Abstract
Background:
Hereditary-alpha tryptasemia (HαT) is the most common etiology for elevated basal serum tryptase (BST). However, the utility of tryptase genotyping of individuals with elevated BST in general clinical practice remains undefined. Moreover, studies showing associations between elevated BST and chronic kidney disease (CKD), myelodysplastic syndrome (MDS), rheumatoid arthritis (RA), or eosinophilic esophagitis (EoE) did not include tryptase genotyping.
Objective:
To determine the utility of tryptase genotyping among individuals with moderate elevations in BST at a regional health system.
Methods:
Clinical and laboratory data from 109 subjects with basal tryptase values ≥ 7.5 ng/mL who were tested for HαT or had a disorder previously linked to elevated BST were collected retrospectively by chart review.
Results:
58 subjects had elevated BST defined as ≥11.5 ng/mL. 63.8% (n=37/58) had HαT, 12.1% (n=7/58) had CKD, and 20.7% (n=12/58) had clonal myeloid disorders. 6.9% (n=4/58) of subjects with elevated BST had negative testing for HαT, CKD, and myeloid neoplasms. 2 subjects with CKD, 1 subject with MDS, and 1 with myeloid hypereosinophilic syndrome (HES) had negative testing for HαT. Among subjects with elevated BST and more than one tryptase measurement, 41.5% (n=22/53) had BST variability that exceeded the 20% plus 2 formula. Increased BST variability was found in subjects with HαT, all forms of mastocytosis, CKD, MDS, and those with no associated diagnosis.
Conclusions:
HαT, CKD, and clonal myeloid disorders or a combination of the three constitute approximately 90% of individuals with elevated BST in clinical practice. Myeloid neoplasms were over-represented in this cohort relative to population prevalence data suggesting tryptase measurement selection bias by clinicians or higher prevalence. Elevated BST is associated with increased tryptase variability, regardless of etiology.
Keywords: tryptase, elevated basal tryptase algorithm, disease distribution, variability, biomarkers
INTRODUCTION
Elevated basal serum tryptase (BST) is the hallmark of the genetic trait hereditary-alpha tryptasemia (HαT) (1). In addition to HαT, BST elevation - currently defined clinically as ≥ 11.5 ng/mL - has been reported in a variety of other disorders including mastocytosis (2), hypereosinophilic syndrome (HES) (3), other myeloid neoplasms (4–6), chronic kidney disease (CKD) (7), eosinophilic esophagitis (EoE) (8), and RA (9) - though in particular among RA patients, heterophile antibodies have not been ruled out as culprit (10,11). A small number of those with chronic spontaneous urticaria (CSU) have also been reported to have modestly elevated BST levels (12,13). HαT is the most common cause for elevated BST in the general population, but most association studies did not evaluate for its presence. Additionally, HαT has been shown to be over-represented in mastocytosis cohorts (14–16). Thus, while evidence of an independent association between elevated BST and several of these diseases is lacking, more than one cause for elevated BST may also be present. Within-person BST variability in those with elevated BST has only been reported for indolent SM (ISM) and HαT (17,18).
Several studies have assessed tryptase values in the general population (19,20). Yet there is no prior study showing the distribution of disease associations among individuals with elevated BST levels obtained during routine clinical evaluation. The aims of this study were to determine the relative distribution of disorders associated with elevated BST (≥11.5 ng/mL) and high-normal BST (≥7.5 to <11.5 ng/mL) in a regional health system and characterize BST variability among individuals with elevated BST.
METHODS
Study Design
A query for serum tryptase reports was performed using a clinical pathology database called the Laboratory Information System (LIS) portion of the DoD Composite Health Care System (CHCS). The LIS processes, stores, and manages patient data related to laboratory functions and testing, and it is used solely by the laboratory. The query identified all tryptase values from the military health system National Capital Region Market which includes a number of hospitals and clinics (see ref (21) for a full list) from January 1, 2018 to March 30, 2021. We identified individuals with at least one tryptase result ≥ 7.5 ng/mL during this timeframe since the vast majority of those with HαT have values ≥ 8 ng/mL with few notable exceptions (22,23).
A retrospective chart review of the Department of Defense unified global electronic health record system known as the Armed Forces Health Longitudinal Technology Application, or AHLTA, was performed to determine final eligibility. Subjects were included in the study after comprehensive AHLTA chart review if they: a) had been tested (whether positive or negative) for HαT or b) met diagnostic criteria for HES, cutaneous mastocytosis (CM), systemic mastocytosis (SM), monoclonal mast cell activation syndrome (MMAS), other myeloid neoplasms, CKD, EoE, or RA. ICD codes were not used to determine if a subject met diagnostic criteria for any of these disorders. Subjects were excluded if they: i) had not been tested for HαT and ii) did not have a diagnosis associated with elevated BST listed in (b) or iii) the tryptase value did not represent their baseline value. A BST value was defined as a value measured at least 24 hours before or after an episode of anaphylaxis. De-identified data were collected by review of subject charts and recorded into a database.
Data Collected
Demographic variables were collected including age, sex, race, and body mass index. Individual charts were comprehensively reviewed to determine whether each subject could be assigned one or more of the following diagnoses: any variant of HES, mastocytosis (CM, SM, MMAS), other myeloid neoplasms, CKD, EoE, RA, and HαT. Tryptase genotypes were recorded from Gene by Gene (Houston, TX) TPSAB1 copy number variation reports. See online repository methods for full description on tryptase genotyping. Myeloid, lymphoid, overlap, and idiopathic variants of HES were identified as previously described (2,24,25). CM, SM subtypes, and MMAS were identified based on prior guidelines (26,27). Other myeloid neoplasms were identified by their respective WHO criteria (2). Cases of EoE were identified according to consensus criteria (28). CKD was defined as a persistent decrease in GFR < 60 mL/min/1.73 m2 with or without evidence of nephropathy via urine albumin, urine sediment, radiographs, or pathologic findings. CSU and RA were identified according to guidelines (29,30). Laboratory data were recorded including basal tryptase values, absolute eosinophil counts, tryptase genotyping, 24-hour urinary N-methylhistamine (N-MH), KIT p.D816V allele-specific polymerase chain reaction (PCR), JAK2 p.V617F allele-specific PCR, FIP1L1-PDGFRa fusion, bone marrow and gastrointestinal biopsy pathology reports, basophil histamine release assay (Viracor Eurofins, CU Index®), and GFR.
BST values for each subject were recorded along with the date of collection. Tryptase values were excluded if they were measured within 24 hours of an episode of anaphylaxis or any acute changes in health. Concurrent use of medications known to alter tryptase values were noted for each tryptase result. These included systemic corticosteroids (31), cytoreductive agents like hydroxyurea, and tyrosine kinase inhibitors (3,32,33). Stop and start dates of medications known to alter leukocyte counts and tryptase values were recorded if they were taken within 2 weeks of a tryptase measurement. The number of tryptase values measured for each subject, specialty of the clinician ordering the initial tryptase, and indication for the initial tryptase measurement were recorded.
Statistical Analysis
The minimum and maximum tryptase levels were determined for each subject with two or more serial tryptase measurements; the difference between that minimum and maximum (tryptase range) was also established. The mean tryptase range for subjects with 2, 3, 4, 5, or >5 serial tryptase levels was calculated and compared using a Kruskal-Wallis test for the global difference between groups. Pairwise Wilcoxon rank-sum tests were done to compare the distribution shifts of tryptase ranges for each group, controlling for Type 1 error by adjusting for multiple comparisons using Holm’s method (10 comparisons total). The threshold for high tryptase was calculated by taking each subject’s minimum tryptase level, multiplying it by 1.2, and then adding 2 (20+2 rule). Tryptase measurements taken while a subject was on interfering medication were not included when calculating the threshold or tryptase range. The minimum time required for a subject to break the threshold limits was determined by taking all possible pairs of tryptase measurements for each subject, determining if the maximum of the 2 tryptase values exceeded 20+2, calculating the time between the measurements, and considering, for each subject, the minimum time between measurements when the threshold was exceeded.
RESULTS
Indications for serum tryptase testing of individuals with elevated BST in clinical practice
1591 tryptase records from the National Capital Region of the Military Health System were identified from January 1, 2018 to March 31, 2021. 350 tryptase records with values ≥ 7.5 ng/mL were identified. 170 unique individuals were found in this group. 61 individuals were excluded. 13/61 individuals excluded from the study had tryptase values obtained during episodes of anaphylaxis. Of these, 5 subjects did not have BST values and 8 subjects had follow-up values that were < 7.5 ng/mL. The remaining 48 excluded individuals had no record of testing for HαT and did not have a diagnosis previously associated with elevated basal tryptase. The 109 individuals included in the study a) had HαT testing performed (whether positive or negative) or b) had another diagnosis associated with elevated BST including HES, mastocytosis (CM, SM, MMAS), EoE, CKD, RA, or other myeloid neoplasms (Figure E1 online repository).
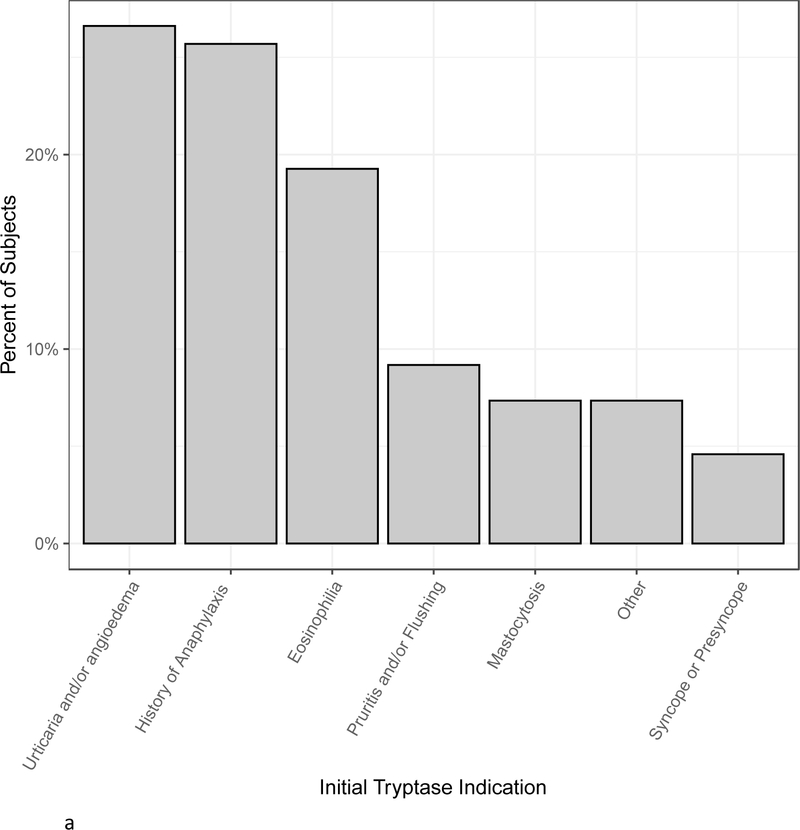
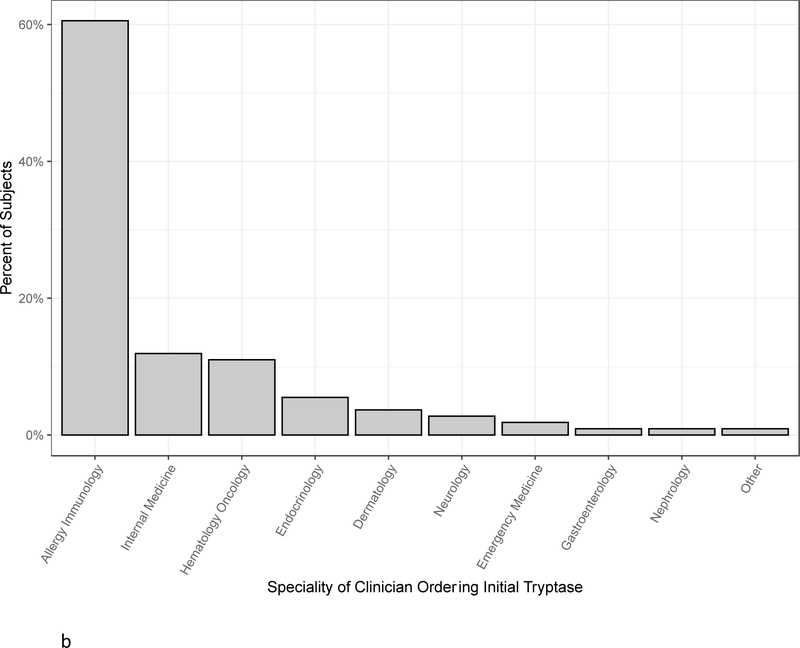
Demographics of all 109 subjects are shown in Table I. There was a slight male predominance. 16% of subjects were black and 70% were white. With regards to medical work up, 51.4% of subjects had basophil histamine release results, 60.5% had 24-hour urinary N-MH results, 72.5% had a blood test for KIT p.D816V, and 84.4% were tested for HαT. Slightly less than one third had bone marrow biopsies performed. The most frequent indications for initial tryptase measurements were urticaria and/or angioedema (27%), history of anaphylaxis (26%), and eosinophilia (19%) (Figure 1a). Pruritis and/or flushing, mastocytosis, and syncope or presyncope were the next most common indications comprising approximately 5–10% of subjects each. Allergy/Immunology clinicians ordered 60% of initial tryptase tests followed by Hematology/Oncology and Internal Medicine clinicians who each ordered approximately 10% of initial tryptase tests (Figure 1b).
TABLE I.
Demographics
| Characteristic | n (%) |
|---|---|
|
| |
| Gender | |
| Male | 59 (54.1) |
| Female | 50 (45.9) |
| Median age at initial tryptase (y) (range) | 47 (1–92) |
| Median BMI (range) | 27.0 (15.3–43.1) |
| Ethnicity | |
| White/Caucasian | 77 (70.6) |
| Black/African American | 18 (16.5) |
| American Indian/Alaskan Native | 1 (0.9) |
| Hispanic | 6 (5.5) |
| Asian | 2 (1.8) |
| Unknown | 5 (4.5) |
| Lab Markers Evaluated | |
| KIT p.D816V allele-specific PCR blood - positive/total (%) | 5/79 (6.3%) |
| N-MH - positive/total (%) | 5/66 (7.6%) |
| Basophil histamine release assay - positive/total (%) | 8/56 (14.3%) |
| Bone marrow biopsies - positive/total (%) | 14/33 (42%) |
| HαT tested - positive/total (%) | 43/92 (47%) |
| Genotypes of subjects with HαT – n/total (%) | |
| 2 alpha 3 beta | 19/43 (44.2%) |
| 3 alpha 2 beta | 17/43 (39.5%) |
| 4 alpha 2 beta | 4/43 (9.3%) |
| 4 alpha 1 beta | 3/43 (7.0%) |
BMI, body mass index
Figure 1.
A, The percent of subjects with various indications for initial tryptase measurements. “Other” category includes neuropathy (1 subject), Ehlers Danlos syndrome (2 subjects), gastrointestinal symptoms (4 subjects), and macrocytic anemia (1 subject). B, Specialty of clinicians ordering initial tryptase tests.
Diagnosis distribution and associations with elevated BST
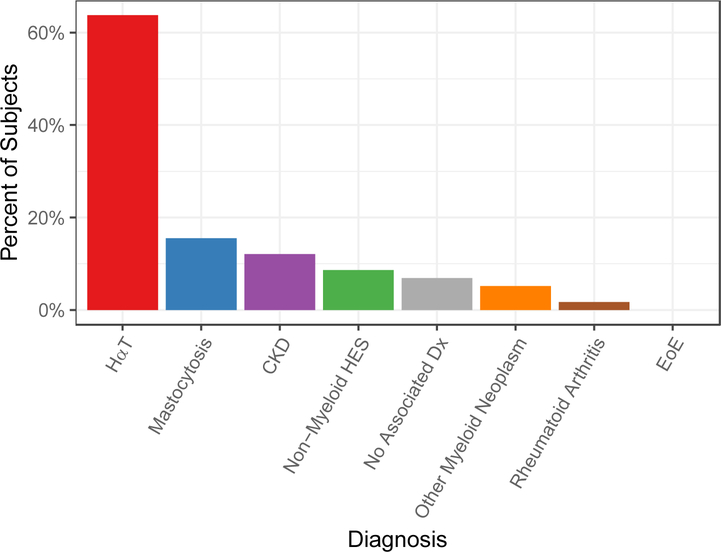
58/109 subjects had at least one elevated BST value defined as ≥ 11.5 ng/mL. A subset of subjects had undergone testing for HαT, KIT p.D816V of blood, N-MH, and bone marrow biopsies (Table E1). The distribution of diagnoses in those with elevated BST includes 37 (63.8%) with HαT, 9 (15.5%) with mastocytosis, 7 (12.1%) with CKD, 5 (8.6%) with non-myeloid HES, 4 (6.9%) with no associated diagnosis, 3 (5.2%) with other myeloid neoplasms, 1 (1.7%) with RA, and 0 with EoE (Figure 2). 12/58 (20.7%) had confirmed clonal disorders including 9 mastocytosis, 1 MDS, 1 SM with polycythemia vera (PV), and 1 FIP1L1-PDGFRa-associated chronic eosinophilic leukemia (CEL). 6/58 subjects were not tested for HαT including: 2 subjects with CM, 1 subject with SM-PV, 1 with idiopathic HES and end stage renal disease (ESRD); and 2 with non-myeloid HES. No subject with RA or non-myeloid HES who was tested for renal impairment and tryptase genotyping had elevated BST in the absence of CKD or HαT. Overall, 52/58 (89.7%) subjects were diagnosed with HαT, myeloid neoplasms, CKD, or a combination of these.
Figure 2.
Diagnosis distribution associated with BST ≥ 11.5 ng/mL. Mastocytosis includes subjects with MCL, ISM, ASM, MMAS, and CM. Other myeloid neoplasms include 1 subject each with FIP1L1-PDGFRa associated CEL, MDS, and SM-PV. Eight subjects had two associated diagnoses (see text for full description of subjects).
Subjects with elevated BST and 1 associated diagnosis
A single diagnosis was identified in 46/58 subjects with BST ≥ 11.5 ng/mL. 32 subjects had HαT with genotypes: 14 (2 alpha 3 beta), 11 (3 alpha 2 beta), 4 (4 alpha 2 beta), and 3 (4 alpha 1 beta). Nine had a single diagnosis of a mast cell-associated neoplasms including: 1 mast cell leukemia (MCL), 1 aggressive SM (ASM), 3 ISM, 1 MMAS, 2 CM, and 1 SM-JAK2 p.V617F-associated PV. Of those with mast cell-associated neoplasms, 3/9 (2 with CM, and 1 with SM-PV) were not tested for HαT and the remaining 6/9 tested negative for HαT.
Three subjects with elevated BST (≥ 11.5 ng/mL) had HES only. One subject had FIP1L1-PDGFRa-associated CEL with a pre-treatment tryptase of 43 ng/mL, tested negative for HαT and KIT p.D816V allele-specific PCR, and had no evidence of mastocytosis on bone marrow. The other 2 subjects had HES with incomplete evaluations at the time of this study - both lacking HαT testing, bone marrow biopsies, FIP1L1-PDGFRa by reverse transcriptase PCR (RT-PCR), and KIT p.D816V allele-specific PCR.
Two subjects with elevated BST (≥ 11.5 ng/mL) had CKD. The first had CKD Stage 4, BST range 17.9 – 25 ng/mL, and tested negative for HαT, N-MH, KIT p.D816V, and had no abnormality on bone marrow. The second subject with CKD Stage 3b and BST range 9.5–13.7 ng/mL, tested negative for HαT, N-MH, and KIT p.D816V.
Subjects with elevated BST and 2 associated diagnoses
Of those with elevated BST, 8/58 subjects (13.8%) had more than one associated diagnosis. One subject with HαT (2 alpha 3 beta) and CKD Stage 3b (GFR of 30 mL/min/1.73 m2), had a BST range 36.2–43 ng/mL, tested negative for N-MH and KIT p.D816V of blood, and had no abnormality on bone marrow examination. A second subject had HαT (3 alpha 2 beta) and CKD Stage 3a (GFR of 56.2 mL/min/1.73 m2), had a BST range 9.8–15.8 ng/mL, and tested negative for KIT p.D816V of blood. The third subject had MDS with del(5q) diagnosed by bone marrow evaluation and CKD Stage 3a (GFR of 56.2 mL/min/1.73 m2), had a BST range 6.4–104 ng/mL, and tested negative for HαT, N-MH, and KIT p.D816V of blood. The fourth subject had ISM and CKD Stage 3a (GFR of 44 mL/min/1.73 m2), had a BST range 17.3–19.5 ng/mL and tested negative for HαT. The fifth subject had HαT (2 alpha 3 beta) and RA with a BST range 10–35 ng/mL and tested negative N-MH and KIT p.D816V of blood. The sixth subject had idiopathic HES and ESRD on intermittent hemodialysis and a single basal tryptase measurement of 24.3 ng/mL without any additional testing. The seventh subject had HαT (2 alpha 3 beta) and HES-DRESS overlap with a BST range 12.9–15.1 ng/mL. The eighth subject had HαT (3 alpha 2 beta) and idiopathic HES with BST range 9.6–16.2 ng/mL, with negative blood testing for KIT p.D816V of blood, FIP1L1-PDGFRa RT-PCR, and JAK2 p.V617F. These data show an independent association of elevated BST with CKD, MDS, and FIP1L1-PDGFRa associated CEL, and demonstrate the possibility of an additive effect of CKD with HαT (see reference ranges reported for HαT genotypes in reference (34)) in one individual but not in another. The data also show that BST elevations identified in those with non-myeloid HES are likely due to HαT or CKD.
Subjects with elevated BST and no associated diagnosis
The remaining 4 subjects with elevated BST did not have an identifiable cause for elevated BST (≥ 11.5 ng/mL); they all tested negative for HαT, had N-MH within normal limits, negative KIT p.D816V in blood, and GFR > 60 mL/min/1.73 m2. One subject had CSU, with BST range 9.6–15.9 ng/mL and no abnormality on bone marrow biopsy. The second subject was diagnosed with Sjögren’s syndrome and had a BST range 10.4–15.9 ng/mL and no abnormality on bone marrow biopsy. The third subject had chronic idiopathic pruritis and BST range 9.1–11.8 ng/mL with no abnormality on bone marrow biopsy. The fourth subject had idiopathic anaphylaxis with BST range 9.2–11.5 ng/mL and bone marrow was not assessed. Mature tryptase levels were not obtainable to determine whether serum elevations were a result from mast cell degranulation.
Diagnosis distribution and associations with high-normal BST
51 individuals had at least one BST ≥ 7.5 but all values < 11.5 ng/mL. A subset of subjects had undergone testing for HαT, KIT p.D816V of blood, N-MH, and bone marrow biopsies (Table E2). The distribution of diagnoses in those with high-normal BST includes 6 (11.8%) with HαT, 5 (10.0%) with mastocytosis, 9 (17.6%) with CKD, 13 (25.5%) with non-myeloid HES, 14 (27.5%) with no associated diagnosis, 3 (5.9%) with other myeloid neoplasms, 1 (2.0%) with RA, and 5 with EoE (9.8%) (Figure E2).
Subjects with high-normal BST and 1 associated diagnosis
6 subjects were identified with HαT, 5 with mastocytosis (3 CM, 2 ISM), 9 with HES (1 lymphoid-variant, 6 idiopathic, 2 HES-EGPA), 3 with EoE, 6 with CKD (2 stage 3a, 1 stage 3b, 1 stage 4, 2 on hemodialysis), 2 with other myeloid neoplasms (1 JAK2-associated neoplasm, 1 MDS-EB-1), 1 with RA.
Subjects with high-normal BST and 2 or no associated diagnoses
5/51 (9.8%) had two associated diagnoses including: 2 patients with HES-EoE-overlap; 1 patient with idiopathic HES and ESRD on hemodialysis; 1 patient with HES-EGPA overlap and CKD Stage 3b; and 1 patient with MDS-EB-1 and CKD Stage 3a. 14/51 subjects with BST range 7.5–11.4 ng/mL had no identifiable associated diagnoses.
Performance of laboratory and genetic studies
Of patients with BST ≥ 7.5, 66/109 subjects were tested for N-MH (reference range for age > 16 years is 30–200 mg/g) and 5 adult subjects had elevated N-MH values including: 1 subject with ASM (561 and 622.4 mg/g); 1 subject with MCL (552 mg/g); 1 subject with ISM (227 mg/g); 1 subject with MMAS (228 mg/g); and 1 subject with EoE (218 and 209 mg/g). The subject with EoE did not have a bone marrow biopsy. Importantly, the subjects with elevated N-MH diagnosed with MCL and MMAS did not have the KIT p.D816V mutation detected in blood or bone marrow. Overall, 4/7 subjects with SM or MMAS who were tested had elevated N-MH.
KIT p.D816V testing of blood was performed in 78/109 of subjects using allele-specific PCR. Five subjects were identified with the somatic variant, and all 5 subjects had SM. Two subjects diagnosed with ISM had negative testing for both N-MH and KIT p.D816V of blood. Their BST ranges were 7.1–8.2 and 11–13.3 ng/mL.
Basophil histamine release was assayed in 56/109 subjects and 8 had elevated values. Six of these subjects had HαT, one having concomitant HES, and two had no identified diagnoses. All 8 subjects had elevated BST. 6/25 (24%) subjects with HαT who were tested for basophil histamine release had increased basophil histamine release.
Baseline Tryptase Variability
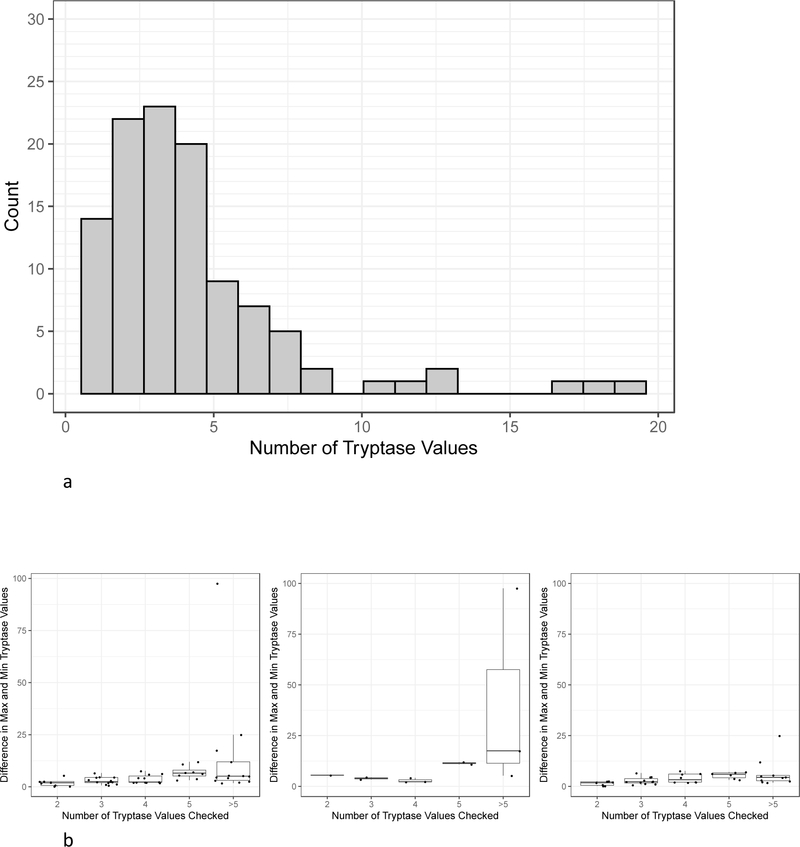
The median number of BST tests resulted per patient was 3 (range 1–19). 12.8% of subjects had only a single tryptase value, 67.9% of subjects had 2–5 tryptase values, 12.8% of subjects had 6–10 tryptase values, and 6.4% of subjects had greater than 10 tryptase values (Figure 3a). The 53/58 subjects with elevated BST and more than one tryptase recorded, excluding those taken while on medication, were binned (Figure 3b) based on their number of tryptase values: 2 (n=8), 3 (n=13), 4 (n=11), 5 (n=8), and greater than 5 (n=13). The mean tryptase ranges and standard deviations (SD) for each bin were 1.96 (SD 1.72), 3.00 (SD 1.70), 3.75 (SD 2.07), 6.96 (SD 6.96), and 14.35 (SD 14.35) ng/mL for subjects with 2,3,4,5, and > 5 BST values recorded, respectively. The tryptase range distribution was significantly different across groups (p =0.001). There were significant differences in the tryptase range distribution between those with 2 and those with 5 tryptase values checked (p = 0.02). There was also a significant difference between those with 3 tryptase values and those with 5 tryptase values checked (p=0.03). Of the 53 subjects with at least one tryptase value ≥ 11.5 and at least 2 tryptase values checked, 11 had confirmed myeloid neoplasms. Of these 11 subjects, 1 subject had 2 tryptase values checked; 2 subjects had 3 tryptase values checked; 3 subjects had 4 tryptase values checked; 2 subjects had 5 tryptase values checked; and 3 subjects had > 5 tryptase values checked. The mean tryptase ranges and SDs for each bin were 5.50, 3.95 (SD 0.78), 2.90 (SD 1.13), 11.45 (SD 0.78), and 40.13 (SD 50.14) ng/mL for subjects with 2,3,4,5, and > 5 BST values recorded, respectively. The tryptase distribution range was not significantly different between groups. 42 of the subjects did not have myeloid neoplasms including those with HαT, CKD, and non-myeloid HES. Of those subjects, 7 subjects had 2 tryptase values checked, 11 subjects had 3 tryptase values checked, 8 subjects had 4 tryptase values checked, 6 subjects had 5 tryptase values checked, and 10 subjects had >5 tryptase values checked. The mean tryptase ranges and SDs for each bin were 1.46 (SD 1.04), 2.83 (SD 1.79), 4.06 (SD 2.30), 5.47 (SD 1.62), and 6.62 (SD 7.08) ng/mL for subjects with 2,3,4,5, and > 5 BST values recorded, respectively. The tryptase range distribution was significantly different across groups (p=0.005). There were significant differences in the tryptase range distribution between those with 2 and those with 5 tryptase values (p=0.03) and those with >5 tryptase values (p=0.04).
Figure 3.
A, Frequency histogram showing subjects with different counts of tryptase values checked. All 109 subjects were included. B, BST range (minimum subtracted from maximum) distribution by total number of tryptase values checked. Boxplots are shown. Left panel: 53 subjects with at least one elevated BST value and at least 2 tryptase values checked. Middle panel: 11/53 subjects with confirmed myeloid neoplasms (middle panel). Right panel: 42/53 subjects with CKD, HαT, non-myeloid HES, and no associated diagnosis.
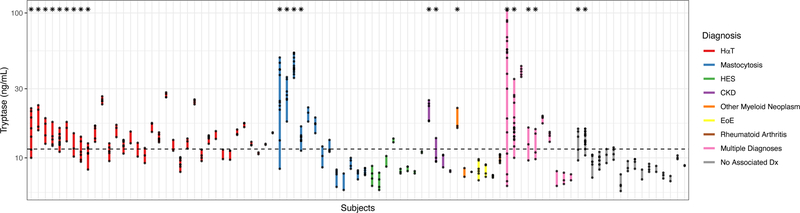
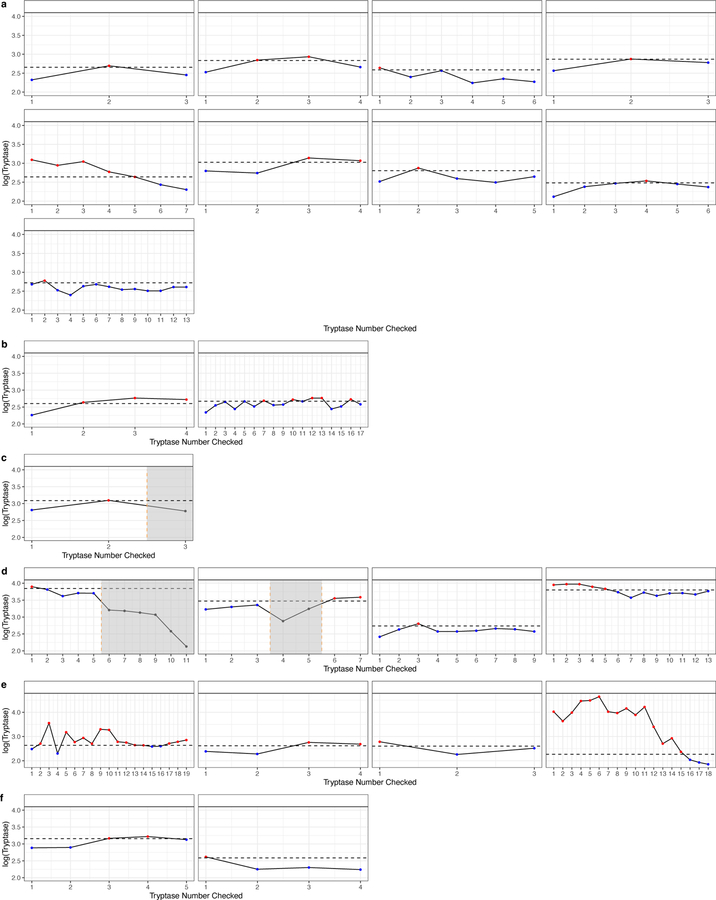
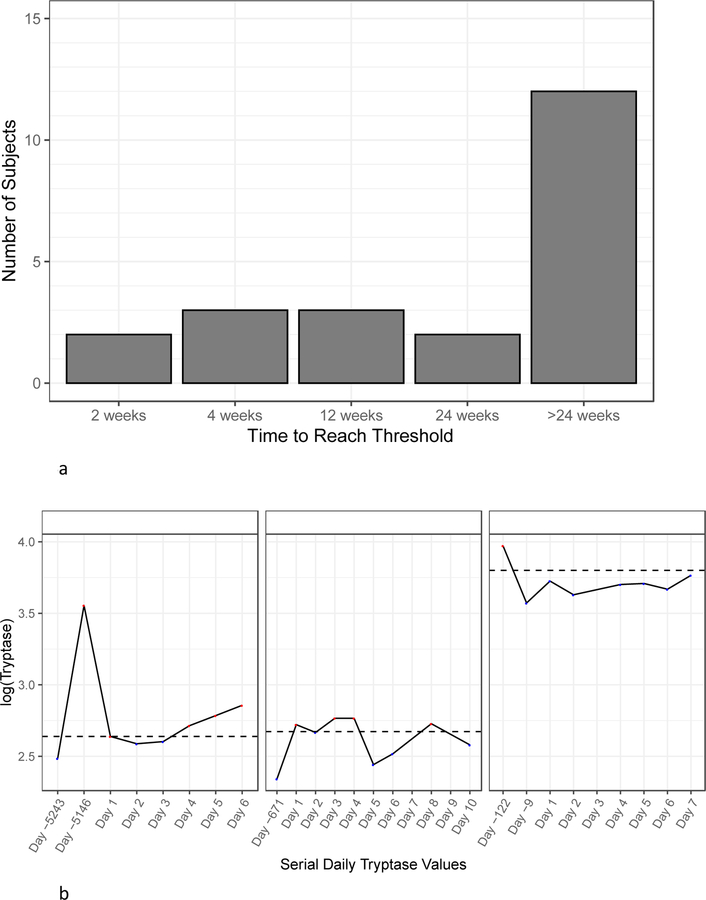
BST ranges were graphed in the 93 subjects with more than one tryptase value when not taking prednisone or imatinib (Figure 4). BST ranges of 22 subjects that exceeded 20+2 were then graphed (Figure 5 and Figure E3 online repository); three had concurrent use of imatinib, prednisone, or hydroxyurea, but each subject had variability outside these timepoints that was greater than 20+2. This is 41.5% of the 53 subjects with elevated BST and multiple tryptase measurements recorded, off medications. Subjects with HαT, mastocytosis, CKD, multiple diagnoses, and no associated diagnosis each demonstrated variability that exceeded 20+2, and all had at least one tryptase value ≥ 11.5 ng/mL. By contrast, only 4/53 (7.5%) subjects, 3 with HαT as well as one with MDS and CKD Stage 3a, exceeded a recently proposed 1.685 threshold ratio (when comparing acute/BST levels) (18) when using their lowest recorded tryptase value. Over one-third of subjects reached the 20+2 within 3 months, and many of those reached the threshold within a shorter time frame (Figure 6a). 3/22 subjects with values exceeding 20+2 were found to have had serial daily tryptase values assessed over a 7–10 day period of time. Tryptase values checked daily showed variation within this short interval (Figure 6b).
Figure 4.
The extremes (minimum to maximum value per subject) of BST variability are shown for the 93 subjects with more than one tryptase value. Subjects are organized by diagnoses and extremes of variability. The horizontal dashed line highlights tryptase ranges that include or rise above 11.5 ng/mL. Only subjects with at least one BST value ≥ 11.5 ng/mL had variability exceeding 20+2. An asterisk above the graph corresponds to subjects with BST variability exceeding 20+2.
Figure 5.
Subjects with variability that exceeded 20+2 had basal tryptase values graphed ordinally. Horizontal dashed lines represent the threshold of the formula using the lowest tryptase value. Shaded regions corresponded to concurrent use of imatinib, prednisone, or hydroxyurea. (a) HαT (b) no associated diagnosis (c) SM-PV (d) mastocytosis (e) multiple diagnoses (f) CKD.
Figure 6.
A, Subjects were binned on the X-axis based on the minimum time required to exceed the threshold between any two tryptase values. The Y-axis corresponds to the number of subjects in each bin. B, Three subjects who had a period of daily tryptase values checked are shown: 1 subject with HαT (left panel), 1 subject with no associated diagnosis (middle panel), and 1 subject with ASM (right panel). Previous minimum and maximum values are shown if they did not occur within the period of daily tryptase checks.
DISCUSSION
Prior studies have assessed the distribution of BST values in the general population (19,20,35) as well as in cohorts with CKD, RA, EoE, myeloid HES, and MDS (4–9). These studies generally did not consider HαT, which is known to be present in approximately 5% of populations with European background. Our data demonstrate that in a general healthcare setting, HαT (63.8%); myeloid neoplasms (20.7%) including mastocytosis, FIP1L1-PDGFRa associated CEL, and MDS; and CKD (12.1%) account for approximately 90% of subjects with elevated BST among patients with a clinical indication to check serum tryptase. Myeloid neoplasms were over-represented relative to HαT and CKD in our study based on reported population prevalence data (22,36,37). The disease distribution we present is likely influenced by selection bias from clinicians who order tryptase since testing is ordered for only a small fraction of patients in a healthcare system. For example, clinical guidelines recommend against checking labs routinely in those with urticaria and angioedema, two phenotypes reported in association with HαT, and frequently absent in those with clonal myeloid neoplasms (38,39). Tryptase measurement is also not part of the standard clinical evaluation of CKD, even when significant pruritus is present (40). Alternatively, myeloid neoplasms may be more common than previously thought given a recent study showing a high frequency of mastocytosis with normal tryptase values among patients with severe venom anaphylaxis (41).
We investigated laboratory markers in those with moderate elevations in serum tryptase. One subject with ISM with BST range of 7.1–8.2 ng/mL and another subject with ISM and BST of 11.0–13.3 ng/mL, had KIT p.D816V detected in marrow but not blood using allele-specific PCR and both had 24-hour urinary N-MH within the reference range. Both subjects had spindled mast cells that expressed CD25 and met 3 minor criteria for SM. 24-hour urinary N-MH was a useful screening test for one person with MCL and another with MMAS who both had negative KIT p.D816V allele-specific PCR of blood and bone marrow. Basophil histamine release (42) after incubation of donor basophils with autologous serum was assessed in 56 subjects and found to be increased in 6 subjects with HαT and 2 with no associated diagnosis. This is notable since a prior study found reduced ex vivo basophil surface CD203c expression in subjects with HαT after stimulation with anti-IgE or N-formyl-methionyl-leucyl-phenylalanine (43) - an affect that has been attributed to histamine exposure in other in vitro studies (44). Interestingly, tryptase has recently been shown to induce histamine release from mast cells and a neutralizing anti-tryptase monoclonal antibody has been shown to both limit IgE-mediated anaphylaxis in a humanized mouse model, and reduce urine histamine metabolites in a nonhuman primate model (45). Further investigation is needed to determine whether increased tryptase production might augment histamine release in HαT. However, N-MH levels were not increased among individuals with HαT in this or another previous study (14), suggesting that at baseline this does not appear to be occurring.
Our data show that the association of advanced CKD, FIP1L1-PDGFRa-associated CEL, and MDS with elevated BST is independent of HαT (3,4,6,7). In contrast to previous studies, we did not find a link between elevated BST and EoE (8) or RA (9) independent of HαT or CKD. We should note the small sample sizes for subjects with EoE (5 subjects) and RA (2 subjects) and a future study with larger samples is needed to confirm these results. It is possible that prior associations were confounded by the presence of either of HαT or CKD. It is also possible that clonal myeloid disease (3,46,47), or heterophile antibody interference with previously employed methods of tryptase measurement (10,11), may have contributed to previously identified associations with EoE and RA, respectively.
Based upon these results, an algorithmic clinical approach (Figure 7) in patients with BST ≥ 11.5 ng/mL could include routine tryptase genotyping, creatinine and GFR calculation, complete blood count with differential, KIT p.D816V allele-specific PCR of blood, and 24-hour urinary N-MH. Bone marrow biopsy should be considered based upon the clinical presentation and results of these tests. Importantly, the presence of HαT or CKD, or negative 24-hour urinary N-MH and KIT p.D816V in blood does not obviate the need for bone marrow evaluation (48).
Figure 7.
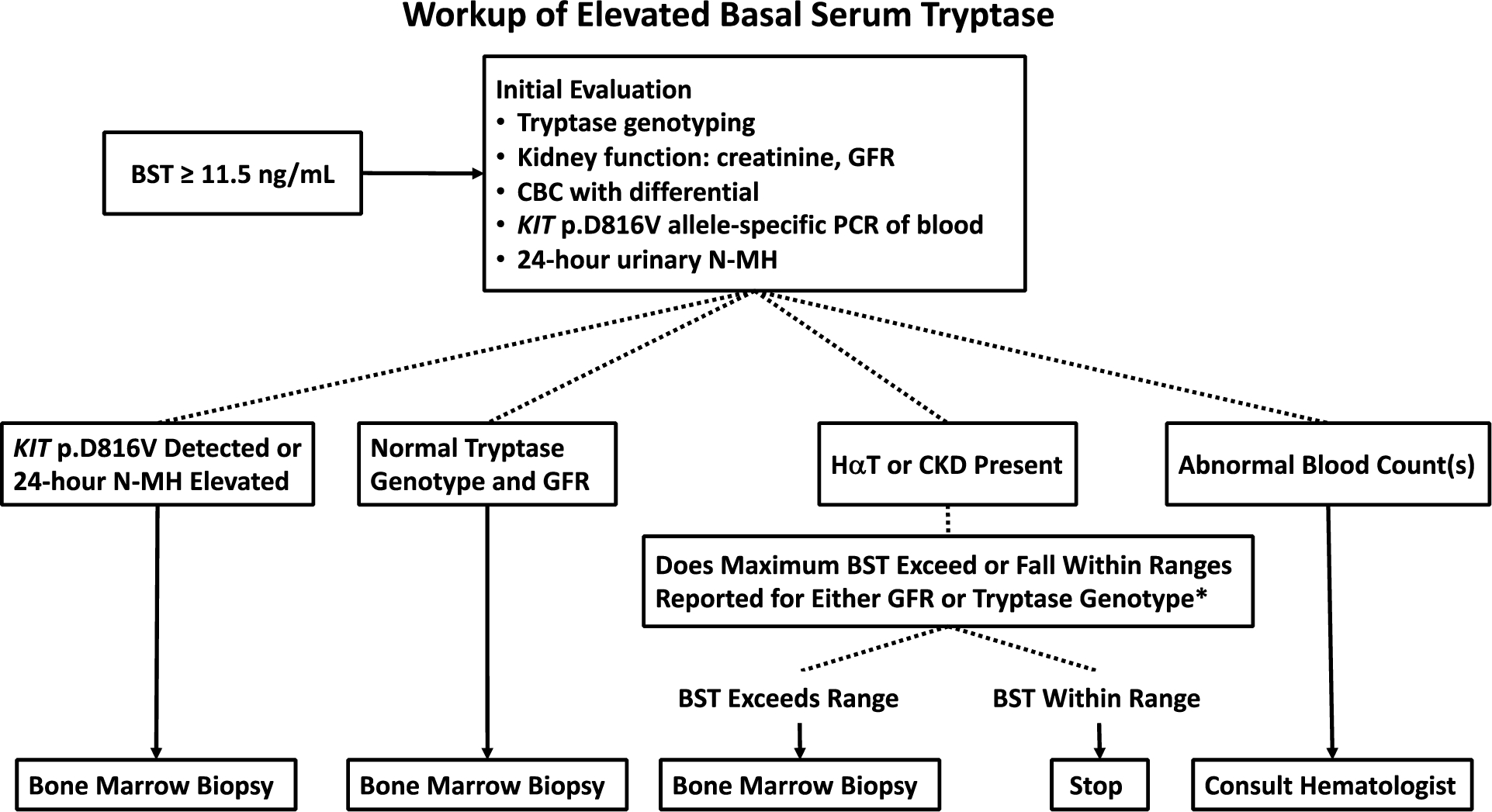
Algorithm for evaluation of patients with elevated BST. Note: Clinical findings such as idiopathic anaphylaxis may prompt bone marrow biopsy independently of basal serum tryptase evaluation * See citations (7) and (34) for reference of BST ranges based on GFR and tryptase genotypes
The 20+2 formula has been used to define a tryptase threshold to aid in confirmation of the diagnoses of anaphylaxis and mast cell activation syndrome (27,49,50). Notably, BST variability has recently been reported to negatively impact the specificity of this algorithm, in particular among individuals with elevated BST caused by HαT and ISM (18). Our data demonstrate that elevated BST resulting from CKD, MDS, MCL, ASM, and other unidentified etiologies negatively impacts the specificity of 20+2 as well. Our data support the use of the recently reported 1.685 threshold ratio (18) rather than 20+2 in order to minimize false-positives when assessing for acute elevations in BST in anaphylaxis. Moreover, a small number of subjects with elevated BST had tryptase ranges that exceeded the optimized threshold of 1.685 if the lowest tryptase value were used, supporting the authors recommendation of employing high sensitivity or high specificity thresholds depending upon clinical judgement.
We found that tryptase variability in people with elevated BST may exceed 20+2 within two weeks and in some with daily measurements over two sequential days. Thus, our data suggest that when clinicians are using this biomarker to aid in clinical decision making, obtaining serial tryptase values over a short period of time may help to better define an individual’s baseline. Why such BST variability occurs remains unknown. Several possibilities exist including cycling proliferation of mast cells in the bone marrow, alterations in pro-tryptase production by mast cells, or factors affecting tryptase degradation.
There were several limitations to this study. First, this was a retrospective study with a lack of standardization of tryptase measurements over time and potentially differing methodologies that may impact the variability that we describe. Second, we did not adjust for age and body mass index which are both associated with relatively small changes in BST (19,20,51). However, the effect of age on BST variability was recently shown to be clinically inconsequential (18). Third, among the 58 individuals with BST ≥ 11.5 ng/mL, 6 individuals did not have tryptase genotyping. Despite this, approximately 90% of individuals with elevated BST were accounted for by HαT, CKD, and myeloid neoplasms.
Results from this study suggest an algorithmic approach would benefit patients with elevated BST to identify the disorder(s) leading to tryptase elevation. Future studies are needed to determine whether those identified in this study without an apparent cause for elevated BST are due to another diagnosis or occult clonal myeloid neoplasia. Studies should also address why some people with HαT, advanced CKD, and myeloid neoplasms have tryptase values < 11.5 ng/mL.
Supplementary Material
Highlights Box.
What is already known about this topic?
Tryptase is a biomarker linked to mast cell number and degranulation. Several disorders have been associated with elevated basal tryptase and a transient increase in tryptase is associated with anaphylaxis.
What does this article add to our knowledge?
HαT is the most common cause for elevated BST in a regional health system, while myeloid neoplasms and CKD are the common acquired causes. BST variability exceeded the 20% plus 2 formula in 41.5% of subjects with elevated BST, suggesting that this algorithm lacks specificity among these individuals.
How does this study impact current management guidelines?
An algorithm starting with tryptase genotyping, kidney function, complete blood count with differential, KIT p.D816V of blood, and 24-hour urinary N-methylhistamine helps guide the differential diagnosis when evaluating individuals with elevated BST.
Funding
This research was funded in whole or in part by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases, NIH and with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. 75N910D00024, Task Order No. 75N91019F00130. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, Uniformed Services University, or Department of Defense; nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. The authors declare no conflict of interest.
Abbreviations
- 20+2
20% of baseline tryptase plus 2 ng/mL
- AEC
Absolute eosinophil count
- ASM
Aggressive systemic mastocytosis
- BST
Basal serum tryptase
- CEL
Chronic eosinophilic leukemia
- CKD
Chronic kidney disease
- CM
Cutaneous mastocytosis
- CSU
Chronic spontaneous urticaria
- EGPA
Eosinophilic granulomatosis with polyangiitis
- EoE
Eosinophilic esophagitis
- GFR
Glomerular filtration rate
- HαT
Hereditary-alpha tryptasemia
- HES
Hypereosinophilic syndrome
- ISM
Indolent systemic mastocytosis
- MCAS
Mast cell activation syndrome
- MCL
Mast cell leukemia
- MDS
Myelodysplastic syndrome
- MMAS
Monoclonal mast cell activation syndrome
- N-MH
24-hour urinary N-methylhistamine
- PCR
Polymerase chain reaction
- PV
Polycythemia vera
- RA
Rheumatoid arthritis
- SM
Systemic mastocytosis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- 1.Lyons JJ, Yu X, Hughes JD, Le QT, Jamil A, Bai Y, et al. Elevated basal serum tryptase identifies a multisystem disorder associated with increased TPSAB1 copy number. Nat Genet. 2016. Dec;48(12):1564–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016. May 19;127(20):2391–405. [DOI] [PubMed] [Google Scholar]
- 3.Klion AD, Noel P, Akin C, Law MA, Gilliland DG, Cools J, et al. Elevated serum tryptase levels identify a subset of patients with a myeloproliferative variant of idiopathic hypereosinophilic syndrome associated with tissue fibrosis, poor prognosis, and imatinib responsiveness. Blood. 2003. Jun 15;101(12):4660–6. [DOI] [PubMed] [Google Scholar]
- 4.Sperr WR, El-Samahi A, Kundi M, Girschikofsky M, Winkler S, Lutz D, et al. Elevated tryptase levels selectively cluster in myeloid neoplasms: a novel diagnostic approach and screen marker in clinical haematology. Eur J Clin Invest. 2009. Oct;39(10):914–23. [DOI] [PubMed] [Google Scholar]
- 5.Sperr WR, Jordan JH, Baghestanian M, Kiener HP, Samorapoompichit P, Semper H, et al. Expression of mast cell tryptase by myeloblasts in a group of patients with acute myeloid leukemia. Blood. 2001. Oct 1;98(7):2200–9. [DOI] [PubMed] [Google Scholar]
- 6.Sperr WR, Stehberger B, Wimazal F, Baghestanian M, Schwartz LB, Kundi M, et al. Serum tryptase measurements in patients with myelodysplastic syndromes. Leuk Lymphoma. 2002. May;43(5):1097–105. [DOI] [PubMed] [Google Scholar]
- 7.Sirvent AE, González C, Enríquez R, Fernández J, Millán I, Barber X, et al. Serum tryptase levels and markers of renal dysfunction in a population with chronic kidney disease. J Nephrol. 2010. Jun;23(3):282–90. [PubMed] [Google Scholar]
- 8.Kutty GR, Downs-Kelly E, Crispin HT, Peterson KA. Elevated Tryptase in EoE Is an Independent Phenomenon Associated with Extra-Esophageal Symptoms. Dig Dis Sci. 2019. Jan;64(1):152–7. [DOI] [PubMed] [Google Scholar]
- 9.Guo Y, Wu Q, Ni B, Mou Z, Jiang Q, Cao Y, et al. Tryptase is a candidate autoantigen in rheumatoid arthritis. Immunology. 2014. May;142(1):67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sargur R, Cowley D, Murng S, Wild G, Green K, Shrimpton A, et al. Raised tryptase without anaphylaxis or mastocytosis: heterophilic antibody interference in the serum tryptase assay. Clin Exp Immunol. 2011. Mar;163(3):339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Toorenenbergen AW, van Daele PLA, Boonstra JG. False-elevated serum tryptase assay result caused by heterophilic antibodies. J Allergy Clin Immunol. 2005. Nov;116(5):1159–60. [DOI] [PubMed] [Google Scholar]
- 12.Siles R, Xu M, Hsieh FH. The utility of serum tryptase as a marker in chronic spontaneous urticaria. Acta Derm Venereol. 2013. May;93(3):354–5. [DOI] [PubMed] [Google Scholar]
- 13.Ferrer M, Nuñez-Córdoba JM, Luquin E, Grattan CE, De la Borbolla JM, Sanz ML, et al. Serum total tryptase levels are increased in patients with active chronic urticaria. Clin Exp Allergy. 2010. Dec;40(12):1760–6. [DOI] [PubMed] [Google Scholar]
- 14.Chollet MB, Akin C. Hereditary alpha tryptasemia is not associated with specific clinical phenotypes. J Allergy Clin Immunol. 2021. Jun 23; [DOI] [PubMed] [Google Scholar]
- 15.Lyons JJ, Chovanec J, O’Connell MP, Liu Y, Šelb J, Zanotti R, et al. Heritable risk for severe anaphylaxis associated with increased α-tryptase-encoding germline copy number at TPSAB1. J Allergy Clin Immunol. 2021. Feb;147(2):622–32. [DOI] [PubMed] [Google Scholar]
- 16.Greiner G, Sprinzl B, Górska A, Ratzinger F, Gurbisz M, Witzeneder N, et al. Hereditary α tryptasemia is a valid genetic biomarker for severe mediator-related symptoms in mastocytosis. Blood. 2021. Jan 14;137(2):238–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carter MC, Clayton ST, Komarow HD, Brittain EH, Scott LM, Cantave D, et al. Assessment of clinical findings, tryptase levels, and bone marrow histopathology in the management of pediatric mastocytosis. J Allergy Clin Immunol. 2015. Dec;136(6):1673–1679.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mateja A, Wang Q, Chovanec J, Kim J, Wilson KJ, Schwartz LB, et al. Defining baseline variability of serum tryptase levels improves accuracy in identifying anaphylaxis. J Allergy Clin Immunol. 2021. Aug 20; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez-Quintela A, Vizcaino L, Gude F, Rey J, Meijide L, Fernandez-Merino C, et al. Factors influencing serum total tryptase concentrations in a general adult population. Clin Chem Lab Med. 2010. May;48(5):701–6. [DOI] [PubMed] [Google Scholar]
- 20.Fenger RV, Linneberg A, Vidal C, Vizcaino L, Husemoen LL, Aadahl M, et al. Determinants of serum tryptase in a general population: the relationship of serum tryptase to obesity and asthma. Int Arch Allergy Immunol. 2012;157(2):151–8. [DOI] [PubMed] [Google Scholar]
- 21.Defense Health Agency D of D. National Capital Region Market | Health.mil [Internet]. National Capital Region Market. [cited 2021 Nov 15]. Available from: https://www.health.mil/About-MHS/OASDHA/Defense-Health-Agency/NCR-Medical-Directorate [Google Scholar]
- 22.Robey RC, Wilcock A, Bonin H, Beaman G, Myers B, Grattan C, et al. Hereditary Alpha-Tryptasemia: UK Prevalence and Variability in Disease Expression. J Allergy Clin Immunol Pract. 2020. Jun 15;8(10):3549–56. [DOI] [PubMed] [Google Scholar]
- 23.Giannetti MP, Weller E, Bormans C, Novak P, Hamilton MJ, Castells M. Hereditary alpha-tryptasemia in 101 patients with mast cell activation-related symptomatology including anaphylaxis. Ann Allergy Asthma Immunol. 2021. Jan 17; [DOI] [PubMed] [Google Scholar]
- 24.Klion AD, Bochner BS, Gleich GJ, Nutman TB, Rothenberg ME, Simon H-U, et al. Approaches to the treatment of hypereosinophilic syndromes: a workshop summary report. J Allergy Clin Immunol. 2006. Jun;117(6):1292–302. [DOI] [PubMed] [Google Scholar]
- 25.Valent P, Klion AD, Horny H-P, Roufosse F, Gotlib J, Weller PF, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol. 2012. Sep 1;130(3):607–612.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valent P, Akin C, Metcalfe DD. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood. 2017. Mar 16;129(11):1420–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valent P, Akin C, Arock M, Brockow K, Butterfield JH, Carter MC, et al. Definitions, criteria and global classification of mast cell disorders with special reference to mast cell activation syndromes: a consensus proposal. Int Arch Allergy Immunol. 2012;157(3):215–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dellon ES, Liacouras CA, Molina-Infante J, Furuta GT, Spergel JM, Zevit N, et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: proceedings of the AGREE conference. Gastroenterology. 2018. Oct;155(4):1022–1033.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018. Jul;73(7):1393–414. [DOI] [PubMed] [Google Scholar]
- 30.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010. Sep;62(9):2569–81. [DOI] [PubMed] [Google Scholar]
- 31.Jhun BW, Kim SJ, Kim K, Lee JE, Hong DJ. Clinical implications of correlation between peripheral eosinophil count and serum levels of IL-5 and tryptase in acute eosinophilic pneumonia. Respir Med. 2014. Nov;108(11):1655–62. [DOI] [PubMed] [Google Scholar]
- 32.Gotlib J, Kluin-Nelemans HC, George TI, Akin C, Sotlar K, Hermine O, et al. Efficacy and Safety of Midostaurin in Advanced Systemic Mastocytosis. N Engl J Med. 2016. Jun 30;374(26):2530–41. [DOI] [PubMed] [Google Scholar]
- 33.van Anrooij B, Oude Elberink JNG, Span LFR, de Monchy JGR, Rosati S, Mulder AB, et al. Midostaurin in patients with indolent systemic mastocytosis: An open-label phase 2 trial. J Allergy Clin Immunol. 2018. Jun 8;142(3):1006–1008.e7. [DOI] [PubMed] [Google Scholar]
- 34.Glover SC, Carter MC, Korošec P, Bonadonna P, Schwartz LB, Milner JD, et al. Clinical relevance of inherited genetic differences in human tryptases: Hereditary alpha-tryptasemia and beyond. Ann Allergy Asthma Immunol. 2021. Aug 13; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fellinger C, Hemmer W, Wöhrl S, Sesztak-Greinecker G, Jarisch R, Wantke F. Clinical characteristics and risk profile of patients with elevated baseline serum tryptase. Allergol Immunopathol (Madr). 2014. Dec;42(6):544–52. [DOI] [PubMed] [Google Scholar]
- 36.Epidemiology Brockow K., prognosis, and risk factors in mastocytosis. Immunol Allergy Clin North Am. 2014. May;34(2):283–95. [DOI] [PubMed] [Google Scholar]
- 37.Murphy D, McCulloch CE, Lin F, Banerjee T, Bragg-Gresham JL, Eberhardt MS, et al. Trends in prevalence of chronic kidney disease in the united states. Ann Intern Med. 2016. Oct 4;165(7):473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaker M, Oppenheimer J, Wallace D, Lang DM, Rambasek T, Dykewicz M, et al. Optimizing Value in the Evaluation of Chronic Spontaneous Urticaria: A Cost-Effectiveness Analysis. J Allergy Clin Immunol Pract. 2020;8(7):2360–2369.e1. [DOI] [PubMed] [Google Scholar]
- 39.American Academy of Allergy, Asthma & Immunology | Choosing Wisely [Internet]. [cited 2021 Aug 10]. Available from: https://www.choosingwisely.org/societies/american-academy-of-allergy-asthma-immunology/
- 40.Vessal G, Sagheb MM, Shilian S, Jafari P, Samani SM. Effect of oral cromolyn sodium on CKD-associated pruritus and serum tryptase level: a double-blind placebo-controlled study. Nephrol Dial Transplant. 2010. May;25(5):1541–7. [DOI] [PubMed] [Google Scholar]
- 41.Šelb J, Rijavec M, Eržen R, Zidarn M, Kopač P, Škerget M, et al. Routine KIT p.D816V screening identifies clonal mast cell disease in Hymenoptera allergic patients regularly missed using baseline tryptase levels alone. J Allergy Clin Immunol. 2021. Mar 19; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Biagtan MJ, Viswanathan RK, Evans MD, Mathur SK. Clinical utility of the Chronic Urticaria Index. J Allergy Clin Immunol. 2011. Jun;127(6):1626–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lyons JJ, Sun G, Stone KD, Nelson C, Wisch L, O’Brien M, et al. Mendelian inheritance of elevated serum tryptase associated with atopy and connective tissue abnormalities. J Allergy Clin Immunol. 2014. May;133(5):1471–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chirumbolo S, Brizzi M, Ortolani R, Vella A, Bellavite P. Inhibition of CD203c membrane up-regulation in human basophils by high dilutions of histamine: a controlled replication study. Inflamm Res. 2009. Nov;58(11):755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maun HR, Jackman JK, Choy DF, Loyet KM, Staton TL, Jia G, et al. An Allosteric Anti-tryptase Antibody for the Treatment of Mast Cell-Mediated Severe Asthma. Cell. 2019. Oct 3;179(2):417–431.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Constantine GM, Ware J, Brown T, Thumm L, Kamal N, Kumar S, et al. Platelet-derived growth factor receptor-alpha-positive myeloid neoplasm presenting as eosinophilic gastrointestinal disease. J Allergy Clin Immunol Pract. 2020. Jun;8(6):2089–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuang FL, Curtin BF, Alao H, Piligian B, Berry A, Holland-Thomas N, et al. Single-Organ and Multisystem Hypereosinophilic Syndrome Patients with Gastrointestinal Manifestations Share Common Characteristics. J Allergy Clin Immunol Pract. 2020. Sep;8(8):2718–2726.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boggs NA, Rao VK. The role of bone marrow evaluation in clinical allergy and immunology practice: when and why. J Allergy Clin Immunol Pract. 2020. Jun 9;8(10):3356–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weiler CR, Austen KF, Akin C, Barkoff MS, Bernstein JA, Bonadonna P, et al. AAAAI Mast Cell Disorders Committee Work Group Report: Mast cell activation syndrome (MCAS) diagnosis and management. J Allergy Clin Immunol. 2019. Oct;144(4):883–96. [DOI] [PubMed] [Google Scholar]
- 50.Valent P, Bonadonna P, Hartmann K, Broesby-Olsen S, Brockow K, Butterfield JH, et al. Why the 20% + 2 tryptase formula is a diagnostic gold standard for severe systemic mast cell activation and mast cell activation syndrome. Int Arch Allergy Immunol. 2019. Jun 28;180(1):44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Belhocine W, Ibrahim Z, Grandné V, Buffat C, Robert P, Gras D, et al. Total serum tryptase levels are higher in young infants. Pediatr Allergy Immunol. 2011. Sep;22(6):600–7. [DOI] [PubMed] [Google Scholar]
- 52.Lyons JJ. Hereditary alpha tryptasemia: genotyping and associated clinical features. Immunol Allergy Clin North Am. 2018. Aug;38(3):483–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.