Abstract
Objective
To determine the effectiveness of booster vaccinations on the risk of hospitalization with coronavirus disease 2019 (COVID-19) and how it varies by enrollee characteristics and interval from the initial vaccination to receipt of a booster.
Patients and Methods
This cohort study used 100% Medicare claims from January 1, 2020, through December 31, 2021, and matched 3,940,475 individuals who received boosters to 3,940,475 controls based on week and type of original COVID-19 vaccine and demographic and clinical characteristics. We compared the association of booster vs no booster with COVID-19 hospitalization using Cox proportional hazards regression models controlling for patient characteristics. We also determined the association of time from original vaccine to booster with COVID-19 hospitalization.
Results
Over a maximum of 130 days of follow-up, boosted enrollees had 8.20 (95% CI, 7.81 to 8.60) COVID-19 hospitalizations per million days vs 43.70 (95% CI, 42.79 to 44.64) for controls (81% effectiveness). Effectiveness varied by race, prior hospitalizations, and certain comorbidities, for example, leukemia/lymphoma (53% effectiveness), autoimmune disease (73%), and dementia (73%). Boosters received between 6 and 9 months after original vaccination varied between 81% and 85% effectiveness, while boosters received at 5 to 6 months (62%) or less than 5 months (58%) were less effective.
Conclusion
Boosters are highly effective in the Medicare population. Approximately 69,225 hospitalizations would be prevented by boosters in the 15 million individuals aged 65 years or older currently not boosted in a period similar to the September 2020 through January 2021 period studied. Boosters provided the greatest benefits if they were received between 6 and 9 months following original vaccinations. However, boosters were associated with substantial decreases in COVID-19 hospitalizations in all categories of enrollees.
To protect against the diminishing immunity from the coronavirus disease 2019 (COVID-19) primary vaccine, the US Food and Drug Administration (FDA) has authorized COVID-19 booster vaccinations for individuals aged 18 and older. As of June 2022, nearly half of all adults and one-third of individuals aged 65 years or older have yet to receive a booster.1 Studies from Israel,2 , 3 England,4 Spain,5 and the United States6 , 7 have found that boosters were effective in reducing COVID-19–associated hospitalization, severe disease, and death.
There is a little information on the optimal interval between the original vaccine and a booster. Originally, the FDA recommended a minimum 6-month interval between the primary vaccine and a booster.8 However, in January 2022, the FDA-recommended interval was reduced to a 5-month minimum.9
The study had 2 objectives. First, we determined the effectiveness of booster vaccinations on the risk of hospitalization with COVID-19 among Medicare beneficiaries and how it varied by demographic characteristics and specific comorbidities. Second, we assessed the risk of hospitalization with COVID-19 as a function of interval between primary vaccination and a booster dose.
Patients and Methods
Study Design and Data Source
In this retrospective cohort study, we used 100% national Medicare claims from January 1, 2020, through December 31, 2021, last updated on May 15, 2022. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines.10
Study Cohort and Identification of Boosted Individuals and Unboosted Controls
This cohort included fee-for-service Medicare beneficiaries who received 2 doses of Pfizer or 2 doses of Moderna vaccine as the primary vaccination series from December 11, 2020, through December 31, 2021 (Supplemental Figures 1 and 2, available online at http://www.mayoclinicproceedings.org).
We emulated a target trial11 each week and matched individuals who received a booster to those who did not receive boosters starting on August 12, 2021, the date the Healthcare Common Procedure Coding System/Current Procedural Terminology code for the third dose of Pfizer was approved.12 All individuals who did not receive a booster that week or prior to that date were eligible for matching. We matched without replacement one unboosted individual to one boosted individual based on the week and type of the original vaccine, age (±2 years), sex, race (non-Hispanic White, non-Hispanic Black, Asian, Hispanic, and other), Medicaid eligibility, number of prior COVID-19 hospitalizations, prior COVID-19 infection, residence (community vs nursing facility), and tertile of summary Elixhauser comorbidity index score. Of 4,731,607 boosted individuals, 4,016,231 were matched to controls for an 84.9% match rate. We assigned the index date of the receipt of a booster for the boosted individual to the unboosted controls. Use of a target trial reduces the risk of selection bias and immortal time bias, clearly assigns time zero, and avoids common methodological pitfalls of observational studies.11 The Supplemental Methods section (available online at http://www.mayoclinicproceedings.org) contains additional details.
For sensitivity analyses, we used a traditional matching method. We identified individuals who received boosters from August 12, 2021, through November 30, 2021. Of all the eligible individuals who did not receive boosters from August 12, 2021, to December 31, 2021, we identified one unboosted control for each boosted individual matched on the week and type of the original vaccination, age (±2 years), sex, race (non-Hispanic White, non-Hispanic Black, Asian, Hispanic, and other), Medicaid eligibility, number of prior COVID-19 hospitalizations, prior COVID-19 infection, residence (community vs nursing facility), and tertile of summary Elixhauser comorbidity index score. The match rate was 48.5% (of 4,731,607 boosted individuals, 2,293,887 were matched to controls).
Identification of Interval Between Original Vaccine and Booster
We classified the interval between primary vaccination and a booster dose as 4 to less than 5, 5 to less than 6, 6 to less than 7, 7 to less than 8, 8 to less than 9, and 9 months or longer.
Study Outcome
We identified a COVID-19 hospitalization if the International Classification of Diseases, Tenth Revision, Clinical Modification code of U07.1 was listed as the primary admission or first or second discharge diagnosis.13 In a sensitivity analysis, we limited the outcome to those with a primary admission diagnosis of COVID-19.14
Covariates
Age, sex, race (non-Hispanic White, non-Hispanic Black, Asian, Hispanic, and other), and Medicaid eligibility were from Medicare’s Master Beneficiary Summary File; the type of primary vaccine from the Carrier File; the number of prior hospitalizations from the hospital Standard Analytic File; residence (community vs nursing facility/institution) prior to vaccination date from the skilled nursing facility Standard Analytic File and the Minimum Data Set15; and prior COVID-19 diagnosis from all claims files.16 We used Elixhauser comorbidities and combined comorbidities in some analyses.17
Statistical Analyses
We used descriptive statistics to characterize the boosted individuals and unboosted controls and Kaplan-Meier estimator to generate cumulative incidence curves for COVID-19 hospitalization for boosted and unboosted exposure groups18 using joinpoint regression to identify any inflection point.19 We used standardized differences to evaluate balance of covariates among boosted and unboosted exposure groups, where a standardized difference of less than 0.10 suggests acceptable balance.20 Incidence rates and 95% CIs of COVID-19 hospitalizations were expressed as hospitalizations per 1 million person-days. We constructed Cox proportional hazards regression models to determine the association of booster receipt with the risk of COVID-19 hospitalization, controlling for the prior vaccine and patient characteristics. Individuals were censored if they died, dropped out of fee-for-service Medicare, or on December 31, 2021. We performed symmetric censoring and censored both the unboosted and boosted individuals of the matched pair at the same date when the unboosted individual received a booster.11 Residual plots and the supremum test for all covariates suggested that the proportional hazard assumption was not violated.21 We tested for an interaction of each patient characteristic with booster and performed stratified analyses where significant. We computed vaccine effectiveness as (1 − hazard ratio) × 100. We multiplied risk difference per 1 million person-days with 130 to calculate risk difference over 130 days per 1 million persons and again multiplied this with 15 to estimate the number of COVID-19 hospitalizations prevented by boosters in approximately 15 million currently unboosted persons aged 65 years or older (also see Supplemental Methods section).22 , 23
For the analysis of the optimal interval between original vaccination and booster, we used the Cox regression model to estimate the risk of hospitalization for boosted individuals compared with unboosted controls for different intervals between vaccination and receipt of boosters, controlling for all patient characteristics, original vaccination type, and the type of booster. We used cohorts from the target trial method for the main analysis and the traditional matched cohorts for a sensitivity analysis.
All analyses were performed with SAS Enterprise Guide statistical software, version 7.1 (SAS Institute) at the Centers for Medicare and Medicaid Services Virtual Research Data Center. The study was approved by the University of Texas Medical Branch Institutional Review Board, which waived the need for informed consent because of the use of deidentified data.
Results
Characteristics of the Study Cohort
Table 1 presents the characteristics of 3,940,475 enrollees who received boosters between August 12, 2021, and November 30, 2021, and 3,940,475 matched controls. The mean age of the cohort was 74 years (interquartile range, 70 to 80 years), and the majority were female (4,595,366 of the 7,880,950 patients [58.3%]) and non-Hispanic White (6,807,760 of the 7,880,950 patients [86.4%]). In the target trial approach, all covariates were well balanced across boosted and unboosted exposure groups (all standardized differences were <0.03 or 3%). The median follow-up duration was identical in boosted and unboosted groups (49 days).
Table 1.
| Variable | Total cohort (N=7,880,950 [100%]) | Boosted individuals (n=3,940,475 [50.0%]) | Unboosted controls (n=3,940,475 [50.0%]) | Standardized difference |
|---|---|---|---|---|
| Follow-up (d) Median (p25-p75) [min-max] |
49 (29-67) [10-141] | 49 (29-67) [10-141] | 49 (29-66) [10-141] | 0.0091 |
| Age (y), median (p25-p75) | 74 (70-80) | 74 (70-80) | 74 (70-80) | −0.0235 |
| COVID-19 original vaccine | 0 | |||
| Pfizer | 4,035,158 (51.2) | 2,017,579 (50.0) | 2,017,579 (50.0) | |
| Moderna | 3,845,792 (48.8) | 1,922,896 (50.0) | 1,922,896 (50.0) | |
| Age group (y) | 0 | |||
| 66-70 | 2,248,536 (28.5) | 1,142,336 (50.8) | 1,106,200 (49.2) | |
| 71-75 | 2,169,531 (27.5) | 1,087,005 (50.1) | 1,082,526 (49.9) | |
| 76-80 | 1,526,810 (19.4) | 757,068 (49.6) | 769,742 (50.4) | |
| 81-85 | 1,006,481 (12.8) | 496,829 (49.4) | 509,652 (50.6) | |
| ≥86 | 929,592 (11.8) | 457,237 (49.2) | 472,355 (50.8) | |
| Sex | 0 | |||
| Male | 3,285,584 (41.7) | 1,642,792 (50.0) | 1,642,792 (50.0) | |
| Female | 4,595,366 (58.3) | 2,297,683 (50.0) | 2,297,683 (50.0) | |
| Race | 0 | |||
| Non-Hispanic White | 6,807,760 (86.4) | 3,403,880 (50.0) | 3,403,880 (50.0) | |
| Non-Hispanic Black | 337,824 (4.3) | 168,912 (50.0) | 168,912 (50.0) | |
| Asian | 225,590 (2.9) | 112,795 (50.0) | 112,795 (50.0) | |
| Hispanic | 218,912 (2.8) | 109,456 (50.0) | 109,456 (50.0) | |
| Otherd | 290,864 (3.7) | 145,432 (50.0) | 145,432 (50.0) | |
| Medicaid eligibility | 0 | |||
| No | 7,388,256 (93.7) | 3,694,128 (50.0) | 3,694,128 (50.0) | |
| Yes | 492,694 (6.3) | 246,347 (50.0) | 246,347 (50.0) | |
| No. of prior hospitalizations | 0 | |||
| 0 | 6,986,464 (88.6) | 3,493,232 (50.0) | 3,493,232 (50.0) | |
| 1 | 675,388 (8.6) | 337,694 (50.0) | 337,694 (50.0) | |
| ≥2 | 219,098 (2.8) | 109,549 (50.0) | 109,549 (50.0) | |
| Prior COVID-19 | 0 | |||
| No | 7,398,502 (93.9) | 3,699,251 (50.0) | 3,699,251 (50.0) | |
| Yes | 482,448 (6.1) | 241,224 (50.0) | 241,224 (50.0) | |
| Residence prior to original vaccination | 0 | |||
| Community | 7,754,612 (98.4) | 3,877,306 (50.0) | 3,877,306 (50.0) | |
| Nursing facility | 126,338 (1.6) | 63,169 (50.0) | 63,169 (50.0) | |
| Elixhauser comorbidity summary scoree | 0 | |||
| <0 | 2,126,332 (27.0) | 1,063,166 (50.0) | 1,063,166 (50.0) | |
| 0 | 3,235,364 (41.0) | 1,617,682 (50.0) | 1,617,682 (50.0) | |
| >0 | 2,519,254 (32.0) | 1,259,627 (50.0) | 1,259,627 (50.0) | |
| Comorbidity | ||||
| AIDS/HIV | 8855 (0.1) | 5291 (59.8) | 3564 (40.2) | 0.0131 |
| Alcohol abuse | 54,863 (0.7) | 26,185 (47.7) | 28,678 (52.2) | −0.0076 |
| Deficiency anemia | 828,691 (10.5) | 412,475 (49.8) | 416,216 (50.2) | −0.0031 |
| Autoimmune | 320,615 (4.1) | 172,836 (53.9) | 147,779 (46.1) | 0.0322 |
| Blood loss anemia | 61,522 (0.8) | 30,784 (50.0) | 30,738 (50.0) | 0.0001 |
| Leukemia | 50,294 (0.6) | 28,846 (57.4) | 21,448 (42.6) | 0.0326 |
| Lymphoma | 84,920 (1.1) | 48,371 (57.0) | 36,549 (43.0) | 0.0291 |
| Metastatic cancer | 86,287 (1.1) | 46,931 (54.4) | 39,356 (45.6) | 0.0185 |
| Solid tumor without metastasis, malignant | 643,506 (8.2) | 336,386 (52.3) | 307,120 (47.7) | 0.0271 |
| Solid tumor without metastasis, in situ | 65,541 (0.8) | 34,878 (53.2) | 30,663 (46.8) | 0.0118 |
| Cerebrovascular disease | 450,634 (5.7) | 218,597 (48.5) | 232,037 (51.5) | −0.0147 |
| Congestive heart failure | 542,345 (6.9) | 259,542 (47.9) | 282,803 (52.1) | −0.0233 |
| Coagulopathy | 182,966 (2.3) | 95,037 (51.9) | 87,929 (48.1) | 0.0120 |
| Dementia | 384,887 (4.9) | 164,584 (42.8) | 220,303 (57.2) | −0.0656 |
| Depression | 707,782 (9.0) | 340,174 (48.1) | 367,608 (51.9) | −0.0244 |
| Diabetes, complicated | 1,008,282 (12.8) | 488,161 (48.4) | 520,121 (51.6) | −0.0243 |
| Diabetes, uncomplicated | 666,844 (8.5) | 326,880 (49.0) | 339,964 (51.0) | −0.0119 |
| Drug abuse | 45,357 (0.6) | 21,245 (46.8) | 24,112 (53.2) | −0.0096 |
| Hypertension, complicated | 768,245 (9.8) | 377,091 (49.1) | 391,154 (50.9) | −0.0120 |
| Hypertension, uncomplicated | 3,602,908 (45.7) | 1,793,937 (49.8) | 1,808,971 (50.2) | −0.0077 |
| Liver disease, mild | 162,274 (2.1) | 83,002 (51.1) | 79,272 (48.8) | 0.0069 |
| Liver disease, moderate to severe | 22,436 (0.3) | 12,046 (53.7) | 10,390 (46.3) | 0.0079 |
| Chronic pulmonary disease | 859,326 (10.9) | 423,950 (49.3) | 435,376 (50.7) | −0.0093 |
| Neurologic disorders affecting movement | 207,529 (2.6) | 95,499 (46.0) | 109,030 (52.5) | −0.0167 |
| Seizures and epilepsy | 102,966 (1.3) | 48,370 (47.0) | 54,596 (53.0) | −0.0139 |
| Other neurologic disorders | 156,293 (2.0) | 73,808 (47.2) | 82,485 (52.8) | −0.0158 |
| Obesity | 844,919 (10.7) | 415,172 (49.1) | 429,747 (50.9) | −0.0120 |
| Paralysis | 88,903 (1.1) | 40,611 (45.7) | 48,292 (54.3) | −0.0185 |
| Peripheral vascular disease | 813,238 (10.3) | 400,660 (49.3) | 412,578 (50.7) | −0.0099 |
| Psychoses | 217,295 (2.8) | 105,050 (48.3) | 112,245 (51.7) | −0.0112 |
| Pulmonary circulation disease | 123,823 (1.6) | 61,804 (49.9) | 62,019 (50.1) | −0.0004 |
| Renal failure, moderate | 676,910 (8.6) | 333,376 (49.2) | 343,534 (50.8) | −0.0092 |
| Renal failure, severe | 148,570 (1.9) | 75,428 (50.8) | 73,142 (49.2) | 0.0043 |
| Hypothyroidism | 1,256,574 (15.9) | 631,890 (50.3%) | 624,684 (49.7) | 0.0050 |
| Other thyroid disorders | 224,160 (2.8) | 116,517 (52.0) | 107,643 (48.0) | 0.0135 |
| Peptic ulcer with bleeding | 40,476 (0.5) | 20,084 (49.6) | 20,392 (50.4) | −0.0011 |
| Valvular disease | 543,637 (6.9) | 276,922 (50.9) | 266,715 (49.1) | 0.0102 |
| Weight loss | 166,927 (2.1) | 80,917 (48.5) | 86,010 (51.5) | −0.0090 |
COVID-19, coronavirus disease 2019; max, maximum; min, minimum.
Data are presented as No. (percentage) of patients unless indicated otherwise. Percentages may not equal 100 because of rounding.
Values in parentheses in the first column are column percentages; values in the second, and third columns are row percentages.
Other race includes Pacific Islander, American Native, and all other races and ethnicities.
We applied weights to 38 Elixhauser conditions to calculate Elixhauser summary score.17
COVID-19 Hospitalization in Boosted Enrollees vs Nonboosted Controls
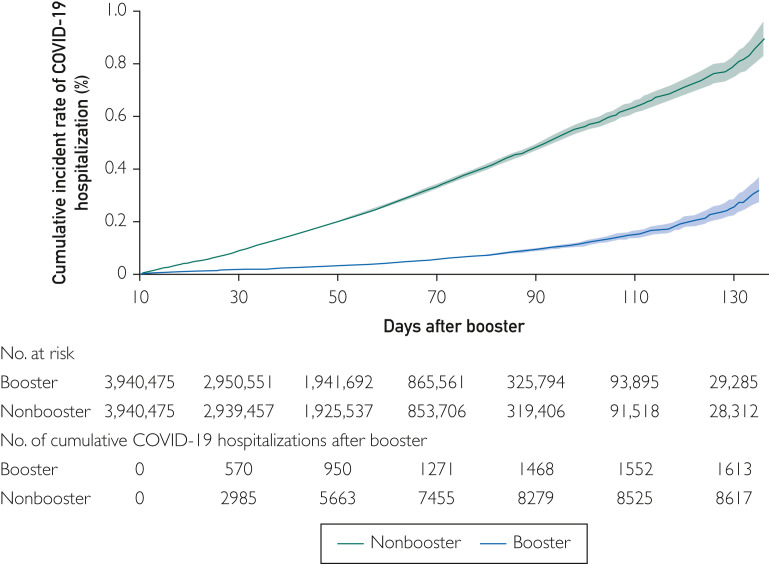
Table 2 presents unadjusted hospitalization rates and adjusted hazard ratios for COVID-19 hospitalization as a function of vaccine and enrollee characteristics. Boosted enrollees had 8.20 (95% CI, 7.81 to 8.60) COVID-19 hospitalizations per million days vs 43.70 (95% CI, 42.79 to 44.64) for controls. In the adjusted analyses, boosted individuals had a hazard of COVID-19 hospitalization of 0.19 (95% CI, 0.18 to 0.20) compared with unboosted controls. The booster effectiveness for COVID-19 hospitalization was 81% (95% CI, 80% to 82%). Figure 1 presents the cumulative incidence of hospitalization in the boosted and nonboosted groups, showing a divergence of the 2 curves throughout the 130 days of follow-up. We estimated that approximately 69,225 hospitalizations (risk difference of 35.50 per 1 million person-days x130 x 15) would be prevented by boosters over 130 days of follow-up among 15 million individuals aged 65 years or older currently not boosted in a period similar to the September 2020 through January 2021 period studied.
Table 2.
Unadjusted COVID-19 Hospitalization Rates and Adjusted Hazards of Hospitalization From a Cox Proportional Hazards Regression Model Including Receipt of Booster and All Patient Characteristicsa,b
| Variable | No. (%) of patients (N=7,880,950) |
Hospitalization rate per 1,000,000 person-days | Adjusted HR (95% CI) |
|---|---|---|---|
| Boosterc | |||
| No | 3,940,475 (50.0) | 43.70 (42.79-44.64) | Reference |
| Yes | 3,940,475 (50.0) | 8.20 (7.81-8.60) | 0.19 (0.18-0.20) |
| COVID-19 original vaccine | |||
| Pfizer | 4,035,158 (51.2) | 29.85 (29.14-30.58) | Reference |
| Moderna | 3,845,792 (48.8) | 20.90 (20.23-21.59) | 0.81 (0.77-0.84) |
| Age group | |||
| 66-70 | 2,248,536 (28.5) | 13.44 (12.78-14.14) | Reference |
| 71-75 | 2,169,531 (27.5) | 19.48 (18.67-20.32) | 1.31 (1.23-1.40) |
| 76-80 | 1,526,810 (19.4) | 29.50 (28.31-30.74) | 1.78 (1.67-1.91) |
| 81-85 | 1,006,481 (12.8) | 39.14 (37.46-40.91) | 2.28 (2.13-2.44) |
| ≥86 | 929,592 (11.8) | 50.60 (48.60-52.69) | 3.06 (2.85-3.29) |
| Sex | |||
| Male | 3,285,584 (41.7) | 30.69 (29.86-31.54) | Reference |
| Female | 4,595,366 (58.3) | 22.45 (21.84-23.07) | 0.73 (0.70-0.76) |
| Race | |||
| Non-Hispanic White | 6,807,760 (86.4) | 26.75 (26.20-27.30) | Reference |
| Non-Hispanic Black | 337,824 (4.3) | 27.64 (25.24-30.25) | 0.85 (0.77-0.94) |
| Asian | 225,590 (2.9) | 10.83 (9.06-12.94) | 0.37 (0.31-0.45) |
| Hispanic | 218,912 (2.8) | 24.03 (21.34-27.07) | 0.77 (0.68-0.88) |
| Other | 290,864 (3.7) | 17.22 (15.22-19.47) | 0.83 (0.73-0.94) |
| Medicaid eligibility | |||
| No | 7,388,256 (93.8) | 24.88 (24.38-25.39) | Reference |
| Yes | 492,694 (6.3) | 41.01 (38.59-43.59) | 1.49 (1.39-1.60) |
| No. of prior hospitalizations | |||
| 0 | 6,986,464 (88.6) | 21.21 (20.73-21.70) | Reference |
| 1 | 675,388 (8.6) | 50.82 (48.52-53.23) | 1.16 (1.09-1.23) |
| ≥2 | 219,098 (2.8) | 91.19 (85.88-96.81) | 1.23 (1.12-1.34) |
| Prior COVID-19 | |||
| No | 7,398,502 (93.9) | 26.31 (25.80-26.84) | Reference |
| Yes | 482,448 (6.1) | 19.92 (18.25-21.74) | 0.43 (0.39-0.48) |
| Residence prior to original vaccination | |||
| Community | 7,754,612 (98.4) | 25.80 (25.30-26.31) | Reference |
| Nursing facility | 126,338 (1.6) | 31.72 (27.79-36.21) | 0.51 (0.44-0.60) |
| Comorbidity status | |||
| AIDS/HIV | 8855 (0.1) | 17.50 (9.10-33.63) | 0.72 (0.37-1.39) |
| Alcohol abuse | 54,863 (0.7) | 39.81 (32.60-47.21) | 0.84 (0.70-1.02) |
| Deficiency anemia | 828,691 (10.5) | 61.62 (59.32-64.01) | 1.20 (1.13-1.26) |
| Autoimmune | 320,615 (4.1) | 47.95 (44.79-51.33) | 1.72 (1.60-1.85) |
| Blood loss anemia | 61,552 (0.8) | 71.85 (63.16-81.74) | 1.07 (0.93-1.22) |
| Leukemia | 50,294 (0.6) | 91.56 (81.14-103.33) | 2.70 (2.38-3.07) |
| Lymphoma | 84,920 (1.1) | 65.36 (58.52-73.01) | 1.91 (1.70-2.15) |
| Metastatic cancer | 86,287 (1.1) | 46.07 (40.43-52.50) | 1.39 (1.21-1.59) |
| Solid tumor without metastasis, malignant | 643,506 (8.2) | 32.23 (30.37-34.21) | 1.01 (0.95-1.08) |
| Solid tumor without metastasis, in situ | 65,541 (0.8) | 33.77 (28.09-40.61) | 1.12 (0.93-1.35) |
| Cerebrovascular disease | 450,634 (5.7) | 24.33 (23.84-54.01) | 1.05 (0.98-1.12) |
| Congestive heart failure | 542,345 (6.9) | 79.97 (76.74-93.34) | 1.35 (1.27-1.44) |
| Coagulopathy | 182,966 (2.3) | 68.65 (63.65-70.05) | 1.14 (1.05-1.25) |
| Dementia | 384,887 (4.9) | 61.68 (58.36-65.19) | 1.29 (1.20-1.39) |
| Depression | 707,782 (9.0) | 45.52 (43.39-47.76) | 1.13 (1.06-1.19) |
| Diabetes, complicated | 1,008,282 (12.8) | 58.42 (56.37-60.54) | 1.66 (1.58-1.75) |
| Diabetes, uncomplicated | 666,844 (8.5) | 29.95 (28.15-31.86) | 1.37 (1.28-1.47) |
| Drug abuse | 45,357 (0.6) | 43.55 (35.90-52.83) | 0.97 (0.79-1.17) |
| Hypertension, complicated | 768,245 (9.8) | 68.88 (66.35-71.51) | 1.16 (1.07-1.25) |
| Hypertension, uncomplicated | 3,602,908 (45.7) | 27.33 (26.58-28.10) | 1.21 (1.15-1.28) |
| Liver disease, mild | 162,274 (2.1) | 44.25 (39.99-48.97) | 1.22 (1.10-1.36) |
| Liver disease, moderate to severe | 22,436 (0.3) | 75.65 (62.13-92.13) | 1.74 (1.41-2.13) |
| Chronic pulmonary disease | 859,326 (10.9) | 57.10 (54.93-59.37) | 1.61 (1.53-1.69) |
| Neurologic disorders affecting movement | 207,529 (2.6) | 50.14 (46.07-54.55) | 1.18 (1.08-1.29) |
| Seizures and epilepsy | 102,966 (1.3) | 57.89 (51.81-64.68) | 1.35 (1.20-1.51) |
| Other neurologic disorders | 156,293 (2.0) | 73.02 (67.45-79.04) | 1.08 (0.98-1.18) |
| Obesity | 844,919 (10.7) | 46.59 (44.58-48.67) | 1.31 (1.24-1.39) |
| Paralysis | 88,903 (1.1) | 59.34 (52.78-66.72) | 1.02 (0.89-1.17) |
| Peripheral vascular disease | 813,238 (10.3) | 54.33 (52.15-56.61) | 1.23 (1.17-1.29) |
| Psychoses | 217,295 (2.8) | 45.39 (41.63-49.49) | 1.23 (1.20-1.35) |
| Pulmonary circulation disease | 123,823 (1.6) | 91.30 (84.21-98.99) | 1.30 (1.19-1.43) |
| Renal failure, moderate | 676,910 (8.6) | 58.13 (55.64-60.72) | 1.35 (1.28-1.44) |
| Renal failure, severe | 148,570 (1.9) | 115.82 (108.53-123.60) | 2.32 (2.13-2.52) |
| Hypothyroidism | 1,256,574 (15.9) | 34.58 (33.17-36.05) | 1.01 (0.97-1.07) |
| Other thyroid disorders | 224,160 (2.8) | 28.24 (35.32-31.50) | 0.95 (0.85-1.07) |
| Peptic ulcer with bleeding | 40,476 (0.5) | 53.72 (44.68-64.60) | 0.90 (0.75-1.09) |
| Valvular disease | 543,637 (6.9) | 48.40 (45.89-51.06) | 0.94 (0.88-1.00) |
| Weight loss | 166,927 (2.1) | 55.46 (50.77-60.59) | 0.93 (0.85-1.03) |
COVID-19, coronavirus disease 2019; HR, hazard ratio.
The hazard ratio and 95% CI for booster was identical in a model that combined some comorbidities as in Table 3.
Booster vaccine effectiveness was 82% (95% CI, 81%-83%), computed as (1 − HR) × 100.
Figure 1.
Cumulative incidence curve of coronavirus disease 2019 (COVID-19) hospitalization among individuals who received boosters vs those who did not receive boosters. Joinpoint analyses of the curves found no significant changes in slope (P>0.30).
Association of Booster With COVID-19 Hospitalization, Stratified by Patient Characteristics
We repeated the analyses in Table 2 and added interaction terms between receipt of booster and the different enrollee characteristics, conducting stratified analyses in which the interactions were significant. Table 3 presents the key stratified analyses; complete results are shown in Supplemental Table 1 (available online at http://www.mayoclinicproceedings.org). The booster effectiveness ranged from 79% to 83% for different age groups. The absolute impact of boosters, assessed by the difference in COVID-19 hospitalization rates between the boosted and nonboosted cohorts, increased substantially with age in those over 65, from 17.4 fewer hospitalizations per million person-days in those aged 66 to 70 years to 68.3 fewer hospitalizations in those older than 85. Both the relative (vaccine effectiveness) and absolute impact of booster shots were more pronounced among White enrollees (vaccine effectiveness, 82% [95% CI, 81% to 83%] and 37.2 fewer hospitalizations per million patient-days) than in Black enrollees (vaccine effectiveness, 76% [95% CI, 70% to 81%] and 33.5 fewer hospitalizations) or Hispanic enrollees (vaccine effectiveness, 72% [95% CI, 63% to 79%] and 26.5 fewer hospitalizations). Booster effectiveness was greater among individuals with no prior COVID-19 infection (vaccine effectiveness, 82% [95% CI, 81% to 83%] and 36.6 fewer hospitalizations) than those with prior infection (vaccine effectiveness, 67% [95% CI, 60% to 73%] and 19.8 fewer hospitalizations).
Table 3.
Unadjusted COVID-19 Hospitalization Rates and Adjusted Booster Vaccine Effectiveness, Stratified by Patient Characteristicsa
| Variable | No. (%) of patients (N=7,880,950) |
Hospitalization rate per 1,000,000 person-days among boosted individuals | Hospitalization rate per 1,000,000 person-days among unboosted individuals | Hospitalization rate difference (95% CI), boosted individuals vs unboosted individuals | Booster vaccine effectiveness, % (95% CI)b |
|---|---|---|---|---|---|
| All patients | 7,880,950 | 8.20 (7.81-8.60) | 43.70 (42.79-44.64) | 35.51 (24.50-36.51) | 81 (80-82) |
| COVID-19 original vaccine | |||||
| Pfizer | 4,035,158 (51.2%) | 9.69 (9.13-10.29) | 50.14 (48.83-51.47) | 40.44 (39.00-41.88) | 81 (79-82) |
| Moderna | 3,845,792 (48.8%) | 6.30 (5.79-6.84) | 35.56 (34.33-36.83) | 29.26 (27.91-30.62) | 82 (81-83) |
| Age group (y) | |||||
| 66-70 | 2,248,536 (28.53) | 4.89 (4.35-5.50) | 22.27 (21.06-23.55) | 17.38 (16.01-18.75) | 79 (76-81) |
| 71-75 | 2,169,531 (27.5) | 6.21 (5.59-6.91) | 32.83 (31.35-34.39) | 26.62 (24.96-28.27) | 81 (79-83) |
| 76-80 | 1,526,810 (19.4) | 8.36 (7.50-9.33) | 50.42 (48.24-52.70) | 42.06 (39.65-44.47) | 83 (81-85) |
| 81-85 | 1,006,481 (12.8) | 12.38 (11.07-13.83) | 65.49 (62.43-68.71) | 53.11 (49.69-56.54) | 81 (79-83) |
| ≥86 | 929,592 (11.8) | 16.22 (14.66-17.95) | 84.50 (80.86-88.30) | 68.28 (64.21-72.35) | 81 (79-83) |
| Race | |||||
| Non-Hispanic White | 6,807,760 (86.4) | 8.21 (7.80-8.65) | 45.37 (44.37-46.40) | 37.16 (36.06-38.26) | 82 (81-83) |
| Non-Hispanic Black | 337,824 (4.3) | 10.94 (8.92-13.41) | 44.39 (40.12-49.11) | 33.45 (28.44-38.46) | 76 (70-81) |
| Asian | 225,590 (2.9) | 3.96 (2.61-6.02) | 17.71 (14.53-21.59) | 13.75 (9.87-17.63) | 79 (66-87) |
| Hispanic | 218,912 (2.8) | 10.80 (8.40-13.88) | 37.31 (32.59-42.71) | 26.51 (20.78-32.24) | 72 (63-79) |
| Other | 290,864 (3.7) | 5.82 (4.31-7.85) | 28.65 (25.03-32.79) | 22.83 (18.59-27.07) | 80 (72-85) |
| Medicaid eligibility | |||||
| No | 7,388,256 (93.8) | 7.66 (7.28-8.07) | 42.18 (41.25-43.12) | 34.52 (33.50-35.53) | 82 (81-83) |
| Yes | 492,694 (6.2) | 16.03 (13.97-18.40) | 66.16 (61.81-70.81) | 50.12 (45.12-55.13) | 76 (72-79) |
| No. of prior hospitalizations | |||||
| 0 | 6,986,464 (88.6) | 6.05 (5.70-6.43) | 36.43 (35.54-37.34) | 30.38 (29.41-31.35) | 83 (82-84) |
| 1 | 675,388 (8.6) | 19.39 (17.44-21.56) | 82.57 (78.41-86.94) | 63.18 (58.45-67.91) | 76 (73-79) |
| ≥2 | 219,098 (2.8) | 38.34 (33.66-43.66) | 144.92 (135.46-155.03) | 106.60 (95.61-117.60) | 73 (69-77) |
| Prior COVID-19 | |||||
| No | 7,398,502 (93.9) | 8.07 (7.67-8.49) | 44.64 (43.96-33.02) | 36.57 (35.53-37.62) | 82 (81-83) |
| Yes | 482,448 (6.1) | 10.07 (8.46-11.98) | 29.84 (26.96-33.02) | 19.77 (16.28-23.27) | 67 (60-73) |
| Residence prior to original vaccination | |||||
| Community | 7,754,612 (98.4) | 8.03 (7.64-8.44) | 43.66 (42.74-44.60) | 35.63 (34.62-36.64) | 82 (81-82) |
| Nursing facility | 126,338 (1.6) | 17.59 (13.68-22.60) | 45.99 (39.36-53.76) | 28.41 (19.99-36.83) | 62 (49-72) |
| Comorbidity status | |||||
| Anemia | |||||
| No | 7,052,259 (89.5) | 6.23 (5.87-6.61) | 36.91 (36.03-37.82) | 30.68 (29.71-31.65) | 83 (82-84) |
| Yes | 828,691 (10.5) | 24.20 (22.22-26.36) | 99.59 (95.45-103.91) | 75.39 (70.68-80.10) | 76 (74-78) |
| Autoimmune | |||||
| No | 7,560,335 (95.9) | 7.49 (7.11-7.89) | 42.21 (41.30-43.14) | 34.72 (33.71-35.72) | 82 (81-83) |
| Yes | 320,615 (4.1) | 21.93 (19.17-25.09) | 81.16 (74.99-87.83) | 59.23 (52.17-66.29) | 73 (68-77) |
| Leukemia/lymphoma | |||||
| No | 7,750,947 (98.4) | 7.17 (6.81-7.56) | 42.76 (41.85-43.69) | 35.59 (34.59-36.58) | 83 (82-84) |
| Yes | 130,003 (1.6) | 52.95 (46.60-60.17) | 106.59 (95.39-119.10) | 53.64 (40.01-67.26) | 53 (44-61) |
| Cancer | |||||
| No | 7,151,157 (90.7) | 7.58 (7.19-8.00) | 42.31 (41.37-43.28) | 34.73 (33.70-35.77) | 82 (81-83) |
| Yes | 729,793 (9.3) | 13.50 (12.01-15.18) | 57.70 (54.28-61.34) | 44.20 (40.34-48.06) | 77 (74-80) |
| Congestive heart failure | |||||
| No | 7,338,605 (93.1) | 6.83 (6.47-7.22) | 36.88 (36.01-37.77) | 30.05 (29.09, 31.00) | 82 (80, 83) |
| Yes | 542,345 (6.9) | 26.94 (24.31-29.86) | 128.50 (122.83-134.42) | 101.60 (95.13-108.01) | 79 (77-81) |
| Dementia | |||||
| No | 7,496,063 (95.1) | 7.55 (7.17-7.95) | 40.79 (39.88-41.72) | 33.24 (32.24-34.24) | 82 (81-83) |
| Yes | 384,887 (4.9) | 22.81 (19.81-26.27) | 89.41 (84.19-94.96) | 66.60 (60.33-72.87) | 73 (68-77) |
| Depression | |||||
| No | 7,173,168 (91.0) | 7.46 (7.08-7.87) | 40.57 (39.65-41.52) | 33.11 (32.09-34.12) | 82 (81-83) |
| Yes | 707,782 (9.0) | 15.74 (13.99-17.70) | 72.97 (69.24-76.90) | 57.23 (52.98-61.49) | 78 (75-81) |
| Diabetes | |||||
| No | 6,205,824 (78.7) | 6.28 (5.90-6.68) | 34.20 (33.29-35.13) | 27.92 (26.92-28.93) | 82 (81-83) |
| Yes | 1,675,126 (21.3) | 15.50 (14.35-16.75) | 77.13 (74.58-79.78) | 61.63 (58.76-64.50) | 80 (78-82) |
| Hypertension | |||||
| No | 3,509,797 (44.5) | 4.39 (3.97-4.85) | 25.12 (24.09-26.20) | 20.73 (19.59-21.88) | 83 (81-84) |
| Yes | 4,371,153 (55.5) | 11.25 (10.64-11.89) | 58.13 (56.73-59.56) | 46.88 (45.33-48.43) | 81 (79-82) |
| Renal failure | |||||
| No | 7,055,470 (89.5) | 5.92 (5.57-6.29) | 35.66 (34.79-36.55) | 29.74 (28.79-30.69) | 83 (81-84) |
| Yes | 825,480 (10.5) | 27.18 (25.05-29.49) | 110.20 (105.83-114.74) | 83.01 (78.04-87.99) | 76 (73-78) |
COVID-19, coronavirus disease 2019; HR, hazard ratio.
We derived HRs for COVID-19 hospitalization for each subcohort, adjusted for all the other patient characteristics. Vaccine effectiveness was computed as (1 − HR) × 10. In this analysis, we combined some patient characteristics. Supplemental Table 1 shows vaccine effectiveness for all individual patient characteristics.
Comorbidities found to be associated with greater hospitalizations from COVID-19 tended to have lower relative but greater absolute booster effects. For example, the vaccine effectiveness was only 53% (95% CI, 44% to 61%) in enrollees with leukemia/lymphoma compared with 83% (95% CI, 82% to 84%) for those without. However, the estimated absolute decrease in hospitalizations was 53.6 (95% CI, 40.0 to 67.3) in the leukemia/lymphoma group vs 35.6 (95% CI, 34.6 to 36.6) in the controls. A similar pattern was seen for enrollees with anemia, cancer, heart failure, autoimmune disease, dementia, depression, diabetes, and renal failure (Table 3).
Association of Booster Interval With COVID-19 Hospitalization
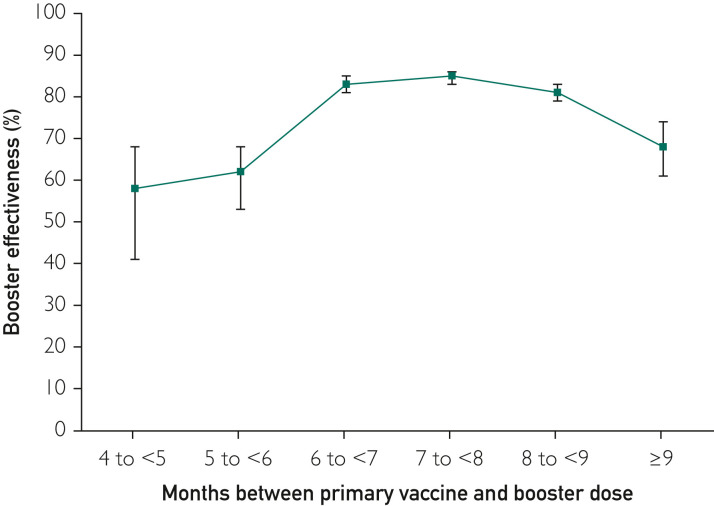
We assessed the effectiveness of the booster vaccination compared with unboosted controls stratified by the number of months between the original vaccination and the booster. The unadjusted rate of hospitalization per 1 million person-days was 26.2 (95% CI, 20.7 to 33.2) for those who received boosters before 5 months, 17.1 (95% CI, 14.6 to 20.0) for those who received boosters between 5 to 6 months, and 6.1 to 8.4 for those who received boosters between 6 to 7, 7 to 8, and 8 to 9 months (Supplemental Table 2, available online at http://www.mayoclinicproceedings.org). Figure 2 illustrates the booster effectiveness by months between original vaccination and booster, generated from a Cox proportional model adjusting for enrollee characteristics. Boosters received between 6 and 9 months after original vaccination varied between 81% and 85% effectiveness, while boosters received at 5 to 6 months (62%) or less than 5 months (58%) were less effective.
Figure 2.
Adjusted vaccine effectiveness for boosted enrollees compared with those not boosted as a function of the months between primary vaccine and receipt of booster. The highest effectiveness was in boosters received 6 to 7, 7 to 8, or 8 to 9 months after the original vaccination, while intervals of less than 6 months or greater than 9 months had lower effectiveness. Error bars indicate 95% CIs. The number of enrollees, hospitalizations, hospitalization rates, and adjusted hazard ratios for each interval are given in Supplemental Table 2.
Sensitivity Analyses
In sensitivity analyses, we analyzed cohorts using a traditional matching method rather than the target trial approach (Supplemental Figure 2). In this method, boosted and unboosted enrollees were well balanced, and the median follow-up was 64 days (Supplemental Table 3, available online at http://www.mayoclinicproceedings.org). Booster effectiveness results were similar to the main analysis (Supplemental Figure 3 and Supplemental Table 4, available online at http://www.mayoclinicproceedings.org). Because the outcome measure might include those hospitalized with COVID-19 in addition to those hospitalized because of COVID-19,14 in sensitivity analyses we restricted the outcome to those hospitalized with an admitting diagnosis of COVID-19 (Supplemental Tables 5 and 6, available online at http://www.mayoclinicproceedings.org). We also added analyses of the optimal interval between vaccination and booster using cohorts generated by traditional matching methods (Supplemental Table 7, available online at http://www.mayoclinicproceedings.org) and by comparing the hospitalization rates within the boosted enrollees stratified by length of the interval between vaccination and receipt of booster (Supplemental Table 8, available online at http://www.mayoclinicproceedings.org). All sensitivity analyses produced results similar to the main analyses.
Discussion
Using national Medicare claims data, we studied over 7 million previously vaccinated enrollees and estimated that boosters were 81% effective in further reducing the risk of COVID-19 hospitalizations. The effectiveness of boosters varied by individuals’ demographic and clinical characteristics, ranging from 50% to 83%. Receipt of boosters within 6 to 9 months from the original vaccination was associated with the largest reductions in risk of COVID-19 hospitalizations.
Our study findings contribute to the growing body of literature documenting the effectiveness of COVID-19 boosters. Studies from Israel and England found that boosters were 85% to 95% effective against symptomatic COVID-19, 93% to 97% against hospitalization, and 81% to 99% against death during a Delta variant–dominant period.2, 3, 4 A Centers for Disease Control and Prevention analysis assessed the effectiveness of the third dose of messenger RNA booster as 90% to 94% in preventing COVID-19–associated hospitalization during both the Delta variant– and Omicron variant–dominant period.7 Our estimates of effectiveness were lower. The sensitivity of Medicare claims data to capture booster administration may be low, as was found with initial vaccinations.24 This factor would bias our estimates of effectiveness toward the null.
Although relative effectiveness of boosters was similar across different age groups over 65 years of age, the absolute number of hospitalizations prevented due to boosters increased substantially with age. Lower effectiveness of boosters among Black and Hispanic patients compared with White patients may be due to differences in genetic makeup and how immunogenicity is built up among different racial/ethnic groups.25 , 26 Consistent with prior studies, individuals with immunocompromised conditions had lower relative vaccine effectiveness, but the absolute number of hospitalizations prevented was greater, underscoring the role of vaccines in reducing the risk of hospitalization among high-risk people.27
In January 2022, the FDA-recommended interval between primary vaccination and booster was reduced from 6 months to a 5-month minimum.9 Our findings of reduced effectiveness of boosters received before 6 months from the original vaccination support the original FDA recommendation. A recent study also noted higher effectiveness of boosters if received after 6 months compared with before 6 months.5 A longer duration between the first 2 doses of the original vaccination also produced greater antibody response.28 The effectiveness of the first 2-dose of vaccine against COVID-19 hospitalization and death remained high, at approximately 89% to 93%, until 6 months after the vaccine receipt and started declining after 7 months.29 , 30 Receiving a booster between 6 to 9 months may have extended this immunity for a longer time and offered the greatest protection against COVID-19 hospitalizations. In contrast, if a booster was received before 6 months, individuals may not have benefited as much due to the greater immunity offered by original vaccine and booster.
This study has some limitations. As noted previously, there may be underrecording of COVID-19 boosters in Medicare claims data. The sensitivity of claims to identify boosters may also vary across patient characteristics, which would affect estimates of vaccine effectiveness for subgroups. Some individuals are hospitalized for other conditions and are incidentally diagnosed with COVID-19.14 To address this issue, we restricted the outcome to those with a primary admitting diagnosis of COVID-19. Use of this strict definition of COVID-19 hospitalization resulted in findings that were similar to the main analyses, indicating the robustness of our study findings. Our estimates of vaccine effectiveness among individuals 65 years and older cannot be generalized to younger people. Most of our data came from a period when the Delta variant of COVID-19 was dominant.31 Analyses of more recent data are needed to determine the effectiveness of boosters during the Omicron variant–dominant period. We monitored patients for a maximum follow-up of 4 months. Longer follow-up is needed to determine boosters’ duration of effectiveness against COVID-19 hospitalizations. We did not estimate booster effectiveness against COVID-19 diagnosis because of poor sensitivity of claims in identifying COVID-19 diagnosis.32
Conclusion
This real-world study found that boosters are highly effective in reducing the risk of COVID-19–associated hospitalizations. Boosters provided the greatest benefits if they were received between 6 and 9 months following original vaccinations. As per the most recent Centers for Disease Control and Prevention data, nearly 15 million individuals aged 65 years or older have not yet received a booster.22 Our results suggest that approximately 69,225 COVID-19 hospitalizations could be prevented by boosters in that population in a 4-month period similar to the one studied. Evidence from this study and others may help improve people’s confidence in boosters’ effectiveness and increase booster uptake to combat the COVID-19 pandemic.
Potential Competing Interests
The authors report no competing interests.
Acknowledgments
The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging.
Author Contributions: Dr Mehta—conceptualization, methodology, writing/original draft, review, and editing, visualization; Dr Li—methodology, software, formal analysis, data curation, writing/review and editing, visualization; Dr Goodwin—conceptualization, methodology, writing/review and editing, supervision, funding acquisition.
Footnotes
Grant Support: This work was supported in part by grants 1K01AG070329 (H.B.M.) and P30-AG024832 (J.S.G.) from the National Institute on Aging.
Supplemental material can be found online at http://www.mayoclinicproceedings.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
References
- 1.Centers for Disease Control and Prevention COVID Data Tracker: COVID-19 vaccinations in the United States. https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-total-admin-rate-total
- 2.Arbel R., Hammerman A., Sergienko R., et al. BNT162b2 vaccine booster and mortality due to Covid-19. N Engl J Med. 2021;385(26):2413–2420. doi: 10.1056/NEJMoa2115624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barda N., Dagan N., Cohen C., et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398(10316):2093–2100. doi: 10.1016/S0140-6736(21)02249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrews N., Stowe J., Kirsebom F., et al. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat Med. 2022;28(4):831–837. doi: 10.1038/s41591-022-01699-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monge S., Rojas-Benedicto A., Olmedo C., et al. The effectiveness of mRNA vaccine boosters for laboratory-confirmed COVID-19 during a period of predominance of the Omicron variant of SARS-CoV-2. SSRN website. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4035396 Preprint posted online February 15, 2022. Accessed September 1, 2022.
- 6.Ferdinands J.M., Rao S., Dixon B.E., et al. Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance – VISION Network, 10 states, August 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(7):255–263. doi: 10.15585/mmwr.mm7107e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson M.G., Natarajan K., Irving S.A., et al. Effectiveness of a third dose of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance – VISION Network, 10 states, August 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(4):139–145. doi: 10.15585/mmwr.mm7104e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.FDA authorizes booster dose of Pfizer-BioNTech COVID-19 vaccine for certain populations [press release] US Food and Drug Administration website. Published September 22, 2021 https://www.fda.gov/news-events/press-announcements/fda-authorizes-booster-dose-pfizer-biontech-covid-19-vaccine-certain-populations Published September 22, 2021. [Google Scholar]
- 9.Coronavirus (COVID-19) update: FDA takes multiple actions to expand use of Pfizer-BioNTech COVID-19 vaccine [press release] US Food and Drug Administration website. Published January 3, 2022 https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-takes-multiple-actions-expand-use-pfizer-biontech-covid-19-vaccine Published January 3, 2022. [Google Scholar]
- 10.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P., STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies [published correction appears in Ann Intern Med. 2008;148(2):168] Ann Intern Med. 2007;147(8):573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 11.Hernán M.A., Robins J.M. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. 2016;183(8):758–764. doi: 10.1093/aje/kwv254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Medicare and Medicaid Services COVID-19 vaccines and monoclonal antibodies. https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/covid-19-vaccines-and-monoclonal-antibodies Updated July 13, 2022.
- 13.Kadri S.S., Gundrum J., Warner S., et al. Uptake and accuracy of the diagnosis code for COVID-19 among US hospitalizations. JAMA. 2020;324(24):2553–2554. doi: 10.1001/jama.2020.20323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray S.G., Croci R., Wachter R.M. Is a patient hospitalized ‘with’ covid or ‘for’ covid? it can be hard to tell. The Washington Post website. Published January 7, 2022 https://www.washingtonpost.com/outlook/2022/01/07/hospitalization-covid-statistics-incidental/ Published January 7, 2022. [Google Scholar]
- 15.Goodwin J.S., Li S., Zhou J., Graham J.E., Karmarkar A., Ottenbacher K. Comparison of methods to identify long term care nursing home residence with administrative data. BMC Health Serv Res. 2017;17(1):376. doi: 10.1186/s12913-017-2318-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta H.B., Li S., Goodwin J.S. Risk factors associated with SARS-CoV-2 infections, hospitalization, and mortality among US nursing home residents. JAMA Netw Open. 2021;4(3) doi: 10.1001/jamanetworkopen.2021.6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agency for Healthcare Research and Quality, Healthcare Cost and Utilization Project Elixhauser Comorbidity Software Refined for ICD-10-CM. https://www.hcup-us.ahrq.gov/toolssoftware/comorbidityicd10/comorbidity_icd10.jsp Updated October 29, 2021.
- 18.SAS Institute Nonparametric estimation and comparison of cumulative incidence functions with competing risks data (%CIF Macro) https://support.sas.com/kb/45/997.html
- 19.US Department of Health and Human Services, National Institutes of Health, National Cancer Institute Joinpoint trend analysis software. https://surveillance.cancer.gov/joinpoint/
- 20.Austin P.C. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38(6):1228–1234. [Google Scholar]
- 21.Klein J.P., Moeschberger M.L. 2nd ed. Springer; 2003. Survival Analysis: Techniques for Censored and Truncated Data. Statistics for Biology and Health. [Google Scholar]
- 22.Austin P.C. Absolute risk reductions and numbers needed to treat can be obtained from adjusted survival models for time-to-event outcomes. J Clin Epidemiol. 2010;63(1):46–55. doi: 10.1016/j.jclinepi.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention COVID Data Tracker: COVID-19 vaccinations in the United States. https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-people-onedose-pop-5yr
- 24.Centers for Medicare and Medicaid Services Assessing the completeness of Medicare claims data for measuring COVID-19 vaccine administration. https://www.cms.gov/files/document/assessing-completeness-medicare-claims-data-measuring-covid-19-vaccine-administration.pdf
- 25.Jethwa H., Wong R., Abraham S. Covid-19 vaccine trials: ethnic diversity and immunogenicity. Vaccine. 2021;39(27):3541–3543. doi: 10.1016/j.vaccine.2021.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu G., Carter B., Gifford D.K. Predicted cellular immunity population coverage gaps for SARS-CoV-2 subunit vaccines and their augmentation by compact peptide sets. Cell Syst. 2021;12(1):102–107.e4. doi: 10.1016/j.cels.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Embi P.J., Levy M.E., Naleway A.L., et al. Effectiveness of 2-dose vaccination with mRNA COVID-19 vaccines against COVID-19-associated hospitalizations among immunocompromised adults – nine states, January-September 2021. MMWR Morb Mortal Wkly Rep. 2021;70(44):1553–1559. doi: 10.15585/mmwr.mm7044e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parry H., Bruton R., Stephens C., et al. Extended interval BNT162b2 vaccination enhances peak antibodygeneration. NPJ Vaccines. 2022;7(1):14. doi: 10.1038/s41541-022-00432-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chemaitelly H., Tang P., Hasan M.R., et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med. 2021;385(24):e83. doi: 10.1056/NEJMoa2114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tartof S.Y., Slezak J.M., Fischer H., et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398(10309):1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention COVID Data Tracker: Variant proportions. https://covid.cdc.gov/covid-data-tracker/#variant-proportions Updated July 12, 2022.
- 32.Wu S.L., Mertens A.N., Crider Y.S., et al. Substantial underestimation of SARS-CoV-2 infection in the United States. Nat Commun. 2020;11(1):4507. doi: 10.1038/s41467-020-18272-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.