1. Introduction
In recent years, the incidence of pancreatic cancer has increased significantly both at home and abroad. The statistical data in 2021 showed that among all malignancies in the United States, the incidence of pancreatic cancer ranked tenth in males and ninth in females, and the mortality of pancreatic cancer accounted for the fourth place. According to the statistical data from National Cancer Center of China in 2021, the incidence of pancreatic cancer ranked the seventh in males and the eleventh in females, and the mortality of pancreatic cancer accounted for the sixth among all malignant tumors. (The pancreatic cancers described herein is specifically referred to the pancreatic ductal adenocarcinoma).
Recently, with the development of radiology, endoscopy and pathology, the diagnosis of pancreatic cancer has been improved; with the development of new concept and technology of surgery (such as laparoscopy, robotics), locoregional therapy methods (such as stereotactic body radiation therapy, nanoknife ablation, radioactive particles implantation), and antitumor drugs (such as gemcitabine, nano albumin-bound paclitaxel, S-1, capecitabine, irinotecan, oxaliplatin and nimotuzumab), new opportunities and progress have been made for the management of pancreatic cancer.
In order to further standardize the diagnosis and treatment behaviors of pancreatic cancer in China, improve the diagnosis and treatment levels of pancreatic cancer in medical institutions, improve the prognosis of pancreatic cancer patients, and ensure the medical quality and medical safety, this guideline is formulated. Although the guideline is intended to aid in clinical decision-making, it does not incorporate all possible clinical scenarios. This guideline applies only to malignancies originating from pancreatic ductal epithelium.
2. Diagnostic techniques and applications
2.1. Risk factors for pancreatic cancer
The precise etiology for pancreatic cancer has never been elucidated, however, epidemiological investigation discovered many risk factors. Nonhereditary factors included long-term smoking, old age, high-fat diet, high body mass index (BMI), chronic pancreatitis or concomitant diabetes. About 10% pancreatic cancer was hereditary, and these risk factors included hereditary pancreatitis, Peutz-Jeghers syndrome, familial malignant melanoma syndrome, and so on. CDKN2A, BRCA1/2 and PALB2 mutation has also been proved related to familial pancreatic cancer.
2.2. Clinical manifestations
Pancreatic cancer is a very malignant tumor and progresses rapidly, but its onset is occult and its early symptoms are atypical, and most patients are diagnosed at middle to late stage. The initial symptoms often depend on the location and extent of the tumor, for example, obstructive jaundice may occur in early pancreatic head cancer, however, jaundice generally does not occur in early pancreatic body and tail tumors. Major clinical manifestations include:
(1) Abdominal discomfort or pain: It’s a common initial symptom. The majority of pancreatic cancer patients only presented with epigastric discomfort or dull pain, blunt pain and flatulence, which were often confused with symptoms of gastrointestinal and hepatobiliary diseases. If there is an obstruction of pancreatic juice outlet, pain or discomfort may be aggravated after food intaking. Persistent severe abdominal pain may occur in the middle and late stage of tumor when invading the celiac plexus.
(2) Weight loss and fatigue: At the initial stage of the disease, 80%−90% of pancreatic cancer patients would experience wasting, fatigue and weight loss, which was related to lack of appetite, anxiety and tumor-induced debilitation.
(3) Alimentary symptoms: When the tumor blocks the lower portion of the common bile duct and pancreatic duct, bile and pancreatic fluid cannot flow into the duodenum, then patients often present with dyspepsia. Impairment of pancreatic exocrine function may lead to diarrhea. When advanced pancreatic cancer invades the duodenum, it could lead to gastrointestinal obstruction or bleeding.
(4) Jaundice: It’s the primary clinical manifestation of pancreatic head cancer, and it’s related to the obstruction of bile duct outlet. It might be accompanied by skin itching, deep brown urine and clay stool.
(5) Other symptoms: Such as persistent or intermittent low-grade fever and abnormal blood glucose, but generally without biliary tract infection.
2.3. Physical examination
(1) Weight loss: Most patients would experience cachexia at late stage.
(2) Jaundice: It often occurred in pancreatic head cancer, and it’s often caused by the obstruction of bile duct outlet.
(3) Hepatomegaly: As a result of cholestasis or liver metastasis, the liver is hard, mostly painless, smooth or nodular.
(4) Enlarged gallbladder: A cystic, smooth and removable gallbladder without tenderness may be touched in some patients, known as Courvoisier sign, which is a characteristic of periampullary carcinoma.
(5) Abdominal lump: The abdominal mass can be touched at the late stage, and it’s mostly located in the upper abdomen, deep, nodular, hard and irremovable.
(6) Other signs: Such as supraclavicular lymph nodes enlargement, ascites, periumbilical nodes, or nodes in Douglas pouch in the late-stage pancreatic cancer patients.
2.4. Radiological examination
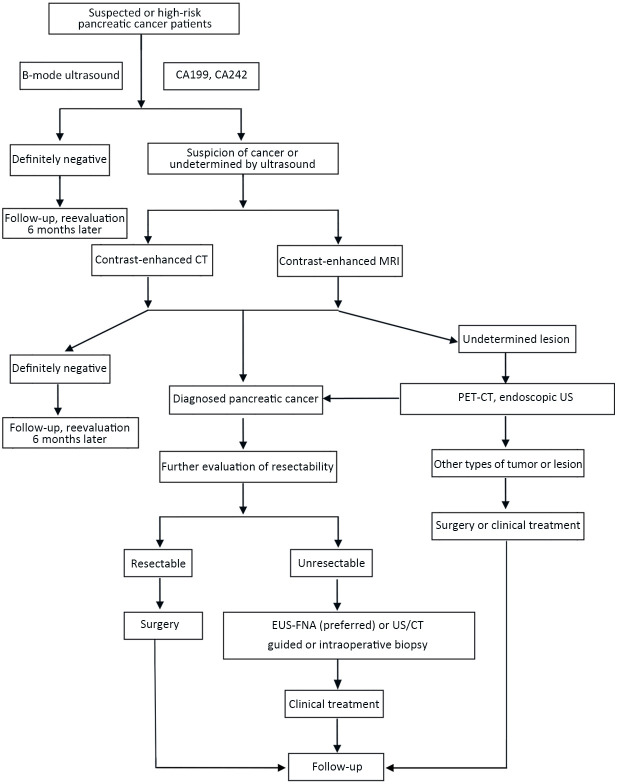
To choose the optimal radiological techniques according to patient’s situation is the prerequisite for the accurate diagnosis of pancreatic lesions. The radiological examination should obey the rule of entire (including the whole pancreas), dedicate (1−2 mm thin slice), dynamic (dynamic enhancement, regular follow-up), three-dimensional (multiplanar reconstruction, to evaluate the relationship with neighboring tissues). The pretreatment and posttreatment radiological examination flow charts were shown in Figure A1 ( Appendix 1 ) and Figure A2 (Appendix 2 ) in detail.
2.4.1. Ultrasound
It’s simple, noninvasive and nonradioactive, and it’s an important examination method for pancreatic cancer.
Conventional ultrasound can display the internal structure of pancreas, observe the obstruction of bile duct and the location of obstruction, and find out the cause of obstruction. Color Doppler ultrasound can help to determine whether the tumor has compressed or invaded the surrounding big vessels. Real-time contrast-enhanced ultrasound can reveal the hemodynamic changes of tumors, help to differentiate and diagnose tumors of different properties, and relying on the flexibility of real-time imaging and multi-section imaging, it has advantages in evaluating tumor microvascular perfusion and guiding interventional therapy.
The limitations of ultrasonography include small vision field, and interference by gastrointestinal tract gas and patient body shape, which makes it difficult to observe the pancreas completely, especially the tail of pancreas.
2.4.2. Computed tomography (CT)
With good spatial and temporal resolution, CT is the best noninvasive imaging method for pancreatic cancer, which is mainly used in the diagnosis, differential diagnosis and staging of pancreatic cancer. Plain scan can show the size and location of the lesion, but it cannot accurately diagnose pancreatic lesions and the relationship between the tumor and the surrounding structure is shown poorly. Three-phase contrast-enhanced scan can better display the size, location, shape, internal structure and relationship with surrounding structures of the pancreatic tumor, and can accurately judge if there are liver metastases or enlarged lymph nodes.
2.4.3. Magnetic resonance imaging (MRI) and magnetic resonance cholangiopancreatography (MRCP)
MRI is not the first choice for diagnosis of pancreatic cancer. It is a complementary method for CT when it’s difficult to diagnose the pancreatic lesions and it’s also the alternative to CT when the patient is allergic to CT contrast medium. MRCP and multi-phase enhanced scan have advantages in qualitative diagnosis and differential diagnosis of pancreatic cancer. It has been reported that MRI can be used to diagnose occult pancreatic head carcinoma using specific tissue contrast agents. MRI can also be used to monitor pancreatic cancer and predict the recurrence, vascular invasion and the invasiveness of pancreatic cancer, which could be a predictor of survival. MRCP can clearly display the panorama of the pancreaticobiliary system and help to judge the location of the lesion, thus helping to detect and differentiate the tumors around the ampulla. Compared to endoscopic retrograde cholangiopancreatography (ERCP) and percutaneous transhepatic cholangiography (PTC), MRCP had the advantage of noninvasiveness. In addition, MR functional imaging can quantitatively reflect tumor metabolic information from a microscopic perspective, including diffusion-weighted imaging (DWI), perfusion-weighted imaging (PWI) and magnetic resonance spectroscopy (MRS), which should be closely combined with conventional MR sequences to play a greater role on diagnosis, differential diagnosis and treatment response evaluation of pancreatic cancer.
2.4.4. Positron emission tomography-CT (PET-CT)
It could show the tumor metabolic activity and burden. It has obvious advantages in detecting extrapancreatic metastasis and evaluating systemic tumor load. It is not recommended as the routine radiological examination method for the diagnosis of pancreatic cancer and it has little efficacy in the diagnosis of small pancreatic cancer. PET-CT has an advantage in excluding and detecting distant metastatic lesions. It is recommended for patients with large primary lesions, suspected regional lymph node metastasis and significantly increased carbohydrate antigen 199 (CA199). During the follow-up after treatment of pancreatic cancer, PET-CT could differentiate between postoperative or postradiotherapy change and local tumor recurrence. PET-CT could also help to diagnose and localize the recurrence and metastases when CA199 level is increased, but the conventional imaging was negative. Early monitoring of the response can be achieved by changes in tumor glucose metabolism in patients who are unable to be operated and receive chemoradiation, which could provide evidences for timely changes of treatment plan and adoption of more active treatment methods.
2.4.5. Endoscopic ultrasonography (EUS)
It could be used to improve the sensitivity and specifity of diagnosis of pancreatic cancer, especially the EUS guided fine needle aspiration (EUS-FNA) biopsy, which is the most accurate method for the localization and qualitative diagnosis of pancreatic cancer. In addition, EUS is also helpful in tumor staging. Recently, the tumor elastic strain rate detection based on EUS elastography can assist in the judgment of the stromal content in pancreatic cancer and guide the selection of clinical drugs.
EUS is an invasive operation, and its accuracy is greatly affected by the operator’s technical level and experiences. It is more common to obtain tissue specimens under the guidance of EUS. For patients with specific diagnosis and surgical indication, routine EUS is not required before surgery
2.4.6. Role of ERCP in diagnosis of pancreatic cancer
The most common ERCP manifestations of pancreatic cancer were proximal stenosis and distal dilatation of the main pancreatic duct. ERCP cannot directly show tumors, it mainly makes the diagnosis of pancreatic cancer depending on the change of pancreatic duct and the morphology of the common bile duct, which has great value for the lower portion of the biliary duct and pancreatic duct obstruction or abnormal changes. In addition, intra-pancreaticobiliary duct cell brush or tissue clamping biopsy and pancreatic fluid or bile cytology or pathological diagnosis can be performed. Especially for inoperable patients with obstructive jaundice, biliary drainage and pathological and cytological examinations can be completed at one time.
2.4.7. Bone scans
Bone scans is the most widely used, cost-effective, and highly sensitive method for detection of malignant bone metastasis. Preoperative bone scans can be routinely performed in patients with pancreatic cancer who are highly suspected of bone metastasis
2.5. Blood immunologic and biochemical examinations
2.5.1. Blood biochemical examination
No specific blood biochemical changes are detected at the early stage. Elevated serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), bile acids and bilirubin levels would be detected when pancreatic cancer invades liver and the bile duct is obstructed. At the late stage of the tumor, electrolyte disturbances and hypoalbuminemia may occur with cachexia. In addition, changes in blood glucose are also associated with the onset or progression of pancreatic cancer.
2.5.2. Serum tumor markers
The commonly used serum tumor markers clinically are CA199, carcinoembryonic antigen (CEA) and CA125, of which CA199 is the most common used one for pancreatic diagnosis, treatment response evaluation, and recurrence surveillance. Serum CA199 higher than 37 U/mL is positive, and repeat testing is generally superior to the single one, which should be measured at least 14 d later. CA199 in untreated pancreatic ductal adenocarcinoma patients increases gradually, possibly up to 1,000 U/mL, and the sensitivity is related to tumor stage, size and location, with a specificity of 72%−90%. However, it should be pointed out that about 10% of the pancreatic cancer patients have a Lewis antigen-negative blood group and do not express CA199. Therefore, abnormal CA199 could not be detected in such patients, and other tumor markers, such as CEA and CA125, are needed to assist in the diagnosis. Moreover, CA199 may be false positive in cases of biliary infection (cholangitis), inflammation, or biliary obstruction (regardless of the cause), which could not indicate a tumor or an advanced lesion. Therefore, preoperative detection of CA199 levels is best performed after biliary tract decompression until serum bilirubin returns to normal level. CA199 is usually correlated with the clinical course. Increased CA199 could return to normal level 2 to 4 weeks after radical surgery (stage I); CA199 may rise again with tumor recurrence or metastasis. Serum CA199 level may also partly reflect tumor burden or the presence of minimal metastatic lesions. Although the increased serum CA199 after pancreatic cancer resection can indicate recurrence or metastasis, a comprehensive evaluation using imaging evidences is needed.
2.6. Histologic and cytological diagnosis
2.6.1. Cytological pathology diagnosis
Cytological diagnosis of pancreatic cancer is composed by sampling techniques, slice preparation techniques and diagnosis reports.
The cell specimen sampling techniques: Commonly used sampling techniques include 1) image (CT or ultrasound) guided percutaneous FNA; 2) EUS-FNA; 3) intraoperative FNA; and 4) cell brush of the pancreatic duct and the lower portion of common bile duct by ERCP.
Cell specimen preparation techniques: Cell specimen preparation techniques include routine smears, liquid-based slices and cell-block sections. Conventional smear is the most commonly used method of slice preparation. FNA or brushed cells are directly smeared on the glass, then wet dried and fixed by 95% alcohol. If the cystic fluid is acquired by FNA puncture, the liquid-based method will enrich the cells in the cystic fluid, thus obtaining a more abundant smear than the conventional smear. The main purpose of cell block preparation is to perform immunocytochemical staining. In addition, some small tissue structures can be restored in the slice of cell block, which is helpful for morphological diagnosis.
According to their own conditions and the nature of the lesion, each center chooses different methods of slice preparation, and it can help to improve the accuracy of diagnosis if the three methods are taken at the same time. Some centers can also carry out site assessment of cell samples if possible to improve the rate of sampling satisfaction.
Cytological diagnosis reports: The six-grade report system recommended by American Papanicolaou Society of Cytopathology was taken. In this report system cytopathology diagnosis was divided into the following six grades: Grade I, cannot diagnosis; Grade II, no sign of malignancy; Grade III, untypical; Grade IVA, benign neoplasm; Grade IVB, neoplastic lesions, other; Grade V, suspected malignancy; Grade VI, malignancy. The most challenging diagnostic grading is “neoplastic lesions, other (IVB),” in which capsular coated cells of intraductal papillary mucinous neoplasms and mucinous cystic tumor can be mild, moderate, or even severe atypical. Cells with severe atypical changes are difficult to differentiate from adenocarcinoma cells. In addition, the diagnosis of some small round cell tumors, such as solid-pseudopapillary tumor, neuroendocrine tumor and acinar cell carcinoma often need to be detected by cell block immunocytochemistry. Cytological diagnosis details are shown in Appendix 3 (Table A1 ).
2.6.2. Histopathology diagnosis of pancreatic cancer
(1) Pathological diagnosis criteria of pancreatic cancer: Pancreatic carcinoma is diagnosed by histopathology and/or cytology of the biopsy tissue or resected specimen of the pancreatic lesion or the metastatic lesion. Pathological diagnosis should be combined with clinical evidences, including understanding the clinical manifestations and imaging findings of the patients thoroughly.
(2) Pathological diagnosis guidelines of pancreatic cancer: It is composed by specimen processing, specimen sampling, pathologic examination and pathologic report.
1) Key points of specimen processing
The surgeon should mark the location, type and number of the specimens on the pathological application sheet, and mark the surgical margins and important lesions with dye staining or suture.
The tumor specimen should be delivered completely to the pathology department for incision and fixation within 30 min after resection as far as possible.
Specimen should be fixed with 10% neutral formalin solution for 12−24 h.
2) Specimen sampling and examination
Specimen after pancreaticoduodenectomy: The tumor is opened perpendicular to the common bile duct from the duodenal papilla to the common bile duct with a probe, and the relationship between the tumor and the common bile duct and the duodenal wall is observed. The margins of stomach, pylorus, small intestine, pancreas and common bile duct are taken respectively. As to the tumor mass (including the deepest portion of infiltration, the relationship with surrounding tissues or organs), according to the tumor size, at least one block is taken per 1 cm; According to the color of each surface, areas of different texture should also be sampled.
Specimen after splenectomy and resection of the body and tail of the pancreas: The tumor mass is opened in the form of a leaf, and at least one block per 1 cm is taken according to the tumor size, including the pancreatic capsule, the pancreatic duct, the margin of the pancreas, the surrounding pancreas, the relationship between the pancreas and the spleen, and so on. All lymph nodes are sampled including peripancreatic lymph nodes and splenic hilar lymph nodes. Pancreatic tissues between tumors are required to be sampled if there are multiple tumors.
2.6.3. Immunohistochemistry
Commonly used immunohistochemical markers for differential diagnosis include Vimentin, CK, EMA, CEA, CA199, CK19, CK7, CK20, MUC1, MUC4, CDX2, PR, CD10, syn, CgA, CD56, ACT, AAT, β-cantenin and Ki-67. A reasonable combination of immunohistochemical markers should be used for differential diagnosis of pancreatic endocrine tumors and various types of pancreatic cancer.
2.6.4. Pathologic reports of pancreatic cancer
The pathologic diagnosis report of pancreatic cancer is composed by gross specimen description, microscopic description, immunohistochemical staining results, pathologic diagnosis names, invasion areas (especially the relationship between the tumor and the common bile duct, duodenum and spleen. If the portal vein margin is involved, it should be reported), lymphatic and vascular tumor embolus, neural invasion, the invasion of pancreatic capsule,lymph node metastases and TNM staging. The gross specimen description is demonstrated in Appendix 4 and Appendix 5 in detail. In addition, the results of molecular pathology related to drug target detection, biological behavior evaluation and prognosis judgement are attached for clinical reference.
2.7. Differential diagnosis of pancreatic cancer
2.7.1. Chronic pancreatitis
(1) Chronic pancreatitis patients have a long history; serum amylase will increase when acute attack, but these patients seldom have jaundice.
(2) Abdominal CT showed irregular pancreatic contour, nodular bulge and heterogeneous pancreatic parenchyma.
(3) Abdominal plain scan and CT showed calcification in pancreas in patients with chronic pancreatitis.
(4) Elevated serum IgG4 level is the characteristic of autoimmune pancreatitis, which is one special type of chronic pancreatitis. In case of equivocal radiological diagnosis, pathologic biopsy is often needed.
2.7.2. Carcinoma of ampulla
(1) Intermittent jaundice might happen due to tumor necrosis and subsequent remission of bile duct obstruction.
(2) Hypotonic duodenography might show “double contour sign” due to duodenal papilla filling defect and mucosal destruction.
(3) Ultrasound, CT, MRI and ERCP might reveal bile duct and pancreatic duct dilation, distant bile duct obstruction, “double duct sign” and ampulla occupation.
(4) EUS: As a new diagnostic technique, EUS is of special value to differentiate carcinoma of pancreas from carcinoma of ampulla, for it could catch sight of small lesions and detect the invasion depth and extent and peripheral enlarged lymph nodes and so on.
2.7.3. Pancreatic cystadenoma and cystadenocarcinoma
Clinically pancreatic cystic neoplasms are rare, and more common in females. Radiologic examinations are important methods to differentiate them from pancreatic cancer, and the tumor marker CA199 is often normal. Ultrasound, CT and EUS could show intra-pancreatic cystic lesions with regular cavity, however, cystic degeneration with irregular cavity only happened after central necrosis of pancreatic cancer.
2.7.4. Choledocholithiasis
Patients with choledocholithiasis often have a long disease history of recurrence, and serum bilirubin level fluctuates greatly. Patients with acute cholangitis often experience the triad of abdominal pain, chills and fever and jaundice.
2.7.5. Other lesions of pancreas
They include pancreatic pseudocyst, insulinoma, solid pseudopapillary tumor, and so on. Clinically these lesions often grow slowly, and patients often have a long disease history and specific clinical manifestations, such as insulinoma with paroxysmal hypoglycemic symptoms, and pancreatic pseudocyst with acute pancreatitis history. It’s not difficult to differentiate them from pancreatic cancer when combined with radiological examinations such as CT and definite biopsy pathology if necessary.
3. Classification and staging of pancreatic cancer
3.1. Histologic classification of pancreatic cancer
Refer to 2019 World Health Organization (WHO) histologic classification of pancreatic cancer (Appendix 6 ).
3.2. Staging of pancreatic cancer (AJCC, the 8th edition)
3.2.1. Definition of T, N and M in pancreatic TNM staging
(1) Primary tumor (pT)
pTx: Primary tumor cannot be assessed
pT0: No evidence of primary tumor
pTis: Carcinoma in situ. This includes high-grade pancreatic intraepithelial neoplasia (PanIn-3), intraductal papillary mucinous neoplasm with high-grade dysplasia, intraductal tubulopapillary neoplasm with high-grade dysplasia, and mucinous cystic neoplasm with high-grade dysplasia.
pT1: Tumor ≤ 2 cm in greatest dimension
pT1a: Tumor ≤0.5 cm in greatest dimension
pT1b: Tumor >0.5 cm and <1 cm in greatest dimension
pT1c: Tumor 1−2 cm in greatest dimension
pT2: Tumor >2 cm and ≤4 cm in greatest dimension
pT3: Tumor >4 cm in greatest dimension
pT4: Tumor involves the celiac axis, superior mesenteric artery, and/or common hepatic artery, regardless of size
(2) Regional lymph nodes (pN)
pNx: Regional lymph nodes cannot be assessed
pN0: No regional lymph node metastases
pN1: Metastases in 1−3 regional lymph nodes
pN2: Metastases in ≥4 regional lymph nodes
(3) Distant metastasis (pM)
pMx: Distant metastasis cannot be assessed
pM0: No distant metastasis
pM1: Distant metastasis
3.2.2. TNM staging for pancreatic cancer
Table 1 shows the TNM staging for pancreatic cancer.
Table 1. TNM staging for pancreatic cancer (AJCC, the 8th edition).
| Staging | TNM |
| AJCC, American Joint Committee on Cancer. | |
| 0 | Tis, N0, M0 |
| IA | T1, N0, M0 |
| IB | T2, N0, M0 |
| IIA | T3, N0, M0 |
| IIB | T1, N1, M0 |
| IIB | T2, N1, M0 |
| IIB | T3, N1, M0 |
| III | T1, N2, M0 |
| III | T2, N2, M0 |
| III | T3, N2, M0 |
| III | T4, any N, M0 |
| IV | Any T, any N, M1 |
4. Management of pancreatic cancer
4.1. Principles
Multidisciplinary comprehensive diagnosis and treatment is the treatment basis of pancreatic cancer of any stage. According to physical condition, tumor location, invasion extent and clinical symptoms of different patients, it can be applied as a multi-disciplinary consultation model, and be planned to reasonably use existing diagnostic and therapeutic methods. It could acquire maximum cure and tumor control, reduce complications and improve the quality of life of patients. The management of pancreatic cancer includes surgery, radiotherapy, chemotherapy, intervention therapy and best supportive care. The performance status of patients who are going to receive chemoradiation should be evaluated according to Karnofsky score (Table A2 in Appendix 7 ) or ECOG score (Table A3 in Appendix 8 ).
4.2. Surgery
4.2.1. Principles of surgery
Surgical resection is the only effective method for patients with pancreatic cancer to get the chance of cure and long-term survival. However, more than 80% of pancreatic cancer patients do not have the chance to receive surgery for late stage diseases. Radical resection (R0) should be carried out as far as possible. The surgical margin is evaluated by “1 mm principle”, which means tumor-free resection margin ≥1 mm is R0 resection, and margin <1 mm is otherwise R1 resection. Before the patients receive treatment, they should complete the necessary radiological examinations, systemic assessment, and the multi-disciplinary consultation, which includes departments of radiological diagnosis, pathology, chemotherapy, radiotherapy and so on.
Evaluation of the tumor before surgical treatment is of great clinical significance. According to radiological manifestations, pancreatic cancer patients could be classified into three categories: resectable, potentially resectable and unresectable. Detailed management principles are made according to these three different categories. The major radiological evaluation criteria of resectability include: whether the tumor has distant metastasis, whether the superior mesenteric vein or portal vein is invaded, whether there is fat space around the celiac artery trunk, hepatic artery, superior mesenteric artery, etc. (Refer to Table A4 in Appendix 9 for detailed information). Standard surgical treatment is the best way to achieve a good prognosis. The following principles should be followed.
(1) Tumor-free principle: including no-touch principle, en bloc resection principle and occlusion of tumor blood supply principle.
(2) Adequate resection range: 1) Standard pancreaticoduodenectomy: Resection range includes 1/3−1/2 of the distal stomach, the whole common bile duct and gallbladder, resection margin at the left side of superior mesenteric vein/3 cm away from tumor, total duodenum and 15 cm of proximal jejunum. Adequate resection of fascia in front of the pancreas and retropanreatic soft tissue is mandated. Resection of the uncinate, tissue of regional lymphatic drainage area, regional nerve plexus, and loose connective tissue around large vessles is required; 2) standard distal pancreatectomy: Resection area includes the body and tail of pancreas, spleen, splenic artery and vein, regional lymphadenectomy, and sometimes includes the left Gerota fascia and partial mesocolon, but does not include the colon itself; and 3) standard total pancreatectomy. Resecton area includes pancreatic head, neck, body and tail, duodenum and the first part of jejunum, gallbladder and common bile duct, spleen and splenic artery and vein and regional lymphadenectomy, and sometimes includes the antrum and pylorus, the Gerota fascia and partial mesocolon, but does not include the colon itself.
(3) Safe resection margins: The following six margins for pancreaticoduodenectomy should be guaranteed: pancreatic (pancreatic neck) margin, common bile duct (common hepatic duct) margin, stomach margin, duodenum margin, retroperitoneal [which means superior mesenteric artery (SMA) and superior mesenteric artery (SMV) skeletonization] margin and other soft tissue margin (such as retropancreatic margin), of which pancreatic margin should be at least 1 mm (no residual tumor microscopically). For adequate margins, intraoperative frozen examinations of margins are advised.
(4) Lymphadenectomy: More than 15 lymph nodes should be obtained under standard lymph node dissection. For patients who received neoadjuvant therapy, the lymph nodes obtained could be less than 15. Routine extended retroperitoneal lymphadenectomy is not advised. Standard radical pancreatic cancer lymphadenectomy includes:
1) Standard range of lymph node dissection for pancreaticoduodenectomy for carcinoma of the head of pancreas: Suprapyloric and infrapyloric lymph nodes (No. 5, 6), lymph nodes in front of common hepatic artery (No. 8a), hepatoduodenal ligament lymph nodes (common hepatic duct, common bile duct and cystic duct lymph nodes, No. 12b1, 12b2, 12c), lymph nodes on the posterior aspect of the superior and inferior portion of the head of the pancreas (No. 13a,13b), lymph nodes on the right side of SMA (No. 14a,14b) and lymph nodes on the anterior surface of the superior and inferior portion of the head of the pancreas (No. 17a,17b).
2) Standard range of lymph node dissection for resection of carcinoma of body and tail of pancreas: Lymph nodes at the splenic hilum (No. 10), lymph nodes along the proximal and distal splenic artery (No. 11) and lymph nodes along the inferior margin of the pancreas (No. 18) should be resected with the specimen en bloc. Lymph nodes around the celiac artery (No. 9), partial lymph nodes along the superior mesenteric artery (No. 14) and abdominal periaortic lymph nodes (No. 16) are suggested to be resected when the tumor locates in the body of the pancreas.
4.2.2. Preoperative biliary drainage
(1) The aim of preoperative biliary drainage is to decompress the bile duct, to alleviate cholangitis, to ameliorate live function, correct coagulation abnormality and lower operative mortality. However, the preoperative biliary drainage is not routinely recommended.
(2) It is recommended for patients with severe symptoms, fever, sepsis and suppurative cholangitis.
(3) Biliary drainage could be accomplished by nasobiliary drainage or percutaneous transhepatic cholangial drainage (PTCD). Cholecystostomy is suggested only in hospitals that do not have the appropriate conditions.
(4) Operation could be performed when the bilirubin level has decreased at least 50%, and the liver function, the temperature and white blood cell (WBC) have become normal after 2 weeks of biliary drainage.
4.2.3. Indications for radical resection
(1) Age <80 years old, good performance status, and heart/lung/liver/kidney function can tolerate surgery by multidisciplinary evaluation.
(2) Pancreatic cancer with clinical stage ≤II.
(3) No liver metastases, no ascites.
(4) It is found that the tumor is confined in the pancreas without portal vein and SMV invasion during intraoperative exploration.
(5) No distant dissemination or metastases.
4.2.4. Operation methods
(1) Pancreaticoduodenectomy for tumors in the head and neck of pancreas.
(2) Distal pancreatectomy plus splenectomy for tumors in the body and tail of pancreas.
(3) Total pancreatectomy for large tumors involved in the head, neck and body of the pancreas.
(4) As to the safety, the number of lymph nodes dissected and R0 resection rate, mini-invasive radical pancreatic cancer resection is comparable to open surgery. However, the oncologic benefit of mini-invasive surgery is undetermined, so it is only recommended to be performed in professional large pancreatic centers by experienced pancreatic surgeons.
4.2.5. Pancreatic anastomosis
The purpose of stump management after pancreatectomy is to prevent pancreatic leakage, and pancreaticojejunostomy is commonly used. A variety of pancreaticojejunostomy methods are now being performed by different surgeons, and the appropriate anastomosis should be taken to reduce the occurrence of pancreatic leakage.
4.2.6. Perioperative medical management
Patients undergoing major open surgery, regardless of their nutritional status, are recommended to take immune nutrition for 5 to 7 d before operation and to continue it until 7 d after the operation or when the patient could acquire more than 60% nutrition needs by oral feeding. Immune-enhanced enteral nutrition should contain three substrates: ω-3 PUFA, arginine and nucleotides simultaneously. The effect of adding any one or two of the above three kinds of nutrients alone needs further studies. Oral enteral nutrition support is preferred.
Patients with moderate malnutrition who are going to receive major operations or patients with severe malnutrition are recommended to receive nutrition treatment for one to two weeks before surgery, even if the surgery is delayed. Patients who are not expected to acquire their nutritional needs through normal diet at least 7 d after operation and those who could not acquire 60% of the nutritional requirement by oral feeding for more than 1 week should be given postoperative nutrition treatment.
4.2.7. Management of postoperative complications
(1) Postpancreatectomy hemorrhage (PPH)
Acute PPH occurs in the first 24 h postoperatively, and delayed PPH occurs more than 24 h postoperatively. PPH includes extraluminal and intraluminal hemorrhage. PPH is graded into A, B and C according to ISGPS definition (refer to Table A5 in Appendix 10 for detailed information).
1) Extraluminal hemorrhage: Extraluminal hemorrhage is mainly caused by incomplete intraoperative hemostasis, the false hemostasis of the bleeding point under the condition of intraoperative hypotension, the shedding of the ligature, and the shedding of the electric coagulation scab. Coagulation defect is also the cause of extraluminal hemorrhage. The main prevention methods are strict hemostasis during operation, careful examination before abdominal closure, suture of important blood vessels and correction of coagulation defect before operation. Great attention should be paid to the extraluminal hemorrhage. A small amount of bleeding can be treated with drugs, blood transfusion and other conservative methods. However, a large amount of blood loss in a short time, resulting in hemorrhagic shock, should be operated as soon as possible to stop bleeding.
2) Intraluminal hemorrhage: Stress ulcer often occurs at least 3 d after operation. The prophylaxis of stress ulcer includes nutrition status correction and the reduction of the impact of surgery and anesthesia as far as possible. Stress ulcer is managed mainly by conservative methods, including hemostatic drugs, somatostatin, proton pump inhibitors, gastrointestinal decompression, injection of norepinephrine diluted in iced saline with concentration of 8 mg/dL via the stomach tube, hemostasis by gastroscopy and angiography embolization. If the conservative methods are invalid, relaparotomy can be considered.
(2) Postoperative pancreatic fistula (PPF)
According to 2016 ISGPS criteria, a clinically relevant PPF is now redefined as a drain output of any measurable volume of fluid with an amylase level >3 times the upper limit of institutional normal serum amylase activity, associated with a clinically relevant development/condition related directly to the PPF. Consequently, the former “grade A PPF of 2005 definition” is now redefined and called a “biochemical leak,” because it has no clinical importance and is no longer referred to a true pancreatic fistula. Grade B pancreatic fistula is related to clinical practice and affects the postoperative process, including: continuous drainage for more than 3 weeks; Clinical changes in the treatment of pancreatic fistula; percutaneous or endoscopic puncture and drainage; angiographic interventional therapy for hemorrhage; and signs of infection other than organ failure. Once single or multiple organ dysfunction occurs due to pancreatic fistula infection, the grade of pancreatic fistula is adjusted from B to C. The treatment of pancreatic fistula includes proper fasting, effective and adequate drainage, infection control, nutritional support, acid suppression and enzyme suppression. If there is abdominal bleeding, interventional embolization hemostasis could be considered. Surgical treatment is indicated for the pancreatic fistula with severe abdominal infection or massive bleeding.
(3) Delayed gastric emptying (DGE)
1) No consensus definition of DGE has been reached. The commonly used definition is as follows: nasogastric tube output >800 mL/d lasting for more than 10 d without gastric outlet obstruction, no water-electrolyte disorders or acid-base imbalance, no underlying diseases inducing gastroparesis, and no medication affecting the contaction of smooth muscles.
2) Diagnosis is made mainly by the disease history, symptoms, signs, alimentary tract contrast examination and gastroscope.
3) Management of DGE: Management of DGE includes adequate gastrointestinal decompression, active nutritional support, psychological suggestion, gastrointestinal prokinetic agents and treatment of underlying diseases and nutritional and metabolic disorders. Traditional Chinese Medicine might be helpful.
(4) Other complications: Including abdominal infection, bile leakage, chyle leakage and late postoperative complications.
4.2.8. Surgical management of potentially resectable pancreatic cancer
Patients with potentially resectable tumors often achieve low R0 resection rate, and the best treatment strategy has been controversial. Neoadjuvant therapy (chemotherapy, chemoradiation or induction chemotherapy followed by concurrent chemoradiation) is advocated for patients who might benefit from it after multidisciplinary discussion to downstage the tumor, then surgical resection is performed. If R0 resection is accomplished when combined with portal vein/SMV resection for patients who received neoadjuvant therapy followed by tumor resection, comparable survival benefit as primarily resectable patients could be achieved. However, the survival benefit of pancreatic cancer resection combined with arterial resection is controversial, and it still needs to be evaluated by large prospective data. In view of the lack of high-level evidence-based medicine evidences at present, borderline resectable pancreatic cancer (BRPC) patients are recommended to participate in clinical trials. The surgical exploration could be carried on directly on the patient’s request by himself. Palliative R2 resection is not recommended for this group of patients, except for exceptional cases such as operative hemostasis to save lives.
4.2.9. Surgical management of locally advanced unresectable pancreatic cancer
For these patients, active treatment is still possible to obtain good therapeutic effects. For patients whose life expectancy is ≥3 months do not experience duodenal obstruction temporarily, prophylactic gastrojejunostomy could be performed if indicated clinically. Choledochojejunostomy or hepaticojejunostomy could be performed for patients with unresectable tumors and biliary obstruction or expected biliary obstruction. In patients with duodenal obstruction, gastrojejunostomy could be performed if the expected survival time is more than 3 months. Intraopetative radiotherapy, irreversible electroporation (nanoknife ablation) could also be taken to improve local tumor control rate and relieve pain. Patients should receive postoperative chemotherapy ± radiotherapy.
4.3. Medical treatment
Medical treatment for pancreatic cancer can be applied to patients at various stages, including preoperative neoadjuvant/conversion therapy in resectable and borderline resectable patients, adjuvant therapy in patients after radical surgery, and treatment in patients with locally advanced or metastatic diseases. Medical treatment could not only prolong the survival time of patients, but also reduce the pain and improve the quality of life of patients in the late stage. The drug and dosage should be adjusted timely according to the patient’s condition and performance status. Patients’ quality of life improvement and comorbidity management, including pain, nutrition, spirit and mental psychology, need to be valued. Genetic testing for locally advanced and metastatic pancreatic cancer is recommended before medical treatment, including but not limit to BRCA1/2, NTRK1/2/3, PALB2, ATM/ATR and RAS, which helps to guide the best drug treatment regimen and participate in the clinical research of new drugs. Patients with late-stage metastatic pancreatic cancer who are refractory to the standard treatment may consider to receive high-throughput sequencing at qualified institutions to find suitable clinical studies or medical treatment regimens.
4.3.1. Neoadjuvant/conversion therapy for resectable or BRPC
The purpose of neoadjuvant/conversion therapy for resectable or borderline resectable patients is to increase the R0 resection rate, thereby to prolong disease-free survival and overall survival. However, there is still a lack of high-level clinical research evidences, and it is recommended to participate in relevant clinical studies.
Preoperative neoadjuvant/conversion therapy should be reserved for patients with resectable pancreatic cancer with good performance status and high-risk factors (e. g., high serum CA199 levels, large primary pancreatic tumors, extensive lymph node metastasis, severe wasting and extreme pain), and BRPC. Patients usually receive radical resection 4 to 8 weeks after preoperative chemotherapy. For those patients without evidence of postoperative recurrence or metastasis, adjuvant chemotherapy is recommended after multidisciplinary evaluation. Adjuvant chemotherapy regimen refers to the response of neoadjuvant chemotherapy or other clinical factors such as the performance status of patients and chemotherapy tolerance. Gemcitabine-based two-drug combination regimen, or a three-drug combination regimen mFOLFIRINOX, is recommended. The commonly used regimens are detailed in Table 2 .
Table 2. Commonly used neoadjuvant/conversion chemotherapy regimens for resectable or borderline resectable pancreatic cancer.
| Regimen | Detailed protocol |
| ECOG PS, Eastern Cooperative Oncology Group performance status; 5-FU, 5-fluorouracil. | |
| Gemcitabine + albumin-bound paclitaxel | Albumin-bound paclitaxel 125 mg/m2 ivgtt, d 1, 8
Gemcitabine 1,000 mg/m2 ivgtt, d 1, 8 Repeat every 3 weeks |
| FOLFIRINOX (only for patients with ECOG PS
score 0−1) |
Oxaliplatin 85 mg/m2 ivgtt, d 1
Irinotecan 180 mg/m2 ivgtt, d 1 Calcium folinate 400 mg/m2 ivgtt, d 1 5-FU 400 mg/m2 bolus d 1 Followed by 5-FU 2,400 mg/m2 continuous infusion over 46 h Repeat every 2 weeks |
| mFOLFIRINOX (only for patients with ECOG PS score 0−1) | Oxaliplatin 85 mg/m2 ivgtt, d 1
Irinotecan 150 mg/m2 ivgtt, d 1 Calcium folinate 400 mg/m2 ivgtt, d 1 5-FU 2,400 mg/m2 continuous infusion over 46 h Repeat every 2 weeks |
Puncture biopsy is recommended for patients who cannot tolerate surgical resection for poor performance status. After acquirement of definite diagnosis, patients should receive palliative chemotherapy and best supportive care.
4.3.2. Postoperative adjuvant chemotherapy for resectable pancreatic cancer
Patients with pancreatic cancer after radical surgery should undergo adjuvant chemotherapy if not contraindicated. The recommended adjuvant chemotherapy regimens are based on gemcitabine or fluorouracil (5-FU, capecitabine, or S-1). For patients with good performance status, combination chemotherapy regimens including gemcitabine plus capecitabine and mFOLFIRINOX are recommended. The commonly used regimens are shown in Table 3 . For patients with poor performance status, monotherapy with gemcitabine or fluorouracil plus the best supportive care is recommended. The adjuvant chemotherapy had best be initiated within 12 weeks after operation with a duration of 6 months.
Table 3. Postoperative adjuvant chemotherapy regimens for resectable pancreatic cancer.
| Regimen | Detailed protocol |
| ECOG PS, Eastern Cooperative Oncology Group performance status; 5-FU, 5-fluorouracil; LV, leucovorin. | |
| mFOLFIRINOX (only for patients with ECOG PS score 0−1) | Oxaliplatin 85 mg/m2 ivgtt, d 1
Irinotecan 150 mg/m2 ivgtt, d 1 Calcium folinate 400 mg/m2 ivgtt, d 1 Followed by 5-FU 2,400 mg/m2 continuous infusion over 46 h Repeat every 2 weeks |
| Gemcitabine + capecitabine | Gemcitabine 1,000 mg/m2 ivgtt, d 1, 8
Capecitabine 1,660 mg/(m2·d), divided into two oral doses, d 1−14 Repeat every 3 weeks |
| Gemcitabine | Gemcitabine 1,000 mg/m2 ivgtt, d 1, 8
Repeat every 3 weeks |
| S-1 | S-1 80−120 mg/d, divided into two oral doses, d 1−14
Repeat every 3 weeks |
| Capecitabine | Capecitabine 2,000 mg/(m2·d), divided into two oral doses, d 1−14
Repeat every 3 weeks |
| 5-FU/LV | LV 400 mg/m2 ivgtt, d 1
5-FU 400 mg/m2 ivgtt, d 1 Followed by 5-FU 2,400 mg/m2 continuous infusion over 46 h |
4.3.3. Unresectable locally advanced or metastatic pancreatic cancer
The treatment effect for unresectable locally advanced or metastatic pancreatic cancer is generally unsatisfactory. It is recommended that clinical studies be carried out. Currently, commonly used chemotherapeutic agents for unresectable locally advanced or metastatic pancreatic cancer include gemcitabine, albumin-bound paclitaxel, 5-FU/LV, cisplatin, oxaliplatin, irinotecan, S-1 and capecitabine. The targeted drug includes erlotinib.
The first-line chemotherapy regimen is selected according to the patient’s performance status (Table 4 ). Combination chemotherapy is recommended for patients with good performance status. The commonly used two-drug gemcitabine-based combination regimens include GN (gemcitabine/albumin-bound paclitaxel), GP (gemcitabine/cisplatin), GX (gemcitabine/capecitabine), GS (gemcitabine/S-1), etc. For patients with ECOG PS score 0−1, the three-drug combination regimen FOLFIRINOX or mFOLFIRINOX could be the choice. Those advanced pancreatic cancer patients with BRCA1/2 germline mutation may be sensitive to platinum drugs, and a preferred regimen containing cisplatin or oxaliplatin (GP or FOLFIRINOX, mFOLFIRINOX) may be considered. Others include FOLFOX (oxaliplatin/5-FU/LV), CapeOx (oxaliplatin/capecitabine) and FOLFIRI (irinotecan/5-FU/LV) are often used as second-line treatment regimens.
Table 4. Chemotherapy regimens for unresectable locally advanced or metastatic pancreatic cancer (For good performance status).
| Regimen | Detailed protocol |
| ECOG PS, Eastern Cooperative Oncology Group performance status; 5-FU, 5-fluorouracil; LV, leucovorin; MSI-H, microsatellite instability-high; dMMR, mismatch repair deficient. | |
| GN: Gemcitabine + Albumin-bound paclitaxel | Albumin-bound paclitaxel 125 mg/m2 ivgtt, d 1, 8
Gemcitabine 1,000 mg/m2 ivgtt, d 1, 8 Repeat every 3 weeks |
| GP: Gemcitabine + cisplatin (especially for hereditary cancer patients with potential BRCA1/2 or other DNA repair gene mutations) | Gemcitabine 1,000 mg/m2 ivgtt, d 1, 8
Cisplatin 75 mg/m2 ivgtt, d 1 Repeat every 3 weeks |
| FOLFIRINOX (only for patients with ECOG PS score 0−1) | Oxaliplatin 85 mg/m2 ivgtt, d 1
Irinotecan 180 mg/m2 ivgtt, d 1 LV 400 mg/m2 ivgtt, d 1 5-FU 400 mg/m2 ivgtt, d 1 Followed by 5-FU 2,400 mg/m2 continuous infusion over 46 h Repeat every 2 weeks |
| mFOLFIRINOX (only for patients with ECOG PS score 0−1) | Oxaliplatin 85 mg/m2 ivgtt, d 1
Irinotecan 150 mg/m2 ivgtt, d 1 LV 400 mg/m2 ivgtt, d 1 Followed by 5-FU 2,400 mg/m2 continuous infusion over 46 h Repeat every 2 weeks |
| Gemcitabine + erlotinib | Gemcitabine 1,000 mg/m2 ivgtt, d 1, 8
Erlotinib 150 mg/d po Repeat every 3 weeks |
| GX: Gemcitabine + capecitabine | Gemcitabine 1,000 mg/m2 ivgtt, d 1, 8
Capecitabine 1,660 mg/(m2·d), divided into two oral doses, d 1−14 Repeat every 3 weeks |
| GS: Gemcitabine + S-1 | Gemcitabine 1,000 mg/m2 ivgtt, d 1, 8
S1 80−120 mg/d divided into two oral doses d 1−14 Repeat every 3 weeks |
| Gemcitabine | Gemcitabine 1,000 mg/m2 ivgtt, d 1, 8
Repeat every 3 weeks |
| S-1 | S-1 80−120 mg/d divided into two oral doses d 1−14
Repeat every 3 weeks |
| Olaparib maintenance (for patients with germline BRCA1/2 mutations and good PS, who have not experienced disease progression for ≥16 weeks during the first-line platinum-containing regimen treatment.) | Olaparib 300 mg po bid |
| CapeOx: Oxaliplatin + capecitabine | Oxaliplatin 130 mg/m2 ivgtt, d 1
Capecitabine 2,000 mg/(m2·d), divided into two oral doses, d 1−14 Repeat every 3 weeks |
| 5-FU/LV | LV 400 mg/m2, ivgtt, d 1
5-FU 400 mg/m2, ivgtt, d 1 Followed by 5-FU 2,400 mg/m2 continuous infusion over 46 h Repeat every 2 weeks |
| Nanoliposomal irinotecan + 5-FU/LV | Nanoliposomal irinotecan 80 mg/m2, ivgtt, d 1
LV 400 mg/m2, ivgtt, d1 5-FU 2,400 mg/m2 continuous infusion over 46 h Repeat every 2 weeks |
| FOLFIRI | Irinotecan 180 mg/m2, ivgtt, d 1
LV 400 mg/m2, ivgtt, d 1 5-FU 400 mg/m2, ivgtt, d 1 Followed by 5-FU 2,400 mg/m2 continuous infusion over 46 h Repeat every 2 weeks |
| Pembrolizumab (only for patients with MSI-H or dMMR) | Pembrolizumab 200 mg ivgtt, d 1
Repeat every 3 weeks |
Subsequent treatment strategies for patients who have response to previous combination chemotherapy are continuation of the prior combination chemotherapy, complete cessation of therapy, withdrawal of the more toxic drug of the prior combination regimen, or maintenance therapy with a new drug. For patients with BRCA1/2 germline gene mutation who do not progress ≥16 weeks with first-line treatment with a platinum-containing regimen, maintenance therapy with the polyadenosine diphosphate ribose polymerase inhibitor olaparib alone is recommended. For patients with somatic BRCA1/2 gene mutation or other abnormal homologous recombination repair pathways, they can be treated in the same way as BRCA1/2 gene germline mutation patients. If patients have received the GN regimen previously, they could take the gemcitabine monotherapy as maintenance therapy; if patients have received the (m) FOLFIRINOX regimen previously, they could take capecitabine or 5-FU/LV or FOLFIRI regimen as maintenance therapy (oxaliplatin maintenance therapy is not recommended due to cumulative neurotoxicity of oxaliplatin).
Patients who failed in first-line treatment, if in good performance status, could choose nanoliposomal irinotecan + 5-Fu/LV; or choose non-overlapping drugs as second-line chemotherapy based on first-line drugs, patient comorbidities and toxic side effects; or participate in clinical trials. For advanced pancreatic cancer with specific genetic variants (such as NTRK gene fusion, ALK gene rearrangement, HER2 amplification, and high microsatellite instability), studies have shown that the corresponding targeted drugs or immune checkpoint inhibitors has certain effect. Such patients are first recommended to participate in their corresponding clinical trials, and they are also encouraged to receive special targeted drugs therapy or immunotherapy under the guidance of experienced oncologists.
Patients with poor performance status are recommended to take monotherapy or/and optimal supportive care. It is still controversial whether patients with pancreatic cancer should continue to receive chemotherapy after the failure of the first-line and second-line chemotherapy. There are no clear chemotherapy regimens to recommend, and they are advised to take part in clinical trials. Chemotherapy efficacy could be evaluated with WHO solid tumor response evaluation criteria and RECIST criteria, which are shown in Appendix 11 and Appendix 12 .
4.4. Radiotherapy
Radiotherapy is important for pancreatic cancer throughout the whole stages. Resectable localized pancreatic cancer patients, who are intolerant of or reject surgery, are advised to take radical precision radiotherapy, which is a new option to provide long-term survival for this group of patients. Borderline resectable patients can receive high-dose radiotherapy or chemoradiation directly, and resectability is then evaluated according to the treatment response. Chemoradiation is the first choice in the treatment for locally advanced pancreatic cancer. For pancreatic cancer patients with oligometastasis (limited number of metastatic foci and limited organs), irradiation of the primary tumor and metastases can be used to relieve obstruction, compression or pain and improve local control of tumors. The role of postoperative radiotherapy for pancreatic cancer is still controversial. For pancreatic cancer patients with local residual disease or positive margin, postoperative concurrent chemoradiation can make up for the inadequacy of the operation. Intensity-modulated radiotherapy and stereotactic radiotherapy (SBRT) based on multi-line beams (X-ray or γ-ray) focusing are increasingly used in the treatment of pancreatic cancer. The dose pattern of radiotherapy has gradually changed to high-dose and low-fractionation (large fraction radiotherapy) pattern, local-control rate, pain-relief rate and survival rate have been improved. However, it still needs to be further confirmed by large phase III clinical trials.
4.4.1. Indications for pancreatic cancer radiotherapy
(1) Resectable pancreatic cancer
For resectable pancreatic cancer patients who refuse surgical treatment or are unable to tolerate surgical treatment for medical reasons, it is recommended to receive high-dose, low-fractionation radiotherapy or SBRT with neoadjuvant or concurrent chemoradiotherapy. There are no clear criteria for the total dose and the fractionated dose of SBRT, and the currently recommended fractionated doses are 25−45 Gy every 5 doses or 33−40 Gy every 5 doses, with every irradiation dose 6.6−8.0 Gy.
(2) BRPC
There are currently no standard radiotherapy patterns for BRPC. High-dose, less-fractionation radiotherapy or SBRT can be used directly on the tumor area, and preoperative radiotherapy can improve the R0 resection rate, which is helpful to improve patient survival. In neoadjuvant chemoradiotherapy, the total dose of radiotherapy is 45−50.4 Gy with 1.8−2.0 Gy/f and 5 times per week; or a total dose of 36 Gy with 2.4 Gy/f and 5 times per week. Surgery is recommended for resectable patients about 4 weeks after neoadjuvant chemoradiation; for borderline resectable patients, the optimal time of surgery is 4−8 weeks after neoadjuvant chemoradiation, which makes the tumor to shrink as much as possible. It is also acceptable to perform surgery for borderline resectable patients more than 8 weeks after neoadjuvant chemoradiation, which however induces the operation more difficult than that performed 4−8 weeks after chemoradiation due to radiotherapy-related fibrosis.
(3) Locally advanced pancreatic cancer
For locally advanced pancreatic cancer patients, it is recommended to receive high-dose, low-fractionation IMRT or SBRT combined with neoadjuvant chemotherapy or concurrent chemoradiation, which could result in better prognosis than conventional radiotherapy.
(4) Oligometastatic pancreatic cancer
For metastatic pancreatic cancer patients with good systemic therapy response or relatively slow tumor progression, both the primary and metastatic tumors could be treated with high-dose radiotherapy, and the local control rate could be transformed into survival prolongation.
(5) Recurrent pancreatic cancer
For recurrent pancreatic cancer patients after resection or other local treatment such as radiofrequency ablation, the risk of radiotherapy is higher than that of primary treatment patients because of previous treatment injury and gastrointestinal diversion, which is not conducive to development.
(6) Postoperative adjuvant radiotherapy
Postoperative adjuvant radiotherapy is controversial and lacks high-level evidence-based medicine evidences. Compared with chemotherapy alone, conventional radiotherapy combined with chemotherapy can reduce the local recurrence rate. The total dose of radiotherapy ranges from 45 to 50.4 Gy with 1.8 to 2 Gy each fractionation, and an additional 5 to 9 Gy can be added to the sites with high risk of recurrence.
4.4.2. Radiotherapy techniques
SBRT and IMRT techniques, including volume rotation intensity modulated radiotherapy (VMAT) and spiral tomography intensity modulated radiotherapy (TOMO), have better dose distribution conformability and focus than three-dimensional conformal radiotherapy (3D-CRT) does. Combined with the simultaneous integrated boost (SIB) dose mode in target or target area, the irradiation dose of pancreatic tumor could be increased without increasing the radiation dose of normal tissues. It is very important to carry out accurate radiotherapy for pancreatic cancer, to refine all aspects of radiotherapy, to improve the accuracy of target drawing, and to reduce the error of positioning and the disturbance of respiratory movement and other factors.
4.4.3. Target area of radiotherapy
For unresected lesions, it is recommended to irradiate the primary pancreatic tumor or the recurrent lesion and metastatic lymph nodes, but excluding the regional lymph node drainage area.
The volume of the target area for postoperative radiotherapy should be determined based on the preoperative CT scan or the silver clip placed during the operation, and should include the primary tumor bed and the regional high-risk lymph node area.
4.4.4. Radiotherapy dose
Increasing the dose of radiotherapy is one of the key factors to improve the local control rate of pancreatic cancer. The dose mode should be determined according to the equipment technology. The dose of 40−70 Gy/5−20 f can be selected, and the higher the biological effective dose (BED) is, the higher the local-control rate is. The premise is to ensure that gastrointestinal radiation injury is avoided or reduced. The common total dose is 45−54 Gy, and the single dose is 1.8−2.0 Gy.
4.4.5. Concurrent chemotherapy
Gemcitabine or fluorouracil (5-FU continuous intravenous infusion, or capecitabine, or S-1) monotherapy or gemcitabine or fluorouracil based multidrug regimens, are preferred for concurrent chemotherapy.
4.4.6. Intraoperative radiotherapy
Intraoperative radiotherapy is usually performed as planned or when the tumor is found unresectable during laparotomy or if the margin of the tumor is close or positive during the operation. It is suggested that the intraoperative electron irradiation dose should be 15−20 Gy, and the external beam radiotherapy (EBRT) (within 1 month after operation) should be supplemented with 30 Gy/10 f or 40 Gy/20 f.
4.5. ERCP and related treatment
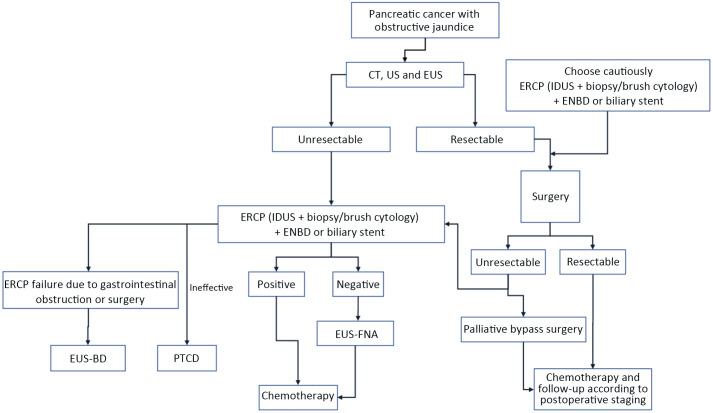
Diagnostic ERCP is not recommended as the first choice for the diagnosis of pancreaticobiliary diseases, however, it is more commonly used in the diagnosis by cholangiopancreatography in the course of therapeutic ERCP. The flowchart of ERCP diagnosis and treatment for pancreatic cancer is shown in Appendix 13 (Figure A3 ).
4.5.1. ERCP for preoperative biliary drainage in pancreatic cancer
Cholestasis caused by the bile duct stricture due to the pancreatic cancer compression can theoretically increase the morbidity after operation and lead to high mortality and disability rate. Preoperative biliary drainage can also improve the synthetic function of liver, improve the clearance of endogenous toxins and ameliorate the mucosal function of digestive tract, thus contributing to the safe operation. However, preoperative biliary drainage in patients with resectable pancreatic cancer should be carefully considered. The results of randomized controlled trials have shown that, within the range of acceptable jaundice (serum bilirubin ≤250 μmol/L), the postoperative effect of the direct operation group was better than that of the preoperative biliary stent group. Therefore, preoperative biliary drainage should be strictly indicated. The indications of preoperative biliary drainage include: 1) Patients with fever, septicemia, obvious cholangitis and other symptoms that should be improved before operation; 2) patients with severe symptoms, itching and suppurative cholangitis; 3) patients whose operations were delayed due to various reasons; and 4) patients who should receive preoperative chemoradiation. Nasobiliary drainage tubes, or removable biliary stents are preferred for biliary drainage; however, non-removable bare metal stents should be avoided.
4.5.2. Application of ERCP in unresectable pancreatic cancer
Primarily, more than 80% of pancreatic cancer patients are not indicated to undergo radical surgery because of local invasion or distant metastasis, so palliative treatment of pancreatic cancer patients is especially important, and its goal is to alleviate symptoms and improve the quality of life. Seventy percent to 80% of patients with advanced pancreatic cancer would experience biliary obstruction, so the main purpose of palliative treatment for these patients is to decompress the bile duct. Compared with PTCD, although endoscopic bile duct drainage has the risk of intubation failure and pancreatitis, the chance of successful drainage is greater, the location of stent is more accurate, and the risk of bleeding and bile leakage is less than that of percutaneous transhepatic bile duct drainage. The overall morbidity is lower than that of PTCD. In general, ERCP is recommended as the first choice for palliative biliary drainage. PTCD is only considered when ERCP is not available, or when the operation fails or when the endoscopic treatment is ineffective. Based on the analysis of efficacy and cost-effectiveness, the plastic biliary stents are recommended for patients whose life expectancy is less than 3 months, and metallic biliary stents for those whose life expectancy are at least 3 months. The nasobiliary drainage tube may be used if necessary before stent implantation.
4.6. Interventional therapy
The interventional therapy of pancreatic cancer mainly includes: interventional therapy for pancreatic cancer and pancreatic cancer metastasis and the treatment of pancreatic cancer related complications. The main treatment methods include transarterial infusion chemotherapy, ablation therapy, PTCD, biliary stent implantation, digestive tract stent implantation, embolization for hemorrhage and celiac plexus neurolysis (CPN).
4.6.1. Principles of intervention therapy
(1) The digital subtraction angiography machine is the prerequisite. Indications and contraindications of intervention therapy should be strictly applied, and standardization and individualized treatment should be emphasized.
(2) Interventional therapy mainly applies to the following situations: 1) locally advanced unresectable pancreatic cancer, evaluated by radiological examinations; 2) unresectable pancreatic cancer for other reasons; 3) the infusion chemotherapy as a special form of neoadjuvant chemotherapy for pancreatic cancer; 4) postoperative prophylactic infusion chemotherapy or adjuvant chemotherapy; 5) pancreatic cancer with hepatic metastasis; and 6) control of pancreatic cancer complications, such as pain, hemorrhage, digestive tract obstruction and obstructive jaundice.
4.6.2. Transarterial infusion chemotherapy
(1) Infusion chemotherapy for pancreatic cancer: When the catheter is placed selectively in the celiac artery and the superior mesenteric artery, arteriography is then performed. If the tumor blood supply vessels are identified, the location, size, number and supply artery of the tumor are determined, then transarterial infusion chemotherapy is performed after the catheter is superselectively inserted into the tumor supply vessels. If there is no clear tumor blood supply artery, the target vessel should be determined according to the tumor location, invasion area and blood supply showed by radiological examinations. In principle, the tumors of the head and neck of the pancreas are infused via the gastroduodenal artery, and the tumors of the body and tail of the pancreas are mostly infused via the celiac artery, superior mesenteric artery or splenic artery.
(2) Infusional chemoembolization for hepatic metastasis of pancreatic cancer: If the pancreatic cancer patient is accompanied with liver metastasis, he/she should be treated with hepatic artery infusion chemotherapy or/and embolization at the same time.
(3) Drugs commonly used for infusion chemotherapy: Commonly used drugs include gemcitabine, fluorouracil, adriamycin (epirubicin), platinum (cisplatin and new chemotherapeutic agents such as lobaplatin), and these drugs could be used alone or in combination. The dosage is determined according to the body surface area, liver and kidney function and routine blood test results of the patients.
4.6.3. Ablation therapy
The operator must undergo strict training and has sufficient practice accumulation. Before treatment, the patient’s general condition, tumor status (size, location, number, etc.) should be comprehensively evaluated, and the relationship between the tumor and adjacent organs should be noted. Finally, the appropriate puncture pathway and ablation range should be planned. The selection of appropriate imaging guidance techniques (ultrasound, CT or MRI) and ablation techniques (e.g. irreversible electroporation) are emphasized.
The range of ablation should include at least 5 mm paracancerous tissue in order to completely necrotize the tumor. It is suggested that the ablation area should be expanded appropriately for the tumors with unclear boundary and irregular shape when the condition of adjacent tissues and structures allows.
4.6.4. Interventional therapy for pancreatic cancer complications
(1) Interventional therapy for jaundice: About 65%−75% of pancreatic cancer patients suffered from the biliary obstruction. Percutaneous transhepatic biliary stent and PTCD could effectively reduce serum bilirubin levels, alleviate itching and other symptoms and prevent other complications such as cholecystitis, which makes surgery and chemotherapy available.
(2) Interventional therapy for digestive tract obstruction: About 5%−10% of the pancreatic cancer patients will have gastrointestinal obstruction symptoms such as the gastric outlet obstruction and the duodenal obstruction. The digestive tract stent implantation can relieve early satiety, nausea, postprandial vomiting, weight loss and other discomfort, and improve the quality of life of the patients.
(3) Interventional therapy for hemorrhage: For patients with bleeding of the pancreatic tumor or the metastatic tumors or with postoperative hemorrhage, if the conservative treatment is ineffective, embolization could be considered. The location of bleeding is determined by interventional angiography, and then embolization of bleeding vessels could be performed to stop bleeding.
(4) CPN: CPN is available for pancreatic patients with persistent epigastric pain who cannot relieve from oral analgesics, or tolerate the adverse reactions of opioid analgesics. CPN is accomplished by injecting drugs (absolute ethanol and local anesthesia drugs) into the celiac plexus under the guidance of CT/MR, or ultrasound/endoscopic ultrasound, so as to relieve the abdominal pain by blocking the sympathetic pathway dominating the viscera.
4.7. Supportive care
Common symptoms in patients with end-stage pancreatic cancer can be divided into four categories: pain, malnutrition, obstructive jaundice and tumor-related thrombosis. Best supportive care should be used throughout the treatment of pancreatic cancer, especially in the end-stage patients, with the aim of preventing or alleviating the clinical symptoms and improving the quality of life of patients.
4.7.1. Pain relief
The pain due to tumor invasion is a major symptom in most patients with pancreatic cancer. The main causes of pancreatic cancer pain include the direct infiltration of pancreatic cancer to the peripheral nerves, the inflammation of nerves around pancreas, the increase of capsule tension caused by pancreatic mass and the increase of pancreatic duct pressure caused by pancreatic head mass. The pain treatment is based on the analgesics, and it often requires the combination of surgery, intervention, nerve block, chemotherapy, radiotherapy and psychology to choose the best pain control method. First of all, it is necessary to identify the reason of pain, and surgical treatment is often needed for non-cancerous pain caused by emergency situations such as the digestive tract obstruction or perforation. Analgesic therapy should follow the WHO “three step analgesic ladder” principle. Patients with mild pain could take indomethacin, paracetamol, aspirin and other non-opioid drugs. Patients with moderate pain could be treated with weak opioid drugs such as codeine, and acetaminophen/codeine and ibuprofen/codeine are commonly used 3−4 times per day. Severe pain should be treated with oral morphine in time. It is necessary to determine the degree of cancer pain, according to which patients are prescribed adequate oral opioid painkillers on time. Intramuscular injection of pethidine only should be avoided. The adverse effects of oral analgesics such as nausea, vomiting, constipation, dizziness and headache should be taken into care.
4.7.2. Nutritional status improvement
Routine nutrition screening and evaluation is necessary for pancreatic cancer patients, if there is a nutritional risk or malnutrition, active nutritional support should be given to prevent or delay the development of cancer cachexia. It is suggested that the total calories of 25−30 kcal/kg and protein of 1.2−1.5 g/kg should be supplied and adjusted according to the changes of nutritional and metabolic status of patients. If there are complications, the calories can be increased to 30−35 kcal/kg. Common nutritional support methods include nutrition education, enteral nutrition, and parenteral nutrition. It is recommended to follow the principle of “five steps for malnutrition” for nutritional support. Patients with anorexia or dyspepsia could take medroxyprogesterone or megestrol with trypsin tablets to improve appetite and promote digestive function.
4.8. Traditional Chinese Medicine treatment for pancreatic cancer
Traditional Chinese Medicine can promote the recovery of body function after pancreatic cancer surgery, reduce the toxic reactions of radiotherapy, chemotherapy and targeted therapy, relieve the symptoms of patients, improve the quality of life of patients, and may prolong the survival. As one of the important treatment methods for pancreatic cancer, it can be used alone or in combination with other anti-tumor drugs.
China Food and Drug Administration has approved a variety of modern Traditional Chinese Medicine preparations for the treatment of pancreatic cancer. However, these drugs have been on the market for many years. Early experimental and clinical studies are relatively weak, and there is still a lack of high-level evidences to fully support them. It needs to be actively studied in depth.
In addition to these listed proprietary traditional Chinese drugs, abiding by the principle of dialectical treatment of Traditional Chinese Medicine, the Traditional Chinese Medicine compound treatment is one of the most commonly used methods. It might reduce tumor-related complications, improve the quality of life and prolong the survival of patients.
5. Diagnosis and treatment flowchart and follow-up
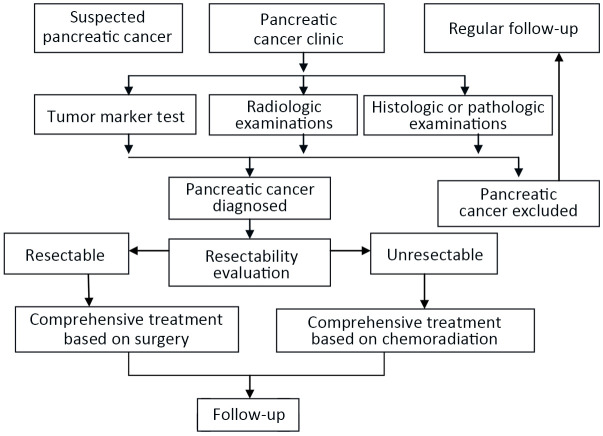
5.1. Diagnosis and treatment flowchart for pancreatic cancer
Refer to Appendix 14 (Figure A4 ) for detailed information.
5.2. Follow-up
The main purpose of follow-up is to find metastasis and recurrence, which can be potentially treated radically, and to find tumor recurrence or the second primary cancer as early as possible, and then to manage them in time to improve the overall survival and the quality of life of the patients. It is recommended that the patients who underwent pancreatic cancer resection should be followed up once every 3 months in the first year, once every 3−6 months in the 2nd and 3rd year, and once every 6 months thereafter. The examination items during follow-up include routine blood test, biochemical test, serum tumor markers such as CA199, CA125 and CEA, ultrasound, X-ray, thin slice chest CT scan, upper abdominal contrast enhanced CT and so on. The follow-up period is at least 5 years. Liver MRI or bone scan is suggested to be performed for patients suspected of liver metastasis or bone metastasis. Patients with advanced disease or distant metastasis should be followed up at least once every 2−3 months. The examination items during follow-up include routine blood test, biochemical test, serum tumor markers such as CA199, CA125 and CEA, chest CT, upper abdominal contrast enhanced CT and PET-CT if necessary. The aim of follow-up is to evaluate the nutritional status and tumor progression of patients and then to adjust the comprehensive treatment regimens in time.
Working group members
Group leader: Yupei Zhao
Associate group leader: Chengfeng Wang
Group members: Liwei Wang, Yi Ba, Susheng Shi, Minghua Cong, Jun Liu, Rong Liu, Xubao Liu, Zhaohui Tang, Chunxia Du, Shengping Li, Jiangtao Li, Yexiong Li, Shu Li, Yinmo Yang, Zhiying Yang, Lin Yang, Liu Yang, Taiping Zhang, Jianwei Zhang, Rufu Chen, Dongmei Lin, Han Ouyang, Gang Jin, Xinming Zhao, Haiping Zhao, Jihui Hao, Renyi Qin, Yufeng Yuan, Li Peng, Bing Peng, Kuirong Jiang, Deliang Fu, Guanghai Dai, Menghua Dai
Secretary: Jianwei Zhang
Translation group members
Baocai Xing Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Peking University Cancer Hospital & Institute
Kemin Jin Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Peking University Cancer Hospital & Institute
Appendix 1
Figure A1.
Optimal selection route chart of radiological examinations for pancreatic cancer before treatment. CA, carbohydrate antigen; CT, computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography; US, ultrasound; EUS, endoscopic ultrasonography; FNA, fine needle aspiration.
Appendix 2
Figure A2.
Optimal selection route chart of radiological examinations for pancreatic cancer after treatment. CT, computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography.
Appendix 3
Table A1. Classification of pancreaticobiliary duct cytological diagnosis.
| Classification | Diagnosis |
| I | Unable to diagnose |
| II | No malignancy |
| III | Atypia |
| IV | Neoplasms |
| A | Benign |
| Serous cystadenoma | |
| Neuroendocrine microadenoma | |
| Lymphangioma | |
| B | Others |
| Intraductal papillary mucinous neoplasm (Cells with mild, moderate or severe dysplasia) | |
| Mucinous cystic neoplasm (Cells with mild, moderate or severe dysplapia) | |
| Well-differentiated neuroendocrine neoplasm | |
| Solid pseudopapillary tumor | |
| V | Suspicious malignancy |
| VI | Malignancy |
| Ductal adenocarcinoma | |
| High-grade (G3) neuroendocrine carcinoma | |
| Acinar cell carcinoma | |
| Pancreatoblastoma | |
| Lymphoma | |
| Secondary tumors |
Appendix 4 General description of gross pancreatic cancer specimens
Pancreaticoduodenectomy
Specimens of pancreaticoduodenectomy, distal stomach, greater curvature length _ cm, lesser curvature length _ cm, duodenum length _ cm, diameter _ cm, common bile duct length _ cm, diameter _cm, pancreas size _×_×_ cm, Tumors (appearance description) were seen in (duodenal papilla/lower common bile duct/pancreatic head), size _×_×_ cm, sectional character _; Depth of invasion (duodenal papilla/lower common bile duct) to_. Involvement/uninvolvement of other organs adjacent to the tumor. What was seen in the mucosa/muscle wall of the paratumoral intestine (polyp/adenoma/ulcerative colitis/necessary negative description), what was seen in the gastric wall (necessary negative description), and what was seen in pancreas (necessary negative description). Duodenum, stomach, common bile duct, transection surface of pancreas and retroperitoneal margins (marked or clinically submited separately). Lymph nodes found (several/many/more than ten/dozens) along the greater curvature, diameters _ to _ cm; Lymph nodes found (several/many/more than ten/dozens) along the lesser curvature, diameters _ to _ cm; Lymph nodes found (several/many/more than ten/dozens) along the intestinal wall, diameters _ to _ cm; Lymph nodes found (several/many/more than ten/dozens) in the mesentery, diameters _ to _ cm; and peripancreatic lymph nodes (several/many/more than ten/dozens), diameters _ to _ cm.
Appendix 5 General description of microscopic findings of pancreatic cancer
1. Tumor
(1) Histological type
(2) Histological grade
(3) Invasion range
(4) Lymphatic/vascular invasion
(5) Perineural invasion
2. Margins
(1) Distal pancreas remnant
(2) Common bile duct
(3) Proximal (stomach)
(4) Distal (duodenum)
3. Other pathological findings
(1) Chronic pancreatitis
(2) Atypical hyperplasia
(3) Metaplasia
(4) Others
4. Regional lymph nodes (including perigastric, periduodenal, peripancreatic lymph nodes and lymph nodes submitted separately)
(1) Total number of lymph nodes acquired
(2) The number of lymph nodes involved
5. Distal metastasis
6. Other tissues/organs
7. Special adjuvant examination results (histochemical staining, immunohistochemical staining, etc.)
8. Difficult pathologic diagnosis should be submitted to the superior hospitals for consultation. (The original pathological reports should be provided to check if the correct sections have been submitted and to reduce possible errors. Sufficient tumor sections or wax blocks and what was observed during the operation should also be provided.)
Appendix 6 Histological classification of pancreatic tumors (WHO 2019)
Benign epithelial tumors and precursor lesions
8441/0 Serous cystadenoma, non-special type
Oligocystic serous cystadenoma
Solid serous cystadenoma
Cerebral retinal angiomatosis-associated serous tumors
Mixed serous-endocrine tumor
8441/3 Serous cystadenocarcinoma, non-specific type
8148/0 Pancreatic intraepithelial neoplasia, low-grade
8148/2 Pancreatic intraepithelial neoplasia, high-grade
8453/0 Intraductal papillary mucinous neoplasm with low-grade dysplasia
8453/2 Intraductal papillary mucinous neoplasm with high-grade dysplasia
8453/3 Intraductal papillary mucinous neoplasm with invasive cancer
8455/2 Intraductal oncocytic papillary tumors, non-specific type
8455/3 Intraductal oncocytic papillary tumors with invasive cancer
8470/0 Mucinous cystic neoplasm with low-grade dysplasia
8470/2 Mucinous cystic neoplasm with high-grade dysplasia
8470/3 Mucinous cystic neoplasm with invasive cancer
Malignant epithelial neoplasm
8500/3 Ductal adenocarcinoma, non-specific type
8480/3 Colloid carcinoma
8490/3 Low adhesive carcinoma
8490/3 Signet ring cell cancer
8510/3 Medullary carcinoma, non-specific type
8560/3 Adenosquamous carcinoma
8576/3 hepatoid cancer
8014/3 Large-cell carcinoma with a rhabdomated muscle phenotype
8020/3 Undifferentiated carcinoma, non-specific type
8035/3 Undifferentiated carcinoma with osteoclastic giant cells
8550/3 Acinar cell carcinoma
8551/3 Acinar cell cystadenocarcinoma
8154/3 Mixed acinar-endocrine carcinoma
8154/3 Mixed acinar-endocrine-ductal carcinoma
8552/3 Mixed acinar-ductal carcinoma
8971/3 Pancreatic blastoma
8452/3 Pancreatic solid-pseudopapillary tumor
Pancreatic solid-pseudopapillary tumor with high-grade carcinoma
Pancreatic neuroendocrine tumors
8150/0 Pancreatic neuroendocrine microadenoma
8240/3 Pancreatic neuroendocrine tumors
8240/3 Neuroendocrine tumor, G1
8249/3 Neuroendocrine tumor, G2
8249/3 Neuroendocrine tumor, G3
8150/3 Pancreatic neuroendocrine tumor, nonfunctional
Oncocytic neuroendocrine tumor, nonfunctional
Pleomorphic neuroendocrine tumor, nonfunctional
Clear-cell neuroendocrine tumor, nonfunctional
Cystic neuroendocrine tumor, nonfunctional
Functional pancreatic neuroendocrine tumor
8241/3 Serotonin-secreting tumors
8153/3 Gastrinoma
8152/3 Glucagonoma
8151/3 Insulinoma
8156/3 Somatostatinoma
5155/3 VIPoma
8158/3 Corticotropin-secreting tumors
8241/3 Enteropheochromocyte carcinoids
8246/3 Neuroendocrine carcinoma
8041/3 Small cell neuroendocrine carcinoma
8013/3 Large cell neuroendocrine carcinoma
8154/3 Mixed neuroendocrine-nonneuroendocrine tumors
8154/3 Mixed acinar-endocrine carcinoma
8154/3 Mixed acinar-neuroendocrine carcinoma
8154/3 Mixed acinar-endocrine-ductal carcinoma
Mature teratoma
Mesenchymoma
Malignant lymphoma
Secondary tumors
Appendix 7
Table A2. Karnofsky score (KPS, percentage method).
| Score | Status |
| 100 | Normal; no complaints; no evidence of disease |
| 90 | Able to carry on normal activity; minor signs or symptoms of disease |
| 80 | Normal activity with effort; some signs or symptoms of disease |
| 70 | Cares for self; unable to carry on normal activity or to do active work |
| 60 | Requires occasional assistance; but is able to care for most of their personal needs |
| 50 | Requires considerable assistance and frequent medical care |
| 40 | Disabled; Requires special care and assistance |
| 30 | Severely disabled; hospital admission is indicated although death not imminent |
| 20 | Very sick; hospital admission necessary; active supportive treatment necessary |
| 10 | Moribund; Fatal processes progressing rapidly |
| 0 | Dead |
Appendix 8
Table A3. Zubrod-ECOG-WHO score (ZPS, 5-point method).
| Score | Status |
| ECOG, Eastern Cooperative Oncology Group; WHO, World Health Organization. | |
| 0 | Fully active; able to carry on all predisease performance without restriction |
| 1 | Slight symptoms; capable of all selfcare; able to carry out work of a light or sedentary nature |
| 2 | Able to tolerate tumor-related symptoms; capable of all selfcare; confined to bed not more than 50% of waking hours |
| 3 | Severe tumor-related symptoms; confined to bed more than 50% of waking hours, but able to stand up; capable of only limited selfcare |
| 4 | Completely disabled; cannot carry on any selfcare; totally confined to bed |
| 5 | Dead |
Appendix 9
Table A4. Criteria of resectability for pancreatic cancer.
| Resectability status | Arterial | Venous |
| Resectable | No arterial tumor contact [celiac axis (CA)], superior mesenteric artery (SMA), or common hepatic artery (CHA)] | No tumor contact with the superior mesenteric vein (SMV) or portal vein (PV) or ≤180° contact without vein contour irregularity |
| Borderline resectable | Pancreatic head and neck:
Solid tumor contact with CHA without extension to CA or hepatic artery bifurcation allowing for safe and complete resection and reconstruction. Solid tumor contact with the SMA of ≤180°. Solid tumor contact with variant arterial anatomy (ex: accessory right hepatic artery, replaced right hepatic artery, replaced CHA, and the origin of replaced or accessory artery) and the presence and degree of tumor contact should be noted if present, as it may affect surgical planning |
Pancreatic head and neck:
Solid tumor contact with the SMV or PV of >180°, contact of ≤180° with contour irregularity of the vein or thrombosis of the vein but with suitable vessel proximal and distal to the site of involvement allowing for safe and complete resection and vein reconstruction. Solid tumor contact with the inferior vena cava (IVC) |
| Pancreatic body and tail:
Solid tumor contact with the CA of ≤180°. Solid tumor contact with the CA of >180° without involvement of the aorta and with intact and uninvolved gastroduodenal artery |
Pancreatic body and tail:
Solid tumor contact with the confluence of splenic vein and PV, or contact with the left side of PV of ≤180° with contour irregularity of the vein or thrombosis of the vein but with suitable vessel proximal and distal to the site of involvement allowing for safe and complete resection and vein reconstruction. Solid tumor contact with the inferior vena cava (IVC) |
|
| Unresectable | ||
| Locally advanced | Pancreatic head and neck:
Solid tumor contact with SMA >180°. Solid tumor contact with the CA >180°. Solid tumor contact with the first jejunal branch of SMA |
Pancreatic head and neck:
Unreconstructible SMV/PV due to tumor involvement or occlusion (can be due to tumor or bland thrombus). Contact with most proximal draining jejunal branch into SMV |
| Pancreatic body and tail:
Solid tumor contact of >180° with the SMA or CA. Solid tumor contact with the CA and aortic involvement |
Pancreatic body and tail:
Unreconstructible SMV/PV due to tumor involvement or occlusion (can be due to tumor or bland thrombus). |
|
| Distant metastasis | Distant metastasis (including nonregional lymph node metastasis) | Distant metastasis (including nonregional lymph node metastasis) |
Appendix 10
Table A5. Clinical grading system of postpancreatectomy hemorrhage (PPH).
| Grade | Time of onset, location, severity and clinical impact of bleeding | Clinical condition | Diagnostic consequence | Therapeutic consequence |
| A | Early, intra- or extra-luminal, mild | Well | Observation, blood count, ultrasonography and, if necessary, computed tomography | No |
| B | Early, intra- or extra-luminal, severe; Late, intra- or extraluminal, mild | Often well/ intermediate, very rarely life-threatening | Observation, blood count, ultrasonography, computed tomography, angiography, endoscopy | Transfusion of fluid/ blood, intermediate care unit (or ICU), therapeutic endoscopy, embolization, relaparotomy for early PPH |
| C | Late, intra- or extra-luminal, severe | Severely impaired, life-threatening | Angiography, computed tomography, endoscopy | Localization of bleeding, angiography and embolization, (endoscopy) or relaparotomy, ICU |
Appendix 11 Response evaluation criteria for solid tumors of WHO
1. Complete response (CR): Disappearance of all lesions, lasting for at least 1 month.
2. Partial response (PR): The product of the maximum diameter and the maximum vertical diameter of the tumor was reduced by 50%, with no increase in other lesions, lasting more than 1 month
3. Stable disease (SD): The product of the maximum diameter and the maximum vertical diameter of the tumor was reduced by no more than 50%, however increased by no more than 25%, lasting for at least 1 month.
4. Progressive disease (PD): The product of the maximum diameter and the maximum vertical diameter of the tumor increased more than 25%.
Appendix 12 Response Evaluation Criteria In Solid Tumors (RECIST)
Evaluation of target lesions:
Complete response (CR): Disappearance of all target lesions.
Partial response (PR): At least a 30% decrease in the sum of the longest diameter (LD) of target lesions, taking as reference the baseline sum LD.
Progressive disease (PD): At least a 20% increase in the sum of the LD of target lesions, taking as reference the smallest sum LD recorded since the treatment started or the appearance of one or more new lesions.
Stable disease (SD): Neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD, taking as reference the smallest sum LD since the treatment started.
Evaluation of non-target lesions:
Complete response (CR): Disappearance of all non-target lesions and normalization of tumor marker level.
Incomplete response/stable disease (IR/SD): Persistence of one or more non-target lesion(s) or/and maintenance of tumor marker level above the normal limits.
Progressive disease (PD): Appearance of one or more new lesions and/or unequivocal progression of existing non-target lesions.
Evaluation of best overall response:
The best overall response is the best response recorded from the start of the treatment until disease progression/recurrence (taking as reference for PD the smallest measurements recorded since the treatment started). In general, the patient's best response assignment will depend on the achievement of both measurement and confirmation criteria.
Appendix 13
Figure A3.
Flow chart of diagnosis and treatment of pancreatic cancer by ERCP. CT, computed tomography; US, ultrasound; EUS, endoscopic ultrasonography; ERCP, endoscopic retrograde cholangiopancreatography; IDUS, intraductal ultrasound; ENBD, endoscopic nasobiliary drainage; EUS-BD, endoscopic ultrasound guided biliary drainage; PTCD, percutaneous transhepatic cholangial drainage; FNA, fine needle aspiration.
Appendix 14
Figure A4.
Flow chart of diagnosis and treatment of pancreatic cancer.