, retroperitoneal area and inguinal region. For non-surgical patients, it can be used to delineate the target volumes, monitor curative effect during treatment, evaluate curative effect at the end of treatment and follow up after treatment.
4.5.3. Abdominal and pelvic CT
CT has low resolution of soft tissues, and the density of lesions on plain scan is similar to that of normal cervix, especially for early cervical cancer limited to the cervix. The contrast ratio of enhanced CT scan is better than that of unenhanced CT scan, but nearly half of the lesions are isodense and difficult to determine the range. The advantages of CT are to display lesions during mid and advanced stages, evaluate the relationship between cervical lesions and peripheral organs, lymph node metastasis, and large-scale scanning for metastasis in other abdominal and pelvic organs. For patients with contraindication to magnetic resonance, CT is preferred.
4.5.4. Chest radiography and CT examination
The main purpose is to exclude lung metastasis and mediastinal lymph node metastasis. Chest radiographs can only exclude obvious lung metastasis and cannot evaluate mediastinal lymph nodes. Therefore, qualified hospitals should still perform chest CT examination.
4.5.5. Nuclear medicine imaging examination
Positron emission tomography-CT (PET-CT) is not recommended to evaluate local infiltration of cervical cancer, but is recommended for patients under the following conditions: 1) staging before treatment for patients with stage IB1 or above in International Federation of Gynecology and Obstetrics (FIGO) staging; 2) when a systemic assessment is needed for patients with cervical cancer found unexpectedly at the time of uterectomy; 3) for the delineation of target areas for radiotherapy; 4) for follow-up monitoring of patients with FIGO stage IB2 or above or other high-risk factors 3−6 months after treatment; and 5) for patients with suspected recurrence and metastasis during follow-up including manifestation of clinical symptoms or elevated tumor markers. Radionuclide bone scans are used only for patients with suspicious bone metastases.
4.5.6. Cystoscopy and rectoscopy examination
Patients with suspected bladder or rectum involvement should undergo endoscopic examination. These patients should be transferred to hospitals capable of performing these procedures.
4.6. Tumor markers examination
Abnormal elevation of tumor markers could assist in the diagnosis, evaluation of therapeutic efficacy, disease monitoring and follow-up monitoring after treatment. Squamous cell carcinoma antigen (SCC) is an important marker for cervical squamous cell carcinoma, SCC level exceeding 1.5 ng/ mL is considered abnormal. Since squamous cell carcinoma is the most common type of cervical cancer, SCC is the most frequently detected serological tumor marker in the diagnosis and treatment of cervical cancer. Carcinoembryonic antigen (CEA), carcinoma antigen 125 (CA125) or CA19-9 are elevated in patients with adenocarcinoma of the uterine cervix.
5. Classification and staging of cervical cancer
5.1. Histological classification of cervical cancer
Histological types for cervical cancer include squamous cell carcinoma, adenocarcinoma, adenosquamous carcinoma and other rare types. Squamous cell carcinoma is the most common, accounting for about 80%, and adenocarcinoma for 15%−20%. With the development of cervical cancer screening, the incidence and mortality of cervical squamous cell carcinoma showed a decreasing trend, but the incidence of adenocarcinoma showed an increasing trend in recent 30 years. The prognosis of squamous cell carcinoma is the best among all pathological types, while the prognosis of cervical adenocarcinoma and adenosquamous carcinoma is relatively poor, and this difference is more obvious in advanced stage. At present, the pathological types of cervical malignant tumors mainly refer to the pathological classification published by the World Health Organization (WHO, 2014) (Table 1 ).
Table 1. WHO classification of tumors of uterine cervix (WHO, 2014).
| Entities | ICD-O
code |
|
| WHO, World Health Organization; NOS, not otherwise specified. | ||
| Epithelial tumors | ||
| Squamous cell tumors and precursors | ||
| Squamous intraepithelial lesions | ||
| Low-grade squamous intraepithelial lesions | 8077/0 | |
| High-grade squamous intraepithelial lesions | 8077/2 | |
| Squamous cell carcinoma, NOS | 8070/3 | |
| Keratinizing | 8071/3 | |
| Non-keratinizing | 8072/3 | |
| Papillary | 8052/3 | |
| Basaloid | 8083/3 | |
| Warty | 8051/3 | |
| Verrucous | 8051/3 | |
| Squamotransitional | 8120/3 | |
| Lymphoepithelioma-like | 8082/3 | |
| Benign squamous cell lesions | ||
| Squamous metaplasia | ||
| Condyloma acuminatum | ||
| Squamous papilloma | 8052/0 | |
| Transitional metaplasia | ||
| Glandular tumors and precursors | ||
| Adenocarcinoma in situ | 8140/2 | |
| Adenocarcinoma | 8140/3 | |
| Endocervical adenocarcinoma, usual type | 8140/3 | |
| Mucinous carcinoma, NOS | 8,480/3 | |
| Gastric type | 8482/3 | |
| Intesitinal type | 8144/3 | |
| Signet-ring cell type | 8490/3 | |
| Villoglandular carcinoma | 8263/3 | |
| Endometrioid carcinoma | 8380/3 | |
| Clear cell carcinoma | 8310/3 | |
| Serous carcinoma | 8441/3 | |
| Mesohephric carcinoma | 9110/3 | |
| Adenocarcinoma admixed with neuroendocrine carcinoma | 8574/3 | |
| Benign glandular tumors and tumor-like lesions | ||
| Endocervical polys | ||
| Müllerian papilloma | ||
| Nabothian cyst | ||
| Tunnel clusters | ||
| Microglandular hyperplasia | ||
| Lobular endocervical gradular hyperplasia | ||
| Diffuse lamina endocervical hyperplasia | ||
| Mesonephric remnants and hyperplasia | ||
| Arias-Stell reaction | ||
| Endocervicosis | ||
| Endometriosisi | ||
| Tuboendometrioid metaplasia | ||
| Ectopic prostate tissues | ||
| Other epithelial tumors | ||
| Adenosquamous carcinoma | 8560/3 | |
| Glassy cell carcinoma | 8015/3 | |
| Adenoid basal carcinoma | 8098/3 | |
| Adenoid cystic carcinoma | 8200/3 | |
| Undifferentiated carcinoma | 8020/3 | |
| Neuroendocrine tumors | ||
| Low-grade neuroendocrine tumors | ||
| Carcinoid tumors | 8240/3 | |
| Atypical carcinoid tumors | 8249/3 | |
| High-grade neuroendocrine tumors | ||
| Small-cell neuroendocrine carcinoma | 8041/3 | |
| Large-cell neuroendocrine carcinoma | 8013/3 | |
| Mesenchymal tumors and tumor-like lesions | ||
| Benign | ||
| Leiomyoma | 8890/0 | |
| Rhabdomyoma | 8905/0 | |
| Others | ||
| Malignant | ||
| Leiomyosarcoma | 8890/3 | |
| Rhabdomyosarcoma | 8910/3 | |
| Alveolar soft-part sarcoma | 9581/3 | |
| Angiosarcoma | 9120/3 | |
| Malignant peripheral nerve sheath tumors | 9540/3 | |
| Other sarcomas | ||
| Liposarcomas | 8850/3 | |
| Undefferentiated endocervical sarcoma | 8805/3 | |
| Ewing sarcoma | 9364/3 | |
| Tumor-like lesions | ||
| Postoperative spindle-cell nodule | ||
| Lymphoma-like lesion | ||
| Mixed epithelial and massenchymal tumors | ||
| Adenomyoma | 8932/0 | |
| Adenosarcoma | 8933/3 | |
| Carcimosarcoma | 8980/3 | |
| Melanocytic tumors | ||
| Blue nevus | 8780/0 | |
| Malignant melanoma | 8720/3 | |
| Germ cell tumors | ||
| Yolk sac tumor | ||
| Lymphoid and myeloid tumors | ||
| Lymphomas | ||
| Myeloid neoplasms | ||
| Secondary tumors | ||
5.2. Staging of cervical cancer
The currently used clinical staging criteria for cervical cancer are modified at FIGO 2018. Clinical staging is determined by gynecological examination (Table 2 ). Compared with the previous version, the staging standard in this edition has been greatly modified. Firstly, in the diagnosis of stage IA, the lateral extend of lesions is no longer considered. The new standard only distinguishes stage IA1 and stage IA2 according to the depth of stromal invasion, mainly considering that the evaluation of lateral extend may be affected by human factors. Secondly, the sub-stages of stage IB were refined, increasing from the original 2 sub-stages to 3 sub-stages, which is more convenient to the selection of postoperative adjuvant therapy and prognosis evaluation. The last important change was the consideration of nodal metastasis has also been revised. Radiology (r) or pathology (p) findings may be used to assess retroperitoneal nodal involvement and are indicated for stage IIIC.
Table 2. Cervical cancer staging based on FIGO 2018 guidelines.
| Stage | Definition |
| FIGO, the International Federation of Gynecology and Obstetrics. | |
| Stage I | The carcinoma is strictly confined to the cervix (extension to the corpus would be disregarded). |
| IA | Invasive carcinoma which can be diagnosed only by microscopy, with maximum depth of invasion ≤5 mm |
| IA1: Measured stromal invasion ≤3 mm in depth | |
| IA2: Measured stromal invasion >3 mm and ≤5 mm in depth | |
| IB | Invasive carcinoma with measured deepest invasion >5 mm (greater than stage IA); lesions limited to the cervix uteri with size measured by maximum tumor diameter |
| IB1: Invasive carcinoma >5 mm depth of stromal invasion and ≤2 cm in greatest dimension | |
| IB2: Invasive carcinoma >2 cm and ≤4 cm in greatest dimension
IB3: Invasive carcinoma >4 cm in greatest dimension |
|
| Stage II | The cervical carcinoma invades beyond the uterus, but has not extended to the lower third of the vagina or the pelvic wall |
| IIA | Involvement limited to the upper two-thirds of the vagina without parametrial invasion |
| IIA1: Invasive carcinoma ≤4 cm in greatest dimension | |
| IIA2: Invasive carcinoma >4 cm in greatest dimension | |
| IIB | With parametrial invasion but not up to the pelvic wall |
| Stage III | The carcinoma involves the lower third of the vagina and/or extends to the pelvic wall and/or causes hydro-nephrosis or non-functioning kidney and/or involves pelvic and/or para-aortic lymph nodes |
| IIIA | Carcinoma involves lower third of the vagina, with no extension to the pelvic wall |
| IIIB | Extension to the pelvic wall and/or hydro-nephrosis or non-functioning kidney (unless known to be due to another cause) |
| IIIC | Involvement of pelvic and/or para-aortic lymph nodes (including micro-metastases), irrespective of tumor size and extent (with r and p notations).
IIIC1: Pelvic lymph node metastasis only IIIC2: Para-aortic lymph node metastasis |
| Stage IV | The carcinoma has extended beyond the true pelvis or has involved (biopsy proven) the mucosa of the bladder or rectum. A bullous edema, as such, does not permit a case to be allotted to stage IV |
| IVA | Spread to adjacent organs |
| IVB | Spread to distant organs |
6. Treatment
6.1. Treatment options of cervical cancer
6.1.1. Microscopic diagnosis for invasive carcinoma
Diagnosis of stage IA tumors is based on microscopic examinations. Cervical biopsy specimens do not reveal all lesions present. Hence conization biopsies are required for accurate diagnosis. For the accurate diagnosis of stage IA cervical cancer, careful pathological examination of conization samples with negative margins is required.
Patients in stage IA1 who have no fertility requirements are advised to receive extrafascial hysterectomy (Type I hysterectomy). If patients wish to preserve fertility, cervical conization could be performed. Patients with negative margins should be followed up regularly. The lymph node metastasis rate of stage IA1 is <1%, hence there is no need for lymph node resection for stage IA1 patients. However, if the lymphovascular space is invaded, cervical conization (negative incision margins) or modified radical hysterectomy with pelvic lymphadenectomy should be performed.
The lymph node metastasis rate for stage IA2 cervical cancer is about 3%−5%. Subradical hysterectomy (type II modified radical hysterectomy) and pelvic lymphadenectomy may be required. If patients have fertility requirements, cervical conization (negative incision margins) or radical trachelectomy with pelvic lymphadenectomy may be selected. For patients who wish to preserve fertility, radical trachelectomy may be advised.
6.1.2. Invasive cervical carcinoma
(1) Stage IB1, IB2 and IIA1 both have good prognosis after surgery or radiotherapy. The surgical procedures are type III radical hysterectomy and pelvic lymphadenectomy ± paraaortic lymph node sampling. Postoperative adjuvant therapy refers to the chapter of radiotherapy. For patients who wish to preserve fertility and the cervical tumor diameter is ≤2 cm, radical trachelectomy and pelvic lymphadenectomy ± para-aortic lymph node sampling could be performed.
(2) Treatment options for stage IB3 and IIA2 include: 1) Concurrent chemoradiation; 2) radical hysterectomy, pelvic lymph node dissection, para-aortic lymph node sampling, and postoperative individualized adjuvant therapy; 3) adjuvant chemotherapy and surgical treatment; 4) hysterectomy after primary chemoradiation. The FIGO Guidelines (2018) also recommended radical hysterectomy and lymphadenectomy following neoadjuvant chemotherapy as another option for the treatment of locally advanced cervical cancer. At present, there is still controversy about the impact of neoadjuvant chemotherapy on the prognosis of cervical cancer patients, so it is generally recommended to be performed in clinical trials or in areas without radiotherapy conditions, especially for pathological types that are relatively insensitive to radiotherapy (such as adenocarcinoma).
The overall 5-year survival rate of stage IB patients is about 80%−90%. Among them, the 5-year survival rate of patients with cervical tumor diameter >4 cm and high-risk factors such as lymph node metastasis, parametrial invasion and/or positive incision margins is between 40% and 70%. For selected newly diagnosed patients with early-stage cervical cancer, chemoradiation may be more beneficial for patients with high-risk factors. At present, the standard treatment for locally advanced cancer patients is concurrent chemoradiation.
(3) Stage IIB and IVA: Concurrent chemoradiotherapy (Detailed treatment plan refers to the chapter of radiotherapy and concurrent chemotherapy).
(4) Stage IVB: Systematic treatment is the principal therapy complemented with supportive treatment, with certain patients having palliative surgery or individualized radiotherapy.
6.2. Surgical treatment
Surgical is the main treatment modality for early cervical cancer (stage IA−IIA). Surgeries include hysterectomy and lymphadenectomy. The extent of surgical excision is different for different stages. In order to describe the extent of surgical excision more properly, many scholars have tried to propose several types of cervical cancer surgery classification systems, among which Piver type and Querleu-Morrow (Q-M) type are accepted and adopted by most scholars at home and abroad.
6.2.1. Piver classification system
The Piver classification system for five types of hysterectomy proposed in 1974 is still widely used today.
Type I: Extrafascial hysterectomy [It is suitable for patients with stage IA1 without lymph-vascular space invasion (LVSI)].
Type II: Modified radical hysterectomy. The resection scope includes 1/2 sacrospinous ligament and main ligament and upper 1/3 of the vagina. (It is suitable for patients with stage IA1 with LVSI and stage IA2).
Type III: Radical hysterectomy. The resection scope includes main ligament adjacent to the pelvic wall, sacrospinous ligament from the sacral attachment, and upper 1/2 of the vagina ((It is a standard radical operation for cervical cancer, suitable for stage IB1, IB2, and selective stage IB3/II A1 patients).
Type IV: Extended radical hysterectomy (applicable to some patients with recurrence).
Type V: Pelvic exenteration (applicable to some patients with recurrence and stage IVA).
6.2.2. Q-M classification system
Q-M classification of hysterectomy published in 2008 is gradually accepted for emphasis on precise anatomy and individualized treatment of surgical resection. The Q-M classification system is a modern surgical classification that describes degree of resection and nerve preservation in three-dimensional (3D) planes of resection. In 2015, the U.S. National Comprehensive Cancer Network (NCCN) guidelines recommended Q-M classification.
Q-M classification system includes two parts: surgical classification of uterus and lymph node dissection. The surgical classification was only related to the extent of paracytomectomy, which was divided by certain anatomical structure (Table 3 ).
Table 3. Querleu-Morrow classification of radical hysterectomy.
| Type | Lateral parametrium | Ventral parametrium | Dorsal parametrium | Vaginectomy |
| A | Halfway between cervix and ureter | Minimal excision | Minimal excision | Less than 1 cm |
| B1 | At the ureter | Partial excision of the vesicouterine ligament | Partial resection of the rectouterine-rectovaginal ligament | Excision of 1 cm
|
| B2 | Identical to B1 plus paracervical lymphadenectomy without resection of vascular structure | Identical to B1 | Identical to B1 | Identical to B1 |
| C1 | At the iliac vessels transversally, caudal part is preserved | Excision of the vesicouterine ligament at the bladder (bladder nerves are dissected and spared) | At the rectum (hypogastric nerve is dissected and spared) | Excision of 2 cm or according to demand |
| C2 | At the level of medial aspect of iliac vessels completely (including the caudal part) | At the bladder (bladder nerves are sacrificed) | At the sacrum (hypogastric nerve is sacrificed) | Identical to C1 |
| D1 | At the pelvic wall, including resection of internal iliac vessels | At the bladder | At the sacrum | According to demand |
| D2 | Identical to D1, including resection of pelvic sidewall | According to demand | According to demand | According to demand |
The classification of lymph node dissection: The extent of retroperitoneal lymphadenectomy was divided into four grades according to the anatomical marker of the artery. The obturator lymph nodes are routinely resected by defaults. Grade 1: removal of nodal tissues of external and internal iliac artery, the boundary with grade 2 is marked by the bifurcation of the internal and external iliac artery. Grade 2: removal of nodal tissues of common iliac artery, the boundary with grade 3 is marked by the bifurcation of abdominal aorta. Grade 3: removal of paraaortic nodal tissues to the level of the inferior mesenteric artery. Grade 4: removal of paraaortic nodal tissues to the level of renal veins.
As pelvic autonomic nerve injury caused by radical hysterectomy results in abnormal bladder function, abnormal colorectal peristalsis and sexual dysfunction, nerve-sparing radical hysterectomy (NSRH) for cervical cancer has been developed. NSRH can be performed through laparotomy, laparoscopy and robotic laparoscopy.
Extrafascial hysterectomy (type I or type A) can be performed vaginally, with open abdominal approach or with minimally invasive (laparoscopy or robotic laparoscopy) approaches. Prospective randomized controlled trials have shown that minimally invasive radical hysterectomy has lower disease-free survival and overall survival compared with abdominal radical hysterectomy.
Lymph node resection for cervical cancer involves pelvic lymph nodes and para-aortic lymph nodes. Pelvic lymphadenectomy ± para-aortic lymph nodes sampling should be performed for stage IA1 (combined with LVSI)−IIA. The postoperative pelvic lymph node metastasis rate for patients with stage I and stage II cervical cancer is 0−16.0% and 24.5%−31.0%. Based on the status of sentinel lymph node metastasis, sentinel lymph node biopsy could reduce the incidence of postoperative complications in patients with cervical cancer. The tracers used for sentinel lymph node mapping include biological dyes, radioisotopes and fluorescent dyes, which can be identified by the naked eye, nuclide detection or infrared detection. Systemic lymphadenectomy and sentinel lymphadenectomy could be performed through laparotomy, laparoscopy and robotic laparoscopy.
The ovarian metastasis rate for stage I−IIA cervical squamous cell carcinoma is less than 1%. For premenopausal patients who require ovarian preservation, the healthy ovary could be preserved during surgery. At present, the risk of occult ovarian metastasis for cervical adenocarcinoma is high, so preserving the ovary should be carefully considered. The retained ovary could be translocated during surgery (i.e., into the abdominal cavity or at a high position in the retroperitoneal paracolon sulcus) to avoid damage to ovarian function induced by postoperative pelvic radiotherapy.
For younger patients with early-stage cervical cancer without lymph node metastasis and who have fertility requirements, fertility-preserving surgery should be performed. For stage IA1 patients without LVSI, cervical conization with negative margins could be performed. If the lesions are wide, trachelectomy should be performed. For stage IA1 patients with LVSI and stage IA2 patients, cervical conization/trachelectomy (the width for negative margins should be 3 mm) + transabdominal/laparoscopic pelvic lymphadenectomy ± para-abdominal aortic lymph node sampling or transabdominal, transvaginal or laparoscopic radical trachelectomy + pelvic lymphadenectomy ± para-abdominal aortic lymph node sampling should be performed. For stage IB1 patients (<2 cm), radical trachelectomy + pelvic lymphadenectomy ± para-abdominal aortic lymph node sampling should be performed. For stage IA2−IB1 patients with LVSI and stage IB1 patients with tumor diameter >2 cm, there is no consensus for fertility-sparing surgery, and has to be carefully considered.
Postoperative adjuvant therapy for patients with cervical cancer should be selected according to recurrence risk factors to reduce recurrence rate and improve prognosis. See the principles of radiotherapy for details.
6.3. Radiotherapy
Medical institutions that do not have radiotherapy qualification should refer patients who need radiotherapy to qualified medical units for treatment without delay. For medical institutions do not equip with brachytherapy equipment, cervical cancer patients requiring brachytherapy should be advised to consult with other institutions before receiving external beam radiation therapy (EBRT), and two-way referral should be done to avoid interruption.
Radiotherapy is suitable for all stages of cervical cancer. Radiotherapy includes EBRT and brachytherapy or the combined application of the two methods. Studies have shown that concurrent chemoradiation improves the efficacy and reduces the risk of recurrence compared to radiotherapy alone. Postoperative adjuvant radiotherapy is indicated for patients with early cervical cancer who are found to have high-risk factors (positive surgical margin, positive parametrium, lymph node metastasis, etc.) or medium-risk factors (such as large tumor size, deep stromal invasion and/or lymphovascular space invasion) in pathological examination after surgery.
6.3.1. Principles of radiotherapy
Radiotherapy is a treatment strategy for malignant tumors. It kills cancer cells to the maximum extent while preserving normal tissues and critical organs. Therefore, appropriate therapeutic equipment, appropriate irradiation range, adequate irradiation dose, uniform dose distribution, reasonable irradiation volume and individual treatment are the basic requirements of radiotherapy.
The deadline for completion of radiotherapy is an essential factor for optimal efficacy. Patients treated with radiotherapy for more than 9 weeks had a higher rate of pelvic control failure than those treated for less than 7 weeks. It has been recommended that all EBRT and brachytherapy should be completed within 8 weeks.
During radical radiotherapy, patients are treated with a radical dose. Due to the large radiation range and high radiation dose, it is necessary to take into account the normal tissues and organs surrounding the tumor, especially the protection of some tissues and organs sensitive to radiation. Palliative radiation therapy aims to relieve symptoms and reduce pain, but does not prolong survival. Radical and palliative radiotherapy is relative, in the process of treatment it can be converted according to the patients and tumor conditions.
If the combination of radiotherapy and surgery is used for systemic treatment, postoperative radiotherapy should be decided according to the condition of tumor and patient. Preoperative radiotherapy is planned. Preoperative radiation therapy is intended to reduce tumor size and improve the surgical resection rate. Postoperative radiotherapy is usually considered after review of postoperative pathologic results together with several other adverse prognostic factors. If high-risk factors are present such as positive surgical margins, parametrial invasion and lymph node metastasis, postoperative adjuvant chemoradiation will be necessary. Based on the Sedlis criteria (Table 4 ) of NCCN guidelines in 2015, postoperative adjuvant pelvic radiotherapy or chemoradiation is required if mid-risk factors such as large tumor size, deep stromal invasion and/or lymphovascular space invasion are observed during or after surgery. This can reduce local recurrence and improve therapeutic efficacy. However, the combination of surgery and radiotherapy also increases complications.
Table 4. Indications for postoperative pelvic radiotherapy for cervical cancer patients with middle-risk factors.
| LVSI | Stromal invasion | Tumor size (cm)
(Determined by clinical palpation) |
| LVSI, lymph-vascular space invasion. | ||
| + | Deep 1/3 | any |
| + | Middle 1/3 | ≥2 |
| + | Superficial 1/3 | ≥5 |
| − | Middle or deep 1/3 | ≥4 |
6.3.2. EBRT
(1) Conventional radiotherapy
Conventional radiotherapy describes the positioning of the simulator or CT simulator. Target volume should generally include the uterus, cervix, parametria and upper 1/2 of the vagina, pelvic lymphatic drainage areas such as internal iliac, obturator, external iliac, and common iliac lymph nodes. Target volume for stage IIIA patients includes all of the vagina and the inguinal region if necessary. Four-field box radiation or isocenter anterior and posterior penetrating radiation is generally used. High-energy 6−12 MV X rays were applied. The EBRT dose is approximately 45 Gy in conventional fractionation of 1.8−2.0 Gy daily, 5 d/week. Stage I−II: 45 Gy/4.5−5.0 weeks, stage III−IV: 45−50 Gy/5−6 weeks.
(2) 3D conformal radiation therapy and intensity-modulated radiation therapy (IMRT)
CT- or MRI-based treatment and conformal blocking are considered the standard of care for EBRT. The gross target volume (GTV) is determined based on rectovaginal examination and imaging results and the clinical target volume (CTV) is determined by direct diffusion of cervical cancer and lymph node metastasis. The target volume for EBRT should include the gross tumor area, parametria, uterosacral ligament, presacral lymph nodes and other potentially involved lymph nodes and sufficiently long vaginal tissues (the lower margin is at least 3 cm from the tumor). If no positive lymph nodes are found during surgery or by imaging, the radiation should include the external iliac lymph nodes, internal iliac lymph nodes, obturator lymph nodes and pre-sacral lymph node drainage area. If the risk of lymph node metastasis is high (such as for bulkier tumors, suspected or confirmed low true pelvic lymph node metastasis), radiation should include the common iliac lymph node areas as well. In patients with documented common iliac and/or para-aortic nodal involvement, extended-field pelvic and para-aortic radiotherapy is recommended, up to the level of the renal vessels (or even more cephalad as directed by involved nodal distribution). If the lesion has invaded the lower 1/3 of the vagina, bilateral inguinal lymph nodes should also be included for radiation therapy. Planning target volume (PTV) is set based on a distance out of CTV (0.5−1.0 cm). The radiotherapy dose is 45−50 Gy/1.8−2.0 Gy/5−6 weeks. For unresectable gross lesions or lymph nodes of limited size, IMRT could be used to treat lesions with an additional dose of 10−20 Gy.
6.3.3. Brachytherapy
With intracavitary brachytherapy, the sealed radioactive source is placed directly into the natural lumen of the human body (such as uterine cavity, vagina, etc.). With interstitial brachytherapy, the radioactive source is placed directly into the tumor tissue. Brachytherapy for cervical cancer has its natural advantages, such as high radiation tolerance of cervix, uterine body and vagina, the shortest distance from radiation source to the tumor, and smaller radiation volume can achieve greater radiotherapy effect.
(1) Frequently-used radioactive sources are listed in Table 5 .
Table 5. Radioactive sources of brachytherapy.
| Radioactive sources | Radium 226 | Cobalt 60 | Cesium 137 | Iridium 192 |
| Specific activity (Ci/cm3) | 2.1 (3.8 at most) | 1,900 | 27.5 | 9,000 |
| Half-life (year) | 1,590 | 5.3 | 33 | 0.2 (74 d) |
(2) Traditional brachytherapy: Stockholm method, Paris method, Manchester method and Beijing method, etc., the commonly used radioactive sources are radium and cesium, which are now rarely used at present.
(3) The dosing prescription regimen of brachytherapy.
Afterloading intracavitary brachytherapy is a technique to perform intracavitary brachytherapy using a remote-controlled afterloader. In this technique, an empty intracavitary applicator is first placed into a body cavity in close proximity to the target tissue. Then, the radiation therapist remotely controls the treatment in the condition of protective shielding and the radioactive source is driven through the transfer tubes and into the applicator.
Intracavitary brachytherapy is a critical component of definitive radiotherapy for cervical cancer. The most commonly used implants were the combination of tandem and vaginal applicators. Radiation oncologists should select appropriate vaginal applicators based upon the characteristics of individual anatomy, the location and the extent of disease. Brachytherapy usually begins in the latter half of external beam radiotherapy (EBRT). With tumor regression, it is easier for brachytherapy to achieve ideal geometrical dose distribution. According to the speed of radiation administrated in cGy, three categories of brachytherapy are defined: low-dose rate (0.667−3.33 cGy/min), medium-dose rate (3.33−20 cGy/min) and high-dose rate (over 20 cGy/min). If intensity modulated radiotherapy was employed, weekly cone-beam CT is recommended during the treatment. At the end of the third week of EBRT, radiological examination should be performed to evaluate the necessity of conducting a second radiotherapy planning.
Generally, brachytherapy is delivered once or twice weekly with a dose of 5−10 Gy to point A weekly. The prescribed dose for point A would be 20−45 Gy and the total dose with the combination of EBRT plus brachytherapy should be at least 75 Gy with an equivalent dose at 2 Gy per fraction (EQD2). Depending on the individualized clinical stage and tumor size, the total dose would be 75−90 Gy. The ICRU rectum and bladder reference point should be restricted under 60%−70% of the dose to point A, or at least not exceed 80%. If these parameters cannot be achieved, reducing dwell position of the radioactive source or prescribed dose may be considered. The recommendation of point A in the NCCN guideline was based on the traditional and widely validated dose fractionation for brachytherapy with LDR. In this dosimetry system, the external dose is delivered at 1.8−2.0 Gy per fraction with an LDR brachytherapy delivery of 40−70 cGy/h to point A. If HDR brachytherapy is used, the nomial HDR dose should be converted to biologically equivalent LDR dose by means of linear-quadratic model equation: EQD2=D×(d+α/β)/(2+α/β). In this formula: D represents the total dose; d is dose per fraction; an α/β ratio of 10 Gy is used for tumor; and an α/β of 3 Gy is used for normal tissues (rectum, bladder and sigmoid) to evaluate the late complications. For brachytherapy in combination with EBRT, the most commomly used HDR fractionation schedules are 30 Gy in 5 fractions and 28 Gy in 4 fractions, which are nearly biologically equivalent to 40 Gy using LDR brachytherapy. 3-dimentional image-guided brachytherapy is recommended for qualified medical institutions to improve clinical outcomes and reduce radiation-related complications.
Limitations of the point A dosing system include the fact that it dose not take into account the 3-dimentioal shape of tumors, nor tumor to normal tissue structures correlations. Studies have shown that image-guided brachytherapy improves survival as well as decreases toxicities. MRI is the optimal imaging modality to evaluate residual diseases at the time of brachytherapy. In the absence of MRI, CT can be used but is inferior for determination of residual diseases and contouring is less accurate. A lower total dose may be considered for small residual tumors and those demonstrating a good response. Recommendation from the Groupe European de Curietherapie and European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) described the definition of GTV and CTV for 3-dimentional image-guided brachytherapy with the guidance of MRI images. GTV is macroscopic tumor extension visualized on the T2 weighted MRI. Three CTVs are proposed based on tumor load and represent risk of recurrence: high-risk CTV (HR-CTV) includes the whole cervix and presumed extracervical tumor extension; intermediate-risk CTV (IR-CTV) represents significant microscopic disease, including initial tumor extent before EBRT; and low-risk CTV (LR-CTV) includes potential microscopic tumor spread, which is generally treated by surgery and/or by external beam radiotherapy. It is recommended to use D90 and D100 for the dose evaluation of GTV, HR-CTV and IR-CTV; V150 and V200 for overall assessment of high-dose volumes; D1cc, D2cc for the dose evaluation of organs at risk. The dose at point A is still needed for reporting as a reference for prescribed dose to target volume. The goal of dose delivery to HR-CTV is 80 Gy; however, with large disease or poor response, HR-CTV should be ≥87 Gy. According to published guidelines, normal tissues should be limited with rectum 2 cm3 dose ≤65−75 Gy, sigmoid 2 cm3 dose ≤70−75 Gy and bladder 2 cm3 dose ≤80−90 Gy. If those parameters cannot be achieved, supplementing dosing with interstitial brachytherapy should be considered.
6.3.4. Combination of brachytherapy and EBRT
Except in a few cases for early-stage cervical cancer that only requires brachytherapy, the combination of brachytherapy and EBRT is necessary. Combined irradiation is effective for the target volume of cervical cancer with uniform dose distribution. The total duration of radiotherapy should be limited to 8 weeks.
6.3.5. Complications of radiotherapy
Due to the different factors such as the type of radiation source, radiation method, radiation area, radiation site, unit dose, total dose, total number of fraction and total treatment time, as well as the difference in patients’ sensitivity to radiation, the incidence and severity of radiotherapy complications are also different. On the one hand, the doctors involved with radiotherapy should understand the complications of radiotherapy, on the other hand, they should be familiar with the dose tolerance of abdominal and pelvic organs to radiation, so as to reduce the complications of radiotherapy.
(1) Acute complications occur during and shortly after treatment, such as infection, vaginitis, vulvitis, dry and wet skin reactions, bone marrow suppression, gastrointestinal reactions, rectal reactions, bladder reactions, mechanical injuries, and others.
(2) Long-term complications include radiation proctitis, radiation cystitis, skin and subcutaneous tissue changes, reproductive organ changes and radiation enteritis. Radiation proctitis is the most common long-term complication and usually occurs 1−1.5 years after radiotherapy. The main manifestations are increased stool frequency, mucous stool, hematochezia, and rectovaginal fistula in severe cases. Radiation cystitis is the second most common late complication and usually occurs 1.5 years after radiotherapy. Its main manifestations are frequent micturition, odynuria, hematuria, dysuria, and vesicovaginal fistula in severe cases.
6.3.6. Normal tissue considerations
Radiotherapy of cervical cancer has potential impact on surrounding critical organs, such as bladder, rectum, colon, bone, skin, small bowel and ureters. TD5/5 which presenting the incidence of severe complications is less than 5% in 5 years after treatment is used to describe the minimum tolerable dose of radiation. TD5/5 of different organs is showed in Table 6 .
Table 6. TD5/5 of impacted tissues.
| Organs | Side effects | TD5/5 | Length or area of radiation |
| Skin | Ulceration, severe fibrosis | 55 | 100 cm2 |
| Bowel | Ulceration, perforation, bleeding | 50 | 100 cm2 |
| Colon | Ulceration, stenosis | 45 | 100 cm2 |
| Rectum | Ulceration, stenosis | 60 | 100 cm2 |
| Kidney | Acute or chronic nephritis | 20 | Kidney |
| Bladder | Contracture | 60 | Entire bladder |
| Ureter | Stenosis | 75 | 5−10 cm |
| Ovary | Permanent sterility | 2−3 | Entire ovary |
| Uterus | Necrosis, perforation | >100 | Entire uterus |
| Vagina | Ulceration, fistula | 90 | Entire vaginal |
| Bones | Necrosis, fracture, sclerosis | 60 | Bone or 10 cm2 |
| Spinal marrow | Infarct necrosis | 45 | 10 cm |
| Muscles | Fibrosis | 60 | Muscles |
| Bone marrow | Hypoplasia | 2 | General |
| 30 | Local | ||
| Lymph nodes | Atrophy sclerosis | 50 | Lymph node |
| Fetus | Death | 2 | Fetus |
| Peripheral nervous | Neuritis | 60 | 10 cm2 |
6.4. Chemotherapy
The efficacy of chemotherapy for cervical cancer treatment has attracted increasing attention. It is mainly used with radiotherapy with chemotherapy (single-agent or combination therapy) for radiotherapy sensitization. It is also used as a preoperative neoadjuvant as well as for palliative treatment for patients with late distant metastasis and recurrence. Effective regimens for the treatment of cervical cancer include cisplatin, paclitaxel, 5-fluorouracil, ifosfamide, gemcitabine, topotecan, etc.
6.4.1. Concurrent chemoradiation
Concurrent chemoradiation refers to simultaneous chemotherapy with radiotherapy, also known as sensitization chemotherapy. The regimens for sensitization chemotherapy during radiotherapy recommend by current NCCN treatment guidelines are weekly therapy of cisplatin 30−40 mg/m2. If the toxicity of cisplatin is intolerant, carboplatin will be preferred.
Concurrent chemoradiation with cisplatin combined regimen is also included in clinical studies: cisplatin 50−70 mg/m2, paclitaxel 135−175 mg/m2, d 1 and d 29 during radiotherapy; cisplatin + paclitaxel weekly, cisplatin 25−30 mg/m2, paclitaxel 60−80 mg/m2, d 1, d 8, d 15, d 22, d 29 and d 36 during radiotherapy. The dose should be adjusted according to the adverse events of chemoradiation in patients, and the general principle is not to affect the process of radiotherapy.
6.4.2. Neoadjuvant chemotherapy
Neoadjuvant chemotherapy refers to 2−3 courses of chemotherapy before surgery. The aim is to reduce the tumor volume, eliminate micrometastases and subclinical lesions, and make patients eligible for surgery. Several non-randomized studies have demonstrated that neoadjuvant chemotherapy reduced the probability of intraoperative dissemination and postoperative metastasis. At present, it is mainly used in patients with early-stage locally advanced cervical cancer. Preferred regimens for neoadjuvant chemotherapy is a platinum-based combination therapy, such as cisplatin + paclitaxel regimen, PVB regimen (cisplatin + vincristine + bleomycin), BIP regimen (cisplatin + bleomycin + ifosfamide + mesisodium), etc. The methods of administration include intravenous systemic chemotherapy or arterial intubation interventional chemotherapy. Currently the most commonly used is paclitaxel + cisplatin regimen.
6.4.3. Systemic chemotherapy
It is mainly used in patients with recurrent or metastatic cervical cancer who did not have surgery or radiotherapy. The first-line chemotherapy regimens recommended by the 2020 NCCN guidelines for cervical cancer are cisplatin combined with paclitaxel, cisplatin combined with paclitaxel and bevacizumab, paclitaxel combined with topotecan and bevacizumab. Carboplatin combined with paclitaxel and bevacizumab is preferred in patients who have received cisplatin. In addition, cisplatin combined with topotecan and topotecan combined with paclitaxel are also alternatives. Available first-line single-agent chemotherapy agents are carboplatin, cisplatin and paclitaxel.
Pembrolizumab has been added as a preferred regimen for second-line option for treating PD-L1-positive or microsatellite instability-high/mismatch repair deficient (MSI-H/dMMR) cervical tumors in NCCN guidelines since 2018. The objective response rate was 14.3% and the complete response rate was 2.6%. Ninety one percent of patients experienced remission for more than six months. The clinical trial in 2021, Keynote-826 (NCT03635567) found that among patients with PD-L1 positive cervical cancer treated with first-line therapy, pembrolizumab combined with chemotherapy ± bevacizumab reduced the risk of death by 36%, and significantly prolonged overall survival and progression-free survival, compared with chemotherapy ± bevacizumab. Based on this, the Food and Drug Administration (FDA) approved pebolizumab + chemotherapy + bevacizumab for first-line treatment of recurrent or metastatic cervical cancer patients whose tumors express PD-L1 (CPS≥1). Second-line chemotherapy regimens include bevacizumab, docetaxel, albumin-bound paclitaxel, gemcitabine, epirubicin, 5-fluorouracil, isocyclophosphoamine, irinotecan, mitomycin, pemetrexel, topotecan, vincristine, etc.
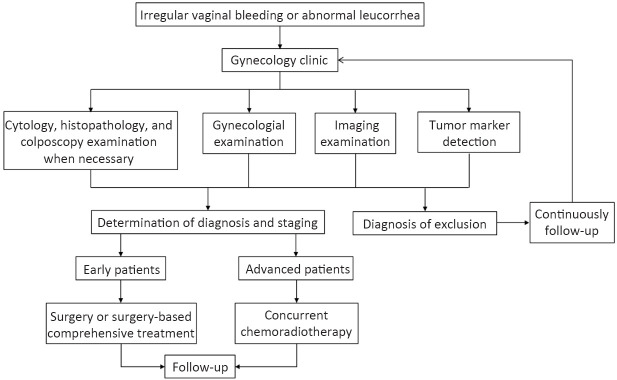
Several immunocheckpoint inhibitors are currently used in clinical trials in combination with targeted agents, chemotherapy or radiotherapy, but more clinical data are needed to support the combination of immunocheckpoint inhibitors. Patients with recurrent and persistent cervical cancer are encouraged to participate in clinical trials. Diagnosis and treatment procedure for cervical cancer is shown in Figure 1 .
Figure 1.
Diagnosis and treatment procedure for cervical cancer
7. Follow-up
For newly diagnosed cervical cancer patients, complete medical records and associated patient characteristics and clinical information should be collected. Regular follow-up after treatment should be monitored. Patients should be followed up every 3 months for the first 2 years after treatment, every 6 months after 3−5 years, and then once a year after 5 years after treatment. After 5 years of continuous follow-ups, follow-up durations should be continued based on patients’ condition. After radiotherapy, regular vaginal irrigation, the use of vaginal dilators when necessary, and early resumption of sexual life are beneficial to reduce vaginal adhesion.
Working group members
Group leader: Ding Ma
Group members: Keqin Hua, Yang Xiang, Congrong Liu, Aijun Liu, Bin Li, Xiaohua Wu, Lingying Wu, Fuquan Zhang, Yan Chen, Zhongqiu Lin, Qi Zhou, Jinyi Lang, Ge Lou, Shuzhong Yao, Guangyi Yuan, Manni Huang, Mei Dong
Translation group members
Naiyi Zhang Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Peking University Cancer Hospital & Institute
Yidi Yuan Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Peking University Cancer Hospital & Institute