Abstract
Background
The role of programmed cell death, especially pyroptosis and apoptosis, in unfavorable immune responses in COVID-19 remains to be elucidated.
Methods
We conducted a cross-sectional analysis to investigate the association between the serum gasdermin D (GSDMD) levels, a pyroptotic marker, and caspase-cleaved cytokeratin 18 fragment (M30), an apoptotic marker, and the clinical status and abnormal chest computed tomography (CT) findings in patients with COVID-19.
Results
In this study, 46 patients diagnosed with COVID-19 were divided into the following three groups according to the disease severity: mild to moderate group (n = 10), severe group (n = 14), and critical group (n = 22). The serum GSDMD levels were higher in the critical group than in the mild to moderate group (P = 0.016). In contrast, serum M30 levels were lower in the critical group than in the severe group (P = 0.048). Patients who required mechanical ventilation or died had higher serum GSDMD levels than those who did not (P = 0.007). Area of consolidation only and of ground glass opacity plus consolidation positively correlated with serum GSDMD levels (r = 0.56, P < 0.001 and r = 0.53, P < 0.001, respectively).
Conclusion
Higher serum GSDMD levels are associated with critical respiratory status and the consolidation area on chest CT in patients with COVID-19, suggesting that excessive activation of pyroptosis may affect the clinical manifestations in patients with COVID-19.
Keywords: COVID-19, Pyroptosis, Apoptosis, Gasdermin D, Caspase-cleaved cytokeratin 18 fragment
Abbreviations: COVID-19, coronavirus disease 2019; ELISA, enzyme-linked immunosorbent assay; GSDMD, gasdermin D; GGO, ground glass opacity; ICU, intensive care unit; IFN, interferon; IL, interleukin; LDH, lactate dehydrogenase; MV, mechanical ventilation; M30, caspase-cleaved cytokeratin 18 fragment; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TGF, transforming growth factor; TNF, tumor necrosis factor
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was discovered in China at the end of 2019 [1], and the disease caused by it is called COVID-19 by the World Health Organization. Most COVID-19 patients experience mild or moderate symptoms, but some present with acute respiratory distress syndrome, multiple organ failure, and death [2]. The mechanism by which SARS-CoV-2 causes fatal outcomes remains unclear.
Programmed cell death, especially pyroptosis and apoptosis, might play a pivotal role in the unfavorable immune responses in COVID-19 [3]. Apoptosis is a non-inflammatory process that eliminates damaged cells to maintain homeostasis, and it is considered immunologically silent. In contrast, pyroptosis comprises inflammatory cell death processes characterized by cell lysis with the release of cytokines and other inflammatory factors. Pyroptosis is a caspase-dependent reaction that leads to gasdermin D (GSDMD) pore formation [4] and outflux of cytoplasm accompanied by lactate dehydrogenase (LDH) and inflammatory cytokines, such as interleukin (IL)-1β [5], eventually causing cell membrane rupture. Pyroptosis may be closely associated with the pathogenesis of autoimmune diseases [6] and SARS-CoV-2 infection [7]. To date, researchers have investigated the surrogate markers of programmed cell death in several diseases; IL-1β and IL-18 levels reflect the degree of inflammasome activation associated with pyroptosis [8], similar to how caspase-cleaved cytokeratin 18 fragments (M30) levels reflect that associated with apoptosis [9,10]. Recent studies revealed that SARS-CoV-2 infection induces the undesirable activation of programmed cell death, leading to a cytokine storm [11]. Hyperferritinemia is one of the factors indicating critical illness in patients with COVID-19 [12]. The secretion of ferritin requires pyroptosis through the caspase-11–GSDMD pathway in murine models [13], suggesting that hyperferritinemia in COVID-19 might account for the activation of pyroptosis. Furthermore, the unique regulation of apoptosis-related signaling in SARS-CoV-2-infected cells may be related to the virus's ability to replicate, affecting the severity of the clinical course of COVID-19 [14].
Few reports have examined the association between the clinical features and quantified markers of programmed cell death, such as GSDMD and M30, using clinical samples from patients with COVID-19. In this study, we investigated the association between the severity of lung injury and serum GSDMD and M30 levels in patients with COVID-19 at our tertiary care hospital to clarify the extent to which programmed cell death contributes to the pathogenesis of COVID-19 lung injury.
2. Patients and methods
2.1. Patients
We established a retrospective cohort in our tertiary care hospital comprising COVID-19 patients aged ≥18 years who tested positive for SARS-CoV-2 with nucleic acid amplification test or enzyme immunoassay between March 1, 2020, and August 31, 2020. Moreover, we obtained their demographic, clinical, laboratory, and imaging data from the medical records. The severity of COVID-19 was assessed and classified as follows according to the care needed for each patient at the first visit with reference to the Infectious Diseases Society of America guidelines [15]: mild to moderate, no need for oxygen supplementation; severe, need for oxygen supplementation with no ventilation assistance; and critical, need for ventilation assistance. Laboratory results were recorded at the first time point after the hospital visit. This study was approved by the ethics committee of St. Marianna University School of Medicine on April 24, 2020 (Approval No.: 4785). Informed consent was obtained in writing or orally from the patient or the patient's family, especially when the patient was intubated.
2.2. Detection of serum programmed cell-death markers and cytokine molecules
Blood samples were collected to measure the required clinical laboratory values during the first visit to the hospital. The remaining serum samples were stored at −80 °C and brought to room temperature (24 °C) for use in subsequent experiments; the use of multiple freeze-thawed samples was avoided. Enzyme-linked immunosorbent assay (ELISA) was performed to assess the serum GSDMD (LSbio, USA), M30 (VLVbio, Sweden), and IL-18 (MBL, Japan) levels. Interferon (IFN) γ, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-17A, IFN-inducible protein-10, monocyte chemotactic protein-1, transforming growth factor (TGF) β, and tumor necrosis factor (TNF) α were assessed in the serum samples using a cytometric bead assay (LEGENDplex™ HU Essential Immune Response Panel Standard, Biolegend, USA).
2.3. Evaluation of abnormal chest computed tomography findings
All computed tomography (CT) images were obtained at the first visit and reviewed retrospectively by two radiologists (SM and HT), who were unaware of the clinical and laboratory cytokine data. The final assessment was achieved by consensus using a previously reported method [16]. CT analysis included the detection and location of the ground glass opacity (GGO) and consolidation. GGO is defined as an area showing a hazy increase in lung attenuation with visible vessels. Consolidation is defined as opacities that conceal the underlying vessels. The observers visually scored the total extent of abnormal parenchyma at the following four preselected levels: (a) upper level, aortic arch; (b) middle level, carina; (c) lower level, between the aortic arch and carina; and (d) bottom level, 1 cm above the dome of the right hemidiaphragm. The extent of the abnormal findings was assessed independently for each of the four zones of each lung and then calculated by multiplying the percentage of GGO and consolidation in each zone using a predetermined ratio. The estimated volumetric ratio of the upper, middle, and lower (or lower and bottom) lung zones was 1:1.6:1.3, based on a previously published article [17]. Finally, summing the scores calculated for each zone, we presented the percentage of abnormal findings in the entire lung field as the total CT score.
2.4. Statistical analysis
We compared the routine laboratory test results, serum GSDMD levels, M30 levels, and other cytokine markers between the three severity groups at admission using the analysis of variance or Kruskal–Wallis test, depending on the normality of distribution. Furthermore, the serum levels of the patients who required mechanical ventilation (MV) or died and those who did not require MV and survived were compared using t-test or Mann–Whitney U test, depending on the normality of distribution. The correlation between the GSDMD or M30 levels and the CT score and the significance of the correlation were examined using Pearson correlation coefficient test. The extent to which GSDMD, M30, and the covariates associated with disease severity contributed to abnormal CT findings was estimated using linear regression analysis. The serum GSDMD values were logarithmically transformed to adapt to the Pearson correlation coefficient test and linear regression analysis. The level of significance was set as a two-tailed P-value <0.05, and Holm correction was used for multiple comparison. All statistical analyses were conducted using EZR statistical software, which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of R commander (version 1.40) designed to add statistical functions frequently used in biostatistics.
3. Results
3.1. Baseline clinical characteristics, treatment, and outcomes
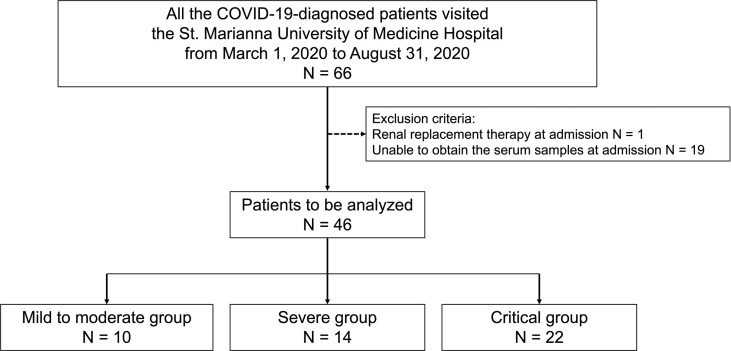
This study initially included 66 Japanese patients diagnosed with COVID-19 at St. Marianna University of Medicine Hospital, between March 1, 2020 and August 31, 2020, whose medical records were available retrospectively. One patient undergoing renal replacement therapy at the time of admission was excluded, because the effect of hemodialysis on the measurement of serum GSDMD and M30 levels remains unclear. Additionally, 19 patients were excluded due to the lack of serum samples at the first visit. Thus, 46 patients (41 males, 89.1%) with a mean age of 63.8 ± 12.5 years were finally included in the analysis. None of the patients had been vaccinated against SARS-CoV-2. Based on the disease severity at the first visit, the patients were divided into the following three groups: mild to moderate group (n = 10, 21.7%), severe group (n = 14, 30.4%), and critical group (n = 22, 47.8%) (Fig. S1). Obesity could not be assessed due to the missing height and weight data. Table 1 presents detailed information on the baseline characteristics. Eventually, we found that 63% (n = 29/46) of the total patients included in this study required MV, and 30% (n = 3/10) of the patients of the mild to moderate group, 28.6% (n = 4/14) of the severe group, and 100% (n = 22/22) of the critical group required MV. The mortality rates were 0% (n = 0/10) in the mild to moderate group, 14.3% (n = 2/14) in the severe group, and 27.3% (n = 6/22) in the critical group. Table 2 and Table S1 present the detailed outcomes and treatment of the patients in this study.
Table 1.
Overall baseline clinical features and biomarkers of patients with COVID-19 in this study.
| Characteristics | Total (n = 46) | Mild to moderate group (n = 10) | Severe group (n = 14) | Critical group (n = 22) | P-value |
|---|---|---|---|---|---|
| Age (years) | 62.83 (12.52) | 62.40 (9.35) | 60.93 (15.41) | 64.23 (12.13) | 0.746 |
| Male sex, n (%) | 41 (89.1) | 8 (80.0) | 12 (85.7) | 21 (95.5) | 0.380 |
| Weight (kg) | 71.22 (22.74) (n = 25) |
62.25 (11.44) | 83.00 (37.05) | 72.28 (20.58) | 0.282 |
| Comorbidities | |||||
| Hypertension, n (%) | 13 (28.2) | 2 (20.0) | 2 (14.3) | 9 (42.9) | 0.147 |
| Diabetes, n (%) | 15 (32.6) | 1 (10.0) | 5 (35.7) | 9 (42.9) | 0.188 |
| Cardiovascular diseases, n (%) | 8 (17.4) | 1 (10.0) | 1 (7.1) | 6 (28.6) | 0.205 |
| Chronic kidney disease, n (%) | 5 (10.9) | 0 (0.0) | 2 (14.3) | 3 (14.3) | 0.448 |
| Liver disease, n (%) | 1 (2.2) | 0 (0.0) | 1 (7.1) | 0 (0.0) | 0.322 |
| Malignancy, n (%) | 6 (13.0) | 1 (10.0) | 3 (21.4) | 2 (9.5) | 0.562 |
| Connective tissue disease, n (%) | 3 (6.5) | 1 (10.0) | 0 (0.0) | 2 (9.5) | 0.483 |
| Duration from disease onset to the first visit (days) | 9.02 (6.01) | 5.20 (3.49) | 8.29 (3.69) | 11.23 (7.17) | 0.023 |
| Albumin, g/dL | 2.78 (0.56) (n = 44) |
3.39 (0.60) | 2.75 (0.40) | 2.54 (0.44) | <0.001a |
| Lactate dehydrogenase, U/L | 474.09 (294.27) | 279.90 (93.00) | 627.14 (387.02) | 464.95 (235.51) | 0.014b |
| C-reactive protein, mg/dL | 10.19 (8.50) (n = 45) |
5.63 (6.19) | 9.44 (5.70) | 12.71 (9.98) | 0.056 |
| Ferritin, ng/mL | 1271.73 (1455.47) (n = 34) | 595.48 (710.65) | 1143.33 (969.30) | 1546.11 (1756.63) | 0.364 |
| KL-6, U/mL | 466.05 (365.99) (n = 39) |
232.80 (51.20) | 451.92 (332.40) | 530.33 (411.84) | 0.260 |
| PaO2/FIO2 | 249.83 (114.95) | 394.30 (81.42) | 206.19 (76.99) | 211.93 (95.34) | <0.001c |
Binary variables are expressed as n (%), and continuous variables as means (standard deviations).
Mild to moderate group, COVID-19 patients not needing oxygen supplementation.
Severe group, COVID-19 patients needing oxygen supplementation with no ventilation assistance.
Critical group, COVID-19 patients needing ventilation assistance.
(PaO2/FIO2, ratio of arterial oxygen partial pressure to fractional inspired oxygen).
Statistically significant differences between the mild to moderate and severe groups and between the mild to moderate and critical groups (P-value after Holm correction <0.007 and < 0.001, respectively).
Statistically significant difference only between the mild to moderate and severe groups (P-value after Holm correction = 0.011).
Statistically significant differences between the mild to moderate and severe groups and between the mild to moderate and critical groups (P-value after Holm correction <0.001 for both).
Table 2.
Outcomes in patients with COVID-19.
| Total (n = 46) | Mild to moderate group (n = 10) | Severe group (n = 14) | Critical group (n = 22) | |
|---|---|---|---|---|
| Death, n (%) | 8 (17.4) | 0 (0.0) | 2 (14.3) | 6 (27.3) |
| Duration from disease onset to death (days) | 47.00 (48.40) | – | 11.50 (3.54) | 58.83 (51.03) |
| Discharge within 28 days, n (%) | 8 (17.4) | 4 (40.0) | 1 (7.1) | 3 (13.6) |
| Death within 28 days, n (%) | 5 (10.9) | 0 (0.0) | 2 (14.3) | 3 (13.6) |
| Invasive mechanical ventilation, n (%) | 29 (63.0) | 3 (30.0) | 4 (28.6) | 22 (100.0) |
| Duration from disease onset to ventilation (days) | 9.83 (5.41) | 9.33 (7.37) | 10.50 (3.70) | 9.77 (5.63) |
| Duration under ventilation (days) | 21.25 (17.2) | 32.00 (28.84) | 32.25 (19.26) | 16.76 (13.36) |
| Extracorporeal membrane oxygenation, n (%) | 4 (8.7) | 0 (0.0) | 0 (0.0) | 4 (18.2) |
Binary variables are expressed as numbers (percentages), and continuous variables as means (standard deviations).
Mild to moderate group, COVID-19 patients not needing oxygen supplementation.
Severe group, COVID-19 patients needing oxygen supplementation with no ventilation assistance.
Critical group, COVID-19 patients needing ventilation assistance.
3.2. Overall baseline laboratory data, programmed cell-death markers, and various cytokines in patients with COVID-19
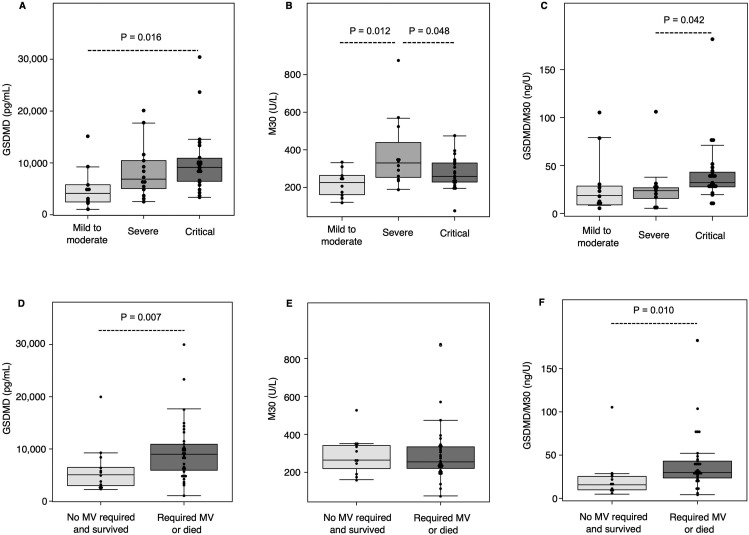
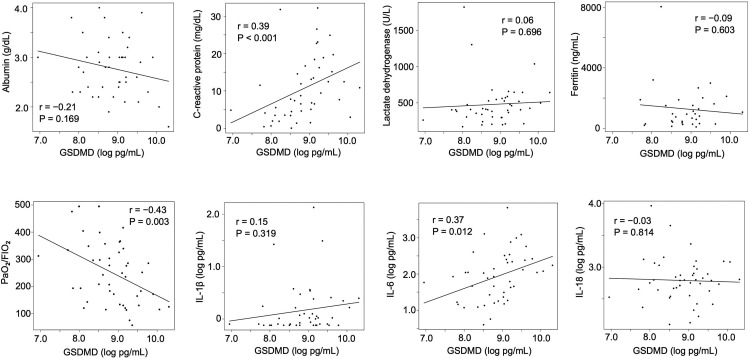
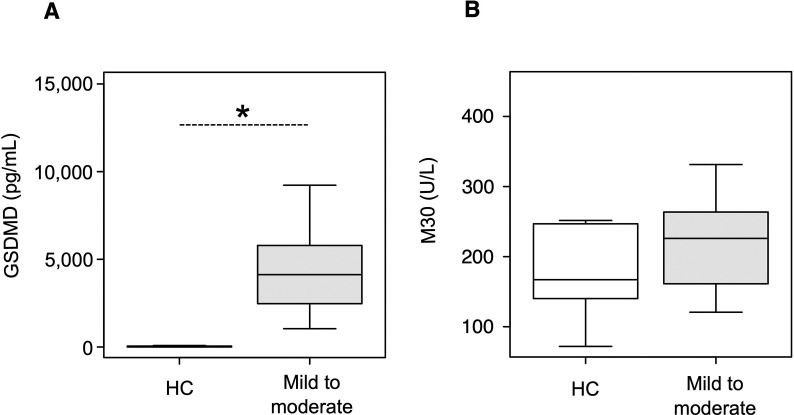
The baseline laboratory data of the patients with COVID-19 in this study are shown in Table 1 and Table S2. The serum albumin levels were significantly lower in the severe and critical groups than in the mild to moderate group (P = 0.007 and < 0.001 after multiple comparison, respectively); LDH levels were higher in the severe group than in the mild to moderate group (P = 0.011 after multiple comparison). First, we examined the expression profile of serum GSDMD, a surrogate marker of pyroptosis, in the healthy volunteers and COVID-19 patients of the mild to moderate group. Serum GSDMD levels were significantly higher in the mild to moderate group than in the healthy controls (P < 0.001). Subsequently, we measured the serum M30 levels in the healthy volunteers and COVID-19 patients of the mild to moderate group, but there was no statistically significant difference between them (P = 0.569) (Fig. S2). We then investigated the serum GSDMD, M30, and various cytokine markers in the COVID-19 patients of the three disease severity groups using serum samples collected at the first visit (Fig. 1 and Table 3 ). The median (interquartile range) serum level of GSDMD was 4126 (2597–5605) pg/mL in the mild to moderate group, 6868 (5169–10147) pg/mL in the severe group, and 9135 (6477–10684) pg/mL in the critical group. The mean (standard deviation) serum level of M30 was 221.0 (70.3) U/L in the mild to moderate group, 379.7 (190.7) U/L in the severe group, and 276.2 (86.6) U/L in the critical group. The serum GSDMD level in the critical group was significantly higher than that in the mild to moderate group (P = 0.016 after multiple comparison) (Fig. 1A). On the other hand, the serum levels of M30 in the critical and mild to moderate groups were significantly lower than those in the severe group (P = 0.012 and 0.048 after multiple comparison, respectively) (Fig. 1B). To consider the imbalance between pyroptosis and apoptosis further in the patients with COVID-19, we investigated the ratio of serum GSDMD to M30 levels. The ratio of serum GSDMD to M30 levels in the critical group was significantly higher than that in the severe group (P = 0.042 after multiple comparison) (Fig. 1C). Patients who required MV or died during the observation period had significantly higher levels of serum GSDMD and ratio of serum GSDMD to M30 than those who did not require MV and survived (P = 0.007 and 0.010, respectively) (Fig. 1D and F); however, there was no statistical difference in the serum M30 levels between these two groups (Fig. 1E). This result indicated that serum GSDMD levels might predict the MV requirement or death in COVID-19 patients. Furthermore, we examined the correlation between serum GSDMD levels and some of the baseline laboratory data and inflammatory cytokines including IL-1β, IL-6, and IL-18. The level of C-reactive protein and (log-transformed) IL-6 positively correlated with the log-transformed levels of serum GSDMD (r = 0.39, P < 0.001 and r = 0.37, P = 0.012, respectively). In contrast, the ratio of arterial oxygen partial pressure to fractional inspired oxygen negatively correlated with the log-transformed levels of serum GSDMD (r = – 0.43, P = 0.003), suggesting that pyroptosis might be induced in some inflammatory and hypoxic conditions in COVID-19 (Fig. 2 ). There was no statistical difference in the various cytokine markers between the three groups, although the serum IL-18 levels tended to be higher in the severe and critical groups than in the mild to moderate group (Table 3).
Fig. 1.
Levels of serum gasdermin D and caspase-cleaved cytokeratin 18 fragment in the different severity and outcome groups. The box-and-whisker plots represent the serum GSDMD (A) and M30 (B) levels and the ratio of GSDMD to M30 levels (C) in the COVID-19 patients stratified into the mild to moderate group (no need for oxygen supplementation; n = 10), severe group (need for oxygen supplementation with no ventilation assistance; n = 14), and critical group (need for ventilation assistance, n = 22). The box-and-whisker plots represent the serum GSDMD (D) and M30 (E) levels and the ratio of GSDMD to M30 levels (F) in the COVID-19 patients classified into the group that did not require MV and survived (n = 13) and the group that required MV or died (n = 33). Data are shown as medians (A, C, D, F) or means (B, E), and error bars represent the 10–90 percentile range values. Statistical significance of differences between the three groups was calculated using the Kruskal–Wallis test for GSDMD levels (A) and ratio of GSDMD to M30 (C) and using ANOVA for M30 (B), followed by Holm correction for multiple comparisons. Statistical significance of the difference between the two groups was calculated using Mann–Whitney U test for GSDMD levels (D) and ratio of GSDMD to M30 (F) and using t-test for M30 (E). (ANOVA, analysis of variance; GSDMD, gasdermin D; MV, mechanical ventilation; M30, caspase-cleaved cytokeratin 18 fragment).
Table 3.
Concentration of cytokines in the serum of patients with COVID-19.
| Total (n = 46) | Mild to moderate group (n = 10) | Severe group (n = 14) | Critical group (n = 22) | P-value | |
|---|---|---|---|---|---|
| IL-1β, pg/mL | 1.01 (0.76, 1.75) (n = 45) |
0.80 (0.77, 1.49) | 0.95 (0.77, 2.36) | 1.02 (0.76, 1.65) | 0.816 |
| IL-2, pg/mL | 2.03 (1.50, 3.37) (n = 45) |
2.04 (1.50, 3.77) | 2.33 (1.56, 2.79) | 1.50 (1.50, 3.80) | 0.830 |
| IL-4, pg/mL | 9.85 (2.54, 9.85) (n = 45) |
9.85 (3.52, 19.09) | 4.46 (2.78, 9.85) | 9.85 (2.28, 9.85) | 0.722 |
| IL-6, pg/mL | 85.1 (23.16, 240.71) (n = 45) |
95.52 (34.54, 462.82) | 93.38 (24.94, 189.54) | 85.09 (23.16, 252.06) | 0.891 |
| IL-8, pg/mL | 19.00 (11.61, 34.77) (n = 45) |
14.80 (9.93, 18.52) | 18.07 (12.49, 31.61) | 21.54 (12.60, 39.57) | 0.389 |
| IL-10, pg/mL | 8.26 (3.24, 17.05) (n = 45) |
11.14 (4.22, 34.23) | 9.27 (3.76, 13.09) | 6.48 (3.23, 12.61) | 0.455 |
| IL-12p70, pg/mL | 1.70 (1.01, 1.70) (n = 45) |
1.54 (1.01, 1.70) | 1.35 (1.01, 1.70) | 1.70 (1.01, 1.77) | 0.528 |
| IL-17A, pg/mL | 2.29 (1.27, 5.09) (n = 45) |
3.43 (2.57, 9.41) | 2.29 (1.65, 5.03) | 2.29 (1.23, 3.69) | 0.389 |
| IL-18, pg/mL | 615.8 (425.6, 899.4) | 391.7 (277.0, 611.5) | 590.9 (475.2, 764.8) | 692.6 (483.7, 1057.7) | 0.187 |
| IFNγ, pg/mL | 4.94 (1.31, 26.72) (n = 45) |
7.75 (2.39, 49.85) | 9.20 (2.70, 19.55) | 2.51 (1.22, 13.84) | 0.187 |
| IP-10, pg/mL | 2003 (971, 3035) (n = 45) |
1653 (1002, 2174) | 2356 (1786, 2836) | 1745 (505, 3907) | 0.272 |
| MCP-1, pg/mL | 359.2 (205.8, 591.4) (n = 45) |
288.4 (229.8, 383.5) | 375.2 (230.3, 486.0) | 268.3 (171.4, 652.3) | 0.809 |
| TGFβ, pg/mL | 47.45 (28.22, 105.65) (n = 45) | 89.63 (48.41, 232.29) | 63.08 (23.87, 151.27) | 47.45 (28.22, 69.39) | 0.162 |
| TNFα, pg/mL | 0.74 (0.52, 1.18) (n = 45) |
1.31 (0.74, 2.70) | 0.64 (0.44, 0.74) | 0.74 (0.64, 0.74) | 0.246 |
Data are presented as medians (interquartile ranges), and the Kruskal–Wallis method was used for statistical analysis.
Mild to moderate group, COVID-19 patients not needing oxygen supplementation.
Severe group, COVID-19 patients needing oxygen supplementation with no ventilation assistance.
Critical group, COVID-19 patients needing ventilation assistance.
(IFN, interferon; IL, interleukin; IP, interferon inducible protein; MCP, monocyte chemotactic protein; TGF, transforming growth factor; TNF, tumor necrosis factor).
Fig. 2.
Correlation between the serum gasdermin D levels and the baseline laboratory data and inflammatory cytokines. The levels of serum GSDMD, IL-1β, IL-6, and IL-18 were log-transformed. Data were analyzed using Pearson correlation coefficient. (GSDMD, gasdermin D; IL, interleukin; PaO2/FIO2, ratio of arterial oxygen partial pressure to fractional inspired oxygen).
3.3. Abnormal chest CT findings in patients with COVID-19 and their correlation with serum GSDMD and M30 levels
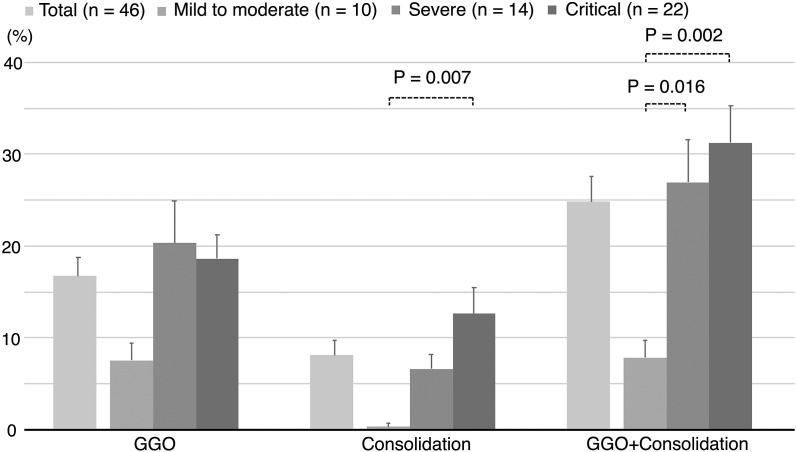
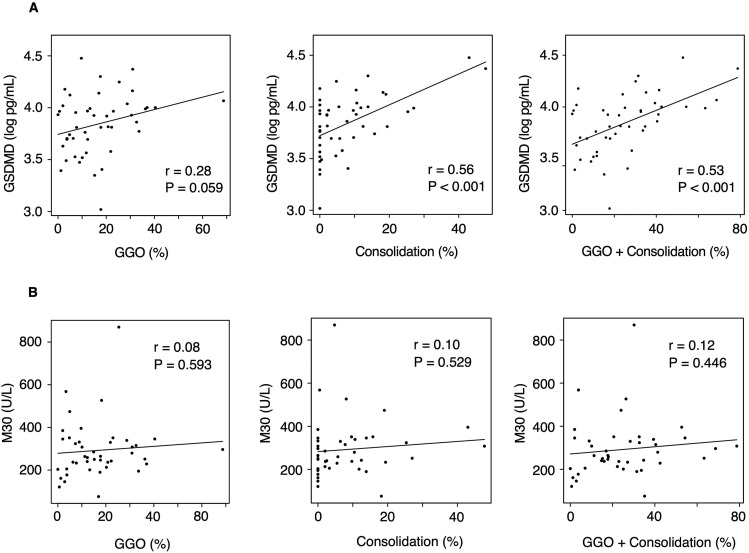
Using the chest CT images of patients with COVID-19 from their first visit, we compared the severity of the respiratory status with the extent of the abnormal chest CT findings. The extent of consolidation and GGO plus consolidation in the critical group was significantly higher than that in the mild to moderate group (P = 0.007 and 0.002, respectively) (Fig. 3 and Table S3). We then examined the correlation between these abnormal CT findings and the serum GSDMD and M30 levels. The areas of consolidation as well as GGO plus consolidation positively correlated with the log-transformed levels of serum GSDMD (r = 0.56, P < 0.001 and r = 0.53, P < 0.001, respectively) (Fig. 4 A). The same comparison was performed for serum M30, but no correlation was observed (Fig. 4B).
Fig. 3.
Abnormal chest computed tomography findings during the first visit in COVID-19 patients of different severity groups. Data of abnormal CT findings are presented as mean percentages ± standard errors (error bars) in the total patients and the three groups. ANOVA was used to compare the scores between the three groups, and Holm correction was used to test for multiple comparisons. Mild to moderate group, no need for oxygen supplementation; severe group, need for oxygen supplementation with no ventilation assistance; critical group, need for ventilation assistance. (ANOVA, analysis of variance; CT, computed tomography; GGO, ground glass opacity).
Fig. 4.
Correlation between the area of abnormal chest computed tomography findings and the levels of serum gasdermin D (A) and caspase-cleaved cytokeratin 18 fragment (B) in patients with COVID-19. The percentage on the horizontal axis indicates the proportion of the area of abnormal CT findings to the total lung field. Data were analyzed using Pearson correlation coefficient. (CT, computed tomography; GSDMD, gasdermin D; GGO, ground glass opacity; M30, caspase-cleaved cytokeratin 18 fragment).
3.4. Factors affecting abnormal chest CT findings in patients with COVID-19
To investigate the factors affecting abnormal chest CT findings in patients with COVID-19, we performed linear regression analysis considering the extent of the abnormal imaging findings as the objective variable and the serum GSDMD, M30, albumin, and LDH levels as covariates. In the adjusted linear regression analysis, higher levels of serum GSDMD were strongly associated with the extent of consolidation only and of GGO plus consolidation (β = 18.32, P < 0.001 and β = 26.54, P = 0.002, respectively) (Table 4 ). Although the influence of the markers, such as albumin and LDH, correlated with disease severity in COVID-19 patients, serum GSDMD can be considered an independent factor affecting the spread of consolidation on chest CT.
Table 4.
Unadjusted and adjusted liner regression models for factors affecting the abnormal computed tomography findings in patients with COVID-19.
| Unadjusted |
Adjusted |
|||
|---|---|---|---|---|
| Regression coefficient | P-value | Regression coefficient | P-value | |
| GGO (%) | ||||
| GSDMD, log pg/mL | 13.12 [−0.52, 26.75] | 0.059 | 8.22 [−4.92, 21.37] | 0.213 |
| M30, U/L | 0.0087 [−0.024, 0.041] | 0.593 | −0.00012 [−0.031, 0.029] | 0.935 |
| Albumin, g/dL | −4.99 [−11.12, 1.24] | 0.113 | −3.96 [−10.98, 3.06] | 0.260 |
| LDH, U/L | 0.0087 [−0.0053, 0.023] | 0.219 | 0.0043 [−0.0084, 0.017] | 0.498 |
| Consolidation (%) | ||||
| GSDMD, log pg/mL | 20.64 [11.25, 30.04] | <0.001 | 18.32 [8.76, 27.87] | <0.001 |
| M30, U/L | 0.0082 [−0.018, 0.035] | 0.529 | −0.0083 [−0.030, 0.013] | 0.443 |
| Albumin, g/dL | −10.43 [−15.62, −5.24] | <0.001 | −8.88 [−13.99, −3.78] | 0.001 |
| LDH, U/L | 0.0049 [−0.0064, 0.016] | 0.384 | 0.0018 [−0.0074, 0.011] | 0.697 |
| GGO plus consolidation (%) | ||||
| GSDMD, log pg/mL | 33.76 [17.20, 50.32] | <0.001 | 26.54 [10.01, 43.07] | 0.002 |
| M30, U/L | 0.017 [−0.028, 0.062] | 0.446 | −0.0095 [−0.047, 0.028] | 0.610 |
| Albumin, g/dL | −15.41 [−24.17, −6.66] | <0.001 | −12.84 [−21.67, −4.01] | 0.006 |
| LDH, U/L | 0.014 [−0.0055, 0.033] | 0.159 | 0.0061 [−0.0099, 0.022] | 0.446 |
β coefficient (presented as measured values with 95% confidence intervals) and P-values were calculated using a linear regression model. The final model was adjusted for GSDMD, M30, albumin, and LDH levels.
(GGO, ground glass opacity; GSDMD, gasdermin D; LDH, lactose dehydrogenase; M30, caspase-cleaved cytokeratin 18 fragment).
4. Discussion
We reported that serum GSDMD levels were significantly higher in critical COVID-19 cases than in the mild to moderate cases, whereas serum M30 levels were significantly lower in the critical group than in the severe group. Furthermore, patients who required MV or died had elevated serum GSDMD levels at admission as compared to those who did not require MV and survived. In addition, the extent of abnormal CT findings, such as consolidation only and GGO plus consolidation, positively correlated with serum GSDMD, independent of the M30 levels and factors indicating COVID-19 severity, including albumin and LDH.
Pyroptosis is a type of GSDM-mediated inflammatory programmed cell death mainly occurring in macrophages and dendritic cells and is triggered by two pathways: the canonical pathway executed by the GSDM family through inflammasome activation-mediated caspase-1 cleavage of GSDMD and the non-canonical pathway through caspase-4,5,11 cleavage of GSDMD, which is directly activated by lipopolysaccharides [18,19]. Cleaved GSDMD moves to the plasma membrane during pyroptosis and forms pores [4], which mediate the release of inflammatory cytokines, cellular alarmins, and other cleaved GSDMs, and then amplifies the immune response to infection and cellular damage. Several studies reported that pyroptosis has an important role in acute lung injury [20]. For instance, Xingying et al. [21] showed that excessive pyroptosis in murine alveolar macrophages exacerbated lung inflammation by recruiting neutrophil infiltration in the lung tissues and amplified the alveolar concentration of inflammatory cytokines including IL-1β, IL-6, and TNFα.
Pyroptosis is associated with bacterial infection [22], acute respiratory distress syndrome [23], and tumors [24]. Nagai et al. [6] showed that serum GSDMD levels in patients with adult-onset Still's disease and systemic juvenile idiopathic arthritis in the active phase, which often result in cytokine storms and are also found in critically ill COVID-19 patients, were higher than those in the inactive phase. The serum GSDMD levels in the active and inactive phases were similar between the present study and that by Nagai et al. [6], although the ELISA kits used for GSDMD detection differed between the two studies.
Innate immunity in COVID-19 plays an important role in the viral entry process, signaling pathways, cytokine release, and cell death [25]. Recent reports have shown that inflammasome activation and pyroptosis are linked to the pathogenesis of COVID-19. Ferreira et al. showed that pyroptosis was observed in human monocytes infected with SARS-CoV-2 in vitro, accompanied by elevation of biological markers that indicated inflammasome activation [26]. Paul et al. [27] reported that intense expression of Nod-like receptor family pyrin domain-containing 3 inflammasome and caspase-1, which are the key components in the pyroptosis pathway, were observed in the lung tissues of patients with COVID-19. The expression of pyroptosis-related inflammatory cytokines, including IL-1β, IL-6, and IL-18, and of GSDMD in the pulmonary macrophages was higher in COVID-19 patients than in controls without COVID-19 [28]. Consistent with these results, we demonstrated that the serum GSDMD levels were elevated in patients with critical COVID-19 and those who required MV or died. Furthermore, the serum GSDMD levels positively correlated with inflammatory markers including IL-6 and C-reactive protein. This suggests that serum GSDMD levels could be a novel biomarker reflecting disease severity and predicting disease progression in patients with COVID-19.
Although the role of apoptosis in the field of viral infections has been studied [29], its physiological role in COVID-19 remains unclear. Most viral infections lead to the suppression of apoptosis to facilitate the replication of viruses, and apoptosis plays an important role in the host's immune defense against viral invasion [30], indicating that viral infection may be based on a fine balance of apoptosis. Henry et al. reported that serum M30 levels were higher in the intensive care unit (ICU) patients with COVID-19 than in the non-ICU patients with COVID-19 [31], whereas serum M30 levels were unexpectedly low in the critically ill patients in our study. These conflicting results could be attributed to the differences between the two studies in the classification of critically ill patients with COVID-19. Moreover, apoptosis may not function properly to eliminate virus-infected cells in critically ill patients with COVID-19.
Previous studies have addressed the bidirectional crosstalk between apoptosis and pyroptosis. Apoptosis itself may block pyroptosis by cleaving GSDMD at a site different from the inherent pyroptotic process [32], and the combination of TNFα and IFNγ induces an integrated programmed cell-death pathway, including pyroptosis, apoptosis, and necroptosis, during SARS-CoV-2 infection [33]. Thus, the imbalance between the activation of pyroptosis and apoptosis may be involved in the pathogenesis of COVID-19 progression.
Consolidation in the lungs is known to be associated with disease severity [34] and specific pathophysiology [35] and may predict disease progression in COVID-19 [36]. However, there is limited knowledge about the relationship between programmed cell-death markers and CT findings. We reported that the area of consolidation tended to be larger in cases of higher severity and increased serum GSDMD levels, independent of the other biological markers (serum albumin, LDH, and M30), suggesting that pyroptosis may contribute partially to the formation of consolidation in COVID-19 cases.
Our study focused on GGO and consolidation in the CT findings and did not examine the other COVID-19-related manifestations identified on CT, such as vascular enhancement signs [37]. A chest CT pattern of organizing pneumonia in patients with COVID-19 showed favorable outcome [38]. However, we could not analyze how much GGO was replaced by consolidation, such as organizing pneumonia pattern, due to the limited follow-up chest CT conducted in this study.
Considering that an excessive host inflammatory response is involved in the critical course of COVID-19 and related mortality, immunosuppressants, such as dexamethasone [39], and IL-6 blocking agents seem to be a promising therapy [40]. However, these therapies only slightly improved the outcomes in patients with severe COVID-19, and no breakthrough treatment has been found. Drugs that act on inflammasome-related immune reactions are currently under investigation, including anakinra [41], a recombinant human IL-1 receptor antagonist, canakinumab [42], a human anti-IL-1β monoclonal antibody, and colchicine [43], an inhibitor of inflammasome activation that affects the cytokine network by blocking IL-1β. Disulfiram [44,45] or dimethyl fumarate [46], which inhibit pyroptosis by blocking GSDMD pore formation, are worth evaluating for their efficacy against COVID-19.
Our study has several limitations. First, most of the patients referred to our tertiary care hospital had been critically ill, and the number of patients with mild to moderate COVID-19 was lower than that in general city hospitals, leading to a selection bias in this study population. Our study included a large number of male participants, although this was not an intentional selection. Second, it remains unclear whether we detected whole GSDMD or cleaved GSDMD in the sera of patients with COVID-19. The oligomerization of N-terminal cleavage GSDMD, resulting from cleavage of whole GSDMD by the activated inflammatory caspases, leads to pyroptosis [47]. Thus, it would be ideal to quantify cleaved GSDMD to obtain a precise picture of pyroptosis activation. Third, our study did not reveal which organs, tissues, or cells underwent programmed cell death. Pyroptosis in COVID-19 has been observed in bronchial epithelial cells [48], alveolar endothelial cells [49], and monocytes [26] infected with SARS-CoV-2 in vitro. However, we could not reveal whether serum GSDMD was derived from the epithelial, endothelial, or immune cells in this study. Apoptosis has been identified in the lymphocytes [50] and endothelial cells [51] of COVID-19 patients as well as in vitro experiments [52]. Additional research should investigate which cells and tissues play a central role in the programmed cell death in COVID-19 and contribute to the secretion of GSDMD or M30 detected in peripheral blood. Finally, we could not analyze sequential data on chest CT scoring, serum GSDMD, and M30. This study was conducted in the early phase of the COVID-19 pandemic, and only limited cases were available to repeat the chest CT imaging in order to curb the risk of nosocomial infection. The lack of predetermined schedules for blood sampling and chest CT resulted in discrepancies in the timings. Further studies are warranted to investigate the successive clinical data in a larger cohort.
5. Conclusion
This study found that higher levels of serum GSDMD were associated with the critical respiratory status and area of consolidation on chest CT in patients with COVID-19. This suggests that excessive activation of pyroptosis may affect the clinical manifestations in patients with COVID-19. Further studies are needed to elucidate in detail how pyroptosis and apoptosis are regulated and how they affect the clinical course of COVID-19.
Author contributions
SS, MI, and KK developed the research idea and wrote the manuscript. MI, MK, TS, TK and KK supervised the study. HT and SM reviewed all the CT images in this study. TT, MT, KK, KS, KY, HK, TM, MM, HT, SF and SO treated patients with COVID-19 and provided peripheral blood samples from the patients included in this study. SS, TK, MM, and MI collected and processed blood samples. SS, MM, and MI were responsible for most of the experiments. SS and MI participated in the creation of a retrospective clinical database.
Conflict of Interest
The authors have no conflicts of interest.
Acknowledgments
We sincerely thank Keiko Yamauchi and Sanae Iwata for collecting the serum samples and recording the clinical data in this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.resinv.2022.06.007.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China. N Engl J Med. 2020;382:727–733. doi: 10.1056/nejmoa2001017. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Ivanisenko N.V., Seyrek K., Kolchanov N.A., Ivanisenko V.A., Lavrik I.N. The role of death domain proteins in host response upon SARS-CoV-2 infection: modulation of programmed cell death and translational applications. Cell Death Discov. 2020;6:101. doi: 10.1038/s41420-020-00331-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ding J., Wang K., Liu W., She Y., Sun Q., Shi J., et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111–116. doi: 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- 5.Kayagaki N., Stowe I.B., Lee B.L., O'Rourke K., Anderson K., Warming S., et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 6.Nagai H., Kirino Y., Nakano H., Kunishita Y., Henmi R., Szymanski A.M., et al. Elevated serum gasdermin D N-terminal implicates monocyte and macrophage pyroptosis in adult-onset Still's disease. Rheumatology. 2021 doi: 10.1093/rheumatology/keaa814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi C.-S., Nabar N.R., Huang N.-N., Kehrl J.H. SARS-Coronavirus Open Reading Frame-8b triggers intracellular stress pathways and activates NLRP3 inflammasomes. Cell Death Discov. 2019;5:101. doi: 10.1038/s41420-019-0181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lucas C., Wong P., Klein J., Castro T.B.R., Silva J., Sundaram M., et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584:463–469. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kramer G., Schwarz S., Hägg M., Havelka A.M., Linder S. Docetaxel induces apoptosis in hormone refractory prostate carcinomas during multiple treatment cycles. Br J Cancer. 2006;94:1592–1598. doi: 10.1038/sj.bjc.6603129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koch A., Yagmur E., Linka J., Schumacher F., Bruensing J., Buendgens L., et al. High circulating caspase-cleaved keratin 18 fragments (M30) indicate short-term mortality in critically ill patients. Dis Markers. 2018;2018:1–8. doi: 10.1155/2018/8583121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X., et al. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27:1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/s0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang D., Yu S., Zhang Y., Huang L., Luo R., Tang Y., et al. Caspse-11-GSDMD pathway is required for serum ferritin secretion in sepsis. Clin Immunol. 2019;205:148–152. doi: 10.1016/j.clim.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Kessel C., Vollenberg R., Masjosthusmann K., Hinze C., Wittkowski H., Debaugnies F., et al. Discrimination of COVID-19 from inflammation-induced cytokine storm syndromes by disease-related blood biomarkers. Arthritis Rheumatol. 2021 doi: 10.1002/art.41763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Executive Summary and Background, IDSA Guidelines on the Treatment and Management of Patients with COVID-19. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/.
- 16.Matsuoka S., Kurihara Y., Yagihashi K., Okamoto K., Niimi H., Nakajima Y. Thin-section CT assessment of spontaneous pneumomediastinum in interstitial lung disease: correlation with serial changes in lung parenchymal abnormalities. Resp Med. 2006;100:11–19. doi: 10.1016/j.rmed.2005.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Müller N.L., Mawson J.B., Mathieson J.R., Abboud R., Ostrow D.N., Champion P. Sarcoidosis: correlation of extent of disease at CT with clinical, functional, and radiographic findings. Radiology. 1989;171:613–618. doi: 10.1148/radiology.171.3.2717730. [DOI] [PubMed] [Google Scholar]
- 18.Shi J., Zhao Y., Wang K., Shi X., Wang Y., Huang H., et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 19.Ding J., Shao F. SnapShot: the noncanonical inflammasome. Cell. 2017;168 doi: 10.1016/j.cell.2017.01.008. 544-544.e1. [DOI] [PubMed] [Google Scholar]
- 20.Liu B., He R., Zhang L., Hao B., Jiang W., Wang W., et al. Inflammatory caspases drive pyroptosis in acute lung injury. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.631256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He X., Qian Y., Li Z., Fan E.K., Li Y., Wu L., et al. TLR4-upregulated IL-1β and IL-1RI promote alveolar macrophage pyroptosis and lung inflammation through an autocrine mechanism. Sci Rep. 2016;6 doi: 10.1038/srep31663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Broz P., Ruby T., Belhocine K., Bouley D.M., Kayagaki N., Dixit V.M., et al. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature. 2012;490:288–291. doi: 10.1038/nature11419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H., Li Y., Song C., Hu Y., Dai M., Liu B., et al. Neutrophil extracellular traps augmented alveolar macrophage pyroptosis via AIM2 inflammasome activation in LPS-Induced ALI/ARDS. J Inflamm Res. 2021;14:4839–4858. doi: 10.2147/jir.s321513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu P., Zhang X., Liu N., Tang L., Peng C., Chen X. Pyroptosis: mechanisms and diseases. Signal Transduct Target Ther. 2021;6:128. doi: 10.1038/s41392-021-00507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diamond M.S., Kanneganti T.-D. Innate immunity: the first line of defense against SARS-CoV-2. Nat Immunol. 2022;23:165–176. doi: 10.1038/s41590-021-01091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferreira A.C., Soares V.C., Azevedo-Quintanilha IG de, Dias S. da SG., Fintelman-Rodrigues N., Sacramento C.Q., et al. SARS-CoV-2 engages inflammasome and pyroptosis in human primary monocytes. Cell Death Discov. 2021;7:43. doi: 10.1038/s41420-021-00428-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paul O., Tao J.Q., West E., Litzky L., Feldman M., Montone K., et al. Pulmonary vascular inflammation with fatal coronavirus disease 2019 (COVID-19): possible role for the NLRP3 inflammasome. Respir Res. 2022;23:25. doi: 10.1186/s12931-022-01944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J., Wu H., Yao X., Zhang D., Zhou Y., Fu B., et al. Pyroptotic macrophages stimulate the SARS-CoV-2-associated cytokine storm. Cell Mol Immunol. 2021;18:1305–1307. doi: 10.1038/s41423-021-00665-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee S., Channappanavar R., Kanneganti T.-D. Coronaviruses: innate immunity, inflammasome activation, inflammatory cell death, and cytokines. Trends Immunol. 2020;41:1083–1099. doi: 10.1016/j.it.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaminskyy V., Zhivotovsky B. To kill or be killed: how viruses interact with the cell death machinery. J Intern Med. 2010;267:473–482. doi: 10.1111/j.1365-2796.2010.02222.x. [DOI] [PubMed] [Google Scholar]
- 31.Henry B.M., Cheruiyot I., Benoit S.W., Sanchis-Gomar F., Lippi G., Benoit J. Cytokeratin 18 cell death assays as biomarkers for quantification of apoptosis and necrosis in COVID-19: a prospective, observational study. J Clin Pathol. 2021 doi: 10.1136/jclinpath-2020-207242. jclinpath-2020-207242. [DOI] [PubMed] [Google Scholar]
- 32.Taabazuing C.Y., Okondo M.C., Bachovchin D.A. Pyroptosis and apoptosis pathways engage in bidirectional crosstalk in monocytes and macrophages. Cell Chem Biol. 2017;24:507–514. doi: 10.1016/j.chembiol.2017.03.009. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karki R., Sharma B.R., Tuladhar S., Williams E.P., Zalduondo L., Samir P., et al. Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell. 2021;184:149–168. doi: 10.1016/j.cell.2020.11.025. e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salaffi F., Carotti M., Tardella M., Borgheresi A., Agostini A., Minorati D., et al. The role of a chest computed tomography severity score in coronavirus disease 2019 pneumonia. Medicine. 2020;99 doi: 10.1097/md.0000000000022433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao L., Wang X., Xiong Y., Fan Y., Zhou Y., Zhu W. Correlation of autopsy pathological findings and imaging features from 9 fatal cases of COVID-19 pneumonia. Medicine. 2021;100 doi: 10.1097/md.0000000000025232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y., Shang K., Bian W., He L., Fan Y., Ren T., et al. Prediction of disease progression in patients with COVID-19 by artificial intelligence assisted lesion quantification. Sci Rep. 2020;10 doi: 10.1038/s41598-020-79097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aoki R., Iwasawa T., Hagiwara E., Komatsu S., Utsunomiya D., Ogura T. Pulmonary vascular enlargement and lesion extent on computed tomography are correlated with COVID-19 disease severity. Jpn J Radiol. 2021;39:451–458. doi: 10.1007/s11604-020-01085-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y., Jin C., Wu C.C., Zhao H., Liang T., Liu Z., et al. Organizing pneumonia of COVID-19: time-dependent evolution and outcome in CT findings. PLoS One. 2020;15 doi: 10.1371/journal.pone.0240347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.WHO rapid evidence appraisal for COVID-19 therapies (REACT) working group, association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19. JAMA. 2020;324 doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghosn L., Chaimani A., Evrenoglou T., Davidson M., Graña C., Schmucker C., et al. Interleukin-6 blocking agents for treating COVID-19: a living systematic review. Cochrane Database Syst Rev. 2021;3:CD013881. doi: 10.1002/14651858.cd013881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.CORIMUNO-19 Collaborative group Effect of anakinra versus usual care in adults in hospital with COVID-19 and mild-to-moderate pneumonia (CORIMUNO-ANA-1): a randomised controlled trial. Lancet Respir Med. 2021;9:295–304. doi: 10.1016/s2213-2600(20)30556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caricchio R., Abbate A., Gordeev I., Meng J., Hsue P.Y., Neogi T., et al. Effect of canakinumab vs placebo on survival without invasive mechanical ventilation in patients hospitalized with severe COVID-19. JAMA. 2021;326:230–239. doi: 10.1001/jama.2021.9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kow C.S., Lee L., Ramachandram D.S., Hasan S.S., Ming L.C., Goh H.P. The effect of colchicine on mortality outcome and duration of hospital stay in patients with COVID-19: a meta-analysis of randomized trials. Immun Inflamm Dis. 2021;10:255–264. doi: 10.1002/iid3.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu J.J., Liu X., Xia S., Zhang Z., Zhang Y., Zhao J., et al. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat Immunol. 2020;21:736–745. doi: 10.1038/s41590-020-0669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fillmore N., Bell S., Shen C., Nguyen V., La J., Dubreuil M., et al. Disulfiram use is associated with lower risk of COVID-19: a retrospective cohort study. PLoS One. 2021;16 doi: 10.1371/journal.pone.0259061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Humphries F., Shmuel-Galia L., Ketelut-Carneiro N., Li S., Wang B., Nemmara V.V., et al. Succination inactivates gasdermin D and blocks pyroptosis. Science. 2020;369:1633–1637. doi: 10.1126/science.abb9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu X., Zhang Z., Ruan J., Pan Y., Magupalli V.G., Wu H., et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–158. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gowda P., Patrick S., Joshi S.D., Kumawat R.K., Sen E. Glycyrrhizin prevents SARS-CoV-2 S1 and Orf3a induced high mobility group box 1 (HMGB1) release and inhibits viral replication. Cytokine. 2021;142 doi: 10.1016/j.cyto.2021.155496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nagashima S., Mendes M.C., Martins A.P.C., Borges N.H., Godoy T.M., Miggiolaro AFR dos S, et al. Endothelial dysfunction and thrombosis in patients with COVID-19—brief Report. Arterioscler Thromb Vasc Biol. 2020;40:2404–2407. doi: 10.1161/atvbaha.120.314860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cizmecioglu A., Cizmecioglu H.A., Goktepe M.H., Emsen A., Korkmaz C., Tasbent F.E., et al. Apoptosis-induced T-cell lymphopenia is related to COVID-19 severity. J Med Virol. 2021;93:2867–2874. doi: 10.1002/jmv.26742. [DOI] [PubMed] [Google Scholar]
- 51.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li S., Zhang Y., Guan Z., Li H., Ye M., Chen X., et al. SARS-CoV-2 triggers inflammatory responses and cell death through caspase-8 activation. Signal Transduct Target Ther. 2020;5:235. doi: 10.1038/s41392-020-00334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.